Whey Proteins Isolate-Based Biopolymeric Combinations to Microencapsulate Supercritical Fluid Extracted Oleoresins from Sea Buckthorn Pomace
Abstract
:1. Introduction
2. Results and Discussion
2.1. Advanced Phytochemical Characterizations of the Extract and Powders
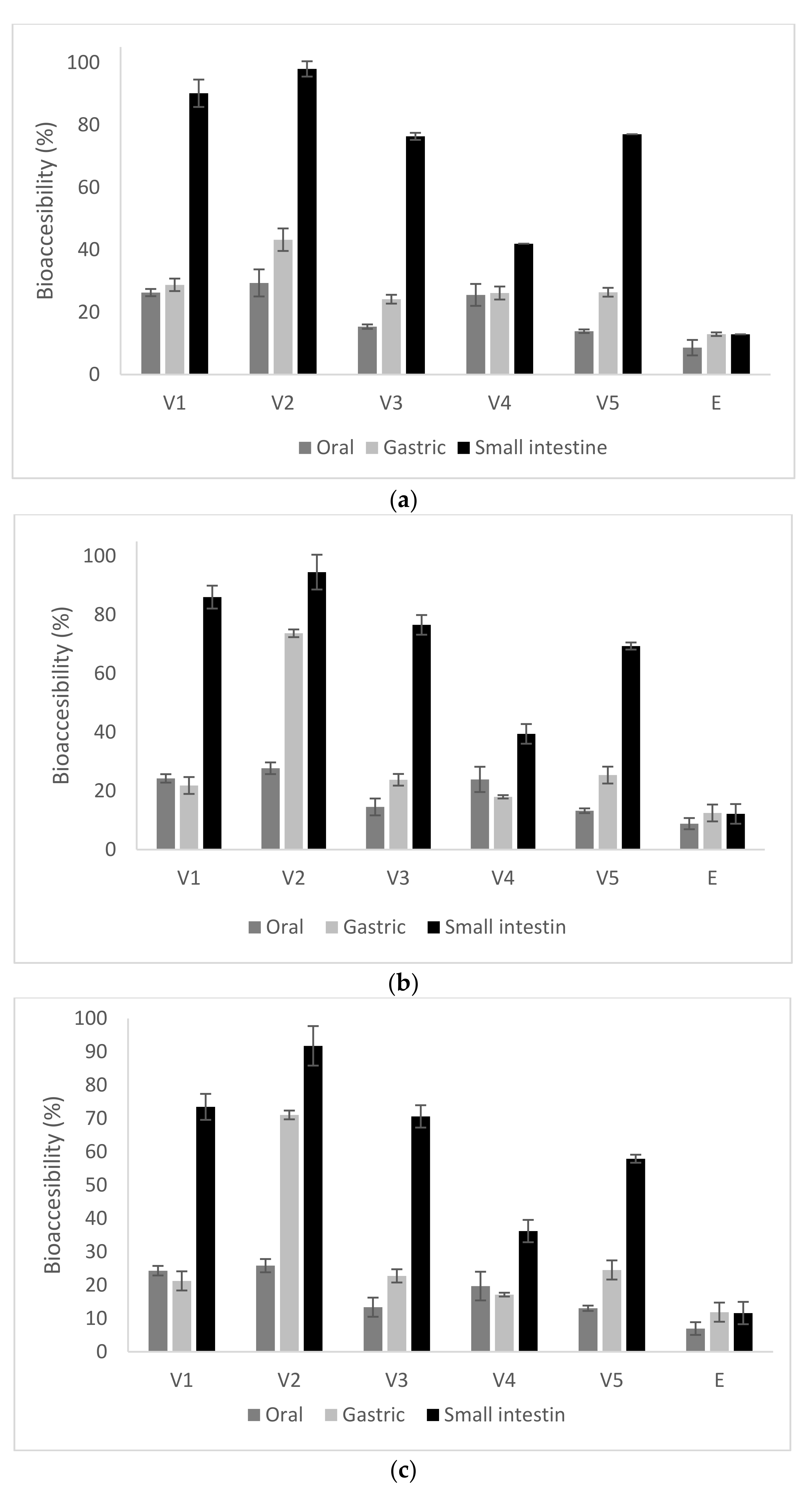
2.2. In Vitro Digestion
2.3. Inhibitory Effect on Metabolic Syndrome-Associated Enzymes


2.4. Evaluation of Physical Characteristics of the Microcapsule Powders
2.5. Structural and Morphological Analysis of the Powders
3. Materials and Methods
3.1. Materials
3.2. SFE-CO2 Extraction
3.3. Microencapsulation of SBP Oleoresins
3.4. Microencapsulation Efficiency
3.5. Global Phytochemicals Profiling and Antioxidant Activity
3.6. Fatty Acids, Phytosterols and Tocopherols Content of the Extract and Powders
3.7. In Vitro Digestion
3.8. Inhibitory Effect on Metabolic Syndrome-Associated Enzymes
3.9. Molecular Modelling Investigation on Phytochemical Binding to the Enzymes
3.10. Evaluation of Physical Characteristics of the Microcapsule Powders
3.11. Structural and Morphological Analysis of the Powders
3.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, I.P.; Ahmad, F.; Gore, D.D.; Tikoo, K.; Bansal, A.; Jachak, S.M.; Jena, G. Therapeutic potential of seabuckthorn: A patent review (2000–2018). Expert Opin. Ther. Pat. 2019, 29, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Attri, S.; Goel, G. Influence of polyphenol rich seabuckthorn berries juice on release of polyphenols and colonic microbiota on exposure to simulated human digestion model. Food Res. Int. 2018, 111, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, D.B.; Mariutti, L.R.B.; Mercadante, A.Z. An in vitro digestion method adapted for carotenoids and carotenoid esters: Moving forward towards standardization. Food Func. 2016, 7, 4992–5001. [Google Scholar] [CrossRef] [PubMed]
- Ciesarová, Z.; Murkovic, M.; Cejpek, K.; Kreps, F.; Tobolková, B.; Koplík, R.; Belajová, E.; Kukurová, K.; Daško, L.; Panovská, Z.; et al. Why is sea buckthorn (Hippophaë rhamnoides L.) so exceptional? A review. Food Res. Int. 2020, 133, 109170. [Google Scholar] [CrossRef]
- Tkacz, K.; Wojdyło, A.; Turkiewicz, I.P.; Nowicka, P. Triterpenoids, phenolic compounds, macro- and microelements in anatomical parts of sea buckthorn (Hippophaë rhamnoides L.) berries, branches and leaves. J. Food Comp. Anal. 2021, 103, 104107. [Google Scholar] [CrossRef]
