Fenofibrate Protects against Retinal Dysfunction in a Murine Model of Common Carotid Artery Occlusion-Induced Ocular Ischemia
Abstract
1. Introduction
2. Results
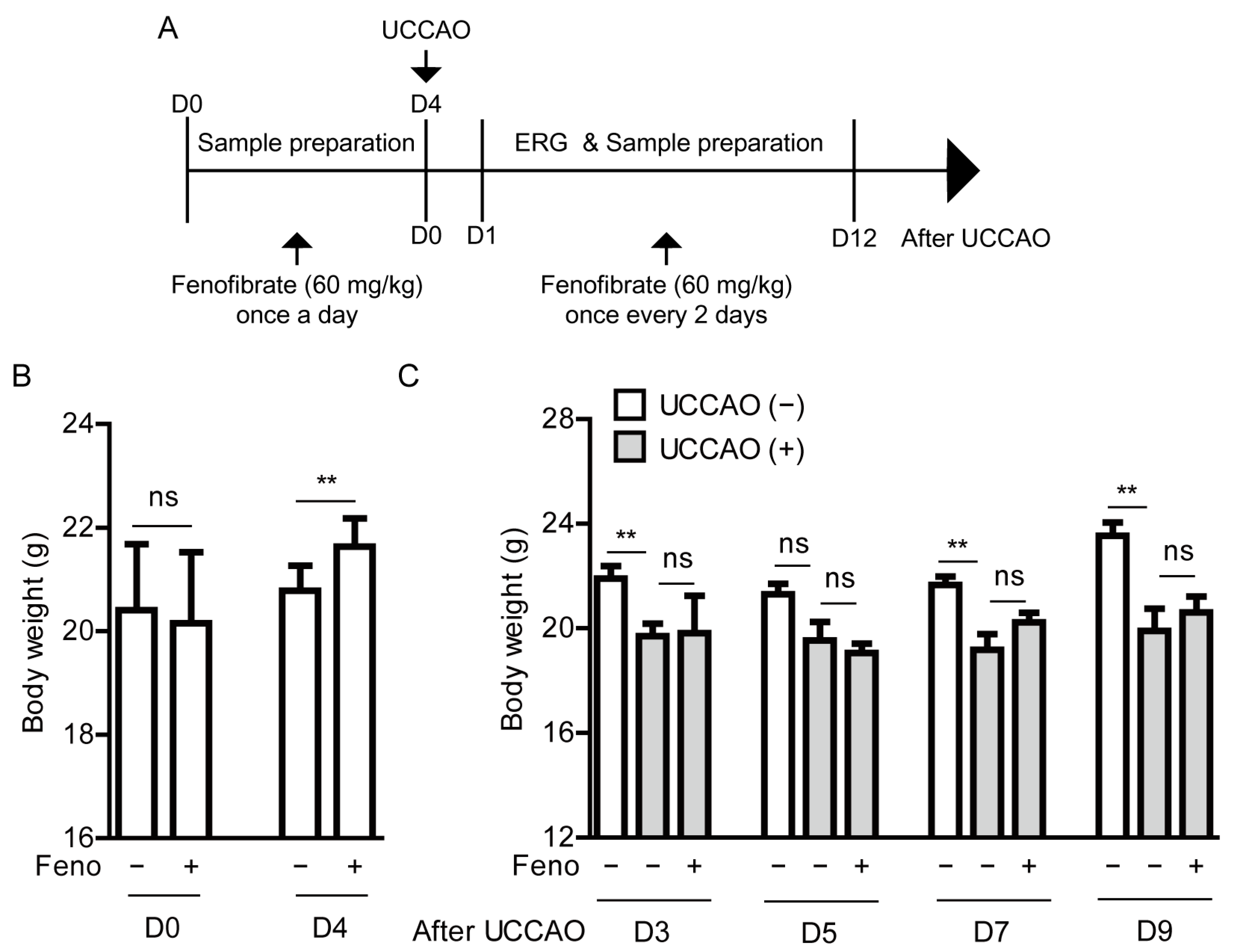
2.1. UCCAO Induces Ocular Ischemia in Adult Mice
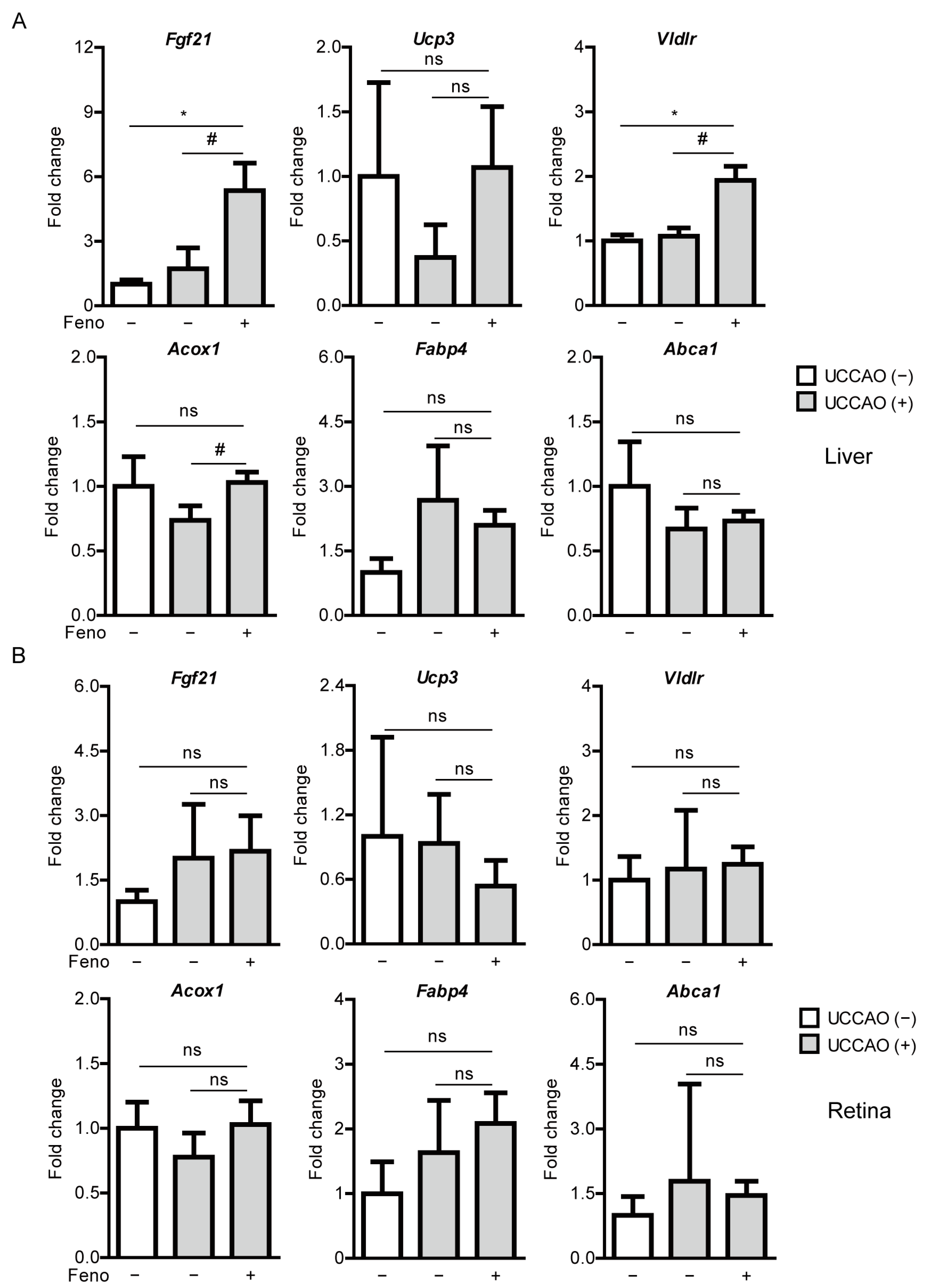
2.2. Fenofibrate Leads to PPARα Target Gene Expressions in Adult Mice
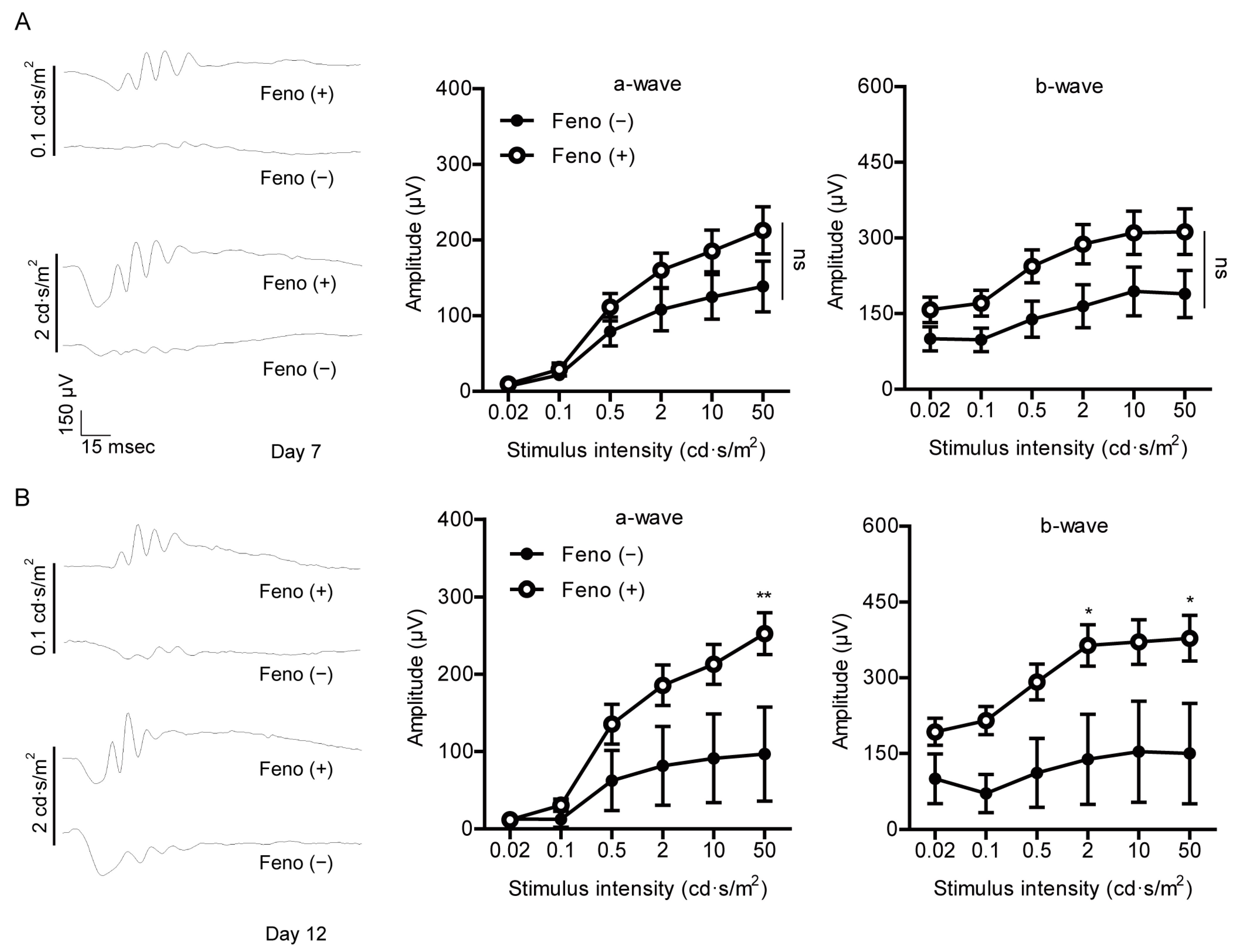
2.3. Fenofibrate Suppresses Retinal Dysfunction in a Mouse Model of UCCAO-Induced Ocular Ischemia
2.4. Fenofibrate Induces PPARα Target Gene Expressions in a Mouse Model of UCCAO-Induced Ocular Ischemia
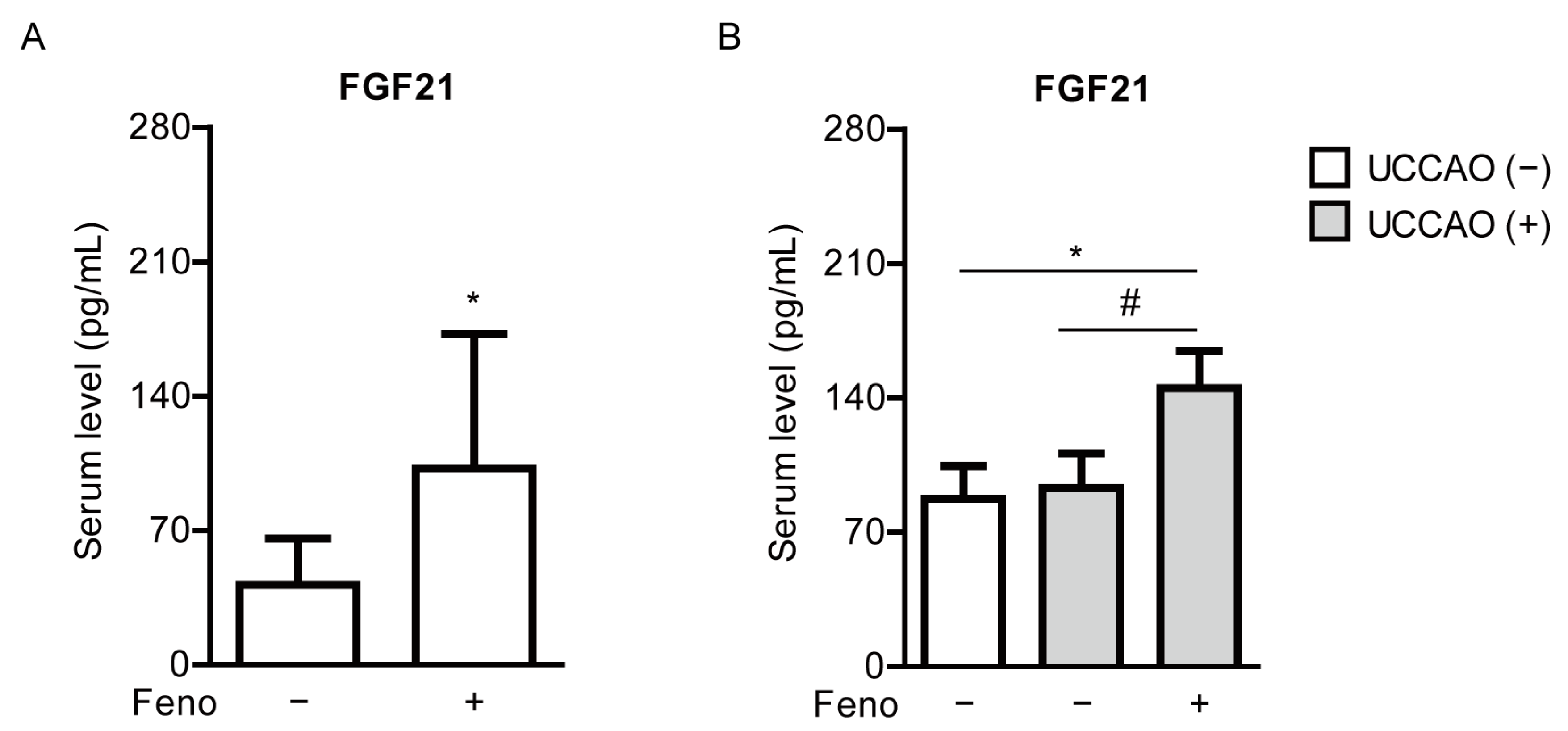
2.5. Fenofibrate Increases Serum FGF21 Levels in a Mouse Model of UCCAO-Induced Ocular Ischemia
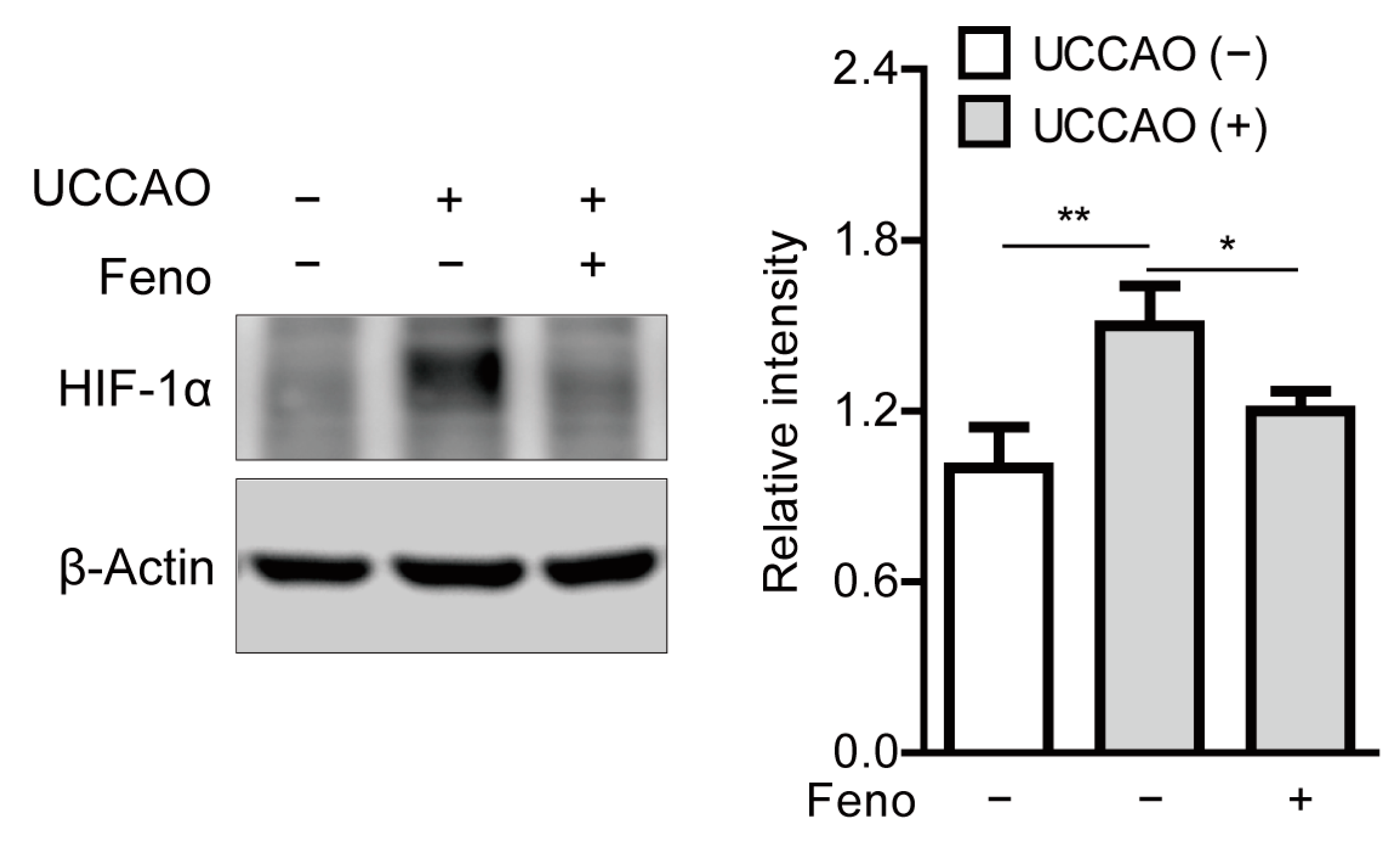
2.6. Fenofibrate Suppresses Stabilization of Hypoxia-Inducible Factor in a Mouse Model of UCCAO-Induced Ocular Ischemia
2.7. Fenofibrate Modulates Hypoxia-Responsive Gene Expressions in a Mouse Model of UCCAO-Induced Ocular Ischemia
3. Discussion
4. Materials and Methods
4.1. Animal
4.2. A Murine Model of UCCAO-Induced Ocular Ischemia and Oral Administration of Fenofibrate
4.3. Optical Coherence Tomography (OCT)
4.4. Electroretinography (ERG)
4.5. Measurement of Serum FGF21 Levels
4.6. Quantitative PCR
4.7. Western Blotting
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
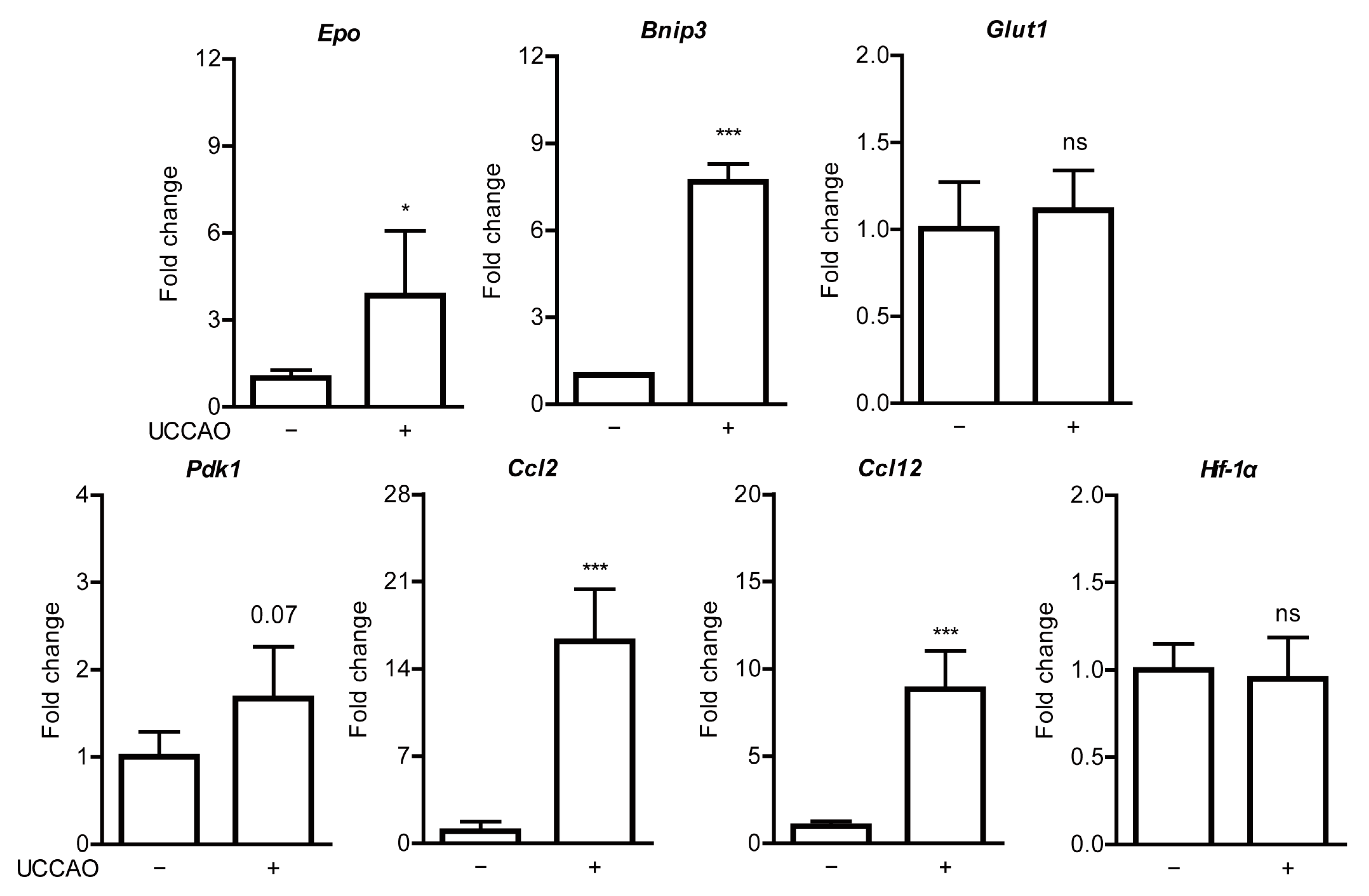
Appendix B
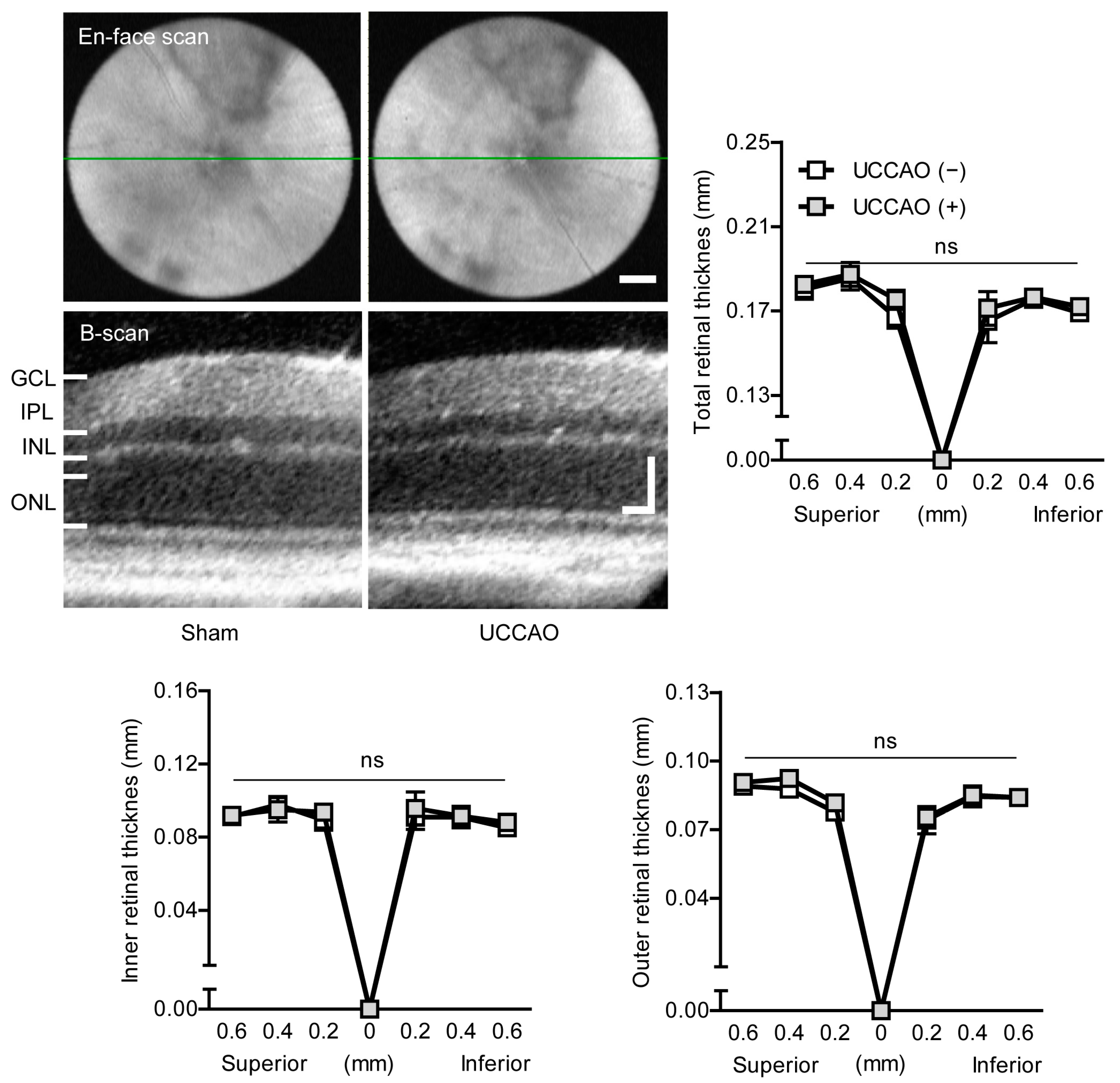
Appendix C
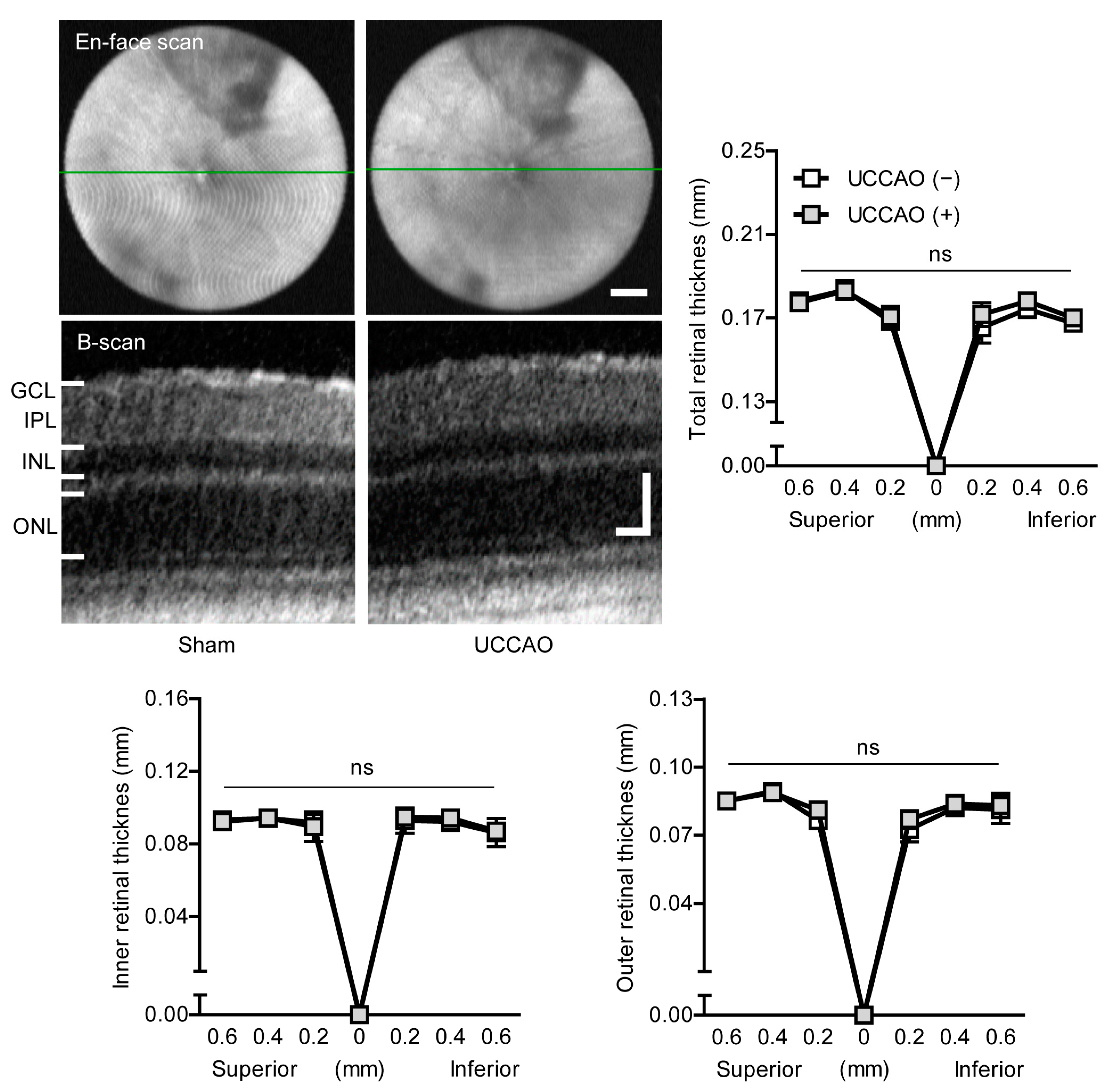
Appendix D
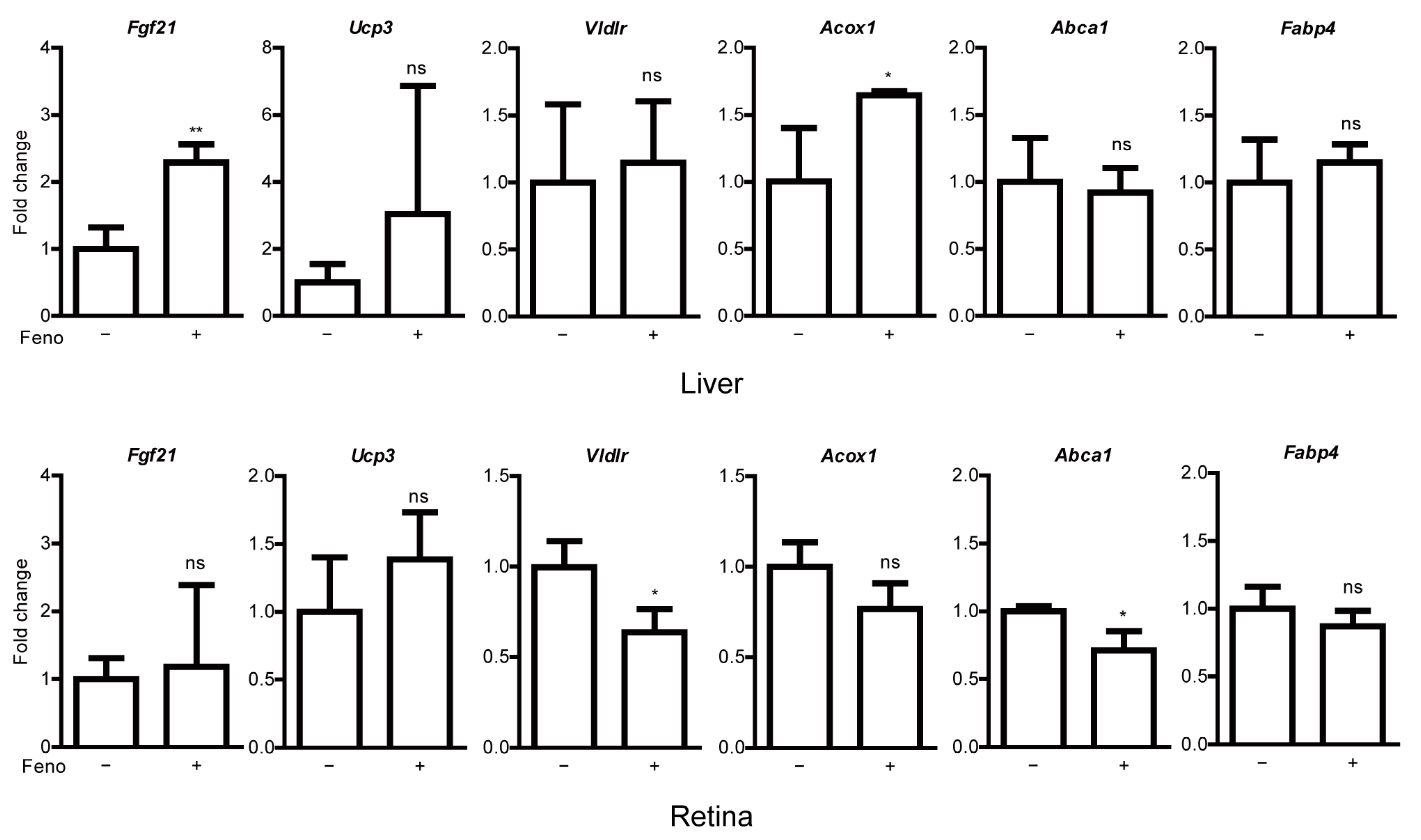
Appendix E

Appendix F

Appendix G

References
- Varma, D.D.; Cugati, S.; Lee, A.W.; Chen, C.S. A review of central retinal artery occlusion: Clinical presentation and management. Eye 2013, 27, 688–697. [Google Scholar] [CrossRef]
- Hayreh, S.S.; Zimmerman, M.B. Central retinal artery occlusion: Visual outcome. Am. J. Ophthalmol. 2005, 140, 376–391. [Google Scholar] [CrossRef] [PubMed]
- Leavitt, J.A.; Larson, T.A.; Hodge, D.O.; Gullerud, R.E. The incidence of central retinal artery occlusion in Olmsted County, Minnesota. Am. J. Ophthalmol. 2011, 152, 820–823.e2. [Google Scholar] [CrossRef] [PubMed]
- Rumelt, S.; Dorenboim, Y.; Rehany, U. Aggressive systematic treatment for central retinal artery occlusion. Am. J. Ophthalmol. 1999, 128, 733–738. [Google Scholar] [CrossRef]
