Quantification of Degradation Products Formed during Heat Sterilization of Glucose Solutions by LC-MS/MS: Impact of Autoclaving Temperature and Duration on Degradation
Abstract
:1. Introduction
2. Results
2.1. Autoclaving under Germicidal Control
2.2. Measuring of the pH Values of the Autoclaved and Non-Autoclaved Glucose Solutions
2.3. Content of GDPs in Autoclaved 10% (w/v) Glucose Solutions in PP Bottles
2.4. Content of GDPs in Commercially Available Aqueous Glucose Solutions (5–50%) in Different Types of Vessels from Three Different Manufacturers
2.5. Method Validation via LC-MS/MS
2.5.1. Selectivity
2.5.2. Linearity
2.5.3. Range
2.5.4. LOD
2.5.5. LOQ
2.5.6. Accuracy
2.5.7. Precision
2.6. Statistical Analysis
3. Discussion
4. Materials and Methods
4.1. Reagents and Chemicals
4.2. Experimental Overview
4.2.1. Preparation of Glucose Solutions
4.2.2. Calculation of the Required Steam Sterilization Time
4.2.3. Autoclaving under Germicidal Control
4.2.4. Measuring of the pH Values
4.2.5. Preparation of Calibration Solutions
4.2.6. Derivatization of Autoclaved Glucose Solutions
4.2.7. LC-MS/MS Analysis
4.2.8. Method Validation
4.2.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haybrard, J.; Simon, N.; Danel, C.; Pinçon, C.; Barthélémy, C.; Tessier, F.J.; Décaudin, B.; Boulanger, E.; Odou, P. Factors Generating Glucose Degradation Products in Sterile Glucose Solutions for Infusion: Statistical Relevance Determination of Their Impacts. Sci. Rep. 2017, 7, 11932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Council of Europe. 5.1.1 Methods of Preparation of Sterile Products. In European Pharmacopoeia 10.0; EDQM: Strasbourg, France, 2020; pp. 995–1000. [Google Scholar]
- Boehringer Ingelheim Pharma KG F0-Concept in Steam Sterilization and the Connected Sterilization Safety. Available online: https://ecv.de/suse_item.php?suseId=Z%7Cpi%7C1762&susePattern (accessed on 19 October 2021).
- Council of Europe. 5.1.5. Application of the F0 concept to Steam Sterilization of Aqueous Preparations. In European Pharmacopoeia 10.0; EDQM: Strasbourg, France, 2020; p. 1009. [Google Scholar]
- Bryland, A.; Broman, M.; Erixon, M.; Klarin, B.; Linden, T.; Friberg, H.; Wieslander, A.; Kjellstrand, P.; Ronco, C.; Carlsson, O.; et al. Infusion fluids contain harmful glucose degradation products. Intensive Care Med. 2010, 36, 1213–1220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linden, T.; Forsbäck, G.; Deppisch, R.; Henle, T.; Wieslander, A. 3-Deoxyglucosone, a promoter of advanced glycation end products in fluids for peritoneal dialysis. Perit. Dial. Int. 1998, 18, 290–293. [Google Scholar]
- Kjellstrand, P.; Erixon, M.; Wieslander, A.; Lindén, T.; Martinson, E. Temperature: The Single Most Important Factor for Degradation of Glucose Fluids during Storage. Perit. Dial. Int. 2004, 24, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Mittelmaier, S.; Fünfrocken, M.; Fenn, D.; Berlich, R.; Pischetsrieder, M. Quantification of the six major α-dicarbonyl contaminants in peritoneal dialysis fluids by UHPLC/DAD/MSMS. Anal. Bioanal. Chem. 2011, 401, 1183–1193. [Google Scholar] [CrossRef]
- Ledebo, I.; Wieslander, A.; Kjellstrand, P. Can we prevent the degradation of glucose in peritoneal dialysis solutions? Perit. Dial. Int. 2000, 20, 48–51. [Google Scholar] [CrossRef]
- Frischmann, M.; Spitzer, J.; Fünfrocken, M.; Mittelmaier, S.; Deckert, M.; Fichert, T.; Pischetsrieder, M. Development and validation of an HPLC method to quantify 3,4-dideoxyglucosone-3-ene in peritoneal dialysis fluids. Biomed. Chromatogr. 2009, 23, 843–851. [Google Scholar] [CrossRef]
- Nakayama, M.; Kawaguchi, Y.; Yamada, K.; Hasegawa, T.; Takazoe, K.; Katoh, N.; Hayakawa, H.; Osaka, N.; Yamamoto, H.; Ogawa, A.; et al. Immunohistochemical detection of advanced glycosylation end-products in the peritoneum and its possible pathophysiological role in CAPD. Kidney Int. 1997, 51, 182–186. [Google Scholar] [CrossRef] [Green Version]
- Mannermaaa, J.P.; Yliruusic, J.; Kanerva, U. Optimization of Moist Heat Sterilization of Glucose Infusions—The effect of different Fo-values on the pH and 5-hydroxymethyl 2-furaldehyde content of the solutions. Pharm. Ind. 1992, 54, 729–732. [Google Scholar]
- Mannermaaa, J.P.; Yliruusic, J.; Muttonen, E. Optimization of Moist Heat Sterilization of Glucose Infusions—The effect of sterilization parameters on the number of particles released from different rubber stoppers. Pharm. Ind. 1992, 54, 639–642. [Google Scholar]
- Mannermaa, J.P.; Muttonen, E.; Yliruusi, J.; Määttänen, L. The use of different time/temperature combinations in the optimization of sterilization of Ringers/glucose infusion solution. J. Pharm. Sci. Technol. 1992, 46, 184–191. [Google Scholar]
- Hung, C.T.; Selkirk, A.B.; Taylor, R.B. A chromatographic quality control procedure based on HPLC for 5-hydroxymethylfurfural in autoclaved D-glucose infusion fluids. J. Clin. Pharm. Ther. 1982, 7, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Cook, A.P.; Macleod, T.M.; Appleton, J.D.; Fell, A.F. Reversed-phase high-performance liquid chromatographic method for the quantification of 5-hydroxymethylfurfural as the major degradation product of glucose in infusion fluids. J. Chromatogr. A 1989, 467, 395–401. [Google Scholar] [CrossRef]
- Postaire, E.; Pradier, F.; Postaire, M.; Pradeau, D.; Matchoutsky, L.; Prognon, P.; Hamon, M. Various techniques for the routine evaluation of the degradation of glucose in parenteral solutions—A critical study. J. Pharm. Biomed. Anal. 1987, 5, 309–318. [Google Scholar] [CrossRef]
- Sigma-Aldrich. Taufkirchen (Germany), Safety Data Sheet. Glyoxal Solution. Product Number 128465. 2020. Available online: https://www.sigmaaldrich.com/DE/de/sds/sial/128465 (accessed on 27 October 2021).
