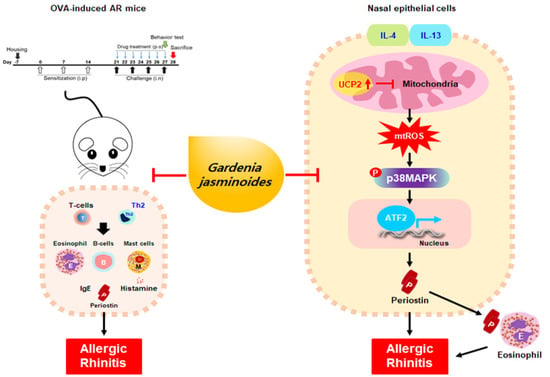
Gardenia jasminoides Attenuates Allergic Rhinitis-Induced Inflammation by Inhibiting Periostin Production
Abstract
:1. Introduction
2. Results
2.1. Quantitative Analysis of the Three Compounds Comprising GJ
2.2. GJW and GJE Inhibit Allergy Symptoms and Reduce NALF Cell Number and Serum Markers in Ovalbumin-Induced AR Mice
2.3. GJW and GJE Inhibit Periostin Levels and Eosinophil Infiltration in the Nasal Tissue of Ovalbumin-Induced AR Mice
2.4. GJW and GJE Inhibit the Activation of Differentiated Th2 Cells
2.5. GJW and GJE Inhibit IL-4/IL-13-Induced Periostin Generation via the Regulation of Mitochondrial ROS Production in HNEpCs
2.6. GJW and GJE Inhibit IL-4/IL-13-Induced Periostin Production via the Activation of UCP2 and p38 MAPK-ATF2 in HNEpCs
3. Discussion
4. Materials and Methods
4.1. Preparation of GJ Extracts
4.2. High-Performance Liquid Chromatography (HPLC) Analysis
4.2.1. Chemicals and Reagents
4.2.2. Preparation of Sample and Standard Solutions
4.2.3. Apparatus and Chromatographic Conditions
4.3. Animals
4.4. AR Mouse Model and Treatments
4.5. Allergic Symptoms of AR Mouse Model
4.6. Analysis of Serum and Nasal Lavage Fluid (NALF)
4.7. Immunohistochemistry
4.8. Histological Analysis
4.9. Isolation of Naïve CD4+ T Cells and Differentiation of Th2 Cells
4.10. Evaluation of Th2 Cell Differentiation
4.11. Analysis of Th2 Cell Activation and Viability
4.12. Cell Culture
4.13. Determining Cell Viability
4.14. Determination of Protein Levels
4.15. Immunofluorescence and Confocal Microscopy
4.16. Measurement of Periostin
4.17. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heffler, E.; Brussino, L.; Del Giacco, S.; Paoletti, G.; Minciullo, P.L.; Varricchi, G.; Scadding, G.; Malvezzi, L.; De Virgilio, A.; Spriano, G.; et al. New drugs in early-stage clinical trials for allergic rhinitis. Expert Opin. Investig. Drugs 2019, 28, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, J.; Van Cauwenberge, P.; Khaltaev, N.; Aria Workshop, G.; World Health, O. Allergic rhinitis and its impact on asthma. J. Allergy Clin. Immunol. 2001, 108, S147–S334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeffery, P.K.; Haahtela, T. Allergic rhinitis and asthma: Inflammation in a one-airway condition. BMC Pulm. Med. 2006, 6 (Suppl. 1), S5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corsico, A.G.; De Amici, M.; Ronzoni, V.; Giunta, V.; Mennitti, M.C.; Viscardi, A.; Marseglia, G.L.; Ciprandi, G. Allergen-specific immunoglobulin E and allergic rhinitis severity. Allergy Rhinol. 2017, 8, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Bousquet, J.; Anto, J.M.; Bachert, C.; Baiardini, I.; Bosnic-Anticevich, S.; Walter Canonica, G.; Melen, E.; Palomares, O.; Scadding, G.K.; Togias, A.; et al. Allergic rhinitis. Nat. Rev. Dis. Primers 2020, 6, 95. [Google Scholar] [CrossRef]
- Ebihara, N.; Takahashi, K.; Takemura, H.; Akanuma, Y.; Asano, K.; Sunagawa, M. Suppressive Effect of Quercetin on Nitric Oxide Production from Nasal Epithelial Cells In Vitro. Evid. Based Complement. Alternat. Med. 2018, 2018, 6097625. [Google Scholar] [CrossRef] [Green Version]
- Irie, S.; Kashiwabara, M.; Yamada, A.; Asano, K. Suppressive Activity of Quercetin on Periostin Functions in Vitro. Vivo 2016, 30, 17–25. [Google Scholar]
- Pawankar, R.; Mori, S.; Ozu, C.; Kimura, S. Overview on the pathomechanisms of allergic rhinitis. Asia Pac. Allergy 2011, 1, 157–167. [Google Scholar] [CrossRef] [Green Version]
