In Vitro Studies on Nasal Formulations of Nanostructured Lipid Carriers (NLC) and Solid Lipid Nanoparticles (SLN)
Abstract
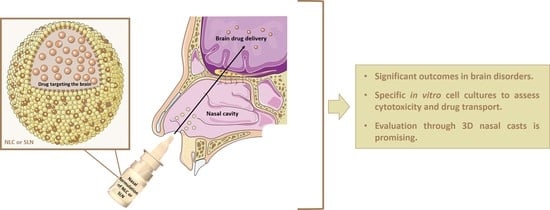
:1. Introduction
2. Nasal Route
2.1. Anatomical and Physiological Considerations
2.2. Nose-to-Brain Delivery
2.3. Requisites of Nasal Formulations
3. In Vitro Cell Models to Evaluate the Effectiveness of Nasal Formulations
3.1. Human Nasal Epithelial Cells (HNEpC)
3.2. Human Nasal Septum Quasidiploid Tumour Cells (RPMI 2650)
3.3. Human Lung Cancer Cells (Calu-3)
3.4. Human Epithelial Colorectal Adenocarcinoma Cells (Caco-2)
3.5. Others
4. Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC) for Nasal Delivery
4.1. In Vitro Studies with Nasal Formulations of NLC and SLN
4.1.1. Liquid Formulations
4.1.2. Semisolid Formulations
5. Nasal Cavity Models
Computational Models
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cunha, S.; Amaral, M.H.; Lobo, J.M.S.; Silva, A.C. Lipid Nanoparticles for Nasal/Intranasal Drug Delivery. Crit. Rev. Ther. Drug. Carrier. Syst. 2017, 34, 257–282. [Google Scholar] [CrossRef]
- Costa, C.P.; Moreira, J.N.; Sousa Lobo, J.M.; Silva, A.C. Intranasal delivery of nanostructured lipid carriers, solid lipid nanoparticles and nanoemulsions: A current overview of in vivo studies. Acta Pharm. Sin. B 2021, 11, 925–940. [Google Scholar] [CrossRef]
- Costa, C.; Moreira, J.N.; Amaral, M.H.; Sousa Lobo, J.M.; Silva, A.C. Nose-to-brain delivery of lipid-based nanosystems for epileptic seizures and anxiety crisis. J. Control. Release 2019, 295, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Shen, Z.; Anraku, Y.; Kataoka, K.; Chen, X. Nanomaterial-based blood-brain-barrier (BBB) crossing strategies. Biomaterials 2019, 224, 119491. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, M.C.; Alhamami, M.; Miedema, S.B.; Yun, Y.; Ruiz-Cardozo, M.; Vannier, M.W. Imaging of intranasal drug delivery to the brain. Am. J. Nucl. Med. Mol. Imaging 2020, 10, 1–31. [Google Scholar] [PubMed]
- Islam, S.U.; Shehzad, A.; Ahmed, M.B.; Lee, Y.S. Intranasal Delivery of Nanoformulations: A Potential Way of Treatment for Neurological Disorders. Molecules 2020, 25, 1929. [Google Scholar] [CrossRef] [Green Version]
- Binda, A.; Murano, C.; Rivolta, I. Innovative Therapies and Nanomedicine Applications for the Treatment of Alzheimer’s Disease: A State-of-the-Art (2017-2020). Int. J. Nanomed. 2020, 15, 6113–6135. [Google Scholar] [CrossRef]
- Wang, Z.; Xiong, G.; Tsang, W.C.; Schätzlein, A.G.; Uchegbu, I.F. Nose-to-Brain Delivery. J. Pharmacol. Exp. Ther. 2019, 370, 593–601. [Google Scholar] [CrossRef] [Green Version]
- Bahadur, S.; Pardhi, D.M.; Rautio, J.; Rosenholm, J.M.; Pathak, K. Intranasal Nanoemulsions for Direct Nose-to-Brain Delivery of Actives for CNS Disorders. Pharmaceutics 2020, 12, 1230. [Google Scholar] [CrossRef]
- Küçüktürkmen, B.; Bozkır, A. A New Approach for Drug Targeting to the Central Nervous System: Lipid Nanoparticles. In Nanoarchitectonics in Biomedicine; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2019; Chapter 10; pp. 335–369. [Google Scholar]
- Akhtar, A.; Andleeb, A.; Waris, T.S.; Bazzar, M.; Moradi, A.-R.; Awan, N.R.; Yar, M. Neurodegenerative diseases and effective drug delivery: A review of challenges and novel therapeutics. J. Control. Release 2021, 330, 1152–1167. [Google Scholar] [CrossRef]
