The Impact of Normalization Procedures on Surface Electromyography (sEMG) Data Integrity: A Study of Bicep and Tricep Muscle Signal Analysis
Abstract
:1. Introduction
| Normalization Technique | Description | Challenges |
|---|---|---|
| MVC | Involves recording the EMG signal during a maximum voluntary isometric contraction and using this value as a reference. | Requires maximum effort and may not be feasible for individuals with motor impairments or pain [13,32]. |
| Peak | Normalizes the EMG signal to the peak electrical activity observed during a specific task. | Task-specific and may not be suitable for comparing muscle activation across different tasks [33]. |
| SMVC | Normalizes the EMG signal to a submaximal reference value, such as 60% of the maximum voluntary contraction. | May not account for inter-individual variability in muscle activation levels [32]. |
| Dynamic | Uses a reference value derived from the task itself, such as the first repetition of a set or the peak activation during a movement. | Provides more reliable results in task-specific applications [30,31]. |
2. Materials and Methods
- Exercise 1 (MVC): Maximal voluntary contraction (MVC) was assessed using isometric contractions of the biceps and triceps. Participants maintained their elbow at a 90-degree angle with their upper arms stationary and aligned naturally. Each contraction was held for 5 s and repeated three times, with 2 min of rest between repetitions. This exercise required 100% of the muscle’s maximal force, and the protocol ensured that compensatory movements, such as bending the back or moving the shoulders, were avoided.The MVC exercises for the biceps and triceps were conducted under controlled conditions to ensure accurate and consistent data collection. For the biceps, participants maintained their arms at a 90-degree elbow flexion, with a neutral wrist position. They performed an isometric contraction by flexing their biceps against a stationary dynamometer, exerting maximal force for 5 s.For the triceps, participants extended their elbows from a similar starting position, pushing against a rigid resistance to achieve maximal force output. Proper stabilization of the shoulder joint was ensured throughout both exercises to isolate muscle activity and prevent compensatory movements. Each MVC trial was repeated three times, with a two-minute rest period between repetitions to minimize fatigue and enhance reliability. The peak sEMG signal from each trial was recorded and used as a reference for normalization.
- Exercise 2 (SMVC): Submaximal voluntary contraction (SMVC) was performed similarly to Exercise 1 but at 70% of the maximal force, measured using a dynamometer. Volunteers followed the same positioning and contraction protocol, with 5-s contractions repeated three times and 2 min of rest between repetitions. This exercise was designed to assess muscle activity under moderate force levels, which are more representative of submaximal effort in real-life tasks.
- Exercise 3 (Isokinetic): Isokinetic contractions were recorded using an isokinetic dynamometer to control the contraction speed and range of motion. Sequential contractions of the right biceps, left biceps, right triceps, and left triceps were performed. The movement was constrained to a predefined range of motion and performed at a constant angular velocity. This exercise aimed to capture muscle activation patterns during dynamic, controlled movements under standardized conditions.
- Exercise 4 (Dynamic and RVC): A combination of dynamic and remote voluntary contraction (RVC) assessments was employed. Dynamic contractions involved controlled bicep curls and tricep extensions using a 4 kg weight, with the range of motion standardized between 30 and 120 degrees of elbow flexion. Simultaneously, isometric contractions of the digitorum and carpi radialis were recorded. Each dynamic contraction was performed slowly and consistently to minimize variability, while the isometric contractions were held for 5 s. This combined approach allows for the evaluation of muscle activity in dynamic tasks while capturing neuromuscular control of remote muscle groups.
2.1. Instrumentation
2.2. Data Analysis
- Cross-Correlation Coefficient (CCC): A measure of the similarity between two signals, defined in Equation (1):
- Linear Correlation Coefficient (LCC): Evaluates the strength and direction of the linear relationship between two variables, as shown in Equation (2):
- Spearman Correlation Coefficient (SPCC): A non-parametric measure for assessing monotonic relationships between two variables, defined in Equation (3):Here, D is the difference in the ranks of corresponding variables, and N is the number of data points.
- R-squared (R2): Indicates how well the regression model explains the variability in the observed data, as defined in Equation (4):
3. Results
4. Discussion and Conclusions
4.1. Discussion of Key Findings
- 1.
- CARA and DIGI Normalizations: The CARA (remote voluntary contraction from carpi radialis) method demonstrated the most consistent results across metrics for the left biceps and the left triceps, achieving the highest CCC and SPCC values. This aligns with prior studies suggesting that CARA normalization is particularly effective for tasks involving coordinated upper-limb activities [55]. Similarly, DIGI normalization was most effective for the right biceps, indicating its potential utility in asymmetric or dynamic tasks. These methods may be advantageous in rehabilitation scenarios where maximal effort is difficult to achieve, such as for individuals with neuromuscular impairments.
- 2.