- Sigma-Aldrich. Taufkirchen (Germany), Safety Data Sheet. Methylglyoxal Solution. Product Number M0252. 2019. Available online: https://www.sigmaaldrich.com/DE/de/sds/sigma/m0252 (accessed on 27 October 2021).
- Braun, R. Glucose-Lösungen 5 bis 50%. In Standardzulassungen für Fertigarzneimittel; Deutscher Apotheker Verlag: Eschborn, Germany, 2010; Volume 16. [Google Scholar]
- National Library of Medicine. Compund Summary. Bromocresol Purple. PubChem CID 8273. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Bromocresol-purple (accessed on 27 October 2021).
- European Medicines Agency ICH Topic Q 2 (R1) Validation of Analytical Procedures: Text and Methodology. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-q-2-r1-validation-analytical-procedures-text-methodology-step-5_en.pdf (accessed on 19 October 2021).
- Pohloudek-Fabini, R.; Martin, E. The effect of the gas permeability of plastics on the stability of thiomersal. Part 49: Contributions to problems concerning the use of plastic receptacles for liquid pharmaceuticals (author’s transl). Pharmazie 1981, 36, 683–685. [Google Scholar]
- Allwood, M.C.; Kearney, M.C. Compatibility and stability of additives in parenteral nutrition admixtures. Nutrition 1998, 14, 697–706. [Google Scholar] [CrossRef]
- Ball, C.O. Thermal process time for canned food. Bull. Natl. Res. Counc. 1923, 7, 1–76. [Google Scholar]
- Reuter, H. Bewertung der thermischen Wirksamtkeit von UHT-Anlagen, Teil I: Reaktionskinetische Grundlagen. Dtsch. Molk.-Ztg. 1980, 101, 362–370. [Google Scholar]
- Burton, H. Ultra-High-Temperature Processing of Milk and Milk Products; Elsevier Applied Science: London, UK, 1988. [Google Scholar]
- Miorini, T. Grundlagen der Sterilisation. Available online: https://wfhss.com/wp-content/uploads/wfhss-training-2-03_de.pdf (accessed on 19 October 2021).
- Tao, F.-R.; Zhuang, C.; Cui, Y.-Z.; Xu, J. Dehydration of glucose into 5-hydroxymethylfurfural in SO3H-functionalized ionic liquids. Chin. Chem. Lett. 2014, 25, 757–761. [Google Scholar] [CrossRef]
- Sturgeon, R.J.; Athanikar, N.K.; Harbison, H.A.; Henry, R.S.; Jurgens, R.W.; Welco, A.D. Degradation of Dextrose during Heating under Simulated Sterilization. PDA J. Pharm. Sci. Technol. 1980, 34, 175–182. [Google Scholar]
- Qian, X.; Nimlos, M.R.; Johnson, D.K.; Himmel, M.E. Acidic sugar degradation pathways. Appl. Biochem. Biotechnol. 2005, 124, 989–997. [Google Scholar] [CrossRef] [Green Version]
- Kjellstrand, P.; Martinson, E.; Wieslander, A.; Kjellstrand, K.; Jeppsson, E.; Svensson, E.; Järkelid, L.; Linden, T.; Olsson, L.F. Degradation in peritoneal dialysis fluids may be avoided by using low pH and high glucose concentration. Perit. Dial. Int. 2001, 21, 338–344. [Google Scholar] [CrossRef]
- Capuano, E.; Fogliano, V. Acrylamide and 5-hydroxymethylfurfural (HMF): A review on metabolism, toxicity, occurrence in food and mitigation strategies. LWT Food Sci. Technol. 2011, 44, 793–810. [Google Scholar] [CrossRef]
- Janzowski, C.; Glaab, V.; Samimi, E.; Schlatter, J.; Eisenbrand, G. 5-Hydroxymethylfurfural: Assessment of mutagenicity, DNA-damaging potential and reactivity towards cellular glutathione. Food Chem. Toxicol. 2000, 38, 801–809. [Google Scholar] [CrossRef]
- Ulbricht, R.J.; Northup, S.J.; Thomas, J.A. A review of 5-hydroxymethylfurfural (HMF) in parenteral solutions. Fundam. Appl. Toxicol. 1984, 4, 843–853. [Google Scholar] [CrossRef]
- Bundesinstitut für Risikobewertung 5-HMF-Gehalte in Lebensmitteln Sind Nach Derzeitigem Wissenschaftlichen Kenntnisstand Gesundheitlich Unproblematisch. Available online: https://www.bfr.bund.de/cm/343/5_hmf_gehalte_in_lebensmitteln_sind_nach_derzeitigem_wissenschaftlichen_kenntnisstand_gesundheitlich_unproblematisch.pdf (accessed on 19 October 2021).
