Prediction of Microvascular Adaptation to Hypoxia Based on Myogenic Microcirculation Oscillations
Abstract
1. Introduction
2. Materials and Methods
2.1. Brief Description of the Analyzed Groups
2.2. The FMSF Methodology
2.3. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nilsson, H.; Aalkjaer, C. Vasomotion: Mechanisms and Physiological Importance. Mol. Interv. 2003, 3, 79–89. [Google Scholar] [CrossRef]
- Aalkjær, C.; Boedtkjer, D.; Matchkov, V. Vasomotion—What Is Currently Thought? Acta Physiol. 2011, 202, 253–269. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.-S.; Lee, M.-G. Rescue Effect of Exercise on Impaired Arteriolar Myogenic Response with Advancing Age. Exerc. Sci. 2017, 26, 8–16. [Google Scholar] [CrossRef]
- Fredriksson, I.; Larsson, M.; Strömberg, T.; Iredahl, F. Vasomotion Analysis of Speed Resolved Perfusion, Oxygen Saturation, Red Blood Cell Tissue Fraction, and Vessel Diameter: Novel Microvascular Perspectives. Ski. Res. Technol. 2022, 28, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Choi, J.Y.; Kim, S.M.; Son, S.-M.; Choi, S.-Y.; Koo, B.; Rah, C.-S.; Nam, J.H.; Ju, M.J.; Lee, J.S.; et al. Vasomotion in Human Arteries and Their Regulations Based on Ion Channel Regulations: 10 Years Study. J. Cell. Physiol. 2023, 238, 2076–2089. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Carpi, A.; Galetta, F.; Franzoni, F.; Santoro, G. The Investigation of Skin Blood Flowmotion: A New Approach to Study the Microcirculatory Impairment in Vascular Diseases? Biomed. Pharmacother. 2006, 60, 437–442. [Google Scholar] [CrossRef]
- Bruning, R.S.; Kenney, W.L.; Alexander, L.M. Altered Skin Flowmotion in Hypertensive Humans. Microvasc. Res. 2015, 97, 81–87. [Google Scholar] [CrossRef]
