Abstract
The role of nuclear medicine in the management of oncological patients has expanded during last two decades. The number of radiopharmaceuticals contributing to the realization of theranostics/radiotheranostics in the context of personalized medicine is increasing. This review is focused on the examples of targeted (radio)pharmaceuticals for the imaging and therapy of neuroendocrine neoplasms (NENs), prostate cancer, and breast cancer. These examples strongly demonstrate the tendency of nuclear medicine development towards personalized medicine.
1. Introduction
The number of nuclear medicine examinations and therapeutic procedures is increasing with acceleration worldwide reflecting the growing importance of the field in the modern healthcare system. The growth and expansion of nuclear medicine relies on the development and availability of radiopharmaceuticals. High demand for radiopharmaceuticals with biological activity specific for a certain disease yielded personalized patient treatment approaches, in particular theranostics using molecular imaging for the disease staging and prediction of the efficacy of specific therapeutic interventions on individual basis as well as for the monitoring response to the treatment. Molecular imaging in nuclear medicine is presented by positron emission tomography (PET) and single photon emission computed tomography (SPECT), and in combination with endoradiotherapy it can be defined as radiotheranostics [1].
Ideally the pre-therapeutic imaging (PET, SPECT) and subsequent endoradiotherapy should be conducted using the radioactive isotopes of the same chemical element, e.g., 123I/124I/131I [1]. However, the development of the respective radiopharmaceuticals is not always possible to achieve in practice. Metal radionuclides offer an advantage in terms of similarities in labelling chemistry, e.g., 68Ga(III), 111In(III), and 177Lu(III), while providing variation in the radiation mode relevant for both diagnostic imaging (PET, SPECT) and endoradiotherapy. Moreover, 68Ga(III), 111In(III), and 177Lu(III) can use the same chelator (DOTA) thus introducing the least possible difference in molecular structure and consequently specific target, e.g., receptor, binding properties.
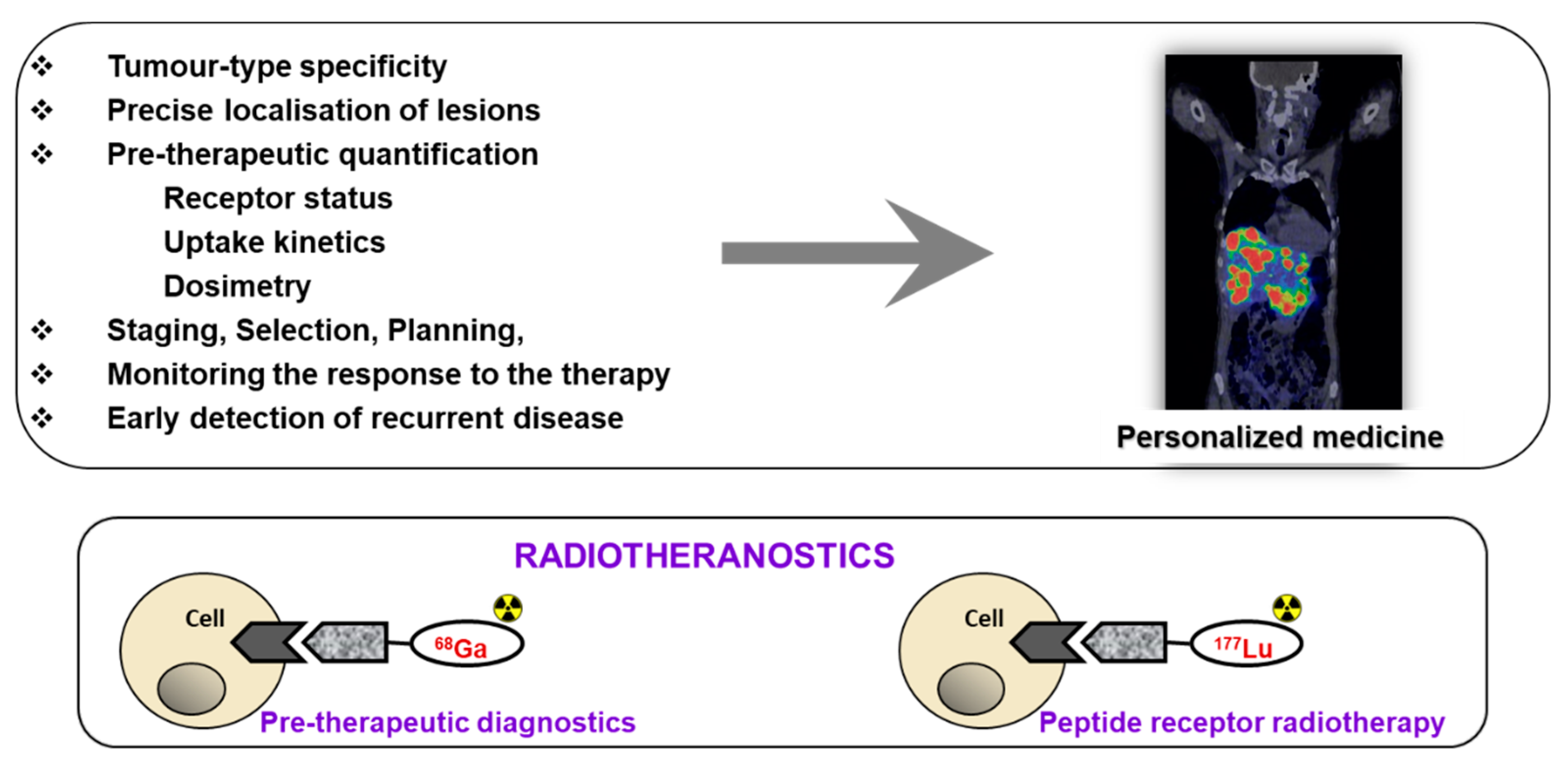
Receptor targeted chemo- and radiotherapeutics gain treatment precision and efficiency due to the prior quantification using molecular imaging for the stratification of the patients, adjustment of the therapeutic dose, and monitoring response (Figure 1). The treatment regimen depends on the stage of the disease at initial diagnosis and thus whole-body quantitative imaging reflecting heterogeneity of receptor expression and variability amongst patients is of utmost importance [2,3,4,5]. Further crucial advantage is possibility to monitor response to the therapy to introduce the treatment changes if necessary, as early as possible. Theranostics/radiotheranostics has strong potential not only for the optimized treatment but also for the exclusion of futile treatments that otherwise would cause unnecessary costs and patient distress. Apart from non-invasive imaging, radiation offers possibility of intraoperative detection for more accurate lesion resection.
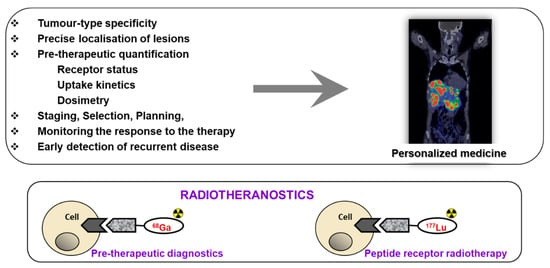
Figure 1.
Upper panel: The targeted imaging in oncology provides tumor-type specific non-invasive diagnosis, precise localization of tumors and metastases that most importantly have the potential for pre-therapeutic quantification of receptor status, uptake kinetics and dosimetry that enables accurate therapy selection and planning, as well as monitoring response to the therapy resulting in personalized medicine. Lower panel: Some imaging and therapeutic radionuclides have similar coordination chemistry thus allowing for the radiotheranostic development wherein the pre-therapeutic imaging and radiotherapy are conducted with the same vector molecule exchanging the imaging and therapeutic radionuclides. Drawing presents the interaction of an agent, either imaging if labeled with, e.g., 68Ga (left) or radiotherapeutic if labeled with 177Lu (right), with the cell receptor. Reproduced from [8].
Targeting specific biomarkers turns PET and SPECT imaging technologies into whole-body, non-invasive “biopsy”. It allows to overcome such disadvantages of a conventional biopsy such as sampling error, inability of taking multiple and repeated biopsies, inability to collect biopsies from certain areas, e.g., bone or brain, receptor expression heterogeneity with discordance of primary tumor and metastases as well as infection, hemorrhage, and patient discomfort. The variability in receptor density and subtype is a very crucial factor influencing the accuracy of diagnosis based on the pathological evaluation of a biopsy, and thus the imaging that reveals such variation in a single examination globally and quantitatively is of utmost important for individualized treatment [5,6].
The pioneer and most prominent example of the receptor targeted (radio)theranostics that has already been introduced into clinical practice is management of patients with neuroendocrine neoplasms (NENs) using somatostatin (SST) analogue based radiopharmaceuticals. Following the footsteps of SST receptor (SSTR) targeted (radio)theranostics, prostate specific membrane antigen (PSMA) targeting radiopharmaceuticals spread around the world with unprecedented acceleration. Two reporting and data system classifications for PSMA- and SSTR-targeted PET imaging have been introduced in order to navigate molecular imaging-guided treatment strategies [7]. Another current example of theranostics that evoked strong clinical interest was the management of breast cancer targeting human epidermal growth factor receptor type 2 (HER2) wherein the quantitative PET navigates the anti-HER2 targeting chemotherapy with antibody-based pharmaceuticals. This review demonstrates tendency of nuclear medicine development towards personalized medicine based on the abovementioned examples in the context of (radio)theranostics with its possibilities and challenges.
2. Targeting SSTR on Neuroendocrine Neoplasms
The clinical use of radiolabeled somatostatin analogues for imaging and radiotherapy has been accepted globally. Interestingly, the incidence of NENs increased from 1.09 to 6.98 per 100,000 individuals what might partly be explained by earlier detection and diagnosis due to availability of imaging technologies in clinical practice [9]. NENs are heterogeneous tumors and it is of utmost importance to identify patients who might benefit from the SST receptor (SSTR) targeted therapy [10]. PET/CT with 68Ga-labeled somatostatin ligand analogues ([68Ga]Ga-SST/PET) is recommended for the diagnosis, staging, and patient selection for endoradiotherapy [11]. Mono- and multi-center clinical trials demonstrated benefits of SSTR targeted pre-therapeutic imaging and radiotherapy in terms of patient management efficiency, efficacy, safety, and survival [12,13,14]. [68Ga]Ga-SST was found valuable not only for pre-operative assessment of resectable lesions and multiple unresectable lesions but also for intraoperative radio-guided resection [15,16]. [68Ga]Ga-SST/PET is also efficient tool for the scheduling of the treatment combining long-acting somatostatin analogue (SSA) therapy and endoradiotherapy [17] since the amount of the administered peptide influences the biodistribution pattern and might enhance lesion uptake while reducing uptake in the healthy tissue and organs with physiological expression of the target [2,18,19]. Guidelines for the standard care of NENs include radionuclide SSTR targeted imaging and therapy [20,21].
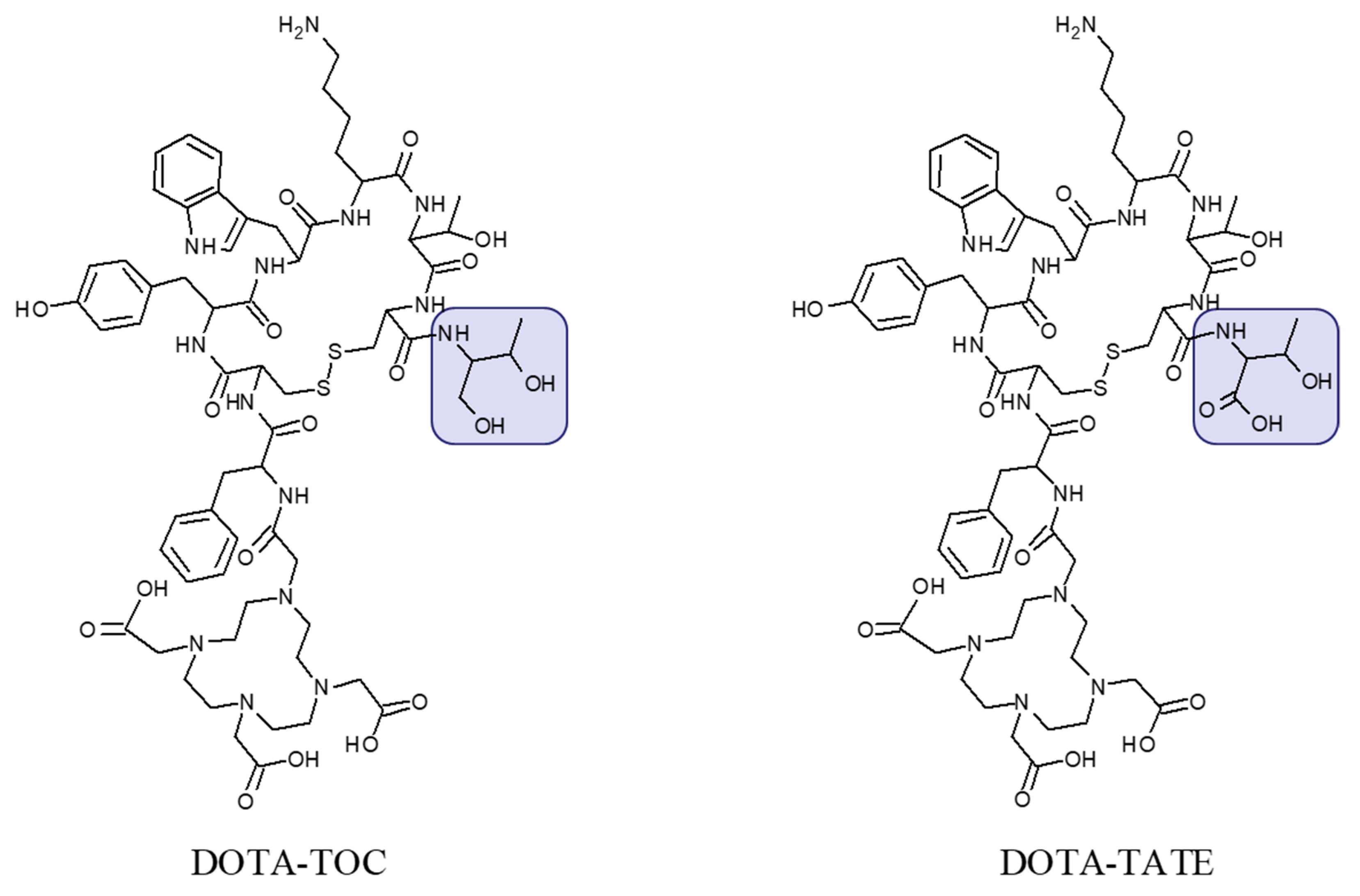
The most commonly used analogues are based on DOTA-Tyr3-octreotide (DOTA-TOC) and DOTA-(Tyr3, Thr8)-octreotate (DOTA-TATE) (Figure 2) labeled with 68Ga and 177Lu, respectively for PET imaging and endoradiotherapy. Advances in regulation, marketing of pharmaceutical grade 68Ge/68Ga generators, commercial availability of precursor peptides, radiopharmaceuticals and kits for the preparation of the radiopharmaceuticals under radiopharmacy conditions facilitate the dissemination of the technology. [68Ga]Ga-DOTA-TATE/[68Ga]Ga-DOTA-TOC and [177Lu]Lu-DOTA-TATE have been approved by Food and Drug Administration (FDA) and European Medicines Agency (EMA) for PET examination and treatment of SSTR positive gastroenteropancreatic NETs in adults.
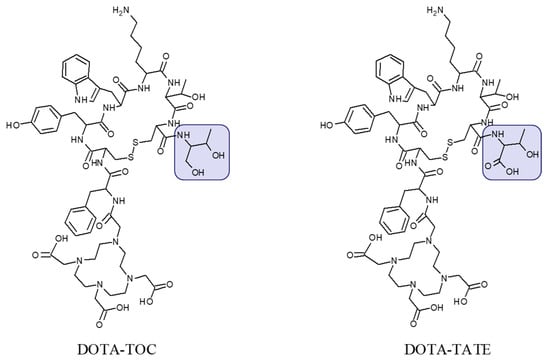
Figure 2.
Chemical structure of DOTA-Tyr3-octreotide (DOTA-TOC) and DOTA-(Tyr3, Thr8)-octreotate (DOTA-TATE) presenting difference in C-terminal where the carboxyl group in threonine amino acid residue (DOTA-TATE) is exchanged to hydroxyl group (DOTA-TOC).
The [68Ga]Ga-SST/PET-CT has become the most promising non-invasive technique to study NENs and demonstrated superiority over such imaging agents as [123I]MIBG, [11C]-HTP, [18F]FDG, [18F]FDOPA, [111In]-pentetreotide, and [99mTc]-SST analogues [22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45]. However, the combination of these imaging agents might provide higher diagnostic accuracy, e.g., [18F]FDG is used for measuring the tumor metabolic rate whereas [68Ga]Ga-SST provides information on SSTR expression guiding the biopsy [12,46,47,48,49,50,51,52,53]. Combination of a high SUV on [68Ga]Ga-SST/PET-CT and minor/no [18F]FDG uptake was associated with better prognosis [54].
Advantages in terms of sensitivity and detection rate of [68Ga]Ga-SST/PET-CT were demonstrated over MRI and CT [55,56,57,58,59,60]. Comparison of [68Ga]Ga-SST/PET-CT and [68Ga]Ga-SST/PET-MR showed similar PET image quality however uptake quantification was found more accurate on PET/CT and detection rate for bone metastases was higher [61,62,63]. However, these technologies can also be complementary [64,65,66].
The vast experience with [68Ga]Ga-SST analogues demonstrated necessity for accurate discrimination between cancerous and benign lesions, physiological and inflammatory uptake [23,67,68,69,70,71,72,73,74]. Studies on biodistribution and radiation dosimetry of [68Ga]Ga-SST analogues in patients and healthy volunteers revealed low total effective dose allowing multiple examinations per year and no immediate or delayed toxicity [40,75].
Impact of SST Radiopharmaceuticals on Patient Treatment Management
Selection of patients who might benefit from endoradiotherapy [35,76,77,78,79,80,81] or are legible for surgery relies on accurate staging. The quantification of marginal differences between baseline and follow-up [68Ga]Ga-SST PET images require high accuracy. The (semi)-quantitative assessment of the response, using [68Ga]Ga-SST PET/CT, to the endoradiotherapy with 177Lu- or 90Y-based somatostatin analogues and re-staging of the disease have entered clinical practice. Patient selection, prognosis, prediction of absorbed dose for radiotherapy, and treatment response based on [68Ga]Ga-SST PET/CT have been performed using various parameters such as maximum standardized uptake value (SUVmax), tumor-to-background SUVmax ratio (TBR), tumor-to-liver SUVmax ratio (TLR), tumor-to spleen SUVmax ratio (TSR), functional volume (FV), K-Patlak and Ki [60,76,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97]. SUVmax cut-off of 16.4 was proposed for patient stratification for endoradiotherapy [84]. The cut-off of 15.0 was significantly associated with survival [54]. Inverse correlation between SUVmax and Ki-67 index indicates that [68Ga]Ga-SST PET reflects cell proliferation and helps guide disease management [98,99]. Nevertheless the criteria for the assessment are still to be refined [85] and standardized.
