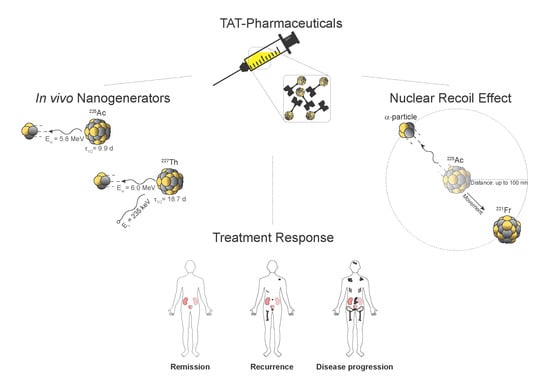
Atomic Nanogenerators in Targeted Alpha Therapies: Curie’s Legacy in Modern Cancer Management
Abstract
:1. Introduction
2. Physicochemical Properties of 225Ac and 227Th
3. Coordination Chemistry
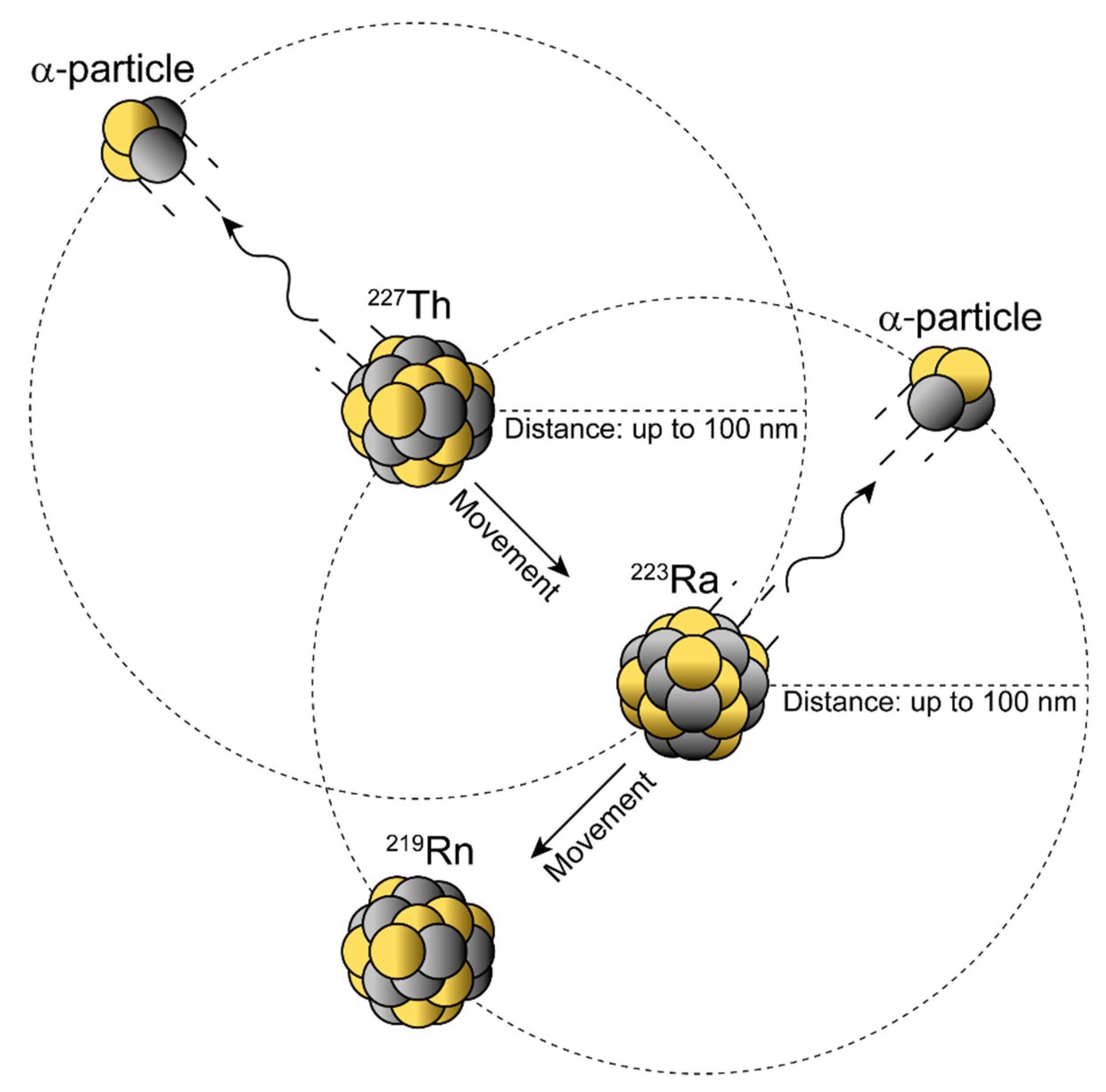
4. Nanogenerators and the Nuclear Recoil Effect
5. Radiobiological Considerations Using α- and β–-Emitters for Endoradiotherapy
6. Exploiting the Indirect Radiation Effects for Tumor Control
7. Atomic Nanogenerators in Modern Cancer Management
8. Outlook
Funding
Acknowledgments
Conflicts of Interest
References
- Curie, È. Madame Curie: A Biography; Da Capo Press: Cambridge, MA, USA, 1936. [Google Scholar]
- Grammaticos, P.C. Pioneers of nuclear medicine, Madame Curie. Hell. J. Nucl. Med. 2004, 7, 30–31. [Google Scholar] [PubMed]
- Reed, A.B. The history of radiation use in medicine. J. Vasc. Surg. 2011, 53, 3s–5s. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levi, H. George von Hevesy memorial lecture. George Hevesy and his concept of radioactive indicators--in retrospect. Eur. J. Nucl. Med. 1976, 1, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Guerra, F.; Leone, M.; Robotti, N. The Discovery of Artificial Radioactivity. Phys. Perspect. 2012, 14, 33–58. [Google Scholar] [CrossRef]
- Lawrence, E.O. Method and Apparatus for the Acceleration of Ions. US Patent 1,943,384, 26 January 1932. [Google Scholar]
- Croll, M.N. Nuclear medicine instrumentation. Historic perspective. Semin. Nucl. Med. 1994, 24, 3–10. [Google Scholar] [CrossRef]
- Pasteau, O.; Degrais. The Radium Treatment of Cancer of the Prostate. Arch. Roentgen Ray 1914, 18, 396–410. [Google Scholar] [CrossRef]
- Tod, M.C. The Treatment of Cancer of the Maxillary Antrum by Radium. Br. J. Radiol. 1948, 21, 270–275. [Google Scholar] [CrossRef]
- Clark, W.L. Cancer of the oral cavity, jaws and throat: Treatment by electrothermic methods or in combination with surgery, the roentgen ray and radium, with an analysis of two hundred cases so treated. JAMA 1918, 71, 1365–1369. [Google Scholar] [CrossRef] [Green Version]
- Ward, G.E. Radium in the treatment of cancer of the cervix uteri. JAMA 1926, 87, 1697–1700. [Google Scholar] [CrossRef]
- Kluetz, P.G.; Pierce, W.; Maher, V.E.; Zhang, H.; Tang, S.; Song, P.; Liu, Q.; Haber, M.T.; Leutzinger, E.E.; Al-Hakim, A.; et al. Radium Ra 223 dichloride injection: U.S. Food and Drug Administration drug approval summary. Clin. Cancer Res. 2014, 20, 9–14. [Google Scholar] [CrossRef] [Green Version]
- Helal, M.; Dadachova, E. Radioimmunotherapy as a Novel Approach in HIV, Bacterial, and Fungal Infectious Diseases. Cancer Biother. Radiopharm. 2018, 33, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Dadachova, E. Future Vistas in Alpha Therapy of Infectious Diseases. J. Med. Imaging Radiat. Sci. 2019, 50, S49–S52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poty, S.; Francesconi, L.C.; McDevitt, M.R.; Morris, M.J.; Lewis, J.S. alpha-Emitters for Radiotherapy: From Basic Radiochemistry to Clinical Studies-Part 1. J. Nucl. Med. 2018, 59, 878–884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haberkorn, U.; Giesel, F.; Morgenstern, A.; Kratochwil, C. The Future of Radioligand Therapy: Alpha, beta, or Both? J. Nucl. Med. 2017, 58, 1017–1018. [Google Scholar] [CrossRef] [Green Version]
