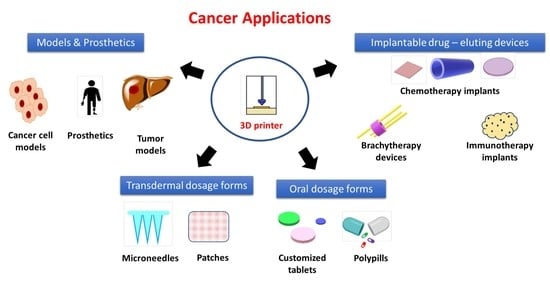
Three-Dimensional Printing for Cancer Applications: Research Landscape and Technologies
Abstract
:1. Introduction
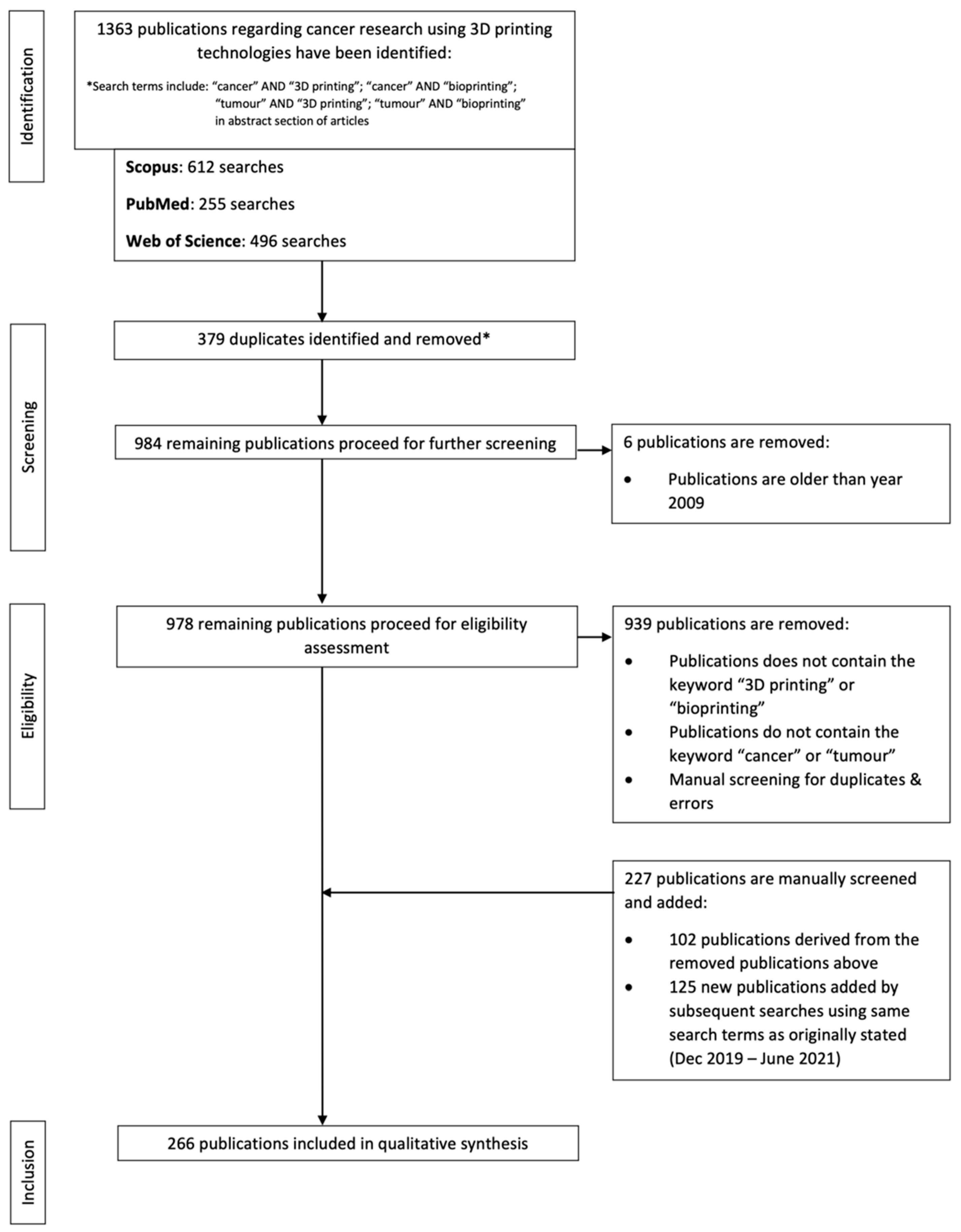
2. Papers and Patents Related to Cancer Research Using 3D-Printing Technologies
3. Clinical and Market Use of 3D-Printed Products for Cancer Treatment
4. 3D Printing of Anticancer Dosage Forms
5. 3D Printing of Implantable Drug Delivery Devices
5.1. 3D Printing of Local Chemotherapy or Thermotherapy Implants
5.2. 3D Printing of Brachytherapy Devices
5.3. 3D Printing of Local Immunotherapy Implant
6. 3D Printing of Oral Solid Dosage Forms
7. 3D Printing of Transdermal Dosage Forms
8. 3D Bioprinting of Cancer Cell Models
8.1. 2D Model vs. Animal Model vs. 3D Model vs. 3D-Bioprinted Model
8.2. 3D Bioprinters
9. 3D Bioprinting of Cancer Cell Model
9.1. Angiogenesis Model
9.2. Tumour Microenvironment
9.3. Metastasis
9.4. Tumour Spheroids
9.5. Organs-On-Chips: Microfluidics System
9.6. 3D Bioprinting for Anticancer Drug Development and Therapeutic Screening
10. The Limitation of 3D-Bioprinted Cancer Models
11. 3D Printing of Nonbiological Medical Devices
11.1. 3D-Printed Models for Training and Planning of Cancer-Related Procedures
11.2. 3D Printing of Prosthetics after Tumour Surgery
11.3. Limitations of 3D-Printed Nonbiological Medical Devices
| Organ | Purpose | Printing Material | Reference |
|---|---|---|---|
| Kidney | Surgical planning and patient counselling of a partial nephrectomy procedure | Thermoplastics | [147] |
| Liver, Kidney, Lung, Prostate and Arteries | Medical education | Polyamide | [149] |
| Liver | Liver transplant procedure | TangoPlus, VeroClear, TangoBlack and VeroBlue | [115] |
| Liver | Medical education | Nylon | [150] |
| Liver | Surgical planning for hepatectomy for colorectal cancer metastases | Silicon | [151] |
| Liver | Pre-operative planning | TangoPlus and TangoBlack | [152] |
| Bladder and Urethra | Robotic vesicourethral anastomosis | Silicon | [40] |
| Brain | Surgical planning in pediatric glioma | VeroClear | [153] |
| Brain | Neurosurgical planning | - | [154] |
| Mandible | Mandibular reconstruction | Photopolymer | [155] |
| Lung | Patient counselling in stage I cancer | Photopolymer | [156] |
| Thorax | Surgical planning for removal of the thoracic tumour | - | [153] |
12. Challenges and Future Orientations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Me, J.F.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serrano, D.R.; Terres, M.C.; Lalatsa, A. Applications of 3D printing in cancer. J. 3D Print. Med. 2018, 2, 115–127. [Google Scholar] [CrossRef]
- Cho, S.-H.; Jeon, J.; Kim, S.I. Personalized Medicine in Breast Cancer: A Systematic Review. J. Breast Cancer 2012, 15, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Król, M.; Pawłowski, K.M.; Majchrzak, K.; Szyszko, K.; Motyl, T. Why chemotherapy can fail? Pol. J. Vet. Sci. 2010, 13, 399–406. [Google Scholar] [PubMed]
- Mitrus, I.; Szala, S. Chemotherapy—Main causes of failure. Nowotwory 2009, 59, 368–376. [Google Scholar]
- Lyman, G.H. Impact of chemotherapy dose intensity on cancer patient outcomes. J. Natl. Compr. Cancer Netw. 2009, 7, 99–108. [Google Scholar] [CrossRef]
- Cho, J.Y. Molecular diagnosis for personalized target therapy in gastric cancer. J. Gastric Cancer 2013, 13, 129–135. [Google Scholar] [CrossRef]
- Diamandis, M.; White, N.M.; Yousef, G.M. Personalized medicine: Marking a new epoch in cancer patient management. Mol. Cancer Res. 2010, 8, 1175–1187. [Google Scholar] [CrossRef] [Green Version]
- Kamel, H.F.M.; Al-Amodi, H.S.A.B. Exploitation of gene expression and cancer biomarkers in paving the path to era of personalized medicine. Genom. Proteom. Bioinform. 2017, 15, 220–235. [Google Scholar] [CrossRef] [PubMed]
- Berman, B. 3-D printing: The new industrial revolution. Bus. Horizons 2012, 55, 155–162. [Google Scholar] [CrossRef]
- Li, Y.Y.; Jones, S.J. Drug repositioning for personalized medicine. Genome Med. 2012, 4, 27. [Google Scholar] [CrossRef] [Green Version]
- Fitzpatrick, A.P.; Mohanned, M.I.; Collins, P.K.; Gibson, I. Design of a patient specific, 3D printed arm cast. KnE Eng. 2017, 2, 135–142. [Google Scholar] [CrossRef]
- Abrahams, E.; Ginsburg, G.S.; Silver, M. The personalized medicine coalition. Am. J. Pharm. 2005, 5, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Zema, L.; Melocchi, A.; Maroni, A.; Gazzaniga, A. Three-dimensional printing of medicinal products and the challenge of personalized therapy. J. Pharm. Sci. 2017, 106, 1697–1705. [Google Scholar] [CrossRef] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. The PRISMA group preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Prima, M.; Coburn, J.; Hwang, D.; Kelly, J.; Khairuzzaman, A.; Ricles, L. Additively manufactured medical products—The FDA perspective. 3D Print. Med. 2016, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oxford Performance Materials. Oxford Performance Materials Receives FDA Clearance for SpineFab VBR Implant System. 2015. Available online: Oxfordpm.com/news-events/opm-press-releases?id=339756/oxford-performance-materials-receives-fda-clearance-for-spinefab-vbr-implant-system (accessed on 9 December 2019).