Non-linear correlation between SUV and Ki indicated that SUV most likely did not reflect SSTR density accurately at higher SUVs [100]. While high correlation found between Ki and TBR indicated that the latter might be more accurate metrics than SUV for semi-quantitative assessment of [68Ga]Ga-SST lesion uptake and treatment response monitoring [101]. Total functional tumor volume (TFTV) measured on [68Ga]Ga-SST PET and computed by summing the volumes of all pathological foci was suggested as prognostic biomarker with cut-off of 13.8 cm3 [102]. Somatostatin receptor expressing tumor volume (SRETV), defined as tumor volume with higher [68Ga]Ga-SST uptake than 50% of SUVmax within the volume of interest for each lesion, demonstrated prognostic value of survival [103]. Visual assessment of SSTR heterogeneity on [68Ga]Ga-SST PET/CT images was found valuable for prediction and prognosis with heterogeneity leading to lower survival, even though it is difficult to quantify (Figure 3) [104]. [68Ga]Ga-SST PET uptake heterogeneity determined based on intratumoral textural features predicted endoradiotherapy outcome more accurately than SUVmax [105]. A threshold of 2.5–4.46 or higher for probe TBR was found a sensitive parameter for guided surgical resection (Figure 4) [15,16].
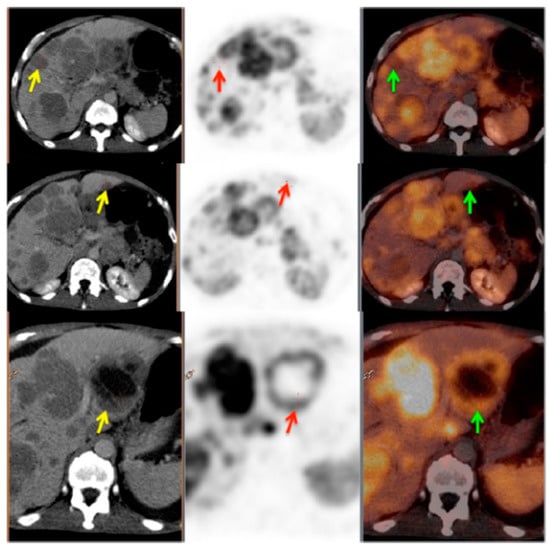
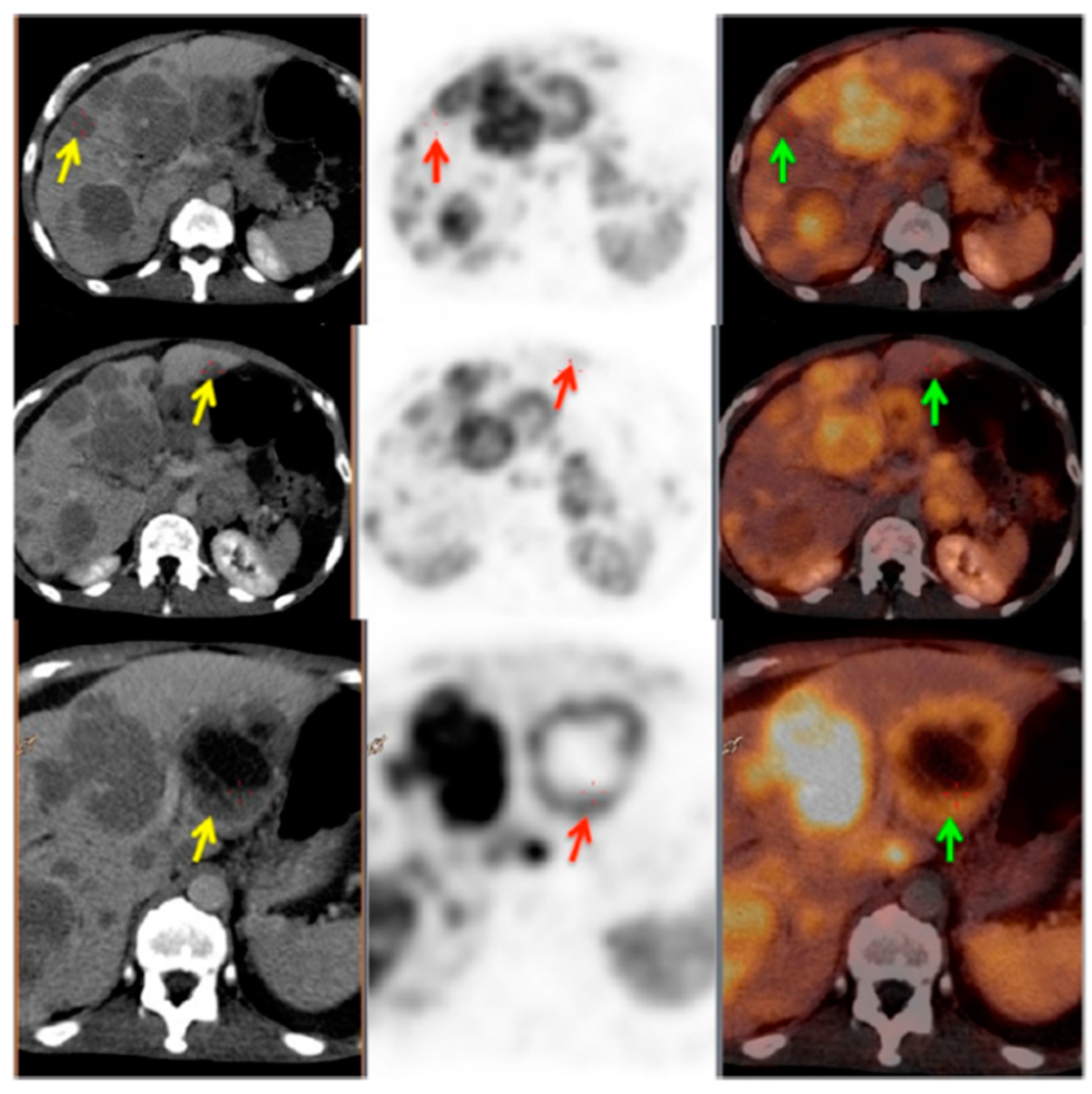
Figure 3.
PET/CT image showing somatostatin receptor heterogeneity. Left panel: CT images; middle panel: PET images; right panel: PET/CT fused images. Yellow arrows show the CT lesions in liver; red and green arrows show corresponding lesions on PET and PET/CT fused images, respectively. This patient was characterized as heterogeneous as more than 50% of the target lesions showed heterogeneous somatostatin receptor (SSTR) expression. Reproduced from [104].
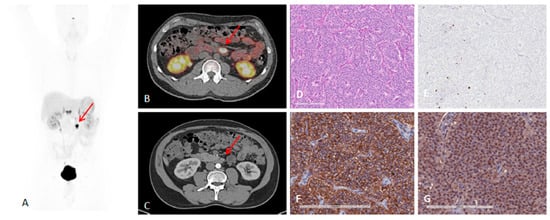
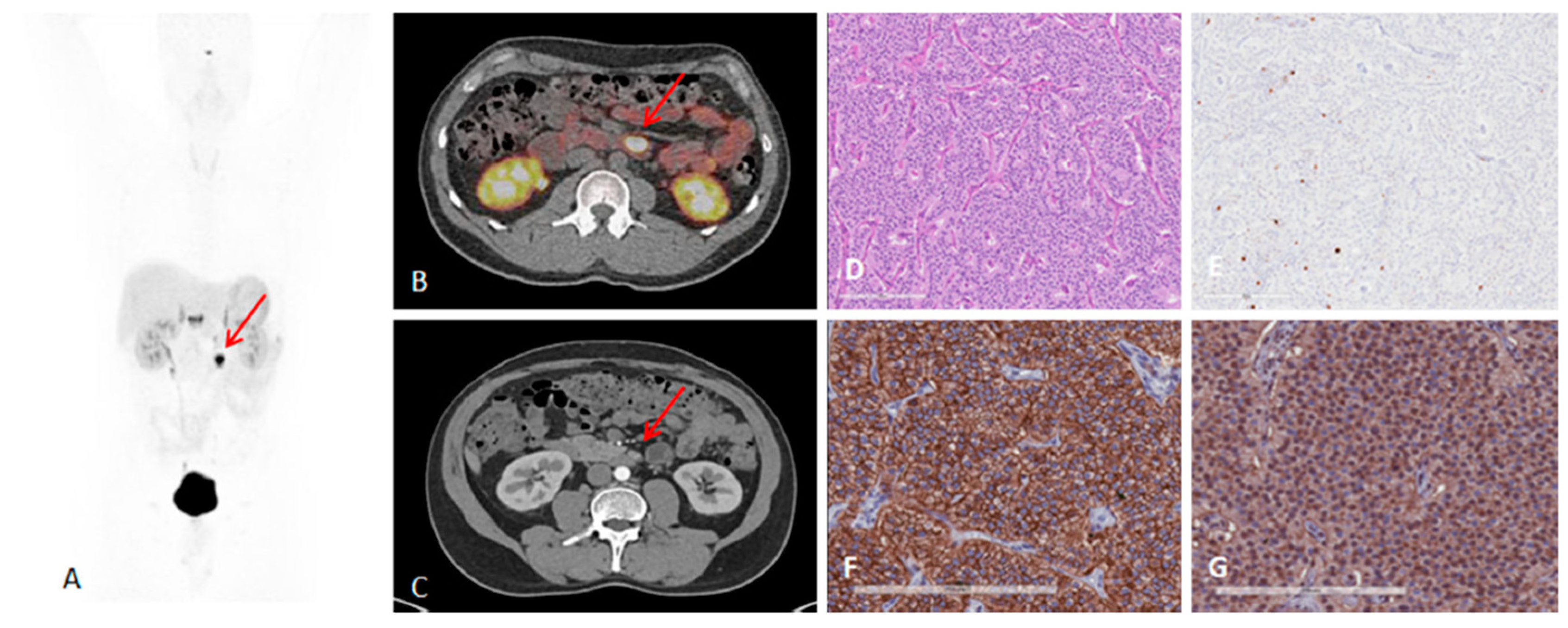
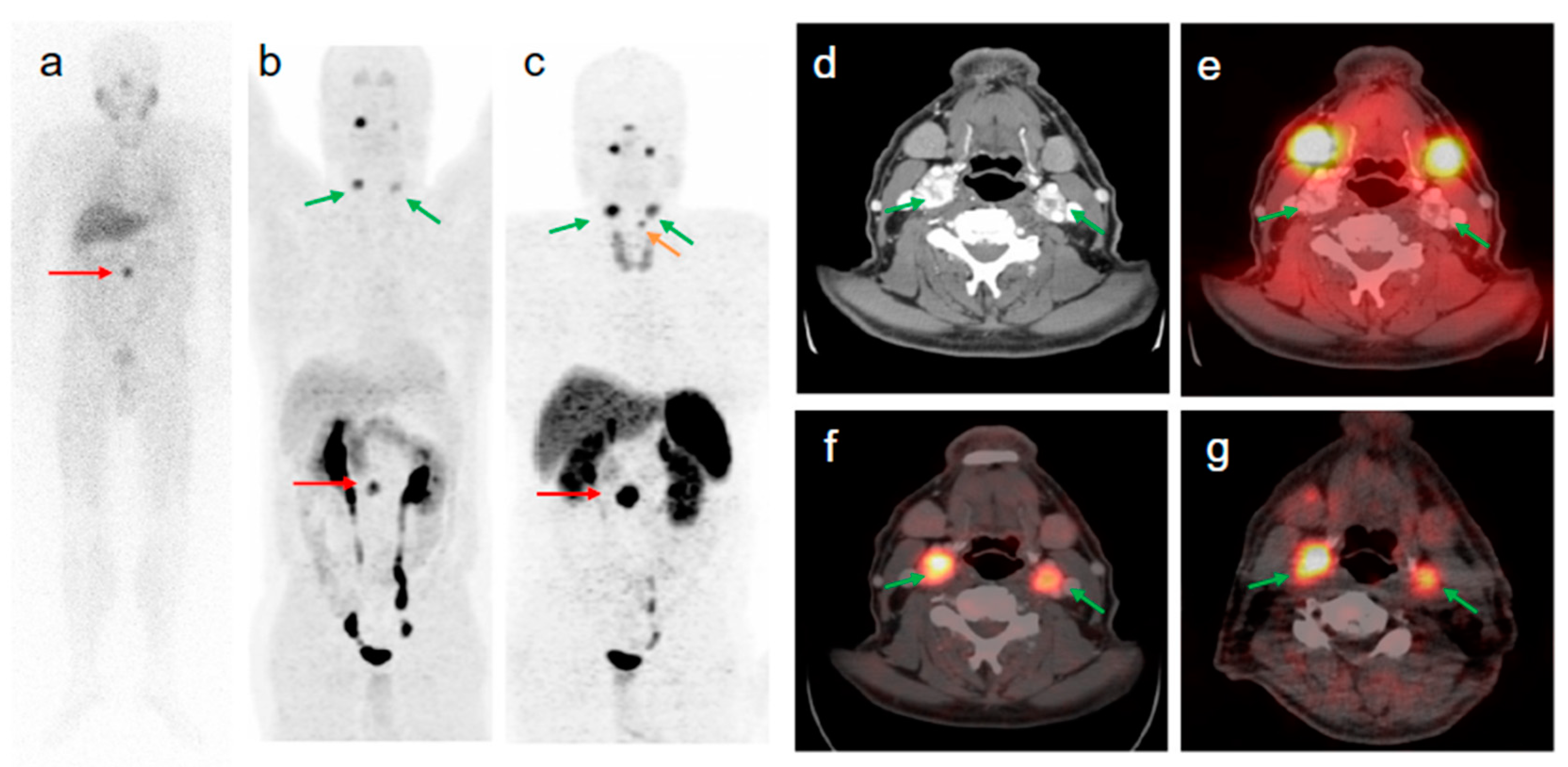
Figure 4.
A 41-year-old man with metastatic lymph nodes on [68Ga]Ga-DOTA-TATE PET/CT. He had undergone previous surgery for pancreatic NET (patient 5). Mesenteric lymph node from primary pancreatic NET was found with probe with TBR of 3.4 for this 0.8 cm lesion (WHO grade 1). (A) [68Ga]Ga-DOTA-TATE PET maximum intensity projection image showing mesenteric/duodenal lesion in segment 3 (red arrow) and multiple retroperitoneal lymph nodes. (B) [68Ga]Ga-DOTA-TATE PET/CT image showing mesenteric mass with SUVmax of 72.8 (red arrow). (C) Arterial phase CT showing corresponding mesenteric mass (red arrow). (D) Representative hematoxylin and eosin staining of tumor showing NET in lymph node, WHO grade 1. (E) Representative immunohistochemistry for MIB-1 staining showing <2% of cells positive. (F) Immunohistochemistry for SSTR2 showing representative image for score 3 (membranous pattern of SSTR2 staining in >50% of tumor cells) (original magnification, ×20). (G) Immunohistochemistry for SSTR5 showing representative image of tumor with >10% of tumor cells positive (original magnification, ×20). Reproduced from [15].
The tumor SUVmax reaches plateau 5 min post injection and remains unchanged within the range of 5–90 min [106] providing freedom of the examination logistics. However, it should be taken into consideration that the washout from the normal tissue and blood requires longer time influencing detection rate in the areas of high background uptake.
The fraction of patient treatments that were changed or adjusted based on [68Ga]Ga-SST/PET-CT examination was considerable and varied dependent on the patient cohort size and stratification [18,107,108]. The meta-analysis of clinical studies demonstrated that [68Ga]Ga-SST/PET-CT was vital for patient management leading to the regimen change in more than one third of patients (16%–71%) [109]. The treatment regimen was changed for 60% [110] and 50% [111] of the patients after [68Ga]Ga-SST/PET-CT. In the patient sub-group re-evaluated for recurrence, the treatment management was changed after [68Ga]Ga-SST/PET-CT in up to 25% of the patients [44]. Operative plans and diagnosis/management were adjusted, respectively in one-third and half of the patients after [68Ga]Ga-SST/PET-CT [112]. [68Ga]Ga-SST/PET-CT led to the treatment change in staggering 90.9% of patients with suspected recurrence [113]. [68Ga]Ga-SST/PET-CT changed the tumor staging from non-malignant to metastatic disease (Figure 5) [43].
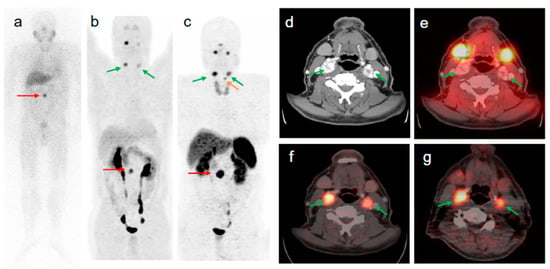
Figure 5.
58-year old male patient with malignant extra-adrenal PGL. The intense focal [123I]MIBG uptake of the abdomen seen on anterior planar image ((a) red arrow) was confirmed by [68Ga]Ga-DOTA-TOC- and [18F]FDOPA PET/CT as being a soft tissue extra-adrenal PGL ((b,c) red arrows). After retrospective software-based image fusion of SPECT images with diagnostic CT images (d), symmetrical physiological [123I]MIBG uptake was observed in the parotid and submandibular glands (a,e). All verified head and neck lesions with focal [68Ga]Ga-DOTA-TOC ((c,f) green arrows) and [18F]FDOPA uptake ((b,g) green arrows) were [123I]MIBG negative (a,e). The small focus of [68Ga]Ga-DOTA-TOC uptake superior to the left upper lobe of the thyroid gland (c orange arrow) is a PGL lesion of the larynx (confirmed by diagnostic CT) which was negative in [18F]FDOPA PET (b). Compared to [123I]MIBG imaging (including SPECT/CT), [68Ga]Ga-DOTA-TOC PET/CT changed the tumor staging from non-malignant to metastatic disease. Reproduced from [43].
