Performance of Continuous Digital Monitoring of Vital Signs with a Wearable Sensor in Acute Hospital Settings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Ethics
2.3. Recruitment and Eligibility
- Hospital Stay Requirement—Patients admitted to a general medical or surgical ward and expected to remain in the hospital for at least 24 h were eligible for recruitment.
- Consultant Identification Process—Each morning, the research team approached the admitting consultants in both medicine and surgery after their ward rounds to obtain a list of eligible patients. Surgical ward rounds typically concluded around 10:00 AM, while the medical board round took place later in the day, around 12:00 PM.
- Age Criteria—Eligible participants were aged 18 years or older, with an upper age limit of 95 years.
- Informed Consent—All eligible patients received an information sheet outlining the sensor technology and study details. Written informed consent was mandatory before participation, ensuring that all patients understood the study requirements. The patient consent form was completed before recruitment.
- Exclusion Criteria—Patients who were unable to provide informed consent or who later withdrew their consent were excluded from this study.
2.4. Sensium Vital Sign Sensor
2.5. Sensor Data Collection and Algorithm
2.5.1. Heart Rate (HR)
2.5.2. Respiratory Rate (RR)
2.5.3. Temperature
2.6. Data Transmission
2.7. Current Ward Monitoring
2.8. Comparison of Sensor with Standard Practice
2.9. Statistical Analysis
- 0.00–0.39: poor to fair agreement;
- 0.40–0.59: moderate agreement;
- 0.60–0.74: good agreement;
- 0.75–1.00: excellent agreement;
3. Results
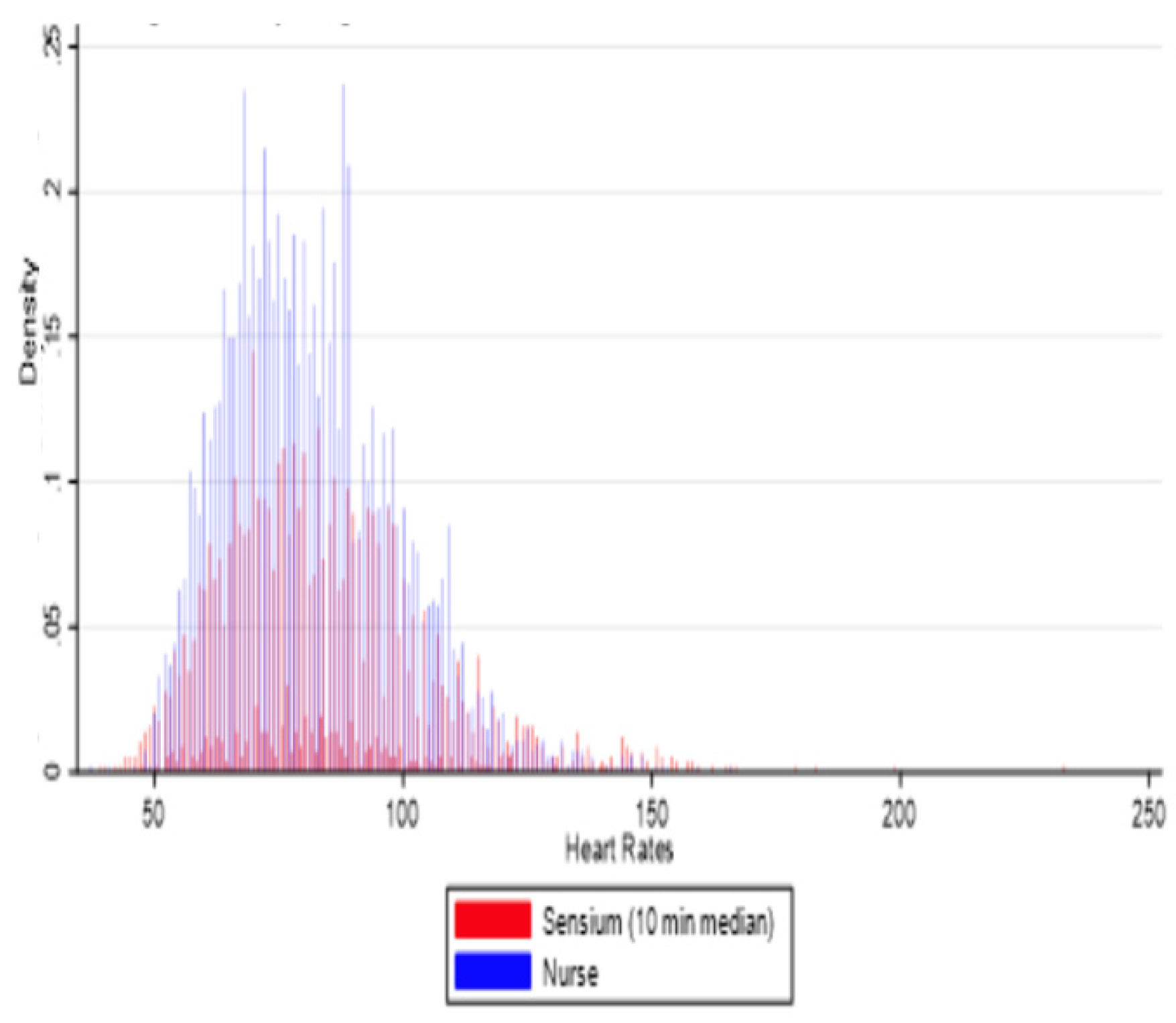
