A Wirelessly Controlled Scalable 3D-Printed Microsystem for Drug Delivery
Abstract
:1. Introduction
2. Results
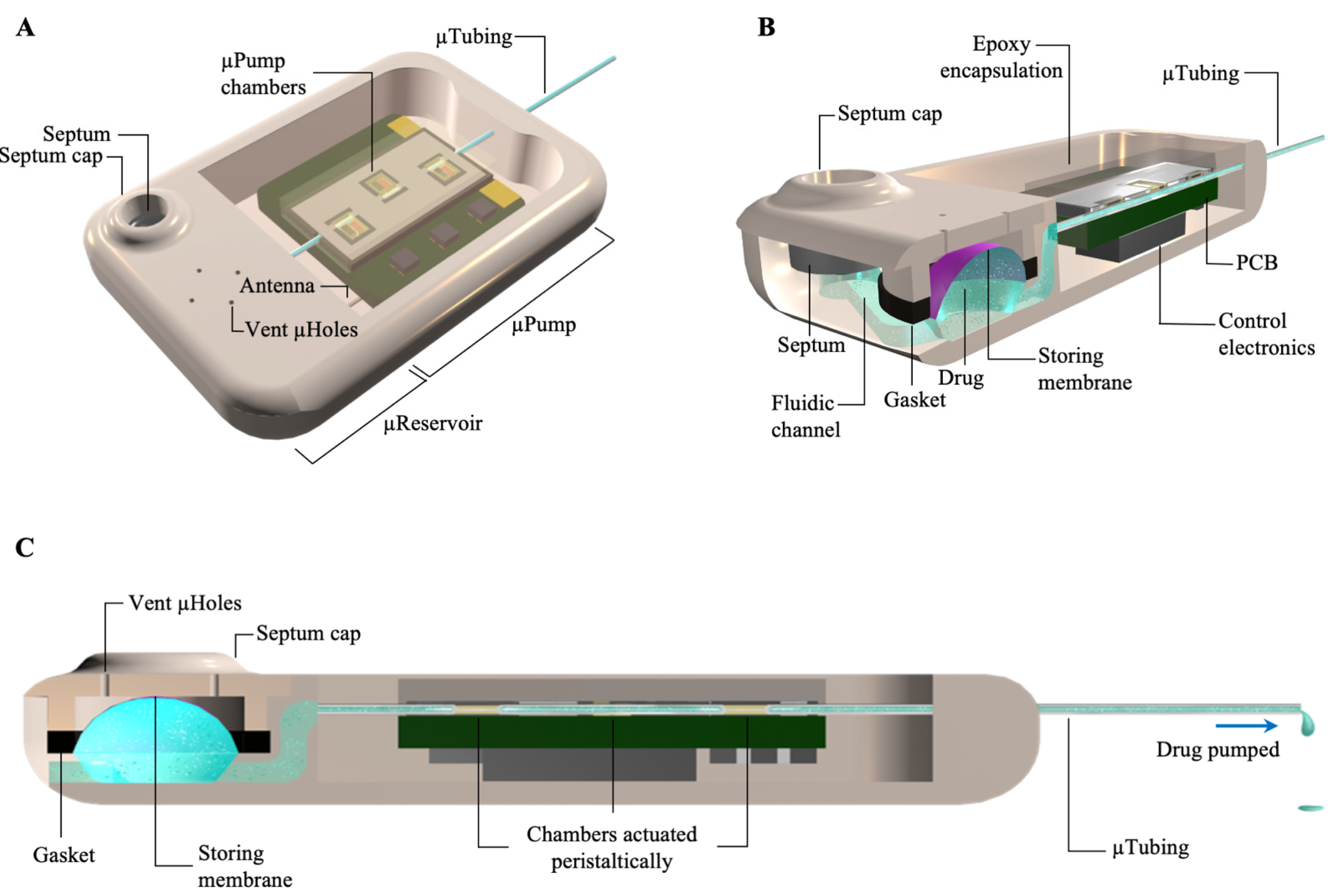
2.1. Benchtop Experiments
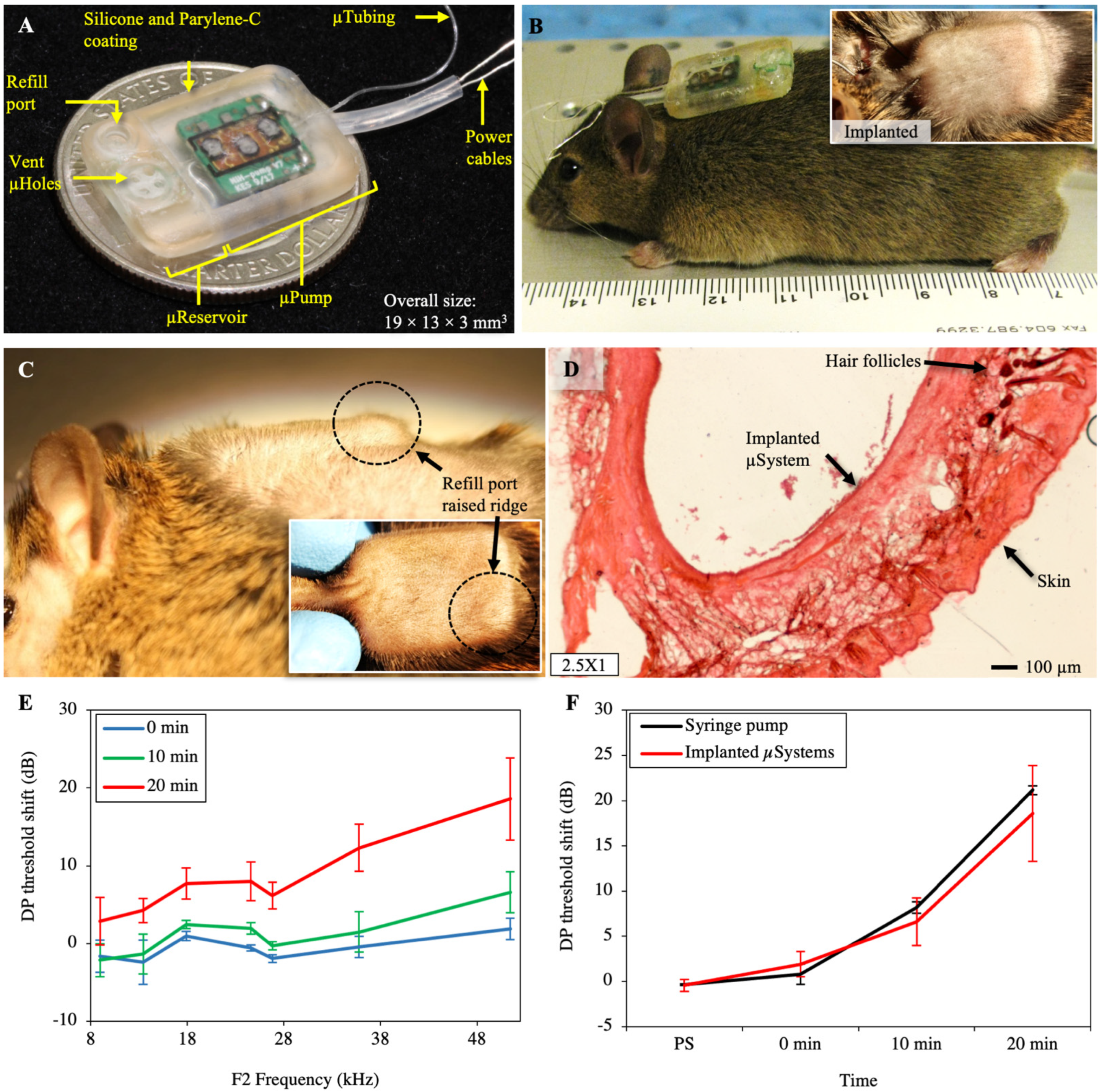
2.2. Implantation and Long-Term Biocompatibility
2.3. Inner-Ear Drug Delivery
3. Discussion
4. Materials and Methods
4.1. Fabrication Process
4.2. Benchtop Experimental Methods
4.3. In Vivo Experimental Methods
4.3.1. Subcutaneous Implantation and Biocompatibility
4.3.2. Inner-Ear Drug Delivery
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Temperature Controller
Appendix B. Thermal Simulation of Subcutaneous Environment

References
- Stevenson, C.L.; Santini, J.T.; Langer, R. Reservoir-based drug delivery systems utilizing microtechnology. Adv. Drug Deliv. Rev. 2012, 64, 1590–1602. [Google Scholar] [CrossRef] [Green Version]
- Nagai, N.; Kaji, H.; Onami, H.; Katsukura, Y.; Ishikawa, Y.; Nezhad, Z.K.; Sampei, K.; Iwata, S.; Ito, S.; Nishizawa, M.; et al. A Platform for Controlled Dual-Drug Delivery to the Retina: Protective Effects against Light-Induced Retinal Damage in Rats. Adv. Health Mater. 2014, 3, 1555–1560. [Google Scholar] [CrossRef] [PubMed]
- Chung, A.; Huh, Y.S.; Erickson, D. A robust, electrochemically driven microwell drug delivery system for controlled vasopressin release. Biomed. Microdevices 2009, 11, 861–867. [Google Scholar] [CrossRef]
