Therapeutic Effects of Hydroalcoholic Extracts from the Ancient Apple Mela Rosa dei Monti Sibillini in Transient Global Ischemia in Rats
Abstract
:1. Introduction
2. Results
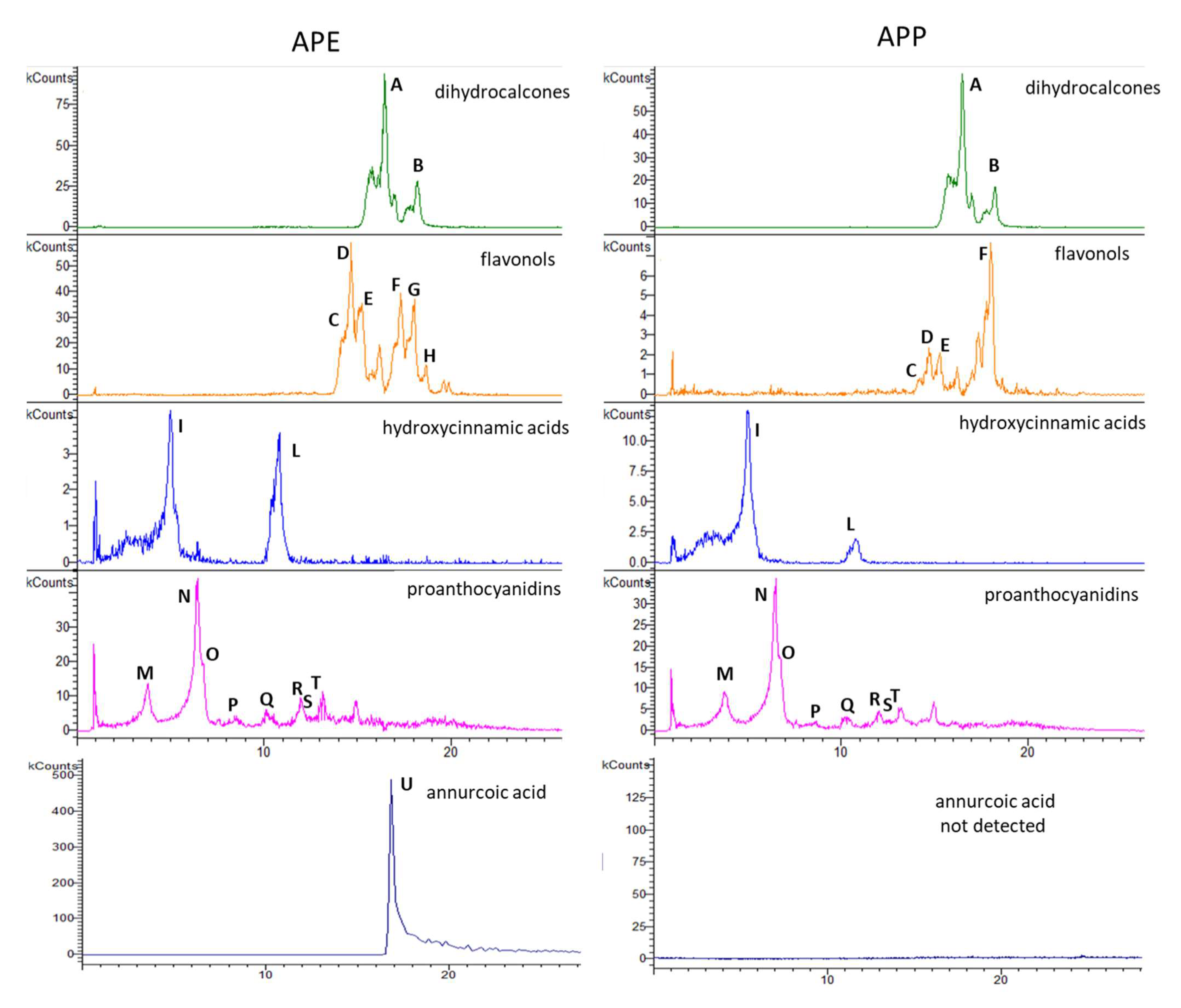
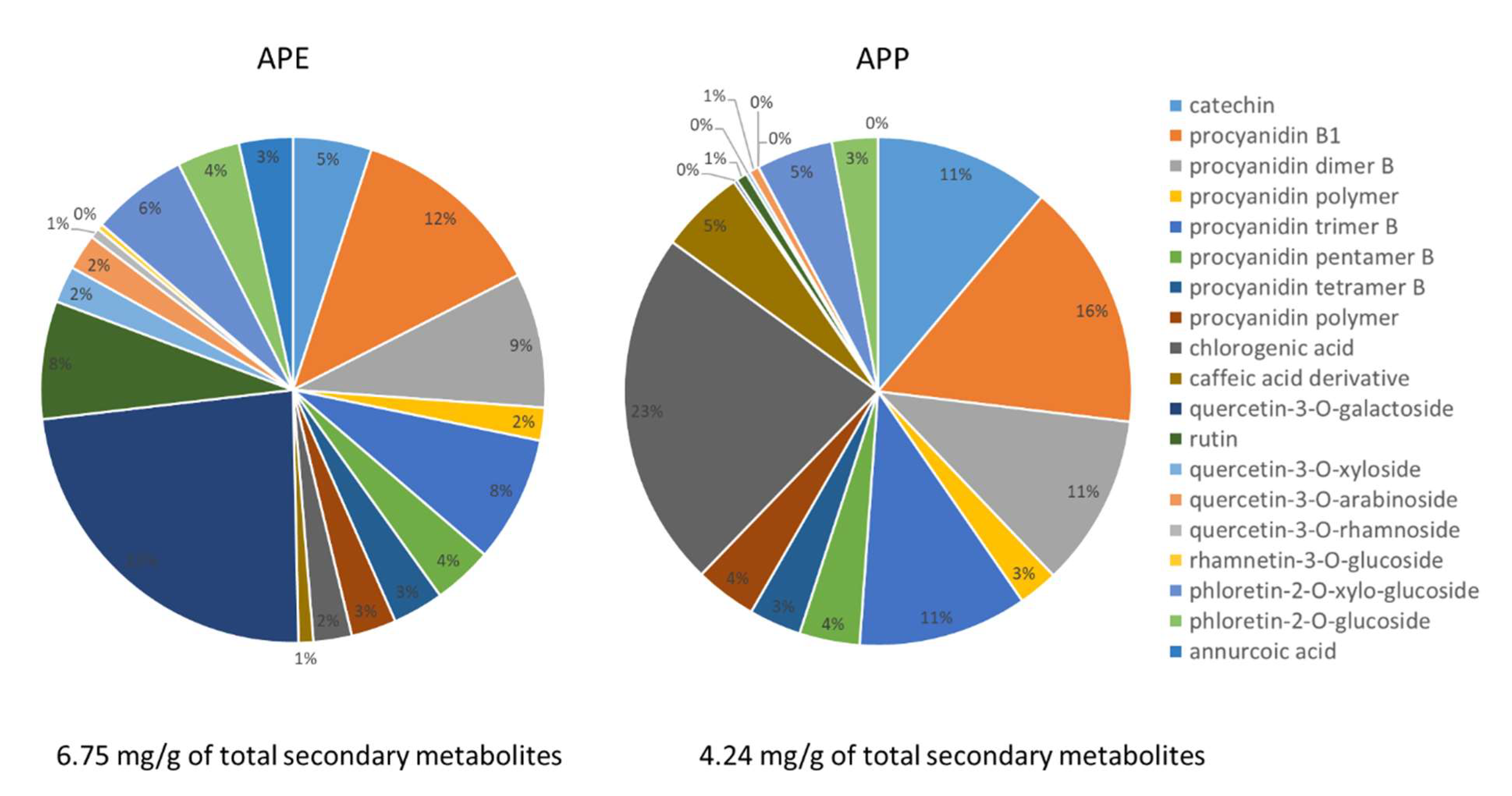
2.1. Chemical Constituents of Apple Peel Extract (APE) and Apple Pulp Extract (APP)