- Tikhonova, I.V.; Kosyakova, N.I.; Tankanag, A.V.; Chemeris, N.K. Oscillations of Skin Microvascular Blood Flow in Patients with Asthma. Microcirculation 2016, 23, 33–43. [Google Scholar] [CrossRef]
- Mizeva, I.; Makovik, I.; Dunaev, A.; Krupatkin, A.; Meglinski, I. Analysis of Skin Blood Microflow Oscillations in Patients with Rheumatic Diseases. J. Biomed. Opt. 2017, 22, 070501. [Google Scholar] [CrossRef]
- Katarzynska, J.; Cholewinski, T.; Sieron, L.; Marcinek, A.; Gebicki, J. Flowmotion Monitored by Flow Mediated Skin Fluorescence (FMSF): A Tool for Characterization of Microcirculatory Status. Front. Physiol. 2020, 11, 702. [Google Scholar] [CrossRef]
- Stefanovska, A.; Bracic, M.; Kvernmo, H.D. Wavelet Analysis of Oscillations in the Peripheral Blood Circulation Measured by Laser Doppler Technique. IEEE Trans. Biomed. Eng. 1999, 46, 1230–1239. [Google Scholar] [CrossRef]
- Hellmann, M.; Roustit, M.; Cracowski, J.L. Skin Microvascular Endothelial Function as a Biomarker in Cardiovascular Diseases? Pharmacol. Rep. 2015, 67, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Cracowski, J.-L.; Roustit, M. Current Methods to Assess Human Cutaneous Blood Flow: An Updated Focus on Laser-Based-Techniques. Microcirculation 2016, 23, 337–344. [Google Scholar] [CrossRef]
- Cracowski, J.-L.; Roustit, M. Human Skin Microcirculation. In Comprehensive Physiology; Prakash, Y.S., Ed.; Wiley: New York, NY, USA, 2020; Chapter 3; Volume 10, pp. 1105–1154. [Google Scholar] [CrossRef]