- European Parliament and of the Council Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on Classification, Labelling and Packaging of Substances and Mixtures, Amending and Repealing Directives 67/548/EEC and 1999/45/EC, and Amending Regulation (EC) No 1907/2006 (Text with EEA Relevance). Available online: https://eur-lex.europa.eu/eli/reg/2008/1272/oj (accessed on 19 October 2021).
- Carbosynth Ltd. Compton (UK), Safety Data Sheet. 3,4-Dideoxyglucosone-3-ene. Product Number MD44643. 2021. Available online: https://www.carbosynth.com/80257AD2003D1CDB/0/64654136FF2B356548257F64000E36D2/$file/MSDS_MD44643_5000_EN.pdf (accessed on 27 October 2021).
- Sigma-Aldrich. Taufkirchen (Germany), Safety Data Sheet. 5-(Hydroxymethyl)-furfural. Product Number 53407. 2019. Available online: https://www.sigmaaldrich.com/DE/de/sds/sial/53407 (accessed on 27 October 2021).
- Sigma-Aldrich. Taufkirchen (Germany), Safety Data Sheet. 2-Keto-D-glucose. Product Number 61793. 2019. Available online: https://www.sigmaaldrich.com/DE/de/sds/sigma/61793 (accessed on 27 October 2021).
- Cayman Chemical. Ann Arbor. Michigan (US), Safety Data Sheet. 3-deoxy Galactosone. Product Number 16801. 2020. Available online: https://www.caymanchem.com/msdss/16801m.pdf (accessed on 27 October 2021).
- Sigma-Aldrich. Taufkirchen (Germany), Safety Data Sheet. 3-Deoxyglucosone. Product Number 75762. 2019. Available online: https://www.sigmaaldrich.com/DE/de/sds/sigma/75762 (accessed on 27 October 2021).
- Zeier, M.; Schwenger, V.; Deppisch, R.; Haug, U.; Weigel, K.; Bahner, U.; Wanner, C.; Schneider, H.; Henle, T.; Ritz, E. Glucose degradation products in PD fluids: Do they disappear from the peritoneal cavity and enter the systemic circulation? Kidney Int. 2003, 63, 298–305. [Google Scholar] [CrossRef] [Green Version]
- García–López, E.; Carrero, J.J.; Suliman, M.E.; Lindholm, B.; Stenvinkel, P. Risk Factors for Cardiovascular Disease in Patients Undergoing Peritoneal Dialysis. Perit. Dial. Int. 2007, 27, 205–209. [Google Scholar] [CrossRef]
- Kandarakis, S.A.; Piperi, C.; Topouzis, F.; Papavassiliou, A.G. Emerging role of advanced glycation-end products (AGEs) in the pathobiology of eye diseases. Prog. Retin. Eye Res. 2014, 42, 85–102. [Google Scholar] [CrossRef] [PubMed]
- Himmele, R.; Sawin, D.-A.; Diaz-Buxo, J.A. GDPs and AGEs: Impact on cardiovascular toxicity in dialysis patients. Adv. Perit. Dial. 2011, 27, 22–26. [Google Scholar]