- Elman, N.M.; Duc, H.L.H.; Cima, M.J. An implantable MEMS drug delivery device for rapid delivery in ambulatory emergency care. Biomed. Microdevices 2009, 11, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Humayun, M.; Santos, A.; Altamirano, J.C.; Ribeiro, R.; Gonzalez, R.; De La Rosa, A.; Shih, J.; Pang, C.; Jiang, F.; Calvillo, P.; et al. Implantable MicroPump for Drug Delivery in Patients with Diabetic Macular Edema. Transl. Vis. Sci. Technol. 2014, 3, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salt, A.; Hartsock, J.; Gill, R.; Smyth, D.; Kirk, J.; Verhoeven, K. Perilymph pharmacokinetics of marker applied through a cochlear implant in guinea pigs. PLoS ONE 2017, 12, e0183374. [Google Scholar] [CrossRef]
- Sanjay, S.T.; Zhou, W.; Dou, M.; Tavakoli, H.; Ma, L.; Xu, F.; Li, X. Recent advances of controlled drug delivery using microfluidic platforms. Adv. Drug Deliv. Rev. 2018, 128, 3–28. [Google Scholar] [CrossRef]
- Mousoulis, C.; Ochoa, M.; Papageorgiou, D.; Ziaie, B. A Skin-Contact-Actuated Micropump for Transdermal Drug Delivery. IEEE Trans. Biomed. Eng. 2011, 58, 1492–1498. [Google Scholar] [CrossRef]
- Cantwell, C.T.; Wei, P.; Ziaie, B.; Rao, M.P. Modular reservoir concept for MEMS-based transdermal drug delivery systems. J. Micromech. Microeng. 2014, 24, 117001. [Google Scholar] [CrossRef]
- Oka, K.; Aoyagi, S.; Arai, Y.; Isono, Y.; Hashiguchi, G.; Fujita, H. Fabrication of a micro needle for a trace blood test. Sens. Actuators A Phys. 2002, 97–98, 478–485. [Google Scholar] [CrossRef]
- Roxhed, N.; Samel, B.; Nordquist, L.; Griss, P.; Stemme, G. Painless Drug Delivery Through Microneedle-Based Transdermal Patches Featuring Active Infusion. IEEE Trans. Biomed. Eng. 2008, 55, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Stoeber, D.; Liepmann, B. Design, Fabrication and Testing of a MEMS Syringe. In Proceedings of the Solid-State Sensor and Actuator Workshop, Hilton Head Island, SC, USA, 2–6 June 2002. [Google Scholar]