- MEAN and MVC Normalizations: The MEAN normalization method excelled in dynamic tasks, with near-perfect scores for the left biceps. This result highlights its ability to preserve the overall variance and energy of the signal, making it a reliable choice for activities involving fluctuating muscle activation. On the other hand, MVC normalization consistently yielded high correlations across all metrics, reinforcing its position as a gold standard in sEMG analysis, especially in controlled, isometric conditions [5]. However, the reliability and validity of MVC as a normalization reference can be influenced by various factors, even in healthy individuals. The force produced during an MVC can exhibit variability within the same individual across different trials due to motivation levels, perceived pain, learning effects, and variations in testing protocols [12]. Achieving a consistent and true MVC can be challenging, affecting the reliability of normalized EMG data and the detection of genuine changes in muscle activation [12]. Furthermore, the characteristics of the MVC task, such as the contraction type and joint angle, can significantly impact the recorded MVC value, potentially leading to inaccurate normalization when applied to different activities [9,10]. A notable limitation is the potential for exceeding 100.
- 3.
- Variability Across Muscles and Sides: Significant differences were observed between muscles and body sides. The triceps exhibited lower CCC and SPCC values compared to the biceps, particularly for the right side. This could reflect inherent differences in muscle architecture or activation patterns, as well as greater sensitivity to electrode placement and inter-individual variability. These findings emphasize the need to tailor normalization methods to the specific muscle group under analysis.
- 4.
- Implications for Sports and Rehabilitation: In sports performance analysis, where dynamic and repetitive movements are common, MEAN and CARA methods emerge as practical choices due to their robustness in preserving signal characteristics under varying conditions. In rehabilitation, particularly for clinical populations unable to perform maximal contractions, DIGI and CARA methods offer viable alternatives, allowing for meaningful comparisons without necessitating exhaustive effort.
4.2. Limitations and Future Directions
- Sample Size: The study was conducted on a limited number of volunteers. Expanding the sample size and including individuals from diverse populations (e.g., athletes, elderly individuals, and patients with musculoskeletal disorders) would enhance the generalizability of the findings.
- Exercise Variability: The analysis focused on specific tasks involving the biceps and triceps. Incorporating a broader range of exercises, including those targeting lower-limb muscles, would provide a more comprehensive assessment of normalization methods.
- Dynamic Noise and Artifacts: Despite filtering, residual noise, or cross-talk may have influenced the results. Future work could explore advanced signal processing techniques, such as adaptive filtering or machine learning-based noise reduction.
- Real-World Applications: Although the methods were tested under controlled conditions, further studies are needed to validate their effectiveness in real-world scenarios, such as during sports training or clinical assessments.
- Limitations of MVC: As discussed in the previous section, the reliability of MVC can be compromised by factors such as participant motivation, pain, learning effects, and the specific testing conditions [9,10,12,23]. This is particularly relevant in cases of muscle fatigue [8] and neuromuscular disorders [5], where the ability to generate a true maximal contraction is often impaired. Future research should further investigate alternative normalization methods that are less reliant on maximal effort, especially for clinical populations.
- Antagonistic Muscle Co-activation: While the study design aimed to isolate agonist muscle activation during specific exercises, the potential influence of low-level antagonistic co-activation (biceps and triceps) on the recorded sEMG signals and normalization effectiveness was not explicitly analyzed. Future research should investigate the co-activation patterns and their impact on sEMG normalization across different contraction conditions by simultaneously recording and analyzing both agonist and antagonist muscle activity.
- Performance of Normalization Methods and Physiological Considerations: The effectiveness of different sEMG normalization methods was evaluated by comparing their statistical properties against the original, unnormalized signals across various muscles and contraction types. Table 2 provides a comprehensive summary of these comparisons, highlighting the strengths and weaknesses of each method. The Concordance Correlation Coefficient (CCC), which assesses both the correlation and the agreement in means between the normalized and original signals, revealed that CARA (remote voluntary contraction from carpi radialis) generally provided the highest agreement, particularly for the left biceps and triceps. This suggests that normalizing to a remote, submaximal isometric contraction of a forearm muscle might better preserve the overall shape and amplitude characteristics of the sEMG signal during both isometric and dynamic tasks of the upper arm, potentially because it is less susceptible to the variability and fatigue associated with maximal voluntary efforts of the target muscles themselves [16,56,57].The Spearman’s Correlation Coefficient (SPCC) consistently indicated strong linear relationships for most normalization methods, suggesting that the scaling applied by these techniques generally preserved the monotonic relationship within the signals. Notably, SMVC (submaximal voluntary contraction) and ISOK (isokinetic) often showed perfect correlations, implying a highly consistent scaling relative to the original signal’s trends [54,58]. Similarly, MEAN normalization, which scales the signal by its own average, also demonstrated near-perfect correlations, particularly for the left biceps and triceps, indicating a strong preservation of the signal’s temporal dynamics relative to its own central tendency [31].The Coefficient of Determination (), representing the proportion of variance in the original signal explained by the normalized signal, was highest for MEAN normalization, especially for the left biceps and triceps. This indicates that scaling by the mean of the dynamic signal was most effective in capturing the overall variability inherent in the muscle activity [54]. MVC (maximal voluntary contraction) also showed good variance explanation, particularly for the triceps, suggesting that scaling to the maximal isometric capacity can effectively account for a substantial portion of the signal’s variability, especially in that muscle group [16]. The Linear Correlation Coefficient (LCC) exhibited the highest variability across methods and muscle groups, suggesting that the linear scaling factor between normalized and original signals was not consistent. This variability highlights the potential for different normalization methods to alter the absolute amplitude relationships within and between signals. Considering the potential physiological rationales, the consistent performance of CARA might be attributed to its provision of a stable, submaximal reference that is less influenced by the task-specific recruitment patterns and fatigue associated with maximal efforts of the biceps and triceps. The strong linear relationships observed with SMVC and ISOK suggest that scaling by a consistent submaximal or controlled dynamic reference can effectively preserve the overall signal trends. The high variance explained by MEAN normalization is likely due to its inherent adaptation to the specific characteristics of the dynamic signal itself. Conversely, the variability in LCC underscores that different normalization strategies can lead to different interpretations of the absolute levels of muscle activation [57,59]. Ultimately, the choice of normalization method should be carefully considered based on the specific research question and the characteristics of the muscles and tasks being investigated.