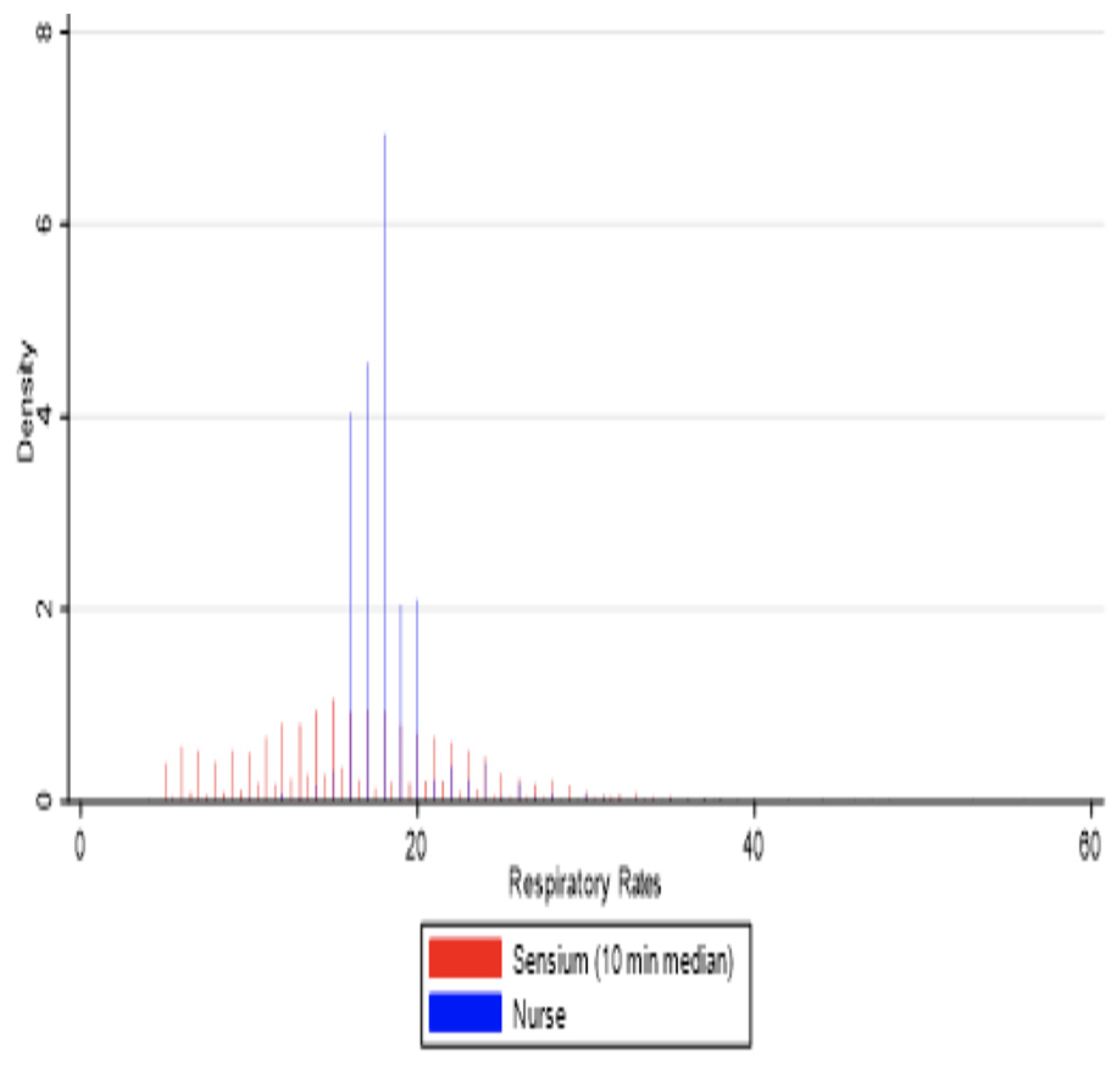
3.1. Reliability, Data Points, and Time Windows
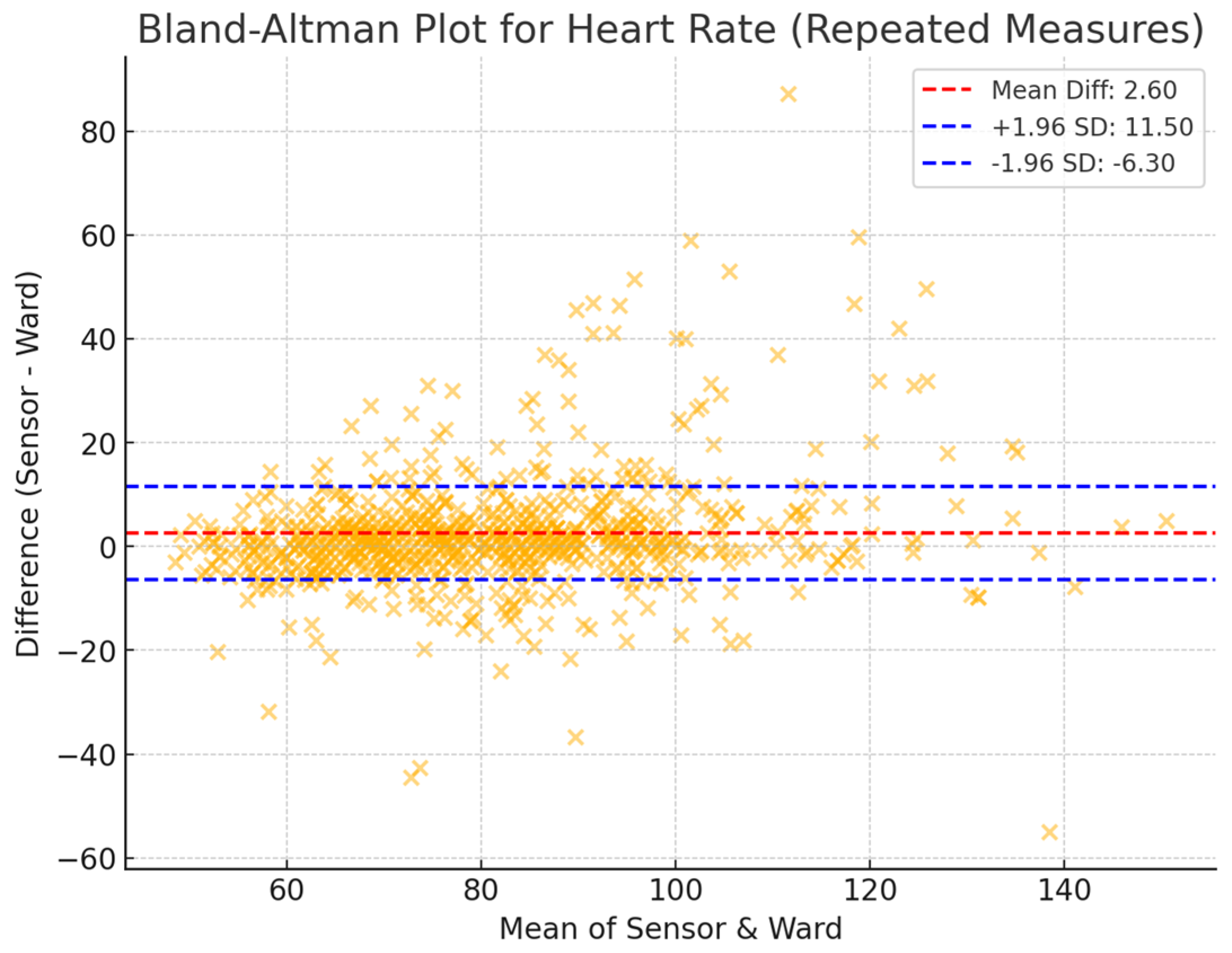
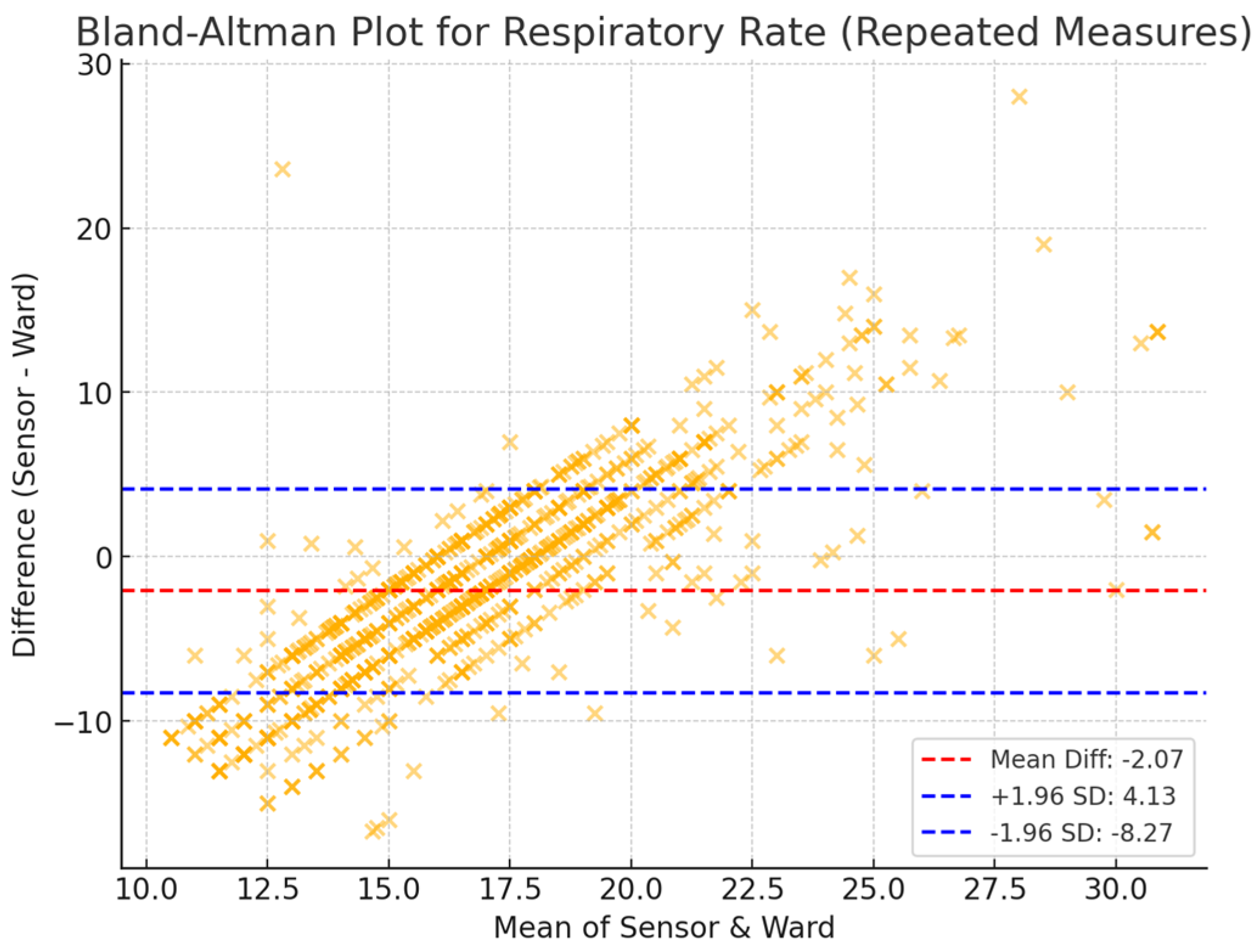
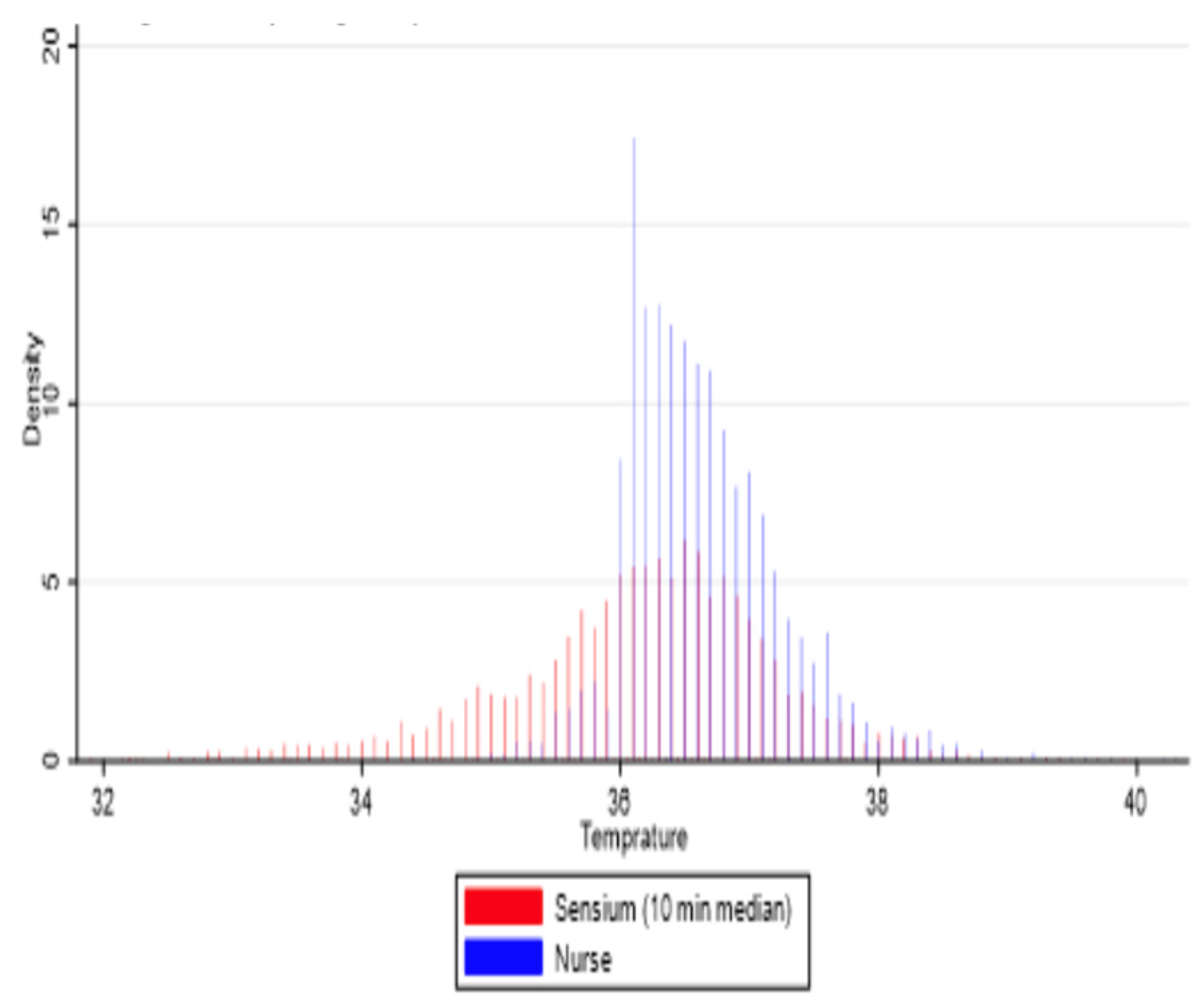
3.2. Agreement Between Sensor-Derived and Ward-Based Measurements
3.3. Reliability and Validity of Sensor-Derived Measurements
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RR | Respiratory rate |
| HR | Heart rate |
| NICE | National Institute for Health and Clinical Excellence |
| ICU | Intensive care unit |
References
- National Confidential Enquiry into Patient Outcomes and Death. An Acute Problem? National Confidential Enquiry into Patient Outcome and Death: London, UK, 2005. [Google Scholar]
- National Patient Safety Agency. Safer Care for the Acutely Ill Patient: Learning from Serious Incidents; The Fifth Report from the Patient Safety Observatory; National Patient Safety Agency: London, UK, 2007; pp. 1–48.
- National Patient Safety Agency. Recognising and Responding Appropriately to Early Signs of Deterioration in Hospitalised Patients; NPSA: London, UK, 2007; pp. 1–39.