- Müller-Krebs, S.; Kihm, L.; Zeier, B.; Gross, M.; Wieslander, A.; Haug, U.; Zeier, M.; Schwenger, V. Glucose degradation products result in cardiovascular toxicity in a rat model of renal failure. Perit. Dial. Int. 2010, 30, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Simm, A.; Wagner, J.; Gursinsky, T.; Nass, N.; Friedrich, I.; Schinzel, R.; Czeslik, E.; Silber, R.E.; Scheubel, R.J. Advanced glycation endproducts: A biomarker for age as an outcome predictor after cardiac surgery? Exp. Gerontol. 2007, 42, 668–675. [Google Scholar] [CrossRef]
- Cho, Y.; Johnson, D.W.; Vesey, D.A.; Hawley, C.M.; Pascoe, E.M.; Clarke, M.; Topley, N. Baseline serum interleukin-6 predicts cardiovascular events in incident peritoneal dialysis patients. Perit. Dial. Int. 2015, 35, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Zimmerman, G.A.; Meistrell, M.; Bloom, O.; Cockroft, K.M.; Bianchi, M.; Risucci, D.; Broome, J.; Farmer, P.; Cerami, A.; Vlassara, H. Neurotoxicity of advanced glycation endproducts during focal stroke and neuroprotective effects of aminoguanidine. Proc. Natl. Acad. Sci. USA 1995, 92, 3744–3748. [Google Scholar] [CrossRef] [Green Version]
- Chawla, D.; Bansal, S.; Banerjee, B.; Madhu, S.; Kalra, O.P.; Tripathi, A. Role of advanced glycation end product (AGE)-induced receptor (RAGE) expression in diabetic vascular complications. Microvasc. Res. 2014, 95, 1–6. [Google Scholar] [CrossRef]
- Srikanth, V.; Maczurek, A.; Phan, T.; Steele, M.; Westcott, B.; Juskiw, D.; Münch, G. Advanced glycation endproducts and their receptor RAGE in Alzheimer’s disease. Neurobiol. Aging 2011, 32, 763–777. [Google Scholar] [CrossRef] [PubMed]
- Sarafidis, P.A.; Whaley-Connell, A.; Sowers, J.R.; Bakris, G.L. Cardiometabolic syndrome and chronic kidney disease: What is the link? J. CardioMetab. Syndr. 2006, 1, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Vogel, M.; Piper, T.; Krug, O.; Beuck, S.; Schänzer, W.; Thevis, M. Quantification of AICAR-ribotide concentrations in red blood cells by means of LC-MSMS. Anal. Bioanal. Chem. 2013, 405, 9703–9709. [Google Scholar] [CrossRef]
- MesaLabs EZ-Test® Steam. Geobacillus Stearothermophilus. Technical Report. Available online: https://biologicalindicators.mesalabs.com/wp-content/uploads/sites/31/2013/11/EZTest-Steam-TIR-003.pdf (accessed on 19 October 2021).