- Scheinberg, D.A.; Strand, M.; Gansow, O.A. Tumor imaging with radioactive metal chelates conjugated to monoclonal antibodies. Science 1982, 215, 1511–1513. [Google Scholar] [CrossRef] [Green Version]
- Sgouros, G. Radiopharmaceutical Therapy. Health Phys. 2019, 116, 175–178. [Google Scholar] [CrossRef]
- Brechbiel, M.W. Targeted alpha-therapy: Past, present, future? Dalton Trans. 2007, 4918–4928. [Google Scholar] [CrossRef] [Green Version]
- de Kruijff, R.M.; Wolterbeek, H.T.; Denkova, A.G. A Critical Review of Alpha Radionuclide Therapy-How to Deal with Recoiling Daughters? Pharmaceuticals 2015, 8, 321–336. [Google Scholar] [CrossRef]
- Targeted Alpha Therapy Working Group; Parker, C.; Lewington, V.; Shore, N.; Kratochwil, C.; Levy, M.; Linden, O.; Noordzij, W.; Park, J.; Saad, F. Targeted Alpha Therapy, an Emerging Class of Cancer Agents: A Review. JAMA Oncol. 2018, 4, 1765–1772. [Google Scholar] [CrossRef]
- Bosch, F.; Rosich, L. The contributions of Paul Ehrlich to pharmacology: A tribute on the occasion of the centenary of his Nobel Prize. Pharmacology 2008, 82, 171–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fani, M.; Maecke, H.R.; Okarvi, S.M. Radiolabeled peptides: Valuable tools for the detection and treatment of cancer. Theranostics 2012, 2, 481–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaturvedi, S.; Mishra, A.K. Small Molecule Radiopharmaceuticals - A Review of Current Approaches. Front. Med. 2016, 3, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carter, L.M.; Poty, S.; Sharma, S.K.; Lewis, J.S. Preclinical optimization of antibody-based radiopharmaceuticals for cancer imaging and radionuclide therapy-Model, vector, and radionuclide selection. J. Label. Compd. Radiopharm. 2018, 61, 611–635. [Google Scholar] [CrossRef] [PubMed]
- McDevitt, M.R.; Sgouros, G.; Sofou, S. Targeted and Nontargeted alpha-Particle Therapies. Annu. Rev. Biomed. Eng. 2018, 20, 73–93. [Google Scholar] [CrossRef] [PubMed]
- Holzwarth, U.; Ojea Jimenez, I.; Calzolai, L. A random walk approach to estimate the confinement of alpha-particle emitters in nanoparticles for targeted radionuclide therapy. EJNMMI Radiopharm. Chem. 2018, 3, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farzin, L.; Sheibani, S.; Moassesi, M.E.; Shamsipur, M. An overview of nanoscale radionuclides and radiolabeled nanomaterials commonly used for nuclear molecular imaging and therapeutic functions. J. Biomed. Mater. Res. A 2019, 107, 251–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shirley, M.; McCormack, P.L. Radium-223 dichloride: A review of its use in patients with castration-resistant prostate cancer with symptomatic bone metastases. Drugs 2014, 74, 579–586. [Google Scholar] [CrossRef]
- Coleman, R. Treatment of Metastatic Bone Disease and the Emerging Role of Radium-223. Semin. Nucl. Med. 2016, 46, 99–104. [Google Scholar] [CrossRef]
