Characteristics of Trunk Acceleration and Angular Velocity in Turning Movement in Post-Stroke Patients with High Risk of Falling
Abstract
Highlights
- Post-stroke patients with a high fall risk showed significantly slower trunk rotation during turning movements compared to low-risk patients and healthy controls.
- Although movement speed was controlled during turning movement, high-risk patients exhibited increased trunk disturbance in the flexion direction.
- An assessment of trunk angular velocity during turning movements can provide valuable insights for target.
- Assessment of trunk kinematics during turning movement based on fall risk could contribute to enhancing postural control.
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Clinical Assessment
2.3. Turning Movement Task
2.4. Data Analysis
2.5. Statistical Analysis
3. Results
3.1. Demographic Data and Clinical Characteristics
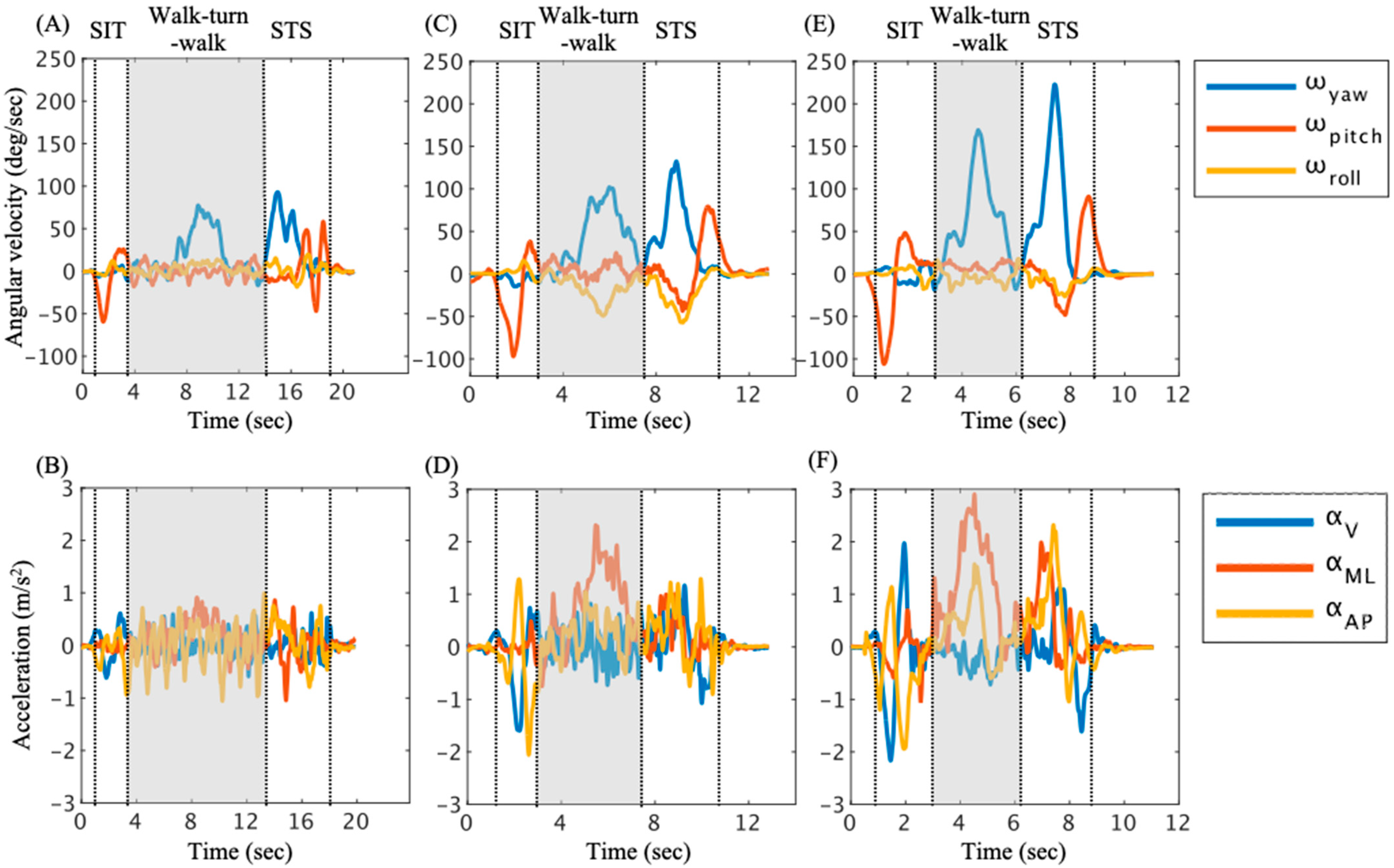
3.2. Trunk Angular Velocity and Acceleration in Walk–Turn–Walk Phase
3.3. The Relationship Between the SIAS Score and Trunk Angular Velocity and Acceleration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Walk–Turn–Walk | ICC (1, 3) | ||
|---|---|---|---|
| Maximum | Minimum | RMS | |
| ωyaw | 0.85 | 0.16 | 0.97 |
| ωpitch | 0.82 | 0.53 | 0.94 |
| ωroll | 0.73 | 0.94 | 0.97 |
| αZ | 0.78 | 0.74 | 0.62 |
| αML | 0.85 | 0.68 | 0.92 |
| αAP | 0.85 | 0.60 | 0.92 |
| High-Risk Group | Low-Risk Group | Control | Effect Size () | Power (1 − β) | |
|---|---|---|---|---|---|
| Time (s) | 2.43 (0.52) ac | 1.99 (0.24) a | 1.90 (0.36) c | 0.18 | 0.65 |
| Maximum | |||||
| ωyaw | 10.38 (6.70) b | 11.56 (6.52) c | 33.73 (22.63) bc | 0.20 | 0.66 |
| ωpitch | 31.44 (11.97) b | 38.17 (9.79) c | 51.82 (10.98) bc | 0.31 | 0.95 |
| ωroll | 11.63 (4.53) | 10.82 (5.29) | 16.00 (5.14) | ||
| αV | 1.93 (0.57) ab | 3.27 (1.26) ac | 7.09 (3.62) bc | 0.60- | 0.86 |
| αML | 1.39 (0.64) ab | 2.31 (1.12) ac | 4.97 (2.31) bc | 0.44 | 0.91 |
| αAP | 1.68 (0.81) ab | 4.03 (2.23) ac | 10.78 (7.19) bc | 0.62 | 0.76 |
| Minimum | |||||
| ωyaw | −15.12 (4.66) b | −15.60 (6.99) c | −27.28 (13.56) bc | 0.19 | 0.53 |
| ωpitch | −66.00 (15.59) a | −88.39 (12.18) ac | −100.72 (21.05) c | 0.40 | 0.95 |
| ωroll | −7.50 (1.75) b | −7.86 (3.87) | −13.91 (7.57) b | 0.18 | 0.48 |
