The Separation of Cannabinoids on Sub-2 µm Immobilized Polysaccharide Chiral Stationary Phases
Abstract
:1. Introduction
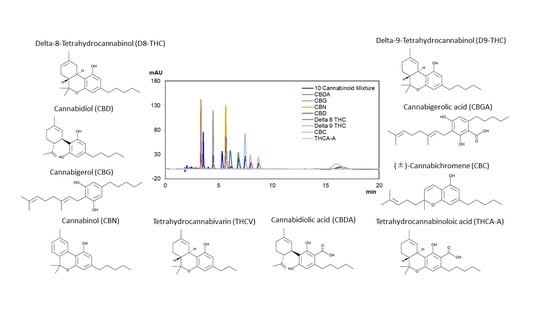
2. Results
2.1. Normal Phase
2.2. Reversed Phase
2.3. A Note on Optimization
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pourseyed Lazarjani, M.; Torres, S.; Hooker, T.; Fowlie, C.; Young, O.; Seyfoddin, A. Methods for Quantification of Cannabinoids: A Narrative. Rev. J. Cannabis Res. 2020, 2, 35. [Google Scholar] [CrossRef] [PubMed]
- Atakan, Z. Cannabis, a Complex Plant: Different Compounds and Different Effects on Individuals. Ther. Adv. Psychopharmacol. 2012, 2, 241–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanuš, L.O.; Meyer, S.M.; Muñoz, E.; Taglialatela-Scafati, O.; Appendino, G. Phytocannabinoids: A Unified Critical Inventory. Nat. Prod. Rep. 2016, 33, 1357–1392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denicola, C.; Barendt, J.M. Cannabinoid Isolation Models Utilizing Immobilized Chiral Stationary Phases and SFC. Cannabis. 2018. Available online: https://chiraltech.com/wp-content/uploads/2019/02/chiral-technologies-new-products-emerald-conference-cannabis-poster.pdf (accessed on 29 March 2019).
- Geiser, F.O.; Keenan, J.J.; Rossi, R.; Sanchez, A.; Whelan, J.M. Process for Purifying (-)-Delta 9 Trans-Tetrahydrocannabinol. U.S. Patent No. 7,449,589, 7 July 2005. [Google Scholar]
- Felletti, S.; De Luca, C.; Buratti, A.; Bozza, D.; Cerrato, A.; Capriotti, A.L.; Laganà, A.; Cavazzini, A.; Catani, M. Potency Testing of Cannabinoids by Liquid and Supercritical Fluid Chromatography: Where We Are, What We Need. J. Chromatogr. A 2021, 1651, 462304. [Google Scholar] [CrossRef] [PubMed]
- Umstead, W. Separation of the Enantiomers of (±) Δ8-THC and (±) Δ9-THC. In Application Note; Daicel Chiral Technologies: West Chester, PA, USA, 2019. [Google Scholar]
- Zivovinovic, S.; Alder, R.; Allenspach, M.D.; Steuer, C. Determination of cannabinoids in Cannabis sativa L. samples for recreational, medical, and forensic purposes by reversed-phase liquid chromatography-ultraviolet detection. J. Anal. Sci. Technol. 2018, 9, 27. [Google Scholar] [CrossRef] [Green Version]
- Saingam, W.; Sakunpak, A. Development and validation of reverse phase high performance liquid chromatography method for the determination of delta-9-tetrahydrocannabinol and cannabidiol in oromucosal spray from cannabis extract. Rev. Bras. Farm. 2018, 28, 669–672. [Google Scholar] [CrossRef]
- Williams, J.B.; Calati, K.B.; Hering, K.W.; Franckowski, R.E.; Umstead§, W.J.; Iula, D.M. HPLC Method to Differentiate Four THC Stereoisomers Formed from Δ9-THC Degradation: (6aR,9R)-Δ10-THC, (6aR,9S)-Δ10-THC, 9(R)-Δ6a,10a-THC, and 9(S)-Δ6a,10a-THC. Application Note. Cayman Chemical and Daicel Chiral Technologies: 2021. Available online: https://thecannabisscientist.com/fileadmin/tas/app-notes/2021/Differentiate_Four_THC_Stereoisomers_2021JUN.pdf (accessed on 29 March 2019).
- Fast and Easy Achiral & Chiral Analysis of Cannabinoids; Application Note; YMC Europe GmbH: Dinslaken, Germany, 2019; Available online: https://www.chromatographytoday.com/news/hplc-uhplc/31/ymc-europe-gmbh/fast-and-easy-achiral-chiral-analysis-of-cannabinoids/49213 (accessed on 29 March 2019).