- Kratochwil, C.; Bruchertseifer, F.; Giesel, F.L.; Weis, M.; Verburg, F.A.; Mottaghy, F.; Kopka, K.; Apostolidis, C.; Haberkorn, U.; Morgenstern, A. 225Ac-PSMA-617 for PSMA-Targeted alpha-Radiation Therapy of Metastatic Castration-Resistant Prostate Cancer. J. Nucl. Med. 2016, 57, 1941–1944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kratochwil, C.; Bruchertseifer, F.; Rathke, H.; Hohenfellner, M.; Giesel, F.L.; Haberkorn, U.; Morgenstern, A. Targeted Alpha Therapy of mCRPC with (225)Actinium-PSMA-617: Swimmer-Plot analysis suggests efficacy regarding duration of tumor-control. J. Nucl. Med. 2018. [Google Scholar] [CrossRef] [Green Version]
- Kratochwil, C.; Giesel, F.L.; Stefanova, M.; Benesova, M.; Bronzel, M.; Afshar-Oromieh, A.; Mier, W.; Eder, M.; Kopka, K.; Haberkorn, U. PSMA-Targeted Radionuclide Therapy of Metastatic Castration-Resistant Prostate Cancer with 177Lu-Labeled PSMA-617. J. Nucl. Med. 2016, 57, 1170–1176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kratochwil, C.; Bruchertseifer, F.; Rathke, H.; Bronzel, M.; Apostolidis, C.; Weichert, W.; Haberkorn, U.; Giesel, F.L.; Morgenstern, A. Targeted alpha-Therapy of Metastatic Castration-Resistant Prostate Cancer with Ac-225-PSMA-617: Dosimetry Estimate and Empiric Dose Finding. J. Nucl. Med. 2017, 58, 1624–1631. [Google Scholar] [CrossRef] [Green Version]
- Makvandi, M.; Dupis, E.; Engle, J.W.; Nortier, F.M.; Fassbender, M.E.; Simon, S.; Birnbaum, E.R.; Atcher, R.W.; John, K.D.; Rixe, O.; et al. Alpha-Emitters and Targeted Alpha Therapy in Oncology: From Basic Science to Clinical Investigations. Target Oncol. 2018, 13, 189–203. [Google Scholar] [CrossRef] [PubMed]
- Adloff, J.P. The centenary of a controversial discovery: Actinium. Radiochim. Acta 2000, 88, 123–127. [Google Scholar] [CrossRef]
- Ferrier, M.G.; Batista, E.R.; Berg, J.M.; Birnbaum, E.R.; Cross, J.N.; Engle, J.W.; La Pierre, H.S.; Kozimor, S.A.; Pacheco, J.S.L.; Stein, B.W.; et al. Spectroscopic and computational investigation of actinium coordination chemistry. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgenstern, A.; Apostolidis, C.; Kratochwil, C.; Sathekge, M.; Krolicki, L.; Bruchertseifer, F. An Overview of Targeted Alpha Therapy with (225)Actinium and (213)Bismuth. Curr. Radiopharm. 2018, 11, 200–208. [Google Scholar] [CrossRef]
- McDevitt, M.R.; Ma, D.; Lai, L.T.; Simon, J.; Borchardt, P.; Frank, R.K.; Wu, K.; Pellegrini, V.; Curcio, M.J.; Miederer, M.; et al. Tumor therapy with targeted atomic nanogenerators. Science 2001, 294, 1537–1540. [Google Scholar] [CrossRef]
- Tichacek, C.J.; Budzevich, M.M.; Wadas, T.J.; Morse, D.L.; Moros, E.G. A Monte Carlo Method for Determining the Response Relationship between Two Commonly Used Detectors to Indirectly Measure Alpha Particle Radiation Activity. Molecules 2019, 24, 3397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, M.C. Photoatomic Data Library MCPLIB04: A New Photoatomic Library Based on Data from ENDF/B-VI Release 8; Los Alamos National Laboratory: Los Alamos, NM, USA, 2003.