- Szczepanek, E.; Marczyk, B.; Chukwu, O.; Chlopicki, S.; Sacha, T. Endothelial Function in Patients with Chronic Myeloid Leukemia Treated with Tyrosine Kinase Inhibitors Is Not Related to Cardiovascular Risk Assessed by the Systematic Coronary Risk Estimation 2 Algorithm. Polish. Arch. Intern. Med. 2024, 134, 16719. [Google Scholar] [CrossRef]
- Mizeva, I.A.; Podolyan, N.P.; Mamontov, O.V.; Sakovskaia, A.V.; Kamshilin, A.A. Study of 0.1-Hz Vasomotion in Microcirculation under Local Heating by Means of Imaging Photoplethysmography. Biomed. Signal Process. Control 2025, 100, 107188. [Google Scholar] [CrossRef]
- Kvernmo, H.D.; Stefanovska, A.; Kirkebøen, K.A.; Kvernebo, K. Oscillations in the Human Cutaneous Blood Perfusion Signal Modified by Endothelium-Dependent and Endothelium-Independent Vasodilators. Microvasc. Res. 1999, 57, 298–309. [Google Scholar] [CrossRef]
- Kvandal, P.; Landsverk, S.A.; Bernjak, A.; Stefanovska, A.; Kvernmo, H.D.; Kirkebøen, K.A. Low-Frequency Oscillations of the Laser Doppler Perfusion Signal in Human Skin. Microvasc. Res. 2006, 72, 120–127. [Google Scholar] [CrossRef]
- Söderström, T.; Stefanovska, A.; Veber, M.; Svensson, H. Involvement of Sympathetic Nerve Activity in Skin Blood Flow Oscillations in Humans. Am. J. Physiol. Circ. Physiol. 2003, 284, H1638–H1646. [Google Scholar] [CrossRef]
- Aalkjaer, C.; Nilsson, H. Vasomotion: Cellular Background for the Oscillator and for the Synchronization of Smooth Muscle Cells. Br. J. Pharmacol. 2005, 144, 605–616. [Google Scholar] [CrossRef]
- Cracowski, J.-L.; Minson, C.T.; Salvat-Melis, M.; Halliwill, J.R. Methodological Issues in the Assessment of Skin Microvascular Endothelial Function in Humans. Trends Pharmacol. Sci. 2006, 27, 503–508. [Google Scholar] [CrossRef]
