Harnessing the Power of Eph/ephrin Biosemiotics for Theranostic Applications
Abstract
:1. Introduction
2. Eph/ephrin is a Ubiquitous Therapeutic Target
3. Eph/ephrin Signaling Dynamics
4. Small Molecule, Peptide, Protein and RNA Targeting of Ephrins
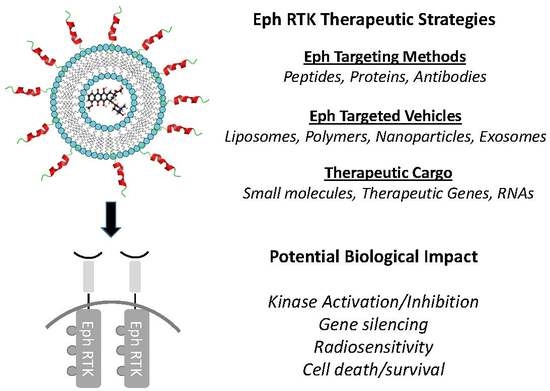
5. Targeted Delivery Strategies for Ephrins
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arvanitis, D.; Davy, A. Eph/ephrin signaling networks. Genome Res. 2008, 22, 416–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davy, A.; Soriano, P. Ephrin signaling in vivo: Look both ways. Dev. Dyn. 2004, 232, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Niethamer, T.K.; Bush, J.O. Getting direction(s): The Eph/ephrin signaling system in cell positioning. Dev. Boil. 2019, 447, 42–57. [Google Scholar] [CrossRef] [PubMed]
- Coulthard, M.; Morgan, M.; Woodruff, T.M.; Arumugam, T.V.; Taylor, S.M.; Carpenter, T.C.; Lackmann, M.; Boyd, A.W. Eph/Ephrin Signaling in Injury, and Inflammation. Am. J. Pathol. 2012, 181, 1493–1503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Funk, S.; Orr, A.W. Ephs and ephrins resurface in inflammation, immunity, and atherosclerosis. Pharmacol. Res. 2013, 67, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Kania, A.; Klein, R. Mechanisms of ephrin–Eph signalling in development, physiology, and disease. Nat. Rev. Mol. Cell Boil. 2016, 17, 240–256. [Google Scholar] [CrossRef]
- Kou, C.-T.J.; Kandpal, R.P. Differential Expression Patterns of Eph Receptors and Ephrin Ligands in Human Cancers. BioMed Res. Int. 2018, 2018, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Sun, Y.; Zhang, L.; Kang, W.; Li, N.; Li, Y. The RhoA/ROCK pathway mediates high glucose-induced cardiomyocyte apoptosis via oxidative stress, JNK, and p38MAPK pathways. Diabetes Metab. Res. Rev. 2018, 34, e3022. [Google Scholar] [CrossRef]
- Darling, T.K.; Lamb, T.J. Emerging Roles for Eph Receptors and Ephrin Ligands in Immunity. Front. Immunol. 2019, 10, 1473. [Google Scholar] [CrossRef] [Green Version]
- Wu, B.; Rockel, J.S.; Lagares, D.; Kapoor, M. Ephrins and Eph Receptor Signaling in Tissue Repair and Fibrosis. Curr. Rheumatol. Rep. 2019, 21, 23. [Google Scholar] [CrossRef]
- Barquilla, A.; Pasquale, E.B. Eph receptors and ephrins: Therapeutic opportunities. Annu. Rev. Pharmacol. Toxicol. 2014, 55, 465–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Himanen, J.P.; Yermekbayeva, L.; Janes, P.W.; Walker, J.R.; Xu, K.; Atapattu, L.; Rajashankar, K.R.; Mensinga, A.; Lackmann, M.; Nikolov, D.B.; et al. Architecture of Eph receptor clusters. Proc. Natl. Acad. Sci. USA 2010, 107, 10860–10865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saha, N.; Robev, D.; Mason, E.; Himanen, J.; Nikolov, D.B. Therapeutic potential of targeting the Eph/ephrin signaling complex. Int. J. Biochem. Cell Boil. 2018, 105, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Tognolini, M.; Hassan-Mohamed, I.; Giorgio, C.; Zanotti, I.; Lodola, A. Therapeutic perspectives of Eph–ephrin system modulation. Drug Discov. Today 2014, 19, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.-Y.; Patel, O.; Janes, P.W.; Murphy, J.M.; Lucet, I.S. Eph receptor signalling: From catalytic to non-catalytic functions. Oncogene 2019, 38, 6567–6584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasquale, E.B. Eph receptors and ephrins engage in cellular cannibalism. J. Cell Boil. 2019, 218, 3168–3170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyd, A.W.; Bartlett, P.F.; Lackmann, M. Therapeutic targeting of EPH receptors and their ligands. Nat. Rev. Drug Discov. 2013, 13, 39–62. [Google Scholar] [CrossRef]
- Hirai, H.; Maru, Y.; Hagiwara, K.; Nishida, J.; Takaku, F. A novel putative tyrosine kinase receptor encoded by the eph gene. Science 1987, 238, 1717–1720. [Google Scholar] [CrossRef]
- Halloran, M.C.; A Wolman, M. Repulsion, or adhesion: Receptors make the call. Curr. Opin. Cell Boil. 2006, 18, 533–540. [Google Scholar] [CrossRef]
- Hamada, K.; Oike, Y.; Ito, Y.; Maekawa, H.; Miyata, K.; Shimomura, T.; Suda, T. Distinct Roles of Ephrin-B2 Forward and EphB4 Reverse Signaling in Endothelial Cells. Arter. Thromb. Vasc. Boil. 2003, 23, 190–197. [Google Scholar] [CrossRef] [Green Version]
- Pasquale, E.B. The Eph family of receptors. Curr. Opin. Cell Boil. 1997, 9, 608–615. [Google Scholar] [CrossRef]
- Zhou, R. The Eph Family Receptors and Ligands. Pharmacol. Ther. 1998, 77, 151–181. [Google Scholar] [CrossRef]
- Pasquale, E.B. Eph receptor signalling casts a wide net on cell behaviour. Nat. Rev. Mol. Cell Boil. 2005, 6, 462–475. [Google Scholar] [CrossRef] [PubMed]
- Pasquale, E.B. Eph-Ephrin Bidirectional Signaling in Physiology and Disease. Cell 2008, 133, 38–52. [Google Scholar] [CrossRef] [Green Version]
- Ojosnegros, S.; Cutrale, F.; Rodríguez, D.; Otterstrom, J.; Chiu, C.L.; Hortigüela, V.; Tarantino, C.; Seriola, A.; Mieruszynski, S.; Martinez, E.; et al. Eph-ephrin signaling modulated by polymerization and condensation of receptors. Proc. Natl. Acad. Sci. USA 2017, 114, 13188–13193. [Google Scholar] [CrossRef] [Green Version]
- Rozbesky, D.; Jones, E.Y. Cell guidance ligands, receptors, and complexes, orchestrating signalling in time and space. Curr. Opin. Struct. Boil. 2020, 61, 79–85. [Google Scholar] [CrossRef]
- Seiradake, E.; Harlos, K.; Sutton, G.; Aricescu, A.R.; Jones, E.Y. An extracellular steric seeding mechanism for Eph-ephrin signaling platform assembly. Nat. Struct. Mol. Boil. 2010, 17, 398–402. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Shang, Y.; Li, J.; Chen, W.; Li, G.; Wan, J.; Liu, W.; Zhang, M. Specific Eph receptor-cytoplasmic effector signaling mediated by SAM–SAM domain interactions. eLife 2018, 7, e35677. [Google Scholar] [CrossRef]