- Kochhar, J.S.; Lim, W.X.S.; Zou, S.; Foo, W.Y.; Pan, J.; Kang, L. Microneedle Integrated Transdermal Patch for Fast Onset and Sustained Delivery of Lidocaine. Mol. Pharm. 2013, 10, 4272–4280. [Google Scholar] [CrossRef]
- Forouzandeh, F.; Arevalo, A.; Alfadhel, A.; Borkholder, D.A. A review of peristaltic micropumps. Sensors Actuators A: Phys. 2021, 326, 112602. [Google Scholar] [CrossRef]
- Johnson, D.G.; Frisina, R.D.; Borkholder, D.A. In-Plane Biocompatible Microfluidic Interconnects for Implantable Microsystems. IEEE Trans. Biomed. Eng. 2010, 58, 943–948. [Google Scholar] [CrossRef] [Green Version]
- Andrews, J.C.; Walker-Andrews, S.C.; Ensminger, W.D. Long-term central venous access with a peripherally placed subcutaneous infusion port: Initial results. Radiology 1990, 176, 45–47. [Google Scholar] [CrossRef]
- Strum, S.; McDermed, J.; Korn, A.; Joseph, C. Improved methods for venous access: The Port-A-Cath, a totally implanted catheter system. J. Clin. Oncol. 1986, 4, 596–603. Available online: https://www.researchgate.net/profile/Stephen_Strum/publication/284291764_The_Port-a-cath_PAC_A_totally_implanted_catheter_system/links/56aa5b0008aef6e05df46165/The-Port-a-cath-PAC-A-totally-implanted-catheter-system.pdf (accessed on 1 April 2019). [CrossRef]
- iPRECIO®. Available online: http://www.iprecio.com (accessed on 3 July 2020).