- Berzelius, J.J. Untersuchung eines neuen Minerals und einer darin enthaltenen zuvor unbekannten Erde. Ann. Der Phys. Und Chem. 1829, 61, 385–415. [Google Scholar] [CrossRef] [Green Version]
- Cotton, S. Lanthanide and Actinide Chemistry; John Wiley & Sons, Ltd.: Chichester, West Sussex, England, 2006. [Google Scholar]
- Frantellizzi, V.; Cosma, L.; Brunotti, G.; Pani, A.; Spanu, A.; Nuvoli, S.; De Cristofaro, F.; Civitelli, L.; De Vincentis, G. Target Alpha Therapy with Thorium-227. Cancer Biother. Radiopharm. 2020. [Google Scholar] [CrossRef]
- Henriksen, G.; Hoff, P.; Larsen, R.H. Evaluation of potential chelating agents for radium. Appl. Radiat. Isot. 2002, 56, 667–671. [Google Scholar] [CrossRef]
- Borrebaek, J.; Larsen, A.; Brevik, E.; Dahle, J.; Abbas, N.; Hjelmerud, A.K. An improved labeling method for Thorium-227 labeled antibodies for targeted alpha therapy. J. Nucl. Med. 2009, 50, 1821. [Google Scholar]
- Carithers, W.; Li, L. New Bifunctional Chelators for Ac-225 and 227Th Radioimmunotherapy. In Proceedings of the 11th International Symposium on Targeted Alpha Therapy (TAT-10), Ottawa, ON, Canada, 1–4 April 2019. [Google Scholar]
- Price, E.W.; Orvig, C. Matching chelators to radiometals for radiopharmaceuticals. Chem. Soc. Rev. 2014, 43, 260–290. [Google Scholar] [CrossRef] [PubMed]
- Boros, E.; Holland, J.P. Chemical aspects of metal ion chelation in the synthesis and application antibody-based radiotracers. J. Label. Compd. Radiopharm. 2018, 61, 652–671. [Google Scholar] [CrossRef]
- Kozempel, J.; Mokhodoeva, O.; Vlk, M. Progress in Targeted Alpha-Particle Therapy. What We Learned about Recoils Release from In Vivo Generators. Molecules 2018, 23, 581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDevitt, M.R.; Scheinberg, D.A. Ac-225 and her daughters: The many faces of Shiva. Cell Death Differ. 2002, 9, 593–594. [Google Scholar] [CrossRef]
- Mamat, C.; Reissig, F.; Bauer, D.; Pietzsch, H.-J.; Steinbach, J. Sulfonated calix-baskets for complexation of Barium and Radium. In Proceedings of the RadChem—18th Radiochemical Conference, Mariánské Lázne, Czech Republic, 13–18 May 2018; pp. 228–229. [Google Scholar]
- Piotrowska, A.; Meczynska-Wielgosz, S.; Majkowska-Pilip, A.; Kozminski, P.; Wojciuk, G.; Cedrowska, E.; Bruchertseifer, F.; Morgenstern, A.; Kruszewski, M.; Bilewicz, A. Nanozeolite bioconjugates labeled with Ra-223 for targeted alpha therapy. Nucl. Med. Biol. 2017, 47, 10–18. [Google Scholar] [CrossRef]
- Khabibullin, A.R.; Karolak, A.; Budzevich, M.M.; McLaughlin, M.L.; Morse, D.L.; Woods, L.M. Structure and properties of DOTA-chelated radiopharmaceuticals within the (225)Ac decay pathway. Medchemcomm 2018, 9, 1155–1163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thiele, N.A.; Brown, V.; Kelly, J.M.; Amor-Coarasa, A.; Jermilova, U.; MacMillan, S.N.; Nikolopoulou, A.; Ponnala, S.; Ramogida, C.F.; Robertson, A.K.H.; et al. An Eighteen-Membered Macrocyclic Ligand for Actinium-225 Targeted Alpha Therapy. Angew. Chem. Int. Edit. 2017, 56, 14712–14717. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, O.R.; Zalutsky, M.R. Radiopharmaceutical chemistry of targeted radiotherapeutics, Part 3: Alpha-particle-induced radiolytic effects on the chemical behavior of (211)At. J. Nucl. Med. 2007, 48, 1190–1196. [Google Scholar] [CrossRef] [Green Version]
- Ryan, D.E.; Brooks, R.R.; Zhang, H.F. The analytical chemistry of thorium. Rev. Anyltical Chem. 1981, 5, 281–318. [Google Scholar] [CrossRef]
- Ramdahl, T.; Bonge-Hansen, H.T.; Ryan, O.B.; Larsen, A.; Herstad, G.; Sandberg, M.; Bjerke, R.M.; Grant, D.; Brevik, E.M.; Cuthbertson, A.S. An efficient chelator for complexation of thorium-227. Bioorg. Med. Chem. Lett. 2016, 26, 4318–4321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benjamin, R.O.; Cuthbertson, A.; Karlsson, J.; Bjerke, R.M.; Indrevoll, B.; Hagemann, U.B.; Kristian, A. In-vivo Comparison of Thorium-227 and Zirconium-89 Labeled 3,2-HOPO Mesothelin Antibody-chelator Conjugate. J. Med Imaging Radiat. Sci. 2019, 50, S26. [Google Scholar] [CrossRef] [Green Version]
- Morgenstern, A.; Bruchertseifer, F.; Apostolidis, C. Bismuth-213 and actinium-225 -- generator performance and evolving therapeutic applications of two generator-derived alpha-emitting radioisotopes. Curr. Radiopharm. 2012, 5, 221–227. [Google Scholar] [CrossRef]
- Essler, M.; Gartner, F.C.; Neff, F.; Blechert, B.; Senekowitsch-Schmidtke, R.; Bruchertseifer, F.; Morgenstern, A.; Seidl, C. Therapeutic efficacy and toxicity of 225Ac-labelled vs. 213Bi-labelled tumour-homing peptides in a preclinical mouse model of peritoneal carcinomatosis. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 602–612. [Google Scholar] [CrossRef]