2.2. APP and APE Improved the Behavioral Tests after the Brain Transient Global Ischemia
2.3. APP and APE Decreased Brain Tissue Injury in Hematoxylin and Eosin (H and E) Staining
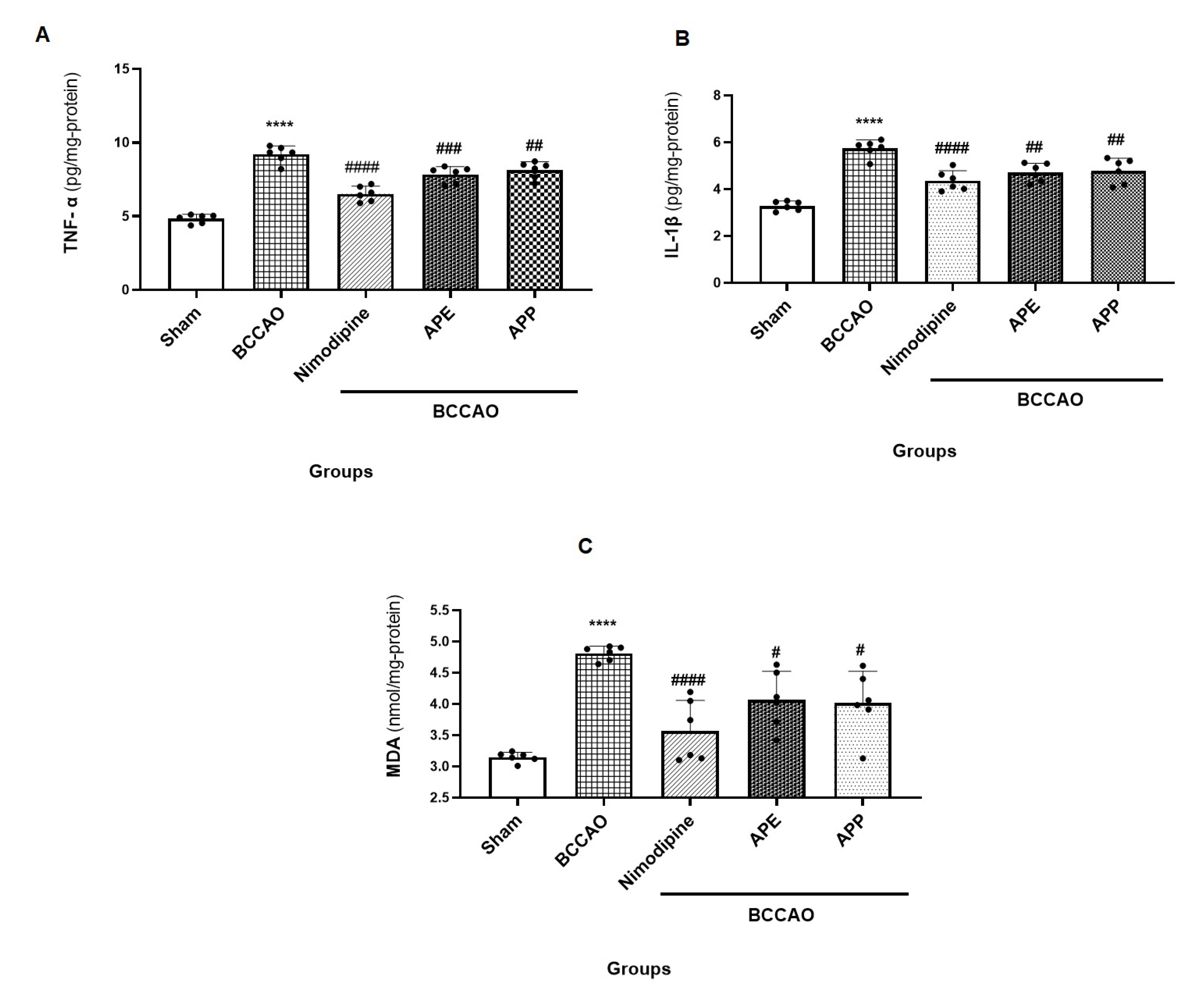
