Simple Approach to Enhance Green Tea Epigallocatechin Gallate Stability in Aqueous Solutions and Bioavailability: Experimental and Theoretical Characterizations
Abstract
:1. Introduction
2. Results and Discussion
2.1. Approach to Stabilize EGCG in Aqueous Solution
2.2. Characterization of the Phenomena Involved in the Formula
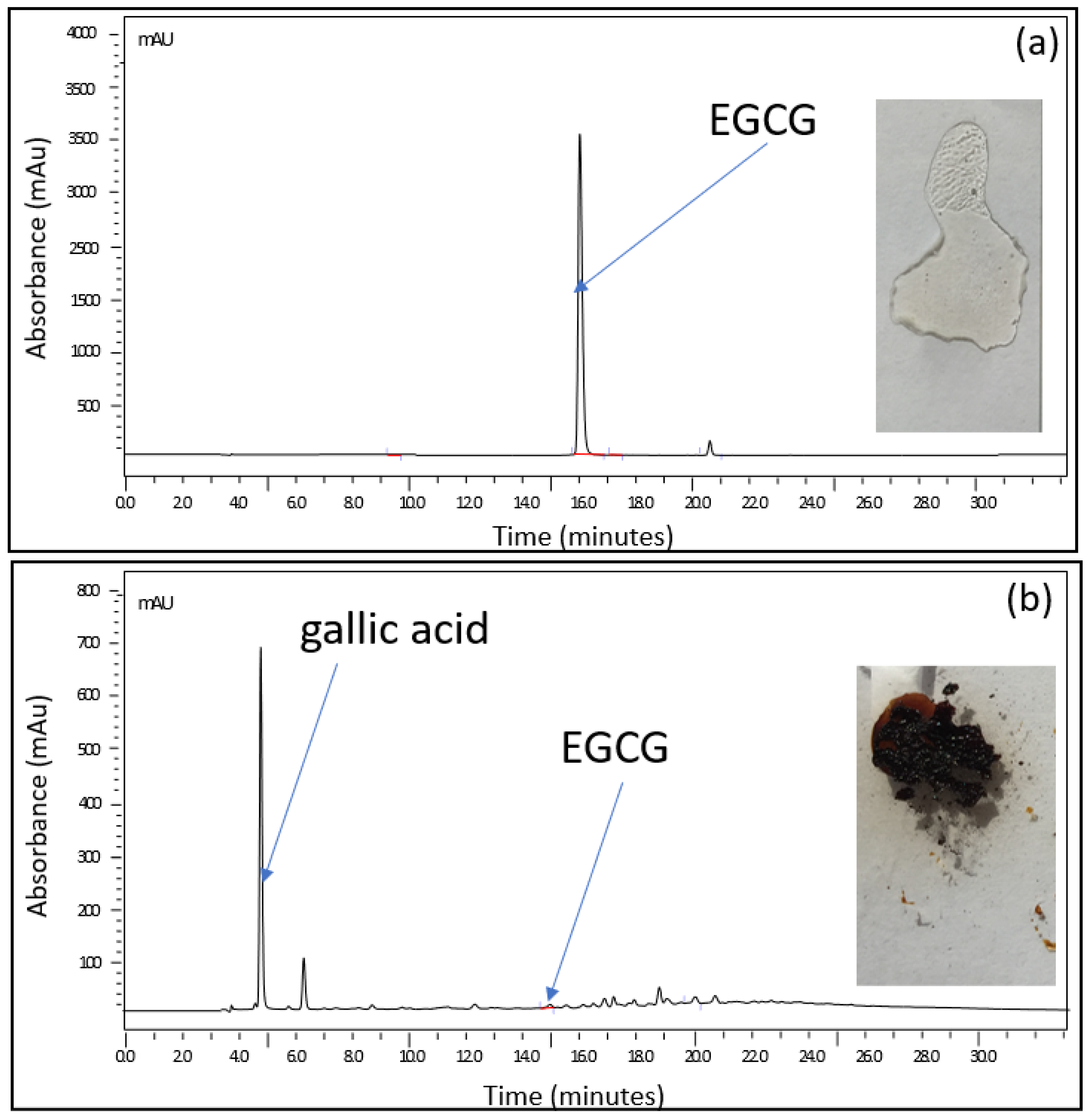
2.2.1. Experimental Studies of the EGCG–Sucrose Complex
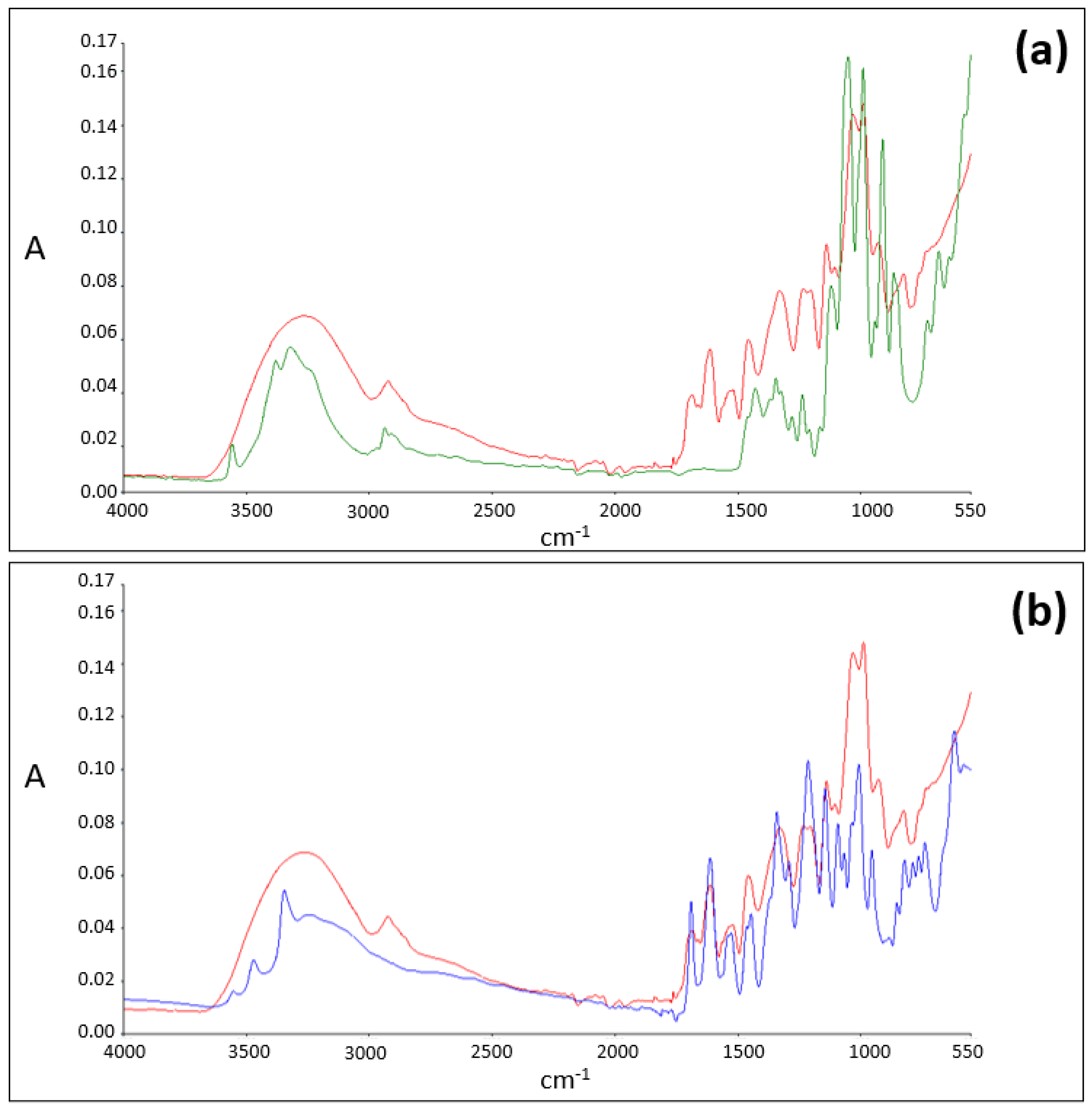
ATR-FTIR Analysis
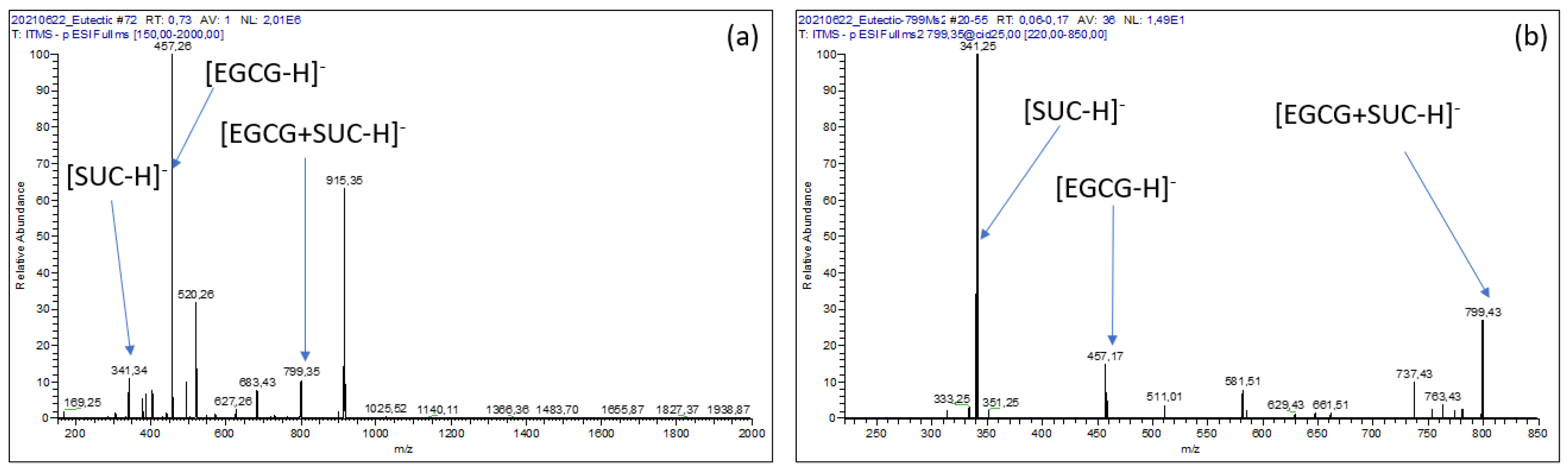
Mass Spectrometry Analysis
2.2.2. Theoretical Studies (DFT) of the EGCG–Sucrose Complex
Possible Interactions Sites of EGCG and Sucrose
Structures and Interaction Energies of Epigallocatechin Gallate–Sucrose Complexes
2.3. Stability of the Oral Formulation
2.3.1. Evolution of EGCG’s Content in the Formulation as a Function of Temperature
2.3.2. Investigation on Detected Degradation Products
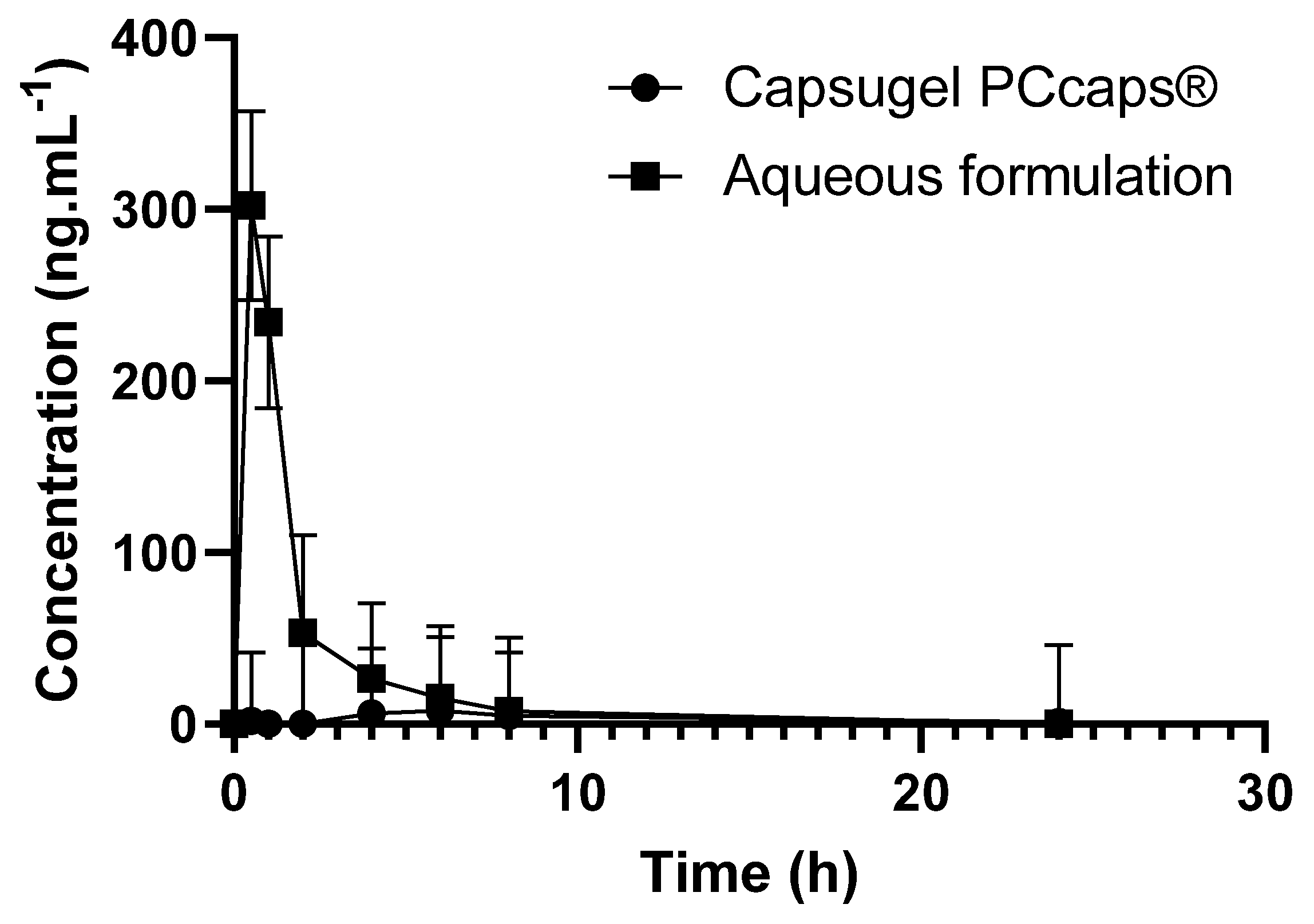
2.4. Bioavailability of the Formulation in Comparison to EGCG Solid Dosage Form (EGCG in Hard-Gel Capsule)
3. Materials and Methods
3.1. Materials and Reagents
3.2. Manufacturing Process
3.3. Long-Term and Accelerated Stability Protocol
3.4. Analytical Conditions
3.4.1. Infrared Conditions
3.4.2. Mass Spectrometry Conditions
3.4.3. Chromatographic Conditions
3.5. Computational
3.6. Bioavailability Studies
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, H.-S.; Quon, M.J.; Kim, J. New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox Biol. 2014, 2, 187–195. [Google Scholar] [CrossRef] [Green Version]
- Du, G.-J.; Zhang, Z.; Wen, X.-D.; Yu, C.; Calway, T.; Yuan, C.-S.; Wang, C.-Z. Epigallocatechin Gallate (EGCG) Is the Most Effective Cancer Chemopreventive Polyphenol in Green Tea. Nutrients 2012, 4, 1679–1691. [Google Scholar] [CrossRef]