- Deal, K.A.; Davis, I.A.; Mirzadeh, S.; Kennel, S.J.; Brechbiel, M.W. Improved in vivo stability of actinium-225 macrocyclic complexes. J. Med. Chem. 1999, 42, 2988–2992. [Google Scholar] [CrossRef]
- Jaggi, J.S.; Kappel, B.J.; McDevitt, M.R.; Sgouros, G.; Flombaum, C.D.; Cabassa, C.; Scheinberg, D.A. Efforts to control the errant products of a targeted in vivo generator. Cancer Res. 2005, 65, 4888–4895. [Google Scholar] [CrossRef] [Green Version]
- Jaggi, J.S.; Seshan, S.V.; McDevitt, M.R.; LaPerle, K.; Sgouros, G.; Scheinberg, D.A. Renal tubulointerstitial changes after internal irradiation with alpha-particle-emitting actinium daughters. J. Am. Soc. Nephrol. 2005, 16, 2677–2689. [Google Scholar] [CrossRef] [Green Version]
- Beninson, D.J.; Dunster, H.J.; Jacobi, W.; Jammet, H.P.; Liniecki, J.; Meinhold, C.B.; Moiseev, A.A.; Rowley, K.A.; Sinclair, W.K.; Takahashi, S.; et al. Limits for Intakes of Radionuclides by Workers. In Annals of the ICRP; Snowby, F.D., Ed.; Pergamon Press: Oxford, UK, 1980; p. 67. [Google Scholar]
- Beninson, D.; Dunster, H.J.; Ilyin, I.A.; Jacobi, W.; Jammet, H.P.; Kaul, A.; Li, D.; Liniecki, J.; Matsudaira, H.; Mettler, F.; et al. Age-dependent Doses to Members of the Public from Intake of Radionuclides: Part 2 Ingestion Dose Coefficients. In Annals of the ICRP; Pergamon Press: Oxford, UK, 1993; pp. 75–87. [Google Scholar]
- Keverling Buisman, A.S. Handboek Radionucliden; BetaText: Bergen, NL, The Netherlands, 1996; pp. 226–227. [Google Scholar]
- Kumar, A.; Mishra, P.; Ghosh, S.; Sharma, P.; Ali, M.; Pandey, B.N.; Mishra, K.P. Thorium-induced oxidative stress mediated toxicity in mice and its abrogation by diethylenetriamine pentaacetate. Int. J. Radiat. Biol. 2008, 84, 337–349. [Google Scholar] [CrossRef]
- Nilsson, S.; Larsen, R.H.; Fossa, S.D.; Balteskard, L.; Borch, K.W.; Westlin, J.E.; Salberg, G.; Bruland, O.S. First clinical experience with alpha-emitting radium-223 in the treatment of skeletal metastases. Clin. Cancer Res. 2005, 11, 4451–4459. [Google Scholar] [CrossRef] [Green Version]
- Lassmann, M.; Nosske, D.; Reiners, C. Therapy of ankylosing spondylitis with 224Ra-radium chloride: Dosimetry and risk considerations. Radiat. Environ. Biophys. 2002, 41, 173–178. [Google Scholar] [CrossRef]
- Henriksen, G.; Fisher, D.R.; Roeske, J.C.; Bruland, O.S.; Larsen, R.H. Targeting of osseous sites with alpha-emitting 223Ra: Comparison with the beta-emitter 89Sr in mice. J. Nucl. Med. 2003, 44, 252–259. [Google Scholar] [PubMed]
- Kiess, A.P.; Minn, I.; Vaidyanathan, G.; Hobbs, R.F.; Josefsson, A.; Shen, C.; Brummet, M.; Chen, Y.; Choi, J.; Koumarianou, E.; et al. (2S)-2-(3-(1-Carboxy-5-(4-211At-Astatobenzamido)Pentyl)Ureido)-Pentanedioic Acid for PSMA-Targeted alpha-Particle Radiopharmaceutical Therapy. J. Nucl. Med. 2016, 57, 1569–1575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.K.; Sugyo, A.; Tsuji, A.B.; Morokoshi, Y.; Minegishi, K.; Nagatsu, K.; Kanda, H.; Harada, Y.; Nagayama, S.; Katagiri, T.; et al. alpha-particle therapy for synovial sarcoma in the mouse using an astatine-211-labeled antibody against frizzled homolog 10. Cancer Sci. 2018, 109, 2302–2309. [Google Scholar] [CrossRef]
- Atkins, H.L.; Budinger, T.F.; Lebowitz, E.; Ansari, A.N.; Greene, M.W.; Fairchild, R.G.; Ellis, K.J. Thallium-201 for medical use. Part 3: Human distribution and physical imaging properties. J. Nucl. Med. 1977, 18, 133–140. [Google Scholar] [PubMed]
- Dahle, J.; Jonasdottir, T.J.; Heyerdahl, H.; Nesland, J.M.; Borrebaek, J.; Hjelmerud, A.K.; Larsen, R.H. Assessment of long-term radiotoxicity after treatment with the low-dose-rate alpha-particle-emitting radioimmunoconjugate (227)Th-rituximab. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 93–102. [Google Scholar] [CrossRef]
- Henriksen, G.; Breistol, K.; Bruland, O.S.; Fodstad, O.; Larsen, R.H. Significant antitumor effect from bone-seeking, alpha-particle-emitting (223)Ra demonstrated in an experimental skeletal metastases model. Cancer Res. 2002, 62, 3120–3125. [Google Scholar]
- Kennel, S.J.; Chappell, L.L.; Dadachova, K.; Brechbiel, M.W.; Lankford, T.K.; Davis, I.A.; Stabin, M.; Mirzadeh, S. Evaluation of 225Ac for vascular targeted radioimmunotherapy of lung tumors. Cancer Biother. Radiopharm. 2000, 15, 235–244. [Google Scholar] [CrossRef]
- Robertson, A.K.H.; Ramogida, C.F.; Schaffer, P.; Radchenko, V. Development of (225)Ac Radiopharmaceuticals: TRIUMF Perspectives and Experiences. Curr. Radiopharm. 2018, 11, 156–172. [Google Scholar] [CrossRef]
- Ramogida, C.F.; Orvig, C. Tumour targeting with radiometals for diagnosis and therapy. Chem. Commun. 2013, 49, 4720–4739. [Google Scholar] [CrossRef]