- Vodopivec, I.; Rizzo, J.F., III. Ophthalmic manifestations of giant cell arteritis. Rheumatology 2018, 57, ii63–ii72. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.; Marco, R.D.; Goldhardt, R.; Modi, Y. Central Retinal Artery Occlusion: Acute Management and Treatment. Curr. Ophthalmol. Rep. 2017, 5, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Babikian, V.; Wijman, C.A.C.; Koleini, B.; Malik, S.N.; Goyal, N.; Matjucha, I.C.A. Retinal Ischemia and Embolism. Cerebrovasc. Dis. 2001, 12, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, R.M.; Chaturvedi, S.; Eliott, D.; Joshi, N.; Puklin, J.E.; Abrams, G.W. Mechanisms of retinal arterial occlusive disease in African American and Caucasian patients. Stroke 1999, 30, 1506–1509. [Google Scholar] [CrossRef][Green Version]
- Sun, W.; Geng, Y.; Chen, Y.T.; Tang, X.H.; Zhang, Y.J.; Gu, S.H.; Xie, J.J.; Zhang, Z.A.; Tian, X.S. Differences of brain pathological changes and cognitive function after bilateral common carotid artery occlusion between Sprague-Dawley and Wistar rats. Acta Phys. Sin. 2019, 71, 705–716. [Google Scholar]
- Qin, Y.; Ji, M.; Deng, T.; Luo, D.; Zi, Y.; Pan, L.; Wang, Z.; Jin, M. Functional and morphologic study of retinal hypoperfusion injury induced by bilateral common carotid artery occlusion in rats. Sci. Rep. 2019, 9, 80. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Garcia, S.; Reichhart, N.; Skosyrski, S.; Foddis, M.; Wu, J.; Figura, A.; Herrspiegel, C.; Fuchtemeier, M.; Sassi, C.; Dirnagl, U.; et al. Individual and temporal variability of the retina after chronic bilateral common carotid artery occlusion (BCCAO). PLoS ONE 2018, 13. [Google Scholar] [CrossRef]
- Lavinsky, D.; Sarmento Arterni, N.; Achaval, M.; Netto, C.A. Chronic bilateral common carotid artery occlusion: A model for ocular ischemic syndrome in the rat. Graefe Arch. Clin. Exp. Ophthalmol. 2006, 244, 199–204. [Google Scholar] [CrossRef]
- Kalesnykas, G.; Tuulos, T.; Uusitalo, H.; Jolkkonen, J. Neurodegeneration and cellular stress in the retina and optic nerve in rat cerebral ischemia and hypoperfusion models. Neuroscience 2008, 155, 937–947. [Google Scholar] [CrossRef]
- Lee, D.; Kang, H.; Yoon, K.Y.; Chang, Y.Y.; Song, H.B. A mouse model of retinal hypoperfusion injury induced by unilateral common carotid artery occlusion. Exp. Eye Res. 2020, 201, 108275. [Google Scholar] [CrossRef] [PubMed]
- Hayreh, S.S.; Zimmerman, M.B. Ocular Arterial Occlusive Disorders and Carotid Artery Disease. Ophthalmol. Retina 2017, 1, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Weymouth, W.; Pedersen, C. Central Retinal Artery Occlusion Associated with Carotid Artery Occlusion. Clin. Pract. Cases Emerg. Med. 2019, 3, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Schmidt-Kastner, R.; Hamasaki, D.I.; Yamamoto, H.; Parel, J.M. Complex neurodegeneration in retina following moderate ischemia induced by bilateral common carotid artery occlusion in Wistar rats. Exp. Eye Res. 2006, 82, 767–779. [Google Scholar] [CrossRef]