- Mollinedo, F.; Gajate, C. Lipid rafts as signaling hubs in cancer cell survival/death and invasion: Implications in tumor progression and therapy. J. Lipid Res. 2020. [Google Scholar] [CrossRef] [Green Version]
- Chavent, M.; Seiradake, E.; Jones, E.Y.; Sansom, M.S. Structures of the EphA2 Receptor at the Membrane: Role of Lipid Interactions. Structure 2015, 24, 337–347. [Google Scholar] [CrossRef] [Green Version]
- Sakamoto, A.; Sugamoto, Y.; Tokunaga, Y.; Yoshimuta, T.; Hayashi, K.; Konno, T.; Kawashiri, M.; Takeda, Y.; Yamagishi, M. Expression Profiling of the Ephrin (EFN) and Eph Receptor (EPH) Family of Genes in Atherosclerosis-Related Human Cells. J. Int. Med. Res. 2011, 39, 522–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jellinghaus, S.; Poitz, D.M.; Ende, G.; Augstein, A.; Weinert, S.; Stutz, B.; Braun-Dullaeus, R.C.; Pasquale, E.B.; Strasser, R.H. Ephrin-A1/EphA4-mediated adhesion of monocytes to endothelial cells. Biochim. Biophys. Acta 2013, 1833, 2201–2211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheleg, M.; Yu, Q.; Go, C.; Wagner, G.C.; Kusnecov, A.W.; Zhou, R. Decreased maternal behavior and anxiety in ephrin-A5(-/-) mice. Genes. Brain Behav. 2017, 16, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Sheleg, M.; Yochum, C.L.; Richardson, J.; Wagner, G.C.; Zhou, R. Ephrin-A5 regulates inter-male aggression in mice. Behav. Brain Res. 2015, 286, 300–307. [Google Scholar] [CrossRef] [Green Version]
- Gamble, J.A.; Karunadasa, D.K.; Pape, J.-R.; Skynner, M.J.; Todman, M.G.; Bicknell, R.J.; Allen, J.P.; Herbison, A.E. Disruption of Ephrin Signaling Associates with Disordered Axophilic Migration of the Gonadotropin-Releasing Hormone Neurons. J. Neurosci. 2005, 25, 3142–3150. [Google Scholar] [CrossRef]
- Cisse, M.; Checler, F. Eph receptors: New players in Alzheimer’s disease pathogenesis. Neurobiol. Dis. 2015, 73, 137–149. [Google Scholar] [CrossRef]
- Dines, M.; Grinberg, S.; Vassiliev, M.; Ram, A.; Tamir, T.; Lamprecht, R. The roles of Eph receptors in contextual fear conditioning memory formation. Neurobiol. Learn. Mem. 2015, 124, 62–70. [Google Scholar] [CrossRef]
- Jain, R.; Jain, D.; Liu, Q.; Bartosinska, B.; Wang, J.; Schumann, D.M.; Kauschke, S.G.; Eickelmann, P.; Piemonti, L.; Gray, N.S.; et al. Pharmacological inhibition of Eph receptors enhances glucose-stimulated insulin secretion from mouse and human pancreatic islets. Diabetologyia 2013, 56, 1350–1355. [Google Scholar] [CrossRef] [Green Version]
- Weiss, A.-C.; Kispert, A. Eph/ephrin signaling in the kidney and lower urinary tract. Pediatr. Nephrol. 2015, 31, 359–371. [Google Scholar] [CrossRef]
- Yucel, S.; Dravis, C.; Garcia, N.; Henkemeyer, M.; Baker, L.A. Hypospadias and anorectal malformations mediated by defective Eph/ephrin signaling. J. Pediatr. Urol. 2007, 3, 354–363. [Google Scholar] [CrossRef] [Green Version]
- Grandi, A.; Zini, I.; Palese, S.; Giorgio, C.; Tognolini, M.; Marchesani, F.; Bruno, S.; Flammini, L.; Cantoni, A.M.; Castelli, R.; et al. Targeting the Eph/Ephrin System as Anti-Inflammatory Strategy in IBD. Front. Pharmacol. 2019, 10, 691. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, R.C.; Rundle, C.H.; Mohan, S. Role of IGF1 and EFN-EPH signaling in skeletal metabolism. J. Mol. Endocrinol. 2018, 61, 87–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fawal, M.-A.; Jungas, T.; Kischel, A.; Audouard, C.; Iacovoni, J.S.; Davy, A. Cross Talk between One-Carbon Metabolism, Eph Signaling, and Histone Methylation Promotes Neural Stem Cell Differentiation. Cell Rep. 2018, 23, 2864–2873. [Google Scholar] [CrossRef] [PubMed]
- Stark, D.A.; Karvas, R.M.; Siegel, A.L.; Cornelison, D. Eph/ephrin interactions modulate muscle satellite cell motility and patterning. Development 2011, 138, 5279–5289. [Google Scholar] [CrossRef] [Green Version]
- Lai, K.-O.; Ip, F.C.; Cheung, J.; Fu, K.Y.; Ip, N.Y. Expression of Eph Receptors in Skeletal Muscle and Their Localization at the Neuromuscular Junction. Mol. Cell. Neurosci. 2001, 17, 1034–1047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dries, J.L.; Kent, S.D.; Virag, J.A. Intramyocardial administration of chimeric ephrinA1-Fc promotes tissue salvage following myocardial infarction in mice. J. Physiol. 2011, 589, 1725–1740. [Google Scholar] [CrossRef] [PubMed]
- Dusablon, A.; Kent, S.; Coburn, A.; Virag, J.A. EphA2-receptor deficiency exacerbates myocardial infarction and reduces survival in hyperglycemic mice. Cardiovasc. Diabetol. 2014, 13, 114. [Google Scholar] [CrossRef] [Green Version]
- O’Neal, W.T.; Griffin, W.F.; Kent, S.D.; Faiz, F.; Hodges, J.; Vuncannon, J.; Virag, J.A. Deletion of the EphA2 receptor exacerbates myocardial injury and the progression of ischemic cardiomyopathy. Front. Physiol. 2014, 5. [Google Scholar] [CrossRef] [Green Version]
- Dusablon, A.; Parks, J.; Whitehurst, K.; Estes, H.; Chase, R.; Vlahos, E.; Sharma, U.; Wert, D.; Virag, J.A. EphrinA1-Fc attenuates myocardial ischemia/reperfusion injury in mice. PLoS ONE 2017, 12, e0189307. [Google Scholar] [CrossRef]
- Lefcoski, S.; Kew, K.; Reece, S.; Torres, M.J.; Parks, J.; Reece, S.; Bras, L.E.D.C.; Virag, J.A. Anatomical-Molecular Distribution of EphrinA1 in Infarcted Mouse Heart Using MALDI Mass Spectrometry Imaging. J. Am. Soc. Mass Spectrom. 2018, 29, 527–534. [Google Scholar] [CrossRef]
- Horton, J.L.; Virag, J.A. Use of Multifactorial Treatments to Address the Challenge of Translating Experimental Myocardial Infarct Reduction Strategies. Int. J. Mol. Sci. 2019, 20, 1449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres, M.J.; McLaughlin, K.L.; Renegar, R.H.; Valsaraj, S.; Whitehurst, K.S.; Sharaf, O.M.; Sharma, U.M.; Horton, J.L.; Sarathy, B.; Parks, J.C.; et al. Intracardiac administration of ephrinA1-Fc preserves mitochondrial bioenergetics during acute ischemia/reperfusion injury. Life Sci. 2019, 239, 117053. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, C.; Chen, Z.W.; Bedirian, A.; Yokota, N.; Nasr, S.H.; Rabb, H.; Lemay, S. Upregulation of EphA2 during in vivo and in vitro renal ischemia-reperfusion injury: Role of Src kinases. Am. J. Physiol. Physiol. 2006, 291, 960–971. [Google Scholar] [CrossRef] [PubMed]