- McLaughlin, M.F.; Woodward, J.; Boll, R.A.; Wall, J.S.; Rondinone, A.J.; Kennel, S.J.; Mirzadeh, S.; Robertson, J.D. Gold coated lanthanide phosphate nanoparticles for targeted alpha generator radiotherapy. PLoS ONE 2013, 8, e54531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, S.B.; Tiffany, L.J.; Garmestani, K.; Gansow, O.A.; Kozak, R.W. Evaluation of dithiol chelating agents as potential adjuvants for anti-IL-2 receptor lead or bismuth alpha radioimmunotherapy. Nucl. Med. Biol. 1996, 23, 105–113. [Google Scholar] [CrossRef]
- Basinger, M.A.; Jones, M.M.; McCroskey, S.A. Antidotes for acute bismuth intoxication. J. Toxicol. Clin. Toxicol. 1983, 20, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Slikkerveer, A.; Jong, H.B.; Helmich, R.B.; de Wolff, F.A. Development of a therapeutic procedure for bismuth intoxication with chelating agents. J. Lab. Clin. Med. 1992, 119, 529–537. [Google Scholar]
- Slikkerveer, A.; Noach, L.A.; Tytgat, G.N.; Van der Voet, G.B.; De Wolff, F.A. Comparison of enhanced elimination of bismuth in humans after treatment with meso-2,3-dimercaptosuccinic acid and D,L-2,3-dimercaptopropane-1-sulfonic acid. Analyst 1998, 123, 91–92. [Google Scholar] [CrossRef] [PubMed]
- Jaggi, J.S.; Seshan, S.V.; McDevitt, M.R.; Sgouros, G.; Hyjek, E.; Scheinberg, D.A. Mitigation of radiation nephropathy after internal alpha-particle irradiation of kidneys. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 1503–1512. [Google Scholar] [CrossRef]
- Chan, H.S.; Konijnenberg, M.W.; Daniels, T.; Nysus, M.; Makvandi, M.; de Blois, E.; Breeman, W.A.; Atcher, R.W.; de Jong, M.; Norenberg, J.P. Improved safety and efficacy of (213)Bi-DOTATATE-targeted alpha therapy of somatostatin receptor-expressing neuroendocrine tumors in mice pre-treated with L-lysine. EJNMMI Res. 2016, 6, 83. [Google Scholar] [CrossRef] [Green Version]
- Hanadate, S.; Washiyama, K.; Yoshimoto, M.; Matsumoto, H.; Tsuji, A.; Higashi, T.; Yoshii, Y. Oral administration of barium sulfate reduces radiation exposure to the large intestine during alpha therapy with radium-223 dichloride. J. Nucl. Med. 2017, 58, 1030. [Google Scholar]
- Gasinska, A. The contribution of women to radiobiology: Marie Curie and beyond. Rep. Pract. Oncol. Radiother. 2016, 21, 250–258. [Google Scholar] [CrossRef] [Green Version]
- Pouget, J.P.; Lozza, C.; Deshayes, E.; Boudousq, V.; Navarro-Teulon, I. Introduction to radiobiology of targeted radionuclide therapy. Front. Med. 2015, 2, 12. [Google Scholar] [CrossRef] [Green Version]
- Pouget, J.P.; Georgakilas, A.G.; Ravanat, J.L. Targeted and Off-Target (Bystander and Abscopal) Effects of Radiation Therapy: Redox Mechanisms and Risk/Benefit Analysis. Antioxid. Redox Signal. 2018, 29, 1447–1487. [Google Scholar] [CrossRef] [PubMed]
- Bethe, H. Bremsformel für Elektronen relativistischer Geschwindigkeit. Z. Phys. 1932, 76, 293–299. [Google Scholar] [CrossRef]
- Grimes, D.R.; Warren, D.R.; Partridge, M. An approximate analytical solution of the Bethe equation for charged particles in the radiotherapeutic energy range. Sci. Rep. 2017, 7, 9781. [Google Scholar] [CrossRef] [PubMed]
- Wulbrand, C.; Seidl, C.; Gaertner, F.C.; Bruchertseifer, F.; Morgenstern, A.; Essler, M.; Senekowitsch-Schmidtke, R. Alpha-particle emitting 213Bi-anti-EGFR immunoconjugates eradicate tumor cells independent of oxygenation. PLoS ONE 2013, 8, e64730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrera, F.G.; Bourhis, J.; Coukos, G. Radiotherapy combination opportunities leveraging immunity for the next oncology practice. CA Cancer J. Clin. 2017, 67, 65–85. [Google Scholar] [CrossRef]
- Rockwell, S. Experimental radiotherapy: A brief history. Radiat. Res. 1998, 150, S157–S169. [Google Scholar] [CrossRef]
- Tang, C.; Wang, X.; Soh, H.; Seyedin, S.; Cortez, M.A.; Krishnan, S.; Massarelli, E.; Hong, D.; Naing, A.; Diab, A.; et al. Combining radiation and immunotherapy: A new systemic therapy for solid tumors? Cancer Immunol. Res. 2014, 2, 831–838. [Google Scholar] [CrossRef] [Green Version]
- Gorin, J.B.; Guilloux, Y.; Morgenstern, A.; Cherel, M.; Davodeau, F.; Gaschet, J. Using alpha radiation to boost cancer immunity? Oncoimmunology 2014, 3. [Google Scholar] [CrossRef] [Green Version]
- Gorin, J.B.; Gouard, S.; Menager, J.; Morgenstern, A.; Bruchertseifer, F.; Faivre-Chauvet, A.; Guilloux, Y.; Cherel, M.; Davodeau, F.; Gaschet, J. Alpha Particles Induce Autophagy in Multiple Myeloma Cells. Front. Med. 2015, 2, 74. [Google Scholar] [CrossRef] [Green Version]
- Keisari, Y.; Hochman, I.; Confino, H.; Korenstein, R.; Kelson, I. Activation of local and systemic anti-tumor immune responses by ablation of solid tumors with intratumoral electrochemical or alpha radiation treatments. Cancer Immunol. Immunother. CII 2014, 63, 1–9. [Google Scholar] [CrossRef]