- Oxford Performance Materials. OsteoFab® Implants. 2019. Available online: Oxfordpm.com/cmf-orthopedics/osteofab-implants (accessed on 10 December 2019).
- Zhu, W.; Holmes, B.; Glazer, R.I.; Zhang, L.G. 3D printed nanocomposite matrix for the study of breast cancer bone metastasis. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 69–79. [Google Scholar] [CrossRef]
- Materialise. Materialise First Company to Receive FDA Clearance for Diagnostic 3D-Printed Anatomical Models. 2018. Available online: www.materialise.com/en/press-releases/materialise-first-company-to-receive-fda-clearance-for-diagnostic-3d-printed-models (accessed on 9 December 2019).
- Shafiee, A.; Atala, A. Printing technologies for medical applications. Trends Mol. Med. 2016, 22, 254–265. [Google Scholar] [CrossRef] [PubMed]
- Norman, J.; Madurawe, R.D.; Moore, C.M.; Khan, M.; Khairuzzaman, A. A new chapter in pharmaceutical manufacturing: 3D-printed drug products. Adv. Drug Deliv. Rev. 2017, 108, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Zilberman, M.; Kraitzer, A.; Grinberg, O.; Elsner, J.J. Drug-Eluting Medical Implants. Drug Deliv. 2009, 197, 299–341. [Google Scholar]
- Lim, S.H.; Kathuria, H.; Tan, J.J.Y.; Kang, L. 3D printed drug delivery and testing systems—A passing fad or the future? Adv. Drug Deliv. Rev. 2018, 132, 139–168. [Google Scholar] [CrossRef] [PubMed]
- Jamróz, W.; Szafraniec-Szczęsny, J.; Kurek, M.; Jachowicz, R. 3D printing in pharmaceutical and medical applications—Recent achievements and challenges. Pharm. Res. 2018, 35, 176. [Google Scholar] [CrossRef] [Green Version]
- Mansour, H.M.; Sohn, M.; Al-Ghananeem, A.; DeLuca, P.P. Materials for pharmaceutical dosage forms: Molecular pharmaceutics and controlled release drug delivery aspects. Int. J. Mol. Sci. 2010, 11, 3298–3322. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Le, D.Q.; Hein, S.; Li, P.; Nygaard, J.V.; Kassem, M.; Kjems, J.; Besenbacher, F.; Bünger, C. Fabrication and characterization of a rapid prototyped tissue engineering scaffold with embedded multicomponent matrix for controlled drug release. Int. J. Nanomed. 2012, 7, 4285–4297. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhao, S.; Zhu, M.; Zhu, Y.; Zhang, Y.; Liu, Z.; Zhang, C. 3D-printed magnetic Fe3O4/MBG/PCL composite scaffolds with multifunctionality of bone regeneration, local anticancer drug delivery and hyperthermia. J. Mater. Chem. B 2014, 2, 7583–7595. [Google Scholar] [CrossRef]
- Ma, H.; Luo, J.; Sun, Z.; Xia, L.; Shi, M.; Liu, M.; Chang, J.; Wu, C. 3D printing of biomaterials with mussel-inspired nanostructures for tumor therapy and tissue regeneration. Biomaterials 2016, 111, 138–148. [Google Scholar] [CrossRef]
- Ma, H.; Li, T.; Huan, Z.; Zhang, M.; Yang, Z.; Wang, J.; Chang, J.; Wu, C. 3D printing of high-strength bioscaffolds for the synergistic treatment of bone cancer. NPG Asia Mater. 2018, 10, 31–44. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Wei, X.; Wan, Y.; Lin, X.; Wang, Z.; Huang, P. 3D printing of hydrogel scaffolds for future application in photothermal therapy of breast cancer and tissue repair. Acta Biomater. 2019, 92, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Zachkani, P.; Jackson, J.K.; Pirmoradi, F.N.; Chiao, M. A cylindrical magnetically-actuated drug delivery device proposed for minimally invasive treatment of prostate cancer. RSC Adv. 2015, 5, 98087–98096. [Google Scholar] [CrossRef]
- Agila, S.; Poornima, J. Magnetically controlled nano-composite based 3D printed cell scaffolds as targeted drug delivery systems for cancer therapy. In Proceedings of the 2015 IEEE 15th International Conference on Nanotechnology (IEEE-NANO), Rome, Italy, 27–30 July 2015. [Google Scholar]
- Yi, H.-G.; Choi, Y.-J.; Kang, K.S.; Hong, J.M.; Pati, R.G.; Park, M.N.; Shim, I.K.; Lee, C.M.; Kim, S.C.; Cho, D.-W. A 3D-printed local drug delivery patch for pancreatic cancer growth suppression. J. Control. Release 2016, 238, 231–241. [Google Scholar] [CrossRef]
- Cho, H.; Jammalamadaka, U.; Tappa, K.; Egbulefu, C.; Prior, J.; Tang, R.; Achilefu, S. 3D printing of poloxamer 407 nanogel discs and their applications in adjuvant ovarian cancer therapy. Mol. Pharm. 2018, 16, 552–560. [Google Scholar] [CrossRef]
- Hosseinzadeh, R.; Mirani, B.; Pagan, E.; Mirzaaghaei, S.; Nasimian, A.; Kawalec, P.; da Silva Rosa, S.; Hamdi, D.; Fernandez, N.P.; Toyota, B.D.; et al. A drug-eluting 3D-printed mesh (GlioMesh) for management of glioblastoma. Adv. Ther. 2019, 11. [Google Scholar] [CrossRef]
- Kim, S.; Jeong, C.; Chang, K.; Ji, Y.; Cho, B.; Lee, D.; Kim, Y.; Song, S.; Lee, S.; Kwak, J. Development of 3D printed applicator in Brachytherapy for gynecologic cancer. Int. J. Radiat. Oncol. 2017, 99, E678. [Google Scholar] [CrossRef]
- Lindegaard, J.C.; Madsen, M.L.; Traberg, A.; Meisner, B.; Nielsen, S.K.; Tanderup, K.; Spejlborg, H.; Fokdal, L.U.; Nørrevang, O. Individualised 3D printed vaginal template for MRI guided brachytherapy in locally advanced cervical cancer. Radiother. Oncol. 2016, 118, 173–175. [Google Scholar] [CrossRef] [PubMed]
- Chmura, J.; Erdman, A.; Ehler, E.; Lawrence, J.; Wilke, C.T.; Rogers, B.; Ferreira, C. Novel design and development of a 3D-printed conformal superficial brachytherapy device for the treatment of non-melanoma skin cancer and keloids. 3D Print. Med. 2019, 5, 10. [Google Scholar] [CrossRef]
- Shee, K.; Koo, K.; Wu, X.; Ghali, F.M.; Halter, R.J.; Hyams, E.S. A novel ex vivo trainer for robotic vesicourethral anastomosis. J. Robot. Surg. 2019, 14, 21–27. [Google Scholar] [CrossRef]
- Arenas, M.; Sabater, S.; Sintas, A.; Arguís, M.; Hernández, V.; Árquez, M.; López, I.; Rovirosa, À.; Puig, D. Individualized 3D scanning and printing for non-melanoma skin cancer brachytherapy: A financial study for its integration into clinical workflow. J. Contemp. Brachyther. 2017, 9, 270–276. [Google Scholar] [CrossRef] [Green Version]
- Canters, R.A.; Lips, I.; Wendling, M.; Kusters, M.; van Zeeland, M.; Gerritsen, R.M.; Poortmans, P.; Verhoef, C.G. Clinical implementation of 3D printing in the construction of patient specific bolus for electron beam radiotherapy for non-melanoma skin cancer. Radiother. Oncol. 2016, 121, 148–153. [Google Scholar] [CrossRef]
- Goldberg, M.S. Immunoengineering: How nanotechnology can enhance cancer immunotherapy. Cell 2015, 161, 201–204. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Du, T.; Zhang, J.; Kang, T.; Luo, L.; Tao, J.; Gou, Z.; Chen, S.; Du, Y.; He, J.; et al. A 3D-engineered conformal implant releases DNA nanocomplexs for eradicating the postsurgery residual glioblastoma. Adv. Sci. 2017, 4, 1600491. [Google Scholar] [CrossRef]
- Ghosh, U.; Ning, S.; Wang, Y.; Kong, Y.L. Addressing unmet clinical needs with 3D printing technologies. Adv. Healthc. Mater. 2018, 7, e1800417. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.; Tan, D.K.; Nokhodchi, A.; Maniruzzaman, M. Drop-on-powder 3D printing of tablets with an anti-cancer drug, 5-fluorouracil. Pharmaceutics 2019, 11, 150. [Google Scholar] [CrossRef] [Green Version]