- Broide, D.H. Allergic rhinitis: Pathophysiology. Allergy Asthma Proc. 2010, 31, 370–374. [Google Scholar] [CrossRef]
- Bjermer, L.; Westman, M.; Holmstrom, M.; Wickman, M.C. The complex pathophysiology of allergic rhinitis: Scientific rationale for the development of an alternative treatment option. Allergy Asthma Clin. Immunol. 2019, 15, 24. [Google Scholar] [CrossRef]
- Sin, B.; Togias, A. Pathophysiology of allergic and nonallergic rhinitis. Proc. Am. Thorac. Soc. 2011, 8, 106–114. [Google Scholar] [CrossRef]
- Min, Y.G. The pathophysiology, diagnosis and treatment of allergic rhinitis. Allergy Asthma Immunol. Res. 2010, 2, 65–76. [Google Scholar] [CrossRef] [Green Version]
- Benam, K.H.; Denney, L.; Ho, L.P. How the Respiratory Epithelium Senses and Reacts to Influenza Virus. Am. J. Resp. Cell Mol. 2019, 60, 259–268. [Google Scholar] [CrossRef]
- Calven, J.; Ax, E.; Radinger, M. The Airway Epithelium-A Central Player in Asthma Pathogenesis. Int. J. Mol. Sci. 2020, 21, 8907. [Google Scholar] [CrossRef]
- Houser, K.R.; Johnson, D.K.; Ishmael, F.T. Anti-inflammatory effects of methoxyphenolic compounds on human airway cells. J. Inflamm. 2012, 9, 6. [Google Scholar] [CrossRef] [Green Version]
- Bals, R.; Hiemstra, P.S. Innate immunity in the lung: How epithelial cells fight against respiratory pathogens. Eur. Respir. J. 2004, 23, 327–333. [Google Scholar] [CrossRef]
- Takayama, G.; Arima, K.; Kanaji, T.; Toda, S.; Tanaka, H.; Shoji, S.; McKenzie, A.N.; Nagai, H.; Hotokebuchi, T.; Izuhara, K. Periostin: A novel component of subepithelial fibrosis of bronchial asthma downstream of IL-4 and IL-13 signals. J. Allergy Clin. Immunol. 2006, 118, 98–104. [Google Scholar] [CrossRef]
- Masuoka, M.; Shiraishi, H.; Ohta, S.; Suzuki, S.; Arima, K.; Aoki, S.; Toda, S.; Inagaki, N.; Kurihara, Y.; Hayashida, S.; et al. Periostin promotes chronic allergic inflammation in response to Th2 cytokines. J. Clin. Investig. 2012, 122, 2590–2600. [Google Scholar] [CrossRef] [Green Version]
- Ishida, A.; Ohta, N.; Suzuki, Y.; Kakehata, S.; Okubo, K.; Ikeda, H.; Shiraishi, H.; Izuhara, K. Expression of pendrin and periostin in allergic rhinitis and chronic rhinosinusitis. Allergol. Int. 2012, 61, 589–595. [Google Scholar] [CrossRef] [Green Version]
- Izuhara, K.; Nunomura, S.; Nanri, Y.; Ono, J.; Takai, M.; Kawaguchi, A. Periostin: An emerging biomarker for allergic diseases. Allergy 2019, 74, 2116–2128. [Google Scholar] [CrossRef] [Green Version]
- Izuhara, K.; Arima, K.; Ohta, S.; Suzuki, S.; Inamitsu, M.; Yamamoto, K. Periostin in allergic inflammation. Allergol. Int. 2014, 63, 143–151. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Gao, P.; Zhi, Y.; Xu, W.; Wu, Y.; Yin, J.; Zhang, J. Periostin: Its role in asthma and its potential as a diagnostic or therapeutic target. Respir. Res. 2015, 16, 57. [Google Scholar] [CrossRef] [Green Version]
- O’Dwyer, D.N.; Moore, B.B. The role of periostin in lung fibrosis and airway remodeling. Cell Mol. Life Sci. 2017, 74, 4305–4314. [Google Scholar] [CrossRef]
- Yu, L.; Wang, J.; Liu, K. Role of periostin in ECRS. Eur. Arch. Otorhinolaryngol. 2020, 278, 1–8. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, Q.; Wang, M.; Jia, J.; Wu, R. Gardenia Decoction Prevent Intestinal Mucosal Injury by Inhibiting Pro-inflammatory Cytokines and NF-kappaB Signaling. Front. Pharmacol. 2019, 10, 180. [Google Scholar] [CrossRef]