- Higdon, J.V.; Frei, B. Tea Catechins and Polyphenols: Health Effects, Metabolism, and Antioxidant Functions. Crit. Rev. Food Sci. Nutr. 2003, 43, 89–143. [Google Scholar] [CrossRef] [PubMed]
- BSingh, N.; Shankar, S.; Srivastava, R.K. Green tea catechin, epigallocatechin-3-gallate (EGCG): Mechanisms, perspectives and clinical applications. Biochem. Pharmacol. 2011, 82, 1807–1821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chow, H.-H.S.; Hakim, I.A.; Vining, D.R.; Crowell, J.A.; Ranger-Moore, J.; Chew, W.M.; Celaya, C.A.; Rodney, S.R.; Hara, Y.; Alberts, D.S. Effects of Dosing Condition on the Oral Bioavailability of Green Tea Catechins after Single-Dose Administration of Polyphenon E in Healthy Individuals. Clin. Cancer Res. 2005, 11, 4627–4633. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.-Q.; Yu, P.; Zhou, W. Combined effect of pH and temperature on the stability and antioxidant capacity of epigallocatechin gallate (EGCG) in aqueous system. J. Food Eng. 2019, 250, 46–54. [Google Scholar] [CrossRef]
- Zeng, J.; Xu, H.; Cai, Y.; Xuan, Y.; Liu, J.; Gao, Y.; Luan, Q. The Effect of Ultrasound, Oxygen and Sunlight on the Stability of (−)-Epigallocatechin Gallate. Molecules 2018, 23, 2394. [Google Scholar] [CrossRef] [Green Version]
- Krupkova, O.; Ferguson, S.J.; Wuertz-Kozak, K. Stability of (−)-epigallocatechin gallate and its activity in liquid formulations and delivery systems. J. Nutr. Biochem. 2016, 37, 1–12. [Google Scholar] [CrossRef]
- Sang, S.; Lambert, J.D.; Ho, C.-T.; Yang, C.S. The chemistry and biotransformation of tea constituents. Pharmacol. Res. 2011, 64, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.-Y.; Li, X.-M.; Liang, J.-P.; Xiang, L.-P.; Wang, K.-R.; Shi, Y.-L.; Yang, R.; Shi, M.; Ye, J.-H.; Lu, J.-L.; et al. Bioavailability of Tea Catechins and Its Improvement. Molecules 2018, 23, 2346. [Google Scholar] [CrossRef] [Green Version]