- Yang, G.; Kitagawa, K.; Matsushita, K.; Mabuchi, T.; Yagita, Y.; Yanagihara, T.; Matsumoto, M. C57BL/6 strain is most susceptible to cerebral ischemia following bilateral common carotid occlusion among seven mouse strains: Selective neuronal death in the murine transient forebrain ischemia. Brain Res. 1997, 752, 209–218. [Google Scholar] [CrossRef]
- Lee, D.; Miwa, Y.; Jeong, H.; Ikeda, S.I.; Katada, Y.; Tsubota, K.; Kurihara, T. A Murine Model of Ischemic Retinal Injury Induced by Transient Bilateral Common Carotid Artery Occlusion. J. Vis. Exp. JoVE 2020. [Google Scholar] [CrossRef]
- Lee, B.J.; Jun, H.O.; Kim, J.H.; Kim, J.H. Astrocytic cystine/glutamate antiporter is a key regulator of erythropoietin expression in the ischemic retina. FASEB J. 2019, 33, 6045–6054. [Google Scholar] [CrossRef] [PubMed]
- Keech, A.C.; Mitchell, P.; Summanen, P.A.; O’Day, J.; Davis, T.M.; Moffitt, M.S.; Taskinen, M.R.; Simes, R.J.; Tse, D.; Williamson, E.; et al. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): A randomised controlled trial. Lancet 2007, 370, 1687–1697. [Google Scholar] [CrossRef]
- Chew, E.Y.; Davis, M.D.; Danis, R.P.; Lovato, J.F.; Perdue, L.H.; Greven, C.; Genuth, S.; Goff, D.C.; Leiter, L.A.; Ismail-Beigi, F.; et al. The effects of medical management on the progression of diabetic retinopathy in persons with type 2 diabetes: The Action to Control Cardiovascular Risk in Diabetes (ACCORD) Eye Study. Ophthalmology 2014, 121, 2443–2451. [Google Scholar] [CrossRef] [PubMed]
- Group, A.S.; Group, A.E.S.; Chew, E.Y.; Ambrosius, W.T.; Davis, M.D.; Danis, R.P.; Gangaputra, S.; Greven, C.M.; Hubbard, L.; Esser, B.A.; et al. Effects of medical therapies on retinopathy progression in type 2 diabetes. N. Engl. J. Med. 2010, 363, 233–244. [Google Scholar] [CrossRef]
- Chew, E.Y.; Ambrosius, W.T. Update of the ACCORD Eye Study. N. Engl. J. Med. 2011, 364, 188–189. [Google Scholar] [CrossRef]
- Chew, E.Y.; Ambrosius, W.T.; Howard, L.T.; Greven, C.M.; Johnson, S.; Danis, R.P.; Davis, M.D.; Genuth, S.; Domanski, M.; Group, A.S. Rationale, design, and methods of the Action to Control Cardiovascular Risk in Diabetes Eye Study (ACCORD-EYE). Am. J. Cardiol. 2007, 99, 103i–111i. [Google Scholar] [CrossRef]
- Shiono, A.; Sasaki, H.; Sekine, R.; Abe, Y.; Matsumura, Y.; Inagaki, T.; Tanaka, T.; Kodama, T.; Aburatani, H.; Sakai, J.; et al. PPARα activation directly upregulates thrombomodulin in the diabetic retina. Sci. Rep. 2020, 10, 10837. [Google Scholar] [CrossRef]
- Moran, E.; Ding, L.; Wang, Z.; Cheng, R.; Chen, Q.; Moore, R.; Takahashi, Y.; Ma, J.X. Protective and antioxidant effects of PPARα in the ischemic retina. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4568–4576. [Google Scholar] [CrossRef] [PubMed]
