Protective Effect of Piplartine against LPS-Induced Sepsis through Attenuating the MAPKs/NF-κB Signaling Pathway and NLRP3 Inflammasome Activation
Abstract
1. Introduction
2. Results
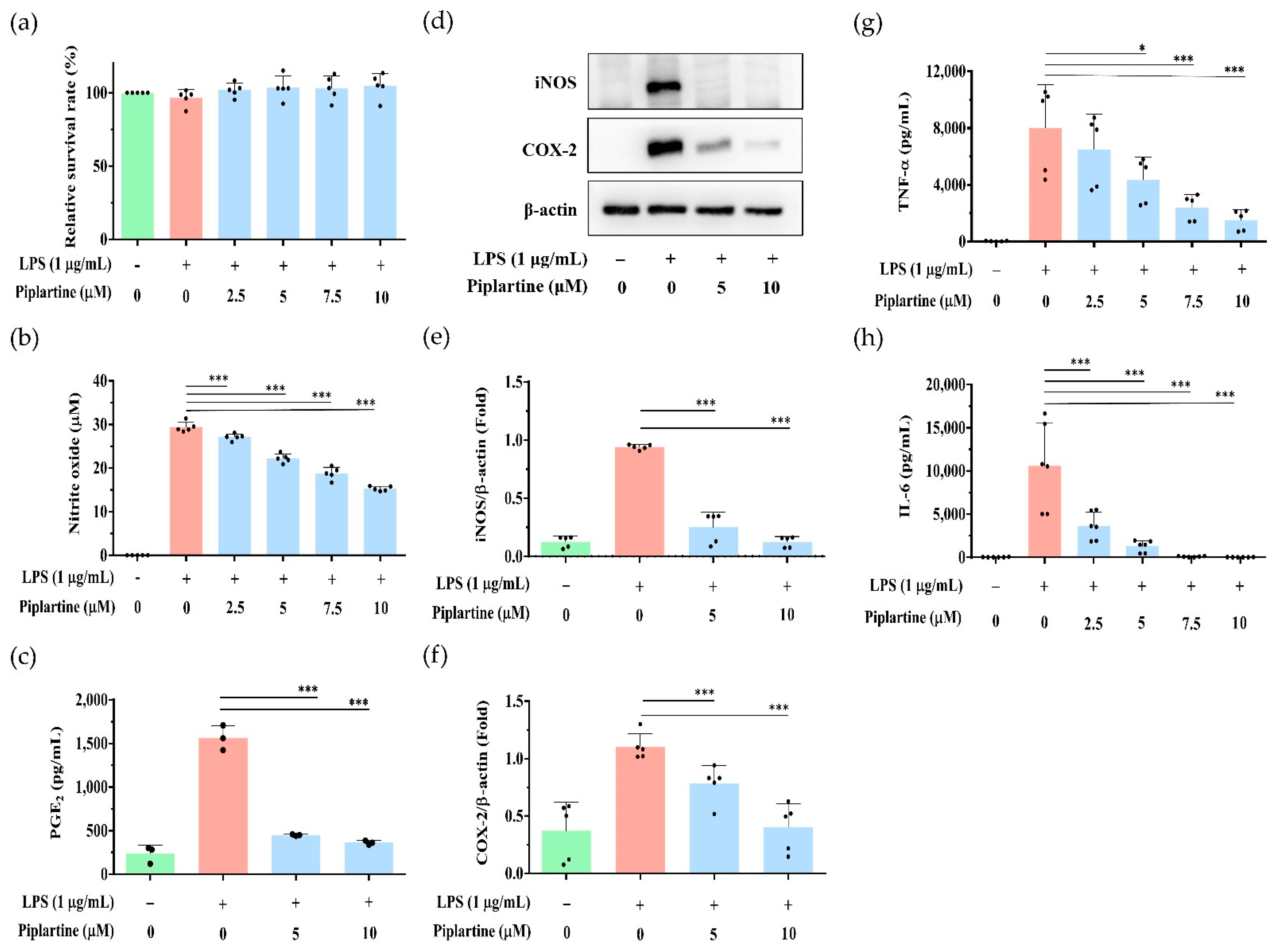
2.1. Piplartine Represses NO and PGE2 Production, Decreases COX-2 and iNOS Expression and Attenuates Proinflammatory Cytokine Secretion by LPS-Activated J774A.1 Cells
2.2. Piplartine Decreases the Phosphorylation of MAPKs, IκB and NF-κB by LPS-Activated J774A.1 Cells and Suppresses the Activation of NF-κB by LPS-Activated J-Blue Cells
2.3. Piplartine Decreases IL-1β Production and Represses NLRP3 Inflammasome Activation by LPS/ATP- and LPS/Nigericin-Activated J774A.1 Cells
2.4. Piplartine Represses NO and Proinflammatory Cytokine Productions by LPS-Activated Murine Peritoneal and Bone Marrow-Derived Macrophages
2.5. Piplartine Exhibits Protective Effects against LPS-Induced Tissue Damages and Increases the Survival Rate of LPS-Challenged Mice
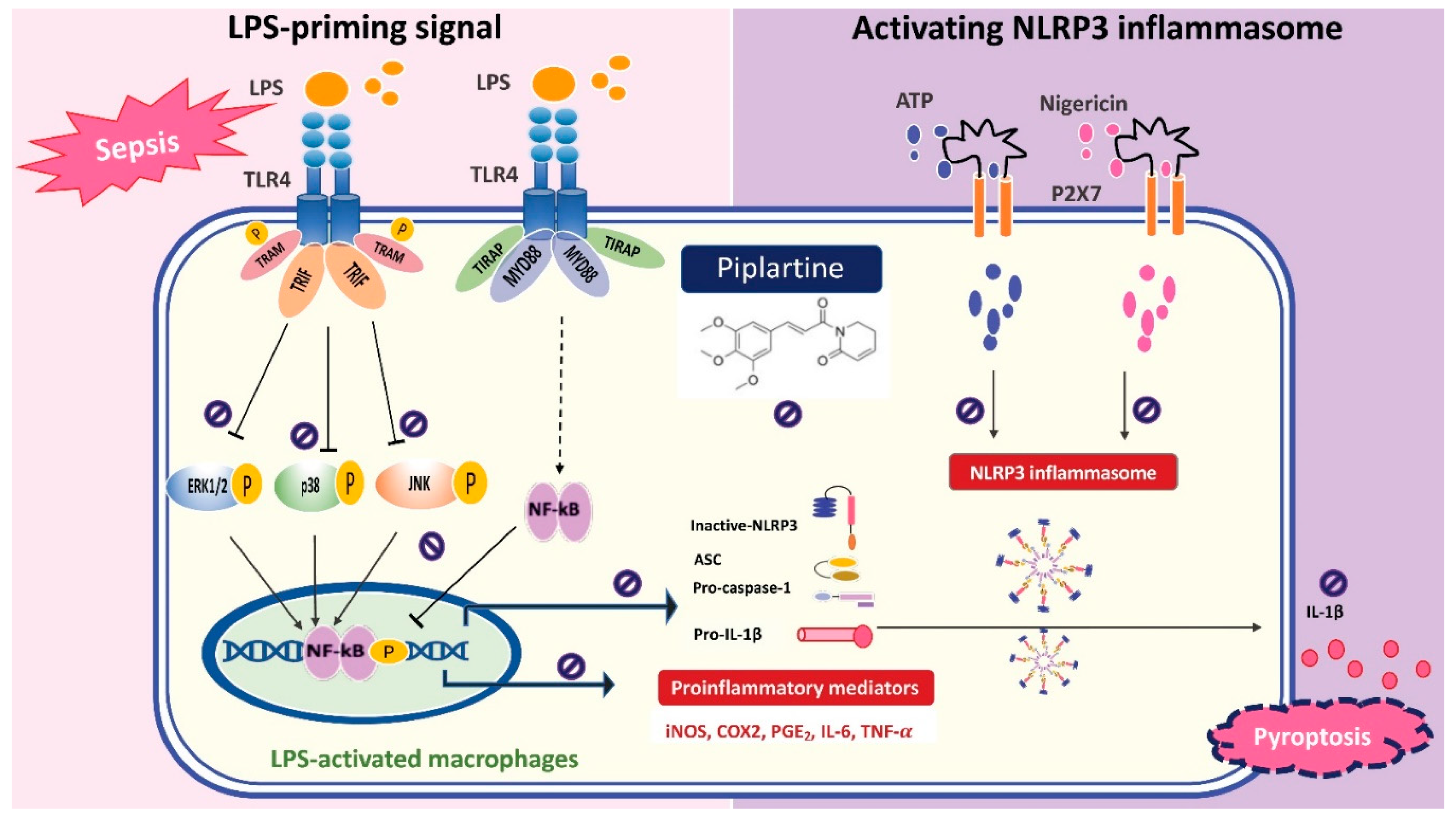
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Preparation of Peritoneal and Bone Marrow-Derived Macrophages
4.3. Animals
4.4. Cytotoxicity Assay
4.5. NO Assay
4.6. Measurement of Cytokine Secretion
4.7. Western Blotting Analysis
4.8. NF-κB Promoter Activity Assay
4.9. Immunofluorescence Staining
4.10. Endotoxemia Animal Model and Histological and Biochemistry Analysis
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Serafini, N.; Dahdah, A.; Barbet, G.; Demion, M.; Attout, T.; Gautier, G.; Arcos-Fajardo, M.; Souchet, H.; Jouvin, M.-H.; Vrtovsnik, F.; et al. The TRPM4 Channel Controls Monocyte and Macrophage, but Not Neutrophil, Function for Survival in Sepsis. J. Immunol. 2012, 189, 3689–3699. [Google Scholar] [CrossRef]
- Rhee, C.; Jones, T.M.; Hamad, Y.; Pande, A.; Varon, J.; O’Brien, C.; Anderson, D.J.; Warren, D.K.; Dantes, R.B.; Epstein, L.; et al. Prevalence, Underlying Causes, and Preventability of Sepsis-Associated Mortality in US Acute Care Hospitals. JAMA Netw. Open 2019, 2, e187571. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, H.; Zhou, J.; Zhong, Y.; Ali, M.M.; McGuire, F.; Nagarkatti, P.S.; Nagarkatti, M. Role of cytokines as a double-edged sword in sepsis. In Vivo 2013, 27, 669–684. [Google Scholar] [PubMed]