- Erdő, F.; Bors, L.A.; Farkas, D.; Bajza, Á.; Gizurarson, S. Evaluation of intranasal delivery route of drug administration for brain targeting. Brain Res. Bull. 2018, 143, 155–170. [Google Scholar] [CrossRef]
- Tan, M.S.A.; Parekh, H.S.; Pandey, P.; Siskind, D.J.; Falconer, J.R. Nose-to-brain delivery of antipsychotics using nanotechnology: A review. Expert. Opin. Drug. Deliv. 2020, 17, 839–853. [Google Scholar] [CrossRef]
- Agrawal, M.; Saraf, S.; Saraf, S.; Dubey, S.K.; Puri, A.; Patel, R.J.; Ajazuddin, V.R.; Murty, U.S.; Alexander, A. Recent strategies and advances in the fabrication of nano lipid carriers and their application towards brain targeting. J. Control. Release 2020, 321, 372–415. [Google Scholar] [CrossRef]
- Pires, A.; Fortuna, A.; Alves, G.; Falcão, A. Intranasal drug delivery: How, why and what for? J. Pharm. Pharm. Sci. 2009, 12, 288–311. [Google Scholar] [CrossRef] [Green Version]
- Hussein, N.R.; Omer, H.; Elhissi, A.; Ahmed, W. Advances in Nasal Drug Delivery Systems. In Advances in Medical and Surgical Engineering; Academic Press: Cambridge, MA, USA, 2020; Chapter 15; pp. 279–311. [Google Scholar]
- Djupesland, P.G.; JMessina, J.C.; Mahmoud, R.A. The nasal approach to delivering treatment for brain diseases: An anatomic, physiologic, and delivery technology overview. Ther. Deliv. 2014, 5, 709–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gänger, S.; Schindowski, K. Tailoring Formulations for Intranasal Nose-to-Brain Delivery: A Review on Architecture, Physico-Chemical Characteristics and Mucociliary Clearance of the Nasal Olfactory Mucosa. Pharmaceutics 2018, 10, 116. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Tao, J.; Wang, J. Design and Application in Delivery System of Intranasal Antidepressants. Front. Bioeng Biotechnol 2020, 8, 626882. [Google Scholar] [CrossRef]
- Javia, A.; Kore, G.; Misra, A. Polymers in Nasal Drug Delivery: An. Overview. In Applications of Polymers in Drug Delivery, 2nd ed.; Misra, A., Shahiwala, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; Chapter 11; pp. 305–332. [Google Scholar]
- Dhas, N.L.; Kudarha, R.R.; Mehta, T.A. Intranasal Delivery of Nanotherapeutics/Nanobiotherapeutics for the Treatment of Alzheimer’s Disease: A Proficient Approach. Crit Rev. Ther Drug Carrier Syst 2019, 36, 373–447. [Google Scholar] [CrossRef] [PubMed]
- Pangua, C.; Reboredo, C.; Camión, R.; Gracia, J.M.; Martínez-López, A.L.; Agüeros, M. Mucus-Penetrating Nanocarriers. In Theory and Applications of Nonparenteral Nanomedicines; Kesharwani, P., Taurin, S., Greish, K., Eds.; Academic Press: Cambridge, MA, USA, 2021; Chapter 7; pp. 137–152. [Google Scholar]
- Agrawal, M.; Saraf, S.; Saraf, S.; Dubey, S.K.; Puri, A.; Gupta, U.; Kesharwani, P.; Ravichandiran, V.; Kumar, P.; Naidu, V.G.M.; et al. Stimuli-responsive In situ gelling system for nose-to-brain drug delivery. J. Control. Release 2020, 327, 235–265. [Google Scholar] [CrossRef]
- Sarma, A.; Das, M.K. Nose to brain delivery of antiretroviral drugs in the treatment of neuroAIDS. Mol. Biomed. 2020, 1, 15. [Google Scholar] [CrossRef]