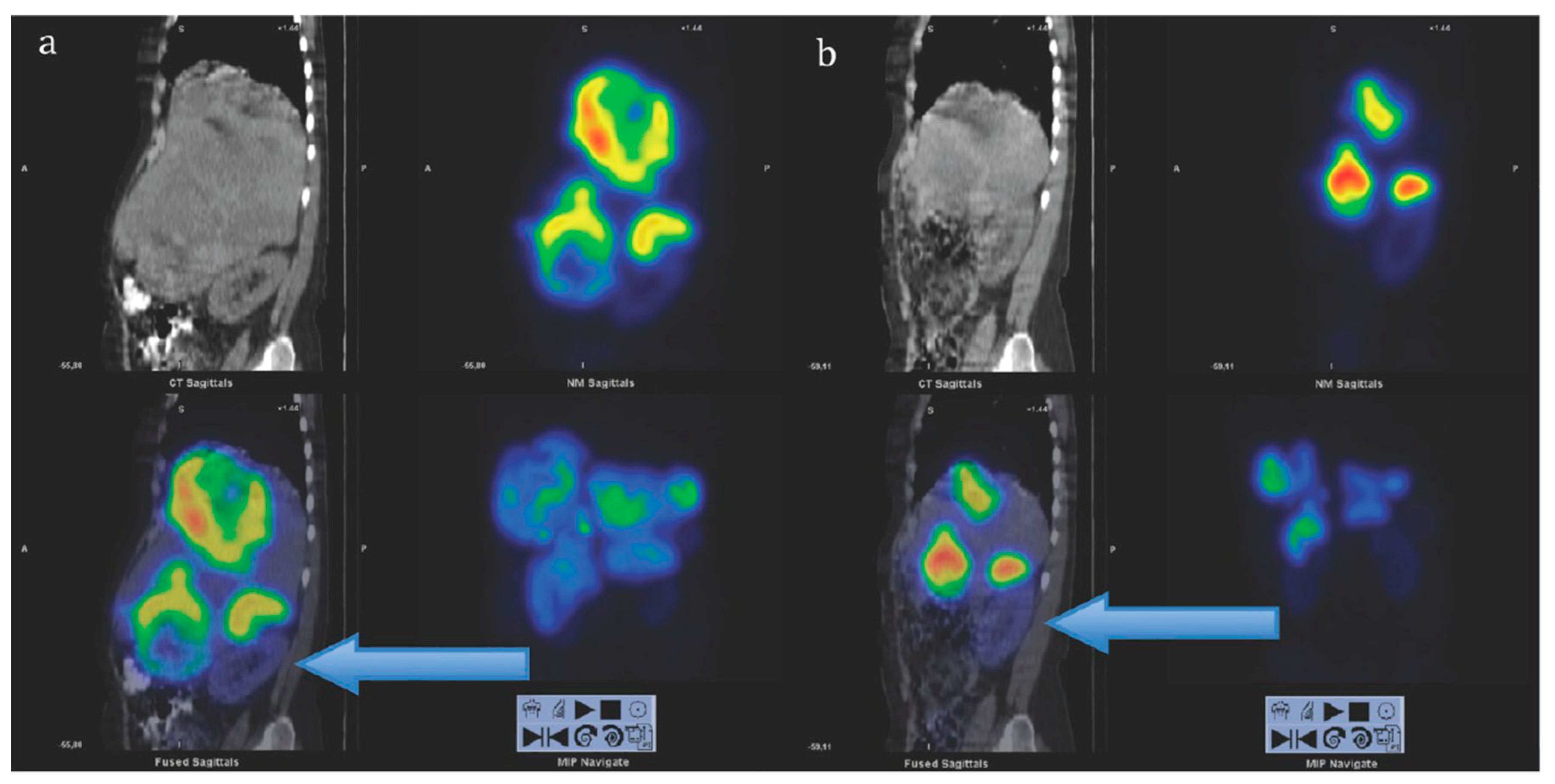
As mentioned above the receptor density and type vary among lesions and within the same lesion contributing to the considerable variation in the individual characteristics of patients with similar clinical presentations. Consequently, optimization of endoradiotherapy in terms of administered radioactivity dose, number of cycles, and time delay between the cycles is required [114]. It could be achieved by individual pre-therapeutic quantitative dosimetry that would allow dose planning: (1) To avoid radiotoxicity to the essential radiosensitive and excretory organs, e.g., bone marrow and kidneys, to organs with physiological uptake of the radiopharmaceutical and healthy tissue surrounding lesions; (2) to avoid undertreatment in case of high tumor burden. 177Lu emits gamma particles that can be detected by SPECT for the dosimetry measurement and calculation, however not prior but during the therapy course. The [177Lu]Lu-DOTA-TATE dosimetry feasibility and impact on radiotherapy efficacy and outcome was demonstrated wherein the survival improved with increased treatment cycle number determined based on dosimetry (Figure 6) [115]. Fractionated [177Lu]Lu-DOTA-TATE therapy based on dosimetry also improved the treatment outcome [116].
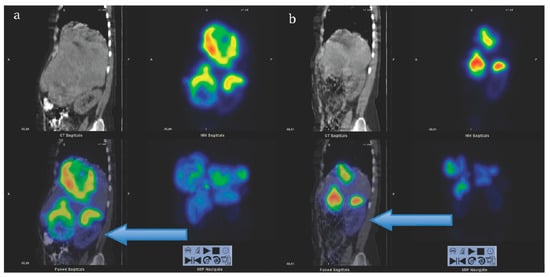
Figure 6.
Sagittal views of single photon emission computed tomography (SPECT)-CT over the abdomen at the level of the right kidney, 24 hrs after infusion of 7.4 GBq of [177Lu]Lu-DOTA-octreotate. (a) Cycle 1, May 2010 (b) cycle 7, August 2011. Left upper corner in each image: Attenuation correction CT, right upper corner attenuation corrected SPECT, left lower corner fused SPECT-CT, right lower corner maximum intensity projection (MIP). Note the position of the right kidney (arrow) and tracer distribution within the tumors. Reproduced from [116].
As mentioned above [177Lu]Lu-DOTA-TATE SPECT dosimetry can earliest be performed after the first treatment cycle and it requires 3–4 examinations within one week making logistics complex and elevating costs. Quantification accuracy, higher spatial resolution, and dynamic scanning of PET are strong advantages over SPECT. [68Ga]Ga-SST/PET by a single examination and with minimal radiation dose to healthy organs would provide the required information prior to the radiotherapy with higher spatial resolution and quantification accuracy, thus allowing for better selection of patients and radiation dose planning. However, the straightforward use is precluded by the difference in physical half-lives of the radionuclides (68 min (68Ga) vs 6.71 d (177Lu)) and thus different pharmacokinetic time window. Kinetic modeling could provide a solution wherein the early distribution time points could be acquired by [68Ga]Ga-SST/PET with high accuracy [2] and possibly extrapolated to match therapeutic radionuclide time window providing higher resolution and quantification accuracy to predict absorbed doses to tumors and healthy organs. However, a prospective clinical study is needed to confirm this hypothesis.
3. Targeting PSMA on Prostate Cancer
Prostate specific membrane antigen (PSMA) is a membrane bound protein overexpressed in prostate cancer, bladder carcinoma, schwannoma, and tumor neovasculature of many solid tumors [117]. The level of its expression is related to androgen independence, tumor aggressiveness, metastases, disease progression and recurrence, and the quantification of the upregulation would provide tool for accurate staging, prediction of aggressiveness and monitoring treatment response.
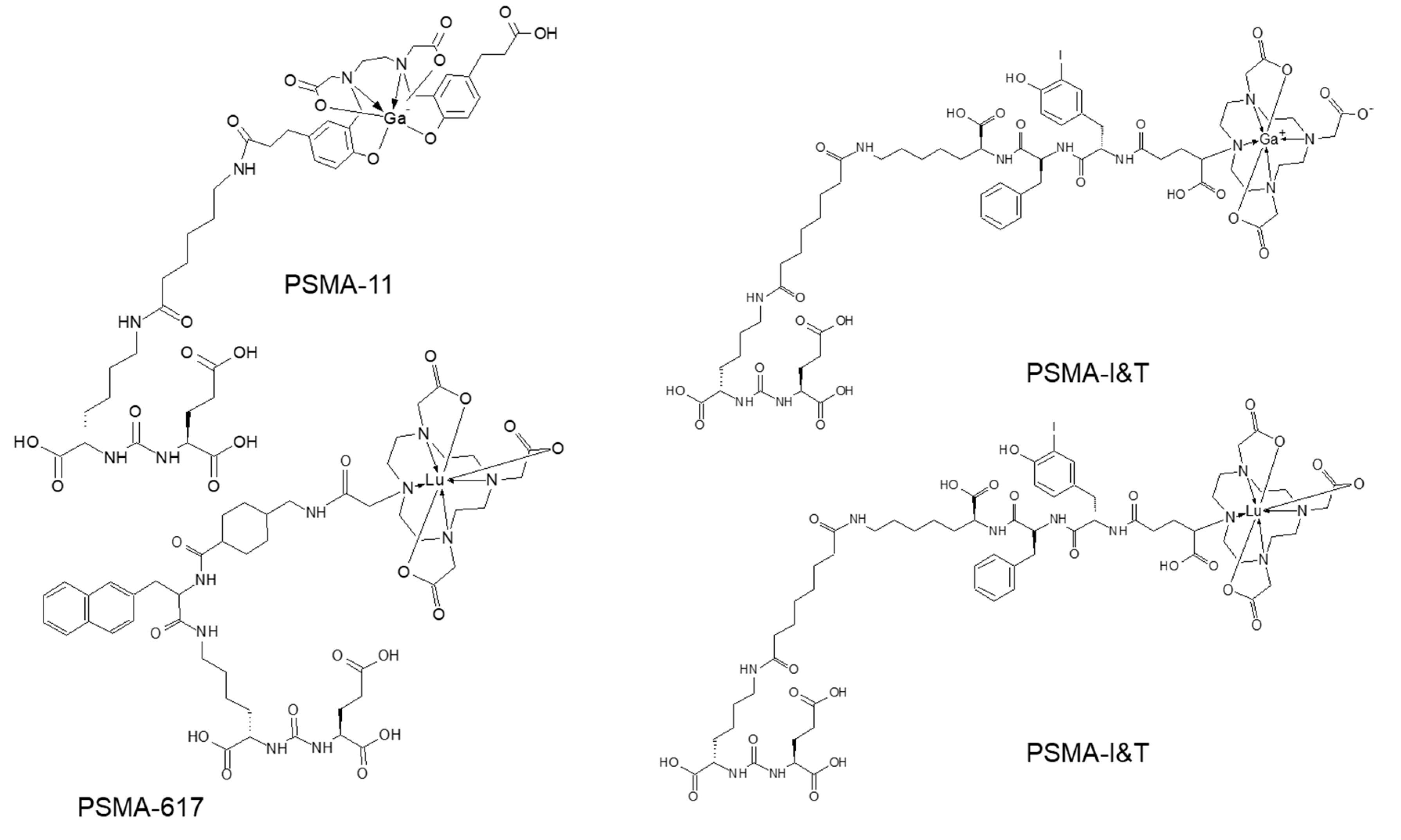
Urea-based inhibitors of prostate specific membrane antigen representing low-molecular-weight peptidomimetics can image PSMA-expressing prostate tumors. Most analogues currently used in nuclear medicine are based on Glu–urea–Glu or Glu–urea–Lys motifs and it has been an explosive clinical use of the analogues labeled with various radionuclides (this review is focused on the analogues presented in Figure 7) [118]. The major radiometal-based analogues in clinical studies comprise HBED (N,N′-bis [2-hydroxy-5-(carboxyethyl)benzyl] ethylenediamine) and DOTA (1,4,7,10-tetraazcyclododecane-N,N′,N″,N‴-tetraacetic acid) chelators.
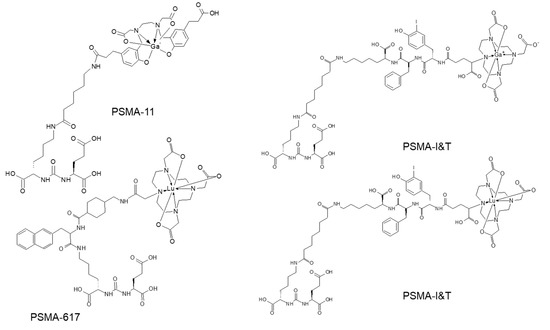
Figure 7.
Molecular structures of prostate specific membrane antigen (PSMA) analogues based on Glu–urea–Lys motif.
Impact of PSMA-Targeted Radiopharmaceuticals on Patient Treatment Management
In the first clinical trials, Glu-NH-CO-NH-Lys-(Ahx)-[68Ga(HBED-CC)] [119] demonstrated promising imaging results [120]. Since then numerous original research and review articles have pointed out that the PSMA imaging using PET/CT is a sensitive, specific, safe, efficient and reproducible diagnostic method allowing visualization of local disease, lymph node, bone, and visceral organ lesions with high detection rate, and it has positive predictive value [121,122,123,124,125,126,127,128]. Retrospective data analysis and prospective studies demonstrated the advantage of PSMA PET/CT compared to CT, MRI, and 99mTc-MDP in terms of sensitivity and specificity that are crucial parameters for staging accuracy and treatment planning. It is relevant for initial staging [129], early detection of biochemical recurrence [130,131], and therapy planning and monitoring [132,133] particularly in patients with metastatic castration-resistant prostate cancer (mCRPC). Significant correlation between SUVmax on [68Ga]Ga-PSMA PET/CT and PSMA expression in primary prostate cancer, determined histopathologically, was found and cut-off for SUVmax of 3.15 to discriminate tumor from normal prostate was recommended [134]. Early imaging 5 min post injection was suggested for distinguishing lesions from urinary bladder [135].
PSMA PET has demonstrated strong impact on therapy planning and clinical decision making with treatment regimen adjustment in 27%–77% of patients [136,137,138,139,140,141,142,143,144]. The most frequently used pair in the context of radiotheranostics is [68Ga]Ga-PSMA-11 and [177Lu]Lu-PSMA-617 (Figure 7). Despite the structural difference and various radionuclides, it was demonstrated that [68Ga]Ga-PSMA-11 PET plays an important role in predicting treatment response to [177Lu]Lu-PSMA-617 and monitoring response for patient treatment management optimization [131,145,146,147,148]. Phase II prospective clinical trial demonstrated correlation of the treatment response with the uptake of [68Ga]Ga-PSMA-11 (Figure 8) [146]. Encouraging results in terms of safety, efficiency, response rate, toxicity, and reduction of pain have been demonstrated by clinical trials assessing the role of [177Lu]Lu-PSMA-617 in patients with mCRPC (Figure 9) [131,132,149,150].
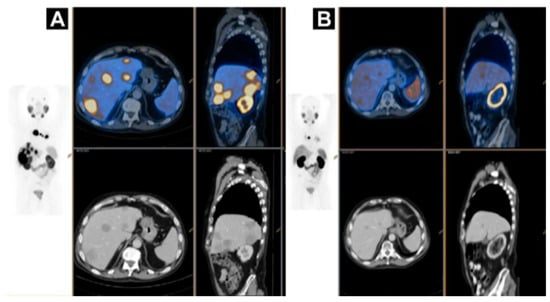
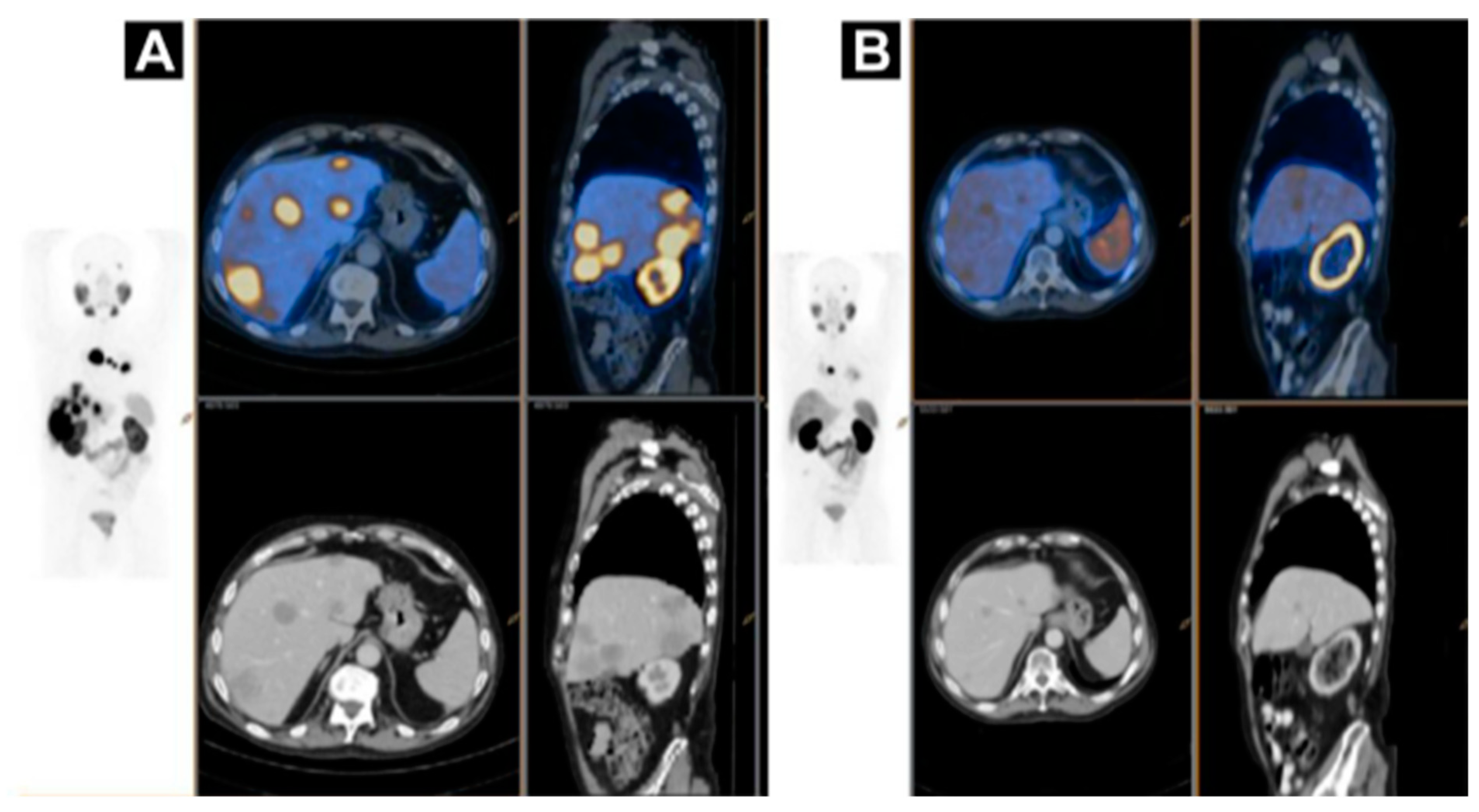
Figure 8.
A case of homogenous treatment response (A) [68Ga]Ga-HBEDD PSMA-11/PET-CT: Highly PSMA-avid liver and mediastinal lymph nodal metastases (SUVmax 70) on screening (PSA 340 ng/mL). (B) [68Ga]Ga-HBEDD PSMA-11/PET-CT: 3 months after 4 cycles of [177Lu]Lu-PSMA therapy (PSA 1.5 ng/mL) exhibited marked biomarker and RECIST response with minimal residual PSMA activity in liver metastases and solitary PSMA-avid mediastinal lymph node. Reproduced from [146].
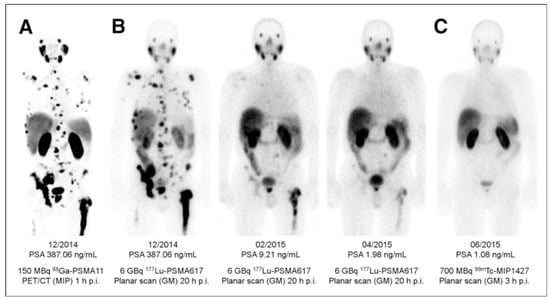
Figure 9.