- Arimboor, R.; Arumughan, C. HPLC-DAD-MS/MS profiling of antioxidant flavonoid glycosides in sea buckthorn (Hippophae rhamnoides L.) seeds. Int. J. Food Sci. Nutr. 2012, 63, 730–738. [Google Scholar] [CrossRef]
- Yang, W.; Laaksonen, O.; Kallio, H.; Yang, B. Effects of latitude and weather conditions on proanthocyanidins in berries of Finnish wild and cultivated sea buckthorn (Hippophaë rhamnoides L. ssp. rhamnoides). Food Chem. 2017, 216, 87–96. [Google Scholar] [CrossRef]
- Dąbrowski, G.; Czaplicki, S.; Szustak, M.; Cichońska, E.; Gendaszewska-Darmach, E.; Konopka, I. Composition of flesh lipids and oleosome yield optimization of selected sea buckthorn (Hippophae rhamnoides L.) cultivars grown in Poland. Food Chem. 2022, 369, 130921. [Google Scholar] [CrossRef]
- Yang, Z.-H.; Takeo, J.; Katayama, M. Oral administration of omega-7 palmitoleic acid induces satiety and the release of appetite-related hormones in male rats. Appetite 2013, 65, 1–7. [Google Scholar] [CrossRef]
- Talbot, N.A.; Wheeler-Jones, C.P.; Cleasby, M.E. Palmitoleic acid prevents palmitic acid-induced macrophage activation and consequent p38 MAPK-mediated skeletal muscle insulin resistance. Mol. Cell. Endocrinol. 2014, 393, 129–142. [Google Scholar] [CrossRef]
- Hu, W.; Fitzgerald, M.; Topp, B.; Alam, M.; O’Hare, T.J. A review of biological functions, health benefits, and possible de novo biosynthetic pathway of palmitoleic acid in macadamia nuts. J. Funct. Foods 2019, 62, 103520. [Google Scholar] [CrossRef]
- Tricò, D.; Mengozzi, A.; Nesti, L.; Hatunic, M.; Gabriel Sanchez, R.; Konrad, T.; Natali, A. Circulating palmitoleic acid is an independent determinant of insulin sensitivity, beta cell function and glucose tolerance in non-diabetic individuals: A longitudinal analysis. Diabetologia 2020, 63, 206–218. [Google Scholar] [CrossRef] [Green Version]
- Gani, A.; Jan, R.; Ashwar, B.A.; Ashraf, Z.; Shah, A.; Gani, A. Encapsulation of saffron and sea buckthorn bioactives: Its utilization for development of low glycemic baked product for growing diabetic population of the world. LWT—Food Sci. Technol. 2021, 142, 111035. [Google Scholar] [CrossRef]
- Mihalcea, L.; Turturică, M.; Ghinea, I.O.; Barbu, V.; Ioniță, E.; Cotărleț, M.; Stănciuc, N. Encapsulation of carotenoids from sea buckthorn extracted by CO2 supercritical fluids method within whey proteins isolates matrices. Innov. Food Sci. Emerg. Technol. 2017, 42, 120–129. [Google Scholar] [CrossRef]