- Hoang, V.D.; Uchenna, A.R.; Mark, J.; Renaat, K.; Norbert, V. Characterization of human nasal primary culture systems to investigate peptide metabolism. Int. J. Pharm. 2002, 238, 247–256. [Google Scholar] [CrossRef]
- Dimova, S.; Brewster, M.E.; Noppe, M.; Jorissen, M.; Augustijns, P. The use of human nasal in vitro cell systems during drug discovery and development. Toxicol. Vitr. 2005, 19, 107–122. [Google Scholar] [CrossRef]
- Merkle, H.P.; Ditzinger, G.; Lang, S.R.; Peter, H.; Schmidt, M.C. In vitro cell models to study nasal mucosal permeability and metabolism. Adv. Drug Deliv. Rev. 1998, 29, 51–79. [Google Scholar] [PubMed]
- Wengst, A.; Reichl, S. RPMI 2650 epithelial model and three-dimensional reconstructed human nasal mucosa as in vitro models for nasal permeation studies. Eur. J. Pharm. Biopharm. 2010, 74, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Yang, T.; Abbruscato, T.J.; Ahsan, F. Evaluation of human nasal RPMI 2650 cells grown at an air-liquid interface as a model for nasal drug transport studies. J. Pharm. Sci. 2008, 97, 1165–1178. [Google Scholar] [CrossRef] [PubMed]
- Kreft, M.E.; Jerman, U.D.; Lasič, E.; Rižner, T.L.; Hevir-Kene, N.; Peternel, L.; Kristan, K. The Characterization of the Human Nasal Epithelial Cell Line RPMI 2650 Under Different Culture Conditions and Their Optimization for an Appropriate in vitro Nasal Model. Pharm. Res. 2015, 32, 665–679. [Google Scholar] [CrossRef]
- Dolberg, A.M.; Reichl, S. Expression of P-glycoprotein in excised human nasal mucosa and optimized models of RPMI 2650 cells. Int. J. Pharm. 2016, 508, 22–33. [Google Scholar] [CrossRef]
- Gil-Martins, E.; Barbosa, D.J.; Silva, V.; Remião, F.; Silva, R. Dysfunction of ABC transporters at the blood-brain barrier: Role in neurological disorders. Pharmacol. Ther. 2020, 213, 107554. [Google Scholar] [CrossRef]
- Sibinovska, N.; Žakelj, S.; Roškar, R.; Kristan, K. Suitability and functional characterization of two Calu-3 cell models for prediction of drug permeability across the airway epithelial barrier. Int. J. Pharm. 2020, 585, 119484. [Google Scholar] [CrossRef]
- Gonçalves, V.S.S.; Matias, A.A.; Poejo, J.; Serra, A.T.; Duarte, C.M.M. Application of RPMI 2650 as a cell model to evaluate solid formulations for intranasal delivery of drugs. Int. J. Pharm. 2016, 515, 1–10. [Google Scholar] [CrossRef]
- Mercier, C.; Perek, N.; Delavenne, X. Is RPMI 2650 a Suitable In Vitro Nasal Model. for Drug Transport. Studies? Eur. J. Drug Metab. Pharm. 2018, 43, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Sibinovska, N.; Žakelj, S.; Kristan, K. Suitability of RPMI 2650 cell models for nasal drug permeability prediction. Eur. J. Pharm. Biopharm. 2019, 145, 85–95. [Google Scholar] [CrossRef]
- Charles, D.D.; Fisher, J.R.; Hoskinson, S.M.; Medina-Colorado, A.A.; Shen, Y.C.; Chaaban, M.R.; Widen, S.G.; Eaves-Pyles, T.D.; Maxwell, C.A.; Miller, A.L.; et al. Development of a Novel ex vivo Nasal Epithelial Cell Model. Supporting Colonization With Human Nasal Microbiota. Front. Cell. Infect. Microbiol. 2019, 9, 165. [Google Scholar] [CrossRef] [Green Version]
- Keller, L.-A.; Merkel, O.; Popp, A. Intranasal drug delivery: Opportunities and toxicologic challenges during drug development. Drug Deliv. Transl. Res. 2021, 1–23. [Google Scholar]