| αV | −2.37 (1.41) ab | −3.66 (1.40) ac | −7.75 (4.51) bc | 0.45 | 0.71 |
| αML | −1.40 (0.59) b | −1.71 (0.90) c | −4.32 (2.16) bc | 0.40 | 0.86 |
| αAP | −1.43 (0.50) ab | −2.58 (0.93) ac | −6.69 (5.95) bc | 0.65 | 0.45 |
| RMS | |||||
| ωyaw | 8.15 (2.29) b | 7.95 (2.43) | 14.48 (6.32) bc | 0.23 | 0.67 |
| ωpitch | 34.58 (8.00) ab | 45.43 (5.17) a | 52.16 (10.22) b | 0.45 | 0.96 |
| ωroll | 6.20 (2.17) | 6.26 (2.92) | 9.16 (3.17) | ||
| αV | 0.78 (0.27) ab | 1.41 (0.49) ac | 2.76 (1.08) bc | 0.68 | 0.97 |
| αML | 0.48 (0.20) b | 0.69 (0.32) c | 1.45 (0.62) bc | 0.46 | 0.93 |
| αAP | 0.58 (0.18) ab | 1.20 (0.51) ac | 2.63 (1.12) bc | 0.68 | 0.97 |
| High-Risk Group | Low-Risk Group | Control | Effect Size () | Power (1 − β) | |
|---|---|---|---|---|---|
| Time (s) | 4.56 (0.91) ac | 3.38 (0.61) ab | 2.13 (0.55) bc | 0.66 | 0.99 |
| Maximum | |||||
| ωyaw | 73.49 (17.39) ab | 110.08 (28.08) ac | 193.60 (25.78) bc | 0.71 | 1.00 |
| ωpitch | 59.28 (10.95) ab | 73.08 (14.86) ac | 87.84 (15.63) bc | 0.36 | 0.97 |
| ωroll | 11.82 (7.55) | 10.29 (6.61) | 10.35 (15.10) | ||
| αV | 3.76 (1.43) | 3.79 (1.29) c | 5.17 (1.81) c | 0.12 | 0.43 |
| αML | 3.53 (1.26) b | 4.15 (1.37) | 5.88 (2.00) b | 0.18 | 0.69 |
| αAP | 4.73 (2.49) | 6.20 (1.79) | 7.66 (3.41) | ||
| Minimum | |||||
| ωyaw | −4.26 (6.51) | −9.02 (12.42) | −8.13 (7.21) | ||
| ωpitch | −28.02 (9.77) b | −36.60 (9.91) | −49.06 (11.86) b | 0.35 | 0.96 |
| ωroll | −23.12 (10.63) | −30.46 (14.02) | −48.09 (33.85) | ||
| αV | −7.07 (4.06) | −6.10 (2.68) | −6.91 (2.54) | ||
| αML | −3.08 (1.21) | −3.12 (0.78) | −3.97 (1.29) | ||
| αAP | −4.24 (2.21) | −3.56 (1.20) | −3.33 (0.82) | ||
| RMS | |||||
| ωyaw | 37.97 (11.34) ab | 54.14 (12.41) ac | 98.65 (16.03) bc | 0.63 | 1.00 |
| ωpitch | 22.78 (4.33) ab | 31.52 (6.60) ac | 44.95 (9.56) bc | 0.60 | 1.00 |
| ωroll | 11.33 (5.41) b | 17.00 (7.77) | 28.15 (17.29) b | 0.17 | 0.51 |
| αV | 1.27 (0.56) b | 1.42 (0.44) c | 2.24 (0.68) bc | 0.28 | 0.89 |
| αML | 1.03 (0.38) b | 1.20 (0.28) c | 1.69 (0.47) bc | 0.29 | 0.84 |
| αAP | 1.00 (0.33) ab | 1.35 (0.38) ac | 1.96 (0.62) bc | 0.39 | 0.90 |
References
- Abey-Nesbit, R.; Schluter, P.J.; Wilkinson, T.J.; Thwaites, J.H.; Berry, S.D.; Allore, H.; Jamieson, H.A. Risk factors for injuries in New Zealand older adults with complex needs: A national population retrospective study. BMC Geriatr. 2021, 21, 630. [Google Scholar] [CrossRef] [PubMed]