- Chang, M.; Guo, Y.; Jiang, Z.; Shi, L.; Zhang, T.; Wang, Y.; Gong, M.; Wang, T.; Lin, R.; Liu, R.; et al. Sea buckthorn pulp oil nanoemulsions fabricated by ultra-high pressure homogenization process: A promising carrier for nutraceutical. J. Food Eng. 2020, 287, 110129. [Google Scholar] [CrossRef]
- Wang, L.; Liu, F.; Wang, A.; Yu, Z.; Xu, Y.; Yang, Y. Purification, characterization and bioactivity determination of a novel polysaccharide from pumpkin (Cucurbita moschata) seeds. Food Hydrocoll. 2017, 66, 357–364. [Google Scholar] [CrossRef]
- Loyeau, P.A.; Spotti, M.J.; Vinderola, G.; Carrara, C.R. Encapsulation of potential probiotic and canola oil through emulsification and ionotropic gelation, using protein/polysaccharides Maillard conjugates as emulsifiers. LWT—Food Sci. Technol. 2021, 150, 111890. [Google Scholar] [CrossRef]
- Shi, L.; Zheng, L.; Zhao, C.; Huang, J.; Jin, Q.; Wang, X. Effects of deacidification methods on high free fatty acid containing oils obtained from sea buckthron (Hippophaë rhamnoides L.) berry. Ind. Crops Prod. 2018, 124, 797–805. [Google Scholar] [CrossRef]
- Fernández-García, E.; Mínguez-Mosquera, M.I.; Pérez-Gálvez, A. Changes in composition of the lipid matrix produce a differential incorporation of carotenoids in micelles. Interaction effect of cholesterol and oil. Inn. Food Sci. Emer. Technol. 2007, 8, 379–384. [Google Scholar] [CrossRef]
- Szabo, K.; Teleky, B.E.; Ranga, F.; Simon, E.; Pop, O.L.; Babalau-Fuss, V.; Kapsalis, N.; Vodnar, D.C. Bioaccessibility of microencapsulated carotenoids, recovered from tomato processing industrial by-products, using in vitro digestion model. LWT—Food Sci. Technol. 2021, 152, 112285. [Google Scholar] [CrossRef]
- Donhowe, E.G.; Flores, F.P.; Kerr, W.L.; Wicker, L.; Kong, F.B. Characterization and in vitro bioavailability of β-carotene: Effects of microencapsulation method and food matrix. LWT—Food Sci. Technol. 2014, 57, 42–48. [Google Scholar] [CrossRef]
- Schmitt, C.; Sanchez, C.; Desobry-Banon, S.; Hardy, J. Structure and techno functional properties of protein–polysaccharide complexes: A review. Crit. Rev. Food Sci. Nutr. 1998, 38, 689–753. [Google Scholar] [CrossRef]
- Gomez-Mascaraque, L.G.; Perez-Masia, R.; Gonzalez-Barrio, R.; Periago, M.J.; Lopez-Rubio, A. Potential of microencapsulation through emulsion-electrospraying to improve the bioaccesibility of β-carotene. Food Hydrocoll. 2017, 73, 1–12. [Google Scholar] [CrossRef]