- Basile, D.P.; Yoder, M.C. Getting the "inside" scoop on ephrinB2 signaling in pericytes and the effect on peritubular capillary stability. J. Am. Soc. Nephrol. 2013, 24, 521–523. [Google Scholar] [CrossRef] [Green Version]
- Ernst, A.-S.; Böhler, L.-I.; Hagenston, A.M.; Hoffmann, A.; Heiland, S.; Sticht, C.; Bendszus, M.; Hecker, M.; Bading, H.; Marti, H.H.; et al. EphB2-dependent signaling promotes neuronal excitotoxicity and inflammation in the acute phase of ischemic stroke. Acta Neuropathol. Commun. 2019, 7, 15. [Google Scholar] [CrossRef]
- Ghori, A.; Freimann, F.B.; Nieminen-Kelhä, M.; Kremenetskaia, I.; Gertz, K.; Endres, M.; Vajkoczy, P.; Nieminen-Kehlä, M. EphrinB2 Activation Enhances Vascular Repair Mechanisms and Reduces Brain Swelling After Mild Cerebral Ischemia. Arter. Thromb. Vasc. Boil. 2017, 37, 867–878. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Tu, P.; Chen, X.; Guo, S.; Wang, J. Eph Receptors: Actors in Tumor Microenvironment. Crit. Rev. Oncog. 2017, 22, 499–505. [Google Scholar] [CrossRef]
- Farnsworth, R.H.; Achen, M.G.; Stacker, S.A. The evolving role of lymphatics in cancer metastasis. Curr. Opin. Immunol. 2018, 53, 64–73. [Google Scholar] [CrossRef]
- Buensuceso, A.V.; Son, A.I.; Zhou, R.; Paquet, M.; Withers, B.M.; de Roo, B.J. Ephrin-A5 is required for optimal fertility and a complete ovulatory response to gonadotropins in the female mouse. Endocrinology 2016, 157, 942–955. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Feng, L.; Zhang, H.; Hachy, S.; Satohisa, S.; Laurent, L.C.; Parast, M.; Zheng, J.; Chen, N.-B. Preeclampsia up-regulates angiogenesis-associated microRNA (i.e., miR-17, -20a, and -20b) that target ephrin-B2 and EPHB4 in human placenta. J. Clin. Endocrinol. Metab. 2012, 97, 1051–1059. [Google Scholar] [CrossRef] [Green Version]
- Aasheim, H.-C.; Patzke, S.; Hjorthaug, H.S.; Finne, E.F. Characterization of a novel Eph receptor tyrosine kinase, EphA10, expressed in testis. Biochim. Biophys. Acta Gen. Subj. 2005, 1723, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gofur, M.R.; Ogawa, K. Compartments with predominant ephrin-B1 and EphB2/B4 expression are present alternately along the excurrent duct system in the adult mouse testis and epididymis. Andrology 2019, 7, 888–901. [Google Scholar] [CrossRef] [PubMed]
- Kida, Y.; Ieronimakis, N.; Schrimpf, C.; Reyes, M.; Duffield, J.S. EphrinB2 Reverse Signaling Protects against Capillary Rarefaction and Fibrosis after Kidney Injury. J. Am. Soc. Nephrol. 2013, 24, 559–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Zheng, X.; Peng, Q.; Zhang, X.; Qin, Z. Eph receptors: The bridge linking host and virus. Cell. Mol. Life Sci. 2019, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Li, Q.; Zheng, Y.; Li, G.; Liu, W. Systematic biochemical characterization of the SAM domains in Eph receptor family from Mus Musculus. Biochem. Biophys. Res. Commun. 2016, 473, 1281–1287. [Google Scholar] [CrossRef]
- Hafner, C.; Schmitz, G.; Meyer, S.; Bataille, F.; Hau, P.; Langmann, T.; Dietmaier, W.; Landthaler, M.; Vogt, T. Differential Gene Expression of Eph Receptors and Ephrins in Benign Human Tissues and Cancers. Clin. Chem. 2004, 50, 490–499. [Google Scholar] [CrossRef] [Green Version]
- Woodruff, T.M.; Wu, M.C.-L.; Morgan, M.; Bain, N.T.; Jeanes, A.; Lipman, J.; Ting, M.J.; Boyd, A.W.; Taylor, S.M.; Coulthard, M. Epha4-Fc Treatment Reduces Ischemia/Reperfusion-Induced Intestinal Injury by Inhibiting Vascular Permeability. Shock 2016, 45, 184–191. [Google Scholar] [CrossRef]
- Ivanov, A.I.; Romanovsky, A.A. Putative dual role of ephrin-Eph receptor interactions in inflammation. IUBMB Life 2006, 58, 389–394. [Google Scholar] [CrossRef]
- Hordijk, P.L. Recent insights into endothelial control of leukocyte extravasation. Cell. Mol. Life Sci. 2016, 73, 1591–1608. [Google Scholar] [CrossRef]
- Tosato, G. Ephrin ligands and Eph receptors contribution to hematopoiesis. Cell. Mol. Life Sci. 2017, 74, 3377–3394. [Google Scholar] [CrossRef]
- Liu, H.; Devraj, K.; Möller, K.; Liebner, S.; Hecker, M.; Korff, T. EphrinB-mediated reverse signalling controls junctional integrity and pro-inflammatory differentiation of endothelial cells. Thromb. Haemost. 2014, 112, 151–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trinidad, E.M.; Ballesteros, M.; Zuloaga, J.; Zapata, A.G.; Alonso-Colmenar, L.M. An impaired transendothelial migration potential of chronic lymphocytic leukemia (CLL) cells can be linked to ephrin-A4 expression. Blood 2009, 114, 5081–5090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sepehri, Z.; Kiani, Z.; Kohan, F.; Alavian, S.M.; Ghavami, S. Toll like receptor 4 and hepatocellular carcinoma; A systematic review. Life Sci. 2017, 179, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Mukai, M.; Suruga, N.; Saeki, N.; Ogawa, K. EphA receptors and ephrin-A ligands are upregulated by monocytic differentiation/maturation and promote cell adhesion and protrusion formation in HL60 monocytes. BMC Cell Boil. 2017, 18, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muñoz, J.J.; Alonso-Colmenar, L.M.; Sacedón, R.; Crompton, T.; Vicente, A.; Jimenez, E.; Varas, A.; Zapata, A.G. Expression and function of the Eph A receptors and their ligands ephrins A in the rat thymus. J. Immunol. 2002, 169, 177–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Luo, H. Recent advances on T-cell regulation by receptor tyrosine kinases. Curr. Opin. Hematol. 2005, 12, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Sharfe, N.; Freywald, A.; Toro, A.; Dadi, H.; Roifman, C.M. Ephrin stimulation modulates T? Cell chemotaxis. Eur. J. Immunol. 2002, 32, 3745–3755. [Google Scholar] [CrossRef]