- Cook, A.P.; MacLeod, T.M.; Appleton, J.D.; Fell, A.F. HPLC studies on the degradation profiles of glucose 5% solutions subjected to heat sterilization in a microprocessor-controlled autoclave. J. Clin. Pharm. Ther. 1989, 14, 189–195. [Google Scholar] [CrossRef]
- Leitzen, S.; Vogel, M.; Engels, A.; Zapf, T.; Brandl, M. Identification and quantification of glucose degradation products in heat-sterilized glucose solutions for parenteral use by thinlayer chromatography. PLoS ONE 2021, 16, e0253811. [Google Scholar] [CrossRef] [PubMed]
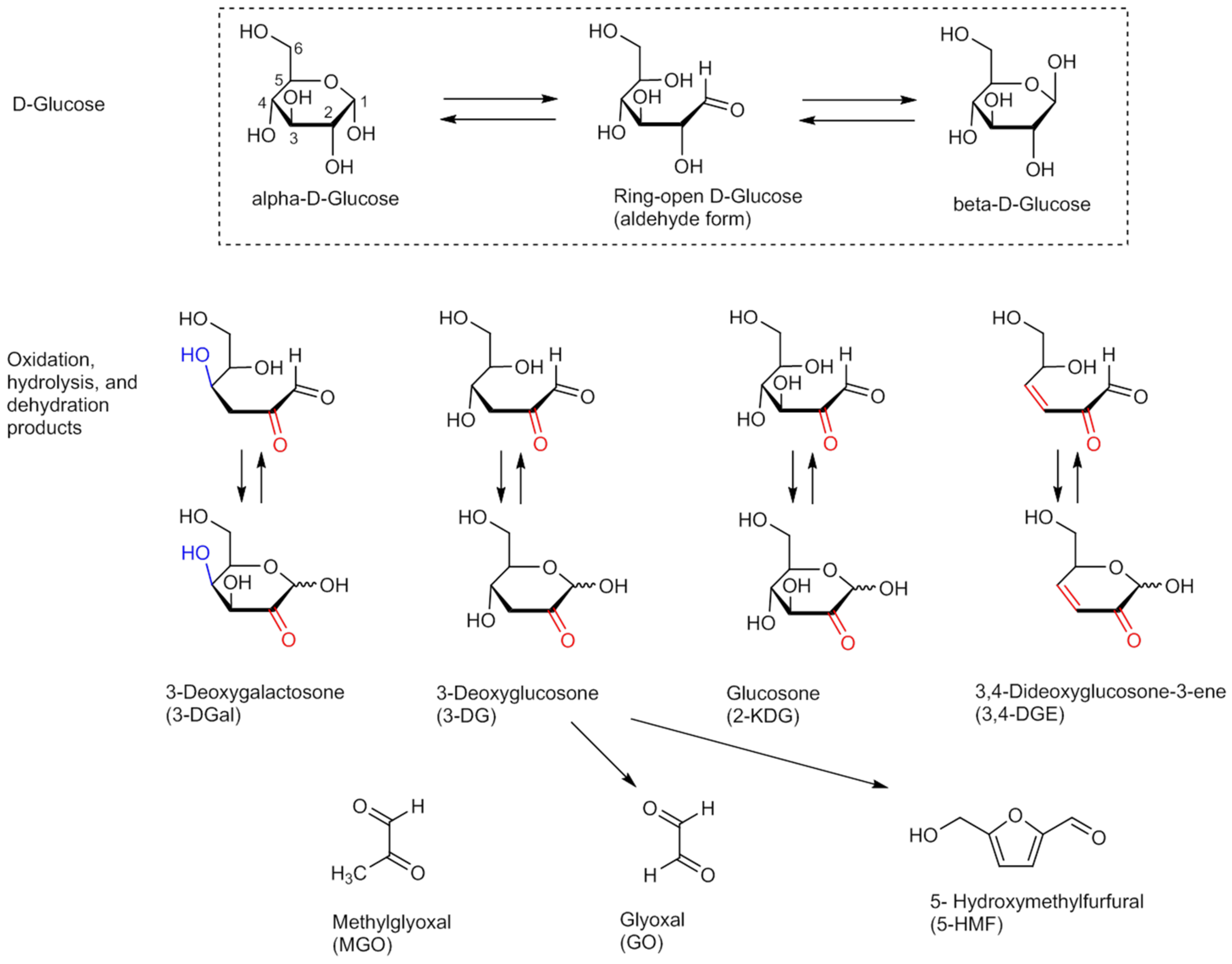

| Temperature [°C] | Scheme A (Overkill Conditions) Autoclaving Time (F0) [min] | Scheme B Autoclaving Time (F0) [min] |
|---|---|---|
| 111 | 180 | 233 |
| 116 | 57 | 85 |
| 121 | 18 | 30 |
| Temperature [°C] | F0 [min] (Scheme A) | Geobacillus stearothermophilus Killed (Scheme A/Scheme B)? |
|---|---|---|
| 111 | 180 | yes |
| 116 | 57 | yes |
| 121 | 18 | yes |
| 10% Glucose Solution (n = 3 for Each Scheme A/B) | Control Values of Autoclaved Water without Glucose (n = 3 for Each Scheme A/B) | |||
|---|---|---|---|---|
| Temperature [°C] | 10% PP Bottle (Scheme A) pH ± SD | 10% PP Bottle (Scheme B) pH ± SD | Water, PP Bottle (Scheme A) pH ± SD | Water, PP Bottle(Scheme B) pH ± SD |
| 111 | 5.17 ± 0.0 | 4.08 ± 0.0 | 7.03 ± 0.0 | 6.80 ± 0.0 |
| 116 | 4.67 ± 0.0 | 4.12 ± 0.0 | 6.77 ± 0.0 | 6.53 ± 0.0 |
| 121 | 4.36 ± 0.0 | 4.15 ± 0.0 | 6.68 ± 0.0 | 6.11 ± 0.0 |
| non-autoclaved (room temperature) | 4.98 ± 0.0 | 6.81 ± 0.0 | ||
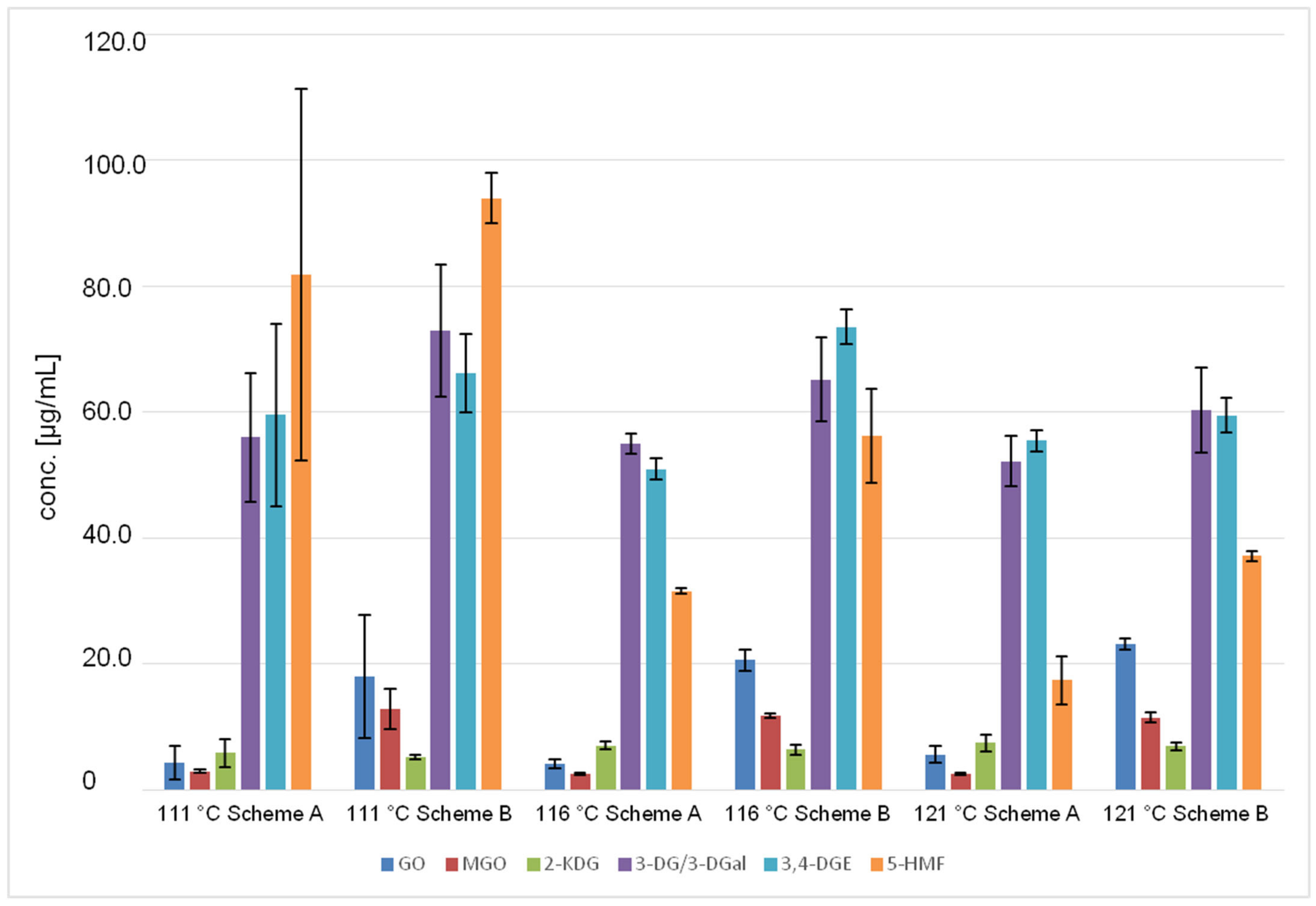
| Temp [°C] | GO [µg/mL] ± SD | MGO [µg/mL] ± SD | 2-KDG [µg/mL] ± SD | 3-DG/3-DGal [µg/mL] ± SD | 3,4-DGE [µg/mL] ± SD | 5-HMF [µg/mL] ± SD | |
|---|---|---|---|---|---|---|---|
| Scheme A | 111 | 4.4 ± 2.7 | 3.0 ± 0.3 | 5.9 ± 2.2 | 56.0 ± 10.2 | 59.6 ± 14.5 | 81.9 ± 29.5 |
| 116 | 4.2 ± 0.7 | 2.5 ± 0.2 | 7.1 ± 0.6 | 55.0 ± 1.6 | 50.9 ± 1.7 | 31.6 ± 0.5 | |
| 121 | 5.6 ± 1.3 | 2.6 ± 0.2 | 7.5 ± 1.4 | 52.2 ± 4.0 | 55.5 ± 1.7 | 17.4 ± 3.9 | |
| Scheme B | 111 | 18.0 ± 9.7 | 12.9 ± 3.2 | 5.2 ± 0.3 | 72.9 ± 10.5 | 66.2 ± 6.2 | 94.0 ± 4.0 |
| 116 | 20.6 ± 1.7 | 11.8 ± 0.4 | 6.5 ± 0.8 | 65.2 ± 6.7 | 73.6 ± 2.8 | 56.3 ± 7.4 | |
| 121 | 23.2 ± 0.9 | 11.5 ± 0.8 | 6.9 ± 0.7 | 60.3 ± 6.8 | 59.5 ± 2.8 | 37.2 ± 0.8 |
| Temp [°C] | GO [µg/mL] ± SD | MGO [µg/mL] ± SD | 2-KDG [µg/mL] ± SD | 3-DG/3-DGal [µg/mL] ± SD | 3,4-DGE [µg/mL] ± SD | 5-HMF [µg/mL] ± SD |