2.4. APP and APE Reduced the Pro-Inflammatory Cytokines and Oxidative Stress in Brain after Ischemic Stroke
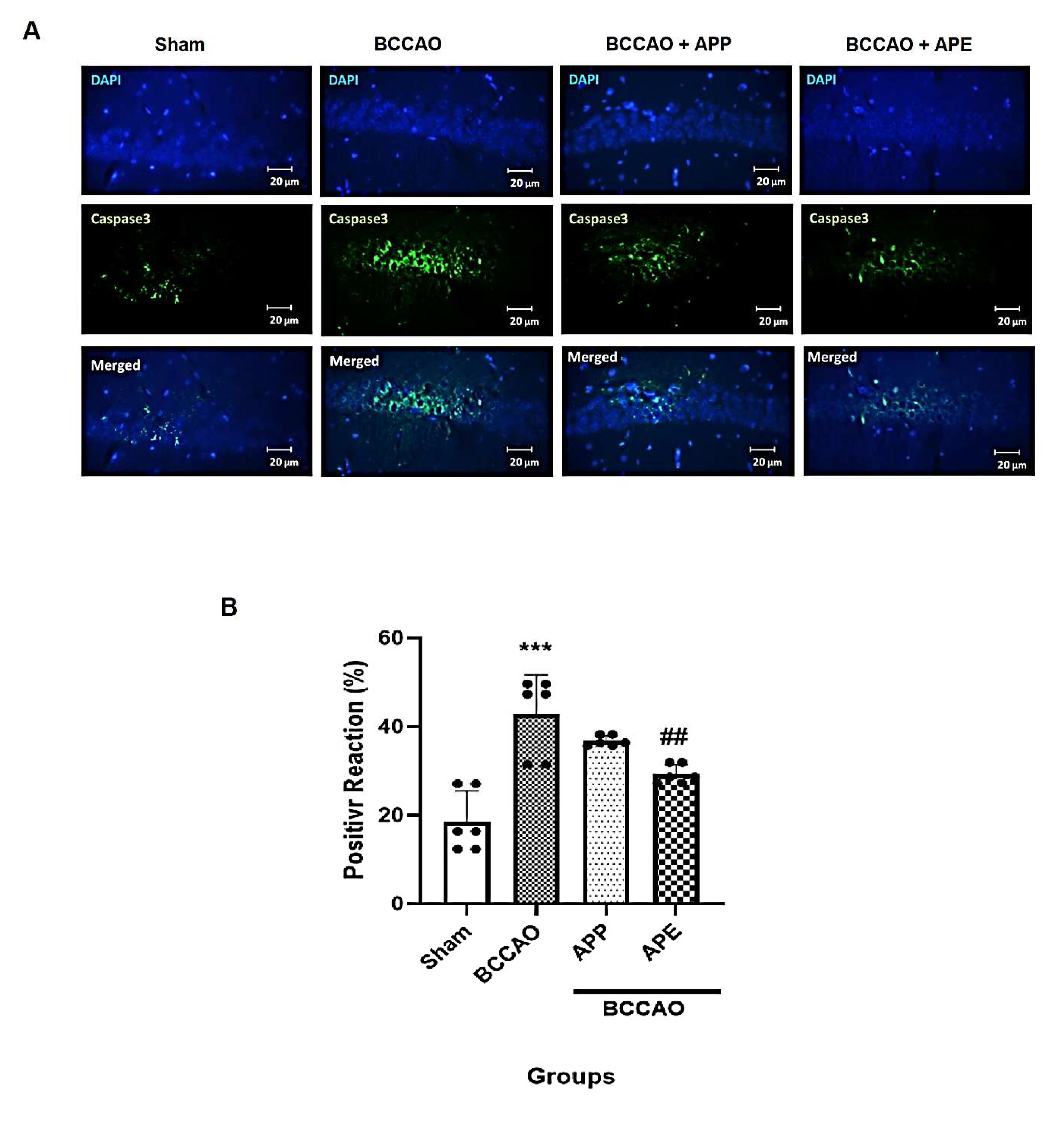
2.5. APP and APE Reduced Caspase 3 Expression in the Brain after Transient Global Ischemia
3. Discussion
4. Materials and Methods
4.1. Apple Sampling
4.2. Hydroalcoholic Extracts Preparation
4.3. High-Performance Liquid Chromatography-Diode Array Detection-Mass Spectrometry (HPLC-DAD-MSn) Analysis
4.4. Animals
4.4.1. Induction of Ischemic Stroke
4.4.2. Animal Groups
- Sham group: the animals were only anesthetized, a midline cut in the neck was made without BCCAO and received normal saline i.p.