- Lopez, D.R.S.; Carda, J.R.; Fernandez-Garcia, R.; Perez-Ballesteros, L.F.; Papantonakis, M.P.B.; Lalatsa, K. Market demands in 3D printing pharmaceuticals products. In 3D Printing Technology in Nanomedicine; Elsevier: Pittsburgh, PA, USA, 2019. [Google Scholar]
- Goyanes, A.; Det-Amornrat, U.; Wang, J.; Basit, A.W.; Gaisford, S. 3D scanning and 3D printing as innovative technologies for fabricating personalized topical drug delivery systems. J. Control. Release 2016, 234, 41–48. [Google Scholar] [CrossRef]
- Muwaffak, Z.; Goyanes, A.; Clark, V.; Basit, A.W.; Hilton, S.T.; Gaisford, S. Patient-specific 3D scanned and 3D printed antimicrobial polycaprolactone wound dressings. Int. J. Pharm. 2017, 527, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Uddin, J.; Scoutaris, N.; Klepetsanis, P.; Chowdhry, B.; Prausnitz, M.; Douroumis, D. Inkjet printing of transdermal microneedles for the delivery of anticancer agents. Int. J. Pharm. 2015, 494, 593–602. [Google Scholar] [CrossRef] [Green Version]
- Maher, S.; Kaur, G.; Lima-Marques, L.; Evdokiou, A.; Losic, D. Engineering of micro- to nanostructured 3d-printed drug-releasing titanium implants for enhanced osseointegration and localized delivery of anticancer drugs. ACS Appl. Mater. Interfaces 2017, 9, 29562–29570. [Google Scholar] [CrossRef] [PubMed]
- Ahangar, P.; Akoury, E.; Luna, A.S.R.G.; Nour, A.; Weber, M.H.; Rosenzweig, D.H. Nanoporous 3D-printed scaffolds for local doxorubicin delivery in bone metastases secondary to prostate cancer. Materials 2018, 11, 1485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salmoria, G.V.; Klauss, P.; Kanis, L.A. Laser printing of PCL/Progesterone tablets for drug delivery Applications in hormone cancer therapy. Lasers Manuf. Mater. Process. 2017, 4, 108–120. [Google Scholar] [CrossRef]
- Yang, N.; Chen, H.; Han, H.; Shen, Y.; Gu, S.; He, Y.; Guo, S. 3D printing and coating to fabricate a hollow bullet-shaped implant with porous surface for controlled cytoxan release. Int. J. Pharm. 2018, 552, 91–98. [Google Scholar] [CrossRef]
- Qiao, X.; Yang, Y.; Huang, R.; Shi, X.; Chen, H.; Wang, J.; Chen, Y.; Tan, Y.; Tan, Z. E-Jet 3D-printed scaffolds as sustained multi-drug delivery vehicles in breast cancer therapy. Pharm. Res. 2019, 36, 182. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, L.; Mei, Z.; Zhang, F.; He, M.; Fletcher, C.; Wang, F.; Yang, J.; Bi, D.; Jiang, Y.; et al. 3D printed biodegradable implants as an individualized drug delivery system for local chemotherapy of osteosarcoma. Mater. Des. 2020, 186, 108336. [Google Scholar] [CrossRef]
- Salmoria, G.V.; Vieira, F.E.; Ghizoni, G.B.; Marques, M.S.; Kanis, L.A. 3D printing of PCL/Fluorouracil tablets by selective laser sintering: Properties of implantable drug delivery for cartilage cancer treatment. Drugs 2017, 4, 6. [Google Scholar]
- Pouliot, J.; Goldberg, K.; Hsu, I.C.; Cunha, J.A.M.; Animesh, G.A.R.G.; Patil, S.; Abbeel, P.; Siauw, T. Patient-Specific Temporary Implants for Accurately Guiding Local Means of Tumor Control Along Patient-Specific Internal Channels to Treat Cancer. U.S. Patent 10286197B2, 29 January 2015. [Google Scholar]
- Lu, Y.; Mantha, S.N.; Crowder, D.C.; Chinchilla, S.; Shah, K.; Yun, Y.H.; Wicker, R.B.; Choi, J.-W. Microstereolithography and characterization of poly(propylene fumarate)-based drug-loaded microneedle arrays. Biofabrication 2015, 7, 045001. [Google Scholar] [CrossRef]
- Uddin, J.; Scoutaris, N.; Economidou, S.N.; Giraud, C.; Chowdhry, B.Z.; Donnelly, R.; Douroumis, D. 3D printed microneedles for anticancer therapy of skin tumours. Mater. Sci. Eng. C 2020, 107, 110248. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.K.; Yun, Y.H.; Choi, J.S.; Choi, Y.C.; Kim, J.D.; Cho, Y.W. Fabrication of drug-loaded polymer microparticles with arbitrary geometries using a piezoelectric inkjet printing system. Int. J. Pharm. 2012, 427, 305–310. [Google Scholar] [CrossRef]
- Metiner, P.S.; Iz, S.G.; Biray-Avci, C. Bioengineering-inspired three-dimensional culture systems: Organoids to create tumor microenvironment. Gene 2019, 686, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Kapałczyńska, M.; Kolenda, T.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, L.; Lamperska, K. 2D and 3D cell cultures—A comparison of different types of cancer cell cultures. Arch. Med. Sci. 2016, 12, 910–919. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Thomas, D. Advances in medical polymer technology towards the panacea of complex 3D tissue and organ manufacture. Am. J. Surg. 2018, 217, 807–808. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, H.; Wu, D.; Chen, Q.; Zhou, Z.; Zhang, R.; Peng, X.; Su, Y.-C.; Sun, D. 3D printed microfluidic chip for multiple anticancer drug combinations. Sens. Actuators B Chem. 2018, 276, 507–516. [Google Scholar] [CrossRef]
- Schachtschneider, K.; Schwind, R.; Newson, J.; Kinachtchouk, N.; Rizko, M.; Mendoza-Elias, N.; Grippo, P.; Principe, D.R.; Park, A.; Overgaard, N.H.; et al. The oncopig cancer model: An innovative large animal translational oncology platform. Front. Oncol. 2017, 7, 190. [Google Scholar] [CrossRef]
- Wang, C.; Tang, Z.; Zhao, Y.; Yao, R.; Li, L.; Sun, W. Three-dimensional in vitro cancer models: A short review. Biofabrication 2014, 6, 022001. [Google Scholar] [CrossRef]
- Ma, X.; Liu, J.; Zhu, W.; Tang, M.; Lawrence, N.; Yu, C.; Gou, M.; Chen, S. 3D bioprinting of functional tissue models for personalized drug screening and in vitro disease modeling. Adv. Drug Deliv. Rev. 2018, 132, 235–251. [Google Scholar] [CrossRef]
- Albritton, J.L.; Miller, J.S. 3D bioprinting: Improving in vitro models of metastasis with heterogeneous tumor microenvironments. Dis. Model. Mech. 2017, 10, 3–14. [Google Scholar] [CrossRef] [Green Version]
- King, S.M.; Presnell, S.C.; Nguyen, D.G. Abstract 2034: Development of 3D bioprinted human breast cancer for in vitro drug screening. Cancer Res. 2014, 74, 2034. [Google Scholar] [CrossRef]
- Choudhury, D.; Anand, S.; Naing, M.W. The arrival of commercial bioprinters—Towards 3D bioprinting revolution! Int. J. Bioprint. 2018, 4, 139. [Google Scholar] [CrossRef] [PubMed]
- CELLINK. World’s First Universal Bioink. 2020. Available online: cellink.com/global/ (accessed on 24 February 2020).
- Jerums, G. World-First 3D Printer Helping Advance Cancer Treatment at Peter Mac Wins Award. 2019. Available online: connection.vic.gov.au/world-first-3d-printer-helping-advance-cancer-treatment-at-peter-mac-wins-award (accessed on 12 February 2020).
- Bredin, E. French Hospital Uses World’s Only Full Color, Multi-Material 3D Printing Technology to Improve Kidney Cancer Surgery. 2019. Available online: blog.stratasys.com/2019/07/18/french-hospital-uses-worlds-only-full-color-multi-material-3d-printing-technology-to-improve-kidney-cancer-surgery/ (accessed on 14 February 2020).
- Bordeaux, U. 3D Printing in Kidney Cancer Treatment. 2019. Available online: www.u-bordeaux.com/News/3D-printing-in-kidney-cancer-treatment (accessed on 14 February 2020).
- CSIRO. World-First Surgery Saves Cancer Patient’s Leg. 2019. Available online: www.csiro.au/en/Research/MF/Areas/Metals/Lab22/Titanium-Heel (accessed on 14 February 2020).