- Reliability and reproducibility of neuromuscular measurements: The reliability and reproducibility of neuromuscular measurements are critical considerations when evaluating muscle performance. While our study focuses on the effects of different normalization methods on EMG data, it is important to acknowledge that the reliability and reproducibility of the underlying muscle performance measurements themselves (upon which normalization is based) also play a significant role. Table 1 provides a summary of some of the challenges associated with these measurements. Maximal voluntary contraction (MVC) normalization, a widely used technique, relies on a measurement that is inherently susceptible to variability. As noted in Table 1 and supported by previous research [13,32], MVC requires maximal effort, which can be influenced by a range of factors, including subject motivation, pain, fatigue, and learning effects. These factors can compromise the reproducibility of MVC measurements, potentially leading to inconsistencies in normalized EMG data. Submaximal voluntary contraction (SMVC) normalization, while avoiding the need for maximal exertion, introduces its own challenges to reproducibility. The consistency of submaximal effort can be difficult to control, both within and between individuals, leading to potential variability in the normalized EMG signals. Factors such as changes in attention or perceived exertion can affect the reproducibility of SMVC-based normalization. Isokinetic testing offers a controlled dynamic measurement, aiming to improve reliability compared to less constrained movements. However, as highlighted in Table 1, isokinetic measurements are task-specific. The results obtained during a particular isokinetic test (e.g., at a specific angular velocity) might not be fully reproducible or generalizable to other dynamic tasks with different movement patterns or speeds. The influence of factors such as muscle fatigue and the specificity of the testing device can also affect reproducibility. In contrast, remote voluntary contraction (RVC), specifically the CARPI method used in our study, seeks to provide a more stable reference by normalizing to a contraction of a muscle that is not the primary mover in the task of interest. This approach aims to minimize the impact of fatigue and task-specific factors that can affect the reproducibility of MVC or dynamic measurements. However, the reproducibility of RVC measurements can still be influenced by factors such as electrode placement and individual variations in muscle activation patterns. In conclusion, each muscle performance measurement method (MVC, SMVC, Isokinetic, and RVC) has inherent limitations that can affect its reliability and reproducibility. A comprehensive evaluation of these factors requires careful consideration of the specific research context and the implementation of standardized measurement protocols.
| Normalization Method | Concordance (CCC) | Linear Relationship (SPCC) | Variance Explained () | Linear Correlation (LCC) | Overall Performance Summary | Potential Physiological Rationale for Performance |
|---|---|---|---|---|---|---|
| MVC | Moderate agreement, high variability in triceps. | Strong linear relationship across muscles. | Good variance explanation, especially for triceps. | High variability, particularly in triceps. | Generally good linear relationship and variance explanation, but agreement (CCC) can be moderate, especially in the triceps. High LCC variability. | May perform well when dynamic recruitment closely scales with maximal isometric capacity. Variability might arise if dynamic tasks involve different synergistic contributions or fatigue patterns not reflected in the MVC. |