- Hood, A.T.; Currie, D.; Garte, S.J. Establishment of a rat nasal epithelial tumor cell line. In Vitro Cell Dev. Biol. 1987, 23, 274–278. [Google Scholar] [CrossRef]
- Kreft, M.E.; Tratnjek, L.; Lasič, E.; Hevir, N.; Rižner, T.L.; Kristan, K. Different Culture Conditions Affect. Drug Transporter Gene Expression, Ultrastructure, and Permeability of Primary Human Nasal Epithelial Cells. Pharm. Res. 2020, 37, 170. [Google Scholar]
- Moore, G.E.; Sandberg, A.A. Studies of a human tumor cell line with a diploid karyotype. Cancer 1964, 17, 170–175. [Google Scholar] [CrossRef]
- Furubayashi, T.; Inoue, D.; Nishiyama, N.; Tanaka, A.; Yutani, R.; Kimura, S.; Katsumi, H.; Yamamoto, A.; Sakane, T. Comparison of Various Cell Lines and Three-Dimensional Mucociliary Tissue Model. Systems to Estimate Drug Permeability Using an In Vitro Transport. Study to Predict Nasal Drug Absorption in Rats. Pharmaceutics 2020, 12, 79. [Google Scholar]
- Volpe, D.A. Drug-permeability and transporter assays in Caco-2 and MDCK cell lines. Future Med. Chem. 2011, 3, 2063–2077. [Google Scholar] [CrossRef]
- Sarmento, B.; Andrade, F.; Baptista da Silva, S.; Rodrigues, F.; Neves, J.; Ferreira, D. Cell-based in vitro models for predicting drug permeability. Expert Opin. Drug Metab. Toxicol. 2012, 8, 607–621. [Google Scholar] [CrossRef]
- Forbes, B.; Shah, A.; Martin, G.P.; Lansley, A.B. The human bronchial epithelial cell line 16HBE14o- as a model system of the airways for studying drug transport. Int. J. Pharm. 2003, 257, 161–167. [Google Scholar] [CrossRef]
- Callaghan, P.J.; Ferrick, B.; Rybakovsky, E.; Thomas, S.; Mullin, J.M. Epithelial barrier function properties of the 16HBE14o- human bronchial epithelial cell culture model. Biosci. Rep. 2020, 40, BSR20201532. [Google Scholar] [CrossRef]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: Applications, advantages and disadvantages. Res. Pharm. Sci. 2018, 13, 288–303. [Google Scholar]
- Cunha, S.; Almeida, H.; Amaral, M.H.; Sousa Lobo, J.M.; Silva, A.C. Intranasal lipid nanoparticles for the treatment of neurodegenerative diseases. Curr. Pharm. Des. 2017, 23, 6553–6562. [Google Scholar] [CrossRef]
- Silva, A.C.; Santos, D.; Ferreira, D.; Lopes, C.M. Lipid-based Nanocarriers As An. Alternative for Oral Delivery of Poorly Water- Soluble Drugs: Peroral and Mucosal Routes. Curr. Med. Chem. 2012, 19, 4495–4510. [Google Scholar]
- Silva, A.C.; González-Mira, E.; Sousa Lobo, J.M.; Amaral, M.H. Current progresses on nanodelivery systems for the treatment of neuropsychiatric diseases: Alzheimer’s and schizophrenia. Curr. Pharm. Des. 2013, 19, 7185–7195. [Google Scholar] [CrossRef]
- Almeida, H.; Amaral, M.H.; Lobão, P.; Silva, A.C.; Sousa Lobo, J.M. Applications of polymeric and lipid nanoparticles in ophthalmic pharmaceutical formulations: Present and future considerations. J. Pharm. Pharm. Sci. 2014, 17, 278–293. [Google Scholar] [CrossRef] [Green Version]
- Silva, A.C.; Amaral, M.H.; Sousa Lobo, J.M.; Lopes, C.M. Lipid nanoparticles for the delivery of biopharmaceuticals. Curr. Pharm. Biotechnol. 2015, 16, 291–302. [Google Scholar] [CrossRef]
