3D Printing of Temporary Prostheses for Controlled-Release of Drugs: Design, Physical Characterization and Preliminary Studies
Abstract
:1. Introduction
2. Results and Discussion
2.1. Design of the Spacers
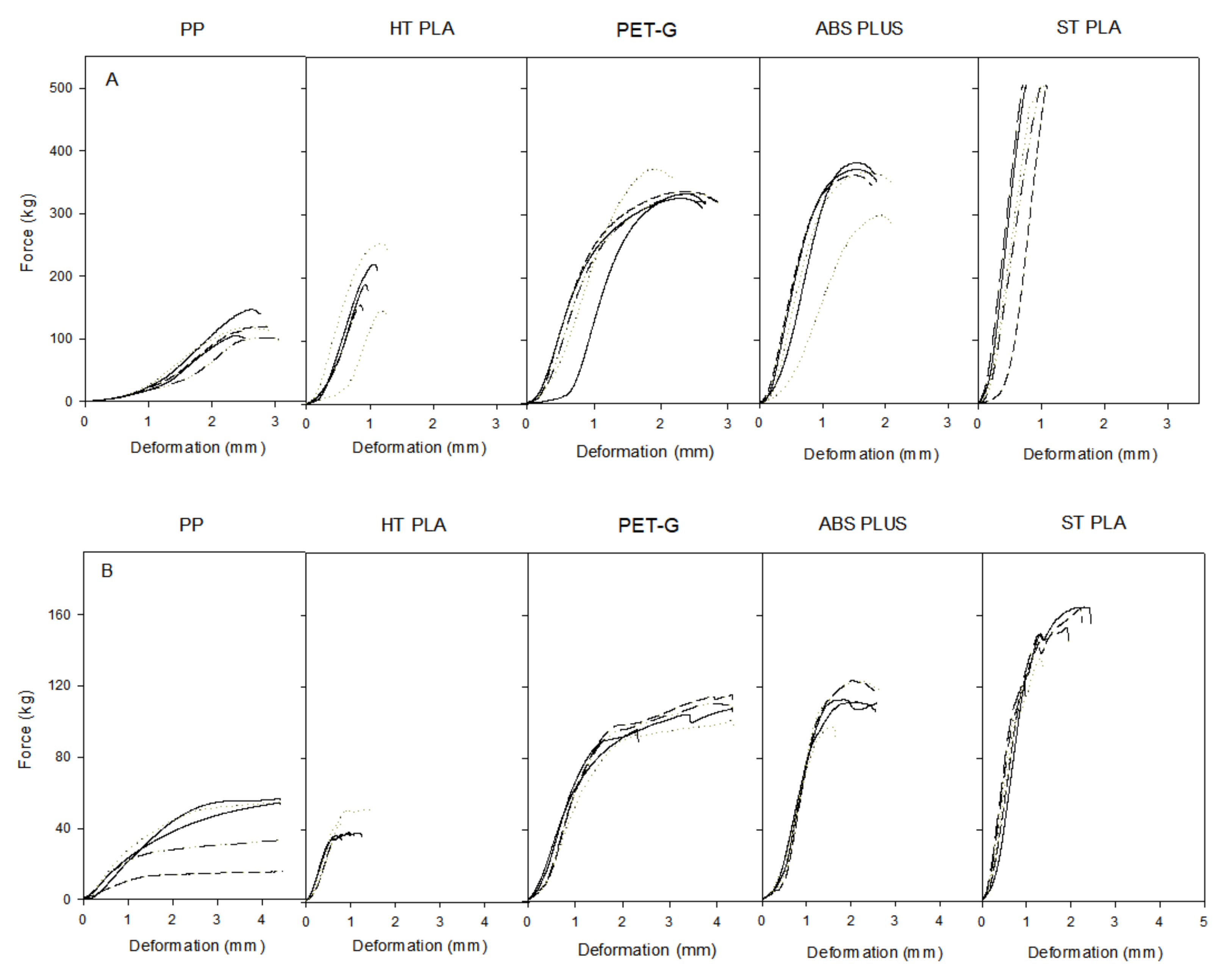
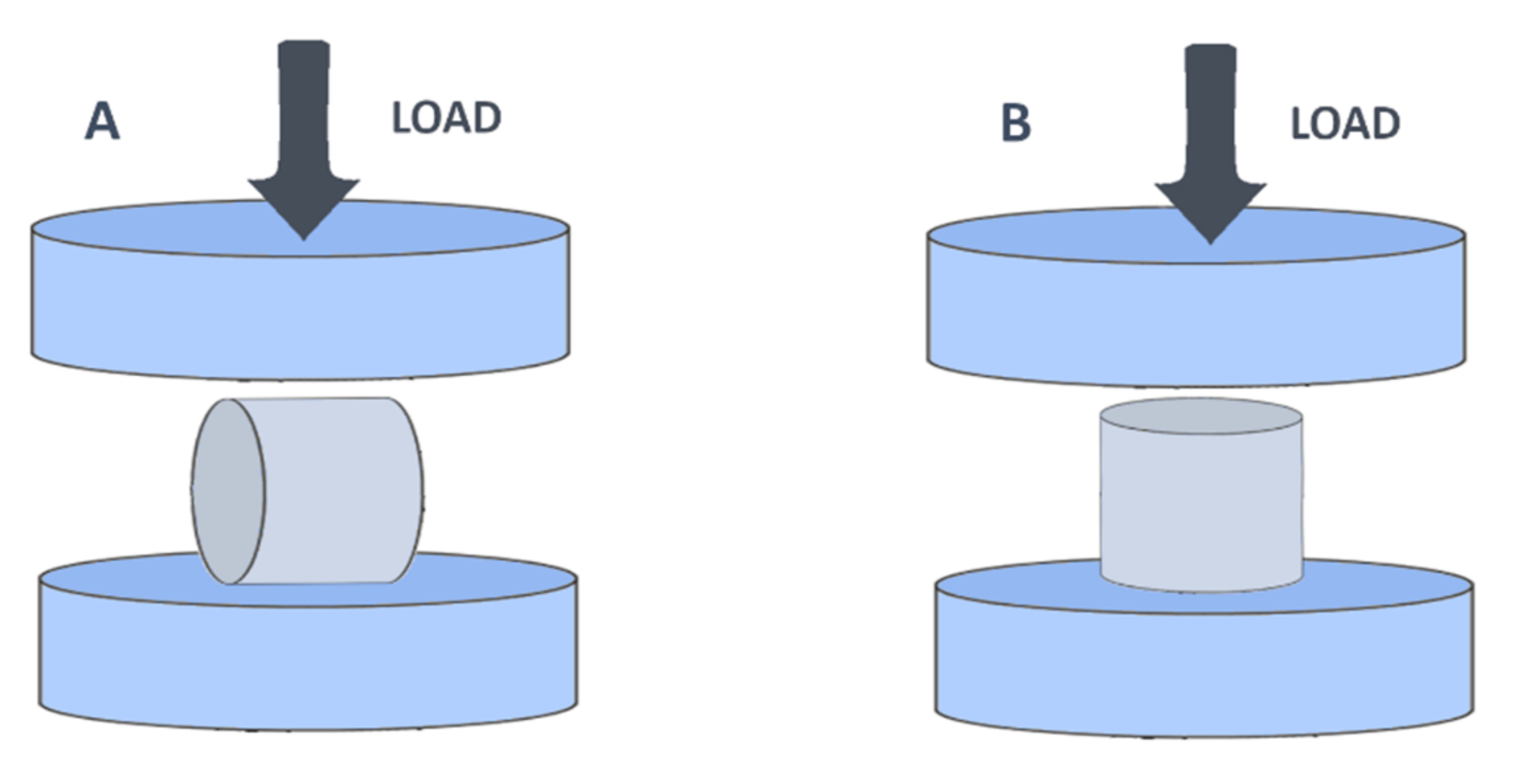
2.2. Material Strength Assessment
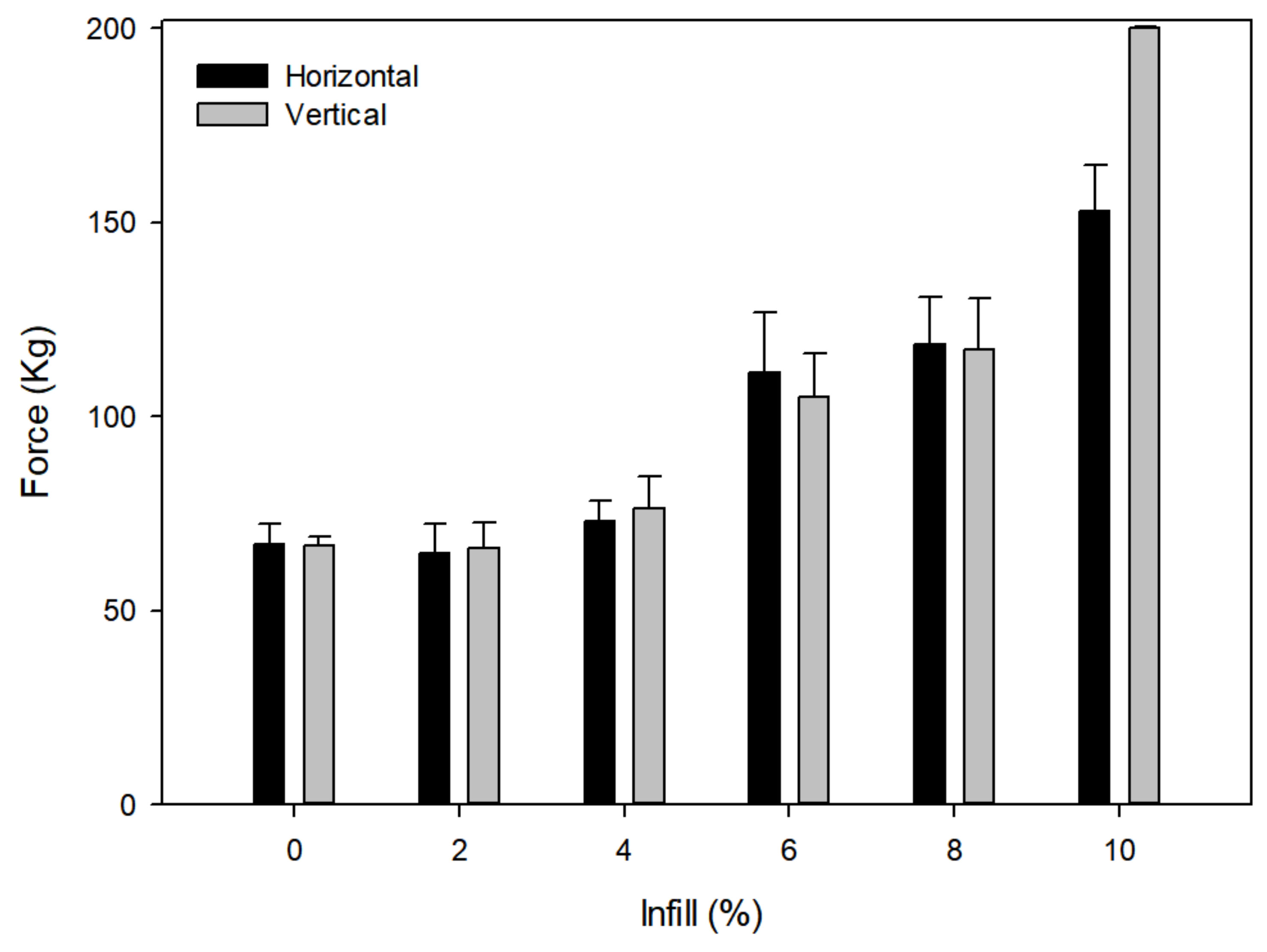
2.3. Influence of Infill on Strength
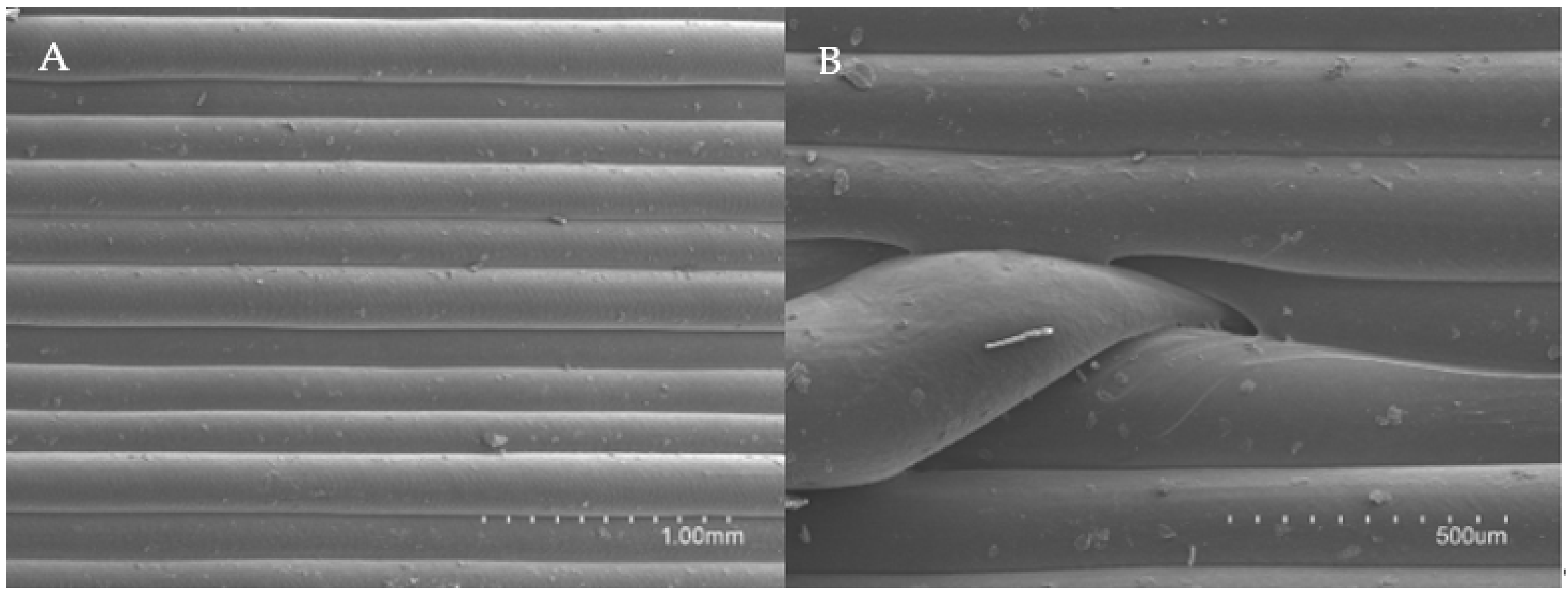
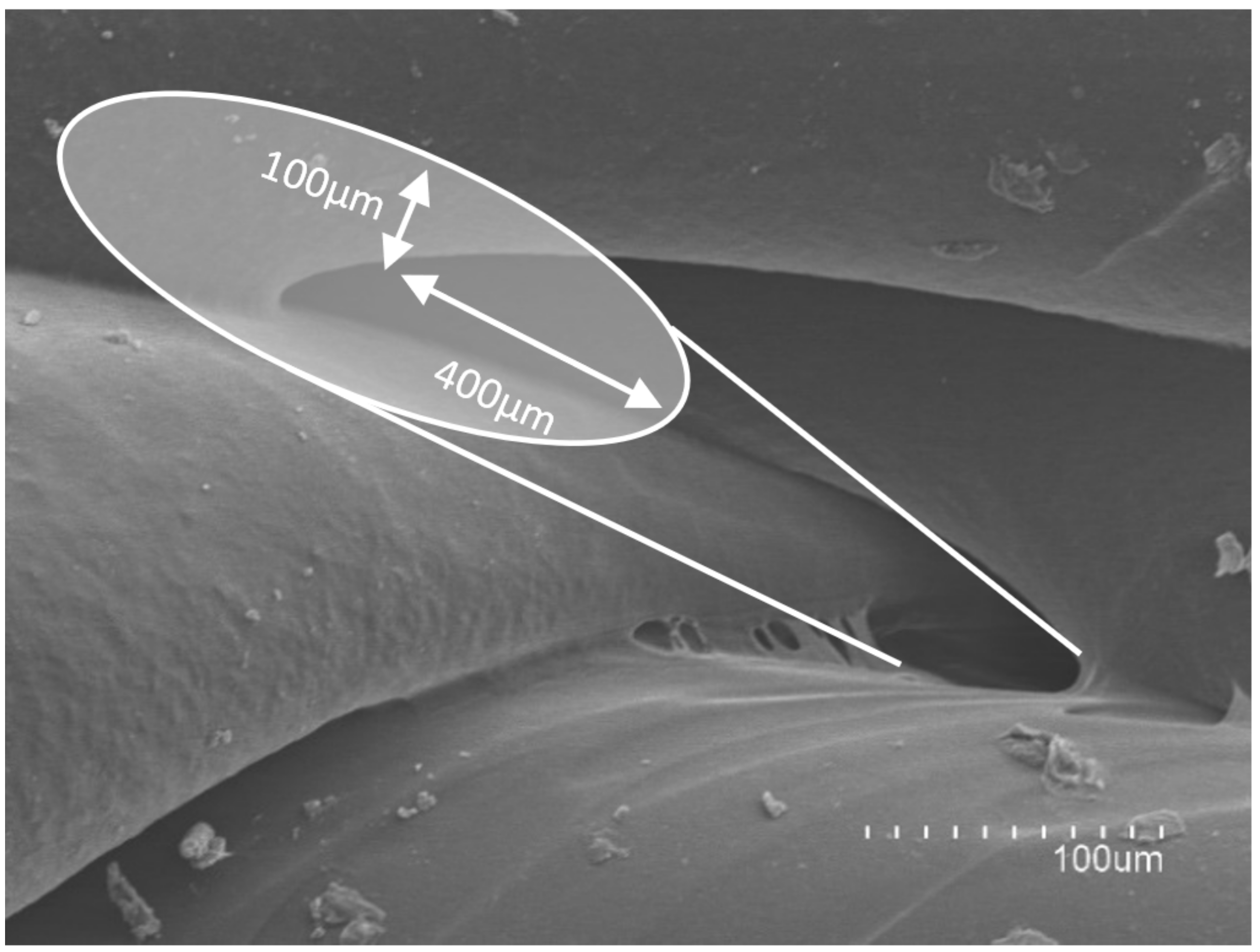
2.4. Release Studies
3. Materials and Methods
3.1. Materials
3.2. Design and 3D Printing
3.3. Physical Characterizations
3.4. In Vitro Release Studies
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Veerachamy, S.; Yarlagadda, T.; Manivasagam, G.; Yarlagadda, P.K. Bacterial adherence and biofilm formation on medical implants: A review. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2014, 228, 1083–1099. [Google Scholar] [CrossRef] [PubMed]
- Zardi, E.M.; Franceschi, F. Prosthetic joint infection. A relevant public health issue. J. Infect. Public Health 2020, 13, 1888–1891. [Google Scholar] [CrossRef]
- Pavithra, D.; Doble, M. Biofilm formation, bacterial adhesion and host response on polymeric implants—Issues and prevention. Biomed. Mater. 2008, 3, 034003. [Google Scholar] [CrossRef] [PubMed]
- Del Pozo, J.L.; Patel, R. Infection Associated with Prosthetic Joints. N. Engl. J. Med. 2009, 361, 787–794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tande, A.J.; Patel, R. Prosthetic joint infection. Clin. Microbiol. Rev. 2014, 27, 302–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marín, M.; Esteban, J.; Meseguer, M.A.; Sánchez-Somolinos, M. Diagnóstico microbiológico de las infecciones osteoarticulares. Enferm. Infecc. Microbiol. Clin. 2010, 28, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Rosteius, T.; Jansen, O.; Fehmer, T.; Baecker, H.; Citak, M.; Schildhauer, T.A.; Geßmann, J. Evaluating the microbial pattern of periprosthetic joint infections of the hip and knee. J. Med. Microbiol. 2018, 67, 1608–1613. [Google Scholar] [CrossRef] [PubMed]