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knowlton, S.; Onal, S.; Yu, C.H.; Zhao, J.J.; Tasoglu, S. Bioprinting for cancer research. Trends Biotechnol. 2015, 33, 504–513. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Duchamp, M.; Oklu, R.; Ellisen, L.W.; Langer, R.; Khademhosseini, A. Bioprinting the cancer microenvironment. ACS Biomater. Sci. Eng. 2016, 2, 1710–1721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.; Delavaux, C.; Zhang, Y.S. 3D bioprinting for oncology applications. J. 3D Print. Med. 2019, 3, 55–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, V.K.; Dai, G.; Zou, H.; Yoo, S.S. Generation of 3-D glioblastoma-vascular niche using 3-D bioprinting. In Proceedings of the 2015 41st Annual Northeast Biomedical Engineering Conference (NEBEC), Troy, NY, USA, 17–19 April 2015. [Google Scholar]
- Charbe, N.; McCarron, P.; Tambuwala, M.M. Three-dimensional bio-printing: A new frontier in oncology research. World J. Clin. Oncol. 2017, 8, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Samavedi, S.; Joy, N. 3D printing for the development of in vitro cancer models. Curr. Opin. Biomed. Eng. 2017, 2, 35–42. [Google Scholar] [CrossRef]
- Zhao, Y.; Yao, R.; Ouyang, L.; Ding, H.; Zhang, T.; Zhang, K.; Cheng, S.; Sun, W. Three-dimensional printing of Hela cells for cervical tumor model in vitro. Biofabrication 2014, 6, 035001. [Google Scholar] [CrossRef] [PubMed]
- Guan, X. Cancer metastases: Challenges and opportunities. Acta Pharm. Sin. B 2015, 5, 402–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, F.; Meyer, C.M.; Joung, D.; Vallera, D.A.; McAlpine, M.C.; Panoskaltsis-Mortari, A. 3D bioprinted in vitro metastatic models via reconstruction of tumor microenvironments. Adv. Mater. 2019, 31, e1806899. [Google Scholar] [CrossRef]
- Knowlton, S.; Joshi, A.; Yenilmez, B.; Ozbolat, I.T.; Chua, C.K.; Khademhosseini, A.; Tasoglu, S. Advancing cancer research using bioprinting for tumor-on-a-chip platforms. Int. J. Bioprint. 2016, 2. [Google Scholar] [CrossRef] [Green Version]
- Nunes, A.S.; Barros, A.S.; Costa, E.C.; Moreira, A.F.; Correia, I.J. 3D tumor spheroids as in vitro models to mimic in vivo human solid tumors resistance to therapeutic drugs. Biotechnol. Bioeng. 2018, 116, 206–226. [Google Scholar] [CrossRef] [Green Version]
- Nath, S.; Devi, G.R. Three-dimensional culture systems in cancer research: Focus on tumor spheroid model. Pharmacol. Ther. 2016, 163, 94–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swaminathan, S.; Hamid, Q.; Sun, W.; Clyne, A.M. Bioprinting of 3D breast epithelial spheroids for human cancer models. Biofabrication 2019, 11. [Google Scholar] [CrossRef]
- Liao, W.; Wang, J.; Xu, J.; You, F.; Pan, M.; Xu, X.; Weng, J.; Han, X.; Li, S.; Li, Y.; et al. High-throughput three-dimensional spheroid tumor model using a novel stamp-like tool. J. Tissue Eng. 2019, 10. [Google Scholar] [CrossRef]
- Yi, H.-G.; Lee, H.; Cho, D.-W. 3D Printing of Organs-On-Chips. Bioengineering 2017, 4, 10. [Google Scholar] [CrossRef]
- Sun, H.; Jia, Y.; Dong, H.; Dong, D.; Zheng, J. Combining additive manufacturing with microfluidics: An emerging method for developing novel organs-on-chips. Curr. Opin. Chem. Eng. 2020, 28, 1–9. [Google Scholar] [CrossRef]
- Mi, S.; Du, Z.; Xu, Y.; Sun, W. The crossing and integration between microfluidic technology and 3D printing for organ-on-chips. J. Mater. Chem. B 2018, 6, 6191–6206. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Choudhury, D. Microfluidic bioprinting for organ-on-a-chip models. Drug Discov. Today 2019, 24, 1248–1257. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.M.B.; Ng, S.H.; Li, H.; Yoon, Y.-J. 3D printed microfluidics for biological applications. Lab Chip 2015, 15, 3627–3637. [Google Scholar] [CrossRef]
- Rużycka, M.; Cimpan, M.R.; Rios-Mondragon, I.; Grudzinski, I.P. Microfluidics for studying metastatic patterns of lung cancer. J. Nanobiotechnol. 2019, 17, 71. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Liu, C.-Y.; Wang, X.; Sweet, E.; Liu, N.; Gong, X.; Lin, L. 3D printed microfluidic devices for circulating tumor cells (CTCs) isolation. Biosens. Bioelectron. 2020, 150, 111900. [Google Scholar] [CrossRef]
- Wang, L.; Cao, T.; Li, X.; Huang, L. Three-dimensional printing titanium ribs for complex reconstruction after extensive posterolateral chest wall resection in lung cancer. J. Thorac. Cardiovasc. Surg. 2016, 152, e5–e7. [Google Scholar] [CrossRef] [Green Version]
- Valente, K.P.; Khetani, S.; Kolahchi, A.R.; Sanati-Nezhad, A.; Suleman, A.; Akbari, M. Microfluidic technologies for anticancer drug studies. Drug Discov. Today 2017, 22, 1654–1670. [Google Scholar] [CrossRef]
- Vanderburgh, J.; Sterling, J.A.; Guelcher, S.A. 3D Printing of tissue engineered constructs for in vitro modeling of disease progression and drug screening. Ann. Biomed. Eng. 2016, 45, 164–179. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Du, S.; Chai, L.M.; Xu, Y.; Liu, L.; Zhou, X.; Wang, J.; Zhang, W.; Liu, C.-H.; Wang, X. Anti-cancer drug screening based on a adipose-derived stem cell/hepatocyte 3D printing technique. J. Stem Cell Res. Ther. 2015, 5, 273. [Google Scholar]
- Lim, K.H.A.; Loo, Z.Y.; Goldie, S.J.; Adams, J.; McMenamin, P.G. Use of 3D printed models in medical education: A randomized control trial comparing 3D prints versus cadaveric materials for learning external cardiac anatomy. Anat. Sci. Educ. 2015, 9, 213–221. [Google Scholar] [CrossRef]
- Mafeld, S.; Nesbitt, C.; McCaslin, J.; Bagnall, A.; Davey, P.; Bose, P.; Williams, R. Three-dimensional (3D) printed endovascular simulation models: A feasibility study. Ann. Transl. Med. 2017, 5, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasan, T. Is dissection humane? J. Med. Ethics Hist. Med. 2011, 4, 4. [Google Scholar]
- Dare, A.J.; Anderson, B.O.; Sullivan, R.; Pramesh, C.S.; Yip, C.-H.; Ilbawi, A.; Adewole, I.F.; Badwe, R.A.; Gauvreau, C.L. Surgical services for cancer care. In Disease Control Priorities; The World Bank: Washington, DC, USA, 2015; Volume 3, pp. 223–238. [Google Scholar]
- van Renterghem, K.; Ghazi, A. 3D pelvic cadaver model: A novel approach to surgical training for penile implant surgery. Int. J. Impot. Res. 2020, 32, 261–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alshomer, F.; AlFaqeeh, F.; Alariefy, M.; Altweijri, I.; Alhumsi, T. Low-cost desktop-based three-dimensional-printed patient-specific craniofacial models in surgical counseling, consent taking, and education of parent of craniosynostosis patients: A comparison with conventional visual explanation modalities. J. Craniofacial Surg. 2019, 30, 1652–1656. [Google Scholar] [CrossRef]
- Knoedler, M.; Feibus, A.H.; Lange, A.; Maddox, M.M.; Ledet, E.; Thomas, R.; Silberstein, J.L. Individualized physical 3-dimensional kidney tumor models constructed from 3-dimensional printers result in improved trainee anatomic understanding. Urology 2015, 85, 1257–1262. [Google Scholar] [CrossRef]