- Garcês, A.; Amaral, M.H.; Sousa Lobo, J.M.; Silva, A.C. Formulations based on solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) for cutaneous use: A review. Eur. J. Pharm. Sci. 2018, 112, 159–167. [Google Scholar] [CrossRef]
- Silva, A.C.; Amaral, M.H.; Sousa Lobo, J.M.; Almeida, H. Editorial: Applications of Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC): State of the Art. Curr. Pharm. Des. 2017, 23, 6551–6552. [Google Scholar] [CrossRef]
- Mehnert, W.; Mäder, K. Solid lipid nanoparticles: Production, characterization and applications. Adv. Drug Deliv. Rev. 2012, 64, 83–101. [Google Scholar] [CrossRef]
- Müller, R.H.; Mäder, K.; Gohla, S. Solid lipid nanoparticles (SLN) for controlled drug delivery–a review of the state of the art. Eur. J. Pharm. Biopharm. 2000, 50, 161–177. [Google Scholar] [CrossRef]
- Müller, R.H.; Radtke, M.; Wissing, S.A. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv. Drug Deliv. Rev. 2002, 54, S131–S155. [Google Scholar] [CrossRef]
- Pardeike, J.; Hommoss, A.; Müller, R.H. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. Int. J. Pharm. 2009, 366, 170–184. [Google Scholar] [CrossRef]
- Muller, R.H.; Shegokar, R.; Keck, C.M. 20 years of lipid nanoparticles (SLN & NLC): Present state of development & industrial applications. Curr. Drug Discov. Technol. 2011, 8, 207–227. [Google Scholar]
- Müller, R.H.; Petersen, R.D.; Hommoss, A.; Pardeike, J. Nanostructured lipid carriers (NLC) in cosmetic dermal products. Adv. Drug Deliv. Rev. 2007, 59, 522–530. [Google Scholar] [CrossRef]
- Weber, S.; Zimmer, A.; Pardeike, J. Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC) for pulmonary application: A review of the state of the art. Eur. J. Pharm. Biopharm. 2014, 86, 7–22. [Google Scholar] [CrossRef]
- Üner, M. Preparation, characterization and physico-chemical properties of Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC): Their benefits as colloidal drug carrier systems. Die Pharm. Int. J. Pharm. Sci. 2006, 61, 375–386. [Google Scholar]
- Wissing, S.A.; Kayser, O.; Müller, R.H. Solid lipid nanoparticles for parenteral drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 1257–1272. [Google Scholar] [CrossRef]
- Almeida, H.; Amaral, M.H.; Lobão, P.; Sousa Lobo, J.M. In situ gelling systems: A strategy to improve the bioavailability of ophthalmic pharmaceutical formulations. Drug Discov. Today 2014, 19, 400–412. [Google Scholar] [CrossRef]
- Almeida, H.; Amaral, M.H.; Lobão, P.; Sousa Lobo, J.M. Applications of poloxamers in ophthalmic pharmaceutical formulations: An overview. Expert Opin. Drug Deliv. 2013, 10, 1223–1237. [Google Scholar] [CrossRef]
- Wang, Q.; Zuo, Z.; Cheung, C.K.C.; Leung, S.S.Y. Updates on thermosensitive hydrogel for nasal, ocular and cutaneous delivery. Int. J. Pharm. 2019, 559, 86–101. [Google Scholar] [CrossRef]
- Karavasili, C.; Fatouros, D.G. Smart materials: In situ gel-forming systems for nasal delivery. Drug Discov. Today 2016, 21, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Nižić, L.; Potás, J.; Winnicka, K.; Szekalska, M.; Erak, I.; Gretić, M.; Jug, M.; Hafner, A. Development, characterisation and nasal deposition of melatonin-loaded pectin/hypromellose microspheres. Eur. J. Pharm. Sci. 2020, 141, 105115. [Google Scholar] [CrossRef] [PubMed]