- Salvucci, O.; Tosato, G. Essential Roles of EphB Receptors and EphrinB Ligands in Endothelial Cell Function and Angiogenesis. Adv. Cancer Res. 2012, 114, 21–57. [Google Scholar] [CrossRef] [Green Version]
- Wolf, K.; Hu, H.; Isaji, T.; Dardik, A. Molecular identity of arteries, veins, and lymphatics. J. Vasc. Surg. 2018, 69, 253–262. [Google Scholar] [CrossRef]
- Henderson, N.T.; Dalva, M.B. EphBs and ephrin-Bs: Trans-synaptic organizers of synapse development and function. Mol. Cell. Neurosci. 2018, 91, 108–121. [Google Scholar] [CrossRef]
- Cibert-Goton, V.; Yuan, G.; Battaglia, A.; Fredriksson, S.; Henkemeyer, M.; Sears, T.; Gavazzi, I. Involvement of EphB1 Receptors Signalling in Models of Inflammatory and Neuropathic Pain. PLoS ONE 2013, 8, e53673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alonso-Colmenar, L.M.; Trinidad, E.M.; Garcillán, B.; Ballesteros, M.; Castellanos, M.; Cotillo, I.; Muñoz, J.J.; Zapata, A.G. Expression profile of Eph receptors and ephrin ligands in healthy human B lymphocytes and chronic lymphocytic leukemia B-cells. Leuk. Res. 2009, 33, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Wybenga-Groot, L.E.; McGlade, C.J. Rtk Slap Down: The emerging role of Src-like adaptor protein as a key player in receptor tyrosine kinase signaling. Cell. Signal. 2015, 27, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Aitsebaomo, J.; Portbury, A.L.; Schisler, J.C.; Patterson, C. Brothers, and sisters: Molecular insights into arterial-venous heterogeneity. Circ. Res. 2008, 103, 929–939. [Google Scholar] [CrossRef] [Green Version]
- Coso, S.; Bovay, E.; Petrova, T.V. Pressing the right buttons: Signaling in lymphangiogenesis. Blood 2014, 123, 2614–2624. [Google Scholar] [CrossRef] [Green Version]
- Makinen, T.; Adams, R.H.; Bailey, J.; Lu, Q.; Ziemiecki, A.; Alitalo, K.; Klein, R.; Wilkinson, G.A. PDZ interaction site in ephrinB2 is required for the remodeling of lymphatic vasculature. Genome Res. 2005, 19, 397–410. [Google Scholar] [CrossRef] [Green Version]
- Lodola, A.; Giorgio, C.; Incerti, M.; Zanotti, I.; Tognolini, M. Targeting Eph/ephrin system in cancer therapy. Eur. J. Med. Chem. 2017, 142, 152–162. [Google Scholar] [CrossRef]
- Magic, Z.; Sandström, J.; Perez-Tenorio, G. Ephrin-B2 inhibits cell proliferation and motility in vitro and predicts longer metastasis-free survival in breast cancer. Int. J. Oncol. 2019, 55. [Google Scholar] [CrossRef] [Green Version]
- Abéngozar, M.A.; de Frutos, S.; Ferreiro, S.; Soriano, J.; Perez-Martinez, M.; Olmeda, D.; Marenchino, M.; Cañamero, M.; Ortega, S.; Megias, D.; et al. Blocking ephrinB2 with highly specific antibodies inhibits angiogenesis, lymphangiogenesis, and tumor growth. Blood 2012, 119, 4565–4576. [Google Scholar] [CrossRef] [Green Version]
- Alfaro, D.; Zapata, A.G. Eph/Ephrin-mediated stimulation of human bone marrow mesenchymal stromal cells correlates with changes in cell adherence and increased cell death. Stem Cell Res. Ther. 2018, 9, 172. [Google Scholar] [CrossRef]
- Nguyen, T.M.; Arthur, A.; Panagopoulos, R.; Paton, S.; Hayball, J.D.; Zannettino, A.C.; Purton, L.; Matsuo, K.; Gronthos, S. EphB4 Expressing Stromal Cells Exhibit an Enhanced Capacity for Hematopoietic Stem Cell Maintenance. Stem Cells 2015, 33, 2838–2849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, T.M.; Arthur, A.; Gronthos, S. The role of Eph/ephrin molecules in stromal–hematopoietic interactions. Int. J. Hematol. 2015, 103, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Alonso, R.; Bustos, F.; Budzyk, M.; Kumar, P.; Helbig, A.O.; Hukelmann, J.; Lamond, A.I.; Lanner, F.; Zhou, H.; Petsalaki, E.; et al. Phosphoproteomics identifies a bimodal EPHA2 receptor switch that promotes embryonic stem cell differentiation. Nat. Commun. 2020, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Nakajima-Koyama, M.; Sone, M.; Koga, M.; Ebisuya, M.; Yamamoto, T. Secreted Ephrin Receptor A7 Promotes Somatic Cell Reprogramming by Inducing ERK Activity Reduction. Stem. Cell Rep. 2015, 5, 480–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, Y.H.; Lee, S.-J.; Oh, S.Y.; Lee, H.J.; Ryu, J.M.; Han, H.J. Oleic acid enhances the motility of umbilical cord blood derived mesenchymal stem cells through EphB2-dependent F-actin formation. Biochim. Biophys. Acta Bioenerg. 2015, 1853, 1905–1917. [Google Scholar] [CrossRef] [Green Version]
- Fujiwara, H.; Ono, M.; Sato, Y.; Imakawa, K.; Iizuka, T.; Kagami, K.; Fujiwara, T.; Horie, A.; Tani, H.; Hattori, A.; et al. Promoting Roles of Embryonic Signals in Embryo Implantation and Placentation in Cooperation with Endocrine and Immune Systems. Int. J. Mol. Sci. 2020, 21, 1885. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Wang, Y.; Jiang, M.; Deng, X.; Pei, Z.; Li, F.; Xia, K.; Zhu, L.; Yang, T.; Chen, M. Downregulation of Profilin-1 Expression Attenuates Cardiomyocytes Hypertrophy and Apoptosis Induced by Advanced Glycation End Products in H9c2 Cells. BioMed Res. Int. 2017, 2017, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, S.; Ridley, A.J.; Lutz, S. The Function of Rho-Associated Kinases ROCK1 and ROCK2 in the Pathogenesis of Cardiovascular Disease. Front. Pharmacol. 2015, 6, 385. [Google Scholar] [CrossRef]
- Liu, Y.; Tejpal, N.; You, J.; Li, X.C.; Ghobrial, R.M.; Kloc, M. ROCK inhibition impedes macrophage polarity and functions. Cell. Immunol. 2016, 300, 54–62. [Google Scholar] [CrossRef]
- Sharfe, N.; Nikolic, M.; Cimpeon, L.; van de Kratts, A.; Freywald, A.; Roifman, C.M. EphA and ephrin-A proteins regulate integrin-mediated T lymphocyte interactions. Mol. Immunol. 2008, 45, 1208–1220. [Google Scholar] [CrossRef]