- Paliwal, R.; Babu, R.J.; Palakurthi, S. Nanomedicine Scale-up Technologies: Feasibilities and Challenges. AAPS PharmSciTech 2014, 15, 1527–1534. [Google Scholar] [CrossRef] [Green Version]
- Do, B.; Paul, M.; Astier, A. Epigallocathechin Gallate Solution, US20200375939A1. 2020. Available online: https://patents.google.com/patent/US20200375939A1/en?q=EPIGALLOCATHECHIN+GALLATE+SOLUTION&oq=EPIGALLOCATHECHIN+GALLATE+SOLUTION (accessed on 24 October 2021).
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant Polyphenols: Chemical Properties, Biological Activities, and Synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef]
- Henning, S.M.; Choo, J.J.; Heber, D. Nongallated Compared with Gallated Flavan-3-ols in Green and Black Tea Are More Bioavailable. J. Nutr. 2008, 138, 1529S–1534S. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, M.; Yamazaki, S.; Kano, K.; Ikeda, T. Kinetic analysis and mechanistic aspects of autoxidation of catechins. Biochim. Biophys. Acta BBA-Gen. Subj. 2002, 1569, 35–44. [Google Scholar] [CrossRef]
- Hagerman, A.E.; Dean, R.T.; Davies, M.J. Radical chemistry of epigallocatechin gallate and its relevance to protein damage. Arch. Biochem. Biophys. 2003, 414, 115–120. [Google Scholar] [CrossRef]
- Wang, R.; Zhou, W.; Jiang, X. Reaction Kinetics of Degradation and Epimerization of Epigallocatechin Gallate (EGCG) in Aqueous System over a Wide Temperature Range. J. Agric. Food Chem. 2008, 56, 2694–2701. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Sano, M.; Yoshida, R.; Degawa, M.; Miyase, T.; Maeda-Yamamoto, M. Epimerization of Tea Catechins and O-Methylated Derivatives of (−)-Epigallocatechin-3-O-gallate: Relationship between Epimerization and Chemical Structure. J. Agric. Food Chem. 2003, 51, 510–514. [Google Scholar] [CrossRef]