- Chonkar, A.; Nayak, U.; Udupa, N. Smart Polymers in Nasal Drug Delivery. Indian J. Pharm. Sci. 2015, 77, 367–375. [Google Scholar]
- Nižić, L.; Ugrina, I.; Špoljarić, D.; Saršon, V.; Kučuk, M.S.; Pepić, I.; Hafner, A. Innovative sprayable in situ gelling fluticasone suspension: Development and optimization of nasal deposition. Int. J. Pharm. 2019, 563, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Li, H.; Tian, B.; Sai, S.; Gao, Y.; Lan, T.; Meng, Y.; Ding, C. Development of nose-to-brain delivery of ketoconazole by nanostructured lipid carriers against cryptococcal meningoencephalitis in mice. Colloids Surf. B Biointerfaces 2019, 183, 110446. [Google Scholar] [CrossRef]
- Jojo, G.M.; Kuppusamy, G.; De, A.; Karri, V.V.S.N.R. Formulation and optimization of intranasal nanolipid carriers of pioglitazone for the repurposing in Alzheimer’s disease using Box-Behnken design. Drug Dev. Ind. Pharm. 2019, 45, 1061–1072. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Marto, J.; Gonçalves, L.; Almeida, A.J.; Vale, N. Formulation, Characterization and Evaluation against SH-SY5Y Cells of New Tacrine and Tacrine-MAP Loaded with Lipid Nanoparticles. Nanomaterials 2020, 10, 2089. [Google Scholar] [CrossRef]
- Trapani, A.; Guerra, L.; Corbo, F.; Castellani, S.; Sanna, E.; Capobianco, L.; Monteduro, A.G.; Manno, D.E.; Mandracchia, D.; Di Giola, S.; et al. Cyto/Biocompatibility of Dopamine Combined with the Antioxidant Grape Seed-Derived Polyphenol Compounds in Solid Lipid Nanoparticles. Molecules 2021, 26, 916. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, X.; Du, J.; Liu, M.; Feng, J.; Hu, K. Improved brain delivery of pueraria flavones via intranasal administration of borneol-modified solid lipid nanoparticles. Nanomedicine 2019, 14, 2105–2119. [Google Scholar] [CrossRef]
- Sadegh Malvajerd, S.; Azadi, A.; Izadi, Z.; Kurd, M.; Dara, T.; Dibaei, M.; Zadeh, M.S.; Javar, H.A.; Hamidi, M. Brain Delivery of Curcumin Using Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Preparation, Optimization, and Pharmacokinetic Evaluation. ACS Chem. Neurosci. 2019, 10, 728–739. [Google Scholar] [CrossRef] [PubMed]
- Khanna, K.; Sharma, N.; Rawat, S.; Khan, N.; Karwasra, R.; Hasan, N.; Kumar, A.; Jain, G.K.; Nishad, D.K.; Khanna, S.; et al. Intranasal solid lipid nanoparticles for management of pain: A full factorial design approach, characterization & Gamma Scintigraphy. Chem. Phys. Lipids 2021, 236, 105060. [Google Scholar]
- Sarma, A.; Das, M.K.; Chakraborty, T.; Das, S. Nanostructured lipid carriers (NLCs)-based intranasal Drug Delivery System of Tenofovir disoproxil fumerate (TDF) for brain targeting. Res. J. Pharm. Technol. 2020, 13, 5411–5424. [Google Scholar]
- Gadhave, D.; Rasal, N.; Sonawane, R.; Sekar, M.; Kokare, C. Nose-to-brain delivery of teriflunomide-loaded lipid-based carbopol-gellan gum nanogel for glioma: Pharmacological and in vitro cytotoxicity studies. Int. J. Biol. Macromol. 2021, 167, 906–920. [Google Scholar] [CrossRef]
- Sun, Y.; Li, L.; Xie, H.; Wang, Y.; Gao, S.; Zhang, L.; Bo, F.; Yang, S.; Feng, A. Primary Studies on Construction and Evaluation of Ion.-Sensitive in situ Gel Loaded with Paeonol-Solid Lipid Nanoparticles for Intranasal Drug Delivery. Int. J. Nanomed. 2020, 15, 3137–3160. [Google Scholar] [CrossRef]