- Stewart, J.M.; Indu, T.; Michael, S.G.; Medow, M.S. Noninvasive Measure of Microvascular Nitric Oxide Function in Humans Using Very Low-Frequency Cutaneous Laser Doppler Flow Spectra. Microcirculation 2007, 14, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Hodges, G.J.; Mallette, M.M.; Martin, Z.T.; Del Pozzi, A.T. Effect of Sympathetic Nerve Blockade on Low-Frequency Oscillations of Forearm and Leg Skin Blood Flow in Healthy Humans. Microcirculation 2017, 24, e12388. [Google Scholar] [CrossRef]
- Zhao, X.; Schalkwijk, C.; Kroon, A.; Schram, M.T.; Stehouwer, C.; Houben, A. Different Measures of Hyperglycemia Are Negatively Associated with Skin Microvascular Flowmotion: The Maastricht Study. Microcirculation 2024, 31, e12882. [Google Scholar] [CrossRef]
- Hellmann, M.; Tarnawska, M.; Dudziak, M.; Dorniak, K.; Roustit, M.; Cracowski, J.L. Reproducibility of Flow Mediated Skin Fluorescence to Assess Microvascular Function. Microvasc. Res. 2017, 113, 60–64. [Google Scholar] [CrossRef]
- Tarnawska, M.; Dorniak, K.; Kaszubowski, M.; Dudziak, M.; Hellmann, M. A Pilot Study with Flow Mediated Skin Fluorescence: A Novel Device to Assess Microvascular Endothelial Function in Coronary Artery Disease. Cardiol. J. 2018, 25, 120–127. [Google Scholar] [CrossRef]
- Marcinek, A.; Katarzynska, J.; Sieron, L.; Skokowski, R.; Zielinski, J.; Gebicki, J. Non-Invasive Assessment of Vascular Circulation Based on Flow Mediated Skin Fluorescence (FMSF). Biology 2023, 12, 385. [Google Scholar] [CrossRef] [PubMed]
- Marcinek, A.; Katarzynska, J.; Gebicki, J. Simultaneous Assessment of Mitochondrial and Vascular Function Using the Flow Mediated Skin Fluorescence Technique. Front. Physiol. 2025, 16, 1509159. [Google Scholar] [CrossRef] [PubMed]