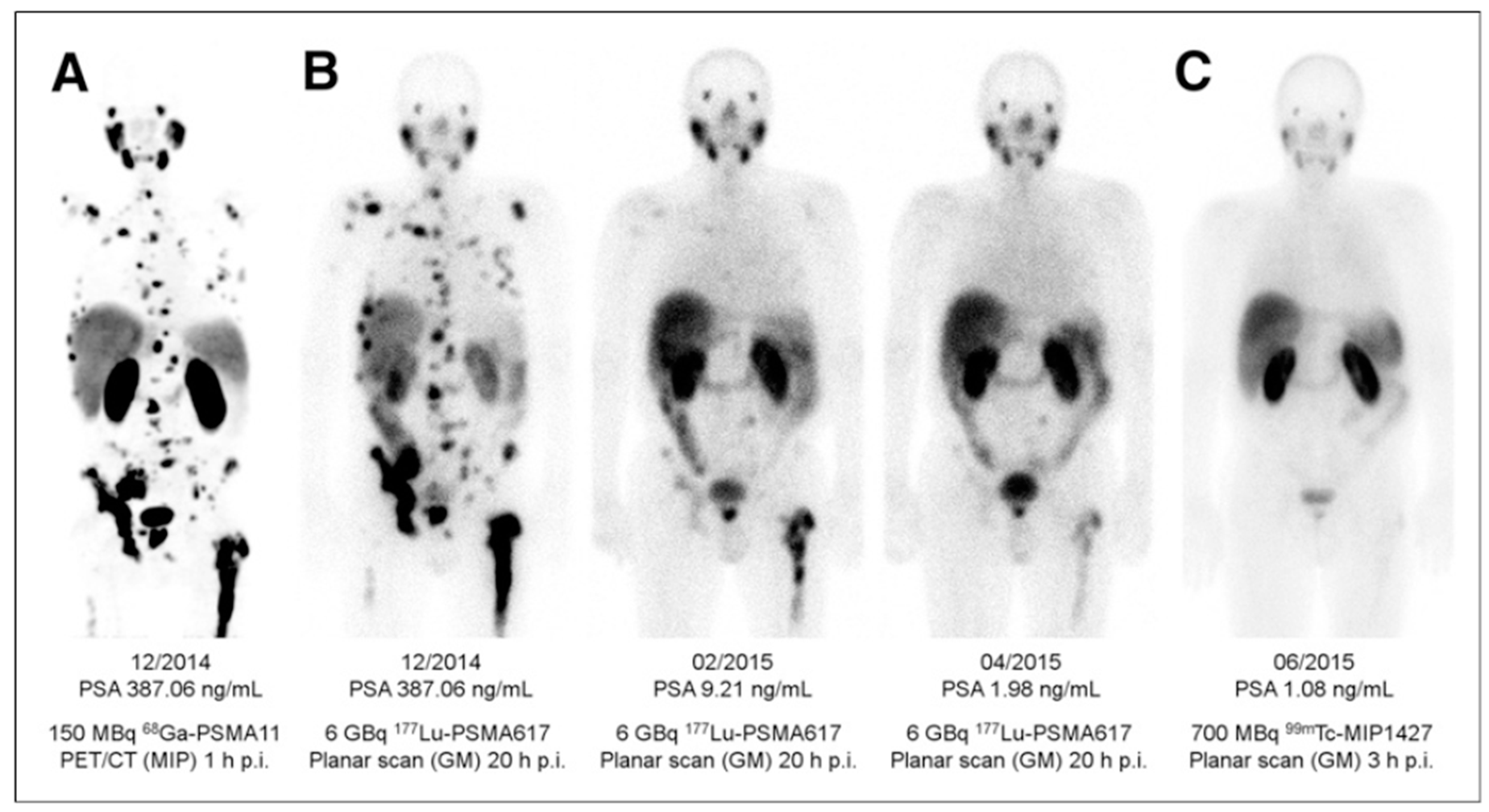
(A) Pre-therapeutic imaging using [68Ga]Ga-PSMA-11 PET/CT delivering highest resolution; (B) Co-emission of γ-rays by Lu-177 enables imaging during therapy with [177Lu]Lu-PSMA-617; (C) 99mTc-PSMA scintigraphy has minimally less noise than posttherapy scanning and can be used for imaging follow-up in out-patient setting. GM 5 geometric mean; MIP 5 maximum-intensity projections; p.i. 5 after injection. Reproduced from [132].
Another Glu–urea–Lys motif based analogue, PSMA I&T, was labeled with 68Ga and 177Lu (Figure 7) respectively for the imaging and radiotherapy and demonstrated safe and effective radiopharmaceutical properties [151,152]. Both [177Lu]Lu-PSMA-617 and [177Lu]Lu-PSMA-I&T are found beneficial for patients in terms of survival and side effects [153,154]. The stratification of the patients, that would benefit from the endoradiotherapy, pre-therapeutic dosimetry, and treatment response monitoring were based on [68Ga]Ga-PSMA-PET/CT examination showing high correlation between PET/SUVmax and absorbed tumor dose of 177Lu analogue [155,156,157]. The radiation sensitive organs such as kidneys, bone marrow, and salivary glands require individual dosimetry assessment due to the inter-patient variance and for the subsequent administered therapeutic dose adjustment [150,156]. The European Association of Nuclear Medicine published guidelines for radionuclide therapy with [177Lu]Lu-labeled PSMA-ligand wherein PSMA-ligand/PET and [18F]FDG/PET are recommended for the selection of patients that would benefit from the therapy [158]. PSMA-radioguided surgery in prostate cancer may further improve the treatment outcome [123,159,160].
4. Targeting HER2 on Breast Cancer
Pre-therapeutic imaging can be combined not only with endoradiotherapy, but also with chemotherapy yielding theranostic approach. HER2 is overexpressed in various malignant tumors, and particularly in 25% of breast cancer cases indicating poor survival [161,162,163,164,165,166]. Therapies based on antibodies and inhibitors targeting HER2 have revolutionized breast cancer treatment wherein the pre-therapeutic invasive biopsy for histopathological confirmation of sufficient HER2 expression (e.g., HercepTest®) for the patient selection and prediction of response is conducted [161,163,167,168,169,170]. However, heterogeneity of receptor expression within a lesion, and between the primary tumor and metastasis leads to such drawback with biopsy as sampling error. Moreover, it is highly invasive or not possible to perform sampling on bone and brain lesions as well as to collect samples from multiple lesions. Repeated sampling to monitor treatment response and receptor expression change over time is rarely possible in clinical practice [6,170,171,172]. Biopsy procedure causes patient distress and potential side effects such as infection and hemorrhage. The solution to provide whole-body HER2 receptor mapping and to overcome the biopsy drawbacks was found in radionuclide imaging, like in the cases of SSTR and PSMA. Various radiolabeled ligands based on antibodies, antibody fragments, EGFR natural ligand, Affibody® molecules, and tyrosine kinase inhibitors targeting HER2 have been developed and studied pre-clinically and clinically [18,173,174,175,176]. Anti-HER2 Affibody® molecule (Figure 10) presents advantages in terms of high affinity for HER2 receptors as well as favorable pharmacokinetics and clearance from non-target tissue [177,178,179,180,181]. The second generation Affibody® molecule, ABY-025, binds selectively to HER2 receptors with picomolar affinity. Importantly, the binding site differs from that of trastuzumab and pertuzumab thus allowing imaging during the respective treatment [182].
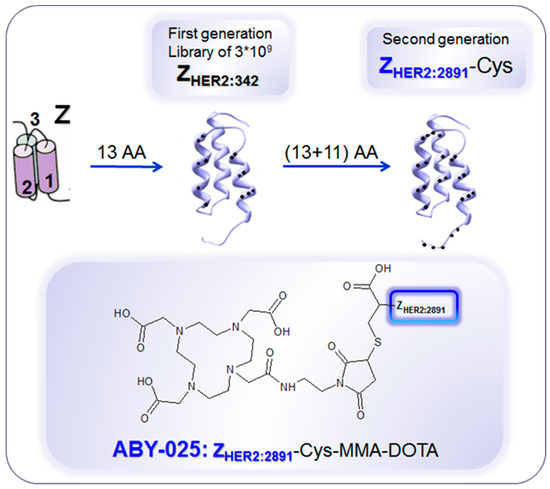
Figure 10.
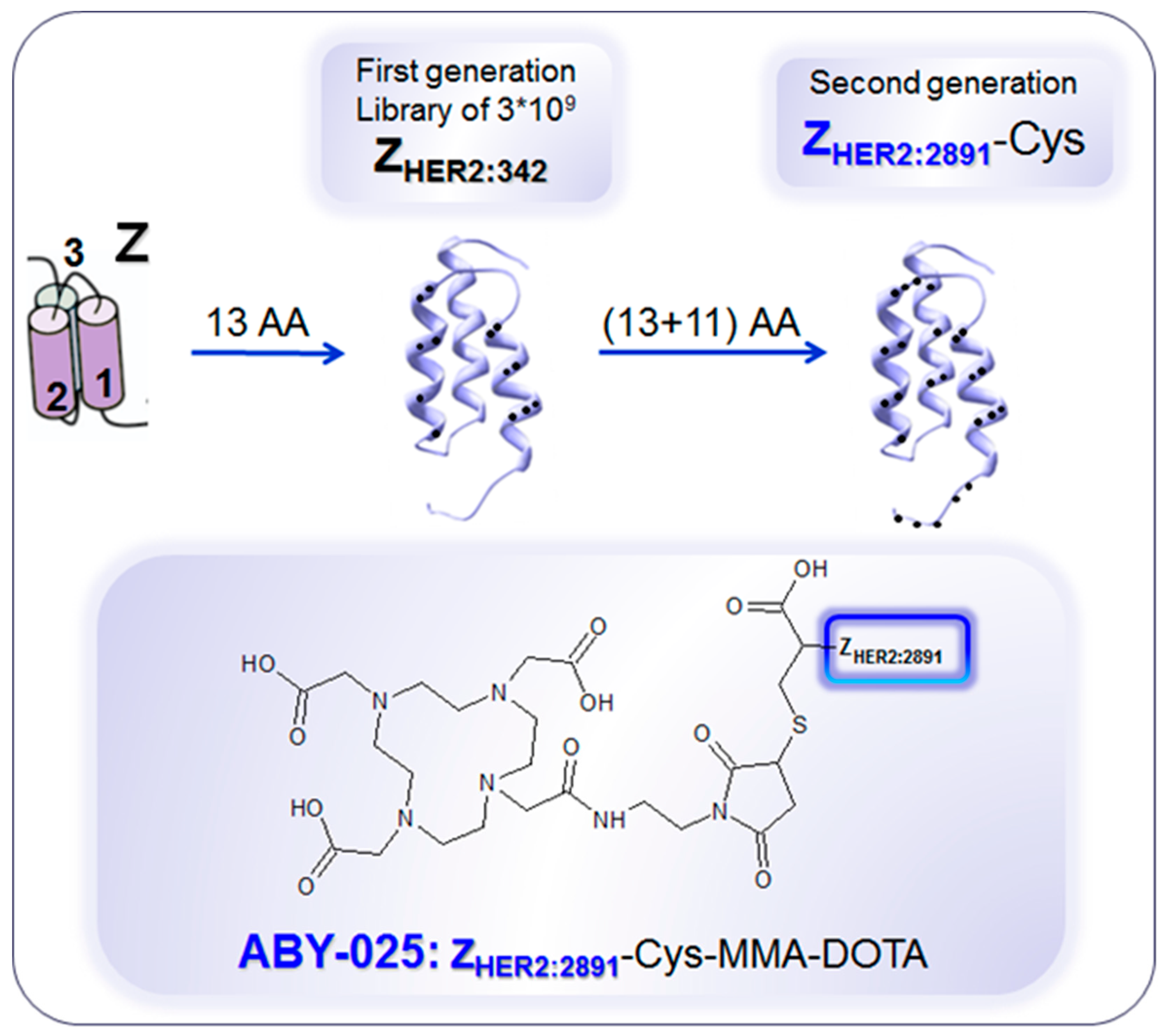
Development of anti-human epidermal growth factor receptor type 2 (HER2) Affibody® molecules. The variation of 13 amino acids (AA) on the binding surface of a 3-helix peptide structure (Z) resulted in a combinatorial library containing billions of variants from which the 1st generation HER2-binding Affibody molecule, ZHER2:342, was selected. Further modification of the non-binding surface resulted in the second generation Affibody molecule, ZHER2:2891, with higher thermal stability and hydrophilicity, diminished background interactions with immunoglobulins and production flexibility by peptide synthesis or recombinant expression as well as fully retained in vitro and in vivo functionality. ZHER2:2891 was modified by addition of a unique terminal cysteine for site-specific conjugation to the bifunctional chelator, 1,4,7,10-tetraaza cyclododecane-1,4,7-tris-acetic acid-10-maleimidoethylacetamide (MMA-DOTA). ZHER2:2891–Cys binds selectively to HER2 with high affinity (KD: 60 pM). Reproduced from [183].
Impact of [68Ga]Ga-ABY-025 PET-CT on Patient Treatment Management
SPECT and PET imaging using anti-HER2 Affibody® molecule [177,178,179,180,181,182] demonstrated the potential of safe, whole-body, and non-invasive “biopsy”, allowing receptor expression heterogeneity profiling (Figure 11), in clinical trials with ongoing multicenter Phase II/III one (NCT03655353) [5,183,184,185,186,187]. [68Ga]Ga-ABY-025 PET-CT presents advantages over [111In]In-ABY-025/SPECT/CT in terms of simpler logistics, higher resolution, higher detection rate, dynamic scanning, and accurate quantification [5,183] potentially allowing staging, prognosis, patient selection, quantification of the receptor expression and therapeutic drug dose estimation, early monitoring of the treatment response and resistance, residual disease, follow-up, and relapse.
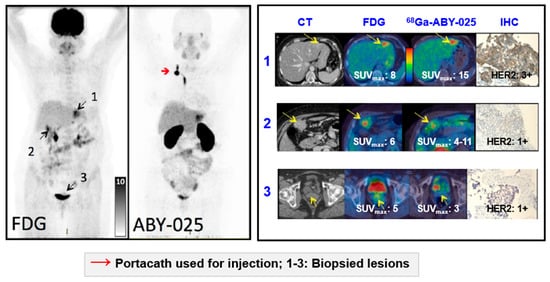
Figure 11.
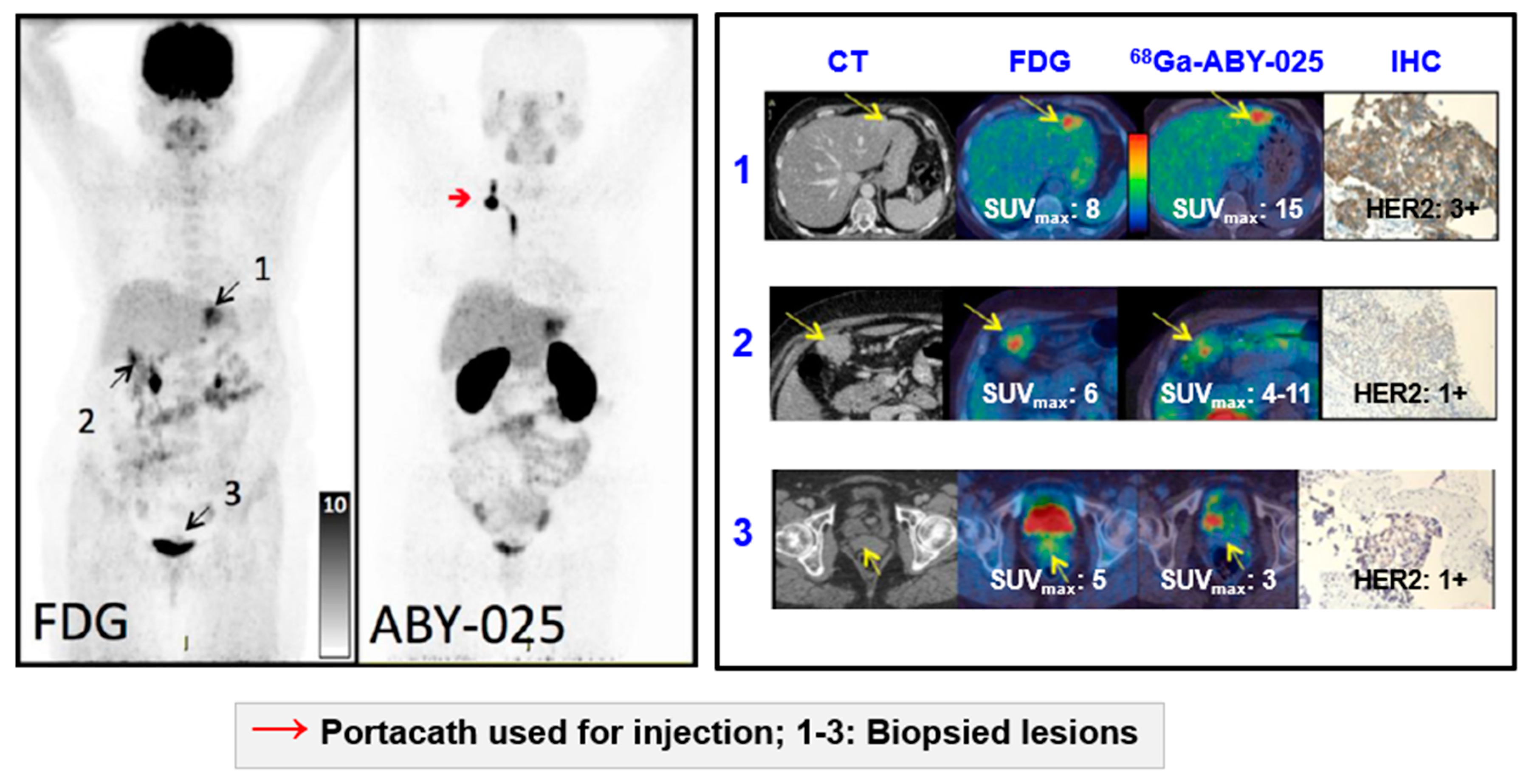
Based on the results from [68Ga]Ga-ABY-025 PET/CT, mixed expression of HER2 in metastatic breast cancer was seen in several patients and confirmed by biopsies in two. Patient 9 had HER2-negative primary tumor and was enrolled as negative control. [18F]FDG-PET/CT showed metastases in left liver lobe, peritoneal lymph nodes, and cervix of uterus. [68Ga]Ga-ABY-025 uptake was high in the liver metastasis, low in peritoneal metastases and absent in the cervical region (not shown). According to IHC, the liver finding was true positive and both other sites were true negative. Adopted from [5].
HER2-targeted treatment was changed as a consequence of [68Ga]Ga-ABY-025 PET examination in 19% (n = 16) of patients [5]. Figure 12 presents a case wherein the prior IHC analysis of the primary tumor biopsy specimen showed a borderline expression of HER2 and consequently the treatment with Trastuzumab was not considered. However, subsequent [68Ga]Ga-ABY-025/PET-CT detected bone metastasis and primary tumor with high SUVmax. The HER2-overexpression in the metastasis was confirmed by IHC. Consequently, the treatment regimen was significantly changed. Moreover, the false positive finding in the axilla by [18F]FDG/PET-CT (Figure 12D,F) was attributed to post-surgical inflammation. Given these examples it is difficult to overestimate importance of the targeting selectivity of [68Ga]Ga-ABY-025/PET-CT. Re-assessment of HER2 status is strongly encouraged due to high probability of the receptor conversion from positive to negative and vice versa [188]. Trastuzumab treatment was stopped after [68Ga]Ga-ABY-025/PET-CT examination that showed HER2 status conversion from positive to negative confirmed also by biopsy [5].
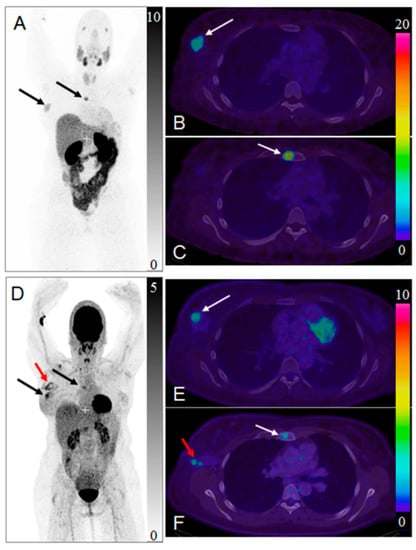
Figure 12.