- Cowan, C.A.; Henkemeyer, M. The SH2/SH3 adaptor Grb4 transduces B-ephrin reverse signals. Nature 2001, 413, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Park, I.; Lee, H.-S. EphB/ephrinB signaling in cell adhesion and migration. Mol. Cells 2014, 38, 14–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prospéri, M.-T.; Lépine, P.; Dingli, F.; Paul-Gilloteaux, P.; Martin, R.; Loew, D.; Knölker, H.-J.; Coudrier, E. Myosin 1b functions as an effector of EphB signaling to control cell repulsion. J. Cell Boil. 2015, 210, 347–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamazaki, T.; Masuda, J.; Omori, T.; Usui, R.; Akiyama, H.; Maru, Y. EphA1 interacts with integrin-linked kinase and regulates cell morphology and motility. J. Cell Sci. 2008, 122, 243–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.-H.; Chang, Z.-F. Regulation of RhoA-dependent ROCKII activation by Shp2. J. Cell Boil. 2008, 181, 999–1012. [Google Scholar] [CrossRef] [Green Version]
- Miao, H.; Burnett, E.; Kinch, M.; Simon, E.; Wang, B. Activation of EphA2 kinase suppresses integrin function and causes focal-adhesion-kinase dephosphorylation. Nature 1999, 2, 62. [Google Scholar] [CrossRef]
- Olgar, Y.; Celen, M.; Yamasan, B.E.; Ozturk, N.; Turan, B.; Ozdemir, S. Rho-kinase inhibition reverses impaired Ca 2+ handling and associated left ventricular dysfunction in pressure overload-induced cardiac hypertrophy. Cell Calcium 2017, 67, 81–90. [Google Scholar] [CrossRef]
- Brand, C.S.; Tan, V.P.; Brown, J.H.; Miyamoto, S. RhoA regulates Drp1 mediated mitochondrial fission through ROCK to protect cardiomyocytes. Cell. Signal. 2018, 50, 48–57. [Google Scholar] [CrossRef] [Green Version]
- He, K.; Yan, L.; Pan, C.-S.; Liu, Y.-Y.; Cui, Y.-C.; Hu, B.-H.; Chang, X.; Li, Q.; Sun, K.; Mao, X.-W.; et al. ROCK-dependent ATP5D modulation contributes to the protection of notoginsenoside NR1 against ischemia-reperfusion-induced myocardial injury. Am. J. Physiol. Circ. Physiol. 2014, 307, 1764–1776. [Google Scholar] [CrossRef]
- Bian, H.; Zhou, Y.; Yu, B.; Shang, D.; Liu, F.; Li, B.; Qi, J. Rho-kinase signaling pathway promotes the expression of PARP to accelerate cardiomyocyte apoptosis in ischemia/reperfusion. Mol. Med. Rep. 2017, 16, 2002–2008. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.; Surma, M.; Yang, Y.; Wei, L. Disruption of both ROCK1 and ROCK2 genes in cardiomyocytes promotes autophagy and reduces cardiac fibrosis during aging. FASEB J. 2019, 33, 7348–7362. [Google Scholar] [CrossRef] [PubMed]
- Pegg, C.; Cooper, L.T.; Zhao, J.; Gerometta, M.; Smith, F.M.; Yeh, M.; Bartlett, P.F.; Gorman, J.J.; Boyd, A.W. Glycoengineering of EphA4 Fc leads to a unique, long-acting and broad spectrum, Eph receptor therapeutic antagonist. Sci. Rep. 2017, 7, 6519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheideler, O.J.; Yang, C.; Kozminsky, M.; Mosher, K.I.; Falcón-Banchs, R.; Ciminelli, E.C.; Bremer, A.W.; Chern, S.A.; Schaffer, D.V.; Sohn, L.L. Recapitulating complex biological signaling environments using a multiplexed, DNA-patterning approach. Sci. Adv. 2020, 6, e5696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noberini, R.; Lamberto, I.; Pasquale, E.B. Targeting Eph receptors with peptides and small molecules: Progress and challenges. Semin. Cell Dev. Boil. 2011, 23, 51–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soler, M.M.G.; Gehring, M.P.; Lechtenberg, B.C.; Zapata-Mercado, E.; Hristova, K.; Pasquale, E.B. Engineering nanomolar peptide ligands that differentially modulate EphA2 receptor signaling. J. Boil. Chem. 2019, 294, 8791–8805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alves, D.S.; Westerfield, J.M.; Shi, X.; Nguyen, V.P.; Stefanski, K.M.; Booth, K.R.; Kim, S.; Morrell-Falvey, J.L.; Wang, B.; Abel, S.; et al. A novel pH-dependent membrane peptide that binds to EphA2 and inhibits cell migration. eLife 2018, 7. [Google Scholar] [CrossRef]
- Gambini, L.; Salem, A.F.; Udompholkul, P.; Tan, X.-F.; Baggio, C.; Shah, N.; Aronson, A.; Song, J.; Pellecchia, M. Structure-Based Design of Novel EphA2 Agonistic Agents with Nanomolar Affinity in Vitro and in Cell. ACS Chem. Boil. 2018, 13, 2633–2644. [Google Scholar] [CrossRef]
- Wu, B.; De, S.K.; Kulinich, A.; Salem, A.F.; Koeppen, J.; Wang, R.; Barile, E.; Wang, S.; Zhang, D.; Ethell, I.; et al. Potent and Selective EphA4 Agonists for the Treatment of ALS. Cell Chem. Boil. 2017, 24, 293–305. [Google Scholar] [CrossRef] [Green Version]
- Heinzlmeir, S.; Kudlinzki, D.; Sreeramulu, S.; Klaeger, S.; Gande, S.L.; Linhard, V.; Wilhelm, M.; Qiao, H.; Helm, D.; Ruprecht, B.; et al. Chemical Proteomics and Structural Biology Define EPHA2 Inhibition by Clinical Kinase Drugs. ACS Chem. Boil. 2016, 11, 3400–3411. [Google Scholar] [CrossRef]
- Miao, B.; Ji, Z.; Tan, L.; Taylor, M.; Zhang, J.; Choi, H.G.; Frederick, D.T.; Kumar, R.; Wargo, J.A.; Flaherty, K.T.; et al. EPHA2 is a mediator of vemurafenib resistance and a novel therapeutic target in melanoma. Cancer Discov. 2014, 5, 274–287. [Google Scholar] [CrossRef] [Green Version]
- Giorgio, C.; Incerti, M.; Corrado, M.; Presta, M.; Chiodelli, P.; Russo, S.; Callegari, D.; Ferlenghi, F.; Ballabeni, V.; Barocelli, E.; et al. Pharmacological evaluation of new bioavailable small molecules targeting Eph/ephrin interaction. Biochem. Pharmacol. 2018, 147, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Giorgio, C.; Incerti, M.; Pala, D.; Russo, S.; Chiodelli, P.; Rusnati, M.; Cantoni, A.; di Lecce, R.; Barocelli, E.; Bertoni, S.; et al. Inhibition of Eph/ephrin interaction with the small molecule UniPR500 improves glucose tolerance in healthy and insulin-resistant mice. Pharmacol. Res. 2019, 141, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Dai, B.; Shi, X.; Ma, N.; Ma, W.; Zhang, Y.; Yang, T.; Zhang, J.; He, L. HMQ-T-B10 induces human liver cell apoptosis by competitively targeting EphrinB2 and regulating its pathway. J. Cell. Mol. Med. 2018, 22, 5231–5243. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Zhu, M.; Zhang, D.; Yang, L.; Yang, T.; Li, X.; Zhang, Y. Berberine inhibits the proliferation and migration of breast cancer ZR-75-30 cells by targeting Ephrin-B2. Phytomedicine 2017, 25, 45–51. [Google Scholar] [CrossRef]