- Isocratic Separation of 18 Cannabinoids. Application Note. Advanced Materials Technology. Available online: https://www.hplc.eu/Downloads/HALO_AN_Cannabinoids.pdf (accessed on 29 March 2019).
- McRae, G.; Melanson, J.E. Quantitative determination and validation of 17 cannabinoids in cannabis and hemp using liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2020, 412, 7381–7393. [Google Scholar] [CrossRef] [PubMed]
- Runco, J.; Aubin, A.; Layton, C. The Separation of Δ8-THC, Δ9-THC, and Their Enantiomers; Application Note; Waters Corporation: Milford, MA, USA, 2019. [Google Scholar]
- Mazzoccanti, G.; Ismail, O.H.; D’Acquarica, I.; Villani, C.; Manzo, C.; Wilcox, M.; Cavazzini, A.; Gasparrini, F. Cannabis through the looking glass: Chemo- and enantio-selective separation of phytocannabinoids by enantioselective ultra high performance supercritical fluid chromatography. Chem. Commun. 2017, 53, 12262–12265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breitenbach, S.; Rowe, W.F.; McCord, B.; Lurie, I.S. Assessment of ultra high performance supercritical fluid chromatography as a separation technique for the analysis of seized drugs: Applicability to synthetic cannabinoids. J. Chromatogr. A 2016, 1440, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Doi, T.; Asada, A.; Takeda, A.; Tagami, T.; Katagi, M.; Kamata, H.; Sawabe, Y. Enantioseparation of the carboxamide-type synthetic cannabinoids N-(1-amino-3-methyl-1-oxobutan-2-yl)-1-(5-fluoropentyl)-1H-indazole-3-carboxamide and methyl [1-(5-fluoropentyl)-1H-indazole-3-carbonyl]-valinate in illicit herbal products. J. Chromatogr. A 2016, 1473, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Tarbox, T.; Dilek, I.; Sreenivasan, U.; Yaser, K. A Validated Chiral HPLC Method for Resolution of Δ8 and Δ9 THC Enantiomers. Application Note. Cerilliant. Available online: https://www.cerilliant.com/newsAndEvents/posterArticle.aspx?ID=12 (accessed on 29 March 2019).
- Levin, S.; Abu-Lafi, S.; Zahalka, J.; Mechoulam, R. Resolution of chiral cannabinoids on amylose tris(3,5-dimethylphenylcarbamate) chiral stationary phase: Effects of structural features and mobile phase additives. J. Chromatogr. A 1993, 654, 53–64. [Google Scholar] [CrossRef]
- Umstead, W.J. The Separation of Several Minor Cannabinoids via Chiral HPLC. Cannabis Sci. Technol. 2021, 4, 44–51. [Google Scholar]
- Ali, I.; Suhail, M.; Asnin, L.; Aboul-Enein, H.Y. Reverse elution order of β-blockers in chiral separation. J. Liq. Chromatogr. Relat. Technol. 2017, 40, 435–441. [Google Scholar] [CrossRef]
- Okamoto, M. Reversal of elution order during the chiral separation in high performance liquid chromatography. J. Pharm. Biomed. Anal. 2002, 27, 401–407. [Google Scholar] [CrossRef]
- Yashima, E.; Yamada, M.; Kaida, Y.; Okamoto, Y. Computational Studied on Chiral Discrimination Mechanism of Cellulose Trisphenylcarbamate. J. Chromatogr. A 1995, 694, 347–354. [Google Scholar] [CrossRef]
- Yashima, E.; Yamamoto, C.; Okamoto, Y. NMR Studies of Chiral Discrimination Relevant to the Liquid Chromatographic Enantioseparation by a Cellulose Phenylcarbamate Derivative. J. Am. Chem. Soc. 1996, 118, 4036–4048. [Google Scholar] [CrossRef]
- Bi, W.; Wang, F.; Han, J.; Liu, B.; Shen, J.; Zhang, L.; Okamoto, Y. Ínfluence of the Substituents on Phenyl Groups on Enantioseparation Property of Amylose Phenylcarbamate. Carb. Polym. 2020, 241, 116372. [Google Scholar] [CrossRef]