- Mera, S.; Tatulescu, D.; Cismaru, C.; Bondor, C.; Slavcovici, A.; Zanc, V.; Carstina, D.; Oltean, M. Multiplex cytokine profiling in patients with sepsis. Apmis 2011, 119, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhao, Y.; Lai, D.; Zhang, P.; Yang, Y.; Li, Y.; Fei, K.; Jiang, G.; Fan, J. Neutrophil extracellular traps promote macrophage pyroptosis in sepsis. Cell Death Dis. 2018, 9, 597. [Google Scholar] [CrossRef] [PubMed]
- Serbina, N.V.; Jia, T.; Hohl, T.M.; Pamer, E.G. Monocyte-Mediated Defense Against Microbial Pathogens. Annu. Rev. Immunol. 2008, 26, 421–452. [Google Scholar] [CrossRef] [PubMed]
- Küper, C.; Beck, F.X.; Neuhofer, W. Toll-like receptor 4 activates NF-κB and MAP kinase pathways to regulate expression of proinflammatory COX-2 in renal medullary collecting duct cells. Am. J. Physiol. Ren. Physiol. 2012, 302, F38–F46. [Google Scholar] [CrossRef]
- Lin, C.Y.; Wang, W.H.; Chen, S.H.; Chang, Y.W.; Hung, L.C.; Chen, C.Y.; Chen, Y.H. Lipopolysaccharide-Induced Nitric Oxide, Prostaglandin E2, and Cytokine Production of Mouse and Human Macrophages Are Suppressed by Pheophytin-b. Int. J. Mol. Sci. 2017, 18, 2637. [Google Scholar] [CrossRef]
- Franchi, L.; Eigenbrod, T.; Muñoz-Planillo, R.; Nuñez, G. The inflammasome: A caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat. Immunol. 2009, 10, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.Y.; Li, L.H.; Rao, Y.K.; Ju, T.C.; Nai, Y.S.; Chen, Y.W.; Hua, K.F. Mechanistic insight into the attenuation of gouty inflammation by Taiwanese green propolis via inhibition of the NLRP3 inflammasome. J. Cell. Physiol. 2019, 234, 4081–4094. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.Y.; Tu, C.E.; Wang, S.C.; Hung, Y.L.; Su, C.C.; Fang, S.H.; Chen, C.S.; Liu, P.L.; Cheng, W.C.; Huang, Y.W.; et al. Corylin inhibits LPS-induced inflammatory response and attenuates the activation of NLRP3 inflammasome in microglia. BMC Complement. Altern. Med. 2018, 18, 221. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Yeon, S.H.; Lee, H.E.; Kang, H.C.; Cho, Y.Y.; Lee, H.S.; Lee, J.Y. Suppression of NLRP3 inflammasome by oral treatment with sulforaphane alleviates acute gouty inflammation. Rheumatology 2018, 57, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.C.; Tseng, J.S.; Hsu, K.H.; Shih, S.J.; Yi, C.Y.; Wu, C.L.; Kou, Y.R. A minimum blood glucose value less than or equal to 120 mg/dL under glycemic control is associated with increased 14-day mortality in nondiabetic intensive care unit patients with sepsis and stress hyperglycemia. J. Crit. Care 2016, 34, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Danielski, L.G.; Giustina, A.D.; Bonfante, S.; Barichello, T.; Petronilho, F. The NLRP3 Inflammasome and Its Role in Sepsis Development. Inflammation 2020, 43, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V. Inflammasomes: Pandora’s box for sepsis. J. Inflamm. Res. 2018, 11, 477–502. [Google Scholar] [CrossRef] [PubMed]
- Da Nóbrega, F.R.; Ozdemir, O.; Nascimento Sousa, S.C.S.; Barboza, J.N.; Turkez, H.; de Sousa, D.P. Piplartine Analogues and Cytotoxic Evaluation against Glioblastoma. Molecules 2018, 23, 1382. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, X.; Zhou, Y.; Jiang, H.; Pan, S.; Sun, B. Piperlongumine Suppresses Growth and Sensitizes Pancreatic Tumors to Gemcitabine in a Xenograft Mouse Model by Modulating the NF-kappa B Pathway. Cancer Prev. Res. 2016, 9, 234–244. [Google Scholar] [CrossRef]
- Hung, Y.L.; Fang, S.H.; Wang, S.C.; Cheng, W.C.; Liu, P.L.; Su, C.C.; Chen, C.S.; Huang, M.Y.; Hua, K.F.; Shen, K.H.; et al. Corylin protects LPS-induced sepsis and attenuates LPS-induced inflammatory response. Sci. Rep. 2017, 7, 46299. [Google Scholar] [CrossRef]
- Liu, X.; Quan, N. Immune Cell Isolation from Mouse Femur Bone Marrow. Bio Protoc. 2015, 5, e1631. [Google Scholar] [CrossRef]
- Tang, R.; Zhang, G.; Chen, S.Y. Response gene to complement 32 protein promotes macrophage phagocytosis via activation of protein kinase C pathway. J. Biol. Chem. 2014, 289, 22715–22722. [Google Scholar] [CrossRef]
- Guha, M.; Mackman, N. LPS induction of gene expression in human monocytes. Cell. Signal. 2001, 13, 85–94. [Google Scholar] [CrossRef]
- Schorey, J.S.; Cooper, A.M. Macrophage signalling upon mycobacterial infection: The MAP kinases lead the way. Cell. Microbiol. 2003, 5, 133–142. [Google Scholar] [CrossRef]