- Acutely Ill Patients in Hospital: Recognition of and Response to Acute Illness in Adults in Hospital; NICE Clinical Guideline 50; National Institute for Health and Care Excellence (NICE): London, UK, 2007.
- Findlay, G.P.; Shotton, H.; Kelly, K.; Mason, M. Time to Intervene? 2012, pp. 1–125. Available online: www.ncepod.org.uk (accessed on 10 April 2025).
- Smith, G.B. In-hospital cardiac arrest: Is it time for an in-hospital ‘chain of prevention’? Resuscitation 2010, 81, 1209–1211. [Google Scholar] [CrossRef]
- DeVita, M.A.; Smith, G.B.; Adam, S.K.; Adams-Pizarro, I.; Buist, M.; Bellomo, R.; Bonello, R.; Cerchiari, E.; Farlow, B.; Goldsmith, D.; et al. “Identifying the hospitalised patient in crisis”—A consensus conference on the afferent limb of Rapid Response Systems. Resuscitation 2010, 81, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Sax, F.L.; Charlson, M.E. Medical patients at high risk for catastrophic deterioration. Crit. Care Med. 1987, 15, 510–515. [Google Scholar] [CrossRef]
- Smith, A.F.; Wood, J. Can some in-hospital cardio-respiratory arrests be prevented? A prospective survey. Resuscitation 1998, 37, 133–137. [Google Scholar] [CrossRef]
- Kause, J.; Smith, G.; Prytherch, D.; Parr, M.; Flabouris, A.; Hillman, K. A comparison of Antecedents to Cardiac Arrests, Deaths and EMergency Intensive care Admissions in Australia and New Zealand, and the United Kingdom—The ACADEMIA study. Resuscitation 2004, 62, 275–282. [Google Scholar] [CrossRef]
- McQuillan, P.; Pilkington, S.; Allan, A.; Taylor, B.; Short, A.; Morgan, G.; Nielsen, M.; Barrett, D.; Smith, G. Confidential inquiry into quality of care before admission to intensive care. BMJ 1998, 316, 1853–1858. [Google Scholar] [CrossRef] [PubMed]
- Breteler, M.J.; Huizinga, E.; van Loon, K.; Leenen, L.P.; Dohmen, D.A.; Kalkman, C.J.; Blokhuis, T.J. Reliability of wireless monitoring using a wearable patch sensor in high-risk surgical patients at a step-down unit in the Netherlands: A clinical validation study. BMJ Open 2018, 8, e020162. [Google Scholar] [CrossRef] [PubMed]
- NICE Clinical Guidelines. In Acutely Ill Adults in Hospital: Recognising and Responding to Deterioration; National Institute for Health and Care Excellence (NICE): London, UK, 2007.
- Evans, D.; Hodgkinson, B.; Berry, J. Vital signs in hospital patients: A systematic review. Int. J. Nurs. Stud. 2001, 38, 643–650. [Google Scholar] [CrossRef]
- Marra, C.; Chen, J.L.; Coravos, A.; Stern, A.D. Quantifying the use of connected digital products in clinical research. Npj Digit. Med. 2020, 3, 50. Available online: https://www.nature.com/articles/s41746-020-0259-x (accessed on 25 February 2024). [CrossRef]
- Masanneck, L.; Gieseler, P.; Gordon, W.J.; Meuth, S.G.; Stern, A.D. Evidence from ClinicalTrials.gov on the growth of Digital Health Technologies in neurology trials. Npj Digit. Med. 2023, 6, 23. Available online: https://www.nature.com/articles/s41746-023-00767-1 (accessed on 25 February 2024). [CrossRef]
- Joshi, M.; Archer, S.; Morbi, A.; Ashrafian, H.; Arora, S.; Khan, S.; Cooke, G.; Darzi, A. Perceptions on the Use of Wearable Sensors and Continuous Monitoring in Surgical Patients: Interview Study Among Surgical Staff. JMIR Form. Res. 2022, 6, e27866. Available online: https://formative.jmir.org/2022/2/e27866 (accessed on 10 April 2022). [CrossRef] [PubMed]