- Del Rio, D.; Borges, G.; Crozier, A. Berry flavonoids and phenolics: Bioavailability and evidence of protective effects. Br. J. Nutr. 2010, 104, 67–90. [Google Scholar] [CrossRef] [Green Version]
- Gopal, S.S.; Maradgi, T.; Ponesakki, G. Antiobese properties of carotenoids: An overview of underlying molecular mechanisms. In Carotenoids: Properties, Processing and Applications; Galanakis, C.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Condurache (Lazăr), N.-N.; Croitoru, C.; Enachi, E.; Bahrim, G.-E.; Stănciuc, N.; Râpeanu, G. Eggplant peels as a valuable source of anthocyanins: Extraction, thermal stability and biological activities. Plants 2021, 10, 577. [Google Scholar] [CrossRef]
- Axer, A.; Jumde, R.P.; Adam, S.; Faust, A.; Schäfers, M.; Fobker, M.; Koehnke, J.; Hirsch, A.K.; Gilmour, R. Enhancing glycan stability via site-selective fluorination: Modulating substrate orientation by molecular design. Chem. Sci. 2021, 12, 1286–1294. [Google Scholar] [CrossRef]
- Hadvary, P.; Sidler, W.; Meister, W.; Vetter, W.; Wolfer, H. The lipase inhibitor tetrahydrolipstatin binds covalently to the putative active site serine of pancreatic lipase. J. Biol. Chem. 1991, 266, 2021–2027. [Google Scholar] [CrossRef]
- Gilbert, N.C.; Bartlett, S.G.; Waight, M.T.; Neau, D.B.; Boeglin, W.E.; Brash, A.R.; Newcomer, M.E. The structure of human 5-lipoxygenase. Science 2011, 331, 217–219. [Google Scholar] [CrossRef] [Green Version]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD—Visual Molecular Dynamics. J. Mol. Graph. Model. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Wolska, E. Fine powder of lipid microparticles—Spray drying process development and optimization. J. Drug Deliv. Sci. Technol. 2021, 64, 102640. [Google Scholar] [CrossRef]
- Bordon, M.G.; Alasino, X.; Villanueva-Lazo, Á.; Carrera-Sánchez, C.; Pedroche-Jiménez, J.; Millan-Linares, M.; Ribotta, P.; Martinez, M. Scale-up and optimization of the spray drying conditions for the development of functional microparticles based on chia oil. Food Bioprod. Proc. 2021, 130, 48–67. [Google Scholar] [CrossRef]
- Kurozawa, L.E.; Gomes Morassi, A.; Vanzo, A.A.; Park, K.J.; Dupas Hubinger, M. Influence of spray drying conditions on physicochemical properties of chicken meat powder. Dry. Technol. 2009, 27, 1248–1257. [Google Scholar] [CrossRef]