| SMVC | Low to moderate agreement, best for right biceps. | Perfect correlation in biceps (right) and triceps. | Perfect variance explanation in triceps, good in biceps. | High LCC in right biceps, variable in triceps. | Strong linear relationship and variance explanation, but agreement (CCC) can be low for some muscles. | Using a submaximal isometric reference might be more reliable if maximal effort is inconsistent or if dynamic tasks are performed at submaximal levels. However, it might not capture the full range of motor unit recruitment during high-intensity dynamic actions. |
| ISOK | Low to moderate agreement, better for right biceps. | Perfect correlation in biceps (right) and triceps. | Perfect variance explanation in triceps, good in biceps. | High LCC in right biceps, variable in triceps. | Similar to SMVC in terms of strong linear relationship and variance explanation, but agreement (CCC) can be low for some muscles. | Normalizing to an isokinetic contraction (dynamic but at constant velocity) might better reflect recruitment during controlled dynamic movements. However, the specific velocity and contraction type of the isokinetic reference might not generalize to other dynamic tasks (like RVC). |
| CARA | Best overall agreement (CCC), especially left. | High linear relationship, consistent in both muscles. | Good variance explanation, robust across muscles. | Moderate variability, relatively consistent. | Most consistent method for signal agreement (CCC), good linear relationship and variance explanation across both biceps and triceps. | A remote, low-intensity isometric contraction might provide a more stable reference that is less influenced by the maximal effort variability and fatigue associated with MVC, potentially leading to better agreement with the shape of dynamic signals, even if the overall activation levels differ. |
| DIGI | Moderate agreement, best for right biceps. | High linear relationship, particularly for triceps. | Good variance explanation for biceps. | Moderate variability. | Good agreement for the right biceps, strong linear relationship, and good variance explanation for the biceps. | Similar rationale to CARA. The choice of a different remote muscle might capture some individual differences in neural drive or overall activation strategies that correlate better with specific dynamic tasks in certain individuals or limbs. |
| MEAN | Low to moderate agreement. | Near-perfect to perfect correlation, especially left biceps and triceps. | Highest variance explanation for left biceps and triceps. | High LCC for left biceps, variable elsewhere. | Strongest linear relationship and variance explanation, particularly for the left side. Agreement (CCC) can be lower compared to CARA. | Normalizing to the mean of the dynamic signal itself inherently captures the characteristics of the dynamic recruitment pattern. The high correlation and variance explained suggest it scales well with the overall activation changes, but it does not reference an external physiological maximum. |
| PEAK | Low agreement, especially left side. | High linear relationship, good for triceps. | Good variance explanation for triceps. | Moderate variability. | Generally good linear relationship and variance explanation for the triceps, but agreement (CCC) is often low, especially on the left side. | Normalizing to the peak of the dynamic signal focuses on the maximal activation achieved during that specific movement. This might be relevant if the research question centers on peak activation levels, but it does not provide a consistent external reference across different tasks or individuals. |
4.3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cram, J.R.; Kasman, G.S.; Holtz, J. Introduction to Surface Electromyography; Aspen Publishing: Aspen, CO, USA, 1998; p. 408. [Google Scholar]
- Piervirgili, G.; Petracca, F.; Merletti, R. A new method to assess skin treatments for lowering the impedance and noise of individual gelled Ag-AgCl electrodes. Physiol. Meas. 2014, 35, 2101–2118. [Google Scholar] [CrossRef] [PubMed]
- Fox, S.R.; Gurchiek, R.D.; Ursiny, A.T.; Meyer, B.M.; Boughton, J.M.; McGinnis, R.S. Adaptive surface electromyography normalization for long-duration recordings. In Proceedings of the 2021 IEEE 17th International Conference on Wearable and Implantable Body Sensor Networks, BSN 2021, Athens, Greece, 27–30 July 2021. [Google Scholar] [CrossRef]