- BCCAO group: the stroke model was induced as described above; animals received normal saline i.p.
- Nimodipine group (as positive group): 30 min before BCCAO, the animals received nimodipine 10 mg/kg (i.p.), then BCCAO was induced [22].
- BCCAO + APP: 30 min before BCCAO, the animals received APP extract 30 mg/kg (i.p.), then BCCAO was induced [17].
- BCCAO + APE: 30 min before BCCAO, the animals received APE extract 30 mg/kg (i.p.), then, BCCAO was induced [17].
4.5. Behavioral Tests
4.6. Caspase-3 Immunostaining
4.7. Histological Assay
4.8. Molecular Analysis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Di Lorenzo, A.; Sobolev, A.P.; Nabavi, S.F.; Sureda, A.; Moghaddam, A.H.; Khanjani, S.; Di Giovanni, C.; Xiao, J.; Shirooie, S.; Tsetegho Sokeng, A.J.; et al. Antidepressive effects of a chemically characterized maqui berry extract (Aristotelia chilensis (molina) stuntz) in a mouse model of Post-stroke depression. Food Chem. Toxicol. 2019, 129, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef] [PubMed]
- Dojo Soeandy, C.; Salmasi, F.; Latif, M.; Elia, A.J.; Suo, N.J.; Henderson, J.T. Endothelin-1-mediated cerebral ischemia in mice: Early cellular events and the role of caspase-3. Apoptosis 2019, 24, 578–595. [Google Scholar] [CrossRef] [PubMed]
- Kamel, H.; Okin, P.M.; Elkind, M.S.; Iadecola, C. Atrial fibrillation and mechanisms of stroke: Time for a new model. Stroke 2016, 47, 895–900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willmot, M.; Leonardi-Bee, J.; Bath, P.M. High blood pressure in acute stroke and subsequent outcome: A systematic review. Hypertension 2004, 43, 18–24. [Google Scholar] [CrossRef]
- Patlolla, S.H.; Lee, H.-C.; Noseworthy, P.A.; Wysokinski, W.E.; Hodge, D.O.; Greene, E.L.; Gersh, B.J.; Melduni, R.M. Impact of diabetes mellitus on stroke and survival in patients with atrial fibrillation. Am. J. Cardiol. 2020, 131, 33–39. [Google Scholar] [CrossRef]
- Chaudhary, D.; Khan, A.; Gupta, M.; Hu, Y.; Li, J.; Abedi, V.; Zand, R. Obesity and mortality after the first ischemic stroke: Is obesity paradox real? PLoS ONE 2021, 16, e0246877. [Google Scholar] [CrossRef]
- Jackova, J.; Sedova, P.; Brown, R.D., Jr.; Zvolsky, M.; Volna, M.; Baluchova, J.; Belaskova, S.; Bednarik, J.; Mikulik, R. Risk Factors in Ischemic Stroke Subtypes: A Community-Based Study in Brno, Czech Republic. J. Stroke Cerebrovasc. Dis. 2020, 29, 104503. [Google Scholar] [CrossRef]
- Yousefi-Manesh, H.; Rashidian, A.; Hemmati, S.; Shirooie, S.; Sadeghi, M.A.; Zarei, N.; Dehpour, A.R. Therapeutic effects of modafinil in ischemic stroke; possible role of NF-κB downregulation. Immunopharmacol. Immunotoxicol. 2019, 41, 558–564. [Google Scholar] [CrossRef]
- Zheng, T.S.; Schlosser, S.F.; Dao, T.; Hingorani, R.; Crispe, I.N.; Boyer, J.L.; Flavell, R.A. Caspase-3 controls both cytoplasmic and nuclear events associated with Fas-mediated apoptosis in vivo. Proc. Natl. Acad. Sci. USA 1998, 95, 13618–13623. [Google Scholar] [CrossRef] [Green Version]