- Willig, R.; Luukinen, H.; Jalovaara, P. Factors related to occurrence of hip fracture during a fall on the hip. Public Health 2003, 117, 25–30. [Google Scholar] [CrossRef]
- Wing, J.J.; Burke, J.F.; Clarke, P.J.; Feng, C.; Skolarus, L.E. The role of the environment in falls among stroke survivors. Arch. Gerontol. Geriatr. 2017, 72, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Schmid, A.A.; Yaggi, H.K.; Burrus, N.; McClain, V.; Austin, C.; Ferguson, J.; Fragoso, C.; Sico, J.J.; Miech, E.J.; Matthias, M.S.; et al. Circumstances and consequences of falls among people with chronic stroke. J. Rehabil. Res. Dev. 2013, 50, 1277–1286. [Google Scholar] [CrossRef]
- Wang, W.; Xiao, Y.; Yue, S.; Wei, N.; Li, K. Analysis of center of mass acceleration and muscle activation in hemiplegic paralysis during quiet standing. PLoS ONE 2019, 14, e0226944. [Google Scholar] [CrossRef] [PubMed]
- Cabanas-Valdés, R.; Bagur-Calafat, C.; Girabent-Farrés, M.; Caballero-Gómez, F.M.; Hernández-Valiño, M.; Cuchí, G.U. The effect of additional core stability exercises on improving dynamic sitting balance and trunk control for subacute stroke patients: A randomized controlled trial. Clin. Rehabil. 2016, 30, 1024–1033. [Google Scholar] [CrossRef]
- Jung, K.S.; In, T.S.; Cho, H.Y. Effects of sit-to-stand training combined with transcutaneous electrical stimulation on spasticity, muscle strength and balance ability in patients with stroke: A randomized controlled study. Gait Posture 2017, 54, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Glaister, B.C.; Bernatz, G.C.; Klute, G.K.; Orendurff, M.S. Video task analysis of turning during activities of daily living. Gait Posture 2007, 25, 289–294. [Google Scholar] [CrossRef]
- Peyer, K.E.; Brassey, C.A.; Rose, K.A.; Sellers, W.I. Locomotion pattern and foot pressure adjustments during gentle turns in healthy subjects. J. Biomech. 2017, 60, 65–71. [Google Scholar] [CrossRef]
- Nolasco, L.A.; Silverman, A.K.; Gates, D.H. Whole-body and segment angular momentum during 90-degree turns. Gait Posture 2019, 70, 12–19. [Google Scholar] [CrossRef]
- Nevitt, M.C.; Cummings, S.R.; Hudes, E.S. Risk Factors for Injurious Falls: A Prospective Study. 1991. Available online: http://geronj.oxfordjournals.org/ (accessed on 14 February 2024).