- Formenti, S.C.; Demaria, S. Combining radiotherapy and cancer immunotherapy: A paradigm shift. J. Natl. Cancer Inst. 2013, 105, 256–265. [Google Scholar] [CrossRef] [Green Version]
- Verbrugge, I.; Hagekyriakou, J.; Sharp, L.L.; Galli, M.; West, A.; McLaughlin, N.M.; Duret, H.; Yagita, H.; Johnstone, R.W.; Smyth, M.J.; et al. Radiotherapy increases the permissiveness of established mammary tumors to rejection by immunomodulatory antibodies. Cancer Res. 2012, 72, 3163–3174. [Google Scholar] [CrossRef] [Green Version]
- Golden, E.B.; Demaria, S.; Schiff, P.B.; Chachoua, A.; Formenti, S.C. An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer. Cancer Immunol. Res. 2013, 1, 365–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiniker, S.M.; Chen, D.S.; Reddy, S.; Chang, D.T.; Jones, J.C.; Mollick, J.A.; Swetter, S.M.; Knox, S.J. A systemic complete response of metastatic melanoma to local radiation and immunotherapy. Transl. Oncol. 2012, 5, 404–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Postow, M.A.; Callahan, M.K.; Barker, C.A.; Yamada, Y.; Yuan, J.; Kitano, S.; Mu, Z.; Rasalan, T.; Adamow, M.; Ritter, E.; et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N. Engl. J. Med. 2012, 366, 925–931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slovin, S.F.; Higano, C.S.; Hamid, O.; Tejwani, S.; Harzstark, A.; Alumkal, J.J.; Scher, H.I.; Chin, K.; Gagnier, P.; McHenry, M.B.; et al. Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: Results from an open-label, multicenter phase I/II study. Ann. Oncol. 2013, 24, 1813–1821. [Google Scholar] [CrossRef]
- Jeggo, P. The role of the DNA damage response mechanisms after low-dose radiation exposure and a consideration of potentially sensitive individuals. Radiat. Res. 2010, 174, 825–832. [Google Scholar] [CrossRef]
- Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fossa, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N. Engl. J. Med. 2013, 369, 213–223. [Google Scholar] [CrossRef] [Green Version]
- Jurcic, J.G.; Larson, S.M.; Sgouros, G.; McDevitt, M.R.; Finn, R.D.; Divgi, C.R.; Ballangrud, A.M.; Hamacher, K.A.; Ma, D.; Humm, J.L.; et al. Targeted alpha particle immunotherapy for myeloid leukemia. Blood 2002, 100, 1233–1239. [Google Scholar] [CrossRef] [Green Version]
- Roscher, M.; Hormann, I.; Leib, O.; Marx, S.; Moreno, J.; Miltner, E.; Friesen, C. Targeted alpha-therapy using [Bi-213]anti-CD20 as novel treatment option for radio- and chemoresistant non-Hodgkin lymphoma cells. Oncotarget 2013, 4, 218–230. [Google Scholar] [CrossRef] [Green Version]
- Friesen, C.; Glatting, G.; Koop, B.; Schwarz, K.; Morgenstern, A.; Apostolidis, C.; Debatin, K.M.; Reske, S.N. Breaking chemoresistance and radioresistance with [213Bi]anti-CD45 antibodies in leukemia cells. Cancer Res. 2007, 67, 1950–1958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kratochwil, C.; Giesel, F.L.; Heussel, C.P.; Kazdal, D.; Endris, V.; Nientiedt, C.; Bruchertseifer, F.; Kippenberger, M.; Rathke, H.; Leichsenring, J.; et al. Patients resistant against PSMA-targeting alpha-radiation therapy often harbor mutations in DNA-repair associated genes. J. Nucl. Med. 2019. [Google Scholar] [CrossRef] [PubMed]
- Fremlin, J. Health Risks of Radon and Other Internally Deposited Alpha-Emitters: Beir IV; National Academies Press: Washington, DC, USA, 1988; pp. 125–126. [Google Scholar] [CrossRef]
- Miederer, M.; Thomas, C.; Beck, J.; Hampel, C.; Krieger, C.; Baque, P.E.; Helisch, A.; Schreckenberger, M. Haematopoietic toxicity of radium-223 in patients with high skeletal tumour burden. Nuklearmedizin. Nucl. Med. 2015, 54, 197–203. [Google Scholar] [CrossRef]
- Zhou, H.; Suzuki, M.; Randers-Pehrson, G.; Vannais, D.; Chen, G.; Trosko, J.E.; Waldren, C.A.; Hei, T.K. Radiation risk to low fluences of alpha particles may be greater than we thought. Proc. Natl. Acad. Sci. USA 2001, 98, 14410–14415. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Gu, Y.; Miki, J.; Hukku, B.; McLeod, D.G.; Hei, T.K.; Rhim, J.S. Malignant transformation of human benign prostate epithelial cells by high linear energy transfer alpha-particles. Int. J. Oncol. 2007, 31, 537–544. [Google Scholar] [PubMed]
- Allen, B.J.; So, T.; Abbas Rizvi, S.M.; Song, E.Y.; Fernandez, H.R.; Lutz-Mann, L. Mutagenesis induced by targeted alpha therapy using 213Bi-cDTPA-9.2.27 in lacZ transgenic mice. Cancer Biol. Ther. 2009, 8, 777–781. [Google Scholar] [CrossRef] [Green Version]