- Schroeter, J.D.; Tewksbury, E.W.; Wong, B.A.; Kimbell, J.S. Experimental measurements and computational predictions of regional particle deposition in a sectional nasal model. J. Aerosol. Med. Pulm. Drug Deliv. 2015, 28, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; Yuan, J.E.; Zhang, Y.; Nevorski, D.; Wang, Z.; Zhou, Y. Visualization and Quantification of Nasal and Olfactory Deposition in a Sectional Adult Nasal Airway Cast. Pharm. Res. 2016, 33, 1527–1541. [Google Scholar] [CrossRef] [PubMed]
- Sosnowski, T.R.; Rapiejko, P.; Sova, J.; Dobrowolska, K. Impact of physicochemical properties of nasal spray products on drug deposition and transport in the pediatric nasal cavity model. Int. J. Pharm. 2020, 574, 118911. [Google Scholar] [CrossRef] [PubMed]
- Djupesland, P.G. Nasal drug delivery devices: Characteristics and performance in a clinical perspective-a review. Drug Deliv. Transl. Res. 2013, 3, 42–62. [Google Scholar] [CrossRef] [Green Version]
- Nižić Nodilo, L.; Ugrina, I.; Špoljarić, D.; Amidžić Klarić, D.; Jakobušić Brala, C.; Perkušić, M.; Pepić, I.; Lovrić, J.; Saršon, V.; Safundžić Kučuk, M.; et al. A Dry Powder Platform for Nose-to-Brain Delivery of Dexamethasone: Formulation Development and Nasal Deposition Studies. Pharmaceutics 2021, 13, 795. [Google Scholar] [CrossRef] [PubMed]
- Setty, Y. EBrain: A Three Dimensional Simulation Tool to Study Drug Delivery in the Brain. Sci. Rep. 2019, 9, 6162. [Google Scholar] [CrossRef] [PubMed]
- Sawant, N.; Donovan, M.D. In Vitro Assessment of Spray Deposition Patterns in a Pediatric (12 Year-Old) Nasal Cavity Model. Pharm. Res. 2018, 35, 108. [Google Scholar] [CrossRef]
- Warnken, Z.N.; Smyth, H.D.C.; Davis, D.A.; Weitman, S.; Kuhn, J.G.; Williams 3rd, R.O. Personalized Medicine in Nasal Delivery: The Use of Patient-Specific Administration Parameters To Improve Nasal Drug Targeting Using 3D-Printed Nasal Replica Casts. Mol. Pharm. 2018, 15, 1392–1402. [Google Scholar] [CrossRef]
- Pu, Y.; Goodey, A.P.; Fang, X.; Jacob, K. A Comparison of the Deposition Patterns of Different Nasal Spray Formulations Using a Nasal Cast. Aerosol. Sci. Technol. 2014, 48, 930–938. [Google Scholar] [CrossRef] [Green Version]
- Deruyver, L.; Rigaut, C.; Lambert, P.; Haut, B.; Goole, J. The importance of pre-formulation studies and of 3D-printed nasal casts in the success of a pharmaceutical product intended for nose-to-brain delivery. Adv. Drug Deliv. Rev. 2021, 175, 113826. [Google Scholar] [CrossRef]
- Gholizadeh, H.; Messerotti, E.; Pozzoli, M.; Cheng, S.; Traini, D.; Young, P.; Kourmatzis, A.; Caramella, C.; Ong, H.X. Application of a Thermosensitive In Situ Gel of Chitosan-Based Nasal Spray Loaded with Tranexamic Acid for Localised Treatment of Nasal Wounds. AAPS PharmSciTech 2019, 20, 299. [Google Scholar] [CrossRef]
- Xi, J.; Wang, J.; Si, X.A.; Zhou, Y. Nasal dilation effects on olfactory deposition in unilateral and bi-directional deliveries: In vitro tests and numerical modeling. Eur. J. Pharm. Sci. 2018, 118, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.; Golshahi, L. An in vitro evaluation of importance of airway anatomy in sub-regional nasal and paranasal drug delivery with nebulizers using three different anatomical nasal airway replicas of 2-, 5- and 50-Year old human subjects. Int. J. Pharm. 2019, 563, 426–436. [Google Scholar] [CrossRef]