- Osada, Y.; Motojima, N.; Kobayashi, Y.; Yamamoto, S. Abnormal Gait Movements Prior to a Near Fall in Individuals After Stroke. Arch. Rehabil. Res. Clin. Transl. 2021, 3, 100156. [Google Scholar] [CrossRef] [PubMed]
- Wüest, S.; Massé, F.; Aminian, K.; Gonzenbach, R.; de Bruin, E.D. Reliability and validity of the inertial sensor-based Timed ‘Up and Go’ test in individuals affected by stroke. J. Rehabil. Res. 2016, 53, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Bonnyaud, C.; Pradon, D.; Vaugier, I.; Vuillerme, N.; Bensmail, D.; Roche, N. Timed Up and Go test: Comparison of kinematics between patients with chronic stroke and healthy subjects. Gait Posture 2016, 49, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Vistamehr, A.; Kautz, S.A.; Bowden, M.G.; Neptune, R.R. Correlations between measures of dynamic balance in individuals with post-stroke hemiparesis. J. Biomech. 2016, 49, 396–400. [Google Scholar] [CrossRef]
- Nott, C.R.; Neptune, R.R.; Kautz, S.A. Relationships between frontal-plane angular momentum and clinical balance measures during post-stroke hemiparetic walking. Gait Posture 2014, 39, 129–134. [Google Scholar] [CrossRef]
- Lima, C.A.; Ricci, N.A.; Nogueira, E.C.; Perracini, M.R. The Berg Balance Scale as a clinical screening tool to predict fall risk in older adults: A systematic review. Physiotherapy 2018, 104, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Fugl-Meyer, A.R.; Jääskö, L.; Leyman, I.; Olsson, S.; Steglind, S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand. J. Rehabil. Med. 1975, 7, 13–31. [Google Scholar] [CrossRef]
- Chino, N.; Sonoda, S.; Domen, K.; Saitoh, E.; Kimura, A. Stroke Impairment Assessment Set (SIAS)-A new evaluation instrument for stroke patients. Jpn. J. Rehabil. Med. 1994, 31, 119–125. [Google Scholar] [CrossRef]
- Verheyden, G.; Nieuwboer, A.; Mertin, J.; Preger, R.; Kiekens, C.; De Weerdt, W. The Trunk Impairment Scale: A new tool to measure motor impairment of the trunk after stroke. Clin. Rehabil. 2004, 18, 326–334. [Google Scholar] [CrossRef]
- Yamada, S.; Aoyagi, Y.; Yamamoto, K.; Ishikawa, M. Quantitative evaluation of gait disturbance on an instrumented timed up-and-go test. Aging Dis. 2019, 10, 23–36. [Google Scholar] [CrossRef]
- Yamada, S.; Aoyagi, Y.; Ishikawa, M.; Yamaguchi, M.; Yamamoto, K.; Nozaki, K. Gait Assessment Using Three-Dimensional Acceleration of the Trunk in Idiopathic Normal Pressure Hydrocephalus. Front. Aging Neurosci. 2021, 13, 653964. [Google Scholar] [CrossRef] [PubMed]