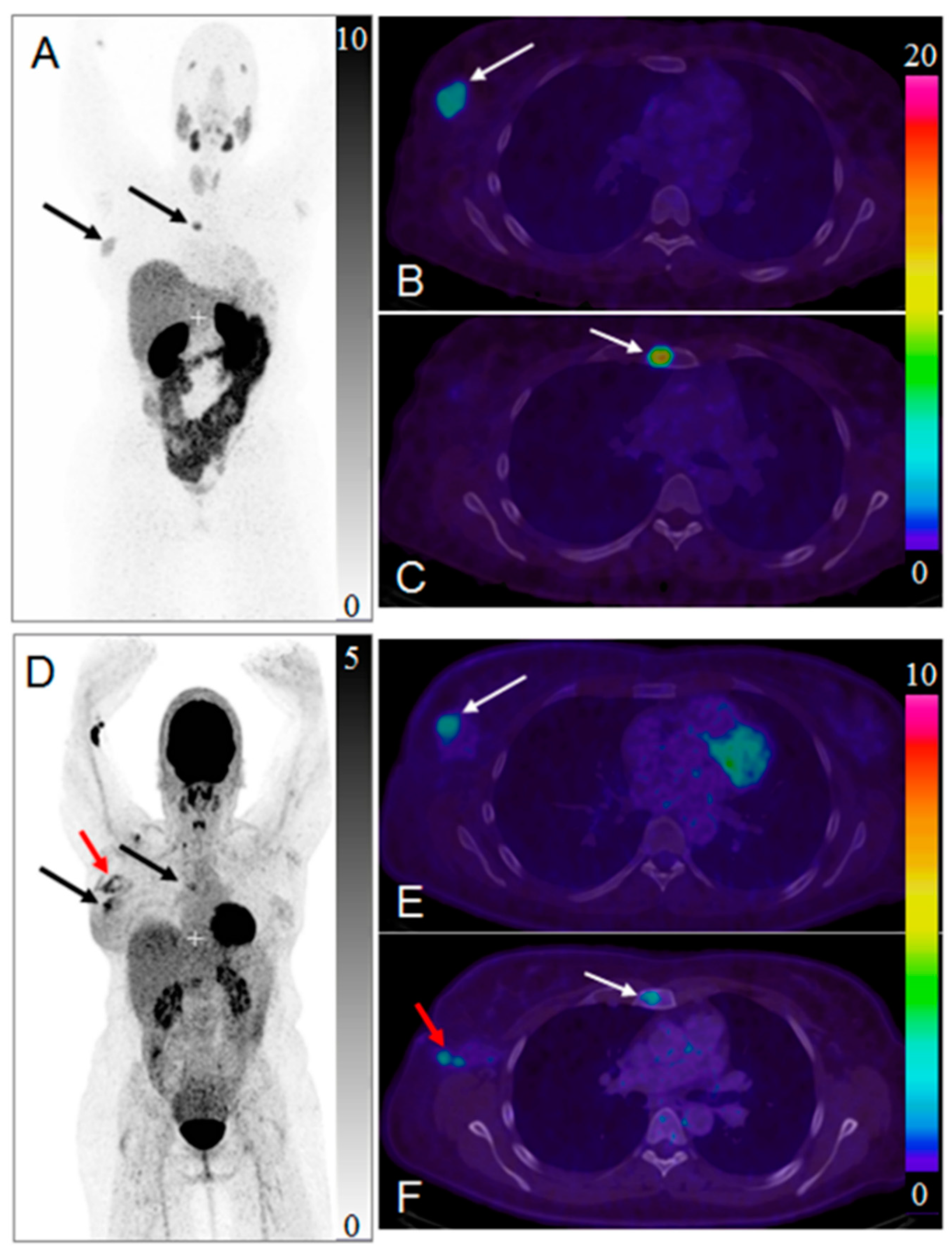
Maximum intensity projection positron emission tomography (PET) images of whole-body scan with [68Ga]Ga-ABY-025 ((A) 2 h) and [18F]FDG (D). Transaxial PET-CT fused images of the primary tumor ((B,E), respectively for [68Ga]Ga-ABY-025 (2 h) and [18F]FDG) and metastasis ((C,F), respectively for [68Ga]Ga-ABY-025 (2 h) and [18F]FDG). The black and white arrows indicate known tumor deposits. The red arrow (D,F) indicate post-surgical inflammation after biopsy wherein sentinel lymph node was tumor- and HER2-negative. Reproduced from [184].
Extraordinary receptor shedding was observed after the start of HER2-targeted therapy wherein most probably the on-going treatment had executed a rapid cytotoxic impact on the metastases with HER2 debris leaking into the blood stream [186]. Liver biopsy after PET examination showed fibrosis and no sign of remaining cancer cells. Serum-HER2 level at the time of scanning was almost one hundred times higher than the normal upper limit. The high serum-HER2 level resulted in drastically altered organ distribution of [68Ga]Ga-ABY-025.
As in the case of SST analogues [2,17,18,19], the strong influence of the administered peptide amount on the organ distribution, tumor uptake and dosimetry was observed in the case of [68Ga]Ga-ABY-025 wherein the higher peptide dose radiopharmaceutical (427 µg vs 78 µg) presented higher detection rate and image contrast, more favorable organ distribution and lower effective and absorbed doses [5,183,187]. These examples strongly advocate for the importance of individualized therapeutical dose determination.
In order to facilitate standardized multicenter trials and to enable dissemination of this diagnostic methodology for routine clinical use, it is necessary to provide data evaluation methods that are independent on the variation of PET scanner characteristics. Intra-image normalization such as tumor-to-reference tissue ratio (T/R) was investigated and spleen was found the most accurate approach providing a simple and robust semi-quantification of HER2 expression [186]. The spleen T/R ratio met the selection criteria such as correlation with biopsy analysis results, low variation of radioactivity uptake, and low probability of hosting metastases from breast cancer on spleen. The suggested cut-off for the discrimination between HER2-positive and HER2-negative lesions was set to 6.5. Another crucial aspect for [68Ga]Ga-ABY-025 PET-CT technology worldwide spreading is production and availability of the radiopharmaceutical [184]. The automated production procedure is currently used in the ongoing phase II/III study, aiming to validate the use of [68Ga]Ga-ABY-025/PET-CT for non-invasive assessment of HER2-status in breast cancers in a multicenter setting (ClinicalTrials.gov: NCT03655353).
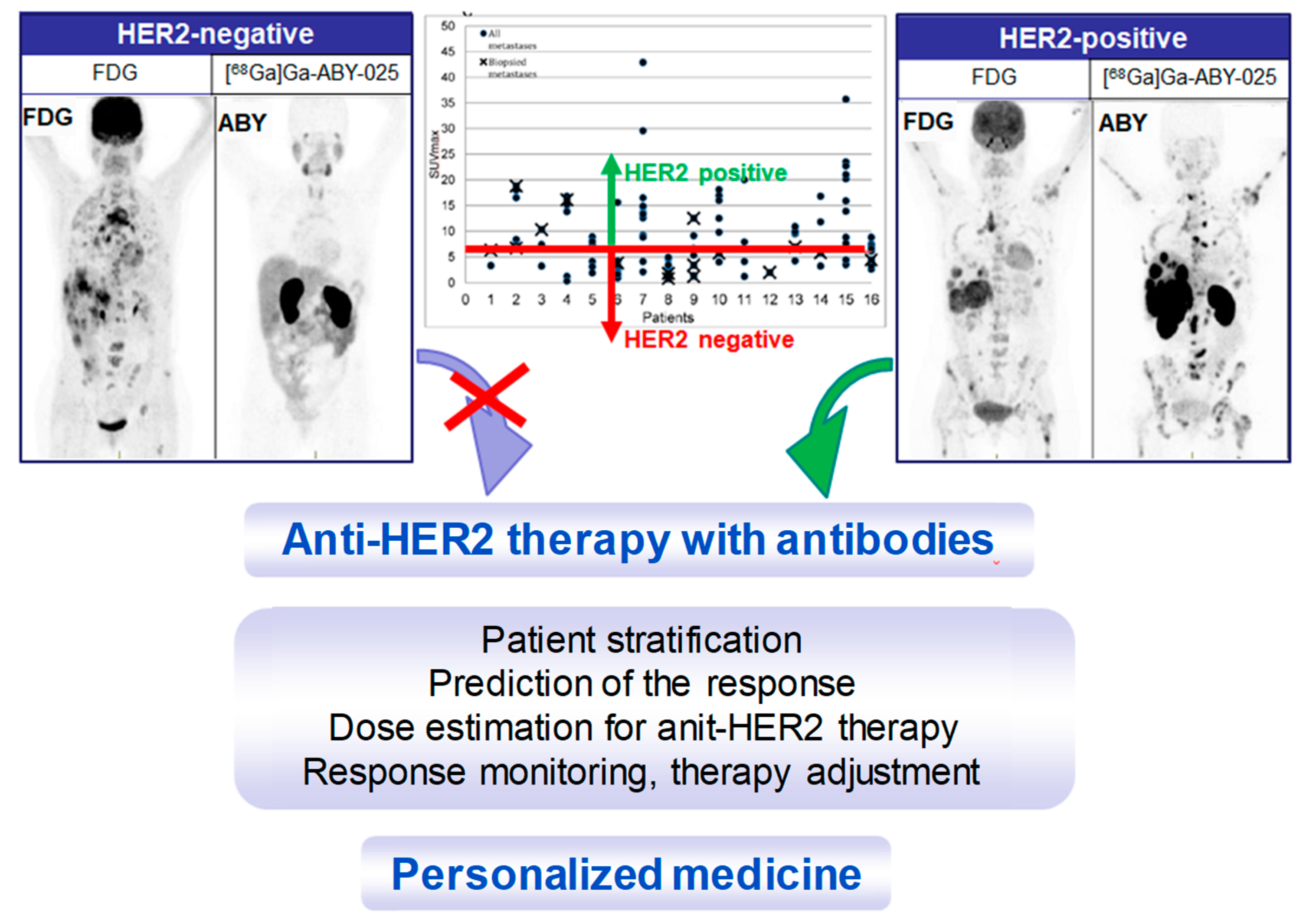
Reliable whole-body, quantitative assessment of HER2-receptor expression is crucial in order to identify patients with HER2-positive tumors that can benefit from HER2 targeted treatments (Figure 13). It is as important to avoid unnecessary cost and potential risk of serious adverse effects related to the treatment of patients with HER2-negative tumors. [68Ga]Ga-ABY-025 PET/CT has potential for therapy planning and treatment response monitoring, enabling adjustment of the treatment very early in the process.
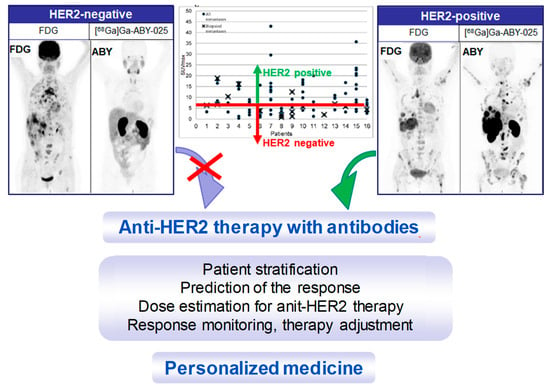
Figure 13.
Maximum intensity projection PET images from two studied patients with wide-spread metastatic breast cancer. Left panel: HER2-negative. Right panel: HER2-positive. All images are normalized to SUV 10. Darker colors indicate higher uptake. The graph presents the range of [68Ga]Ga-ABY-025 SUVmax in all nodular metastases (black dots) identified using [18F]FDG-PET/CT in all 16 patients undergoing [68Ga]Ga-ABY-025 PET/CT at 2 h after injection of tracer with high peptide content. Crosses symbolize biopsied metastases. The red line indicates a proposed threshold at SUVmax = 6 for discriminating HER2-positive and HER2-negative metastases. Adopted from [5].
5. Conclusions
These examples of NENs, prostate cancer, and breast cancer management using targeted imaging and (radio)therapy have proven the concept of (radio)theranostics for clinical practice valid. Numerous publications report on the alteration of treatment regimen based on radionuclide imaging that provides non-invasive, whole body mapping of the specific target in a single examination that can be safely repeated multiple times for monitoring treatment response and disease progression. Pre-therapeutic determination of the absorbed doses to normal organs and lesions is essential for treatment planning. However, the use of short-lived radionuclides for the prediction of dosimetry for long-lived therapeutic radionuclides presents challenge and therapeutic dose planning based on the receptor expression quantification awaits prospective clinical studies to prove the concept.
Nuclear medicine is becoming an important component in personalized patient treatment. Dissemination of the (radio)theranostic technology requires standardization and harmonization of the clinical protocols and data evaluation strategies, as well as accessibility and regulatory approval of radiopharmaceuticals.
Funding
This research received no external funding.
Conflicts of Interest
The author declares no conflict of interest.
References
- Velikyan, I. Radionuclides for imaging and therapy in oncology. In Cancer Theranostics; Chen, X., Wong, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 285–325. [Google Scholar]
- Velikyan, I.; Sundin, A.; Eriksson, B.; Lundqvist, H.; Sorensen, J.; Bergstrom, M.; Langstrom, B. In vivo binding of [68ga]-Dotatoc to somatostatin receptors in neuroendocrine tumours--Impact of peptide mass. Nucl. Med. Biol. 2010, 37, 265–275. [Google Scholar] [CrossRef]
- Sorensen, J.; Velikyan, I.; Wennborg, A.; Feldwisch, J.; Tolmachev, V.; Sandberg, D.; Nilsson, G.; Olofsson, H.; Sandstrom, M.; Lubberink, M.; et al. Measuring her2-Expression in metastatic breast cancer using 68ga-aby025 pet/ct. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, S226. [Google Scholar]
- Velikyan, I.; Wennborg, A.; Feldwisch, J.; Orlova, A.; Tolmachev, V.; Lubberink, M.; Sandstrom, M.; Lindman, H.; Carlsson, J.; Sorensen, J. Gmp compliant preparation of a 68gallium-Labeled affibody analogue for breast cancer patient examination: First-In-Man. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, S228–S229. [Google Scholar]
- Sörensen, J.; Velikyan, I.; Sandberg, D.; Wennborg, A.; Feldwisch, J.; Tolmachev, V.; Orlova, A.; Sandström, M.; Lubberink, M.; Olofsson, H.; et al. Measuring her2-Receptor expression in metastatic breast cancer using [68ga]aby-025 affibody pet/ct. Theranostics 2016, 6, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Zidan, J.; Dashkovsky, I.; Stayerman, C.; Basher, W.; Cozacov, C.; Hadary, A. Comparison of her-2 overexpression in primary breast cancer and metastatic sites and its effect on biological targeting therapy of metastatic disease. Br. J. Cancer 2005, 93, 552–556. [Google Scholar] [CrossRef]
- Werner, R.A.; Thackeray, J.T.; Pomper, M.G.; Bengel, F.M.; Gorin, M.A.; Derlin, T.; Rowe, S.P. Recent updates on molecular imaging reporting and data systems (mi-rads) for theranostic radiotracers-Navigating pitfalls of sstr- and psma-Targeted pet/ct. J. Clin. Med. 2019, 8, 60. [Google Scholar] [CrossRef] [PubMed]
- Velikyan, I. 68ga-Based radiopharmaceuticals: Production and application relationship. Molecules 2015, 20, 12913–12943. [Google Scholar] [CrossRef] [PubMed]
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the united states. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef]
- Pencharz, D.; Gnanasegaran, G.; Navalkissoor, S. Theranostics in neuroendocrine tumours: Somatostatin receptor imaging and therapy. Br. J. Radiol. 2018, 91, 20180108. [Google Scholar] [CrossRef]
- Singh, S.; Poon, R.; Wong, R.; Metser, U. 68ga pet imaging in patients with neuroendocrine tumors: A systematic review and meta-Analysis. Clin. Nucl. Med. 2018, 43, 802–810. [Google Scholar] [CrossRef]
- Sorbye, H.; Kong, G.; Grozinsky-Glasberg, S. Prrt in high-Grade gastroenteropancreatic neuroendocrine neoplasms (who g3). Endocr. Relat. Cancer 2019. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.; Wolin, E.; Chasen, B.; Kulke, M.; Bushnell, D.; Caplin, M.; Baum, R.P.; Kunz, P.; Hobday, T.; Hendifar, A.; et al. Health-Related quality of life in patients with progressive midgut neuroendocrine tumors treated with (177)lu-Dotatate in the phase iii netter-1 trial. J. Clin. Oncol. 2018, 36, 2578–2584. [Google Scholar] [CrossRef]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 trial of (177)lu-Dotatate for midgut neuroendocrine tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Sadowski, S.M.; Millo, C.; Neychev, V.; Aufforth, R.; Keutgen, X.; Glanville, J.; Alimchandani, M.; Nilubol, N.; Herscovitch, P.; Quezado, M.; et al. Feasibility of radio-Guided surgery with (6)(8)gallium-Dotatate in patients with gastro-Entero-Pancreatic neuroendocrine tumors. Ann. Surg. Oncol. 2015, 22 (Suppl. 3), S676–682. [Google Scholar] [CrossRef]
- El Lakis, M.; Gianakou, A.; Nockel, P.; Wiseman, D.; Tirosh, A.; Quezado, M.A.; Patel, D.; Nilubol, N.; Pacak, K.; Sadowski, S.M.; et al. Radioguided surgery with gallium 68 dotatate for patients with neuroendocrine tumors. JAMA Surg. 2019, 154, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Cherk, M.H.; Kong, G.; Hicks, R.J.; Hofman, M.S. Changes in biodistribution on (68)ga-Dota-Octreotate pet/ct after long acting somatostatin analogue therapy in neuroendocrine tumour patients may result in pseudoprogression. Cancer Imaging 2018, 18, 3. [Google Scholar] [CrossRef] [PubMed]
- Velikyan, I. Positron emitting [68ga]ga-Based imaging agents: Chemistry and diversity. Med. Chem. 2011, 7, 338–372. [Google Scholar] [CrossRef]
- Sabet, A.; Nagarajah, J.; Dogan, A.S.; Biersack, H.J.; Sabet, A.; Guhlke, S.; Ezziddin, S. Does prrt with standard activities of 177luoctreotate really achieve relevant somatostatin receptor saturation in target tumor lesions?: Insights from intra-therapeutic receptor imaging in patients with metastatic gastroenteropancreatic neuroendocrine tumors. EJNMMI Res. 2013, 3, 1–6. [Google Scholar]
- Sundin, A.; Arnold, R.; Baudin, E.; Cwikla, J.B.; Eriksson, B.; Fanti, S.; Fazio, N.; Giammarile, F.; Hicks, R.J.; Kjaer, A.; et al. Enets consensus guidelines for the standards of care in neuroendocrine tumors: Radiological, nuclear medicine & hybrid imaging. Neuroendocrinology 2017, 105, 212–244. [Google Scholar]
- Hope, T.A.; Bergsland, E.K.; Bozkurt, M.F.; Graham, M.; Heaney, A.P.; Herrmann, K.; Howe, J.R.; Kulke, M.H.; Kunz, P.L.; Mailman, J.; et al. Appropriate use criteria for somatostatin receptor pet imaging in neuroendocrine tumors. J. Nucl. Med. 2018, 59, 66–74. [Google Scholar] [CrossRef]
- Sollini, M.; Farioli, D.; Froio, A.; Chella, A.; Asti, M.; Boni, R.; Grassi, E.; Roncali, M.; Versari, A.; Erba, P.A. Brief report on the use of radiolabeled somatostatin analogs for the diagnosis and treatment of metastatic small-Cell lung cancer patients. J. Thorac. Oncol. 2013, 8, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Ambrosini, V.; Nanni, C.; Fanti, S. The use of gallium-68 labeled sstrs in pet/ct imaging. PET Clin. 2014, 9, 323–329. [Google Scholar] [CrossRef]
- Maffione, A.M.; Karunanithi, S.; Kumar, R.; Rubello, D.; Alavi, A. Nuclear medicine procedures in the diagnosis of net: A historical perspective. PET Clin. 2014, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.; Schwartz, L. Imaging of neuroendocrine tumors. Semin. Oncol. 2013, 40, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Balogova, S.; Talbot, J.N.; Nataf, V.; Michaud, L.; Huchet, V.; Kerrou, K.; Montravers, F. 18f-fluorodihydroxyphenylalanine vs other radiopharmaceuticals for imaging neuroendocrine tumours according to their type. Eur. J. Nucl. Med. Mol. Imaging 2013, 1–24. [Google Scholar] [CrossRef]
- Kroiss, A.; Putzer, D.; Frech, A.; Decristoforo, C.; Uprimny, C.; Gasser, R.W.; Shulkin, B.L.; Url, C.; Widmann, G.; Prommegger, R.; et al. A retrospective comparison between 68ga-Dota-Toc pet/ct and 18f-Dopa pet/ct in patients with extra-Adrenal paraganglioma. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 1800–1808. [Google Scholar] [CrossRef]
- Yilmaz, S.; Ocak, M.; Asa, S.; Gülsen, F.; Halac, M.; Kabasakal, L. Appearance of intracranial meningioma in FDG and 68ga-DOTATOC PET/CT. Rev. Esp. Med. Nucl. Imagen Mol. 2013, 32, 60–61. [Google Scholar]
- Castaldi, P.; Treglia, G.; Rufini, V. Multifocal head and neck paraganglioma evaluated with different pet tracers: Comparison between fluorine-18-Fluorodeoxyglucose and gallium-68-Somatostatin receptor pet/ct. Nucl. Med. Mol. Imaging 2013, 47, 218–219. [Google Scholar] [CrossRef][Green Version]
- Epstude, M.; Tornquist, K.; Riklin, C.; di Lenardo, F.; Winterhalder, R.; Hug, U.; Strobel, K. Comparison of (18)f-fdg pet/ct and (68)ga-Dotatate pet/ct imaging in metastasized merkel cell carcinoma. Clin. Nucl. Med. 2013, 38, 283–284. [Google Scholar] [CrossRef]
- Venkitaraman, B.; Karunanithi, S.; Kumar, A.; Khilnani, G.C.; Kumar, R. Role of 68ga-Dotatoc pet/ct in initial evaluation of patients with suspected bronchopulmonary carcinoid. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 856–864. [Google Scholar] [CrossRef]
- Rufini, V.; Treglia, G.; Castaldi, P.; Perotti, G.; Giordano, A. Comparison of metaiodobenzylguanidine scintigraphy with positron emission tomography in the diagnostic work-up of pheochromocytoma and paraganglioma: A systematic review. Q. J. Nucl. Med. Mol. Imaging 2013, 57, 122–133. [Google Scholar] [PubMed]
- Damle, N.A.; Kumar, R.; Tripathi, M.; Bal, C. Positive (68)ga-Dotanoc pet/ct with negative (131)i-Metaiodobenzylguanidine scan in a case of glomus jugulare. Indian J. Endocrinol. Metab. 2013, 17, 765–767. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Thakar, A.; Suman, K.C.S.; Dhull, V.S.; Singh, H.; Naswa, N.; Reddy, R.M.; Karunanithi, S.; Kumar, R.; Malhotra, A.; et al. 68ga-Dotanoc pet/ct for baseline evaluation of patients with head and neck paraganglioma. J. Nucl. Med. 2013, 54, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Charron, M. Contemporary approach to diagnosis and treatment of neuroblastoma. Q. J. Nucl. Med. Mol. Imaging 2013, 57, 40–52. [Google Scholar] [PubMed]
- Treglia, G.; Inzani, F.; Campanini, N.; Rindi, G.; Agnes, S.; Giordano, A.; Rufini, V. A case of insulinoma detected by 68ga-Dotanoc pet/ct and missed by 18f-Dihydroxyphenylalanine pet/ct. Clin. Nucl. Med. 2013, 38, e267–e268. [Google Scholar] [CrossRef]
- Breer, S.; Brunkhorst, T.; Beil, F.T.; Peldschus, K.; Heiland, M.; Klutmann, S.; Barvencik, F.; Zustin, J.; Gratz, K.F.; Amling, M. 68ga dota-Tate pet/ct allows tumor localization in patients with tumor-Induced osteomalacia but negative 111in-Octreotide spect/ct. Bone 2014, 64, 222–227. [Google Scholar] [CrossRef]