- Bezerra, D.P.; Pessoa, C.; de Moraes, M.O.; Saker-Neto, N.; Silveira, E.R.; Costa-Lotufo, L.V. Overview of the therapeutic potential of piplartine (piperlongumine). Eur. J. Pharm. Sci. 2013, 48, 453–463. [Google Scholar] [CrossRef]
- Yadav, V.; Krishnan, A.; Vohora, D. A systematic review on Piper longum L.: Bridging traditional knowledge and pharmacological evidence for future translational research. J. Ethnopharmacol. 2020, 247, 112255. [Google Scholar] [CrossRef] [PubMed]
- Baliza, I.R.S.; Silva, S.L.R.; Santos, L.S.; Neto, J.H.A.; Dias, R.B.; Sales, C.B.S.; Rocha, C.A.G.; Soares, M.B.P.; Batista, A.A.; Bezerra, D.P. Ruthenium Complexes with Piplartine Cause Apoptosis through MAPK Signaling by a p53-Dependent Pathway in Human Colon Carcinoma Cells and Inhibit Tumor Development in a Xenograft Model. Front. Oncol. 2019, 9, 582. [Google Scholar] [CrossRef] [PubMed]
- Giacone, D.V.; Dartora, V.; de Matos, J.K.R.; Passos, J.S.; Miranda, D.A.G.; de Oliveira, E.A.; Silveira, E.R.; Costa-Lotufo, L.V.; Maria-Engler, S.S.; Lopes, L.B. Effect of nanoemulsion modification with chitosan and sodium alginate on the topical delivery and efficacy of the cytotoxic agent piplartine in 2D and 3D skin cancer models. Int. J. Biol. Macromol. 2020, 165 Pt A, 1055–1065. [Google Scholar] [CrossRef]
- Oliveira, M.S.; Barbosa, M.I.F.; de Souza, T.B.; Moreira, D.R.M.; Martins, F.T.; Villarreal, W.; Machado, R.P.; Doriguetto, A.C.; Soares, M.B.P.; Bezerra, D.P. A novel platinum complex containing a piplartine derivative exhibits enhanced cytotoxicity, causes oxidative stress and triggers apoptotic cell death by ERK/p38 pathway in human acute promyelocytic leukemia HL-60 cells. Redox Biol. 2019, 20, 182–194. [Google Scholar] [CrossRef]
- Fan, X.; Song, J.; Zhao, Z.; Chen, M.; Tu, J.; Lu, C.; Wu, F.; Zhang, D.; Weng, Q.; Zheng, L.; et al. Piplartine suppresses proliferation and invasion of hepatocellular carcinoma by LINC01391-modulated Wnt/beta-catenin pathway inactivation through ICAT. Cancer Lett. 2019, 460, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Mengarda, A.C.; Mendonca, P.S.; Morais, C.S.; Cogo, R.M.; Mazloum, S.F.; Salvadori, M.C.; Teixeira, F.S.; Morais, T.R.; Antar, G.M.; Lago, J.H.G.; et al. Antiparasitic activity of piplartine (piperlongumine) in a mouse model of schistosomiasis. Acta Trop. 2020, 205, 105350. [Google Scholar] [CrossRef] [PubMed]