- Noonan, J.E.; Jenkins, A.J.; Ma, J.X.; Keech, A.C.; Wang, J.J.; Lamoureux, E.L. An update on the molecular actions of fenofibrate and its clinical effects on diabetic retinopathy and other microvascular end points in patients with diabetes. Diabetes 2013, 62, 3968–3975. [Google Scholar] [CrossRef] [PubMed]
- Inoue, H.; Jiang, X.F.; Katayama, T.; Osada, S.; Umesono, K.; Namura, S. Brain protection by resveratrol and fenofibrate against stroke requires peroxisome proliferator-activated receptor alpha in mice. Neurosci. Lett. 2003, 352, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Wang, G.; Namura, S. Fenofibrate improves cerebral blood flow after middle cerebral artery occlusion in mice. Journal of cerebral blood flow and metabolism. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2010, 30, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Tomita, Y.; Lee, D.; Tsubota, K.; Kurihara, T. PPARα Agonist Oral Therapy in Diabetic Retinopathy. Biomedicines 2020, 8, 433. [Google Scholar] [CrossRef]
- Sasaki, Y.; Raza-Iqbal, S.; Tanaka, T.; Murakami, K.; Anai, M.; Osawa, T.; Matsumura, Y.; Sakai, J.; Kodama, T. Gene Expression Profiles Induced by a Novel Selective Peroxisome Proliferator-Activated Receptor α Modulator (SPPARMα) Pemafibrate. Int. J. Mol. Sci. 2019, 20, 5682. [Google Scholar] [CrossRef] [PubMed]
- Badman, M.K.; Pissios, P.; Kennedy, A.R.; Koukos, G.; Flier, J.S.; Maratos-Flier, E. Hepatic Fibroblast Growth Factor 21 Is Regulated by PPARα and Is a Key Mediator of Hepatic Lipid Metabolism in Ketotic States. Cell Metab. 2007, 5, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, T.; Ozawa, Y.; Shinoda, K.; Nagai, N.; Inoue, M.; Oike, Y.; Tsubota, K.; Ishida, S.; Okano, H. Neuroprotective effects of angiotensin II type 1 receptor (AT1R) blocker, telmisartan, via modulating AT1R and AT2R signaling in retinal inflammation. Investig. Ophthalmol. Vis. Sci. 2006, 47, 5545–5552. [Google Scholar] [CrossRef] [PubMed]
- Tomita, Y.; Lee, D.; Miwa, Y.; Jiang, X.; Ohta, M.; Tsubota, K.; Kurihara, T. Pemafibrate Protects Against Retinal Dysfunction in a Murine Model of Diabetic Retinopathy. Int. J. Mol. Sci. 2020, 21, 6243. [Google Scholar] [CrossRef]
- Tomita, Y.; Ozawa, N.; Miwa, Y.; Ishida, A.; Ohta, M.; Tsubota, K.; Kurihara, T. Pemafibrate Prevents Retinal Pathological Neovascularization by Increasing FGF21 Level in a Murine Oxygen-Induced Retinopathy Model. Int. J. Mol. Sci. 2019, 20, 5878. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.-L.; Januszewski, A.S.; O’Connell, R.; Jenkins, A.J.; Xu, A.; Sullivan, D.R.; Barter, P.J.; Hung, W.-T.; Scott, R.S.; Taskinen, M.-R.; et al. The relationship of fibroblast growth factor 21 with cardiovascular outcome events in the Fenofibrate Intervention and Event Lowering in Diabetes study. Diabetologia 2015, 58, 464–473. [Google Scholar] [CrossRef]