- Ma, W.; Zhu, M.; Yang, L.; Yang, T.; Zhang, Y. Synergistic Effect of TPD7 and Berberine against Leukemia Jurkat Cell Growth through Regulating Ephrin-B2 Signaling. Phytother. Res. 2017, 31, 1392–1399. [Google Scholar] [CrossRef]
- Riedl, S.J.; Pasquale, E.B. Targeting the Eph System with Peptides and Peptide Conjugates. Curr. Drug Targets 2015, 16, 1031–1047. [Google Scholar] [CrossRef]
- Salem, A.F.; Wang, S.; Billet, S.; Chen, J.-F.; Udompholkul, P.; Gambini, L.; Baggio, C.; Tseng, H.; Posadas, E.M.; Bhowmick, N.A.; et al. Reduction of Circulating Cancer Cells and Metastases in Breast-Cancer Models by a Potent EphA2-Agonistic Peptide–Drug Conjugate. J. Med. Chem. 2018, 61, 2052–2061. [Google Scholar] [CrossRef]
- Wu, B.; Wang, S.; De, S.K.; Barile, E.; Quinn, B.A.; Zharkikh, I.; Purves, A.; Stebbins, J.L.; Oshima, R.G.; Fisher, P.B.; et al. Design and Characterization of Novel EphA2 Agonists for Targeted Delivery of Chemotherapy to Cancer Cells. Chem. Boil. 2015, 22, 876–887. [Google Scholar] [CrossRef] [Green Version]
- Barile, E.; Wang, S.; Das, S.K.; Noberini, R.; Dahl, R.; Stebbins, J.L.; Pasquale, E.B.; Fisher, P.B.; Pellecchia, M. Design, synthesis and bioevaluation of an EphA2 receptor-based targeted delivery system. Chem. Med. Chem. 2014, 9, 1403–1412. [Google Scholar] [CrossRef]
- Wang, S.; Placzek, W.J.; Stebbins, J.L.; Mitra, S.; Noberini, R.; Koolpe, M.; Zhang, Z.; Dahl, R.; Pasquale, E.B.; Pellecchia, M. Novel Targeted System To Deliver Chemotherapeutic Drugs to EphA2-Expressing Cancer Cells. J. Med. Chem. 2012, 55, 2427–2436. [Google Scholar] [CrossRef] [Green Version]
- Quinn, B.A.; Wang, S.; Barile, E.; Das, S.K.; Emdad, L.; Sarkar, D.; De, S.K.; Kharagh, S.M.; Stebbins, J.L.; Pandol, S.J.; et al. Therapy of pancreatic cancer via an EphA2 receptor-targeted delivery of gemcitabine. Oncotarget 2016, 7, 17103–17110. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, O.; Li, Q.; Chen, H.; Niu, G.; Kiesewetter, D.O.; Xu, L.; Cook, K.; Yang, G.; Dall’Acqua, W.; Tsui, P.; et al. PET-Guided Evaluation and Optimization of Internalized Antibody-Drug Conjugates Targeting Erythropoietin-Producing Hepatoma A2 Receptor. J. Nucl. Med. 2017, 58, 1838–1844. [Google Scholar] [CrossRef] [PubMed]
- Damelin, M.; Bankovich, A.; Park, A.; Aguilar, J.; Anderson, W.; Santaguida, M.; Aujay, M.; Fong, S.; Khandke, K.; Pulito, V.; et al. Anti-EFNA4 Calicheamicin Conjugates Effectively Target Triple-Negative Breast and Ovarian Tumor-Initiating Cells to Result in Sustained Tumor Regressions. Clin. Cancer Res. 2015, 21, 4165–4173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatia, S.; Hirsch, K.; Sharma, J.; Oweida, A.; Griego, A.; Keysar, S.; Jimeno, A.; Raben, D.; Krasnoperov, V.; Gill, P.S.; et al. Enhancing radiosensitization in EphB4 receptor-expressing Head and Neck Squamous Cell Carcinomas. Sci. Rep. 2016, 6, 38792. [Google Scholar] [CrossRef] [Green Version]
- Ferluga, S.; Tomé, C.M.L.; Herpai, D.M.; D’Agostino, R.; Debinski, W. Simultaneous targeting of Eph receptors in glioblastoma. Oncotarget 2016, 7, 59860–59876. [Google Scholar] [CrossRef] [Green Version]
- Qazi, M.; Vora, P.; Venugopal, C.; Adams, J.; Singh, M.; Hu, A.X.; Gorelik, M.; Subapanditha, M.K.; Savage, N.; Yang, J.; et al. Cotargeting Ephrin Receptor Tyrosine Kinases A2 and A3 in Cancer Stem Cells Reduces Growth of Recurrent Glioblastoma. Cancer Res. 2018, 78, 5023–5037. [Google Scholar] [CrossRef] [Green Version]
- Stephenson, S.-A.; Douglas, E.L.; Mertens-Walker, I.; Lisle, J.; Maharaj, M.S.; Herington, A.C. Anti-tumour effects of antibodies targeting the extracellular cysteine-rich region of the receptor tyrosine kinase EphB4. Oncotarget 2015, 6, 7554–7569. [Google Scholar] [CrossRef]
- Özcan, G.; Ozpolat, B.; Coleman, R.L.; Sood, A.K.; Lopez-Berestein, G. Preclinical and clinical development of siRNA-based therapeutics. Adv. Drug Deliv. Rev. 2015, 87, 108–119. [Google Scholar] [CrossRef] [Green Version]
- Bhatia, S.; Hirsch, K.; Bukkapatnam, S.; Baig, N.A.; Oweida, A.; Griego, A.; Calame, D.; Sharma, J.; Donson, A.; Foreman, N.K.; et al. Combined EphB2 receptor knockdown with radiation decreases cell viability and invasion in medulloblastoma. Cancer Cell Int. 2017, 17, 41. [Google Scholar] [CrossRef] [Green Version]
- Ma, T.; Liu, X.; Cen, Z.; Xin, C.; Guo, M.; Zou, C. MicroRNA-302b negatively regulates IL-1beta production in response to MSU crystals by targeting IRAK4 and EphA2. Arthr. Res.Ther. 2018, 20, 34. [Google Scholar] [CrossRef]
- Jin, Q.; Li, X.J.; Cao, P.G. MicroRNA-26b Enhances the Radiosensitivity of Hepatocellular Carcinoma Cells by Targeting EphA2. Tohoku J. Exp. Med. 2016, 238, 143–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alavi, M.; Hamidi, M. Passive and active targeting in cancer therapy by liposomes and lipid nanoparticles. Drug Metab. Pers. Ther. 2019, 34. [Google Scholar] [CrossRef] [PubMed]
- Su, C.-Y.; Chen, M.; Chen, L.-C.; Ho, Y.-S.; Ho, H.-O.; Lin, S.-Y.; Chuang, K.-H.; Sheu, M.-T. Bispecific antibodies (anti-mPEG/anti-HER2) for active tumor targeting of docetaxel (DTX)-loaded mPEGylated nanocarriers to enhance the chemotherapeutic efficacy of HER2-overexpressing tumors. Drug Deliv. 2018, 25, 1066–1079. [Google Scholar] [CrossRef] [PubMed]
- Ulbrich, K.; Holá, K.; Subr, V.; Bakandritsos, A.; Tucek, J.; Zbořil, R. Targeted Drug Delivery with Polymers and Magnetic Nanoparticles: Covalent and Noncovalent Approaches, Release Control, and Clinical Studies. Chem. Rev. 2016, 116, 5338–5431. [Google Scholar] [CrossRef] [PubMed]
- Ekladious, I.; Colson, Y.L.; Grinstaff, M.W. Polymer–drug conjugate therapeutics: Advances, insights, and prospects. Nat. Rev. Drug Discov. 2018, 18, 273–294. [Google Scholar] [CrossRef] [PubMed]