|---|---|---|---|---|---|---|
| 121 | 8.3 ± 0.0 | 1.2 ± 0.1 | 1.1 ± 0.1 | 13.7 ± 0.1 | 12.5 ± 0.3 | 41.1 ± 0.1 |
| Temp [°C] | GO [µg/mL] ± SD | MGO [µg/mL] ± SD | 2-KDG [µg/mL] ± SD | 3-DG/3-DGal [µg/mL] ± SD | 3,4-DGE [µg/mL] ± SD | 5-HMF [µg/mL] ± SD |
|---|---|---|---|---|---|---|
| 121 | 1.0 ± 0.5 | 0.9 ± 0.1 | 0.1 ± 0.0 | n.d. | n.d. | 0.1 ± 0.0 |
| MAH/Conc | GO [µg/mL] ± SD | MGO [µg/mL] ± SD | 2-KDG [µg/mL] ± SD | 3-DG/3-DGal [µg/mL] ± SD | 3,4-DGE [µg/mL] ± SD | 5-HMF [µg/mL] ± SD |
|---|---|---|---|---|---|---|
| 5% PP | 35.9 ± 2.5 | 0.8 ± 0.3 | 0.5 ± 0.6 | 8.5 ± 0.7 | 1.2 ± 1.0 | 0.4 ± 0.6 |
| 10% PP | 42.1 ± 8.5 | 0.8 ± 0.3 | 1.1 ± 0.9 | 11.3 ± 1.0 | 1.5 ± 1.3 | 0.7 ± 0.6 |
| 20% PP | 39.2 ± 3.4 | 0.8 ± 0.2 | 18.9 ± 4.8 | 1.3 ± 0.1 | 0.1 ± 0.1 | 2.1 ± 0.2 |
| 40% PP | 47.3 ± 2.6 | 0.9 ± 0.2 | 23.0 ± 0.3 | 0.4 ± 0.1 | 0.0 ± 0.0 | 4.6 ± 0.4 |
| 50% Glass | 43.6 ± 3.9 | 0.9 ± 0.4 | 11.1 ± 1.0 | 31.4 ± 2.4 | 3.5 ± 0.3 | 5.6 ± 0.9 |
| MAH/Conc | GO [µg/mL] ± SD | MGO [µg/mL] ± SD | 2-KDG [µg/mL] ± SD | 3-DG/3-DGal [µg/mL] ± SD | 3,4-DGE [µg/mL] ± SD | 5-HMF [µg/mL] ± SD |
|---|---|---|---|---|---|---|
| 5% PP | 0.6 ± 0.1 | 0.5 ± 0.1 | 1 ± 0.9 | 14.2 ± 3.4 | 3.4 ± 1.0 | 1.6 ± 0.3 |
| 10% PP | 11.1 ± 0.5 | 0.6 ± 0.1 | 1.7 ± 0.1 | 18.4 ± 2.1 | 3.6 ± 0.4 | 2.6 ± 0.4 |
| 20% PP | 13.7 ± 1.3 | 0.7 ± 0.3 | 5.1 ± 2.7 | 20.2 ± 0.4 | 3.3 ± 0.3 | 5.0 ± 0.3 |
| 40% PP | 16.1 ± 3.6 | 0.8 ± 0.5 | 17.5 ± 8.8 | 20.4 ± 0.4 | 1.6 ± 0.0 | 9.5 ± 1.2 |
| 50% Glass | 15.9 ± 0.9 | 0.9 ± 0.2 | 10.1 ± 2.6 | 34.6 ± 0.5 | 4.0 ± 0.3 | 7.9 ± 1.0 |
| MAH/Conc | GO [µg/mL] ± SD | MGO [µg/mL] ± SD | 2-KDG [µg/mL] ± SD | 3-DG/3-DGal [µg/mL] ± SD | 3,4-DGE [µg/mL] ± SD | 5-HMF [µg/mL] ± SD |
|---|---|---|---|---|---|---|
| 5% Glass | 20.9 ± 5.1 | 0.7 ± 0.3 | 0.3 ± 0.6 | 12.3 ± 0.7 | 2.9 ± 0.2 | 1.2 ± 0.1 |
| 10% Glass | 31.9 ± 3.2 | 0.7 ± 0.2 | 0.9 ± 0.9 | 13.3 ± 1.4 | 2.5 ± 0.2 | 2.9 ± 0.2 |
| 20% Glass | 33.0 ± 0.8 | 0.7 ± 0.1 | 7.6 ± 4.2 | 14.2 ± 0.6 | 1.2 ± 0.2 | 14.1 ± 1.1 |
| 40% PP | 32.4 ± 6.5 | 0.8 ± 0.4 | 15.3 ± 2.3 | 10.8 ± 1.1 | 0.8 ± 0.1 | 4.6 ± 0.1 |
| 50% Glass | 40.5 ± 3.3 | 0.9 ± 0.3 | 6.7 ± 4.5 | 31.8 ± 3.0 | 2.9 ± 0.2 | 12.9 ± 0.5 |
| Analyte | Regression | R2 | Weighting | Range [µg/mL] | LOD [µg/mL] (1 calc.) | LOQ [µg/mL] (1 calc.) |
|---|---|---|---|---|---|---|
| GO | 0.999 | none | 0.5–100 | 0.078 | 0.236 | |
| MGO | 0.999 | none | 0.5–100 | 0.023 | 0.070 | |