- Zimmerli, W. Clinical presentation and treatment of orthopaedic implant-associated infection. J. Intern. Med. 2014, 276, 111–119. [Google Scholar] [CrossRef]
- Winkler, H. Treatment of chronic orthopaedic infection. EFORT Open Rev. 2017, 2, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Charette, R.S.; Melnic, C.M. Two-Stage Revision Arthroplasty for the Treatment of Prosthetic Joint Infection. Curr. Rev. Musculoskelet. Med. 2018, 11, 332–340. [Google Scholar] [CrossRef]
- Kuzyk, P.R.T.; Dhotar, H.S.; Sternheim, A.; Gross, A.E.; Safir, O.; Backstein, D. Two-stage Revision Arthroplasty for Management of Chronic Periprosthetic Hip and Knee Infection. J. Am. Acad. Orthop. Surg. 2014, 22, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Bistolfi, A.; Massazza, G.; Verné, E.; Massè, A.; Deledda, D.; Ferraris, S.; Miola, M.; Galetto, F.; Crova, M. Antibiotic-loaded cement in orthopedic surgery: A review. ISRN Orthop. 2011, 2011, 290851. [Google Scholar] [CrossRef]
- Struelens, B.; Claes, S.; Bellemans, J. Spacer-related problems in two-stage revision knee arthroplasty. Acta Orthop. Belg. 2013, 79, 422–426. [Google Scholar] [PubMed]
- Wall, V.; Nguyen, T.-H.; Nguyen, N.; Tran, P.A. Controlling Antibiotic Release from Polymethylmethacrylate Bone Cement. Biomedicines 2021, 9, 26. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.-C.; Su, C.-Y.; Nien, W.-H.; Huang, T.-T.; Huang, C.-H.; Lu, Y.-C.; Chen, Y.-J.; Huang, G.-C.; Fang, H.-W. Influence of Antibiotic-Loaded Acrylic Bone Cement Composition on Drug Release Behavior and Mechanism. Polymers 2021, 13, 2240. [Google Scholar] [CrossRef]
- Dunne, N.J.; Hill, J.; McAfee, P.; Kirkpatrick, R.; Patrick, S.; Tunney, M. Incorporation of large amounts of gentamicin sulphate into acrylic bone cement: Effect on handling and mechanical properties, antibiotic release, and biofilm formation. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2008, 222, 355–365. [Google Scholar] [CrossRef]