- Dumitrașcu, L.; Stănciuc, N.; Borda, D.; Neagu, C.; Barbu, V.; Ioniță, E.; Aprodu, I. Microencapsulation of bioactive compounds from cornelian cherry fruits using different biopolymers with soy proteins. Food Biosci. 2021, 41, 101032. [Google Scholar] [CrossRef]
- Katoh, T.; Nagashima, U.; Mimuro, M. Fluorescence properties of the allenic carotenoid fucoxanthin: Implication for energy transfer in photosynthetic pigment systems. Photosyn. Res. 1991, 27, 221–226. [Google Scholar]
- Neagu, C.; Mihalcea, L.; Enachi, E.; Barbu, V.; Borda, D.; Bahrim, G.E.; Stănciuc, N. Cross-Linked Microencapsulation of CO2 Supercritical Extracted Oleoresins from Sea Buckthorn: Evidence of Targeted Functionality and Stability. Molecules 2020, 25, 2442. [Google Scholar] [CrossRef]
- Mihalcea, L.; Turturică, M.; Cucolea, E.I.; Dăanilă, G.-M.; Dumitrașcu, L.; Coman, G.; Constantin, O.E.; Grigore-Gurgu, L.; Stănciuc, N. CO2 Supercritical Fluid Extraction of Oleoresins from Sea Buckthorn Pomace: Evidence of Advanced Bioactive Profile and Selected Functionality. Antioxidants 2021, 10, 1681. [Google Scholar] [CrossRef]
- Santoyo, S.; Jaime, L.; García-Risco, M.R.; Lopez-Hazas, M.; Reglero, G. Supercritical fluid extraction as an alternative process to obtain antiviral agents from thyme species. Ind. Crops Prod. 2014, 52, 475–480. [Google Scholar] [CrossRef]
- Gheonea, I.; Aprodu, I.; Râpeanu, G.; Stănciuc, N. Binding mechanisms between lycopene extracted from tomato peels and bovine β-lactoglobulin. J. Lumin. 2018, 203, 582–589. [Google Scholar]
- Schneidman-Duhovny, D.; Inbar, Y.; Nussinov, R.; Wolfson, H.J. PatchDock and SymmDock: Servers for rigid and symmetric docking. Nucleic Acids Res. 2005, 33, W363–W367. [Google Scholar] [CrossRef] [Green Version]
- Van Tilbeurgh, H.; Sarda, L.; Verger, R.; Cambillau, C. Structure of the pancreatic lipase-procolipase complex. Nature 1992, 359, 159–162. [Google Scholar]


| Phytochemicals | Extract | V1 | V2 | V3 | V4 | V5 |
|---|---|---|---|---|---|---|
| Carotenoids (mg/g DW) | ||||||
| Total carotenoids | 510 ± 8 | 143.0 ± 0.3 c | 120.0 ± 0.6 d | 179 ± 2 b | 199.0 ± 0.4 a | 178 ± 1 b |
| β-carotene | 432 ± 6 | 122.0 ± 0.3 c | 101.0 ± 0.2 d | 152.0 ± 0.8 b | 168 ± 2 a | 151.0 ± 0.4 b |
| Lycopene | 88 ± 2 | 27.0 ± 0.2 c | 23.0 ± 0.4 d | 31.0 ± 0.4 b | 34.0 ± 0.4 a | 35.1 ± 0.8 a |
| Fatty acids (mg/g) | ||||||