- Le, D.A.; Wu, Y.; Huang, Z.; Matsushita, K.; Plesnila, N.; Augustinack, J.C.; Hyman, B.T.; Yuan, J.; Kuida, K.; Flavell, R.A.; et al. Caspase activation and neuroprotection in caspase-3- deficient mice after in vivo cerebral ischemia and in vitro oxygen glucose deprivation. Proc. Natl. Acad. Sci. USA 2002, 99, 15188–15193. [Google Scholar] [CrossRef] [Green Version]
- Akpan, N.; Troy, C.M. Caspase Inhibitors: Prospective Therapies for Stroke. Neuroscientists 2012, 19, 129–136. [Google Scholar] [CrossRef]
- Lo, E.H. Degeneration and repair in central nervous system disease. Nat. Med. 2010, 16, 1205–1209. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Endres, M.; Moskowitz, M.A. Synergistic effects of caspase inhibitors and MK-801 in brain injury after transient focal cerebral ischaemia in mice. Br. J. Pharm. 1998, 124, 756–762. [Google Scholar] [CrossRef] [Green Version]
- Nabavi, S.F.; Sureda, A.; Sanches-Silva, A.; Pandima Devi, K.; Ahmed, T.; Shahid, M.; Sobarzo-Sánchez, E.; Dacrema, M.; Daglia, M.; Braidy, N.; et al. Novel therapeutic strategies for stroke: The role of autophagy. Crit. Rev. Clin. Lab. Sci. 2019, 56, 182–199. [Google Scholar] [CrossRef]
- Yousefi-Manesh, H.; Dehpour, A.R.; Ansari-Nasab, S.; Hemmati, S.; Sadeghi, M.A.; Shahraki, R.H.; Shirooie, S.; Nabavi, S.M.; Nkuimi Wandjou, J.G.; Sut, S.; et al. Hepatoprotective Effects of Standardized Extracts from an Ancient Italian Apple Variety (Mela Rosa dei Monti Sibillini) against Carbon Tetrachloride (CCl4)-Induced Hepatotoxicity in Rats. Molecules 2020, 25, 1816. [Google Scholar] [CrossRef]
- Yousefi-Manesh, H.; Hemmati, S.; Shirooie, S.; Nabavi, S.M.; Bonakdar, A.T.; Fayaznia, R.; Asgardoon, M.H.; Dehnavi, A.Z.; Ghafouri, M.; Wandjou, J.G.N. Protective effects of hydroalcoholic extracts from an ancient apple variety ‘Mela Rosa dei Monti Sibillini’against renal ischemia/reperfusion injury in rats. Food Funct. 2019, 10, 7544–7552. [Google Scholar] [CrossRef]
- Leontowicz, H.; Leontowicz, M.; Gorinstein, S.; Martin-Belloso, O.; Trakhtenberg, S. Apple peels and pulp as a source of bioactive compounds and their influence digestibility and lipid profile in normal and atherogenic rats-in English. Med. Weter. 2007, 63, 1434. [Google Scholar]
- Fathy, S.M.; Drees, E.A. Protective effects of Egyptian cloudy apple juice and apple peel extract on lipid peroxidation, antioxidant enzymes and inflammatory status in diabetic rat pancreas. BMC Complementary Altern. Med. 2015, 16, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Gao, J.-M.; Li, F.; Gong, Q.-H.; Shi, J.-S. Gastrodin attenuates bilateral common carotid artery occlusion-induced cognitive deficits via regulating Aβ-related proteins and reducing autophagy and apoptosis in rats. Front. Pharmacol. 2018, 9, 405. [Google Scholar] [CrossRef] [Green Version]
- Yuan, J. Neuroprotective strategies targeting apoptotic and necrotic cell death for stroke. Apoptosis: Int. J. Program. Cell Death 2009, 14, 469–477. [Google Scholar] [CrossRef] [Green Version]
- Yousefi-Manesh, H.; Dehpour, A.R.; Shirooie, S.; Bagheri, F.; Farrokhi, V.; Mousavi, S.E.; Ricciutelli, M.; Cappellacci, L.; López, V.; Maggi, F.; et al. Isofuranodiene, a Natural Sesquiterpene Isolated from Wild Celery (Smyrnium olusatrum L.), Protects Rats against Acute Ischemic Stroke. Pharmaceuticals 2021, 14, 344. [Google Scholar] [CrossRef]