- Sung, Y.Y.; Lee, A.Y.; Kim, H.K. The Gardenia jasminoides extract and its constituent, geniposide, elicit anti-allergic effects on atopic dermatitis by inhibiting histamine in vitro and in vivo. J. Ethnopharmacol. 2014, 156, 33–40. [Google Scholar] [CrossRef]
- Chen, J.; Tchivelekete, G.M.; Zhou, X.; Tang, W.; Liu, F.; Liu, M.; Zhao, C.; Shu, X.; Zeng, Z. Anti-inflammatory activities of Gardenia jasminoides extracts in retinal pigment epithelial cells and zebrafish embryos. Exp. Ther. Med. 2021, 22, 700. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Li, S.; Wang, S.; Ho, C.T. Chemistry and bioactivity of Gardenia jasminoides. J. Food Drug Anal. 2017, 25, 43–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.H.; An, J.E.; Jang, S.; Kim, J.Y.; Lee, J.W.; Kim, H.K. Gardenia jasminoides extract without crocin improved atopic dermatitis-like skin lesions via suppression of Th2-related cytokines in Dfe-induced NC/Nga mice. J. Ethnopharmacol. 2019, 241, 112015. [Google Scholar] [CrossRef] [PubMed]
- Yosri, H.; Elkashef, W.F.; Said, E.; Gameil, N.M. Crocin modulates IL-4/IL-13 signaling and ameliorates experimentally induced allergic airway asthma in a murine model. Int. Immunopharmacol. 2017, 50, 305–312. [Google Scholar] [CrossRef]
- Sung, Y.Y.; Kim, H.K. Crocin Ameliorates Atopic Dermatitis Symptoms by down Regulation of Th2 Response via Blocking of NF-kappaB/STAT6 Signaling Pathways in Mice. Nutrients 2018, 10, 1625. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.I.; Hong, S.H.; Lee, S.Y.; Ku, J.M.; Kim, M.J.; Ko, S.G. Gardenia Jasminoides Ameliorates Antibiotic-Associated Aggravation of DNCB-Induced Atopic Dermatitis by Restoring the Intestinal Microbiome Profile. Nutrients 2021, 13, 1349. [Google Scholar] [CrossRef]
- Saravanakumar, K.S.; Park, S.J.; Sathiyaseelan, A.Z.; Kim, K.N.; Cho, S.H.; Mariadoss, A.V.A.M.; Wang, M.H. Metabolite Profiling of Methanolic Extract of Gardenia jasminoides by LC-MS/MS and GC-MS and Its Anti-Diabetic, and Anti-Oxidant Activities. Pharmaceuticals 2021, 14, 102. [Google Scholar] [CrossRef]
- Song, J.S.; Shin, J.E.; Kim, J.-H.; Kim, Y. Gardenia jasminoides Exerts Anti-inflammatory Activity via Akt and p38-dependent Heme Oxygenase-1 Upregulation in Microglial Cells. J. Life Sci. 2017, 27, 8–14. [Google Scholar] [CrossRef]
- Li, Z.; Zeng, M.; Deng, Y.; Zhao, J.; Zhou, X.; Trudeau, J.B.; Goldschmidt, E.; Moore, J.A.; Chu, H.; Zhang, W.; et al. 15-Lipoxygenase 1 in nasal polyps promotes CCL26/eotaxin 3 expression through extracellular signal-regulated kinase activation. J. Allergy Clin. Immunol. 2019, 144, 1228–1241.e1229. [Google Scholar] [CrossRef] [Green Version]
- Noguchi, T.; Nakagome, K.; Kobayashi, T.; Uchida, Y.; Soma, T.; Nakamoto, H.; Nagata, M. Periostin upregulates the effector functions of eosinophils. J. Allergy Clin. Immunol. 2016, 138, 1449–1452.e1445. [Google Scholar] [CrossRef] [Green Version]
- Johansson, M.W.; Khanna, M.; Bortnov, V.; Annis, D.S.; Nguyen, C.L.; Mosher, D.F. IL-5-stimulated eosinophils adherent to periostin undergo stereotypic morphological changes and ADAM8-dependent migration. Clin. Exp. Allergy 2017, 47, 1263–1274. [Google Scholar] [CrossRef]
- Patergnani, S.; Bouhamida, E.; Leo, S.; Pinton, P.; Rimessi, A. Mitochondrial Oxidative Stress and “Mito-Inflammation”: Actors in the Diseases. Biomedicines 2021, 9, 216. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.H.; Kim, D.E.; Lee, S.H. Effects of 18beta-Glycyrrhetinic Acid on Fungal Protease-Induced Airway Inflammatory Responses. Mediat. Inflamm. 2018, 2018, 6461032. [Google Scholar] [CrossRef] [Green Version]