- Prometra Pump/Flowonix. Available online: http://www.flowonix.com/healthcare-provider/products/prometra-pump (accessed on 3 July 2020).
- Li, T.; Evans, A.T.; Chiravuri, S.; Gianchandani, R.Y.; Gianchandani, Y.B. Compact, power-efficient architectures using microvalves and microsensors, for intrathecal, insulin, and other drug delivery systems. Adv. Drug Deliv. Rev. 2012, 64, 1639–1649. [Google Scholar] [CrossRef] [PubMed]
- Reynaerts, D.; Peirs, J.; van Brussel, H.; Leuven, K.U. A Sma-Actuated Implantable System for Delivery of Liquid Drugs. In Proceedings of the Fifth International Conference on New Actuators, Bremen, Germany, 26–28 June 1996; pp. 26–28. [Google Scholar]
- Shepherd, R.F.; Stokes, A.; Nunes, R.M.D.; Whitesides, G.M. Soft Machines That are Resistant to Puncture and That Self Seal. Adv. Mater. 2013, 25, 6709–6713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Circuits, L.E.F.; Elast, P. Re-configurable fluid circuits by PDMS elastomer micromachining. In Proceedings of the Technical Digest. IEEE International MEMS 99 Conference. Twelfth IEEE International Conference on Micro Electro Mechanical Systems (Cat. No.99CH36291), Orlando, FL, USA, 21–21 January 1999; pp. 222–227. [Google Scholar]
- Chaudhury, M.K.; Whitesides, G.M. Direct measurement of interfacial interactions between semispherical lenses and flat sheets of poly(dimethylsiloxane) and their chemical derivatives. Langmuir 1991, 7, 1013–1025. [Google Scholar] [CrossRef]
- Lee, M.W.; An, S.; Yoon, S.S.; Yarin, A.L. Advances in self-healing materials based on vascular networks with mechanical self-repair characteristics. Adv. Colloid Interface Sci. 2018, 252, 21–37. [Google Scholar] [CrossRef]
- HamiltonTM GC Septa. Available online: https://www.hamiltoncompany.com/laboratory-products/gc-septa (accessed on 4 June 2021).
- Lo, R.; Li, P.-Y.; Saati, S.; Agrawal, R.N.; Humayun, M.S.; Meng, E. A passive MEMS drug delivery pump for treatment of ocular diseases. Biomed. Microdevices 2009, 11, 959–970. [Google Scholar] [CrossRef] [PubMed]
- Lo, R.; Li, P.-Y.; Saati, S.; Agrawal, R.; Humayun, M.S.; Meng, E. A refillable microfabricated drug delivery device for treatment of ocular diseases. Lab Chip 2008, 8, 1027–1030. [Google Scholar] [CrossRef]
- Lo, R.; Kuwahara, K.; Li, P.-Y.; Agrawal, R.; Humayun, M.S.; Meng, E. A Passive Refillable Intraocular MEMS Drug Delivery Device. In Proceedings of the 2006 International Conference on Microtechnologies in Medicine and Biology, Okinawa, Japan, 9–12 May 2006; pp. 74–77. [Google Scholar] [CrossRef] [Green Version]
- Li, P.-Y.; Shih, J.; Lo, R.; Saati, S.; Agrawal, R.; Humayun, M.S.; Tai, Y.-C.; Meng, E. An electrochemical intraocular drug delivery device. Sens. Actuators Phys. 2008, 143, 41–48. [Google Scholar] [CrossRef]
- Gensler, H.; Sheybani, R.; Li, P.-Y.; Mann, R.L.; Meng, E. An implantable MEMS micropump system for drug delivery in small animals. Biomed. Microdevices 2012, 14, 483–496. [Google Scholar] [CrossRef] [Green Version]
- Hsu, M.-C.; Alfadhel, A.; Forouzandeh, F.; Borkholder, D.A. Biocompatible magnetic nanocomposite microcapsules as microfluidic one-way diffusion blocking valves with ultra-low opening pressure. Mater. Des. 2018, 150, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Trull, F.L.; Rich, B.A. More regulation of rodents. Science 1999, 284, 1463. [Google Scholar] [CrossRef]