- Ince, A.; Schütze, N.; Karl, N.; Löhr, J.F.; Eulert, J. Gentamicin negatively influenced osteogenic function in vitro. Int. Orthop. 2007, 31, 223–228. [Google Scholar] [CrossRef] [Green Version]
- James, A.; Larson, T. Acute renal failure after high-dose antibiotic bone cement: Case report and review of the literature. Ren. Fail. 2015, 37, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Bagaria, V.; Bhansali, R.; Pawar, P. 3D printing- creating a blueprint for the future of orthopedics: Current concept review and the road ahead! J. Clin. Orthop. Trauma 2018, 9, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Lal, H.; Patralekh, M.K. 3D printing and its applications in orthopaedic trauma: A technological marvel. J. Clin. Orthop. Trauma 2018, 9, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Manganaro, M.S.; Morag, Y.; Weadock, W.J.; Yablon, C.M.; Gaetke-Udager, K.; Stein, E.B. Creating Three-dimensional Printed Models of Acetabular Fractures for Use as Educational Tools. Radiographics 2017, 37, 871–880. [Google Scholar] [CrossRef]
- Ryan, J.R.; Almefty, K.K.; Nakaji, P.; Frakes, D.H. Cerebral Aneurysm Clipping Surgery Simulation Using Patient-Specific 3D Printing and Silicone Casting. World Neurosurg. 2016, 88, 175–181. [Google Scholar] [CrossRef]
- Liu, Z.; Jia, J.; Zhang, Y.; Tian, W.; Jin, X.; Hu, Y. Internal Fixation of Complicated Acetabular Fractures Directed by Preoperative Surgery with 3D Printing Models. Orthop. Surg. 2017, 9, 257–260. [Google Scholar] [CrossRef] [Green Version]
- Mobbs, R.J.; Coughlan, M.; Thompson, R.; Sutterlin, C.E.; Phan, K. The utility of 3D printing for surgical planning and patient-specific implant design for complex spinal pathologies: Case report. J. Neurosurg. Spine 2017, 26, 513–518. [Google Scholar] [CrossRef] [Green Version]