- Ellis, K.; Godbole, S.; Marshall, S.; Lanckriet, G.; Staudenmayer, J.; Kerr, J. Identifying active travel behaviors in challenging environments using GPS, accelerometers, and machine learning algorithms. Front. Public Health 2014, 2, 36. [Google Scholar] [CrossRef] [PubMed]
- Cosetti, M.; Arie, L.; Kelly, J.; Ren, J.; Lubetzky, A.V. Dual task iTUG to investigate increased fall risk among older adults with bilateral hearing loss. Am. J. Otolaryngol. Head Neck Med. Surg. 2025, 46, 104536. [Google Scholar] [CrossRef]
- Neťuková, S.; Klempíř, O.; Krupička, R.; Dušek, P.; Kutílek, P.; Szabó, Z.; Růžička, E. The timed up & go test sit-to-stand transition: Which signals measured by inertial sensors are a viable route for continuous analysis? Gait Posture 2021, 84, 8–10. [Google Scholar] [CrossRef]
- van Lummel, R.C.; Walgaard, S.; Hobert, M.A.; Maetzler, W.; van Dieën, J.H.; Galindo-Garre, F.; Terwee, C.B. Intra-rater, inter-rater and test-retest reliability of an instrumented timed up and Go (iTUG) test in patients with Parkinson’s disease. PLoS ONE 2016, 11, e0151881. [Google Scholar] [CrossRef]
- Yu, L.; Zhao, Y.; Wang, H.; Sun, T.L.; Murphy, T.E.; Tsui, K.L. Assessing elderly’s functional balance and mobility via analyzing data from waist-mounted tri-axial wearable accelerometers in timed up and go tests. BMC Med. Inform. Decis. Mak. 2021, 21, 108. [Google Scholar] [CrossRef]
- Menz, H.B.; Lord, S.R.; Fitzpatrick, R.C. Acceleration Patterns of the Head and Pelvis When Walking on Level and Irregular Surfaces. Available online: www.elsevier.com/locate/gaitpost (accessed on 15 April 2025).
- Cohen, J. Quantitative methods in psychology A Power Primer. Psychol. Bull. 1992, 112, 1155–1159. [Google Scholar] [CrossRef] [PubMed]
- Schober, P.; Schwarte, L.A. Correlation coefficients: Appropriate use and interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef]
- Hollands, K.L.; Hollands, M.A.; Zietz, D.; Wing, A.M.; Wright, C.; Van Vliet, P. Kinematics of turning 180° during the timed up and go in stroke survivors with and without falls history. Neurorehabilit. Neural Repair 2010, 24, 358–367. [Google Scholar] [CrossRef]
- Roelofs, J.M.B.; Zandvliet, S.B.; Schut, I.M.; Huisinga, A.C.M.; Schouten, A.C.; Hendricks, H.T.; de Kam, D.; Aerden, L.A.M.; Bussmann, J.B.J.; Geurts, A.C.H.; et al. Mild Stroke, Serious Problems: Limitations in Balance and Gait Capacity and the Impact on Fall Rate, and Physical Activity. Neurorehabilit. Neural Repair 2023, 37, 786–798. [Google Scholar] [CrossRef]