- Cacciamani, G.E.; Okhunov, Z.; Meneses, A.D.; Socarrás, M.R.; Rivas, J.G.; Porpiglia, F.; Liatsikos, E.; Veneziano, D. Impact of three-dimensional printing in urology: State of the art and future perspectives. A systematic review by ESUT-YAUWP group. Eur. Urol. 2019, 76, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Lurje, I.; Czigany, Z.; Bednarsch, J.; Roderburg, C.; Isfort, P.; Neumann, U.P.; Lurje, G. Treatment strategies for hepatocellular carcinoma—A multidisciplinary approach. Int. J. Mol. Sci. 2019, 20, 1465. [Google Scholar] [CrossRef] [Green Version]
- Denizet, G.; Calame, P.; Lihoreau, T.; Kleinclauss, F.; Aubry, S. 3D multi-tissue printing for kidney transplantation. Quant. Imaging Med. Surg. 2018, 9, 101–106. [Google Scholar] [CrossRef]
- Förnvik, D.; Zackrisson, S.; Ljungberg, O.; Svahn, T.M.; Timberg, P.; Tingberg, A.; Andersson, I. Breast tomosynthesis: Accuracy of tumor measurement compared with digital mammography and ultrasonography. Acta Radiol. 2010, 51, 240–247. [Google Scholar] [CrossRef]
- Kusaka, M.; Sugimoto, M.; Fukami, N.; Sasaki, H.; Takenaka, M.; Anraku, T.; Ito, T.; Kenmochi, T.; Shiroki, R.; Hoshinaga, K. Initial experience with a tailor-made simulation and navigation program using a 3-D printer model of kidney transplantation surgery. Transplant. Proc. 2015, 47, 596–599. [Google Scholar] [CrossRef] [PubMed]
- Zein, N.N.; Hanouneh, I.A.; Bishop, P.D.; Samaan, M.; Eghtesad, B.; Quintini, C.; Miller, C.; Yerian, L.; Klatte, R. Three-dimensional print of a liver for preoperative planning in living donor liver transplantation. Liver Transplant. 2013, 19, 1304–1310. [Google Scholar] [CrossRef]
- Adams, F.; Qiu, T.; Mark, A.; Fritz, B.; Kramer, L.; Schlager, D.; Wetterauer, U.; Miernik, A.; Fischer, P. Soft 3D-printed phantom of the human kidney with collecting system. Ann. Biomed. Eng. 2016, 45, 963–972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, C.L.; Looi, T.; Lendvay, T.S.; Drake, J.M.; Farhat, W.A. Use of 3-dimensional printing technology and silicone modeling in surgical simulation: Development and face validation in pediatric laparoscopic pyeloplasty. J. Surg. Educ. 2014, 71, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Nguyen, N.H.; Hwang, S.I.; Lee, H.J.; Hong, S.K.; Byun, S.-S. Personalized 3D kidney model produced by rapid prototyping method and its usefulness in clinical applications. Int. Braz. J. 2018, 44, 952–957. [Google Scholar] [CrossRef]
- Costa, E.F.; Nogueira, T.E.; Lima, N.C.D.S.; Mendonça, E.F.; Leles, C.R. A qualitative study of the dimensions of patients’ perceptions of facial disfigurement after head and neck cancer surgery. Spec. Care Dent. 2013, 34, 114–121. [Google Scholar] [CrossRef]
- Aldaadaa, A.; Owji, N.; Knowles, J. Three-dimensional printing in maxillofacial surgery: Hype versus reality. J. Tissue Eng. 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Vialva, T. Cancer Survivor Receives Facial Prosthesis Made Using 3d Printing. 2020. Available online: 3dprintingindustry.com/news/cancer-survivor-receives-facial-prosthesis-made-using-3d-printing-167048/ (accessed on 3 March 2020).
- Salazar-Gamarra, R.; Seelaus, R.; Da Silva, J.V.L.; Da Silva, A.M.; Dib, L.L. Monoscopic photogrammetry to obtain 3D models by a mobile device: A method for making facial prostheses. J. Otolaryngol. Head Neck Surg. 2016, 45, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Neal, B. Cancer Survivor with 3D Printed Jaw Prosthesis Feels Good About Life Again, Inspires Doctor & 3D Designer. 2016. Available online: 3dprint.com/139868/3d-printed-jaw-prosthesis/ (accessed on 25 April 2020).
- Armstrong, R.; Ellis, M. Orbit reconstruction with 3D printed PEKK implant. J. Head Neck Surg. 2019, 2, 38–41. [Google Scholar]
- Le Clerc, N.; Baudouin, R.; Carlevan, M.; Khoueir, N.; Verillaud, B.; Herman, P. 3D titanium implant for orbital reconstruction after maxillectomy. J. Plast. Reconstr. Aesthetic Surg. 2020, 73, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Sutradhar, A.; Park, J.; Carrau, D.; Miller, M.J. Experimental validation of 3D printed patient-specific implants using digital image correlation and finite element analysis. Comput. Biol. Med. 2014, 52, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Sutradhar, A.; Park, J.; Carrau, D.; Nguyen, T.; Miller, M.J.; Paulino, G.H. Designing patient-specific 3D printed craniofacial implants using a novel topology optimization method. Med. Biol. Eng. Comput. 2015, 54, 1123–1135. [Google Scholar] [CrossRef]
- Han, H.H.; Shim, J.-H.; Lee, H.; Kim, B.Y.; Lee, J.-S.; Jung, J.W.; Yun, W.-S.; Baek, C.H.; Rhie, J.-W.; Cho, D.-W. Reconstruction of complex maxillary defects using patient-specific 3D-printed biodegradable scaffolds. Plast. Reconstr. Surg. Glob. Open 2018, 6, e1975. [Google Scholar] [CrossRef]
- Haidar, Z. 3D printed titanium implants: Colossal FDA-approved leap towards “personalized” maxillo-facial surgery. J. Oral Res. 2017, 6, 282–284. [Google Scholar] [CrossRef]
- Mohammed, M.I.; Fitzpatrick, A.P.; Gibson, I. Customised design and development of patient specific 3D printed whole mandible implant. In Proceedings of the 27th Annual International Solid Freeform Fabrication Symposium, Austin, TX, USA, 8–10 August 2016. [Google Scholar]
- Mitsouras, D.; Liacouras, P.; Imanzadeh, A.; Giannopoulos, A.A.; Cai, T.; Kumamaru, K.K.; George, E.; Wake, N.; Caterson, E.J.; Pomahac, B.; et al. Medical 3D printing for the radiologist. Radiographics 2015, 35, 1965–1988. [Google Scholar] [CrossRef]
- Palade, D.O.; Cobzeanu, B.M.; Zaharia, P.; Dabija, M. 3D Reconstruction role in surgical treatment of sinonasal tumours. Rev. Chim. 2018, 69, 1455–1457. [Google Scholar] [CrossRef]
- Walton, R.L.; Seelaus, R.; Robinson, B.R. Subtotal nasal reconstruction using a custom 3-dimensional porous polyethylene construct. Plast. Reconstr. Surg. Glob. Open 2019, 7, e2568. [Google Scholar] [CrossRef]
- Junn, J. The New 3d-Printed Solution for Breast Cancer Survivors. 2019. Available online: thespinoff.co.nz/business/06-10-2019/the-new-3d-printed-solution-for-breast-cancer-survivors/ (accessed on 25 April 2020).
- Chavoin, J.P.; Taizou, M.; Moreno, B.; Leyx, P.; Grolleau, J.L.; Chaput, B. Correcting poland syndrome with a custom-made silicone implant: Contribution of three-dimensional computer-aided design reconstruction. Plast. Reconstr. Surg. 2018, 142, 109–119. [Google Scholar] [CrossRef]
- Kite-Powell, J. Peking University Implants First 3D Printed Vertebra. 2014. Available online: www.forbes.com/sites/jenniferhicks/2014/08/19/peking-university-implants-first-3d-printed-vertebra/?sh=5ff93c2122c9 (accessed on 25 April 2020).
- Javelosa, J. 3D Printing Is Being Used to Make Prosthetic Bones for Cancer Patients. 2016. Available online: futurism.com/3d-printing-used-make-prosthetic-bones-cancer-patients (accessed on 3 May 2020).
- RMIT University. Orthopedic Design & Technology. ‘Just-in-Time’ 3D Printed Implants Set to Transform Bone Cancer Surgery. 2017. Available online: www.odtmag.com/contents/view_videos/2017-10-30/just-in-time-3d-printed-implants-set-to-transform-bone-cancer-surgery/ (accessed on 26 April 2020).