- Kafle, A.; Luis, E.; Silwal, R.; Pan, H.M.; Shrestha, P.L.; Bastola, A.K. 3D/4D Printing of Polymers: Fused Deposition Modelling (FDM), Selective Laser Sintering (SLS), and Stereolithography (SLA). Polymers 2021, 13, 3101. [Google Scholar] [CrossRef] [PubMed]
- Goyanes, A.; Finab, F.; Martoranab, A.; Sedoughb, D.; Gaisfordab, S.W.; Basit, A. Development of modified release 3D printed tablets (printlets) with pharmaceutical excipients using additive manufacturing. Int. J. Pharm. 2017, 527, 21–30. [Google Scholar] [CrossRef]
- Tan, L.J.; Zhu, W.; Zhou, K. Recent Progress on Polymer Materials for Additive Manufacturing. Adv. Funct. Mater. 2020, 30, 2003062. [Google Scholar] [CrossRef]
- Weisman, J.A.; Ballard, D.H.; Jammalamadaka, U.; Tappa, K.; Sumerel, J.; D’Agostino, H.B.; Mills, D.K.; Woodard, P.K. 3D Printed Antibiotic and Chemotherapeutic Eluting Catheters for Potential Use in Interventional Radiology: In Vitro Proof of Concept Study. Acad. Radiol. 2019, 26, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Haigh, J.N.; Dargaville, T.R.; Dalton, P.D. Additive manufacturing with polypropylene microfibers. Mater. Sci. Eng. C 2017, 77, 883–887. [Google Scholar] [CrossRef] [Green Version]
- Cohen Tervaert, J.W. Autoinflammatory/autoimmunity syndrome induced by adjuvants (Shoenfeld’s syndrome) in patients after a polypropylene mesh implantation. Best Pract. Res. Clin. Rheumatol. 2018, 32, 511–520. [Google Scholar] [CrossRef]
- Haffner, M.; Quinn, A.; Hsieh, T.; Strong, E.B.; Steele, T. Optimization of 3D Print Material for the Recreation of Patient-Specific Temporal Bone Models. Ann. Otol. Rhinol. Laryngol. 2018, 127, 338–343. [Google Scholar] [CrossRef]
- Baqasah, H.; He, F.; Zai, B.A.; Asif, M.; Khan, K.A.; Thakur, V.K.; Khan, M.A. In-situ dynamic response measurement for damage quantification of 3D printed ABS cantilever beam under thermomechanical load. Polymers 2019, 11, 2079. [Google Scholar] [CrossRef] [Green Version]