- Haghiralsadat, F.; Amoabediny, G.; Naderinezhad, S.; Doulabi, B.Z.; Forouzanfar, T.; Helder, M.N. Codelivery of doxorubicin and JIP1 siRNA with novel EphA2-targeted PEGylated cationic nanoliposomes to overcome osteosarcoma multidrug resistance. Int. J. Nanomed. 2018, 13, 3853–3866. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Du, Z.; Pan, S.; Shi, M.; Li, J.; Yang, C.; Hu, H.; Qiao, M.; Chen, D.; Zhao, X. Overcoming Multidrug Resistance by Codelivery of MDR1-Targeting siRNA and Doxorubicin Using EphA10-Mediated pH-Sensitive Lipoplexes: In Vitro and In Vivo Evaluation. ACS Appl. Mater. Interfaces 2018, 10, 21590–21600. [Google Scholar] [CrossRef]
- Guo, Z.; He, B.; Yuan, L.; Dai, W.; Zhang, H.; Wang, X.; Wang, J.; Zhang, X.; Zhang, Q. Dual targeting for metastatic breast cancer and tumor neovasculature by EphA2-mediated nanocarriers. Int. J. Pharm. 2015, 493, 380–389. [Google Scholar] [CrossRef]
- Nasreen, N.; Lee, H.-Y.; A Mohammed, K.; Kaye, F.; Sharma, P.; Moudgil, B.M.; Clapp, W.L. Targeted delivery of let-7a microRNA encapsulated ephrin-A1 conjugated liposomal nanoparticles inhibit tumor growth in lung cancer. Int. J. Nanomed. 2013, 8, 4481–4494. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.R.; Tipparaju, S.K.; Kirpotin, D.B.; Pien, C.; Kornaga, T.; Noble, C.O.; Koshkaryev, A.; Tran, J.; Kamoun, W.S.; Drummond, D.C. Formulation optimization of an ephrin A2 targeted immunoliposome encapsulating reversibly modified taxane prodrugs. J. Control. Release 2019, 310, 47–57. [Google Scholar] [CrossRef]
- Zhang, K.; Geddie, M.L.; Kohli, N.; Kornaga, T.; Kirpotin, D.B.; Jiao, Y.; Rennard, R.; Drummond, D.C.; Nielsen, U.B.; Xu, L.; et al. Comprehensive optimization of a single-chain variable domain antibody fragment as a targeting ligand for a cytotoxic nanoparticle. mAbs 2015, 7, 42–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geddie, M.L.; Kohli, N.; Kirpotin, D.B.; Razlog, M.; Jiao, Y.; Kornaga, T.; Rennard, R.; Xu, L.; Schoerberl, B.; Marks, J.D.; et al. Improving the developability of an anti-EphA2 single-chain variable fragment for nanoparticle targeting. MAB’s 2016, 9, 58–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pearce, A.K.; Fuchs, A.V.; Fletcher, N.L.; Thurecht, K.J. Targeting Nanomedicines to Prostate Cancer: Evaluation of Specificity of Ligands to Two Different Receptors In Vivo. Pharm. Res. 2016, 33, 2388–2399. [Google Scholar] [CrossRef] [PubMed]
- Blevins, K.S.; Jeong, J.H.; Ou, M.; Brumbach, J.H.; Kim, S.W. EphA2 targeting peptide tethered bioreducible poly (cystamine bisacrylamide-diamino hexane) for the delivery of therapeutic pCMV-RAE-1gamma to pancreatic islets. J. Control Release 2012, 158, 115–122. [Google Scholar] [CrossRef] [Green Version]
- Conway, A.; Vazin, T.; Spelke, D.P.; Rode, N.A.; Healy, K.E.; Kane, R.S.; Schaffer, D.V. Multivalent ligands control stem cell behaviour in vitro and in vivo. Nat. Nanotechnol. 2013, 8, 831–838. [Google Scholar] [CrossRef] [Green Version]
- Möser, C.; Lorenz, J.S.; Sajfutdinow, M.; Smith, D.M. Pinpointed Stimulation of EphA2 Receptors via DNA-Templated Oligovalence. Int. J. Mol. Sci. 2018, 19, 3482. [Google Scholar] [CrossRef] [Green Version]
- Patel, A.R.; Chougule, M.; Singh, M. EphA2 targeting pegylated nanocarrier drug delivery system for treatment of lung cancer. Pharm. Res. 2014, 31, 2796–2809. [Google Scholar] [CrossRef] [Green Version]
- Ou, W.; Byeon, J.H.; Soe, Z.C.; Kim, B.K.; Thapa, R.K.; Gupta, B.; Poudel, B.K.; Ku, S.K.; Yong, C.S.; Kim, J.O. Tailored Black Phosphorus for Erythrocyte Membrane Nanocloaking with Interleukin-1α siRNA and Paclitaxel for Targeted, Durable, and Mild Combination Cancer Therapy. Theranostics 2019, 9, 6780–6796. [Google Scholar] [CrossRef]
- Alkilany, A.; Boulos, S.P.; Lohse, S.E.; Thompson, L.; Murphy, C. Homing Peptide-Conjugated Gold Nanorods: The Effect of Amino Acid Sequence Display on Nanorod Uptake and Cellular Proliferation. Bioconjugate Chem. 2014, 25, 1162–1171. [Google Scholar] [CrossRef]
- Hanley, T.; Yin, R.; Mac, J.T.; Tan, W.; Anvari, B. Functionalized erythrocyte-derived optical nanoparticles to target ephrin-B2 ligands. J. Biomed. Opt. 2019, 24, 85002–85009. [Google Scholar] [CrossRef]
- Chu, L.; Wang, A.; Ni, L.; Yan, X.; Song, Y.; Zhao, M.; Sun, K.; Mu, H.; Liu, S.; Wu, Z.; et al. Nose-to-brain delivery of temozolomide-loaded PLGA nanoparticles functionalized with anti-EPHA3 for glioblastoma targeting. Drug Deliv. 2018, 25, 1634–1641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patil, M.; Upadhyay, A.K.; Hernandez-Lagunas, L.; Good, R.; Carpenter, T.C.; Sucharov, C.C.; Nozik-Grayck, E.; Kompella, U.B. Targeted delivery of YSA-functionalized and non-functionalized polymeric nanoparticles to injured pulmonary vasculature. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1059–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Wang, B.; Wu, Z.; Shi, X.; Zhang, J.; Han, S. Treatment of Dutch rat models of glioma using Ephrin-A1-PE38/GM-CSF chitosan nanoparticles by in situ activation of dendritic cells. Tumour Biol. 2015, 36, 7961–7966. [Google Scholar] [CrossRef] [PubMed]
- Pasquale, E.B. Exosomes expand the sphere of influence of Eph receptors and ephrins. J. Cell Boil. 2016, 214, 5–7. [Google Scholar] [CrossRef] [Green Version]
- Gong, J.; Körner, R.; Gaitanos, L.; Klein, R. Exosomes mediate cell contact–independent ephrin-Eph signaling during axon guidance. J. Cell Boil. 2016, 214, 35–44. [Google Scholar] [CrossRef]
- Zhao, Y.; Yin, L.; Zhang, H.; Lan, T.; Li, S.; Ma, P. Eph/ephrin family anchored on exosome facilitate communications between cells. Cell Boil. Int. 2018, 42, 1458–1462. [Google Scholar] [CrossRef]