| 2-KDG | 0.999 | none | 0.5–100 | 0.053 | 0.161 | |
| 3-DG/3-DGal | 0.999 | 0.5–100 | 0.004 | 0.012 | ||
| 3,4-DGE | 0.998 | 0.5–100 | 0.015 | 0.046 | ||
| 5-HMF | 0.993 | 0.5–100 | 0.010 | 0.031 |
| Precision (as% RSD) | Accuracy (% Recovery) | ||||
|---|---|---|---|---|---|
| GDP | GDP conc. [µg/mL] | Mean [µg/mL] ± SD | RSD% | Mean [µg/mL] ± SD | % Recovery |
| GO | 0.5 | 0.5 ± 0.0 | 2.5 | 0.5 ± 0.0 | 98.8 |
| 25 | 23.0 ± 0.3 | 1.2 | 24.6 ± 0.7 | 98.3 | |
| 100 | 98.5 ± 0.7 | 0.7 | 98.2 ± 3.0 | 98.2 | |
| MGO | 0.5 | 0.7 ± 0.0 | 0.8 | 0.5 ± 0.0 | 109.0 |
| 25 | 24.0 ± 0.3 | 1.4 | 25.5 ± 0.7 | 102.1 | |
| 100 | 98.5 ± 0.7 | 0.7 | 99.5 ± 2.4 | 99.5 | |
| 2-KDG | 0.5 | 0.6 ± 0.0 | 2.9 | 0.5 ± 0.0 | 103.7 |
| 25 | 24.7 ± 0.6 | 2.4 | 26.0 ± 0.9 | 103.9 | |
| 100 | 101.5 ± 0.5 | 0.5 | 103.6 ± 3.7 | 103.6 | |
| 3-DG/ 3-DGal | 0.5 | 0.4 ± 0.0 | 4.9 | 0.4 ± 0.0 | 89.8 |
| 25 | 26.4 ± 0.2 | 0.7 | 25.1 ± 1.8 | 100.2 | |
| 100 | 99.3 ± 2.0 | 2.0 | 100.5 ± 3.1 | 100.5 | |
| 3,4-DGE | 0.5 | 0.5 ± 0.0 | 4.3 | 0.5 ± 0.0 | 106.9 |
| 25 | 25.9 ± 0.5 | 2.0 | 26.0 ± 2.0 | 104.2 | |
| 100 | 92.3 ± 1.1 | 1.1 | 96.1 ± 4.0 | 96.1 | |
| 5-HMF | 0.5 | 0.5 ± 0.0 | 3.1 | 0.5 ± 0.0 | 94.8 |
| 25 | 26.9 ± 0.3 | 1.4 | 27.0 ± 0.7 | 107.9 | |
| 100 | 103.4 ± 3.6 | 0.5 | 102.5 ± 4.5 | 102.5 | |
| GDP Temp [°C] | F0 Scheme A vs. F0 Scheme B | |||
|---|---|---|---|---|
| p-Value | Significance Level | Degrees of Freedom | ||
| GO | 111 °C | 0.008012 | * | 16 |
| 116 °C | 1.512 × 10−12 | */** | ||
| 121 °C | 1.516 × 10−13 | */** | ||
| MGO | 111 °C | 0.3065 | not significant | |
| 116 °C | 6.058 × 10−14 | */** | ||
| 121 °C | 2.938 × 10−13 | */** | ||
| 2-KDG | 111 °C | 0.5692 | not significant | |
| 116 °C | 0.1991 | not significant | ||
| 121 °C | 0.4838 | not significant | ||
| 3-DG/ 3-DGal | 111 °C | 0.05203 | not significant | |
| 116 °C | 0.003162 | * | ||
| 121 °C | 0.01666 | * | ||
| 3,4-DGE | 111 °C | 0.4311 | not significant | |
| 116 °C | 6.311 × 10−8 | */** | ||
| 121 °C | 0.02456 | * | ||
| 5-HMF | 111 °C | 0.3691 | not significant | |
| 116 °C | 1.03 × 10−6 | */** | ||
| 121 °C | 4.946 × 10−9 | */** | ||
| GDP | 121 °C versus 111 °C and 116 °C | ||||
|---|---|---|---|---|---|
| Standard Autoclaving Temperature | Alternative Autoclaving Temperature | p-Value | Significance Level | Degrees of Freedom | |
| GO | 121 °C | 111 °C | 0.3051 | not significant | 16 |