- Beslikas, T.; Gigis, I.; Goulios, V.; Christoforides, J.; Papageorgiou, G.Z.; Bikiaris, D.N. Crystallization Study and Comparative in Vitro–in Vivo Hydrolysis of PLA Reinforcement Ligament. Int. J. Mol. Sci. 2011, 12, 6597–6618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weisman, J.A.; Nicholson, J.C.; Tappa, K.; Jammalamadaka, U.; Wilson, C.G.; Mills, D.K. Antibiotic and chemotherapeutic enhanced three-dimensional printer filaments and constructs for biomedical applications. Int. J. Nanomed. 2015, 10, 357–370. [Google Scholar] [CrossRef] [Green Version]
- Huynh, D.; Fadaee, N.; Al-Aufey, B.; Capati, I.; Towfigh, S. Robotic iliopubic tract (r-IPT) repair: Technique and preliminary outcomes of a minimally invasive tissue repair for inguinal hernia. Hernia 2020, 24, 1041–1047. [Google Scholar] [CrossRef]
- Rovner, E.; de Tayrac, R.; Kirschner-Hermanns, R.; Veit-Rubin, N.; Anding, R. Is polypropylene mesh material fundamentally safe for use as a reconstructive material in vaginal surgery: ICI-RS 2019? Neurourol. Urodyn. 2020, 39 (Suppl. 3), S132–S139. [Google Scholar] [CrossRef]
- Goyanes, A.; Buanz, A.B.M.; Basit, A.W.; Gaisford, S. Fused-filament 3D printing (3DP) for fabrication of tablets. Int. J. Pharm. 2014, 476, 88–92. [Google Scholar] [CrossRef]
- Crisan, A.G.; Porfile, A.; Ambrus, R.; Katona, G.; Rus, L.M.; Porav, A.S.; Iyés, K.; Tomuta, I. Polyvinyl Alcohol-Based 3D Printed Tablets: Novel Insight into the Influence of Polymer Particle Size on Filament Preparation and Drug Release Performance. Pharmaceuticals 2021, 14, 418. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release I. Fickian and non-fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J. Control. Release 1987, 5, 23–36. [Google Scholar] [CrossRef]
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Yao, C.; Xie, S. DDSolver: An add-in program for modeling and comparison of drug dissolution profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef] [Green Version]