- Jadhav, S.; Kasaliwal, R.; Lele, V.; Rangarajan, V.; Chandra, P.; Shah, H.; Malhotra, G.; Jagtap, V.S.; Budyal, S.; Lila, A.R.; et al. Functional imaging in primary tumour-Induced osteomalacia: Relative performance of fdg pet/ct vs somatostatin receptor-based functional scans: A series of nine patients. Clin. Endocrinol. 2014, 81, 31–37. [Google Scholar] [CrossRef]
- Gilardi, L.; Colandrea, M.; Fracassi, S.L.; Sansovini, M.; Paganelli, G. 68ga-dota0-tyr3octreotide (dotatoc) positron emission tomography (pet)/ct in five cases of ectopic adrenocorticotropin-Secreting tumours. Clin. Endocrinol. 2014, 81, 152–153. [Google Scholar] [CrossRef]
- Walker, R.C.; Smith, G.T.; Liu, E.; Moore, B.; Clanton, J.; Stabin, M. Measured human dosimetry of 68ga-Dotatate. J. Nucl. Med. 2013, 54, 855–860. [Google Scholar] [CrossRef]
- Toumpanakis, C.; Kim, M.K.; Rinke, A.; Bergestuen, D.S.; Thirlwell, C.; Khan, M.S.; Salazar, R.; Oberg, K. Combination of cross-Sectional and molecular imaging studies in the localization of gastroenteropancreatic neuroendocrine tumors. Neuroendocrinology 2014, 99, 63–74. [Google Scholar] [CrossRef]
- Gabriel, S.; Garrigue, P.; Dahan, L.; Castinetti, F.; Sebag, F.; Baumstark, K.; Archange, C.; Jha, A.; Pacak, K.; Guillet, B.; et al. Prospective evaluation of (68) ga-Dotatate pet/ct in limited disease neuroendocrine tumours and/or elevated serum neuroendocrine biomarkers. Clin. Endocrinol. (Oxf.) 2018, 82, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Kroiss, A.S.; Uprimny, C.; Shulkin, B.L.; Gruber, L.; Frech, A.; Url, C.; Riechelmann, H.; Sprinzl, G.M.; Thome, C.; Treglia, G.; et al. (68)ga-Dotatoc pet/ct in the localization of head and neck paraganglioma compared with (18)f-Dopa pet/ct and (123)i-mibg spect/ct. Nucl. Med. Biol. 2019, 71, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Fallahi, B.; Manafi-Farid, R.; Eftekhari, M.; Fard-Esfahani, A.; Emami-Ardekani, A.; Geramifar, P.; Akhlaghi, M.; Hashemi Taheri, A.P.; Beiki, D. Diagnostic fficiency of (68)ga-Dotatate pet/ct as ompared to (99m)tc-Octreotide spect/ct andonventional orphologic odalities in euroendocrine umors. Asia Ocean J. Nucl. Med. Biol. 2019, 7, 129–140. [Google Scholar] [PubMed]
- Han, S.; Suh, C.H.; Woo, S.; Kim, Y.J.; Lee, J.J. Performance of (68)ga-Dota-Conjugated somatostatin receptor-Targeting peptide pet in detection of pheochromocytoma and paraganglioma: A systematic review and metaanalysis. J. Nucl. Med. 2019, 60, 369–376. [Google Scholar] [CrossRef]
- Naswa, N.; Sharma, P.; Gupta, S.K.; Karunanithi, S.; Reddy, R.M.; Patnecha, M.; Lata, S.; Kumar, R.; Malhotra, A.; Bal, C. Dual tracer functional imaging of gastroenteropancreatic neuroendocrine tumors using 68ga-dota-noc pet-ct and 18f-fdg pet-ct: Competitive or complimentary? Clin. Nucl. Med. 2014, 39, e27–e34. [Google Scholar] [CrossRef]
- Zhao, X.; Xiao, J.; Xing, B.; Wang, R.; Zhu, Z.; Li, F. Comparison of 68ga dotatate to 18f-fdg uptake is useful in the differentiation of residual or recurrent pituitary adenoma from the remaining pituitary tissue after transsphenoidal adenomectomy. Clin. Nucl. Med. 2014, 39, 605–608. [Google Scholar] [CrossRef]
- Lococo, F.; Treglia, G. Which is the best strategy for diagnosing bronchial carcinoid tumours? The role of dual tracer pet/ct scan. Hell. J. Nucl. Med. 2014, 17, 7–9. [Google Scholar]
- Lococo, F.; Cesario, A.; Paci, M.; Filice, A.; Versari, A.; Rapicetta, C.; Ricchetti, T.; Sgarbi, G.; Alifano, M.; Cavazza, A.; et al. Pet/ct assessment of neuroendocrine tumors of the lung with special emphasis on bronchial carcinoids. Tumor Biol. 2014, 35, 8369–8377. [Google Scholar] [CrossRef]
- Treglia, G.; Giovanella, L. Could 68ga-Somatostatin analogues replace other pet tracers in evaluating extra-Adrenal paragangliomas? Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 1797–1799. [Google Scholar] [CrossRef]
- Hindie, E. The netpet score: Combining fdg and somatostatin receptor imaging for optimal management of patients with metastatic well-Differentiated neuroendocrine tumors. Theranostics 2017, 7, 1159–1163. [Google Scholar] [CrossRef]
- Ashwathanarayana, A.G.; Biswal, C.K.; Sood, A.; Parihar, A.S.; Kapoor, R.; Mittal, B.R. Imaging-Guided use of combined (177)lu-Dotatate and capecitabine therapy in metastatic mediastinal paraganglioma. J. Nucl. Med. Technol. 2017, 45, 314–316. [Google Scholar] [CrossRef] [PubMed]
- Waseem, N.; Aparici, C.M.; Kunz, P.L. Evaluating the role of theranostics in grade 3 neuroendocrine neoplasms. J. Nucl. Med. 2019, 60, 882–891. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Kulkarni, H.R.; Singh, A.; Niepsch, K.; Muller, D.; Baum, R.P. Peptide receptor radionuclide therapy in grade 3 neuroendocrine neoplasms: Safety and survival analysis in 69 patients. J. Nucl. Med. 2019, 60, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Schraml, C.; Schwenzer, N.F.; Sperling, O.; Aschoff, P.; Lichy, M.P.; Uller, M.; Brendle, C.; Werner, M.K.; Claussen, C.D.; Pfannenberg, C. Staging of neuroendocrine tumours: Comparison of [68ga]dotatoc multiphase pet/ct and whole-Body mri. Cancer Imaging 2013, 13, 63–72. [Google Scholar] [CrossRef]
- Graf, R.; Nyuyki, F.; Steffen, I.G.; Michel, R.; Fahdt, D.; Wust, P.; Brenner, W.; Budach, V.; Wurm, R.; Plotkin, M. Contribution of 68ga-Dotatoc pet/ct to target volume delineation of skull base meningiomas treated with stereotactic radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 68–73. [Google Scholar] [CrossRef]
- Klinaki, I.; Al-Nahhas, A.; Soneji, N.; Win, Z. 68ga dota-Tate pet/ct uptake in spinal lesions and mri correlation on a patient with neuroendocrine tumor: Potential pitfalls. Clin. Nucl. Med. 2013, 38, e449–e453. [Google Scholar] [CrossRef]
- Mayerhoefer, M.E.; Ba-Ssalamah, A.; Weber, M.; Mitterhauser, M.; Eidherr, H.; Wadsak, W.; Raderer, M.; Trattnig, S.; Herneth, A.; Karanikas, G. Gadoxetate-Enhanced versus diffusion-Weighted mri for fused ga-68-Dotanoc pet/mri in patients with neuroendocrine tumours of the upper abdomen. Eur. Radiol. 2013, 23, 1978–1985. [Google Scholar] [CrossRef]
- Schmid-Tannwald, C.; Schmid-Tannwald, C.M.; Morelli, J.N.; Neumann, R.; Haug, A.R.; Jansen, N.; Nikolaou, K.; Schramm, N.; Reiser, M.F.; Rist, C. Comparison of abdominal mri with diffusion-Weighted imaging to 68ga-Dotatate pet/ct in detection of neuroendocrine tumors of the pancreas. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 897–907. [Google Scholar] [CrossRef]
- Wulfert, S.; Kratochwil, C.; Choyke, P.L.; Afshar-Oromieh, A.; Mier, W.; Kauczor, H.U.; Schenk, J.P.; Haberkorn, U.; Giesel, F.L. Multimodal imaging for early functional response assessment of 90y-/177lu-dotatoc peptide receptor targeted radiotherapy with dw-mri and 68ga-Dotatoc-pet/ct. Mol. Imaging Biol. 2014, 16, 586–594. [Google Scholar] [CrossRef]
- Gaertner, F.C.; Beer, A.J.; Souvatzoglou, M.; Eiber, M.; Furst, S.; Ziegler, S.I.; Brohl, F.; Schwaiger, M.; Scheidhauer, K. Evaluation of feasibility and image quality of 68ga-Dotatoc positron emission tomography/magnetic resonance in comparison with positron emission tomography/computed tomography in patients with neuroendocrine tumors. Invest. Radiol. 2013, 48, 263–272. [Google Scholar] [CrossRef]
- Wiesmüller, M.; Quick, H.H.; Navalpakkam, B.; Lell, M.M.; Uder, M.; Ritt, P.; Schmidt, D.; Beck, M.; Kuwert, T.; Von Gall, C.C. Comparison of lesion detection and quantitation of tracer uptake between pet from a simultaneously acquiring whole-Body pet/mr hybrid scanner and pet from pet/ct. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Beiderwellen, K.J.; Poeppel, T.D.; Hartung-Knemeyer, V.; Buchbender, C.; Kuehl, H.; Bockisch, A.; Lauenstein, T.C. Simultaneous 68ga-dotatoc pet/mri in patients with gastroenteropancreatic neuroendocrine tumors: Initial results. Invest. Radiol. 2013, 48, 273–279. [Google Scholar] [CrossRef]
- Al-Nabhani, K.Z.; Syed, R.; Michopoulou, S.; Alkalbani, J.; Afaq, A.; Panagiotidis, E.; O′Meara, C.; Groves, A.; Ell, P.; Bomanji, J. Qualitative and quantitative comparison of pet/ct and pet/mr imaging in clinical practice. J. Nucl. Med. 2014, 55, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Thorwarth, D.; Müller, A.C.; Pfannenberg, C.; Beyer, T. Combined PET/MR imaging using 68Ga-DOTATOC for radiotherapy treatment planning in meningioma patients. In Theranostics, Gallium-68, and Other Radionuclides; Recent Results in Cancer Research; Springer: Berlin/Heidelberg, Germany, 2013; Volume 194, pp. 425–439. [Google Scholar]
- Afaq, A.; Syed, R.; Bomanji, J. Pet/mri: A new technology in the field of molecular imaging. Br. Med. Bull. 2013, 108, 159–171. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kuyumcu, S.; Özkan, Z.G.; Sanli, Y.; Yilmaz, E.; Mudun, A.; Adalet, I.; Unal, S. Physiological and tumoral uptake of 68ga-Dotatate: Standardized uptake values and challenges in interpretation. Ann. Nucl. Med. 2013, 27, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Todorović-Tirnanić, M.V.; Gajić, M.M.; Obradović, V.B.; Baum, R.P. Gallium-68 dotatoc pet/ct in vivo characterization of somatostatin receptor expression in the prostate. Cancer Biother. Radiopharm. 2014, 29, 108–115. [Google Scholar] [CrossRef]
- Reindl, O.; Loidl, A.; Franz, B.; Hofer, J.F.; Pichler, R. Pitfall in follow-Up imaging of pancreatic neuroendocrine tumor by somatostatin receptor pet. Neuroendocrinol. Lett. 2013, 34, 273–274. [Google Scholar]
- Treglia, G.; Giovanella, L.; Muoio, B.; Caldarella, C. Splenosis mimicking relapse of a neuroendocrine tumor at gallium-68-Dotatoc pet/ct. Nucl. Med. Mol. Imaging 2014, 48, 163–165. [Google Scholar] [CrossRef]
- Kulkarni, H.R.; Prasad, V.; Kaemmerer, D.; Hommann, M.; Baum, R.P. High uptake of (68)ga-Dotatoc in spleen as compared to splenosis: Measurement by pet/ct. Recent Results Cancer Res. 2013, 194, 373–378. [Google Scholar]
- Calissendorff, J.; Sundin, A.; Falhammar, H. 68Ga-DOTA-TOC-PET/CT detects heart metastases from ileal neuroendocrine tumors. Endocrine 2013, 47, 169–176. [Google Scholar] [CrossRef]
- Mapelli, P.; Tam, H.H.; Sharma, R.; Aboagye, E.O.; Al-Nahhas, A. Frequency and significance of physiological versus pathological uptake of 68ga-dotatate in the pancreas: Validation with morphological imaging. Nucl. Med. Commun. 2014, 35, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Brogsitter, C.; Hofmockel, T.; Kotzerke, J. (68)ga dotatate uptake in vertebral hemangioma. Clin. Nucl. Med. 2014, 39, 462–463. [Google Scholar] [CrossRef]
- Sandström, M.; Velikyan, I.; Garske-Román, U.; Sörensen, J.; Eriksson, B.; Granberg, D.; Lundqvist, H.; Sundin, A.; Lubberink, M. Comparative biodistribution and radiation dosimetry of 68ga-Dotatoc and 68ga-Dotatate in patients with neuroendocrine tumors. J. Nucl. Med. 2013, 54, 1755–1759. [Google Scholar] [CrossRef] [PubMed]
- Versari, A.; Sollini, M.; Frasoldati, A.; Fraternali, A.; Filice, A.; Froio, A.; Asti, M.; Fioroni, F.; Cremonini, N.; Putzer, D.; et al. Differentiated thyroid cancer: A new perspective with radiolabeled somatostatin analogues for imaging and treatment of patients. Thyroid 2014, 24, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Taïeb, D.; Varoquaux, A.; Chen, C.C.; Pacak, K. Current and future trends in the anatomical and functional imaging of head and neck paragangliomas. Semin. Nucl. Med. 2013, 43, 462–473. [Google Scholar] [CrossRef] [PubMed]
- Blaickner, M.; Baum, R.P. Relevance of pet for pretherapeutic prediction of doses in peptide receptor radionuclide therapy. PET Clin. 2014, 9, 99–112. [Google Scholar] [CrossRef]
- Kulkarni, H.R.; Baum, R.P. Patient selection for personalized peptide receptor radionuclide therapy using ga-68 somatostatin receptor pet/ct. PET Clin. 2014, 9, 83–90. [Google Scholar] [CrossRef]
- Slavikova, K.; Montravers, F.; Treglia, G.; Kunikowska, J.; Kaliska, L.; Vereb, M.; Talbot, J.N.; Balogova, S. What is currently the best radiopharmaceutical for the hybrid pet/ct detection of recurrent medullary thyroid carcinoma? Curr. Radiopharm. 2013, 6, 96–105. [Google Scholar]
- Treglia, G.; Castaldi, P.; Villani, M.F.; Perotti, G.; Filice, A.; Ambrosini, V.; Cremonini, N.; Versari, A.; Fanti, S.; Giordano, A.; et al. Comparison of different positron emission tomography tracers in patients with recurrent medullary thyroid carcinoma: Our experience and a review of the literature. In Recent Results in Cancer Research; Springer: Berlin/Heidelberg, Germany, 2013; Volume 194, pp. 385–393. [Google Scholar]
- Haug, A.R.; Auernhammer, C.J.; Wangler, B.; Schmidt, G.P.; Uebleis, C.; Goke, B.; Cumming, P.; Bartenstein, P.; Tiling, R.; Hacker, M. 68ga-Dotatate pet/ct for the early prediction of response to somatostatin receptor-Mediated radionuclide therapy in patients with well-Differentiated neuroendocrine tumors. J. Nucl. Med. 2010, 51, 1349–1356. [Google Scholar] [CrossRef]
- Sainz-Esteban, A.; Prasad, V.; Schuchardt, C.; Zachert, C.; Carril, J.M.; Baum, R.P. Comparison of sequential planar 177lu-Dota-Tate dosimetry scans with 68ga-Dota-Tate pet/ct images in patients with metastasized neuroendocrine tumours undergoing peptide receptor radionuclide therapy. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 501–511. [Google Scholar] [CrossRef]
- Kratochwil, C.; Stefanova, M.; Mavriopoulou, E.; Holland-Letz, T.; Dimitrakopoulou-Strauss, A.; Afshar-Oromieh, A.; Mier, W.; Haberkorn, U.; Giesel, F.L. Suv of [68ga]dotatoc-pet/ct predicts response probability of prrt in neuroendocrine tumors. Mol. Imaging Biol. 2015, 17, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Baum, R.P.; Kulkarni, H.R.; Singh, A.; Kaemmerer, D.; Mueller, D.; Prasad, V.; Hommann, M.; Robiller, F.C.; Niepsch, K.; Franz, H.; et al. Results and adverse events of personalized peptide receptor radionuclide therapy with (90)yttrium and (177)lutetium in 1048 patients with neuroendocrine neoplasms. Oncotarget 2018, 9, 16932–16950. [Google Scholar] [CrossRef] [PubMed]
- Haug, A.R.; Cindea-Drimus, R.; Auernhammer, C.J.; Reincke, M.; Beuschlein, F.; Wangler, B.; Uebleis, C.; Schmidt, G.P.; Spitzweg, C.; Bartenstein, P.; et al. Neuroendocrine tumor recurrence: Diagnosis with 68ga-Dotatate pet/ct. Radiology 2014, 270, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Menda, Y.; Ponto, L.L.; Schultz, M.K.; Zamba, G.K.; Watkins, G.L.; Bushnell, D.L.; Madsen, M.T.; Sunderland, J.J.; Graham, M.M.; O′Dorisio, T.M.; et al. Repeatability of gallium-68 dotatoc positron emission tomographic imaging in neuroendocrine tumors. Pancreas 2013, 42, 937–943. [Google Scholar] [CrossRef]
- Kulkarni, H.R.; Baum, R.P. Theranostics with ga-68 somatostatin receptor pet/ct: Monitoring response to peptide receptor radionuclide therapy. PET Clin. 2014, 9, 91–97. [Google Scholar] [CrossRef]
- Giesel, F.L.; Stefanova, M.; Schwartz, L.H.; Afshar-Oromieh, A.; Eisenhut, M.; Haberkorn, U.; Kratochwil, C. Impact of peptide receptor radionuclide therapy on the 68ga-Dotatoc-pet/ct uptake in normal tissue. Q. J. Nucl. Med. Mol. Imaging 2013, 57, 171–176. [Google Scholar]