- Campelo, Y.; Ombredane, A.; Vasconcelos, A.G.; Albuquerque, L.; Moreira, D.C.; Placido, A.; Rocha, J.; Hilarion Fokoue, H.; Yamaguchi, L.; Mafud, A.; et al. Structure(-)Activity Relationship of Piplartine and Synthetic Analogues against Schistosoma mansoni and Cytotoxicity to Mammalian Cells. Int. J. Mol. Sci. 2018, 19, 1802. [Google Scholar] [CrossRef]
- Ticona, J.C.; Bilbao-Ramos, P.; Flores, N.; Dea-Ayuela, M.A.; Bolas-Fernandez, F.; Jimenez, I.A.; Bazzocchi, I.L. (E)-Piplartine Isolated from Piper pseudoarboreum, a Lead Compound against Leishmaniasis. Foods 2020, 9, 1250. [Google Scholar] [CrossRef]
- Moreira, F.L.; Riul, T.B.; Moreira, M.L.; Pilon, A.C.; Dias-Baruffi, M.; Araujo, M.S.S.; Lopes, N.P.; de Oliveira, A.R.M. Leishmanicidal Effects of Piperlongumine (Piplartine) and Its Putative Metabolites. Planta Med. 2018, 84, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- Salomao, R.; Brunialti, M.K.; Rapozo, M.M.; Baggio-Zappia, G.L.; Galanos, C.; Freudenberg, M. Bacterial sensing, cell signaling, and modulation of the immune response during sepsis. Shock 2012, 38, 227–242. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Yoo, H.; Kim, J.A.; Lee, S.; Jee, J.G.; Lee, M.Y.; Lee, Y.M.; Bae, J.S. Barrier protective effects of piperlonguminine in LPS-induced inflammation in vitro and in vivo. Food Chem. Toxicol. 2013, 58, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Liu, C.; Tu, Z.; Xu, Q.; Chen, S.; Zhang, Y.; Wang, X.; Zhang, J.; Hu, C.A.; Liu, Y. Activation of the NF-κB and MAPK Signaling Pathways Contributes to the Inflammatory Responses, but Not Cell Injury, in IPEC-1 Cells Challenged with Hydrogen Peroxide. Oxid. Med. Cell. Longev. 2020, 2020, 5803639. [Google Scholar] [CrossRef] [PubMed]
- Christgen, S.; Place, D.E.; Kanneganti, T.D. Toward targeting inflammasomes: Insights into their regulation and activation. Cell Res. 2020, 30, 315–327. [Google Scholar] [CrossRef]