- Park, J.W.; Song, C.A.; Kang, H.G.; Kim, J.H.; Lim, K.M.; Kim, H.-S. Integration of a three-dimensional-printed titanium implant in human tissues: Case study. Appl. Sci. 2020, 10, 553. [Google Scholar] [CrossRef] [Green Version]
- Manero, A.; Smith, P.; Sparkman, J.; Dombrowski, M.; Courbin, D.; Kester, A.; Womack, I.; Chi, A. Implementation of 3D printing technology in the field of prosthetics: Past, present, and future. Int. J. Environ. Res. Public Health 2019, 16, 1641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taira, A.V.; Merrick, G.S.; Galbreath, R.W.; Andreini, H.; Taubenslag, W.; Curtis, R.; Butler, W.M.; Adamovich, E.; Wallner, K. Performance of transperineal template-guided mapping biopsy in detecting prostate cancer in the initial and repeat biopsy setting. Prostate Cancer Prostatic Dis. 2009, 13, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, X.; Yang, Q.; Wang, H.; Shi, T.; Chang, Y.; Xu, C.; Sun, Y. Three-dimensional printing technique assisted cognitive fusion in targeted prostate biopsy. Asian J. Urol. 2015, 2, 214–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ratinam, R.; Quayle, M.; Crock, J.; Lazarus, M.; Fogg, Q.; McMenamin, P. Challenges in creating dissectible anatomical 3D prints for surgical teaching. J. Anat. 2019, 234, 419–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souzaki, R.; Kinoshita, Y.; Ieiri, S.; Kawakubo, N.; Obata, S.; Jimbo, T.; Koga, Y.; Hashizume, M.; Taguchi, T. Preoperative surgical simulation of laparoscopic adrenalectomy for neuroblastoma using a three-dimensional printed model based on preoperative CT images. J. Pediatr. Surg. 2015, 50, 2112–2115. [Google Scholar] [CrossRef] [PubMed]
- Cantinotti, M.; Valverde, I.; Kutty, S. Three-dimensional printed models in congenital heart disease. Int. J. Cardiovasc. Imaging 2016, 33, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.B.; Lee, S.; Kim, H.; Yang, D.H.; Kim, Y.-H.; Kyung, Y.; Kim, C.-S.; Choi, S.H.; Kim, B.J.; Ha, H.; et al. Three-dimensional printing: Basic principles and applications in medicine and radiology. Korean J. Radiol. 2016, 17, 182–1977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Ge, H.-W.; Li, N.-C.; Yu, C.-F.; Guo, H.-F.; Jin, S.-H.; Liu, J.-S.; Na, Y.-Q. Evaluation of three-dimensional printing for laparoscopic partial nephrectomy of renal tumors: A preliminary report. World J. Urol. 2015, 34, 533–537. [Google Scholar] [CrossRef]
- Glybochko, P.; Rapoport, L.M.; Alyaev, Y.G.; Sirota, E.S.; Bezrukov, E.A.; Fiev, D.N.; Byadretdinov, I.S.; Bukatov, M.D.; Letunovskiy, A.V.; Korolev, D.O. Multiple application of three-dimensional soft kidney models with localized kidney cancer: A pilot study. Urol. J. 2018, 85, 99–105. [Google Scholar] [CrossRef]
- Javan, R.; Herrin, D.; Tangestanipoor, A. Understanding spatially complex segmental and branch anatomy using 3d printing: Liver, lung, prostate, coronary arteries, and circle of willis. Acad. Radiol. 2016, 23, 1183–1189. [Google Scholar] [CrossRef] [PubMed]
- Watson, R.A. A low-cost surgical application of additive fabrication. J. Surg. Educ. 2014, 71, 14–17. [Google Scholar] [CrossRef] [PubMed]
- Witowski, J.S.; Pędziwiatr, M.; Major, P.; Budzyński, A. Cost-effective, personalized, 3D-printed liver model for preoperative planning before laparoscopic liver hemihepatectomy for colorectal cancer metastases. Int. J. Comput. Assist. Radiol. Surg. 2017, 12, 2047–2054. [Google Scholar] [CrossRef] [Green Version]