- Bongiorno, G.; Sisti, G.; Biancuzzi, H.; Dal Mas, F.; Minisini, F.G.; Miceli, L. Training in Roller Speed Skating: Proposal of Surface Electromyography and Kinematics Data for Educational Purposes in Junior and Senior Athletes. Sensors 2024, 24, 7617. [Google Scholar] [CrossRef] [PubMed]
- Hermens, H.J.; Roberto, B.F.; Stegeman, M.D.; Blok, J.; Rau, G.; Disselhorst-Klug, C.; Hägg, G. European Recommendations for Surface ElectroMyoGraphy: Results of the SENIAM Project. 2009. Available online: http://www.seniam.org/pdf/contents8.PDF (accessed on 15 April 2025).
- Burden, A.; Bartlett, R. Normalisation of EMG amplitude: An evaluation and comparison of old and new methods. Med. Eng. Phys. 1999, 21, 247–257. [Google Scholar] [CrossRef]
- Luca, C.J.D. The Use of Surface Electromyography. J. Appl. Biomech. 1997, 13, 135–163. [Google Scholar] [CrossRef]
- Fuglevand, A.J.; Zackowski, K.M.; Huey, K.A.; Enoka, R.M. Impairment of neuromuscular propagation during human fatiguing contractions at submaximal forces. J. Physiol. 1993, 460, 549–572. [Google Scholar] [CrossRef]
- Miaki, H.; Someya, F.; Tachino, K. A comparison of electrical activity in the triceps surae at maximum isometric contraction with the knee and ankle at various angles. Eur. J. Appl. Physiol. Occup. Physiol. 1999, 80, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Ballak, S.B.; Degens, H.; de Haan, A.; Jaspers, R.T. Aging related changes in determinants of muscle force generating capacity: A comparison of muscle aging in men and male rodents. Ageing Res. Rev. 2014, 14, 43–55. [Google Scholar] [CrossRef]
- Karinkanta, S.; Heinonen, A.; Sievänen, H.; Uusi-Rasi, K.; Fogelholm, M.; Kannus, P. Maintenance of exercise-induced benefits in physical functioning and bone among elderly women. Osteoporos. Int. 2009, 20, 665–674. [Google Scholar] [CrossRef]
- Yang, J.F.; Winter, D.A. Electromyography reliability in maximal and submaximal isometric contractions. Arch. Phys. Med. Rehabil. 1983, 64 9, 417–420. [Google Scholar]
- Lanza, M.B.; Frakes, N.; Gray, V.L. Normalization of the hip abductors rate of activation during a voluntary step task in older adults: Reliability and differences among approaches. Gait Posture 2024, 113, 330–336. [Google Scholar] [CrossRef]
- Da Silva, R.A., Jr. Normalização EMG: Considerações da literatura para avaliação da função muscular. ConScientiae Saúde 2013, 12, 470–480. [Google Scholar] [CrossRef]
- Kishimoto, K.C.; Heroux, M.E.; Gandevia, S.C.; Butler, J.E.; Diong, J. Estimation of maximal muscle electromyographic activity from the relationship between muscle activity and voluntary activation. J. Appl. Physiol. 2021, 130, 1352–1361. [Google Scholar] [CrossRef] [PubMed]
- Diong, J.; Kishimoto, K.C.; Butler, J.E.; Héroux, M.E. Muscle electromyographic activity normalized to maximal muscle activity, not to Mmax, better represents voluntary activation. PLoS ONE 2022, 17, e0277947. [Google Scholar] [CrossRef]
- Lin, X.; Hu, Y.; Sheng, Y. The Effect of Electrical Stimulation Strength Training on Lower Limb Muscle Activation Characteristics During the Jump Smash Performance in Badminton Based on the EMS and EMG Sensors. Sensors 2025, 25, 577. [Google Scholar] [CrossRef] [PubMed]
- Amarantini, D.; Bru, B. Training-related changes in the EMG-moment relationship during isometric contractions: Further evidence of improved control of muscle activation in strength-trained men? J. Electromyogr. Kinesiol. 2015, 25, 697–702. [Google Scholar] [CrossRef]
- Jeong, H.J.; Kim, B.K.; Yoo, G.S.; Kim, K.H. The effect of differences in warm-up and muscle contraction methods on muscle activity and total work during the bench press exercise in Republic of Korea: Experimental research. Med. Lasers Eng. Basic Res. Clin. Appl. 2023, 12, 108–115. [Google Scholar] [CrossRef]
- Anderson, K.G.; Behm, D.G. Maintenance of EMG Activity and Loss of Force Output With Instability. J. Strength Cond. Res. 2004, 18, 637–640. [Google Scholar]