- Brillo, V.; Chieregato, L.; Leanza, L.; Muccioli, S.; Costa, R. Mitochondrial Dynamics, ROS, and Cell Signaling: A Blended Overview. Life 2021, 11, 332. [Google Scholar] [CrossRef]
- Pierelli, G.; Stanzione, R.; Forte, M.; Migliarino, S.; Perelli, M.; Volpe, M.; Rubattu, S. Uncoupling Protein 2: A Key Player and a Potential Therapeutic Target in Vascular Diseases. Oxid. Med. Cell Longev. 2017, 2017, 7348372. [Google Scholar] [CrossRef]
- Jezek, P.; Holendova, B.; Garlid, K.D.; Jaburek, M. Mitochondrial Uncoupling Proteins: Subtle Regulators of Cellular Redox Signaling. Antioxid. Redox Signal. 2018, 29, 667–714. [Google Scholar] [CrossRef] [Green Version]
- Ding, Y.; Zheng, Y.; Huang, J.; Peng, W.; Chen, X.; Kang, X.; Zeng, Q. UCP2 ameliorates mitochondrial dysfunction, inflammation, and oxidative stress in lipopolysaccharide-induced acute kidney injury. Int. Immunopharmacol. 2019, 71, 336–349. [Google Scholar] [CrossRef]
- Ma, S.; Yang, D.; Li, D.; Tan, Y.; Tang, B.; Yang, Y. Inhibition of uncoupling protein 2 with genipin exacerbates palmitate-induced hepatic steatosis. Lipids Health Dis. 2012, 11, 154. [Google Scholar] [CrossRef] [Green Version]
- Busceti, C.L.; Cotugno, M.; Bianchi, F.; Forte, M.; Stanzione, R.; Marchitti, S.; Battaglia, G.; Nicoletti, F.; Fornai, F.; Rubattu, S. Brain Overexpression of Uncoupling Protein-2 (UCP2) Delays Renal Damage and Stroke Occurrence in Stroke-Prone Spontaneously Hypertensive Rats. Int. J. Mol. Sci. 2020, 21, 4289. [Google Scholar] [CrossRef]






| Compound | Linear Range (μg/mL) | Regression Equation (y = ax + b) (a) | r2 | LOD (b) (μg/mL) | LOQ (c) (μg/mL) | Content (mg/g) | ||
|---|---|---|---|---|---|---|---|---|
| Slope (a) | Intercept (b) | 70% EtOH | Water | |||||
| Gardenoside | 3.125–100 | 12175 | 7278.8 | 0.9997 | 0.313 | 0.950 | 21.24 ± 0.08 | 3.59 ± 0.02 |
| Geniposide | 25–800 | 15204 | 93731 | 0.9996 | 0.347 | 1.053 | 272.64 ± 0.21 | 129.64 ± 0.49 |
| Crocin | 3.125–100 | 8965.4 | 3561.9 | 0.9999 | 0.124 | 0.375 | 8.89 ± 0.00 | 3.84 ± 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pyun, B.-J.; Lee, J.Y.; Kim, Y.J.; Ji, K.-Y.; Jung, D.H.; Park, K.-S.; Jo, K.; Choi, S.; Jung, M.-A.; Kim, Y.H.; et al. Gardenia jasminoides Attenuates Allergic Rhinitis-Induced Inflammation by Inhibiting Periostin Production. Pharmaceuticals 2021, 14, 986. https://doi.org/10.3390/ph14100986
Pyun B-J, Lee JY, Kim YJ, Ji K-Y, Jung DH, Park K-S, Jo K, Choi S, Jung M-A, Kim YH, et al. Gardenia jasminoides Attenuates Allergic Rhinitis-Induced Inflammation by Inhibiting Periostin Production. Pharmaceuticals. 2021; 14(10):986. https://doi.org/10.3390/ph14100986
Chicago/Turabian StylePyun, Bo-Jeong, Joo Young Lee, Yu Jin Kim, Kon-Young Ji, Dong Ho Jung, Ki-Sun Park, Kyuhyung Jo, Susanna Choi, Myung-A Jung, Yun Hee Kim, and et al. 2021. "Gardenia jasminoides Attenuates Allergic Rhinitis-Induced Inflammation by Inhibiting Periostin Production" Pharmaceuticals 14, no. 10: 986. https://doi.org/10.3390/ph14100986
APA StylePyun, B.-J., Lee, J. Y., Kim, Y. J., Ji, K.-Y., Jung, D. H., Park, K.-S., Jo, K., Choi, S., Jung, M.-A., Kim, Y. H., & Kim, T. (2021). Gardenia jasminoides Attenuates Allergic Rhinitis-Induced Inflammation by Inhibiting Periostin Production. Pharmaceuticals, 14(10), 986. https://doi.org/10.3390/ph14100986