- Cole, W.C.; Gordon, G.R.; Braun, A.P. Cellular and Ionic Mechanisms of Arterial Vasomotion. In Smooth Muscle Spontaneous Activity: Physiological and Pathological Modulation; Hashitani, H., Lang, R.J., Eds.; Springer: Singapore, 2019; Volume 1124, pp. 297–312. [Google Scholar] [CrossRef]
- Liu, P.; Bi, T.; Du, G.; Yan, L.; Hou, H. Dynamic Non-Invasive Detection of NADH Based on Blood Flow-Mediated Skin Fluorescence (FMSF) Method. Open J. Appl. Sci. 2024, 14, 1437–1453. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Zhang, D.; Wang, W.; Xie, H.; Ruan, J.; Jin, Y.; Li, T.; Li, X.; Zhao, B.; et al. Ca2+ Oscillation in Vascular Smooth Muscle Cells Control Myogenic Spontaneous Vasomotion and Counteract Post-Ischemic No-Reflow. Commun. Biol. 2024, 7, 332. [Google Scholar] [CrossRef]
- Thorn, C.E.; Shore, A.C. The Role of Perfusion in the Oxygen Extraction Capability of Skin and Skeletal Muscle. Am. J. Physiol. Circ. Physiol. 2016, 310, H1277–H1284. [Google Scholar] [CrossRef]
- Davies, T.; Gilbert-Kawai, E.; Wythe, S.; Meale, P.; Mythen, M.; Levett, D.; Mitchell, K.; Grocott, M.; Clough, G.; Martin, D.; et al. Sustained Vasomotor Control of Skin Microcirculation in Sherpas versus Altitude-Naive Lowlanders: Experimental Evidence from Xtreme Everest 2. Exp. Physiol. 2018, 103, 1494–1504. [Google Scholar] [CrossRef] [PubMed]
- Carey, D.; Thanaj, M.; Davies, T.; Gilbert-Kawai, E.; Mitchell, K.; Levett, D.Z.H.; Mythen, M.G.; Martin, D.S.; Grocott, M.P.; Chipperfield, A.J.; et al. Enhanced Flow-Motion Complexity of Skin Microvascular Perfusion in Sherpas and Lowlanders during Ascent to High Altitude. Sci. Rep. 2019, 9, 14391. [Google Scholar] [CrossRef]