- Donaldson, M.; Goodchild, J.H.; Epstein, J.B. Sugar content, cariogenicity, and dental concerns with commonly used medications. J. Am. Dent. Assoc. 2015, 146, 129–133. [Google Scholar] [CrossRef]
- Pal, Y.; Deb, P.K.; Bandopadhyay, S.; Bandyopadhyay, N.; Tekade, R.K. Role of Physicochemical Parameters on Drug Absorption and Their Implications in Pharmaceutical Product Development. In Dosage Form Design Considerations; Elsevier: Amsterdam, The Netherlands, 2018; pp. 85–116. [Google Scholar] [CrossRef]
- Balbani, A.P.S.; Stelzer, L.B.; Montovani, J.C. Pharmaceutical excipients and the information on drug labels. Braz. J. Otorhinolaryngol. 2006, 72, 400–406. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.A.; Rahman, M.S.; Roy, R.; Gambill, P.; Raynie, D.E.; Halim, M.A. Structure Elucidation of Menthol-Based Deep Eutectic Solvent using Experimental and Computational Techniques. J. Phys. Chem. A 2021, 125, 2402–2412. [Google Scholar] [CrossRef]
- Dotsikas, Y.; Loukas, Y.L. Efficient determination and evaluation of model cyclodextrin complex binding constants by electrospray mass spectrometry. J. Am. Soc. Mass Spectrom. 2003, 14, 1123–1129. [Google Scholar] [CrossRef] [Green Version]
- Kwon, S.; Lee, W.; Shin, H.-J.; Yoon, S.; Kim, Y.; Kim, Y.-J.; Lee, K.; Lee, S. Characterization of cyclodextrin complexes of camostat mesylate by ESI mass spectrometry and NMR spectroscopy. J. Mol. Struct. 2009, 938, 192–197. [Google Scholar] [CrossRef]
- Li, H.; Zhou, J.; Tang, F.; Yuan, G. Investigation of noncovalent complexes between β-cyclodextrin and polyamide acids containing N-methylpyrrole and N-methylimidazole by electrospray ionization mass spectrometry. J. Am. Soc. Mass Spectrom. 2006, 17, 9–14. [Google Scholar] [CrossRef] [Green Version]