- Ong, K.-L.; O’Connell, R.; Januszewski, A.S.; Jenkins, A.J.; Xu, A.; Sullivan, D.R.; Barter, P.J.; Scott, R.S.; Taskinen, M.-R.; Waldman, B.; et al. Baseline Circulating FGF21 Concentrations and Increase after Fenofibrate Treatment Predict More Rapid Glycemic Progression in Type 2 Diabetes: Results from the FIELD Study. Clin. Chem. 2017, 63, 1261–1270. [Google Scholar] [CrossRef]
- Ong, K.L.; Rye, K.A.; O’Connell, R.; Jenkins, A.J.; Brown, C.; Xu, A.; Sullivan, D.R.; Barter, P.J.; Keech, A.C. Long-term fenofibrate therapy increases fibroblast growth factor 21 and retinol-binding protein 4 in subjects with type 2 diabetes. J. Clin. Endocrinol. Metab. 2012, 97, 4701–4708. [Google Scholar] [CrossRef]
- Enright, J.M.; Zhang, S.; Thebeau, C.; Siebert, E.; Jin, A.; Gadiraju, V.; Zhang, X.; Chen, S.; Semenkovich, C.F.; Rajagopal, R. Fenofibrate Reduces the Severity of Neuroretinopathy in a Type 2 Model of Diabetes without Inducing Peroxisome Proliferator-Activated Receptor Alpha-Dependent Retinal Gene Expression. J. Clin Med. 2021, 10, 126. [Google Scholar] [CrossRef]
- Itoh, N. FGF21 as a Hepatokine, Adipokine, and Myokine in Metabolism and Diseases. Front. Endocrinol. 2014, 5, 107. [Google Scholar] [CrossRef] [PubMed]
- Itoh, N.; Nakayama, Y.; Konishi, M. Roles of FGFs As Paracrine or Endocrine Signals in Liver Development, Health, and Disease. Front. Cell Dev. Biol. 2016, 4, 30. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, T.; Nakatake, Y.; Konishi, M.; Itoh, N. Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochim. Biophys. Acta 2000, 1492, 203–206. [Google Scholar] [CrossRef]
- Tezze, C.; Romanello, V.; Sandri, M. FGF21 as Modulator of Metabolism in Health and Disease. Front. Physiol. 2019, 10, 419. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Gong, Y.; Liegl, R.; Wang, Z.; Liu, C.H.; Meng, S.S.; Burnim, S.B.; Saba, N.J.; Fredrick, T.W.; Morss, P.C.; et al. FGF21 Administration Suppresses Retinal and Choroidal Neovascularization in Mice. Cell Rep. 2017, 18, 1606–1613. [Google Scholar] [CrossRef]
- Fu, Z.; Wang, Z.; Liu, C.H.; Gong, Y.; Cakir, B.; Liegl, R.; Sun, Y.; Meng, S.S.; Burnim, S.B.; Arellano, I.; et al. Fibroblast Growth Factor 21 Protects Photoreceptor Function in Type 1 Diabetic Mice. Diabetes 2018, 67, 974–985. [Google Scholar] [CrossRef] [PubMed]
- Tomita, Y.; Fu, Z.; Wang, Z.; Cakir, B.; Cho, S.S.; Britton, W.; Sun, Y.; Hellstrom, A.; Talukdar, S.; Smith, L.E.H. Long-Acting FGF21 Inhibits Retinal Vascular Leakage in In Vivo and In Vitro Models. Int. J. Mol. Sci. 2020, 21, 1188. [Google Scholar] [CrossRef]