| 121 °C | 116 °C | 0.05448 | not significant | ||
| MGO | 121 °C | 111 °C | 0.1987 | not significant | |
| 121 °C | 116 °C | 0.6584 | not significant | ||
| 2-KDG | 121 °C | 111 °C | 0.2506 | not significant | |
| 121 °C | 116 °C | 0.6102 | not significant | ||
| 3-DG/ 3-DGal | 121 °C | 111 °C | 0.6078 | not significant | |
| 121 °C | 116 °C | 0.1801 | not significant | ||
| 3,4-DGE | 121 °C | 111 °C | 0.6067 | not significant | |
| 121 °C | 116 °C | 0.01357 | * | ||
| 5-HMF | 121 °C | 111 °C | 1.256 × 10−4 | */** | |
| 121 °C | 116 °C | 5.134 × 10−7 | */** | ||
| ID | Q1 [m/z] | Q3 [m/z] | Dwell Time [msec] | CE [eV] | DP [eV] | Rt [min] |
|---|---|---|---|---|---|---|
| GO | 131.1 | 76.7 | 50 | 40 | 100 | 6.95 |
| MGO | 145.1 | 77 | 50 | 40 | 100 | 7.80 |
| 2-KDG | 251.1 | 173.2 | 50 | 20 | 100 | 3.51 |
| 3-DG/3-DGal | 235.1 | 199.1 | 50 | 25 | 100 | 4.79 |
| 3.4-DGE | 217.1 | 169.1 | 50 | 20 | 100 | 6.37 |
| 5-HMF | 215.1 | 197.1 | 50 | 25 | 100 | 6.46 |
| IS | 159.1 | 118.1 | 50 | 40 | 100 | 8.48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leitzen, S.; Vogel, M.; Steffens, M.; Zapf, T.; Müller, C.E.; Brandl, M. Quantification of Degradation Products Formed during Heat Sterilization of Glucose Solutions by LC-MS/MS: Impact of Autoclaving Temperature and Duration on Degradation. Pharmaceuticals 2021, 14, 1121. https://doi.org/10.3390/ph14111121
Leitzen S, Vogel M, Steffens M, Zapf T, Müller CE, Brandl M. Quantification of Degradation Products Formed during Heat Sterilization of Glucose Solutions by LC-MS/MS: Impact of Autoclaving Temperature and Duration on Degradation. Pharmaceuticals. 2021; 14(11):1121. https://doi.org/10.3390/ph14111121
Chicago/Turabian StyleLeitzen, Sarah, Matthias Vogel, Michael Steffens, Thomas Zapf, Christa Elisabeth Müller, and Martin Brandl. 2021. "Quantification of Degradation Products Formed during Heat Sterilization of Glucose Solutions by LC-MS/MS: Impact of Autoclaving Temperature and Duration on Degradation" Pharmaceuticals 14, no. 11: 1121. https://doi.org/10.3390/ph14111121
APA StyleLeitzen, S., Vogel, M., Steffens, M., Zapf, T., Müller, C. E., & Brandl, M. (2021). Quantification of Degradation Products Formed during Heat Sterilization of Glucose Solutions by LC-MS/MS: Impact of Autoclaving Temperature and Duration on Degradation. Pharmaceuticals, 14(11), 1121. https://doi.org/10.3390/ph14111121