- Kunikowska, J.; Krolicki, L.; Hubalewska-Dydejczyk, A.; Mikolajczak, R.; Sowa-Staszczak, A.; Pawlak, D. Clinical results of radionuclide therapy of neuroendocrine tumours with 90Y-DOTATATE and tandem 90Y/177Lu-DOTATATE: Which is a better therapy option? Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1788–1797. [Google Scholar] [CrossRef] [Green Version]
- Arazi, L.; Cooks, T.; Schmidt, M.; Keisari, Y.; Kelson, I. Treatment of solid tumors by interstitial release of recoiling short-lived alpha emitters. Phys. Med. Biol. 2007, 52, 5025–5042. [Google Scholar] [CrossRef]
- Cooks, T.; Arazi, L.; Schmidt, M.; Marshak, G.; Kelson, I.; Keisari, Y. Growth retardation and destruction of experimental squamous cell carcinoma by interstitial radioactive wires releasing diffusing alpha-emitting atoms. Int. J. Cancer 2008, 122, 1657–1664. [Google Scholar] [CrossRef]
- Domankevich, V.; Cohen, A.; Efrati, M.; Schmidt, M.; Rammensee, H.G.; Nair, S.S.; Tewari, A.; Kelson, I.; Keisari, Y. Combining alpha radiation-based brachytherapy with immunomodulators promotes complete tumor regression in mice via tumor-specific long-term immune response. Cancer Immunol. Immunother. CII 2019, 68, 1949–1958. [Google Scholar] [CrossRef] [Green Version]
- Reitkopf-Brodutch, S.; Confino, H.; Schmidt, M.; Cooks, T.; Efrati, M.; Arazi, L.; Rath-Wolfson, L.; Marshak, G.; Kelson, I.; Keisari, Y. Ablation of experimental colon cancer by intratumoral 224Radium-loaded wires is mediated by alpha particles released from atoms which spread in the tumor and can be augmented by chemotherapy. Int. J. Radiat. Biol. 2015, 91, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Popovtzer, A.; Rosenfeld, E.; Mizrachi, A.; Bellia, S.R.; Ben-Hur, R.; Feliciani, G.; Sarnelli, A.; Arazi, L.; Deutsch, L.; Kelson, I.; et al. Initial Safety and Tumor Control Results From a “First-in-Human” Multicenter Prospective Trial Evaluating a Novel Alpha-Emitting Radionuclide for the Treatment of Locally Advanced Recurrent Squamous Cell Carcinomas of the Skin and Head and Neck. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 571–578. [Google Scholar] [CrossRef] [Green Version]
- Yong, K.; Brechbiel, M. Application of (212)Pb for Targeted alpha-particle Therapy (TAT): Pre-clinical and Mechanistic Understanding through to Clinical Translation. AIMS Med. Sci. 2015, 2, 228–245. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, J.C.; Schafer, M.; Bauder-Wust, U.; Lehnert, W.; Leotta, K.; Morgenstern, A.; Kopka, K.; Haberkorn, U.; Mier, W.; Kratochwil, C. Development and dosimetry of (203)Pb/(212)Pb-labelled PSMA ligands: Bringing “the lead” into PSMA-targeted alpha therapy? Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1081–1091. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| 225Ac | 221Fr | 217At | 213Bi | |
|---|---|---|---|---|
| Character | Actinide | Alkali metal | Metalloid | Post-transition metal |
| Oxidation state * | +3 | +1 | −1, +1 & +7 | +3 |
| Effective ionic radius [pm] # | 112 (6) | 180 (6) | 62 (6) $ | 103 (6) |
| 227Th | 223Ra | 219Rn | 215Po | |
|---|---|---|---|---|
| Character | Actinide | Alkaline earth metal | Noble gas | Post-transition metal |
| Oxidation state * | +4 | +2 | 0 | +2 & +4 |
| Effective ionic radius [pm] # | 94 (6) | 148 (8) | n.d. | 94 (6) $ |
| Element | Radioisotope | Targeted Organ | Reference |
|---|---|---|---|
| Actinium | 225Ac | liver, bone, kidney | [62] |
| Francium | 221Fr | kidney | [63] |
| Bismuth | 211Bi and 213Bi | kidney | [64,65] |
| Lead | 209Pb and 211Pb | liver, bone, kidney | [66] |
| Polonium | 211Po * and 213Po | liver, kidney, red bone marrow, spleen | [67] |
| Thorium | 227Th | liver, bone, spleen | [68] |
| Radium | 223Ra | bone, soft tissue, intestine | [69,70,71] |
| Astatine | 217At $ | thyroid, stomach, lung | [72,73] |
| Thallium | 207Tl and 209Tl # | heart, kidney, large bowel, thyroid, testicles | [74] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roscher, M.; Bakos, G.; Benešová, M. Atomic Nanogenerators in Targeted Alpha Therapies: Curie’s Legacy in Modern Cancer Management. Pharmaceuticals 2020, 13, 76. https://doi.org/10.3390/ph13040076
Roscher M, Bakos G, Benešová M. Atomic Nanogenerators in Targeted Alpha Therapies: Curie’s Legacy in Modern Cancer Management. Pharmaceuticals. 2020; 13(4):76. https://doi.org/10.3390/ph13040076
Chicago/Turabian StyleRoscher, Mareike, Gábor Bakos, and Martina Benešová. 2020. "Atomic Nanogenerators in Targeted Alpha Therapies: Curie’s Legacy in Modern Cancer Management" Pharmaceuticals 13, no. 4: 76. https://doi.org/10.3390/ph13040076
APA StyleRoscher, M., Bakos, G., & Benešová, M. (2020). Atomic Nanogenerators in Targeted Alpha Therapies: Curie’s Legacy in Modern Cancer Management. Pharmaceuticals, 13(4), 76. https://doi.org/10.3390/ph13040076