- Murray, J.S.; Seminario, J.M.; Politzer, P.; Sjoberg, P. Average local ionization energies computed on the surfaces of some strained molecules. Int. J. Quantum Chem. 1990, 38, 645–653. [Google Scholar] [CrossRef]
- Armaković, S.; Armaković, S.J.; Šetrajčić, J.P.; Šetrajčić, I.J. Active components of frequently used β-blockers from the aspect of computational study. J. Mol. Model. 2012, 18, 4491–4501. [Google Scholar] [CrossRef]
- Zheng, K.; Guo, K.; Xu, J.; Liu, W.; Chen, J.; Xu, C.; Chen, L. Study on the interaction between catechin and cholesterol by the density functional theory. Open Chem. 2020, 18, 357–368. [Google Scholar] [CrossRef]
- Rozenberg, M.; Loewenschuss, A.; Marcus, Y. An empirical correlation between stretching vibration redshift and hydrogen bond length. Phys. Chem. Chem. Phys. 2000, 2, 2699–2702. [Google Scholar] [CrossRef]
- Cossi, M.; Barone, V.; Cammi, R.; Tomasi, J. Ab initio study of solvated molecules: A new implementation of the polarizable continuum model. Chem. Phys. Lett. 1996, 255, 327–335. [Google Scholar] [CrossRef]
- Shpigelman, A.; Zisapel, A.; Cohen, Y.; Livney, Y.D. Mechanisms of saccharide protection against epigallocatechin-3-gallate deterioration in aqueous solutions. Food Chem. 2013, 139, 1105–1112. [Google Scholar] [CrossRef]
- Lin, L.-C.; Wang, M.-N.; Tseng, T.-Y.; Sung, J.S.; Tsai, T.-H. Pharmacokinetics of (−)-Epigallocatechin-3-gallate in Conscious and Freely Moving Rats and Its Brain Regional Distribution. J. Agric. Food Chem. 2007, 55, 1517–1524. [Google Scholar] [CrossRef]
- Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. Drug Solubility: Importance and Enhancement Techniques. ISRN Pharm. 2012, 2012, 195727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jodoin, J.; Demeule, M.; Béliveau, R. Inhibition of the multidrug resistance P-glycoprotein activity by green tea polyphenols. Biochim. Biophys. Acta BBA-Mol. Cell Res. 2002, 1542, 149–159. [Google Scholar] [CrossRef] [Green Version]