- Madurska, M.J.; Poyade, M.; Eason, D.; Rea, P.; Watson, A.J.M. Development of a patient-specific 3D-printed liver model for preoperative planning. Surg. Innov. 2017, 24, 145–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.P.; Ta, A.H.; Ellsworth, W.A.; Marco, R.A.; Gaur, P.; Miller, J. Three dimensional model for surgical planning in resection of thoracic tumors. Int. J. Surg. Case Rep. 2015, 16, 127–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spottiswoode, B.; Heever, D.V.D.; Chang, Y.; Engelhardt, S.; du Plessis, S.; Nicolls, F.; Hartzenberg, H.; Gretschel, A. Preoperative three-dimensional model creation of magnetic resonance brain images as a tool to assist neurosurgical planning. Ster. Funct. Neurosurg. 2013, 91, 162–169. [Google Scholar] [CrossRef]
- Cohen, A.; Laviv, A.; Berman, P.; Nashef, R.; Abu Tair, J. Mandibular reconstruction using stereolithographic 3-dimensional printing modeling technology. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2009, 108, 661–666. [Google Scholar] [CrossRef]
- Yoon, S.H.; Park, S.; Kang, C.H.; Park, I.K.; Goo, J.M.; Kim, Y.T. Personalized 3D-printed model for informed consent for stage I lung cancer: A randomized pilot trial. Semin. Thorac. Cardiovasc. Surg. 2019, 31, 316–318. [Google Scholar] [CrossRef] [PubMed]
- Minocchieri, S.; Burren, J.M.; Bachmann, M.A.; Stern, G.; Wildhaber, J.; Buob, S.; Schindel, R.; Kraemer, R.; Frey, U.P.; Nelle, M. Development of the premature infant nose throat-model (PrINT-Model)—An upper airway replica of a premature neonate for the study of aerosol delivery. Pediatr. Res. 2008, 64, 141–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FDA. The Drug Development Process/Step 3: Clinical Research. 2020. Available online: www.fda.gov/patients/drug-development-process/step-3-clinical-research#Clinical_Research_Phase_Studies (accessed on 26 April 2020).
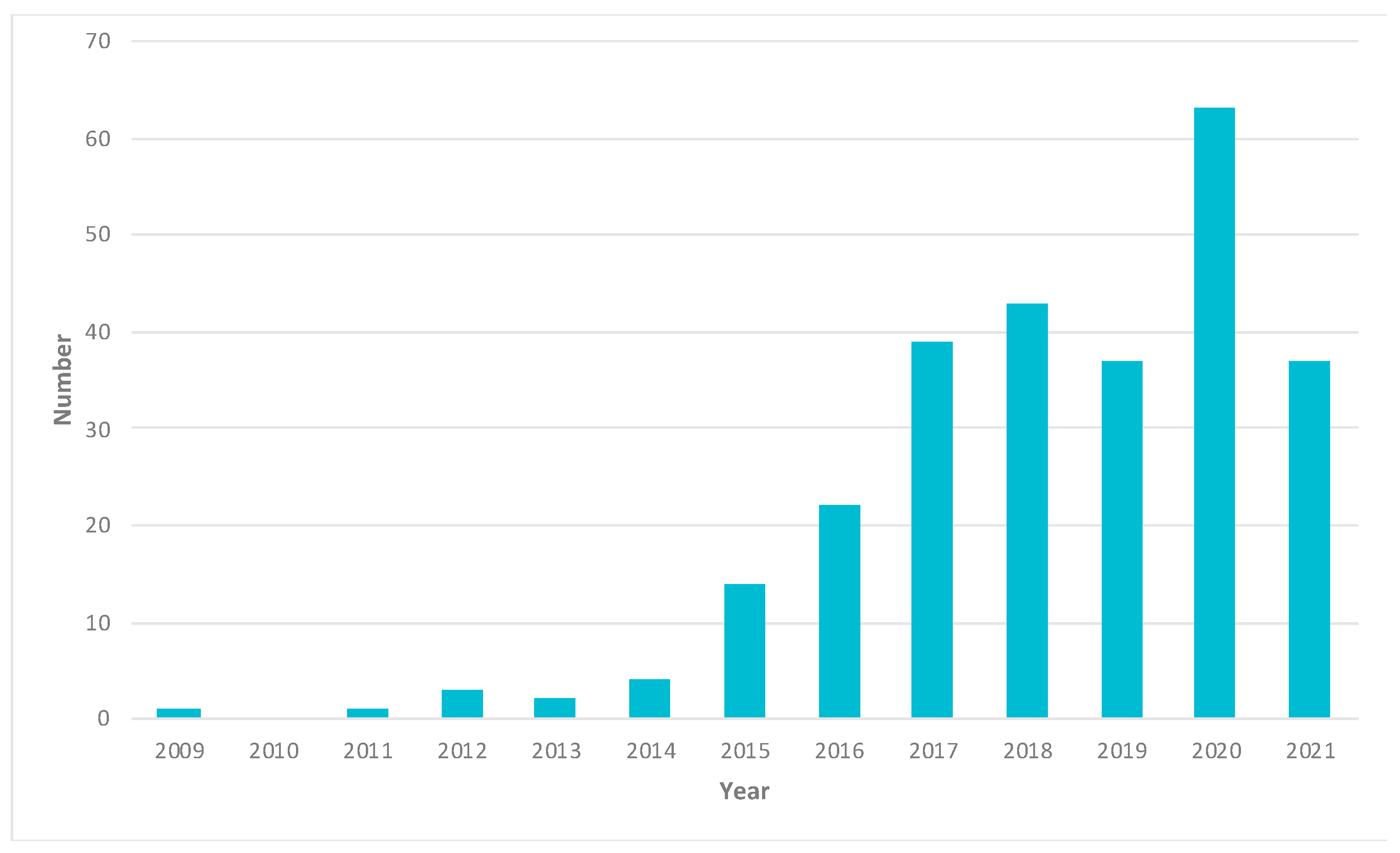
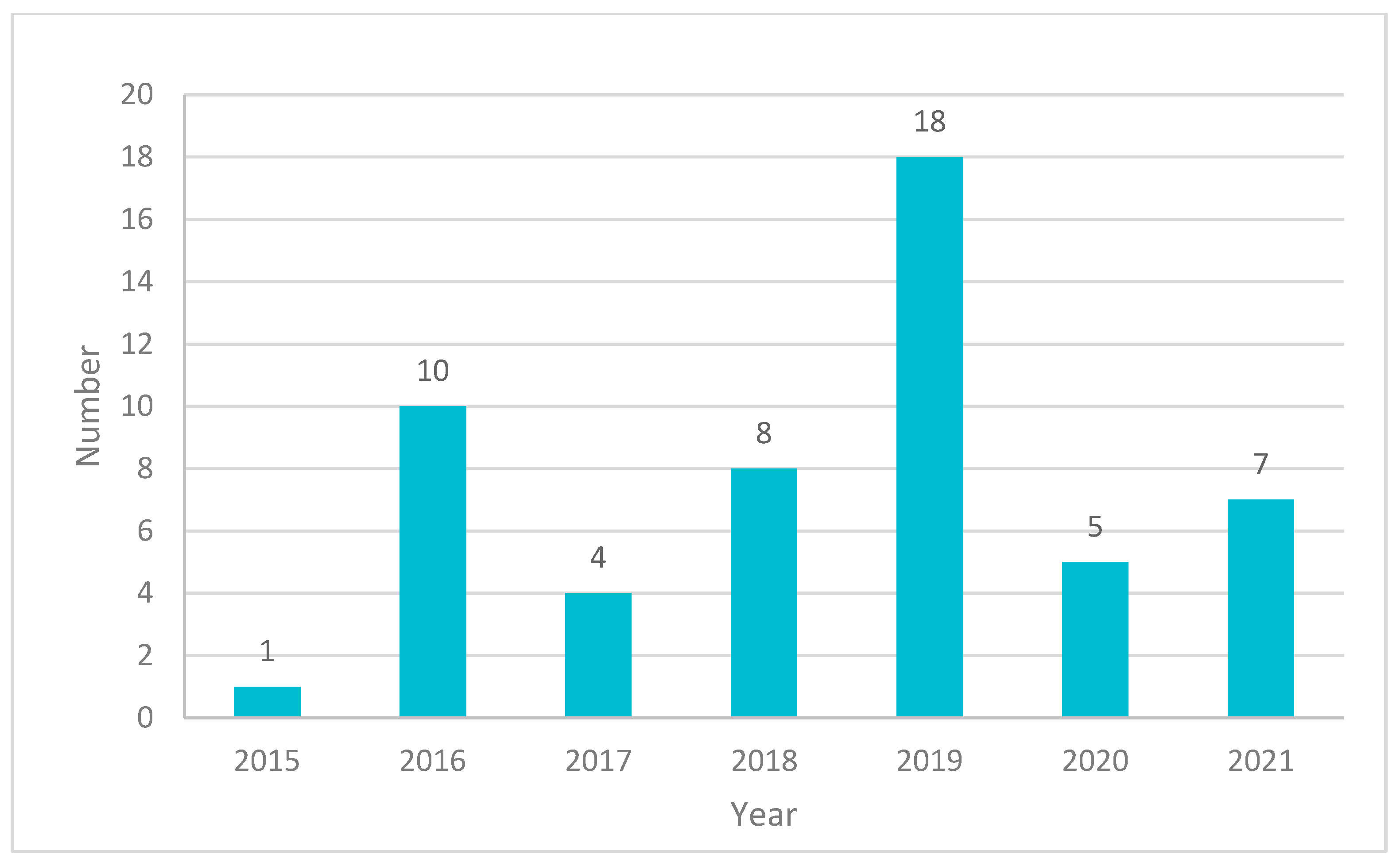
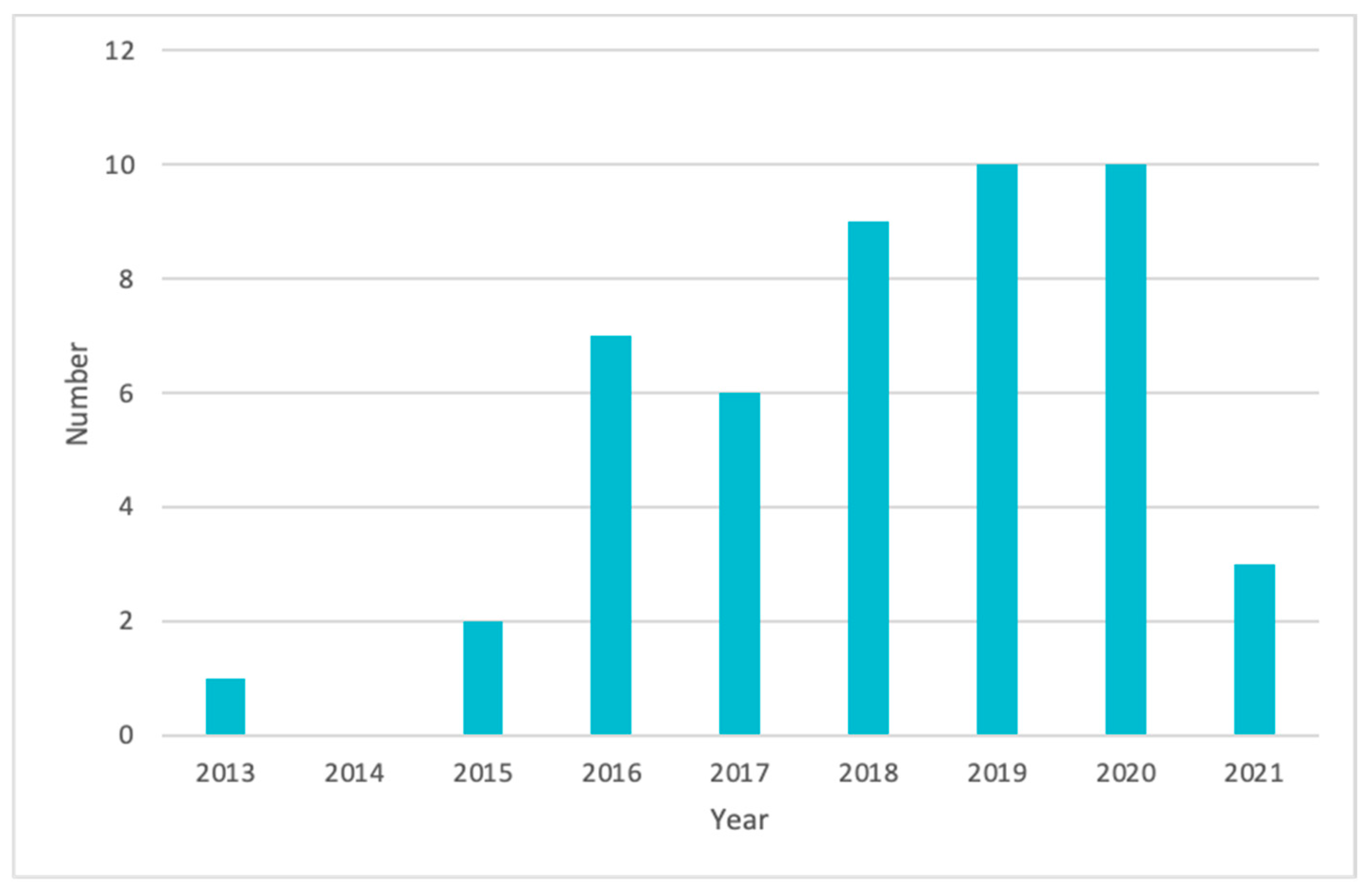
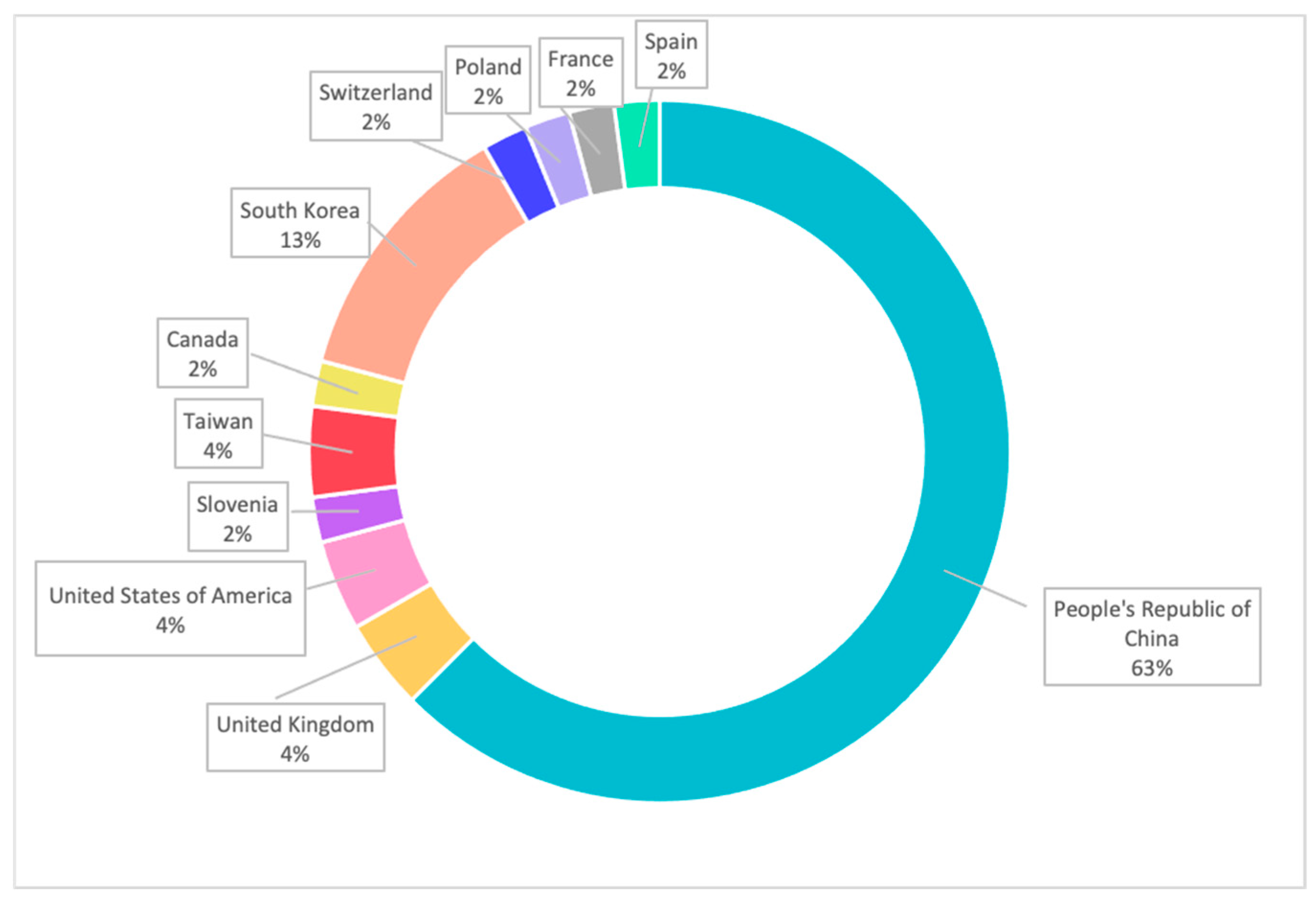
| Type of Cancer | Number of Papers Mentioned | Percentage (%) |
|---|---|---|
| Non-specific | 69 | 25.4% |
| Breast | 40 | 14.7% |
| Brain | 22 | 8.1% |
| Bone | 21 | 7.7% |
| Head and Neck | 16 | 5.9% |
| Gynaecological | 15 | 5.5% |
| Kidney | 13 | 4.8% |
| Lung | 11 | 4.0% |
| Prostate | 11 | 4.0% |
| Colorectal | 10 | 3.7% |
| Liver | 9 | 3.3% |
| Skin | 9 | 3.3% |
| Pelvic | 5 | 1.8% |
| Pancreatic | 4 | 1.5% |
| Spinal | 3 | 1.1% |
| Thoracic | 3 | 1.1% |
| Bladder | 2 | 0.7% |
| Thyroid | 2 | 0.7% |
| Bile duct | 1 | 0.4% |
| Cartilage | 1 | 0.4% |
| Chest wall | 1 | 0.4% |
| Chondrosarcoma | 1 | 0.4% |
| Intestinal | 1 | 0.4% |
| Mandible | 1 | 0.4% |
| Sternal | 1 | 0.4% |
| TOTAL | 272 | 100.0% |
| Types of Dosage Forms | Dosage Forms | APIs | Diseases | Types of Printer | Matrixes | References |
|---|---|---|---|---|---|---|
| Implants for local chemotherapy or thermotherapy | Scaffold | DOX | Bone cancer | FDM | Chitosan, nanoclay and β-tricalcium phosphate, PCL | [27] |
| Drug-eluting implant | DOX and apo2l/trail | Bone cancer | SLM | Ti6A14V | [51] | |
| Magnetic hyperthermia scaffold | DOX | Bone cancer | PE | Fe3O4/MBG/PCL | [28] | |
| Photothermal scaffold | non | Bone cancer | N/A | Ca-P/polydopamine | [29] | |
| Photothermal bioscaffold | non | Bone cancer | N/A | Fe-CaSiO3 | [30] | |
| Photothermal hydrogel scaffolds | PDA | Bone cancer | Bioscaffolder | Alg-PDA | [31] | |
| Nanoporous disc | DOX | Bone metastases secondary to prostate cancer | FDM | TPU | [52] | |
| Tablet | Progesterone | Breast, ovarian, uterus and prostate cancers | SLS | PCL | [53] | |
| Bullet-shaped implant | Cytoxan | N/A | FDM | PLA | [54] | |
| Magnetically actuated implant | Methylene blue (MB), Docetaxel (DTX) | Prostate cancer | N/A | ABS | [32] | |
| Magnetically controlled implant | TNF-related apoptosis-inducing ligand (TRAIL) and DOX | N/A | Bioprinter | graphene oxide and PCL composite | [33] | |