- Benedikz, E.; Kloskowska, E.; Winblad, B. The rat as an animal model of Alzheimer’s disease. J. Cell. Mol. Med. 2009, 13, 1034–1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forouzandeh, F.; Ahamed, N.N.; Hsu, M.-C.; Walton, J.P.; Frisina, R.D.; Borkholder, D.A. A 3D-Printed Modular Microreservoir for Drug Delivery. Micromachines 2020, 11, 648. [Google Scholar] [CrossRef] [PubMed]
- Forouzandeh, F.; Zhu, X.; Alfadhel, A.; Ding, B.; Walton, J.P.; Cormier, D.; Frisina, R.D.; Borkholder, D.A. A nanoliter resolution implantable micropump for murine inner ear drug delivery. J. Control. Release 2019, 298, 27–37. [Google Scholar] [CrossRef]
- Forouzandeh, F.; Borkholder, D.A. Microtechnologies for inner ear drug delivery. Curr. Opin. Otolaryngol. Head Neck Surg. 2020, 28. [Google Scholar] [CrossRef]
- Borkholder, D.A.; Zhu, X.; Frisina, R.D. Round window membrane intracochlear drug delivery enhanced by induced advection. J. Control. Release 2014, 174, 171–176. [Google Scholar] [CrossRef] [Green Version]
- Urquhart, J.; Fara, J.W.; Willis, K.L. Rate-Controlled Delivery Systems in Drug and Hormone Research. Annu. Rev. Pharmacol. Toxicol. 1984, 24, 199–236. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER). Guidance for Industry: Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers; CDER: Rockville, MD, USA, 2005.
- Species Specific Information: Mouse. Available online: http://web.jhu.edu/animalcare/procedures/mouse (accessed on 8 March 2020).
- Sheybani, E.; Cobo, R.; Meng, A. Wireless Programmable Electrochemical Drug Delivery Micropump with Fully Inte-grated Electrochemical Dosing Sensors. Biomed. Microdevices 2015, 17, 74. [Google Scholar] [CrossRef] [PubMed]
- Tandon, V.; Kang, W.S.; Robbins, T.A.; Spencer, A.J.; Kim, E.S.; McKenna, M.J.; Kujawa, S.G.; Fiering, J.; Pararas, E.E.L.; Mescher, M.J.; et al. Microfabricated reciprocating micropump for intracochlear drug delivery with integrated drug/fluid storage and electronically controlled dosing. Lab Chip 2016, 16, 829–846. [Google Scholar] [CrossRef] [PubMed]
- IPRECIO SMP-310R. Available online: https://www.iprecio.com/products/tabid/245/Default.aspx (accessed on 3 July 2020).
- Rogers, C.I.; Qaderi, K.; Woolley, A.T.; Nordin, G.P. 3D printed microfluidic devices with integrated valves. Biomicrofluidics 2015, 9, 016501. [Google Scholar] [CrossRef]
- Oliver, D.; He, D.Z.Z.; Klöcker, N.; Ludwig, J.; Schulte, U.; Waldegger, S.; Ruppersberg, J.P.; Dallos, P.; Fakler, B. Intracellular Anions as the Voltage Sensor of Prestin, the Outer Hair Cell Motor Protein. Science 2001, 292, 2340–2343. [Google Scholar] [CrossRef] [PubMed]
- Frisina, R.D.; Ding, B.; Zhu, X.; Walton, J.P. Age-related hearing loss: Prevention of threshold declines, cell loss and apoptosis in spiral ganglion neurons. Aging 2016, 8, 2081–2099. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forouzandeh, F.; Ahamed, N.N.; Zhu, X.; Bazard, P.; Goyal, K.; Walton, J.P.; Frisina, R.D.; Borkholder, D.A. A Wirelessly Controlled Scalable 3D-Printed Microsystem for Drug Delivery. Pharmaceuticals 2021, 14, 538. https://doi.org/10.3390/ph14060538
Forouzandeh F, Ahamed NN, Zhu X, Bazard P, Goyal K, Walton JP, Frisina RD, Borkholder DA. A Wirelessly Controlled Scalable 3D-Printed Microsystem for Drug Delivery. Pharmaceuticals. 2021; 14(6):538. https://doi.org/10.3390/ph14060538
Chicago/Turabian StyleForouzandeh, Farzad, Nuzhet N. Ahamed, Xiaoxia Zhu, Parveen Bazard, Krittika Goyal, Joseph P. Walton, Robert D. Frisina, and David A. Borkholder. 2021. "A Wirelessly Controlled Scalable 3D-Printed Microsystem for Drug Delivery" Pharmaceuticals 14, no. 6: 538. https://doi.org/10.3390/ph14060538