- Joshi, M.; Archer, S.; Morbi, A.; Arora, S.; Kwasnicki, R.; Ashrafian, H.; Khan, S.; Cooke, G.; Darzi, A. Short-Term Wearable Sensors for In-Hospital Medical and Surgical Patients: Mixed Methods Analysis of Patient Perspectives. JMIR Perioper. Med. 2021, 4, e18836. Available online: http://www.ncbi.nlm.nih.gov/pubmed/33885367 (accessed on 25 March 2023). [CrossRef]
- Joshi, M.; Ashrafian, H.; Aufegger, L.; Khan, S.; Arora, S.; Cooke, G.; Darzi, A. Wearable sensors to improve detection of patient deterioration. Expert Rev. Med. Devices 2019, 16, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Nicolò, A.; Massaroni, C.; Schena, E.; Sacchetti, M. The Importance of Respiratory Rate Monitoring: From Healthcare to Sport and Exercise. Sensors 2020, 20, 6396. Available online: http://www.ncbi.nlm.nih.gov/pubmed/33182463 (accessed on 26 February 2024). [CrossRef]
- Weenk, M.; van Goor, H.; Frietman, B.; Engelen, L.J.; van Laarhoven, C.J.; Smit, J.; Bredie, S.J.; van de Belt, T.H. Continuous Monitoring of Vital Signs Using Wearable Devices on the General Ward: Pilot Study. JMIR Mhealth Uhealth 2017, 5, e91. [Google Scholar] [CrossRef]
- Early Detection of Patient Deterioration. Sensium. Available online: https://www.sensium.co.uk/ (accessed on 27 April 2017).
- Sensium®-Connected Care. Available online: https://www.tsc-cc.com/en/solutions/ (accessed on 10 April 2025).
- Hamilton, P.S.; Tompkins, W.J. Quantitative investigation of QRS detection rules using the MIT/BIH arrhythmia database. IEEE Trans. Biomed. Eng. 1986, 33, 1157–1165. Available online: http://www.ncbi.nlm.nih.gov/pubmed/3817849 (accessed on 1 April 2024). [CrossRef] [PubMed]
- Hernandez-Silveira, M.; Ang, S.S.; Burdett, A. Advances in Ultra-Low-Power Miniaturized Applications for Health Care and Sports. In Novel Advances in Microsystems Technologies and Their Applications; CRC Press: Boca Raton, FL, USA, 2017; pp. 463–495. Available online: https://www.taylorfrancis.com/books/9781466560673/chapters/10.1201/b15283-20 (accessed on 1 April 2024).
- Hernandez-Silveira, M.; Ahmed, K.; Ang, S.-S.; Zandari, F.; Mehta, T.; Weir, R.; Burdett, A.; Toumazou, C.; Brett, S.J. Assessment of the feasibility of an ultra-low power, wireless digital patch for the continuous ambulatory monitoring of vital signs. BMJ Open 2015, 5, e006606. [Google Scholar] [CrossRef]
- Braun ThermoScan PRO 6000. Available online: https://www.hillrom.com/en/products/braun-thermoscan-pro-6000/ (accessed on 10 April 2025).
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef]
- Chan, A.M.; Selvaraj, N.; Ferdosi, N.; Narasimhan, R. Wireless patch sensor for remote monitoring of heart rate, respiration, activity, and falls. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS, Osaka, Japan, 3–7 July 2013; pp. 6115–6118. [Google Scholar]
- Chan, A.M.; Ferdosi, N.; Narasimhan, R. Ambulatory Respiratory Rate Detection using ECG and a Triaxial Accelerometer. In Proceedings of the 35th Annual International Conference of the IEEE EMBS, Osaka, Japan, 3–7 July 2013; pp. 4058–4061. [Google Scholar]
- Cretikos, M.A.; Bellomo, R.; Hillman, K.; Chen, J.; Finfer, S.; Flabouris, A. Respiratory rate: The neglected vital sign. Med. J. Aust. 2008, 188, 657–659. [Google Scholar] [CrossRef]
- Badawy, J.; Nguyen, O.K.; Clark, C.; Halm, E.A.; Makam, A.N. Is everyone really breathing 20 times a minute? Assessing epidemiology and variation in recorded respiratory rate in hospitalised adults. BMJ Qual. Saf. 2017, 26, 832–836. Available online: http://www.ncbi.nlm.nih.gov/pubmed/28652259 (accessed on 26 February 2024). [CrossRef]