- Sato, S.; Vasaikar, S.; Eskaros, A.; Kim, Y.; Lewis, J.S.; Zhang, B.; Zijlstra, A.; Weaver, A.M. EPHB2 carried on small extracellular vesicles induces tumor angiogenesis via activation of ephrin reverse signaling. JCI Insight 2019, 4. [Google Scholar] [CrossRef]
- Yamashita, T.; Kamada, H.; Kanasaki, S.; Nagano, K.; Inoue, M.; Higashisaka, K.; Yoshioka, Y.; Tsutsumi, Y.; Tsunoda, S. Ephrin type-A receptor 2 on tumor-derived exosomes enhances angiogenesis through the activation of MAPK signaling. Pharmazie 2019, 74, 614–619. [Google Scholar]
- Fan, J.; Wei, Q.; Koay, E.J.; Liu, Y.; Ning, B.; Bernard, P.W.; Zhang, N.; Han, H.; Katz, M.H.; Zhao, Z.; et al. Chemoresistance Transmission via Exosome-Mediated EphA2 Transfer in Pancreatic Cancer. Theranostics 2018, 8, 5986–5994. [Google Scholar] [CrossRef]
- Jung, K.O.; Youn, H.; Lee, C.-H.; Kang, K.W.; Chung, J.-K. Visualization of exosome-mediated miR-210 transfer from hypoxic tumor cells. Oncotarget 2016, 8, 9899–9910. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Wang, J.; Chen, Y.; Chen, Y.; Ma, X.; Bihl, J.; Yang, Y. NPC-EXs Alleviate Endothelial Oxidative Stress and Dysfunction through the miR-210 Downstream Nox2 and VEGFR2 Pathways. Oxid. Med. Cell. Longev. 2017, 2017, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Barile, L.; Lionetti, V.; Cervio, E.; Matteucci, M.; Gherghiceanu, M.; Popescu, L.M.; Torre, T.; Siclari, F.; Moccetti, T.; Vassalli, G. Extracellular vesicles from human cardiac progenitor cells inhibit cardiomyocyte apoptosis and improve cardiac function after myocardial infarction. Cardiovasc. Res. 2014, 103, 530–541. [Google Scholar] [CrossRef] [PubMed]


| Target Receptor | Agent Type | Agent Name | Sequence/Description | Reference |
|---|---|---|---|---|
| EphA2 | Peptide | YSA | YSAYPDSVPMMS | [115] |
| Peptide | YSA-GSGSK-bio | YSAYPDSVPMMSGSGSK-bio | [115] | |
| Peptide | SWL | SWLAYPGAVSYR | [115] | |
| Peptide | WLAam | WLAYPDSVPMam | [115] | |
| Peptide | βA-WLA-YRPK-bio | βAWLAYPDSVPYRPK-bio | [115] | |
| Peptide | βA-WLA-YSK-bio | βAWLAYPDSVPYSK-bio | [115] | |
| Peptide | TYPE7 | EFQTLSPEGSGNLAVIGGVAVGVVLELVLAGVEFFIEEEEE | [116] | |
| Peptide | 123B9 | (4-F,3-ClPhOCH2CO)SAYPDSVP(Nle) (hS)S-CONH2 | [117] | |
| Peptide | 135H11 | XLA(4MeTyr)PDA V(Hyp)(4ClPhe)RP-CONH2 X = 3-methyl-6,7-dimethoxy-benzofuranoic acid | [117] | |
| Peptide-small molecule | (123B9)2–L2–PTX | Dimeric 123B9 conjugated to paclitaxel | [127,128,129,130] | |
| Peptide-small molecule | 123B9-L2-Gem YNH/YDH-L2-Gem | 123B9 peptide conjugated to gemcitabine YNH or YDH peptide conjugated to gemcitabine | [131] | |
| Antibody-drug | 3B10-ADC 1C1-ADC | anti-EphA2 monoclonal antibodies fused to tubulysin variant AZ13599185 | [132] | |
| Small molecule | UniPR139, UniPR502 | [121] | ||
| microRNA | miRNA-302B; miRNA-26B | [140,141] | ||
| EphA4 | Peptide | 123C4 | [118] | |
| Antibody-drug | PF-06647263 | hE22 monoclonal antibody fused to calicheamicin | [133] | |
| EphA5 | Small molecule | UniPR500 | [122] | |
| EphB2 | Small molecule | HMQ-T-B10 | [123] | |
| Small molecule | berberine | [124] | ||
| Small molecule | TPD7 | [125] | ||
| siRNA | EphB2 knockdown + radiation | [139] | ||
| EphB4 | Protein | sEphB4-HAS | EphB4 receptor fragment fused to human serum albumin | [134] |
| Antibody | H200 pAb | Polyclonal antibody raised against 200 aa extracellular region of EphB4 | [137] | |
| Multiple targets | Protein fusion | eA5-PE38QQR | EphR ligand eA5 fused to truncated form of Pseudomonas aeruginosa exotoxin A | [135] |
| Antibody | EPHA2/A3 BsAb | Novel bispecific antibody targeting EphA2 and EphA3 | [136] |
| Target Receptor | Agent Type | Description | Reference |
|---|---|---|---|
| EphA2 | Liposome | YSA-liposomes for co-delivery of doxorubicin and JIP1 siRNA | [146] |
| EphA10 | Liposome | EphA10 antibody lipoplex for co-delivery of doxorubicin and MDR1-siRNA | [147] |
| EphA2 | Liposome | YSA-liposomes for delivery of doxorubicin | [148] |
| EphA2 | Liposome | Eph1A-liposomes for delivery of let-7a miRNA | [149] |
| EphA2 | Liposome | Delivery of paclitaxel and docetaxel prodrugs | [150] |
| EphA2 | Lipsome | scFv-liposome for delivery of cytotoxin | [151,152] |
| EphA2 | Polymer | scFV 4B3-pegylated hyperbranched polymer | [153] |
| Multiple targets | Polymer | CHVLWSTRC-peptide labeled cationic polymer delivers therapeutic sRAE-1γ plasmid via EphA2 and EphA4 receptors | [154] |
| EphB4 | Polymer | Biopolymer functionalized with ectodomain of ephrinB2 | [155] |
| EphA2 | DNA | Ephrin-A1 decorated DNA nanostructure | [156] |
| EphA2 | Nanoparticle | Pegylated EphA2 peptide coated nanoparticles | [157] |
| EphA2 | Nanoparticle | YSA-nanoparticle for co-delivery of ILsi RNA and paclitaxel | [158] |
| EphA2 | Nanorod | YSA-gold nanorods | [159] |
| EphB1 | Nanoparticle | EphB1 ligand binding domain-erythrocyte nanoparticles for delivery of phototherapy | [160] |
| EphA3 | Nanoparticle | EphA3 antibody-nanoparticles for delivery of temozolomide | [161] |
| EphA2 | Nanoparticle | YSA-polymeric nanoparticles | [162] |
| EphA2 | Nanoparticle | Chitosan-coated Ephrin-A1-PE38/GM-CSF nanoparticles | [163] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hughes, R.M.; Virag, J.A.I. Harnessing the Power of Eph/ephrin Biosemiotics for Theranostic Applications. Pharmaceuticals 2020, 13, 112. https://doi.org/10.3390/ph13060112
Hughes RM, Virag JAI. Harnessing the Power of Eph/ephrin Biosemiotics for Theranostic Applications. Pharmaceuticals. 2020; 13(6):112. https://doi.org/10.3390/ph13060112
Chicago/Turabian StyleHughes, Robert M., and Jitka A.I. Virag. 2020. "Harnessing the Power of Eph/ephrin Biosemiotics for Theranostic Applications" Pharmaceuticals 13, no. 6: 112. https://doi.org/10.3390/ph13060112
APA StyleHughes, R. M., & Virag, J. A. I. (2020). Harnessing the Power of Eph/ephrin Biosemiotics for Theranostic Applications. Pharmaceuticals, 13(6), 112. https://doi.org/10.3390/ph13060112