- Farina, D.; Merletti, R.; Enoka, R.M. The extraction of neural strategies from the surface EMG: An update. J. Appl. Physiol. 2014, 117, 1215. [Google Scholar] [CrossRef]
- Duchateau, J.; Baudry, S. Insights into the neural control of eccentric contractions. J. Appl. Physiol. 2014, 116, 1418–1425. [Google Scholar] [CrossRef]
- Lehman, G.J.; McGill, S.M. The importance of normalization in the interpretation of surface electromyography: A proof of principle. J. Manip. Physiol. Ther. 1999, 22, 444–446. [Google Scholar] [CrossRef]
- Rainoldi, A.; Galardi, G.; Maderna, L.; Comi, G.; Lo Conte, L.; Merletti, R. Repeatability of surface EMG variables during voluntary isometric contractions of the biceps brachii muscle. J. Electromyogr. Kinesiol. 1999, 9, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Lanza, M.B.; Lacerda, L.T.; Gurgel Simões, M.; Martins-Costa, H.C.; Diniz, R.C.; Chagas, M.H.; Lima, F.V. Normalization of the electromyography amplitude during a multiple-set resistance training protocol: Reliability and differences between approaches. J. Electromyogr. Kinesiol. 2023, 68, 102724. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Baldon, V.S.; de Oliveira, A.B.; Padilha, J.F.; Degani, A.M.; Avila, M.A.; Driusso, P. Reliability of different electromyographic normalization methods for pelvic floor muscles assessment. Neurourol. Urodyn. 2020, 39, 1145–1151. [Google Scholar] [CrossRef]
- Zellers, J.A.; Parker, S.; Marmon, A.; Grävare Silbernagel, K. Muscle activation during maximum voluntary contraction and m-wave related in healthy but not in injured conditions: Implications when normalizing electromyography. Clin. Biomech. 2019, 69, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Hilário, B.E.; de Oliveira, M.L.; Barbosa, P.M.M.; Cunha, D.M.; dos Santos Rigobello, G.; Mendes, J.F.; Nogueira, D.A.; Iunes, D.H.; Carvalho, L.C. Analysis of the use of insoles in the dynamic stability of the lower limbs in recreational runners: An exploratory study. Gait Posture 2022, 92, 435–441. [Google Scholar] [CrossRef]
- Ekstrom, R.A.; Osborn, R.W.; Goehner, H.M.; Moen, A.C.; Ommen, B.M.; Mefferd, M.J.; Bergman, T.R.; Molencamp, T.B.; Kelsey, S.A. Electromyographic normalization procedures for determining exercise intensity of closed chain exercises for strengthening the quadriceps femoris muscles. J. Strength Cond. Res. 2012, 26, 766–771. [Google Scholar] [CrossRef]
- Suydam, S.M.; Manal, K.; Buchanan, T.S. The Advantages of Normalizing Electromyography to Ballistic Rather than Isometric or Isokinetic Tasks. J. Appl. Biomech. 2017, 33, 189–196. [Google Scholar] [CrossRef]
- Korak, J.A.; Bruininks, B.D.; Paquette, M.R. The Influence of Normalization Technique on Between-Muscle Activation during a Back-Squat. Int. J. Exerc. Sci. 2020, 13, 1098–1107. [Google Scholar] [CrossRef]
- Silva, F.P.d.; Santos, J.A.; Meireles, A. Road Accident: Driver Behaviour, Learning and Driving Task. Procedia Soc. Behav. Sci. 2014, 162, 300–309. [Google Scholar] [CrossRef]
- Zacaron, K.A.M.; Dias, J.M.D.; Alencar, M.A.; Almeida, L.L.d.; Alberto Mourão-Júnior, C.; Dias, R.C. Electromyographic normalization of vastus lateralis and biceps femoris co-contraction during gait of elderly females. Fisioterapia em Movimento 2016, 29, 787–794. [Google Scholar] [CrossRef]
- Yang, J.F.; Winter, D.A. Electromyographic amplitude normalization methods: Improving their sensitivity as diagnostic tools in gait analysis. Arch. Phys. Med. Rehabil. 1984, 65, 517–521. [Google Scholar] [PubMed]
- Wang, W.; Stefano, A.D.; Allen, R. A simulation model of the surface EMG signal for analysis of muscle activity during the gait cycle. Comput. Biol. Med. 2006, 36, 601–618. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, H.; Zhou, B.; Wei, C.; Xu, S. Continuous and simultaneous estimation of lower limb multi-joint angles from sEMG signals based on stacked convolutional and LSTM models. Expert Syst. Appl. 2022, 203, 117340. [Google Scholar] [CrossRef]