- Wang, D.; Liu, F.; Zhu, L.; Lin, P.; Han, F.; Wang, X.; Tan, X.; Lin, L.; Xiong, Y. FGF21 alleviates neuroinflammation following ischemic stroke by modulating the temporal and spatial dynamics of microglia/macrophages. J. Neuroinflamm. 2020, 17, 257. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Matei, N.; Pang, J.; Luo, X.; Song, Z.; Tang, J.; Zhang, J.H. Delayed recanalization at 3 days after permanent MCAO attenuates neuronal apoptosis through FGF21/FGFR1/PI3K/Caspase-3 pathway in rats. Exp. Neurol. 2019, 320, 113007. [Google Scholar] [CrossRef]
- Staiger, H.; Keuper, M.; Berti, L.; Hrabe de Angelis, M.; Haring, H.U. Fibroblast Growth Factor 21-Metabolic Role in Mice and Men. Endocr. Rev. 2017, 38, 468–488. [Google Scholar] [CrossRef]
- Liu, M.; Cao, H.; Hou, Y.; Sun, G.; Li, D.; Wang, W. Liver Plays a Major Role in FGF-21 Mediated Glucose Homeostasis. Cell Physiol. Biochem. 2018, 45, 1423–1433. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Qi, J.; Yu, D.; Wu, Y.; Zhu, S.; Li, S.; Wu, Q.; Ren, G.; Li, D. Pharmacological efficacy of FGF21 analogue, liraglutide and insulin glargine in treatment of type 2 diabetes. J. Diabetes Complicat. 2017, 31, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Li, L.; Yang, M.; Liu, H.; Boden, G.; Yang, G. The effects of fibroblast growth factor-21 knockdown and over-expression on its signaling pathway and glucose-lipid metabolism In Vitro. Mol. Cell Endocrinol. 2012, 348, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Cao, S.; Liu, J. Role of angiopoietin-2 in the cardioprotective effect of fibroblast growth factor 21 on ischemia/reperfusion-induced injury in H9c2 cardiomyocytes. Exp. Ther. Med. 2017, 14, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Joyal, J.S.; Sun, Y.; Gantner, M.L.; Shao, Z.; Evans, L.P.; Saba, N.; Fredrick, T.; Burnim, S.; Kim, J.S.; Patel, G.; et al. Retinal lipid and glucose metabolism dictates angiogenesis through the lipid sensor Ffar1. Nat. Med. 2016, 22, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Ray, R.; Dubik, D.; Shi, L.; Cizeau, J.; Bleackley, R.C.; Saxena, S.; Gietz, R.D.; Greenberg, A.H. The E1B 19K/Bcl-2-binding protein Nip3 is a dimeric mitochondrial protein that activates apoptosis. J. Exp. Med. 1997, 186, 1975–1983. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Miwa, Y.; Wu, J.; Shoda, C.; Jeong, H.; Kawagishi, H.; Tsubota, K.; Kurihara, T. A Fairy Chemical Suppresses Retinal Angiogenesis as a HIF Inhibitor. Biomolecules 2020, 10, 1405. [Google Scholar] [CrossRef] [PubMed]