| Design Iterations | Description/Comments | |
|---|---|---|
 | Design I was created with two external layers and a solid thick wall (100% infill). There was an internal chamber where the substance was loaded. | |
| Methylene blue was not released in the 3D constructs corresponding to the design I. This could be due to the number of solid layers between the internal chamber and the exterior. | ||
 | Design II was created with two external layers and a wall with infill of 20% and an internal chamber. The infill of the wall was created with a hexagonal pattern in order to improve resistance. | |
| In order to facilitate drug release in design II, the wall of the cylinder was printed with an infill of 20% instead of solid, but still methylene blue was not released. | ||
 | Design III maintained both the internal chamber and the 20% infill of the wall, as well as the hexagonal pattern, but was created with only one external layer in order to improve the release. | |
| Even though the wall thickness was reduced, methylene blue was not released. It seemed that having to overcome both the internal wall of the chamber and the external wall was stopping the release. | ||
 | In order to achieve release the internal chamber was removed and instead a number of hexagonal chambers were created with 20% infill using the hexagonal pattern. | |
| The result was a massive release of methylene blue as one external layer was proven to be insufficient without the internal chamber and the release was instantaneous. However, after the initial release very little methylene blue was being released. | ||
 | To avoid the first burst of release two external layers were printed instead of one and to increase the overall release infill was reduced to 10% using the hexagonal pattern. | |
| Release was inconsistent and erratic and although some diffusion was observed it was considered to be insufficient. This might be due to de hexagonal pattern creating separated volumes throughout the cylinder preventing a homogeneous internal flow between the chambers. | ||