| Scaffold | DOX and Cisplatin | Breast cancer | E-jet | PLGA | [55] | |
| Scaffold | 5-FU and NVP-BEZ235 | Breast cancer | E-jet | PLGA | [55] | |
| Spherical implant | DOX, ifosfamide, methotrexate, Cisplatin (CDDP) | Osteosarcoma | SLA | PLLA | [56] | |
| Patch | 5-FU | Pancreatic cancer | PE, MHDS | PLGA, PCL | [34] | |
| Tablet | Fluorouracil | Cartilage cancer | SLS | PCL | [57] | |
| Drug delivery implant patent | N/A | Mouth/anal/cervical/vaginal cancer | N/A | N/A | [58] | |
| Nanogel discs | Paclitaxel, rapamycin | Ovarian cancer | FDM | Poloxamer 407 | [35] | |
| Mesh | Temozolomide (TMZ) | Glioblastoma (GBM) | Bioprinter | PLGA | [36] | |
| Brachytherapy device | Vaginal template for brachytherapy | N/A | Cervical cancer | Multi-jet Printing | N/A | [38] |
| Superficial brachytherapy applicator | Radioisotopes of yttrium-90 | Skin cancer | SLA | PLA | [39] | |
| Brachytherapy applicator | Gafchromic ebt3 film | Gynaecologic cancer | FDM | PLA | [37] | |
| Implants for local Immunotherapy | Nanogel | DNA nanocomplex | Glioblastoma | SLA | Gelatin Methacrylamide | [44] |
| Transdermal Dosage forms | Anticancer agent coated metal microneedle | 5-fluorouracil, CUR, cisplatin | Skin cancer | MJ | Metal | [50] |
| Microneedle | Decarbazine | Skin cancer | SLA | Propylene fumarate (PPF)/diethyl fumarate (DEF) | [59] | |
| Microneedle | Cisplatin | Skin cancer | SLA, inkjet printer | Soluplus® | [60] | |
| Oral dosage forms | Tablet | 5-fluorouracil | Colorectal cancer | DOP | Caso4, Soluplus® | [46] |
| Not stated | Microparticles | Paclitaxel (PTX) | Cervical Cancer | Piezoelectric inkjet printer | PLGA | [61] |
| Models | Advantages | Disadvantages | References |
|---|---|---|---|
| 2D culture |
|
| [62,63] |
| Animal (mouse) model |
|
| [21,66] |
| 3D cell culture model |
|
| [63] |
| 3D bio-printed cell model |
|
| [63,67] |
| Model | Tumour Type (Cell line) | Matrix | Drug | Type of Printer | Features | References |
|---|---|---|---|---|---|---|
| Glioma-vascular niche | Brain cancer (Glioblastoma multiforme) | Collagen type with laminin | N/A | N/A | Angiogenesis, Cell–cell/Cell–ECM interaction | [81] |
| In vitro Cell Laden | Cervical cancer (cell line HeLa) | Gelatine, alginate, fibrinogen | Paclitaxel | N/A | Drug toxicity study | [84] |
| 3D bone matrix | Breast cancer (cell line MDA-MB-231, MCF-7, MSC) | PEG PEG-DA HA | N/A | Stereolithography | Metastatic study | [19] |
| 3D Microfluidic device | MCF-7 breast cancer, SW480 colon cancer, (cell line PC3) prostate cancer | N/A | Anti-EpCAM antibodies | Multi-jet printing | CTC isolation | [98] |
| 3D Drug screening | Hepatocyte/ADSC | Gelatine, alginate, fibrinogen | 5-FU, AP, matrine | N/A | Drug screening | [102] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Ting, Y.-H.; Youssef, S.H.; Song, Y.; Garg, S. Three-Dimensional Printing for Cancer Applications: Research Landscape and Technologies. Pharmaceuticals 2021, 14, 787. https://doi.org/10.3390/ph14080787
Li R, Ting Y-H, Youssef SH, Song Y, Garg S. Three-Dimensional Printing for Cancer Applications: Research Landscape and Technologies. Pharmaceuticals. 2021; 14(8):787. https://doi.org/10.3390/ph14080787
Chicago/Turabian StyleLi, Ruixiu, Yu-Huan Ting, Souha H. Youssef, Yunmei Song, and Sanjay Garg. 2021. "Three-Dimensional Printing for Cancer Applications: Research Landscape and Technologies" Pharmaceuticals 14, no. 8: 787. https://doi.org/10.3390/ph14080787
APA StyleLi, R., Ting, Y.-H., Youssef, S. H., Song, Y., & Garg, S. (2021). Three-Dimensional Printing for Cancer Applications: Research Landscape and Technologies. Pharmaceuticals, 14(8), 787. https://doi.org/10.3390/ph14080787