- Hussein, Y.A.; Al-sarraf, A.M.; Alfalluji, W.L. Modulation of oxidative stress, inflammatory and apoptotic response by curcumin against cerebral ischemia reperfusion injury in a mouse model. Interdiscip. Neurosurg. 2020, 21, 100741. [Google Scholar] [CrossRef]
- Fan, W.; Dai, Y.; Xu, H.; Zhu, X.; Cai, P.; Wang, L.; Sun, C.; Hu, C.; Zheng, P.; Zhao, B.Q. Caspase-3 modulates regenerative response after stroke. Stem Cells 2014, 32, 473–486. [Google Scholar] [CrossRef]
- Kerr, L.; McGregor, A.; Amet, L.; Asada, T.; Spratt, C.; Allsopp, T.; Harmar, A.; Shen, S.; Carlson, G.; Logan, N. Mice overexpressing human caspase 3 appear phenotypically normal but exhibit increased apoptosis and larger lesion volumes in response to transient focal cerebral ischaemia. Cell Death Differ. 2004, 11, 1102–1111. [Google Scholar] [CrossRef] [Green Version]
- Awooda, H.A.; Lutfi, M.F. Oxidative/nitrosative stress in rats subjected to focal cerebral ischemia/reperfusion. Int. J. Health Sci. 2015, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-Y., Jr.; Si, Y.-L.; Liao, J.; Yan, G.-T.; Deng, Z.-H.; Xue, H.; Wang, L.-H.; Zhang, K. Leptin administration alleviates ischemic brain injury in mice by reducing oxidative stress and subsequent neuronal apoptosis. J. Trauma Acute Care Surg. 2012, 72, 982–991. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, A.; Meisel, A.; Planas, A.M.; Urra, X.; Van De Beek, D.; Veltkamp, R. The immunology of acute stroke. Nat. Rev. Neurol. 2012, 8, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-Y.; Wang, X.; Liu, C.; Zhang, H.-L. Pharmacological Targeting of Microglial Activation: New Therapeutic Approach. Front. Cell. Neurosci. 2019, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.; Tao, W.; Yu, L.; Jin, J.; Xu, Y. NOX4 negatively regulates memory functions in APP/PS1 mice: Molecular and cell biology/oxidative stress. Alzheimer’s Dement. 2020, 16, e038198. [Google Scholar] [CrossRef]
- Lee, W.-C.; Wong, H.-Y.; Chai, Y.-Y.; Shi, C.-W.; Amino, N.; Kikuchi, S.; Huang, S.-H. Lipid peroxidation dysregulation in ischemic stroke: Plasma 4-HNE as a potential biomarker? Biochem. Biophys. Res. Commun. 2012, 425, 842–847. [Google Scholar] [CrossRef]
- Luo, Y.; Cui, H.-X.; Jia, A.; Jia, S.-S.; Yuan, K. The Protective Effect of the Total Flavonoids of Abelmoschus esculentus L. Flowers on Transient Cerebral Ischemia-Reperfusion Injury Is due to Activation of the Nrf2-ARE Pathway. Oxidative Med. Cell. Longev. 2018, 2018, 8987173. [Google Scholar] [CrossRef]
- Park, D.-J.; Shah, F.-A.; Koh, P.-O. Quercetin attenuates neuronal cells damage in a middle cerebral artery occlusion animal model. J. Vet. Med Sci. 2018, 80, 676–683. [Google Scholar] [CrossRef] [Green Version]
- Vrhovsek, U.; Rigo, A.; Tonon, D.; Mattivi, F. Quantitation of polyphenols in different apple varieties. J. Agric. Food Chem. 2004, 52, 6532–6538. [Google Scholar] [CrossRef]