- Bruick, R.K. Expression of the gene encoding the proapoptotic Nip3 protein is induced by hypoxia. PNAS 2000, 97, 9082. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Moran, E.; Ding, L.; Cheng, R.; Xu, X.; Ma, J.X. PPARα regulates mobilization and homing of endothelial progenitor cells through the HIF-1α/SDF-1 pathway. Investig. Ophthalmol. Vis. Sci. 2014, 55, 3820–3832. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, Y.; Hu, Y.; Lin, M.; Jenkins, A.J.; Keech, A.C.; Mott, R.; Lyons, T.J.; Ma, J.X. Therapeutic effects of PPARα agonists on diabetic retinopathy in type 1 diabetes models. Diabetes 2013, 62, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.F.; Dowling, J.E. Intracellular responses of the Müller (glial) cells of mudpuppy retina: Their relation to b-wave of the electroretinogram. J. Neurophysiol. 1970, 33, 323–341. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, N.K.; Ramamohan, Y.; Raju, T.R. Developmental expression of synaptophysin, synapsin I and syntaxin in the rat retina. Brain Res. Dev. Brain Res. 1997, 102, 267–273. [Google Scholar] [CrossRef]
- Valtorta, F.; Pennuto, M.; Bonanomi, D.; Benfenati, F. Synaptophysin: Leading actor or walk-on role in synaptic vesicle exocytosis? BioEssays 2004, 26, 445–453. [Google Scholar] [CrossRef] [PubMed]
- VanGuilder, H.D.; Brucklacher, R.M.; Patel, K.; Ellis, R.W.; Freeman, W.M.; Barber, A.J. Diabetes downregulates presynaptic proteins and reduces basal synapsin I phosphorylation in rat retina. Eur. J. Neurosci. 2008, 28, 1–11. [Google Scholar] [CrossRef]
- Uejo, F.; Limwikrant, W.; Moribe, K.; Yamamoto, K. Dissolution improvement of fenofibrate by melting inclusion in mesoporous silica. Asian J. Pharm. Sci. 2013, 8, 329–335. [Google Scholar] [CrossRef]
- Ikeda, H.O.; Muraoka, Y.; Hata, M.; Sumi, E.; Ikeda, T.; Nakagawa, T.; Abe, H.; Tada, H.; Morita, S.; Kakizuka, A.; et al. Safety and effectiveness of a novel neuroprotectant, KUS121, in patients with non-arteritic central retinal artery occlusion: An open-label, non-randomized, first-in-humans, phase 1/2 trial. PLoS ONE 2020, 15, e0229068. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Kurihara, T.; Ikeda, S.I.; Kunimi, H.; Mori, K.; Torii, H.; Tsubota, K. Inducement and Evaluation of a Murine Model of Experimental Myopia. J. Vis. Exp. JoVE 2019. [Google Scholar] [CrossRef] [PubMed]
- Miwa, Y.; Hoshino, Y.; Shoda, C.; Jiang, X.; Tsubota, K.; Kurihara, T. Pharmacological HIF inhibition prevents retinal neovascularization with improved visual function in a murine oxygen-induced retinopathy model. Neurochem. Int. 2019, 128, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Ibuki, M.; Lee, D.; Shinojima, A.; Miwa, Y.; Tsubota, K.; Kurihara, T. Rice Bran and Vitamin B6 Suppress Pathological Neovascularization in a Murine Model of Age-Related Macular Degeneration as Novel HIF Inhibitors. Int. J. Mol. Sci. 2020, 21, 8940. [Google Scholar] [CrossRef] [PubMed]