| Myristic acid (C14:0) | 3.1 ± 0.2 | 2.1 ± 0.1 | 2.1 ± 0.1 | 1.5 ± 0.1 | 2.1 ± 0.1 | 2.1 ± 0.1 |
| Pentadecanoic acid (C15:0) | 1.5 ± 0.1 | 0.8 ± 0.1 | 1.01 ± 0.03 | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.01 ± 0.01 |
| Palmitic acid (C16:0) | 92 ± 2 | 16.2 ± 1.5 | 19 ± 1 | 12 ± 1 | 23 ± 1 | 28.1 ± 0.5 |
| Palmitoleic acid (C16:1) | 58 ± 1 | 12 ± 1 | 13 ± 1 | 8 ± 1 | 16 ± 3 | 20.1 ± 0.4 |
| Stearic acid (C18:0) | 15.1 ± 0.7 | 4 ± 1 | 4.0 ± 0.5 | 4 ± 1 | 5 ± 1 | 6 ± 1 |
| Oleic acid (C18:1) | 77 ± 1 | 17 ± 2 | 20 ± 2 | 12 ± 1 | 23 ± 2 | 30 ± 2 |
| Linoleic acid (C18:2) | 158 ± 1 | 16 ± 1 | 34 ± 2 | 21 ± 2 | 22 ± 2 | 46 ± 1 |
| γ-Linolenic acid (C18:3) | 115.0 ± 2.3 | 18 ± 2 | 22 ± 2 | 14 ± 1 | 14 ± 1 | 31 ± 2 |
| Gondoic acid (C20:1) | 1.5 ± 0.1 | 0.40 ± 0.01 | 0.4 ± 0.1 | 0.3 ± 0.1 | 0.5 ± 0.1 | 0.61 ± 0.03 |
| cis-11,14-Eicosadienoic acid (C20:2) | 1.1 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.71 ± 0.05 | 0.8 ± 0.1 | 0.8 ± 0.1 |
| cis-5,8,11,14,17-Eicosapentanoic acid (C20:5) | 1.01 ± 0.06 | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 |
| Tocopherols (µg/g) | ||||||
| α-tocopherol | 1040 ± 22 | 591 ± 12 | 80 ± 2 | 160 ± 5 | 161 ± 5 | 540 ± 11 |
| β-tocopherol | 1290 ± 16 | 101 ± 2 | 93 ± 3 | 57 ± 3 | 107 ± 2 | 163 ± 4 |
| γ-tocopherol | 20 ± 1 | 9 ± 1 | 9 ± 1 | 4 ± 1 | 11 ± 1 | 14 ± 1 |
| Phytosterols (% from total peak) | ||||||
| Campesterol | 2.1 ± 0.2 | 2.1 ± 0.2 | 2.1 ± 0.3 | 2.0 ± 0.1 | 2.0 ± 0.1 | 2.1 ± 0.4 |
| β-Sitosterol | 96 ± 2 | 95 ± 3 | 95 ± 3 | 96 ± 4 | 95 ± 3 | 95 ± 3 |
| β-Amyrin | 1.0 ± 0.1 | 1.1 ± 0.1 | 1.1 ± 0.1 | 1.0 ± 0.1 | 1.1 ± 0.1 | 1.0 ± 0.1 |
| α-Amyrin | 1.1 ± 0.1 | 2.0 ± 0.3 | 2.0 ± 0.1 | 2.0 ± 0.1 | 2.1 ± 0.1 | 2.1 ± 0.1 |
| Antioxidant activity (mMol TEAC/g DW) | ||||||
| 32.2 ± 0.2 | 12 ± 1 ab | 11.1 ± 0.1 b | 11.2 ± 0.1 b | 14 ± 1 ab | 14.0 ± 0.3 a | |
| Microencapsulation Efficiency (%) | Microencapsulated Variants | ||||
|---|---|---|---|---|---|
| V1 | V2 | V3 | V4 | V5 | |
| Total carotenoids | 88.5 ± 0.1 d | 91.5 ± 0.1 a | 87.1 ± 0.1 e | 89.3 ± 0.1 b | 88.5 ± 0.1 c |
| β-carotene | 87.1 ± 0.2 cd | 91 ± 1 a | 87.1 ± 0.1 d | 89.3 ± 0.1 b | 88.4 ± 0.2 c |
| Lycopene | 82.2 ± 0.5 b | 82 ± 1 b | 81.2 ± 0.2 c | 86.5 ± 0.7 a | 83.1 ± 0.7 b |
| Enzyme | IC50 (µg/mL) | ||||
|---|---|---|---|---|---|
| V1 | V2 | V3 | V4 | V5 | |
| α-amylase | 19.1 ± 0.4 b | 0 | 25 ± 2 ab | 28.0 ± 0.2 a | 29.0 ± 0.3 a |