- Rose, J.; McGill, K.C. Neuromuscular activation and motor-unit firing characteristics in cerebral palsy. Dev. Med. Child Neurol. 2005, 47, 329–336. [Google Scholar] [CrossRef]
- Trinidad-Fernández, M.; González-Molina, F.; Moya-Esteban, A.; Roldán-Jiménez, C.; González-Sánchez, M.; Cuesta-Vargas, A.I. Muscle activity and architecture as a predictor of hand-grip strength. Physiol. Meas. 2020, 41, 075008. [Google Scholar] [CrossRef] [PubMed]
- Fuentes del Toro, S. Modelización Biomecánica de Accionamientos mecánicos de Resistencia Variable. Ph.D. Thesis, Universidad Carlos III de Madrid, Madrid, Spain, 2021. [Google Scholar]
- Fuentes del Toro, S.; Santos-Cuadros, S.; Olmeda, E.; San Román, J.L. Study of the Emergency Braking Test with an Autonomous Bus and the sEMG Neck Response by Means of a Low-Cost System. Micromachines 2020, 11, 931. [Google Scholar] [CrossRef]
- Luca, C.D. Electromyography. In Encyclopedia of Medical Devices and Instrumentation; Wiley: Hoboken, NJ, USA, 2006. [Google Scholar] [CrossRef]
- Navarro, X.; Krueger, T.B.; Lago, N.; Micera, S.; Stieglitz, T.; Dario, P. A critical review of interfaces with the peripheral nervous system for the control of neuroprostheses and hybrid bionic systems. J. Peripher. Nerv. Syst. JPNS 2005, 10, 229–258. [Google Scholar] [CrossRef] [PubMed]
- Clancy, E.A.; Morin, E.L.; Merletti, R. Sampling, noise-reduction and amplitude estimation issues in surface electromyography. J. Electromyogr. Kinesiol. 2002, 12, 1–16. [Google Scholar] [CrossRef]
- Tam, H.W.; Webster, J.G. Minimizing electrode motion artifact by skin abrasion. IEEE Trans. Biomed. Eng. 1977, 24, 134–139. [Google Scholar] [CrossRef]
- De Luca, C.J. Myoelectrical manifestations of localized muscular fatigue in humans. Crit. Rev. Biomed. Eng. 1984, 11, 251–279. [Google Scholar]
- Fuentes del Toro, S.; Wei, Y.; Olmeda, E.; Ren, L.; Guowu, W.; Díaz, V. Validation of a Low-Cost Electromyography (EMG) System via a Commercial and Accurate EMG Device: Pilot Study. Sensors 2019, 19, 5214. [Google Scholar] [CrossRef] [PubMed]
- Fuentes del Toro, S.; Santos-Cuadros, S.; Olmeda, E.; Álvarez-Caldas, C.; Díaz, V.; San Román, J.L. Is the Use of a Low-Cost sEMG Sensor Valid to Measure Muscle Fatigue? Sensors 2019, 19, 3204. [Google Scholar] [CrossRef]
- Wei, Y.; Wei, G.; Ren, L. Predict Afferent Tactile Neural Signal for Artificial Nerve Based on Finite Element Human Hand Model. In Proceedings of the Intelligent Robotics and Applications, Shenyang, China, 8–11 August 2019; Yu, H., Liu, J., Liu, L., Ju, Z., Liu, Y., Zhou, D., Eds.; Springer: Cham, Swizterland, 2019; pp. 129–140. [Google Scholar]
- Winter, D.A.; Fuglevand, A.J.; Archer, S.E. Crosstalk in surface electromyography Theoretical and practical estimates. J. Electromyogr. Kinesiol. 1994, 4, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Reaz, M.B.; Hussain, M.S.; Mohd-Yasin, F. Techniques of EMG signal analysis: Detection, processing, classification and applications. Biol. Proced. Online 2006, 8, 11. [Google Scholar] [CrossRef]
- Feng, D.; Baumgartner, R.; Svetnik, V. A robust bayesian estimate of the concordance correlation coefficient. J. Biopharm. Stat. 2015, 25, 490–507. [Google Scholar] [CrossRef]
- Tang, X.; Liu, Y.; Lv, C.; Sun, D. Hand Motion Classification Using a Multi-Channel Surface Electromyography Sensor. Sensors 2012, 12, 1130. [Google Scholar] [CrossRef]
- Gagnat, Y.; Brændvik, S.M.; Roeleveld, K. Surface electromyography normalization affects the interpretation of muscle activity and coactivation in children with cerebral palsy during walking. Front. Neurol. 2020, 11, 202. [Google Scholar] [CrossRef]
- Vigotsky, A.D.; Halperin, I.; Lehman, G.J.; Trajano, G.S.; Vieira, T.M. Interpreting signal amplitudes in surface electromyography studies in sport and rehabilitation sciences. Front. Physiol. 2018, 8, 311624. [Google Scholar] [CrossRef]
- Ebben, W.P.; Leigh, D.H.; Geiser, C.F. The effect of remote voluntary contractions on knee extensor torque. Med. Sci. Sport. Exerc. 2008, 40, 1805–1809. [Google Scholar] [CrossRef]