- Bochevarov, A.D.; Harder, E.; Hughes, T.F.; Greenwood, J.R.; Braden, D.A.; Philipp, D.M.; Rinaldo, D.; Halls, M.D.; Zhang, J.; Friesner, R.A. Jaguar: A high-performance quantum chemistry software program with strengths in life and materials sciences. Int. J. Quantum Chem. 2013, 113, 2110–2142. [Google Scholar] [CrossRef]
- Theilacker, K.; Arbuznikov, A.V.; Bahmann, H.; Kaupp, M. Evaluation of a Combination of Local Hybrid Functionals with DFT-D3 Corrections for the Calculation of Thermochemical and Kinetic Data. J. Phys. Chem. A 2011, 115, 8990–8996. [Google Scholar] [CrossRef] [PubMed]
- Mata-Bilbao, M.D.; Andrés-Lacueva, C.; Roura, E.; Jáuregui, O.; Torre, C.; Lamuela-Raventós, R.M. A New LC/MS/MS Rapid and Sensitive Method for the Determination of Green Tea Catechins and their Metabolites in Biological Samples. J. Agric. Food Chem. 2007, 55, 8857–8863. [Google Scholar] [CrossRef] [PubMed]




| Percentage Formula (% w/w) | |
|---|---|
| EGCG | 2.68 |
| Citric acid | 0.05 |
| Glucose | 23.4 |
| Sucrose | 10.0 |
| Cola flavor | 0.6 |
| Water for injection | ad 100% |
| Complex | Interaction Energy (Vacuum) | Interaction Energy (Water) | R (O-----H)1 | R (O-----H)2 | R (O-----H)3 | Interaction Energy due to H-Bonding (%) | Solvation Energy |
|---|---|---|---|---|---|---|---|
| 1 | −170.24536 | −130.99289 | 1.85 (23) | 1.87 (22) | 1.95 (19) | 37.6 | −140.2895 |
| 2 | −192.98007 | −139.20560 | 1.85 (23) | 2.34 (11) | 3.92 (2.3) | 18.8 | −137.1217 |
| 3 | −250.44187 | −196.03839 | 1.9 (21) | 1.95 (19) | 3.28 (4.0) | 17.6 | −137.7863 |
| Time (min) | A (%) | B (%) |
|---|---|---|
| 0 | 90 | 10 |
| 10 | 80 | 20 |
| 16 | 60 | 40 |
| 20 | 50 | 50 |
| 25 | 50 | 50 |
| 27 | 60 | 40 |