- Salvi, P.; Faini, A.; Castiglioni, P.; Brunacci, F.; Montaguti, L.; Severi, F.; Gautier, S.; Pretolani, E.; Benetos, A.; Parati, G. Increase in Slow-Wave Vasomotion by Hypoxia and Ischemia in Lowlanders and Highlanders. J. Appl. Physiol. 2018, 125, 780–789. [Google Scholar] [CrossRef]
- Mikosiński, J.; Mikosiński, P.; Kwapisz, A.; Katarzynska, J.; Gebicki, J. Conclusions from an Observational Study of Patients with Vascular Diseases Using the FMSF Technique. Vasc. Health Risk Manag. 2023, 19, 755–764. [Google Scholar] [CrossRef]
- Bugaj, O.; Zieliński, J.; Kusy, K.; Kantanista, A.; Wieliński, D.; Guzik, P. The Effect of Exercise on the Skin Content of the Reduced Form of NAD and Its Response to Transient Ischemia and Reperfusion in Highly Trained Athletes. Front. Physiol. 2019, 10, 600. [Google Scholar] [CrossRef]
- Bugaj, O.; Kusy, K.; Kantanista, A.; Korman, P.; Wieliński, D.; Zieliński, J. The Effect of a 7-Week Training Period on Changes in Skin NADH Fluorescence in Highly Trained Athletes. Appl. Sci. 2020, 10, 5133. [Google Scholar] [CrossRef]
- Chudzik, M.; Cender, A.; Mordaka, R.; Zieliński, J.; Katarzyńska, J.; Marcinek, A.; Gebicki, J. Chronic Fatigue Associated with Post-COVID Syndrome versus Transient Fatigue Caused by High-Intensity Exercise: Are They Comparable in Terms of Vascular Effects? Vasc. Health Risk Manag. 2022, 18, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Slowikowska-Hilczer, J.; Walczak-Jedrzejowska, R.; Adamczewska, D.; Byczkiewicz, P.; Marchlewska, K.; Katarzynska, J.; Gebicki, J. A New Approach to the Assessment of Erectile Dysfunction Based on Vasomotion Monitored by the Flow-Mediated Skin Fluorescence (FMSF) Technique—A Preliminary Study. J. Clin. Med. 2024, 13, 3210. [Google Scholar] [CrossRef]