 | In design VI the hexagonal pattern was substituted for a crisscrossed overlapping-beams (image on the right), which allows for a communicated internal space throughout the cylinder. The infill percentage was kept at 10% and it was printed with two external layers. |  |
| Methylene blue was released in a sustained manner over time so further research was conducted using design VI. | ||
| Material | Breaking Load Mean (Kg) | Compression Mean (mm) | Compression (%) |
|---|---|---|---|
| ABS | 338.76 ± 31.34 | 1.94 ± 0.16 | 6.48 ± 0.53 |
| PET-G | 339.83 ± 18.13 | 2.63 ± 0.26 | 8.77 ± 0.88 |
| PP | 114.04 ± 17.00 | 2.84 ± 0.21 | 9.48 ± 0.70 |
| Ht PLA | 184.06 ± 42.55 | 1.10 ± 0.17 | 3.68 ± 0.56 |
| St PLA | 500.00 ± 2.31 | 0.93 ± 0.17 | 3.08 ± 0.57 |
| Material | Breaking Load Mean (Kg) | Compression Mean (mm) | Compression (%) |
|---|---|---|---|
| ABS | 108.77 ± 10.35 | 2.40 ± 0.42 | 8.01 ± 1.42 |
| PET-G | 102.39 ± 9.04 | 3.93 ± 0.90 | 13.12 ± 2.99 |
| PP | 41.91 ± 17.64 | 4.42 ± 0.03 | 14.74 ± 0.09 |
| Ht PLA | 39.90 ± 6.11 | 1.07 ± 0.29 | 3.58 ± 0.98 |
| St PLA | 156.21 ± 9.57 | 1.80 ± 0.61 | 6.02 ± 2.03 |
| Model Equations | Methylene Blue Kinetics |
|---|---|
| Zero order | M (%) = 0.651 (±0.022) + 0.007 (±0.0005) t (r = 0.920, SS = 0.076) |
| First order | M (%) = 1.029 (± 0.031) (1 − e −0.436 (±0.058)t) (r = 0.824, SS = 0.311) |
| Higuchi | M (%) = 0.523 (±0.091) + 0.076 (± 0.012) t 0.5(r = 0.975, SS = 0.034) |
| Korsmeyer-Peppas | M (%) = 0.551 (±0.018) t 0.171 (±0.009) (r = 0.986, SS = 0.020) |
| Polymer | Provider | Density (g/cm3) | Extrusion Temperature (°C) | Printer Bed Temperature (°C) |
|---|---|---|---|---|
| Standard Polylactic Acid (St PLA) | BQ® (Huesca, Spain) | 1.24 | 205 | 50 |
| High Temperature Polylactic Acid (Ht PLA) | Orbi-tech® (Germany) | 1.5 | 220 | 70 |
| Polypropylene (PP) | León 3D® (León, Spain) | 0.9 | 195 | 90 |