| Chiralpak IB-U | Chiralpak IH-U | |
|---|---|---|
| Column Dimension | 3.0 mm i.d. × 100 mm L (2 columns coupled) | 3.0 mm i.d. × 100 mm L (2 columns coupled) |
| Mobile Phase | 96/3/1/0.1 = n-Hexane/Isopropanol/Ethanol/Trifluoroacetic acid (v/v/v/v) | 96/3/1/0.1 = n-Hexane/Isopropanol/Ethanol/Trifluoroacetic acid (v/v/v/v) |
| Flow Rate | 0.21 mL/min | 0.425 mL/min |
| Temperature | 25 °C (controlled) | 25 °C (controlled) |
| Detection | 220 nm UV | 220 nm UV |
| Sample | 10-cannabinoid mixture (1) 0.1 mg/mL in Hexane/IPA/EtOH = 96/3/1 | 10-cannabinoid mixture (1) 0.1 mg/mL in Hexane/IPA/EtOH = 96/3/1 |
| Injection Volume | 0.5 µL | 0.5 µL |
| Chiralpak IG-U | Chiralpak ID-U | Chiralpak ID-U+IC-U | |
|---|---|---|---|
| Column Dimension | 3.0 mm i.d. × 100 mm L | 3.0 mm i.d. × 100 mm L | 3.0 mm i.d. × 100 mm L (2 columns coupled) |
| Mobile Phase | 45/55/0.1 = Water/Acetonitrile/Trifluoroacetic acid (v/v/v) | 55/45/0.1 = Water/Acetonitrile/Trifluoroacetic acid (v/v/v) | 47.5/52.5/0.1 = Water/Acetonitrile/Trifluoroacetic acid (v/v/v) |
| Flow Rate | 0.800 mL/min | 0.600 mL/min | 0.425 mL/min |
| Temperature | 25 °C (controlled) | 25 °C (controlled) | 25 °C (controlled) |
| Detection | 220 nm UV | 220 nm UV | 220 nm UV |
| Sample | 10-cannabinoid mixture (1) 0.1 mg/mL in Water/ACN = 47.5/52.5 | 10-cannabinoid mixture (1) 0.1 mg/mL in Water/ACN = 47.5/52.5 | 10-cannabinoid mixture (1) 0.1 mg/mL in Water/ACN = 47.5/52.5 |
| Injection Volume | 0.5 µL | 0.5 µL | 0.5 µL |
| THCA-A | CBDA | Δ8 THC | CBD | CBC | CBN | Δ9 THC | CBG | Total Time | |
|---|---|---|---|---|---|---|---|---|---|
| Elution Time (min) | 6.12 | 6.80 | 7.38 | 7.66 | 8.49 8.89 | 10.36 | 11.56 | 15.75 | 18.00 |
| THCA-A | CBC | Δ8 THC | Δ9 THC | CBN | CBD | CBDA | CBG | Total Time | |
|---|---|---|---|---|---|---|---|---|---|
| Elution Time (min) | 6.97 | 8.04 8.37 | 9.26 | 9.64 | 10.62 | 12.32 | 13.66 | 22.23 | 25.00 |
| CBDA | CBG | CBN | CBD | Δ8 THC | Δ9 THC | CBC | THCA-A | Total Time | |
|---|---|---|---|---|---|---|---|---|---|
| Elution Time (min) | 2.39 | 4.28 | 5.37 | 6.68 | 7.17 | 8.16 | 8.79 10.74 | 19.81 | 25.00 |
| CBDA | CBD | CBG | CBN | Δ8 THC | Δ9 THC | CBC | THCA-A | Total Time | |
|---|---|---|---|---|---|---|---|---|---|
| Elution Time (min) | 7.56 | 11.71 | 12.35 | 15.05 | 18.21 | 19.59 | 25.34 26.23 | 27.10 | 30.00 |
| CBDA | CBD | CBG | CBN | Δ8 THC | Δ9 THC | CBC | THCA-A | Total Time | |
|---|---|---|---|---|---|---|---|---|---|
| Elution Time (min) | 7.71 | 10.42 | 10.98 | 14.24 | 16.64 | 17.26 | 20.88 22.03 | 24.01 | 30.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onishi, T.; Umstead, W.J. The Separation of Cannabinoids on Sub-2 µm Immobilized Polysaccharide Chiral Stationary Phases. Pharmaceuticals 2021, 14, 1250. https://doi.org/10.3390/ph14121250
Onishi T, Umstead WJ. The Separation of Cannabinoids on Sub-2 µm Immobilized Polysaccharide Chiral Stationary Phases. Pharmaceuticals. 2021; 14(12):1250. https://doi.org/10.3390/ph14121250
Chicago/Turabian StyleOnishi, Takafumi, and Weston J. Umstead. 2021. "The Separation of Cannabinoids on Sub-2 µm Immobilized Polysaccharide Chiral Stationary Phases" Pharmaceuticals 14, no. 12: 1250. https://doi.org/10.3390/ph14121250
APA StyleOnishi, T., & Umstead, W. J. (2021). The Separation of Cannabinoids on Sub-2 µm Immobilized Polysaccharide Chiral Stationary Phases. Pharmaceuticals, 14(12), 1250. https://doi.org/10.3390/ph14121250