- Combs, S.E.; Welzel, T.; Habermehl, D.; Rieken, S.; Dittmar, J.O.; Kessel, K.; Jäkel, O.; Haberkorn, U.; Debus, J. Prospective evaluation of early treatment outcome in patients with meningiomas treated with particle therapy based on target volume definition with mri and 68ga-dotatoc-pet. Acta Oncol. 2013, 52, 514–520. [Google Scholar] [CrossRef]
- Jois, B.; Asopa, R.; Basu, S. Somatostatin receptor imaging in non-131i-Avid metastatic differentiated thyroid carcinoma for determining the feasibility of peptide receptor radionuclide therapy with 177lu-Dotatate: Low fraction of patients suitable for peptide receptor radionuclide therapy and evidence of chromogranin a level-Positive neuroendocrine differentiation. Clin. Nucl. Med. 2014, 39, 505–510. [Google Scholar]
- Oksuz, M.O.; Winter, L.; Pfannenberg, C.; Reischl, G.; Mussig, K.; Bares, R.; Dittmann, H. Peptide receptor radionuclide therapy of neuroendocrine tumors with (90)y-Dotatoc: Is treatment response predictable by pre-Therapeutic uptake of (68)ga-Dotatoc? Diagn. Interv. Imaging 2014, 95, 289–300. [Google Scholar] [CrossRef]
- Kroiss, A.; Putzer, D.; Decristoforo, C.; Uprimny, C.; Warwitz, B.; Nilica, B.; Gabriel, M.; Kendler, D.; Waitz, D.; Widmann, G.; et al. 68ga-dota-toc uptake in neuroendocrine tumour and healthy tissue: Differentiation of physiological uptake and pathological processes in pet/ct. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 514–523. [Google Scholar] [CrossRef]
- Ruf, J.; Schiefer, J.; Kropf, S.; Furth, C.; Ulrich, G.; Kosiek, O.; Denecke, T.; Pavel, M.; Pascher, A.; Wiedenmann, B.; et al. Quantification in ga-68-dota(0)-phe(1)-tyr(3)-Octreotide positron emission tomography/computed tomography: Can we be impartial about partial volume effects? Neuroendocrinology 2013, 97, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Kim, Y.I. Prognostic value of maximum standardized uptake value in 68ga-Somatostatin receptor positron emission tomography for neuroendocrine tumors: A systematic review and meta-Analysis. Clin. Nucl. Med. 2019, 44, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.; Ito, T.; Jensen, R.T. Imaging of pancreatic neuroendocrine tumors: Recent advances, current status, and controversies. Expert Rev. Anticancer Ther. 2018, 18, 837–860. [Google Scholar] [CrossRef] [PubMed]
- Tirosh, A.; Kebebew, E. The utility of (68)ga-Dotatate positron-Emission tomography/computed tomography in the diagnosis, management, follow-Up and prognosis of neuroendocrine tumors. Future Oncol. 2018, 14, 111–122. [Google Scholar] [CrossRef]
- Yu, J.; Li, N.; Li, J.; Lu, M.; Leal, J.P.; Tan, H.; Su, H.; Fan, Y.; Zhang, Y.; Zhao, W.; et al. The correlation between [(68)ga]dotatate pet/ct and cell proliferation in patients with gep-nens. Mol. Imaging Biol. 2019, 21, 984–990. [Google Scholar] [CrossRef]
- Chan, H.; Moseley, C.; Zhang, L.; Bergsland, E.K.; Pampaloni, M.H.; Van Loon, K.; Hope, T.A. Correlation of dotatoc uptake and pathologic grade in neuroendocrine tumors. Pancreas 2019, 48, 948–952. [Google Scholar] [CrossRef]
- Velikyan, I.; Sundin, A.; Sörensen, J.; Lubberink, M.; Sandström, M.; Garske-Román, U.; Lundqvist, H.; Granberg, D.; Eriksson, B. Quantitative and qualitative intrapatient comparison of 68ga-Dotatoc and 68ga-Dotatate: Net uptake rate for accurate quantification. J. Nucl. Med. 2014, 55, 204–210. [Google Scholar] [CrossRef]
- Ilan, E.; Velikyan, I.; Sandstrom, M.; Sundin, A.; Lubberink, M. Tumor-To-Blood ratio for assessment of somatostatin receptor density in neuroendocrine tumors using (68)ga-Dotatoc and (68)ga-Dotatate. J. Nucl. Med. 2019, 61, 217–221. [Google Scholar] [CrossRef]
- Ohnona, J.; Nataf, V.; Gauthe, M.; Balogova, S.; Belissant Benesty, O.; Zhang-Yin, J.; Talbot, J.N.; Montravers, F. Prognostic value of functional tumor burden on 68ga-Dotatoc pet/ct in patients with pancreatic neuro-Endocrine tumors. Neoplasma 2019, 66, 140–148. [Google Scholar] [CrossRef]
- Toriihara, A.; Baratto, L.; Nobashi, T.; Park, S.; Hatami, N.; Davidzon, G.; Kunz, P.L.; Iagaru, A. Prognostic value of somatostatin receptor expressing tumor volume calculated from (68)ga-dotatate pet/ct in patients with well-Differentiated neuroendocrine tumors. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2244–2251. [Google Scholar] [CrossRef]
- Graf, J.; Pape, U.F.; Jann, H.; Denecke, T.; Arsenic, R.; Brenner, W.; Pavel, M.; Prasad, V. Prognostic significance of somatostatin receptor heterogeneity in progressive neuroendocrine tumor treated with lu-177 dotatoc or lu-177 dotatate. Eur. J. Nucl. Med. Mol. Imaging 2019. [Google Scholar] [CrossRef] [PubMed]
- Werner, R.A.; Ilhan, H.; Lehner, S.; Papp, L.; Zsoter, N.; Schatka, I.; Muegge, D.O.; Javadi, M.S.; Higuchi, T.; Buck, A.K.; et al. Pre-Therapy somatostatin receptor-Based heterogeneity predicts overall survival in pancreatic neuroendocrine tumor patients undergoing peptide receptor radionuclide therapy. Mol. Imaging Biol. 2019, 21, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Coura-Filho, G.B.; Hoff, A.; Duarte, P.S.; Buchpiguel, C.A.; Josefsson, A.; Hobbs, R.F.; Sgouros, G.; Sapienza, M.T. 68ga-Dotatate pet: Temporal variation of maximum standardized uptake value in normal tissues and neuroendocrine tumours. Nucl. Med. Commun. 2019, 40, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Velikyan, I. Continued rapid growth in ga applications: Update 2013 to june 2014. J. Labelled. Comp. Radiopharm. 2015, 99–121. [Google Scholar] [CrossRef] [PubMed]
- Paquet, M.; Gauthe, M.; Zhang Yin, J.; Nataf, V.; Belissant, O.; Orcel, P.; Roux, C.; Talbot, J.N.; Montravers, F. Diagnostic performance and impact on patient management of (68)ga-dota-toc pet/ct for detecting osteomalacia-Associated tumours. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1710–1720. [Google Scholar] [CrossRef] [PubMed]
- Barrio, M.; Czernin, J.; Fanti, S.; Ambrosini, V.; Binse, I.; Du, L.; Eiber, M.; Herrmann, K.; Fendler, W.P. The impact of somatostatin receptor-Directed pet/ct on the management of patients with neuroendocrine tumor: A systematic review and meta-Analysis. J. Nucl. Med. 2017, 58, 756–761. [Google Scholar] [CrossRef]
- Herrmann, K.; Czernin, J.; Wolin, E.M.; Gupta, P.; Barrio, M.; Gutierrez, A.; Schiepers, C.; Mosessian, S.; Phelps, M.E.; Allen-Auerbach, M.S. Impact of 68ga-Dotatate pet/ct on the management of neuroendocrine tumors: The referring physician’s perspective. J. Nucl. Med. 2015, 56, 70–75. [Google Scholar] [CrossRef]
- Calais, J.; Czernin, J.; Eiber, M.; Fendler, W.P.; Gartmann, J.; Heaney, A.P.; Hendifar, A.E.; Pisegna, J.R.; Hecht, J.R.; Wolin, E.M.; et al. Most of the intended management changes after (68)ga-Dotatate pet/ct are implemented. J. Nucl. Med. 2017, 58, 1793–1796. [Google Scholar] [CrossRef]
- Crown, A.; Rocha, F.G.; Raghu, P.; Lin, B.; Funk, G.; Alseidi, A.; Hubka, M.; Rosales, J.; Lee, M.; Kennecke, H. Impact of initial imaging with gallium-68 dotatate pet/ct on diagnosis and management of patients with neuroendocrine tumors. J. Surg. Oncol. 2019, 121, 480–485. [Google Scholar] [CrossRef]
- Skoura, E.; Priftakis, D.; Novruzov, F.; Caplin, M.E.; Gnanasegaran, G.; Navalkissoor, S.; Bomanji, J. The impact of ga-68 dotatate pet/ct imaging on management of patients with paragangliomas. Nucl. Med. Commun. 2019, 41, 169–174. [Google Scholar] [CrossRef]
- Kong, G.; Hicks, R.J. Peptide receptor radiotherapy: Current approaches and future directions. Curr. Treat. Options Oncol. 2019, 20, 77. [Google Scholar] [CrossRef] [PubMed]
- Garske-Roman, U.; Sandstrom, M.; Fross Baron, K.; Lundin, L.; Hellman, P.; Welin, S.; Johansson, S.; Khan, T.; Lundqvist, H.; Eriksson, B.; et al. Prospective observational study of (177)lu-Dota-Octreotate therapy in 200 patients with advanced metastasized neuroendocrine tumours (nets): Feasibility and impact of a dosimetry-Guided study protocol on outcome and toxicity. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 970–988. [Google Scholar] [CrossRef] [PubMed]
- Garske, U.; Sandstrom, M.; Johansson, S.; Granberg, D.; Lundqvist, H.; Lubberink, M.; Sundin, A.; Eriksson, B. Lessons on tumour response: Imaging during therapy with (177)lu-Dota-Octreotate. A case report on a patient with a large volume of poorly differentiated neuroendocrine carcinoma. Theranostics 2012, 2, 459–471. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mease, R.C.; Foss, C.A.; Pomper, M.G. Pet imaging in prostate cancer: Focus on prostate-specific membrane antigen. Curr. Top. Med. Chem. 2013, 13, 951–962. [Google Scholar] [CrossRef]
- Kopka, K.; Benesova, M.; Barinka, C.; Haberkorn, U.; Babich, J. Glu-Ureido-Based inhibitors of prostate-Specific membrane antigen: Lessons learned during the development of a novel class of low-Molecular-Weight theranostic radiotracers. J. Nucl. Med. 2017, 58, 17S–26S. [Google Scholar] [CrossRef]
- Eder, M.; Eisenhut, M.; Babich, J.; Haberkorn, U. Psma as a target for radiolabelled small molecules. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 819–823. [Google Scholar] [CrossRef]
- Afshar-Oromieh, A.; Malcher, A.; Eder, M.; Eisenhut, M.; Linhart, H.G.; Hadaschik, B.A.; Holland-Letz, T.; Giesel, F.L.; Kratochwil, C.; Haufe, S.; et al. Pet imaging with a [68ga]gallium-labelled psma ligand for the diagnosis of prostate cancer: Biodistribution in humans and first evaluation of tumour lesions. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 486–495. [Google Scholar] [CrossRef]
- Fendler, W.P.; Calais, J.; Eiber, M.; Flavell, R.R.; Mishoe, A.; Feng, F.Y.; Nguyen, H.G.; Reiter, R.E.; Rettig, M.B.; Okamoto, S.; et al. Assessment of 68ga-psma-11 pet accuracy in localizing recurrent prostate cancer: A prospective single-arm clinical trial. JAMA Oncol 2019, 5, 856–863. [Google Scholar] [CrossRef]
- Bouchelouche, K.; Choyke, P.L. Prostate-specific membrane antigen positron emission tomography in prostate cancer: A step toward personalized medicine. Curr. Opin. Oncol. 2016, 28, 216–221. [Google Scholar] [CrossRef]
- Bouchelouche, K.; Choyke, P.L. Advances in prostate-specific membrane antigen pet of prostate cancer. Curr. Opin. Oncol. 2018, 30, 189–196. [Google Scholar] [CrossRef]
- Alipour, R.; Azad, A.; Hofman, M.S. Guiding management of therapy in prostate cancer: Time to switch from conventional imaging to psma pet? Ther. Adv. Med. Oncol. 2019, 11, 1758835919876828. [Google Scholar] [CrossRef] [PubMed]
- Eissa, A.; Elsherbiny, A.; Coelho, R.F.; Rassweiler, J.; Davis, J.W.; Porpiglia, F.; Patel, V.R.; Prandini, N.; Micali, S.; Sighinolfi, M.C.; et al. The role of 68ga-psma pet/ct scan in biochemical recurrence after primary treatment for prostate cancer: A systematic review of the literature. Minerva Urol. Nefrol. 2018, 70, 462–478. [Google Scholar] [CrossRef] [PubMed]
- Lenzo, N.P.; Meyrick, D.; Turner, J.H. Review of gallium-68 psma pet/ct imaging in the management of prostate cancer. Diagnostics (Basel) 2018, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Maurer, T.; Weirich, G.; Schottelius, M.; Weineisen, M.; Frisch, B.; Okur, A.; Kubler, H.; Thalgott, M.; Navab, N.; Schwaiger, M.; et al. Prostate-Specific membrane antigen-radioguided surgery for metastatic lymph nodes in prostate cancer. Eur. Urol. 2015, 68, 530–534. [Google Scholar] [CrossRef]
- Perera, M.; Papa, N.; Roberts, M.; Williams, M.; Udovicich, C.; Vela, I.; Christidis, D.; Bolton, D.; Hofman, M.S.; Lawrentschuk, N.; et al. Gallium-68 prostate-Specific membrane antigen positron emission tomography in advanced prostate cancer-Updated diagnostic utility, sensitivity, specificity, and distribution of prostate-Specific membrane antigen-Avid lesions: A systematic review and meta-Analysis. Eur. Urol. 2019. [Google Scholar] [CrossRef]
- Yaxley, J.W.; Raveenthiran, S.; Nouhaud, F.X.; Samaratunga, H.; Yaxley, W.J.; Coughlin, G.; Yaxley, A.J.; Gianduzzo, T.; Kua, B.; McEwan, L.; et al. Risk of metastatic disease on (68) gallium-Prostate-Specific membrane antigen positron emission tomography/computed tomography scan for primary staging of 1253 men at the diagnosis of prostate cancer. BJU Int. 2019, 124, 401–407. [Google Scholar] [CrossRef] [PubMed]
- De Visschere, P.J.L.; Standaert, C.; Futterer, J.J.; Villeirs, G.M.; Panebianco, V.; Walz, J.; Maurer, T.; Hadaschik, B.A.; Lecouvet, F.E.; Giannarini, G.; et al. A systematic review on the role of imaging in early recurrent prostate cancer. Eur. Urol. Oncol. 2019, 2, 47–76. [Google Scholar] [CrossRef]
- Barber, T.W.; Singh, A.; Kulkarni, H.R.; Niepsch, K.; Billah, B.; Baum, R.P. Clinical outcomes of (177)lu-Psma radioligand therapy in earlier and later phases of metastatic castration-resistant prostate cancer grouped by previous taxane chemotherapy. J. Nucl. Med. 2019, 60, 955–962. [Google Scholar] [CrossRef]
- Kratochwil, C.; Giesel, F.L.; Stefanova, M.; Benešová, M.; Bronzel, M.; Afshar-Oromieh, A.; Mier, W.; Eder, M.; Kopka, K.; Haberkorn, U. Psma-Targeted radionuclide therapy of metastatic castration-Resistant prostate cancer with 177lu-Labeled psma-617. J. Nucl. Med. 2016, 57, 1170–1176. [Google Scholar] [CrossRef]
- Habl, G.; Sauter, K.; Schiller, K.; Dewes, S.; Maurer, T.; Eiber, M.; Combs, S.E. (68) ga-psma-pet for radiation treatment planning in prostate cancer recurrences after surgery: Individualized medicine or new standard in salvage treatment. Prostate 2017, 77, 920–927. [Google Scholar] [CrossRef]
- Woythal, N.; Arsenic, R.; Kempkensteffen, C.; Miller, K.; Janssen, J.-C.; Huang, K.; Makowski, M.R.; Brenner, W.; Prasad, V. Immunohistochemical validation of psma expression measured by 68ga-psma pet/ct in primary prostate cancer. J. Nucl. Med. 2018, 59, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Kabasakal, L.; Demirci, E.; Ocak, M.; Akyel, R.; Nematyazar, J.; Aygun, A.; Halac, M.; Talat, Z.; Araman, A. Evaluation of psma pet/ct imaging using a 68ga-hbed-cc ligand in patients with prostate cancer and the value of early pelvic imaging. Nucl. Med. Commun. 2015, 36, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Woo, S.; Kim, Y.J.; Suh, C.H. Impact of (68)ga-psma pet on the management of patients with prostate cancer: A systematic review and meta-Analysis. Eur. Urol. 2018, 74, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Davidson, T.; Amit, U.; Saad, A.; Hahiashvili, M.; Goshen, E.; Portnoy, O.; Berger, R.; Goldstein, A.; Sadetsky, I.; Weizman, N.; et al. Gallium-68 prostate-specific membrane antigen pet-ct and the clinical management of prostate cancer. Nucl. Med. Commun. 2019, 40, 913–919. [Google Scholar] [CrossRef]
- Ekmekcioglu, O.; Busstra, M.; Klass, N.D.; Verzijlbergen, F. Bridging the imaging gap: Psma pet/ct has a high impact on treatment planning in prostate cancer patients with biochemical recurrence-a narrative review of the literature. J. Nucl. Med. 2019, 60, 1394–1398. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, D.A.; Garcia Schuler, H.I.; Muehlematter, U.J.; Eberli, D.; Muller, J.; Muller, A.; Gablinger, R.; Kranzbuhler, H.; Omlin, A.; Kaufmann, P.A.; et al. Impact of (68)ga-psma-11 pet staging on clinical decision-Making in patients with intermediate or high-Risk prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2019, 47, 652–664. [Google Scholar] [CrossRef]