| Name | Direction | Sequence (5′→3′) | Accession Number |
|---|---|---|---|
| Hprt | Forward | TCAGTCAACGGGGGACATAAA | NM_013556.2 |
| Reverse | GGGGCTGTACTGCTTAACCAG | ||
| Epo | Forward | GGCCATAGAAGTTTGGCAAG | NM_007942 |
| Reverse | CCTCTCCCGTGTACAGCTTC | ||
| Bnip3 | Forward | GCTCCCAGACACCACAAGAT | NM_009760.4 |
| Reverse | TGAGAGTAGCTGTGCGCTTC | ||
| Pdk1 | Forward | GGCGGCTTTGTGATTTGTAT | NM_172665.5 |
| Reverse | ACCTGAATCGGGGGATAAAC | ||
| Glut1 | Forward | CAGTTCGGCTATAACACTGGTG | NM_011400.3 |
| Reverse | GCCCCCGACAGAGAAGATG | ||
| Ccl2 | Forward | CCCAATGAGTAGGCTGGAGA | NM_011333.3 |
| Reverse | TCTGGACCCATTCCTTCTTG | ||
| Ccl12 | Forward | GCTACAGGAGAATCACAAGCAGC | NM_011331.3 |
| Reverse | ACGTCTTATCCAAGTGGTTTATGG | ||
| Ucp3 | Forward | GGAGTCTCACCTGTTTACTGACAACT | NM_009464.3 |
| Reverse | GCACAGAAGCCAGCTCCAA | ||
| Abca1 | Forward | CGTTTCCGGGAAGTGTCCTA | NM_013454.3 |
| Reverse | GCTAGAGATGACAAGGAGGATGGA | ||
| Fabp4 | Forward | CCGCAGACGACAGGA | NM_024406.3 |
| Reverse | CTCATGCCCTTTCATAAACT | ||
| Fgf21 | Forward | AACAGCCATTCACTTTGCCTGAGC | NM_020013.4 |
| Reverse | GGCAGCTGGAATTGTGTTCTGACT | ||
| Vldlr | Forward | GAGCCCCTGAAGGAATGCC | NM_001161420.1 |
| Reverse | CCTATAACTAGGTCTTTGCAGATATGG | ||
| Acox1 | Forward | TCTTCTTGAGACAGGGCCCAG | AF006688.1 |
| Reverse | GTTCCGACTAGCCAGGCATG | ||
| Hif-1α | Forward | GGTTCCAGCAGACCCAGTTA | NM_001313919.1 |
| Reverse | AGGCTCCTTGGATGAGCTTT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.; Tomita, Y.; Miwa, Y.; Jeong, H.; Mori, K.; Tsubota, K.; Kurihara, T. Fenofibrate Protects against Retinal Dysfunction in a Murine Model of Common Carotid Artery Occlusion-Induced Ocular Ischemia. Pharmaceuticals 2021, 14, 223. https://doi.org/10.3390/ph14030223
Lee D, Tomita Y, Miwa Y, Jeong H, Mori K, Tsubota K, Kurihara T. Fenofibrate Protects against Retinal Dysfunction in a Murine Model of Common Carotid Artery Occlusion-Induced Ocular Ischemia. Pharmaceuticals. 2021; 14(3):223. https://doi.org/10.3390/ph14030223
Chicago/Turabian StyleLee, Deokho, Yohei Tomita, Yukihiro Miwa, Heonuk Jeong, Kiwako Mori, Kazuo Tsubota, and Toshihide Kurihara. 2021. "Fenofibrate Protects against Retinal Dysfunction in a Murine Model of Common Carotid Artery Occlusion-Induced Ocular Ischemia" Pharmaceuticals 14, no. 3: 223. https://doi.org/10.3390/ph14030223
APA StyleLee, D., Tomita, Y., Miwa, Y., Jeong, H., Mori, K., Tsubota, K., & Kurihara, T. (2021). Fenofibrate Protects against Retinal Dysfunction in a Murine Model of Common Carotid Artery Occlusion-Induced Ocular Ischemia. Pharmaceuticals, 14(3), 223. https://doi.org/10.3390/ph14030223