- Barrois, R.P.-M.; Ricard, D.; Oudre, L.; Tlili, L.; Provost, C.; Vienne, A.; Vidal, P.-P.; Buffat, S.; Yelnik, A.P. Observational study of 180° turning strategies using inertial measurement units and fall risk in poststroke hemiparetic patients. Front. Neurol. 2017, 8, 194. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Suzuki, A.; Hokkirigawa, K. Required coefficient of friction in the anteroposterior and mediolateral direction during turning at different walking speeds. PLoS ONE 2017, 12, e0179817. [Google Scholar] [CrossRef] [PubMed]
- Honda, K.; Sekiguchi, Y.; Muraki, T.; Izumi, S.I. The differences in sagittal plane whole-body angular momentum during gait between patients with hemiparesis and healthy people. J. Biomech. 2019, 86, 204–209. [Google Scholar] [CrossRef]
- Negishi, T.; Ogihara, N. Regulation of whole-body angular momentum during human walking. Sci. Rep. 2023, 13, 8000. [Google Scholar] [CrossRef]
- van Mierlo, M.; Ambrosius, J.I.; Vlutters, M.; van Asseldonk, E.H.F.; van der Kooij, H. Recovery from sagittal-plane whole body angular momentum perturbations during walking. J. Biomech. 2022, 141, 111169. [Google Scholar] [CrossRef]
- Liu, J.; Liu, K.; Ge, X.; Zhou, P.; Bao, T.; Gong, W. Flexible exoskeleton-assisted training enhances lower limb motor function after stroke: A systematic review and meta-analysis. J. Neurol. 2025, 272, 274. [Google Scholar] [CrossRef]
- Yu, H.X.; Wang, Z.X.; Liu, C.B.; Dai, P.; Lan, Y.; Xu, G.Q. Effect of Cognitive Function on Balance and Posture Control after Stroke. Neural Plast. 2021, 2021, 6636999. [Google Scholar] [CrossRef] [PubMed]
- Choo, Y.J.; Chang, M.C. Effectiveness of an ankle–foot orthosis on walking in patients with stroke: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 15879. [Google Scholar] [CrossRef]
- Polese, J.C.; Teixeira-Salmela, L.F.; Nascimento, L.R.; Faria, C.D.M.; Kirkwood, R.N.; Laurentino, G.C.; Ada, L. The effects of walking sticks on gait kinematics and kinetics with chronic stroke survivors. Clin. Biomech. 2012, 27, 131–137. [Google Scholar] [CrossRef]
- Bonnyaud, C.; Roche, N.; Van Hamme, A.; Bensmail, D.; Pradon, D. Locomotor trajectories of stroke patients during oriented gait and turning. PLoS ONE 2016, 11, e0149757. [Google Scholar] [CrossRef]