| 30 | 90 | 10 |
| 33 | 90 | 10 |
| 45 | 90 | 10 |
| Reference Compounds | Route of Administration | Vehicle | Concentration (mg/mL) | Volume of Administration (mL/kg) | Selected Dose (mg/kg) | Plasma Sampling Times |
|---|---|---|---|---|---|---|
| EGCG | PO | Administration via 1 PCcaps® | - | - | 27 | 30 min, 1 h, 2 h, 4 h, 6 h, 8 h, 24 h |
| EGCG | PO | Formulation described in Table 1 | 27 | 1 | 27 | 30 min, 1 h, 2 h, 4 h, 6 h, 8 h, 24 h |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Secretan, P.-H.; Thirion, O.; Sadou Yayé, H.; Damy, T.; Astier, A.; Paul, M.; Do, B. Simple Approach to Enhance Green Tea Epigallocatechin Gallate Stability in Aqueous Solutions and Bioavailability: Experimental and Theoretical Characterizations. Pharmaceuticals 2021, 14, 1242. https://doi.org/10.3390/ph14121242
Secretan P-H, Thirion O, Sadou Yayé H, Damy T, Astier A, Paul M, Do B. Simple Approach to Enhance Green Tea Epigallocatechin Gallate Stability in Aqueous Solutions and Bioavailability: Experimental and Theoretical Characterizations. Pharmaceuticals. 2021; 14(12):1242. https://doi.org/10.3390/ph14121242
Chicago/Turabian StyleSecretan, Philippe-Henri, Olivier Thirion, Hassane Sadou Yayé, Thibaud Damy, Alain Astier, Muriel Paul, and Bernard Do. 2021. "Simple Approach to Enhance Green Tea Epigallocatechin Gallate Stability in Aqueous Solutions and Bioavailability: Experimental and Theoretical Characterizations" Pharmaceuticals 14, no. 12: 1242. https://doi.org/10.3390/ph14121242
APA StyleSecretan, P.-H., Thirion, O., Sadou Yayé, H., Damy, T., Astier, A., Paul, M., & Do, B. (2021). Simple Approach to Enhance Green Tea Epigallocatechin Gallate Stability in Aqueous Solutions and Bioavailability: Experimental and Theoretical Characterizations. Pharmaceuticals, 14(12), 1242. https://doi.org/10.3390/ph14121242