- Cho, W.; Barradas, V.R.; Schweighofer, N.; Koike, Y. Design of an Isometric End-Point Force Control Task for Electromyography Normalization and Muscle Synergy Extraction From the Upper Limb Without Maximum Voluntary Contraction. Front. Hum. Neurosci. 2022, 16, 805452. [Google Scholar] [CrossRef]
- Chalard, A.; Belle, M.; Montané, E.; Marque, P.; Amarantini, D.; Gasq, D. Impact of the EMG normalization method on muscle activation and the antagonist-agonist co-contraction index during active elbow extension: Practical implications for post-stroke subjects. J. Electromyogr. Kinesiol. 2020, 51, 102403. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, N.U.; Ahmed, N.; Alqahtani, M.; Altwijri, O.; Badlishah Ahmad, R.; Sundaraj, K. Investigation of the EMG-time relationship of the biceps Brachii muscle during contractions. J. Phys. Ther. Sci. 2015, 27, 39. [Google Scholar] [CrossRef] [PubMed]
- Tabard-Fougère, A.; Rose-Dulcina, K.; Pittet, V.; Dayer, R.; Vuillerme, N.; Armand, S. EMG normalization method based on grade 3 of manual muscle testing: Within- and between-day reliability of normalization tasks and application to gait analysis. Gait Posture 2018, 60, 6–12. [Google Scholar] [CrossRef] [PubMed]




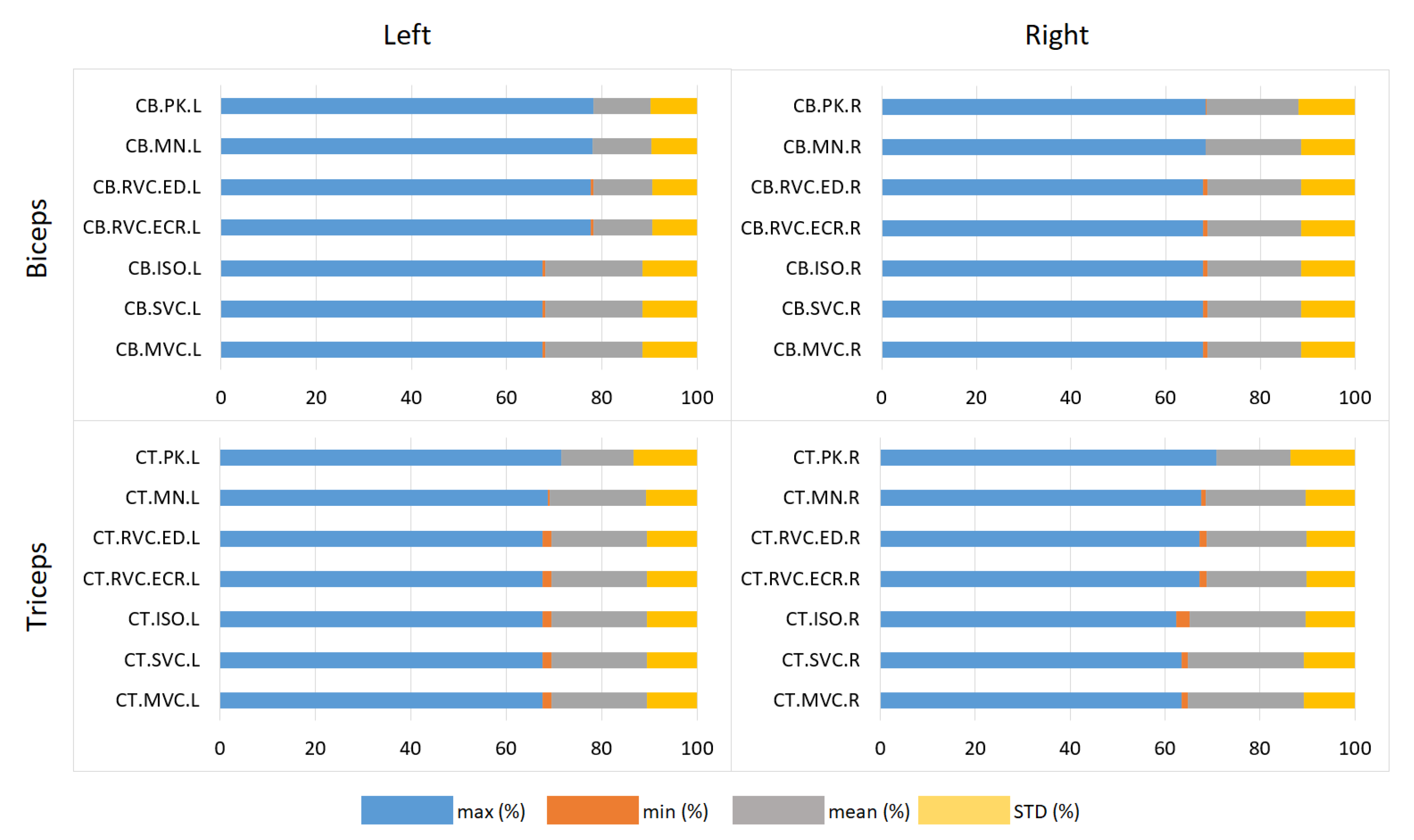
| Metric | Muscle | Right | Left | Overall | Notes |
|---|---|---|---|---|---|
| CCC | Biceps | 0.454 ± 0.402 | 0.587 ± 0.449 | 0.520 ± 0.426 | Best: CARA |
| Triceps | −0.023 ± 0.181 | 0.170 ± 0.366 | 0.074 ± 0.274 | Best: CARA | |
| SPCC | Biceps | 0.897 ± 0.026 | 0.695 ± 0.437 | 0.796 ± 0.232 | Consistent in all |
| Triceps | 0.913 ± 0.058 | 0.865 ± 0.036 | 0.889 ± 0.047 | High for CARA/DIGI | |
| Biceps | 0.793 ± 0.036 | 0.604 ± 0.401 | 0.699 ± 0.219 | Peak: MEAN | |
| Triceps | 0.846 ± 0.107 | 0.748 ± 0.085 | 0.797 ± 0.096 | Robust for all | |
| LCC | Biceps | 33.07 ± 33.42 | 39.81 ± 45.10 | 36.44 ± 39.26 | Variability high |
| Triceps | 91,860 ± 183,600 | 11.52 ± 10.43 | 45,935.76 ± 91,805 | High in MVC |
| Method | Sensitivity (Current Study) | Effectiveness (Current Study) | Comparison to Previous Studies |
|---|---|---|---|
| CARA | (left biceps) | Outperformed MVC in dynamic tasks, aligning with Zeller et al. [27]; reduced fatigue bias vs. MVC [16,54]. | |
| SMVC | (left biceps) | Matched Burden et al. (1999) findings on submaximal protocols preserving linearity; showed lower sensitivity [6]. | |
| ISOK | (right biceps) | Consistent with Burden et al. (1999) on isokinetic reliability; less effective in multi-joint tasks [6]. | |
| MVC | (left biceps) | Aligned with other authors on MVC’s variance explanation; overestimated activation in dynamic tasks [16]. | |
| MEAN | (left biceps) | Superior variance explanation () vs. MVC; similar to dynamic normalization in [31]. | |
| DIGITORUM | (right biceps) | Novel approach; no direct precedent. Highlighted stabilizer interference, as in [54]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuentes del Toro, S.; Aranda-Ruiz, J. The Impact of Normalization Procedures on Surface Electromyography (sEMG) Data Integrity: A Study of Bicep and Tricep Muscle Signal Analysis. Sensors 2025, 25, 2668. https://doi.org/10.3390/s25092668
Fuentes del Toro S, Aranda-Ruiz J. The Impact of Normalization Procedures on Surface Electromyography (sEMG) Data Integrity: A Study of Bicep and Tricep Muscle Signal Analysis. Sensors. 2025; 25(9):2668. https://doi.org/10.3390/s25092668
Chicago/Turabian StyleFuentes del Toro, Sergio, and Josue Aranda-Ruiz. 2025. "The Impact of Normalization Procedures on Surface Electromyography (sEMG) Data Integrity: A Study of Bicep and Tricep Muscle Signal Analysis" Sensors 25, no. 9: 2668. https://doi.org/10.3390/s25092668
APA StyleFuentes del Toro, S., & Aranda-Ruiz, J. (2025). The Impact of Normalization Procedures on Surface Electromyography (sEMG) Data Integrity: A Study of Bicep and Tricep Muscle Signal Analysis. Sensors, 25(9), 2668. https://doi.org/10.3390/s25092668