- Coelho-Junior, H.J.; Rodrigues, B.; de Oliveira Gonçalves, I.; Asano, R.Y.; Uchida, M.C.; Marzetti, E. The physical capabilities underlying timed ‘Up and Go’ test are time-dependent in community-dwelling older women. Exp. Gerontol. 2018, 104, 138–146. [Google Scholar] [CrossRef] [PubMed]
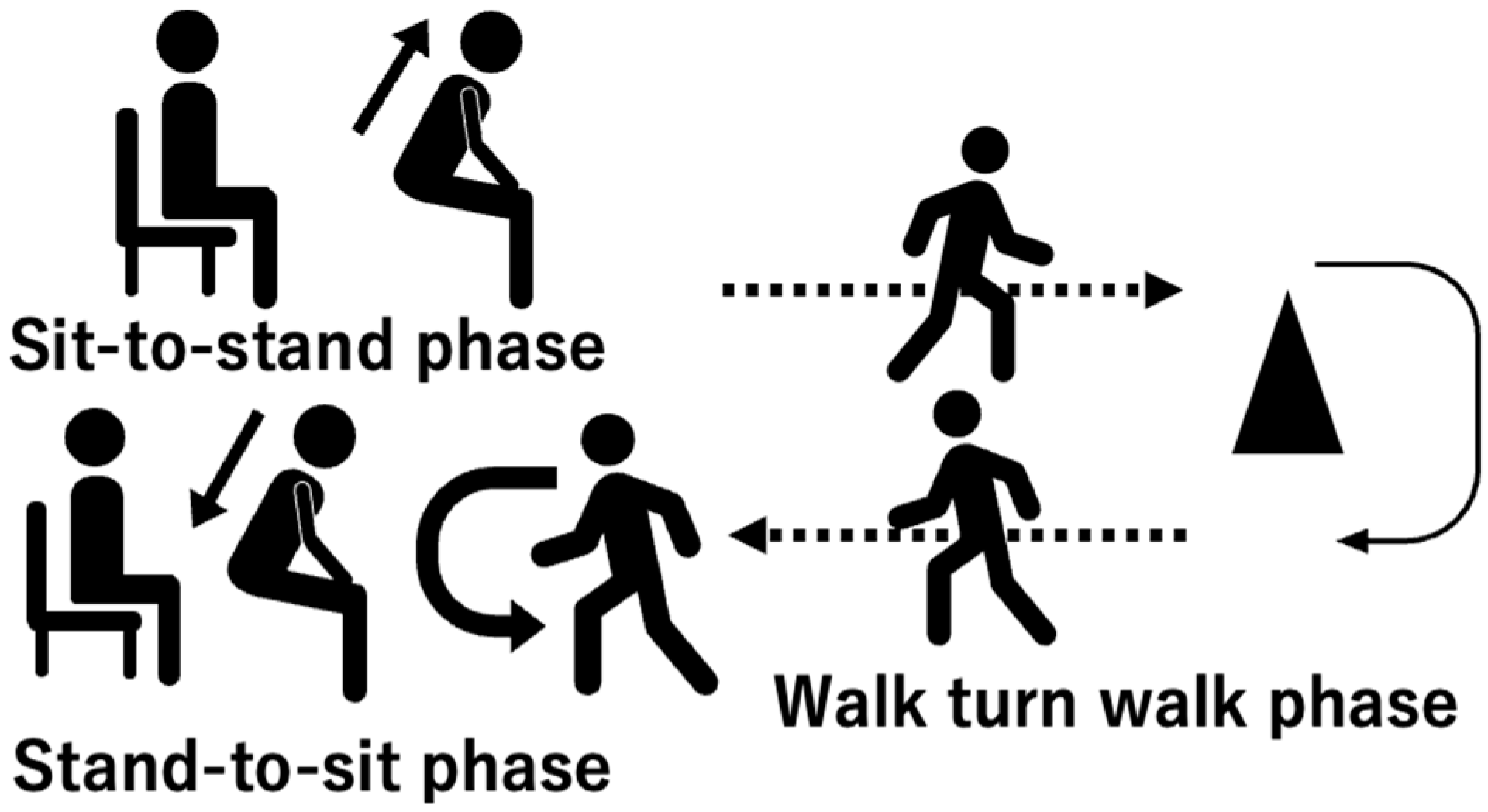
| High-Risk Group | Low-Risk Group | Control | p-Value | |
|---|---|---|---|---|
| Number | 13 | 18 | 10 | |
| Age (years) ac | 69.38 (12.44) | 71.22 (8.50) | 65.90 (11.57) | 0.67 |
| Sex (male/female) be | 7/6 | 12/6 | 6/4 | 0.76 |
| Height (m) ac | 1.57 (0.08) | 1.62 (0.01) | 1.63 (8.00) | 0.23 |
| Weight (kg) ac | 55.01 (11.46) | 51.56 (7.50) | 60.27 (9.49) | 0.11 |
| Time post-stroke (days) ad | 64.84 (36.20) | 78.61 (40.21) | - | 0.32 |
| Diagnosis (hemorrhage/infarction) be | 8/5 | 5/13 | - | 0.11 |
| Paretic side (right/left) be | 3/10 | 10/8 | - | 0.06 |
| Cane (T-cane/none) be | 11/2 | 4/14 | 0/10 | <0.001 |
| Orthosis (ankle foot orthosis/none) be | 8/5 | 1/17 | 0/10 | <0.001 |
| BBS ad | 35.30 (5.52) | 50.94 (3.47) | - | <0.001 |
| FMA-L ad | 24.15 (3.05) | 27.94 (3.05) | - | 0.006 |
| SIAS (1/2/3/4/5) be | ||||
| Hip flexion test | 0/1/2/10/0 | 0/1/0/2/15 | - | <0.001 |
| Knee extension test | 0/3/4/4/2 | 0/0/0/6/12 | - | 0.006 |
| Ankle dorsiflexion test | 2/0/4/3/4 | 0/0/1/6/11 | - | 0.04 |
| TIS ad | 13.62 (4.10) | 16.78 (3.02) | - | 0.07 |
| High-Risk Group | Low-Risk Group | Control | Effect Size () | Power (1 − β) | |
|---|---|---|---|---|---|
| Time (s) | 14.17 (4.71) ac | 6.54 (2.29) ab | 3.31 (0.97) bc | 0.77 | 0.99 |
| Maximum | |||||
| ωyaw (deg/s) | 65.60 (15.56) ab | 103.38 (26.93) ac | 174.29 (26.47) bc | 0.70 | 1.00 |
| ωpitch (deg/s) | 15.65 (4.07) b | 15.79 (3.58) | 25.28 (12.92) b | 0.13 | 0.40 |
| ωroll (deg/s) | 10.22 (3.70) | 9.16 (4.04) | 16.70 (13.95) | ||
| αV (m/s2) | 3.60 (0.85) b | 3.70 (1.00) c | 6.67 (3.29) bc | 0.32 | 0.57 |
| αML (m/s2) | 4.19 (1.25) b | 5.37 (2.09) c | 9.20 (2.29) bc | 0.43 | 1.00 |
| αAP (m/s2) | 5.12 (2.10) ab | 7.12 (1.95) ac | 11.91 (5.42) bc | 0.40 | 0.74 |
| Minimum | |||||
| ωyaw (deg/s) | −16.39 (6.37) | −14.27 (8.40) | −10.72 (12.95) | ||
| ωpitch (deg/s) | −17.65 (6.78) ab | −11.04 (4.95) a | −11.12 (10.26) b | 0.25 | 0.36 |
| ωroll (deg/s) | −12.62 (6.54) b | −16.14 (12.61) c | −38.13 (28.63) bc | 0.19 | 0.49 |
| αV (m/s2) | −5.79 (2.38) b | −6.24 (2.17) c | −10.45 (4.41) bc | 0.22 | 0.66 |
| αML (m/s2) | −3.45 (1.09) | −3.73 (0.84) | −5.27 (2.19) | ||