- Schmidt-Hegemann, N.S.; Eze, C.; Li, M.; Rogowski, P.; Schaefer, C.; Stief, C.; Buchner, A.; Zamboglou, C.; Fendler, W.P.; Ganswindt, U.; et al. Impact of (68)ga-psma pet/ct on the radiotherapeutic approach to prostate cancer in comparison to ct: A retrospective analysis. J. Nucl. Med. 2019, 60, 963–970. [Google Scholar] [CrossRef]
- Rousseau, C.; Le Thiec, M.; Ferrer, L.; Rusu, D.; Rauscher, A.; Maucherat, B.; Frindel, M.; Baumgartner, P.; Fleury, V.; Denis, A.; et al. Preliminary results of a (68) ga-psma pet/ct prospective study in prostate cancer patients with occult recurrence: Diagnostic performance and impact on therapeutic decision-Making. Prostate 2019, 79, 1514–1522. [Google Scholar] [CrossRef]
- Hoffmann, M.A.; Wieler, H.J.; Baues, C.; Kuntz, N.J.; Richardsen, I.; Schreckenberger, M. The impact of 68ga-psma pet/ct and pet/mri on the management of prostate cancer. Urology 2019, 130, 1–12. [Google Scholar] [CrossRef]
- Barbaud, M.; Frindel, M.; Ferrer, L.; Le Thiec, M.; Rusu, D.; Rauscher, A.; Maucherat, B.; Baumgartner, P.; Fleury, V.; Colombie, M.; et al. 68ga-psma-11 pet-ct study in prostate cancer patients with biochemical recurrence and non-Contributive 18f-choline pet-ct: Impact on therapeutic decision-Making and biomarker changes. Prostate 2019, 79, 454–461. [Google Scholar] [CrossRef]
- Farolfi, A.; Ceci, F.; Castellucci, P.; Graziani, T.; Siepe, G.; Lambertini, A.; Schiavina, R.; Lodi, F.; Morganti, A.G.; Fanti, S. (68)ga-psma-11 pet/ct in prostate cancer patients with biochemical recurrence after radical prostatectomy and psa <0.5 ng/ml. Efficacy and impact on treatment strategy. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 11–19. [Google Scholar] [PubMed]
- Roach, P.J.; Francis, R.; Emmett, L.; Hsiao, E.; Kneebone, A.; Hruby, G.; Eade, T.; Nguyen, Q.A.; Thompson, B.D.; Cusick, T.; et al. The impact of (68)ga-psma pet/ct on management intent in prostate cancer: Results of an australian prospective multicenter study. J. Nucl. Med. 2018, 59, 82–88. [Google Scholar] [CrossRef]
- Emmett, L.; Crumbaker, M.; Ho, B.; Willowson, K.; Eu, P.; Ratnayake, L.; Epstein, R.; Blanksby, A.; Horvath, L.; Guminski, A.; et al. Results of a prospective phase 2 pilot trial of (177)lu-psma-617 therapy for metastatic castration-Resistant prostate cancer including imaging predictors of treatment response and patterns of progression. Clin. Genitourin. Cancer 2019, 17, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Haberkorn, U.; Kopka, K.; Giesel, F.; Kratochwil, C. Future trends in prostate cancer theranostics with psma ligands. Clin. Transl. Imaging 2016, 4, 487–489. [Google Scholar] [CrossRef][Green Version]
- van Leeuwen, P.J.; Donswijk, M.; Nandurkar, R.; Stricker, P.; Ho, B.; Heijmink, S.; Wit, E.M.K.; Tillier, C.; van Muilenkom, E.; Nguyen, Q.; et al. Gallium-68-Prostate-Specific membrane antigen ((68) ga-psma) positron emission tomography (pet)/computed tomography (ct) predicts complete biochemical response from radical prostatectomy and lymph node dissection in intermediate- and high-Risk prostate cancer. BJU Int. 2019, 124, 62–68. [Google Scholar] [PubMed]
- Hofman, M.S.; Violet, J.; Hicks, R.J.; Ferdinandus, J.; Thang, S.P.; Akhurst, T.; Iravani, A.; Kong, G.; Ravi Kumar, A.; Murphy, D.G.; et al. [(177)lu]-psma-617 radionuclide treatment in patients with metastatic castration-Resistant prostate cancer (lupsma trial): A single-Centre, single-Arm, phase 2 study. Lancet Oncol. 2018, 19, 825–833. [Google Scholar] [CrossRef]
- Kabasakal, L.; AbuQbeitah, M.; Aygun, A.; Yeyin, N.; Ocak, M.; Demirci, E.; Toklu, T. Pre-Therapeutic dosimetry of normal organs and tissues of (177)lu-psma-617 prostate-Specific membrane antigen (psma) inhibitor in patients with castration-Resistant prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1976–1983. [Google Scholar] [CrossRef]
- Weineisen, M.; Schottelius, M.; Simecek, J.; Baum, R.P.; Yildiz, A.; Beykan, S.; Kulkarni, H.R.; Lassmann, M.; Klette, I.; Eiber, M.; et al. 68ga- and 177lu-Labeled psma i&t: Optimization of a psma-Targeted theranostic concept and first proof-Of-Concept human studies. J. Nucl. Med. 2015, 56, 1169–1176. [Google Scholar]
- Heck, M.M.; Tauber, R.; Schwaiger, S.; Retz, M.; D′Alessandria, C.; Maurer, T.; Gafita, A.; Wester, H.J.; Gschwend, J.E.; Weber, W.A.; et al. Treatment outcome, toxicity, and predictive factors for radioligand therapy with (177)lu-psma-i&t in metastatic castration-Resistant prostate cancer. Eur. Urol. 2019, 75, 920–926. [Google Scholar]
- von Eyben, F.E.; Roviello, G.; Kiljunen, T.; Uprimny, C.; Virgolini, I.; Kairemo, K.; Joensuu, T. Third-Line treatment and (177)lu-psma radioligand therapy of metastatic castration-Resistant prostate cancer: A systematic review. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 496–508. [Google Scholar] [CrossRef]
- Virgolini, I.; Decristoforo, C.; Haug, A.; Fanti, S.; Uprimny, C. Current status of theranostics in prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 471–495. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, S.; Thieme, A.; Allmann, J.; D′Alessandria, C.; Maurer, T.; Retz, M.; Tauber, R.; Heck, M.M.; Wester, H.J.; Tamaki, N.; et al. Radiation dosimetry for (177)lu-psma i&t in metastatic castration-resistant prostate cancer: Absorbed dose in normal organs and tumor lesions. J. Nucl. Med. 2017, 58, 445–450. [Google Scholar] [PubMed]
- Scarpa, L.; Buxbaum, S.; Kendler, D.; Fink, K.; Bektic, J.; Gruber, L.; Decristoforo, C.; Uprimny, C.; Lukas, P.; Horninger, W.; et al. The (68)ga/(177)lu theragnostic concept in psma targeting of castration-Resistant prostate cancer: Correlation of suvmax values and absorbed dose estimates. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 788–800. [Google Scholar] [CrossRef] [PubMed]
- Baum, R.P.; Kulkarni, H.R.; Schuchardt, C.; Singh, A.; Wirtz, M.; Wiessalla, S.; Schottelius, M.; Mueller, D.; Klette, I.; Wester, H.J. 177lu-Labeled prostate-specific membrane antigen radioligand therapy of metastatic castration-Resistant prostate cancer: Safety and efficacy. J. Nucl. Med. 2016, 57, 1006–1013. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Fendler, W.P.; Eiber, M.; Baum, R.; Bozkurt, M.F.; Czernin, J.; Delgado Bolton, R.C.; Ezziddin, S.; Forrer, F.; Hicks, R.J.; et al. Eanm procedure guidelines for radionuclide therapy with (177)lu-Labelled psma-Ligands ((177)lu-psma-rlt). Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2536–2544. [Google Scholar] [CrossRef] [PubMed]
- Derks, Y.H.W.; Lowik, D.; Sedelaar, J.P.M.; Gotthardt, M.; Boerman, O.C.; Rijpkema, M.; Lutje, S.; Heskamp, S. Psma-Targeting agents for radio- and fluorescence-Guided prostate cancer surgery. Theranostics 2019, 9, 6824–6839. [Google Scholar] [CrossRef]
- Maurer, T.; Gschwend, J.E.; Eiber, M. Prostate-Specific membrane antigen-guided salvage lymph node dissection in recurrent prostate cancer: A novel technology to detect lymph node metastases. Curr. Opin. Urol. 2018, 28, 191–196. [Google Scholar] [CrossRef]
- Cortes, J.; Fumoleau, P.; Bianchi, G.V.; Petrella, T.M.; Gelmon, K.; Pivot, X.; Verma, S.; Albanell, J.; Conte, P.; Lluch, A.; et al. Pertuzumab monotherapy after trastuzumab-Based treatment and subsequent reintroduction of trastuzumab: Activity and tolerability in patients with advanced human epidermal growth factor receptor 2-Positive breast cancer. J. Clin. Oncol. 2012, 30, 1594–1600. [Google Scholar] [CrossRef]
- Houssami, N.; Macaskill, P.; Balleine, R.L.; Bilous, M.; Pegram, M.D. Her2 discordance between primary breast cancer and its paired metastasis: Tumor biology or test artefact? Insights through meta-Analysis. Breast Cancer Res. Treat. 2011, 129, 659–674. [Google Scholar] [CrossRef]
- Verma, S.; Miles, D.; Gianni, L.; Krop, I.E.; Welslau, M.; Baselga, J.; Pegram, M.; Oh, D.Y.; Dieras, V.; Guardino, E.; et al. Trastuzumab emtansine for her2-Positive advanced breast cancer. N. Engl. J. Med. 2012, 367, 1783–1791. [Google Scholar] [CrossRef]
- Roskoski, R., Jr. The erbb/her family of protein-Tyrosine kinases and cancer. Pharmacol. Res. 2014, 79, 34–74. [Google Scholar] [CrossRef] [PubMed]
- Citri, A.; Yarden, Y. Egf-erbb signalling: Towards the systems level. Nat. Rev. Mol. Cell Biol. 2006, 7, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Natali, P.G.; Nicotra, M.R.; Bigotti, A.; Venturo, I.; Slamon, D.J.; Fendly, B.M.; Ullrich, A. Expression of the p185 encoded by her2 oncogene in normal and transformed human tissues. Int. J. Cancer 1990, 45, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.J.; Leyland-Jones, B.; Shak, S.; Fuchs, H.; Paton, V.; Bajamonde, A.; Fleming, T.; Eiermann, W.; Wolter, J.; Pegram, M.; et al. Use of chemotherapy plus a monoclonal antibody against her2 for metastatic breast cancer that overexpresses her2. N. Engl. J. Med. 2001, 344, 783–792. [Google Scholar] [CrossRef]
- Nahta, R.; Esteva, F.J. Trastuzumab: Triumphs and tribulations. Oncogene 2007, 26, 3637–3643. [Google Scholar] [CrossRef]
- Molina, R.; Barak, V.; van Dalen, A.; Duffy, M.J.; Einarsson, R.; Gion, M.; Goike, H.; Lamerz, R.; Nap, M.; Soletormos, G.; et al. Tumor markers in breast cancer-European group on tumor markers recommendations. Tumour. Biol. 2005, 26, 281–293. [Google Scholar] [CrossRef]
- Viani, G.A.; Afonso, S.L.; Stefano, E.J.; De Fendi, L.I.; Soares, F.V. Adjuvant trastuzumab in the treatment of her-2-Positive early breast cancer: A meta-Analysis of published randomized trials. BMC Cancer 2007, 7, 153. [Google Scholar] [CrossRef]
- Karlsson, E.; Sandelin, K.; Appelgren, J.; Zhou, W.; Jirstrom, K.; Bergh, J.; Warnberg, F. Clonal alteration of breast cancer receptors between primary ductal carcinoma in situ (dcis) and corresponding local events. Eur. J. Cancer 2014, 50, 517–524. [Google Scholar] [CrossRef]
- Lindstrom, L.S.; Karlsson, E.; Wilking, U.M.; Johansson, U.; Hartman, J.; Lidbrink, E.K.; Hatschek, T.; Skoog, L.; Bergh, J. Clinically used breast cancer markers such as estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 are unstable throughout tumor progression. J. Clin. Oncol. 2012, 30, 2601–2608. [Google Scholar] [CrossRef]
- Corcoran, E.B.; Hanson, R.N. Imaging egfr and her2 by pet and spect: A review. Med. Res. Rev. 2014, 34, 596–643. [Google Scholar] [CrossRef]
- Perik, P.J.; Lub-De Hooge, M.N.; Gietema, J.A.; van der Graaf, W.T.; de Korte, M.A.; Jonkman, S.; Kosterink, J.G.; van Veldhuisen, D.J.; Sleijfer, D.T.; Jager, P.L.; et al. Indium-111-Labeled trastuzumab scintigraphy in patients with human epidermal growth factor receptor 2-Positive metastatic breast cancer. J. Clin. Oncol. 2006, 24, 2276–2282. [Google Scholar] [CrossRef] [PubMed]
- Dijkers, E.C.; Oude Munnink, T.H.; Kosterink, J.G.; Brouwers, A.H.; Jager, P.L.; de Jong, J.R.; van Dongen, G.A.; Schroder, C.P.; Lub-de Hooge, M.N.; de Vries, E.G. Biodistribution of 89zr-Trastuzumab and pet imaging of her2-Positive lesions in patients with metastatic breast cancer. Clin. Pharmacol. Ther. 2010, 87, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Beylergil, V.; Morris, P.G.; Smith-Jones, P.M.; Modi, S.; Solit, D.; Hudis, C.A.; Lu, Y.; O′Donoghue, J.; Lyashchenko, S.K.; Carrasquillo, J.A.; et al. Pilot study of 68ga-dota-f(ab’)2-Trastuzumab in patients with breast cancer. Nucl. Med. Commun. 2013, 34, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, B.; Moks, T.; Jansson, B.; Abrahmsen, L.; Elmblad, A.; Holmgren, E.; Henrichson, C.; Jones, T.A.; Uhlen, M. A synthetic igg-binding domain based on staphylococcal protein a. Protein Eng. 1987, 1, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Nord, K.; Nilsson, J.; Nilsson, B.; Uhlen, M.; Nygren, P.A. A combinatorial library of an alpha-Helical bacterial receptor domain. Protein Eng. 1995, 8, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Nord, K.; Gunneriusson, E.; Ringdahl, J.; Stahl, S.; Uhlen, M.; Nygren, P.A. Binding proteins selected from combinatorial libraries of an alpha-Helical bacterial receptor domain. Nat. Biotechnol. 1997, 15, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Feldwisch, J.; Tolmachev, V.; Lendel, C.; Herne, N.; Sjoberg, A.; Larsson, B.; Rosik, D.; Lindqvist, E.; Fant, G.; Hoiden-Guthenberg, I.; et al. Design of an optimized scaffold for affibody molecules. J. Mol. Biol. 2010, 398, 232–247. [Google Scholar] [CrossRef]
- Ahlgren, S.; Orlova, A.; Wallberg, H.; Hansson, M.; Sandstrom, M.; Lewsley, R.; Wennborg, A.; Abrahmsen, L.; Tolmachev, V.; Feldwisch, J. Targeting of her2-Expressing tumors using 111in-aby-025, a second-Generation affibody molecule with a fundamentally reengineered scaffold. J. Nucl. Med. 2010, 51, 1131–1138. [Google Scholar] [CrossRef]
- Eigenbrot, C.; Ultsch, M.; Dubnovitsky, A.; Abrahmsen, L.; Hard, T. Structural basis for high-Affinity her2 receptor binding by an engineered protein. Proc. Natl. Acad. Sci. USA 2010, 107, 15039–15044. [Google Scholar] [CrossRef]
- Velikyan, I.; Wennborg, A.; Feldwisch, J.; Lindman, H.; Carlsson, J.; Sorensen, J. Good manufacturing practice production of [(68)ga]ga-aby-025 for her2 specific breast cancer imaging. Am. J. Nucl. Med. Mol. Imaging 2016, 6, 135–153. [Google Scholar]
- Velikyan, I.; Schweighofer, P.; Feldwisch, J.; Seemann, J.; Frejd, F.Y.; Lindman, H.; Sorensen, J. Diagnostic her2-binding radiopharmaceutical, ga-68 ga-aby-025, for routine clinical use in breast cancer patients. Am. J. Nucl. Med. Mol. Imaging 2019, 9, 12–23. [Google Scholar] [PubMed]
- Sorensen, J.; Sandberg, D.; Sandstrom, M.; Wennborg, A.; Feldwisch, J.; Tolmachev, V.; Astrom, G.; Lubberink, M.; Garske-Roman, U.; Carlsson, J.; et al. First-In-Human molecular imaging of her2 expression in breast cancer metastases using the 111in-aby-025 affibody molecule. J. Nucl. Med. 2014, 55, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, D.; Tolmachev, V.; Velikyan, I.; Olofsson, H.; Wennborg, A.; Feldwisch, J.; Carlsson, J.; Lindman, H.; Sorensen, J. Intra-Image referencing for simplified assessment of her2-Expression in breast cancer metastases using the affibody molecule aby-025 with pet and spect. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1337–1346. [Google Scholar] [CrossRef] [PubMed]
- Sandstrom, M.; Lindskog, K.; Velikyan, I.; Wennborg, A.; Feldwisch, J.; Sandberg, D.; Tolmachev, V.; Orlova, A.; Sorensen, J.; Carlsson, J.; et al. Biodistribution and radiation dosimetry of the anti-her2 affibody molecule 68ga-aby-025 in breast cancer patients. J. Nucl. Med. 2016, 57, 867–871. [Google Scholar] [CrossRef]
- Schrijver, W.; Suijkerbuijk, K.P.M.; van Gils, C.H.; van der Wall, E.; Moelans, C.B.; van Diest, P.J. Receptor conversion in distant breast cancer metastases: A systematic review and meta-Analysis. J. Natl. Cancer Inst. 2018, 110, 568–580. [Google Scholar] [CrossRef]
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).