- Tomlinson, H.R.; Pimentel, M.A.F.; Gerry, S.; Clifton, D.A.; Tarassenko, L.; Watkinson, P.J. Smoothing Effect in Vital Sign Recordings. Anesth. Analg. 2018, 127, 960–966. [Google Scholar] [CrossRef] [PubMed]
- Sapo, M.; Wu, S.; Asgari, S.; McNair, N.; Buxey, F.; Martin, N.; Hu, X. A comparison of vital signs charted by nurses with automated acquired values using waveform quality indices. J. Clin. Monit. Comput. 2009, 23, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Breteler, M.J.; KleinJan, E.J.; Dohmen, D.A.; Leenen, L.P.; van Hillegersberg, R.; Ruurda, J.P.; van Loon, K.; Blokhuis, T.J.; Kalkman, C.J. Vital Signs Monitoring with Wearable Sensors in High-risk Surgical Patients: A Clinical Validation Study. Anesthesiology 2020, 132, 424–439. [Google Scholar] [CrossRef] [PubMed]


| Time Window (mins) | HR Mean Difference (LoA), bpm | RR Mean Difference (LoA), Breaths per Min | Temp Mean Difference (LoA), Degrees Celsius | HR Agreement | RR Agreement | Temp Agreement | HR Matched Pairs, n | RR Matched Pairs, n | Temp Matched Pairs, n |
|---|---|---|---|---|---|---|---|---|---|
| 6 | 3.55 (+/− 5.11) | −2.03 (−14.95, 10.89) | −0.51 (−2.95, 1.92) | ICC = −0.041 | ICC = 0.069 | Spearman = 0.013, p = 0.701 | 960 (322 patients) | 758 (288 patients) | 877 (307 patients) |
| 10 | 3.63 (−10.87, 18.14) | −2.72 (−10.91, 5.47) | −0.57 (−1.72, 0.58) | ICC = 0.66 | ICC = 0.20 | ICC = 0.30 | 1171 (341 patients) | 1046 (330 patients) | 1083 (335 patients) |
| 12 | 3.20 (−35.86, 42.26) | −2.19 (−14.57, 10.18) | −0.56 (−2.75, 1.63) | ICC = 0.41 | ICC = 0.14 | ICC = 0.23 | 1172 (342 patients) | 1083 (337 patients) | 1095 (335 patients) |
| 16 | 3.40 (−44.28, 51.07) | −2.01 (−14.54, 10.52) | −0.53 (−2.96, 1.90) | Spearman = 0.11 | Spearman = 0.0006 | Spearman = 0.028 | 1181 (336 patients) | 1136 (338 patients) | 1108 (335 patients) |
| 18 | 3.40 (−46.02, 52.82) | −1.97 (−14.39, 10.44) | −0.50 (−2.88, 1.88) | ICC = 0.256 | ICC = −0.021 | ICC = 0.074 | 2265 (355 patients) | 2163 (354 patients) | 2156 (349 patients) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joshi, M.; Iqbal, F.M.; Sharabiani, M.; Ashrafian, H.; Arora, S.; McAndrew, K.; Khan, S.; Cooke, G.; Darzi, A. Performance of Continuous Digital Monitoring of Vital Signs with a Wearable Sensor in Acute Hospital Settings. Sensors 2025, 25, 2644. https://doi.org/10.3390/s25092644
Joshi M, Iqbal FM, Sharabiani M, Ashrafian H, Arora S, McAndrew K, Khan S, Cooke G, Darzi A. Performance of Continuous Digital Monitoring of Vital Signs with a Wearable Sensor in Acute Hospital Settings. Sensors. 2025; 25(9):2644. https://doi.org/10.3390/s25092644
Chicago/Turabian StyleJoshi, Meera, Fahad M. Iqbal, Mansour Sharabiani, Hutan Ashrafian, Sonal Arora, Kenny McAndrew, Sadia Khan, Graham Cooke, and Ara Darzi. 2025. "Performance of Continuous Digital Monitoring of Vital Signs with a Wearable Sensor in Acute Hospital Settings" Sensors 25, no. 9: 2644. https://doi.org/10.3390/s25092644
APA StyleJoshi, M., Iqbal, F. M., Sharabiani, M., Ashrafian, H., Arora, S., McAndrew, K., Khan, S., Cooke, G., & Darzi, A. (2025). Performance of Continuous Digital Monitoring of Vital Signs with a Wearable Sensor in Acute Hospital Settings. Sensors, 25(9), 2644. https://doi.org/10.3390/s25092644