| αAP (m/s2) | −2.47 (0.62) ab | −3.25 (0.69) ac | −4.83 (0.98) bc | 0.57 | 1.00 |
| RMS | |||||
| ωyaw (deg/s) | 25.50 (5.02) ab | 48.00 (12.15) ac | 83.65 (14.16) bc | 0.81 | 1.00 |
| ωpitch (deg/s) | 7.02 (2.20) b | 6.51 (1.57) c | 13.26 (7.82) bc | 0.29 | 0.50 |
| ωroll (deg/s) | 5.37 (2.60) b | 8.13 (5.78) c | 20.04 (11.89) bc | 0.37 | 0.76 |
| αV (m/s2) | 1.14 (0.32) ab | 1.87 (0.80) ac | 3.21 (1.38) bc | 0.50 | 1.00 |
| αML (m/s2) | 1.07 (0.39) ab | 1.42 (0.46) ac | 2.71 (0.82) bc | 0.50 | 0.99 |
| αAP (m/s2) | 1.07 (0.27) ab | 1.68 (0.43) ac | 2.92 (0.89) bc | 0.69 | 0.99 |
| Hip Flexion Test | Knee Extension Test | Foot Pat Test | ||||
|---|---|---|---|---|---|---|
| Correlation Coefficient (r) | p-Value | Correlation Coefficient (r) | p-Value | Correlation Coefficient (r) | p-Value | |
| Maximum ωyaw | 0.591 | <0.001 * | 0.481 | 0.006 | 0.054 | 0.774 |
| Maximum αAP | 0.416 | 0.020 * | 0.220 | 0.234 | −0.095 | 0.609 |
| Minimum ωpitch | 0.520 | 0.003 * | 0.383 | 0.033 * | 0.188 | 0.312 |
| Minimum αAP | −0.511 | 0.003 * | −0.426 | 0.017 * | −0.006 | 0.975 |
| RMS ωyaw | 0.730 | <0.001 * | 0.600 | <0.001 * | 0.244 | 0.185 |
| RMS αV | 0.550 | 0.001 * | 0.337 | 0.064 | 0.007 | 0.971 |
| RMS αML | 0.522 | 0.003 * | 0.438 | 0.014 * | −0.080 | 0.670 |
| RMS αAP | 0.571 | 0.001 * | 0.464 | 0.009 * | 0.024 | 0.899 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naito, D.; Honda, K.; Sekiguchi, Y.; Izumi, S.-I.; Ebihara, S. Characteristics of Trunk Acceleration and Angular Velocity in Turning Movement in Post-Stroke Patients with High Risk of Falling. Sensors 2025, 25, 2689. https://doi.org/10.3390/s25092689
Naito D, Honda K, Sekiguchi Y, Izumi S-I, Ebihara S. Characteristics of Trunk Acceleration and Angular Velocity in Turning Movement in Post-Stroke Patients with High Risk of Falling. Sensors. 2025; 25(9):2689. https://doi.org/10.3390/s25092689
Chicago/Turabian StyleNaito, Daiki, Keita Honda, Yusuke Sekiguchi, Shin-Ichi Izumi, and Satoru Ebihara. 2025. "Characteristics of Trunk Acceleration and Angular Velocity in Turning Movement in Post-Stroke Patients with High Risk of Falling" Sensors 25, no. 9: 2689. https://doi.org/10.3390/s25092689
APA StyleNaito, D., Honda, K., Sekiguchi, Y., Izumi, S.-I., & Ebihara, S. (2025). Characteristics of Trunk Acceleration and Angular Velocity in Turning Movement in Post-Stroke Patients with High Risk of Falling. Sensors, 25(9), 2689. https://doi.org/10.3390/s25092689