- Lewis, A.J.; Seymour, C.W.; Rosengart, M.R. Current Murine Models of Sepsis. Surg. Infect. 2016, 17, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Liu, Q.X.; Liu, T.; Xu, X.E.; Gao, W.; Bai, X.J.; Li, Z.F. Caspase-1-dependent pyroptosis of peripheral blood mononuclear cells predicts the development of sepsis in severe trauma patients: A prospective observational study. Medicine 2018, 97, e9859. [Google Scholar] [CrossRef]
- Su, C.C.; Wang, S.C.; Chen, I.C.; Chiu, F.Y.; Liu, P.L.; Huang, C.H.; Huang, K.H.; Fang, S.H.; Cheng, W.C.; Huang, S.P.; et al. Zerumbone Suppresses the LPS-Induced Inflammatory Response and Represses Activation of the NLRP3 Inflammasome in Macrophages. Front. Pharmacol. 2021, 12, 652860. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.-H.; Wang, S.-C.; Chen, I.-C.; Chen, Y.-T.; Liu, P.-L.; Fang, S.-H.; Huang, S.-P.; Yeh, H.-C.; Liu, C.-C.; Lee, P.-Y.; et al. Protective Effect of Piplartine against LPS-Induced Sepsis through Attenuating the MAPKs/NF-κB Signaling Pathway and NLRP3 Inflammasome Activation. Pharmaceuticals 2021, 14, 588. https://doi.org/10.3390/ph14060588
Huang C-H, Wang S-C, Chen I-C, Chen Y-T, Liu P-L, Fang S-H, Huang S-P, Yeh H-C, Liu C-C, Lee P-Y, et al. Protective Effect of Piplartine against LPS-Induced Sepsis through Attenuating the MAPKs/NF-κB Signaling Pathway and NLRP3 Inflammasome Activation. Pharmaceuticals. 2021; 14(6):588. https://doi.org/10.3390/ph14060588
Chicago/Turabian StyleHuang, Chi-Han, Shu-Chi Wang, I-Chen Chen, Yi-Ting Chen, Po-Len Liu, Shih-Hua Fang, Shu-Pin Huang, Hsin-Chih Yeh, Ching-Chih Liu, Po-Yen Lee, and et al. 2021. "Protective Effect of Piplartine against LPS-Induced Sepsis through Attenuating the MAPKs/NF-κB Signaling Pathway and NLRP3 Inflammasome Activation" Pharmaceuticals 14, no. 6: 588. https://doi.org/10.3390/ph14060588
APA StyleHuang, C.-H., Wang, S.-C., Chen, I.-C., Chen, Y.-T., Liu, P.-L., Fang, S.-H., Huang, S.-P., Yeh, H.-C., Liu, C.-C., Lee, P.-Y., Lin, T.-C., Cheng, W.-C., Su, C.-C., Wu, H.-E., Chen, Y.-R., & Li, C.-Y. (2021). Protective Effect of Piplartine against LPS-Induced Sepsis through Attenuating the MAPKs/NF-κB Signaling Pathway and NLRP3 Inflammasome Activation. Pharmaceuticals, 14(6), 588. https://doi.org/10.3390/ph14060588