- Hultman, M.; Richter, F.; Larsson, M.; Strömberg, T.; Iredahl, F.; Fredriksson, I. Robust Analysis of Microcirculatory Flowmotion during Post-Occlusive Reactive Hyperemia. Microvasc. Res. 2024, 155, 104715. [Google Scholar] [CrossRef]
- Jewell, U.R.; Kvietikova, I.; Scheid, A.; Bauer, C.; Wenger, R.H.; Gassmann, M. Induction of HIF–1α in Response to Hypoxia Is Instantaneous. FASEB J. 2001, 15, 1312–1314. [Google Scholar] [CrossRef]
- Rossi, M.; Carpi, A.; Di Maria, C.; Galetta, F.; Santoro, G. Spectral Analysis of Laser Doppler Skin Blood Flow Oscillations in Human Essential Arterial Hypertension. Microvasc. Res. 2006, 72, 34–41. [Google Scholar] [CrossRef] [PubMed]

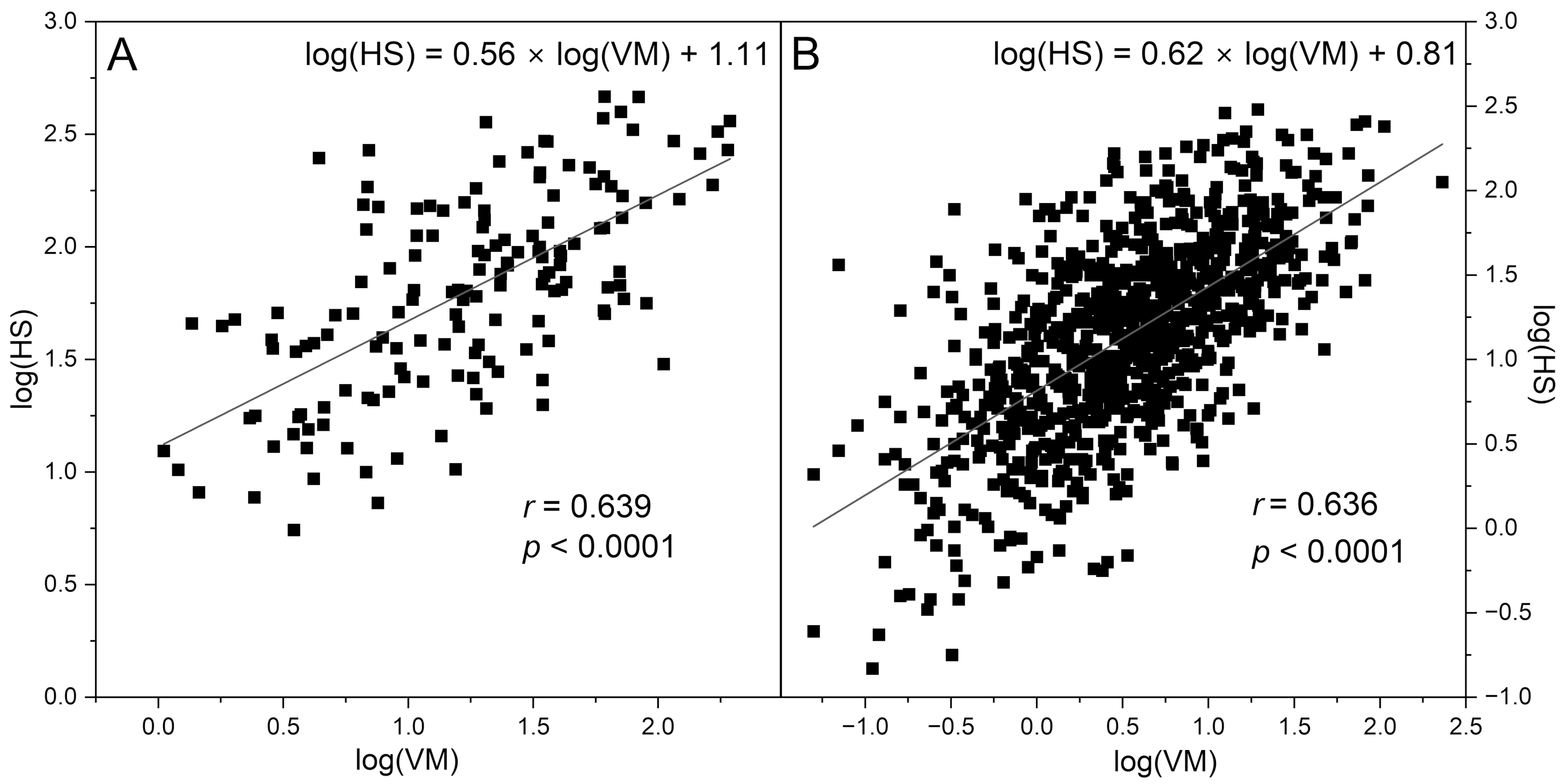
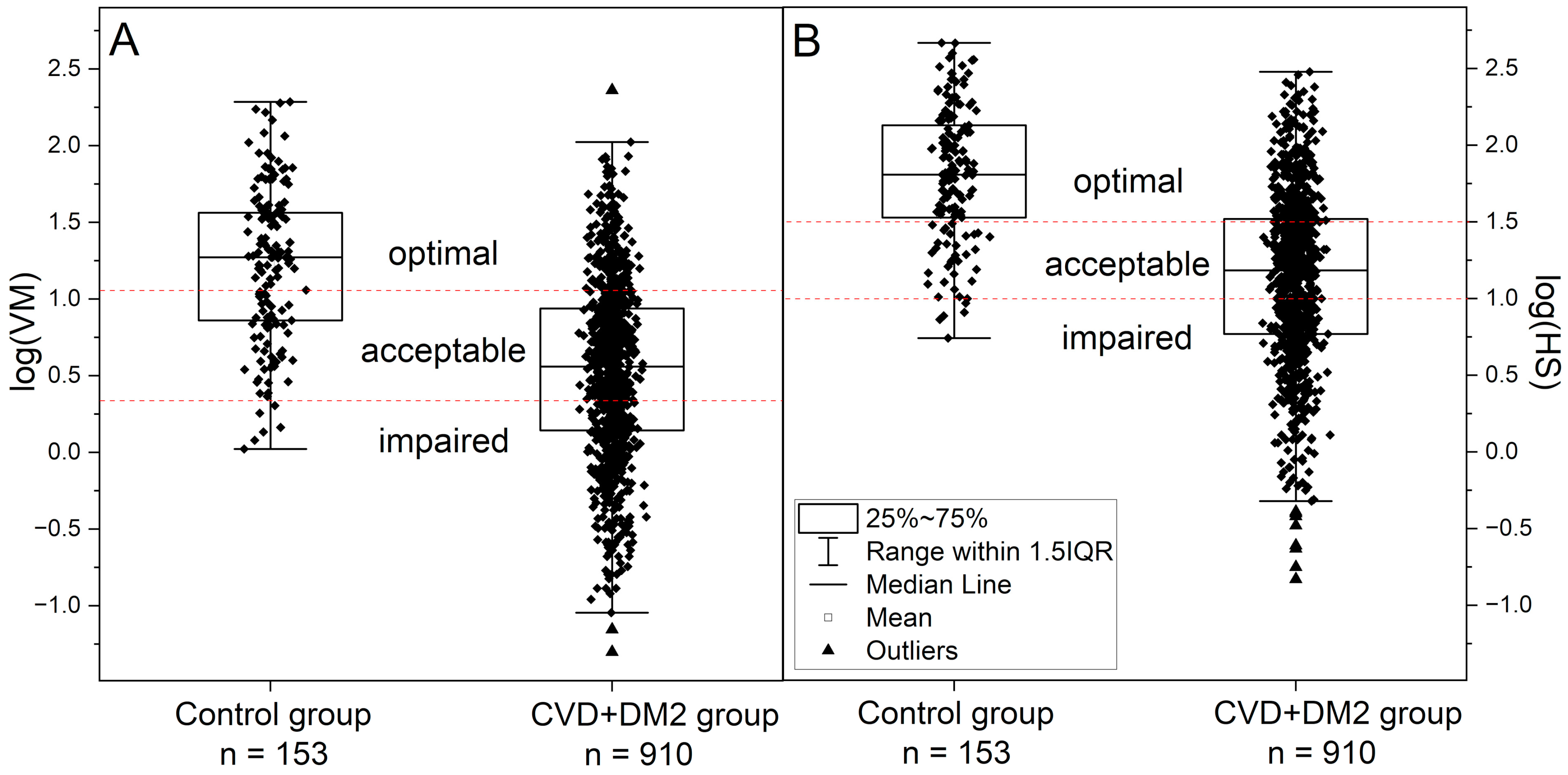

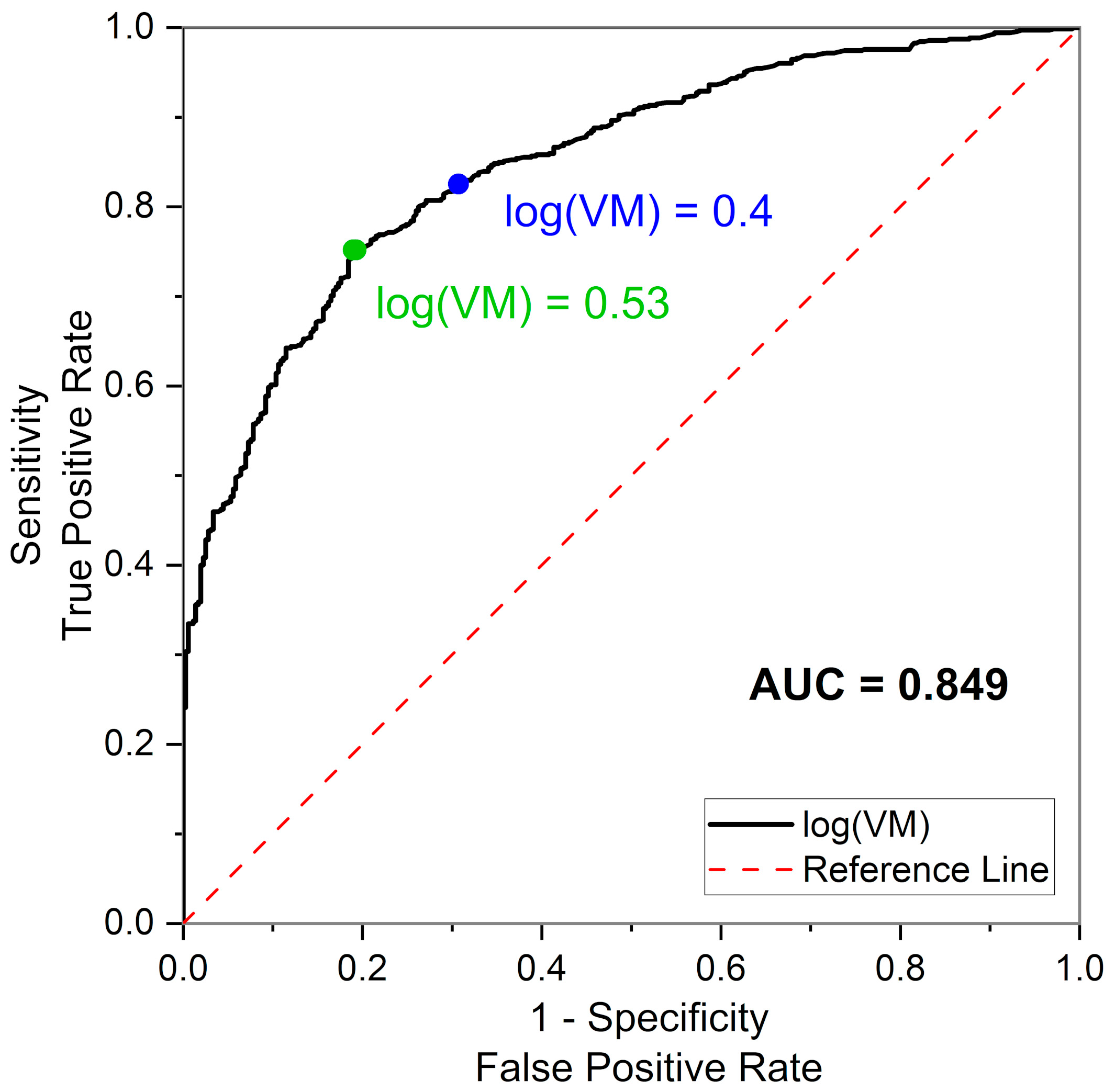
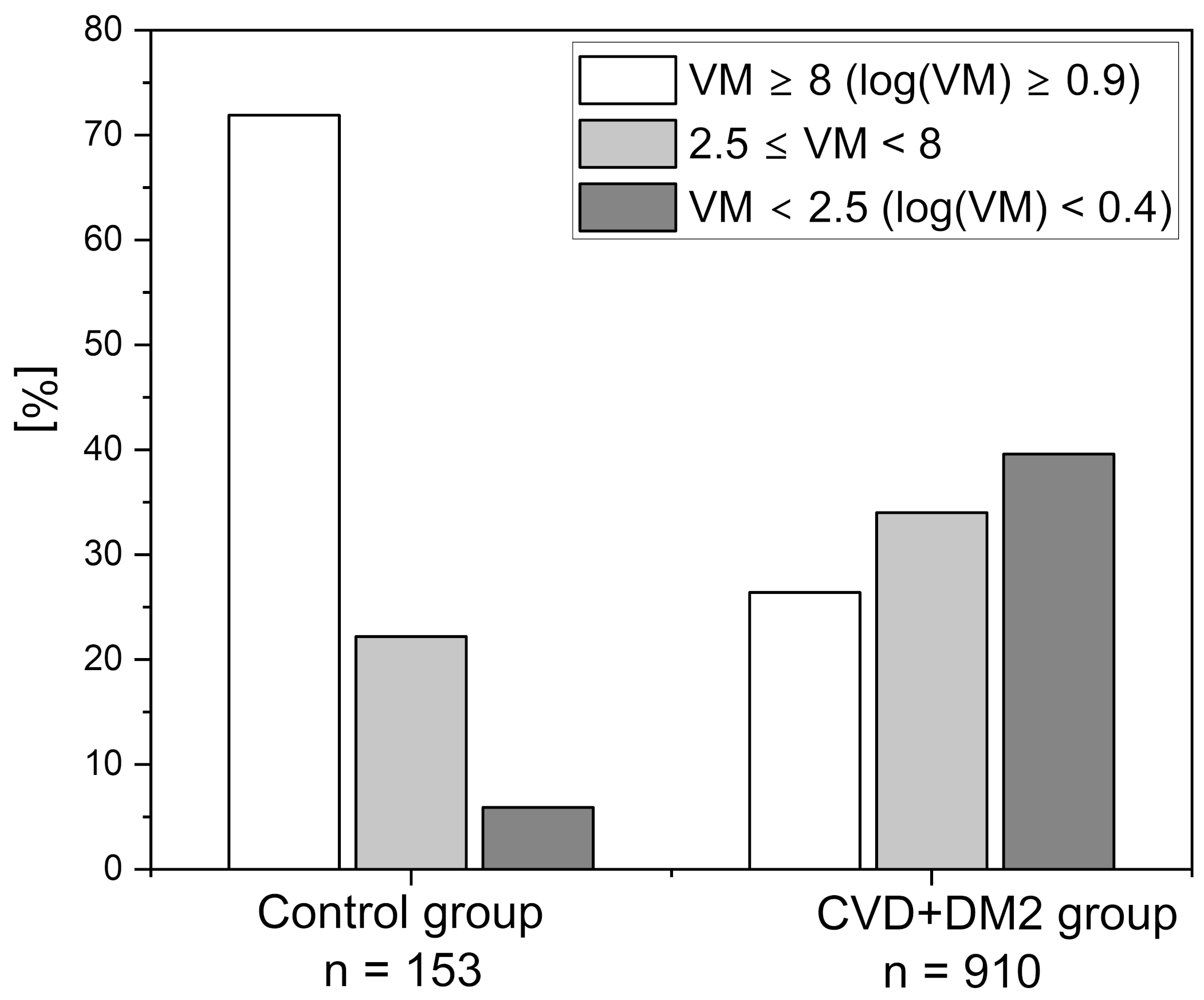
| Control Group | CVD+DM2 Group | Athletes Group | IHT Group | |||
|---|---|---|---|---|---|---|
| Before Exertion to Exhaustion | After Exertion to Exhaustion | Before Intermittent Hypoxia Treatment | After Intermittent Hypoxia Treatment | |||
| N | 153 | 910 AH (508) CVD (475) DM2 (273) | 56 | 9 | ||
| Female/Male | 34/119 | 519/391 | 10/46 | 4/5 | ||
| Age [Years] | 32.6 ± 11.7 | 67.6 ± 12.5 | 25.7 ± 4.8 | 26.2 ± 10.4 | ||
| BMI [kg/m2] | 24.3 ± 4.0 | 28.9 ± 5.8 | 22.4 ± 2.4 | 20.6 ± 1.7 | ||
| DBP [mm Hg] | 77.1 ± 10.3 | 74.9 ± 10.7 | 71.3 ± 7.7 | 72.9 ± 8.1 | 69.2 ± 7.8 | 72.2 ± 7.3 |
| SBP [mm Hg] | 130.4 ± 13.9 | 137.5 ± 18.1 | 129.7 ± 12.2 | 158.4 ± 19.8 | 118.3 ± 12.3 | 112.9 ± 7.8 |