- Keddy, P.G.; Dunlop, K.; Warford, J.; Samson, M.L.; Jones, Q.R.; Rupasinghe, H.V.; Robertson, G.S. Neuroprotective and anti-inflammatory effects of the flavonoid-enriched fraction AF4 in a mouse model of hypoxic-ischemic brain injury. PLoS ONE 2012, 7, e51324. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.-S.; Wang, J.-L.; Feng, D.-Y.; Qin, H.-Z.; Wen, H.; Yin, Z.-M.; Gao, G.-D.; Li, C. Protective effect of quercetin against oxidative stress and brain edema in an experimental rat model of subarachnoid hemorrhage. Int. J. Med. Sci. 2014, 11, 282–290. [Google Scholar] [CrossRef] [Green Version]
- Li, W.-X.; Deng, Y.-Y.; Li, F.; Liu, B.; Liu, H.-Y.; Shi, J.-S.; Gong, Q.-H. Icariin, a major constituent of flavonoids from Epimedium brevicornum, protects against cognitive deficits induced by chronic brain hypoperfusion via its anti-amyloidogenic effect in rats. Pharmacol. Biochem. Behav. 2015, 19, 40–48. [Google Scholar] [CrossRef]
- Chen, S.F.; Hsu, C.W.; Huang, W.H.; Wang, J.Y. Post-injury baicalein improves histological and functional outcomes and reduces inflammatory cytokines after experimental traumatic brain injury. Br. J. Pharm. 2008, 155, 1279–1296. [Google Scholar] [CrossRef] [Green Version]
- Ruan, J.; Yao, Y. Behavioral tests in rodent models of stroke. Brain Hemorrhages 2020, 1, 171–184. [Google Scholar] [CrossRef]
- Liu, S.; Grigoryan, M.M.; Vasilevko, V.; Sumbria, R.K.; Paganini-Hill, A.; Cribbs, D.H.; Fisher, M.J. Comparative analysis of H&E and Prussian blue staining in a mouse model of cerebral microbleeds. J. Histochem. Cytochem. 2014, 62, 767–773. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yousefi-Manesh, H.; Dehpour, A.R.; Nabavi, S.M.; Khayatkashani, M.; Asgardoon, M.H.; Derakhshan, M.H.; Moradi, S.A.; Sheibani, M.; Tavangar, S.M.; Shirooie, S.; et al. Therapeutic Effects of Hydroalcoholic Extracts from the Ancient Apple Mela Rosa dei Monti Sibillini in Transient Global Ischemia in Rats. Pharmaceuticals 2021, 14, 1106. https://doi.org/10.3390/ph14111106
Yousefi-Manesh H, Dehpour AR, Nabavi SM, Khayatkashani M, Asgardoon MH, Derakhshan MH, Moradi SA, Sheibani M, Tavangar SM, Shirooie S, et al. Therapeutic Effects of Hydroalcoholic Extracts from the Ancient Apple Mela Rosa dei Monti Sibillini in Transient Global Ischemia in Rats. Pharmaceuticals. 2021; 14(11):1106. https://doi.org/10.3390/ph14111106
Chicago/Turabian StyleYousefi-Manesh, Hasan, Ahmad Reza Dehpour, Seyed Mohammad Nabavi, Malihe Khayatkashani, Mohammad Hossein Asgardoon, Mohamad Hasan Derakhshan, Sahand Adib Moradi, Mohammad Sheibani, Seyed Mohammad Tavangar, Samira Shirooie, and et al. 2021. "Therapeutic Effects of Hydroalcoholic Extracts from the Ancient Apple Mela Rosa dei Monti Sibillini in Transient Global Ischemia in Rats" Pharmaceuticals 14, no. 11: 1106. https://doi.org/10.3390/ph14111106
APA StyleYousefi-Manesh, H., Dehpour, A. R., Nabavi, S. M., Khayatkashani, M., Asgardoon, M. H., Derakhshan, M. H., Moradi, S. A., Sheibani, M., Tavangar, S. M., Shirooie, S., Nkuimi Wandjou, J. G., Caprioli, G., Sut, S., Dall’Acqua, S., & Maggi, F. (2021). Therapeutic Effects of Hydroalcoholic Extracts from the Ancient Apple Mela Rosa dei Monti Sibillini in Transient Global Ischemia in Rats. Pharmaceuticals, 14(11), 1106. https://doi.org/10.3390/ph14111106