| Lipase | 31.1 ± 0.3 bc | 30.1 ± 0.2 bc | 30 ± 1 c | 31 ± 1 ab | 32.0 ± 0.3 a |
| Lipoxygenase | 33.1 ± 0.1 b | 36.1 ± 0.3 a | 26 ± 1 c | 37 ± 1 a | 23.0 ± 0.1 c |
| Parameter | Microencapsulated Variants | ||||
|---|---|---|---|---|---|
| V1 | V2 | V3 | V4 | V5 | |
| BD (kg/m3) | 118 ± 1 a | 83 ± 2 c | 91 ± 1 b | 62 ± 7 d | 71 ± 5 d |
| TD (kg/m3) | 193 ± 8 a | 132 ± 2 d | 196 ± 8 a | 150 ± 20 c | 177 ± 11 b |
| CI | 38 ± 3 c | 32 ± 1 d | 54 ± 1 b | 58 ± 2 a | 60.1 ± 0.5 a |
| HR | 2.0 ± 0.1 c | 2.01 ± 0.02 c | 2.0 ± 0.1 b | 2.0 ± 0.1 a | 2.01 ± 0.02 a |
| aw | 0.118 ± 0.002 b | 0.084 ± 0.03 e | 0.104 ± 0.009 c | 0.241 ± 0.003 a | 0.088 ± 0.001 d |
| Moisture content (%) | 7 ± 1 b | 7.0 ± 0.5 b | 6.0 ± 0.3 c | 8 ± 1 a | 9 ± 1 a |
| Solubility (%) | 71 ± 6 a | 34 ± 1 d | 60 ± 0.00 c | 33.00 ± 0.00 d | 66 ± 4 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mihalcea, L.; Aprodu, I.; Dumitrașcu, L.; Cucolea, E.I.; Dănilă, G.-M.; Enachi, E.; Barbu, V.; Constantin, O.E.; Grigore-Gurgu, L.; Stănciuc, N. Whey Proteins Isolate-Based Biopolymeric Combinations to Microencapsulate Supercritical Fluid Extracted Oleoresins from Sea Buckthorn Pomace. Pharmaceuticals 2021, 14, 1217. https://doi.org/10.3390/ph14121217
Mihalcea L, Aprodu I, Dumitrașcu L, Cucolea EI, Dănilă G-M, Enachi E, Barbu V, Constantin OE, Grigore-Gurgu L, Stănciuc N. Whey Proteins Isolate-Based Biopolymeric Combinations to Microencapsulate Supercritical Fluid Extracted Oleoresins from Sea Buckthorn Pomace. Pharmaceuticals. 2021; 14(12):1217. https://doi.org/10.3390/ph14121217
Chicago/Turabian StyleMihalcea, Liliana, Iuliana Aprodu, Loredana Dumitrașcu, Elena Iulia Cucolea, George-Mădălin Dănilă, Elena Enachi, Vasilica Barbu, Oana Emilia Constantin, Leontina Grigore-Gurgu, and Nicoleta Stănciuc. 2021. "Whey Proteins Isolate-Based Biopolymeric Combinations to Microencapsulate Supercritical Fluid Extracted Oleoresins from Sea Buckthorn Pomace" Pharmaceuticals 14, no. 12: 1217. https://doi.org/10.3390/ph14121217
APA StyleMihalcea, L., Aprodu, I., Dumitrașcu, L., Cucolea, E. I., Dănilă, G.-M., Enachi, E., Barbu, V., Constantin, O. E., Grigore-Gurgu, L., & Stănciuc, N. (2021). Whey Proteins Isolate-Based Biopolymeric Combinations to Microencapsulate Supercritical Fluid Extracted Oleoresins from Sea Buckthorn Pomace. Pharmaceuticals, 14(12), 1217. https://doi.org/10.3390/ph14121217



.png)