| Acrylonitrile butadiene styrene (ABS) | León 3D® (León, Spain) | 1.04 | 240 | 85 |
| Polyethylene terephthalate (PET-G) | León 3D® (León, Spain) | 1.27 | 220 | 80 |
| Polymer | Base Printing Speed (mm/s) | Exterior Layer Printing Speed (mm/s) | Layer Heigh (mm) | First Layer Printing Heigh (mm) | First Layer Printing Speed (mm/s) |
|---|---|---|---|---|---|
| St PLA | 60 | 30 | 0.2 | 0.4 | 100 |
| Ht PLA | 45 | 20 | |||
| PP | 40 | 20 | |||
| ABS | 60 | 30 | |||
| PET-G | 45 | 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bueno-López, C.; Tamarit-Martínez, C.; Alambiaga-Caravaca, A.M.; Balaguer-Fernández, C.; Merino, V.; López-Castellano, A.; Rodilla, V. 3D Printing of Temporary Prostheses for Controlled-Release of Drugs: Design, Physical Characterization and Preliminary Studies. Pharmaceuticals 2021, 14, 1240. https://doi.org/10.3390/ph14121240
Bueno-López C, Tamarit-Martínez C, Alambiaga-Caravaca AM, Balaguer-Fernández C, Merino V, López-Castellano A, Rodilla V. 3D Printing of Temporary Prostheses for Controlled-Release of Drugs: Design, Physical Characterization and Preliminary Studies. Pharmaceuticals. 2021; 14(12):1240. https://doi.org/10.3390/ph14121240
Chicago/Turabian StyleBueno-López, Carlos, Carlos Tamarit-Martínez, Adrián M. Alambiaga-Caravaca, Cristina Balaguer-Fernández, Virginia Merino, Alicia López-Castellano, and Vicent Rodilla. 2021. "3D Printing of Temporary Prostheses for Controlled-Release of Drugs: Design, Physical Characterization and Preliminary Studies" Pharmaceuticals 14, no. 12: 1240. https://doi.org/10.3390/ph14121240
APA StyleBueno-López, C., Tamarit-Martínez, C., Alambiaga-Caravaca, A. M., Balaguer-Fernández, C., Merino, V., López-Castellano, A., & Rodilla, V. (2021). 3D Printing of Temporary Prostheses for Controlled-Release of Drugs: Design, Physical Characterization and Preliminary Studies. Pharmaceuticals, 14(12), 1240. https://doi.org/10.3390/ph14121240