| IRmax [%] | 16.3 ± 5.8 | 11.4 ± 5.6 | 18.6 ± 5.2 | 13.1 ± 5.4 | 20.1 ± 8.5 | 19.5 ± 4.1 |
| HRmax [%] | 19.8 ± 4.6 | 16.5 ± 5.1 | 20.6 ± 4.7 | 19.9 ± 4.8 | 18.5 ± 5.4 | 19.9 ± 5.6 |
| log(VM) | 1.23 ± 0.50 | 0.53 ± 0.58 | 1.32 ± 0.53 | 0.99 ± 0.52 | 1.06 ± 0.39 | 1.34 ± 0.34 |
| log(HS) | 1.80 ± 0.44 | 1.14 ± 0.56 | 1.91 ± 0.41 | 2.04 ± 0.43 | 1.89 ± 0.20 | 1.95 ± 0.22 |
| Control Group | CVD+DM2 Group | |||||
|---|---|---|---|---|---|---|
| Slope [a.u./year] | r (Pearson Coefficient) | p-Value | Slope [a.u./year] | r (Pearson Coefficient) | p-Value | |
| log(VM) vs. age | −0.017 ± 0.003 | −0.385 | <0.0001 | −0.012 ± 0.002 | −0.264 | <0.0001 |
| log(HS) vs. age | −0.012 ± 0.003 | −0.311 | <0.0001 | −0.013 ± 0.002 | −0.280 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcinek, A.; Katarzynska, J.; Stanek, A.; Gebicki, J. Prediction of Microvascular Adaptation to Hypoxia Based on Myogenic Microcirculation Oscillations. Sensors 2025, 25, 2751. https://doi.org/10.3390/s25092751
Marcinek A, Katarzynska J, Stanek A, Gebicki J. Prediction of Microvascular Adaptation to Hypoxia Based on Myogenic Microcirculation Oscillations. Sensors. 2025; 25(9):2751. https://doi.org/10.3390/s25092751
Chicago/Turabian StyleMarcinek, Andrzej, Joanna Katarzynska, Artur Stanek, and Jerzy Gebicki. 2025. "Prediction of Microvascular Adaptation to Hypoxia Based on Myogenic Microcirculation Oscillations" Sensors 25, no. 9: 2751. https://doi.org/10.3390/s25092751
APA StyleMarcinek, A., Katarzynska, J., Stanek, A., & Gebicki, J. (2025). Prediction of Microvascular Adaptation to Hypoxia Based on Myogenic Microcirculation Oscillations. Sensors, 25(9), 2751. https://doi.org/10.3390/s25092751