- Hosseini, S.; Wei, X.; Wilkins, J.V., Jr.; Fergusson, C.P.; Mohammadi, R.; Vorona, G.; Golshahi, L. In Vitro Measurement of Regional Nasal Drug Delivery with Flonase,(®) Flonase(®) Sensimist™ and MAD Nasal™ in Anatomically Correct Nasal Airway Replicas of Pediatric and Adult Human Subjects. J. Aerosol. Med. Pulm. Drug Deliv. 2019, 32, 374–385. [Google Scholar] [CrossRef]
- Tian, L.; Shang, Y.; Chen, R.; Bai, R.; Chen, C.; Inthavong, K.; Tu, J. Correlation of regional deposition dosage for inhaled nanoparticles in human and rat olfactory. Part. Fibre Toxicol. 2019, 16, 6. [Google Scholar] [CrossRef] [PubMed]




| Region | Surface Area | Location | Characteristics | Vascularization | Epithelium |
|---|---|---|---|---|---|
| Vestibule | 0.6 cm2 | Anterior part |
| Low | Squamous epithelium |
| Respiratory region | 130 cm2 | Middle part and lateral walls |
| High | Respiratory epithelium: ciliated pseudostratified and columnar epithelium |
| Olfactory region | 10 cm2 | Upper part |
| High | Olfactory epithelium |
| Type of Lipid Nanoparticle Formulation | Drug | Targeted Disease | Cell Line | Relevant Results | Reference |
|---|---|---|---|---|---|
| SLN-liquid | Curcumin | CNS disorders | Mouse fetal fibroblasts |
| [76] |
| SLN-liquid | Dopamine and grape seed extract | Parkinson’s disease | SH-SY5Y neuroblastoma and Olfactory ensheathing |
| [74] |
| NLC-liquid | Ketoconazole | Meningoencephalitis | Fungal cells |
| [71] |
| SLN-liquid | Nalbuphine | Pain management | Human embryonic kidney (HEK-293) |
| [77] |
| SLN-semisolid | Paeonol | CNS disorders | RPMI 2650 |
| [80] |
| NLC-liquid | Pioglitazone | Alzheimer’s disease | SH-SY5Y |
| [72] |
| SLN-liquid | Pueraria flavone | CNS disorders | Caco-2 |
| [75] |
| NLC-liquid | Tacrine | Alzheimer’s disease | SH-SY5Y |
| [73] |
| NLC-liquid | Tenofovir disoproxil fumarate | Acquired Immune Deficiency Syndrome (AIDS) | bEnd.3 cerebral cortex |
| [78] |
| NLC-semisolid | Teriflunomide | Glioma | Human U-87 |
| [79] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, C.P.; Barreiro, S.; Moreira, J.N.; Silva, R.; Almeida, H.; Sousa Lobo, J.M.; Silva, A.C. In Vitro Studies on Nasal Formulations of Nanostructured Lipid Carriers (NLC) and Solid Lipid Nanoparticles (SLN). Pharmaceuticals 2021, 14, 711. https://doi.org/10.3390/ph14080711
Costa CP, Barreiro S, Moreira JN, Silva R, Almeida H, Sousa Lobo JM, Silva AC. In Vitro Studies on Nasal Formulations of Nanostructured Lipid Carriers (NLC) and Solid Lipid Nanoparticles (SLN). Pharmaceuticals. 2021; 14(8):711. https://doi.org/10.3390/ph14080711
Chicago/Turabian StyleCosta, Cláudia Pina, Sandra Barreiro, João Nuno Moreira, Renata Silva, Hugo Almeida, José Manuel Sousa Lobo, and Ana Catarina Silva. 2021. "In Vitro Studies on Nasal Formulations of Nanostructured Lipid Carriers (NLC) and Solid Lipid Nanoparticles (SLN)" Pharmaceuticals 14, no. 8: 711. https://doi.org/10.3390/ph14080711
APA StyleCosta, C. P., Barreiro, S., Moreira, J. N., Silva, R., Almeida, H., Sousa Lobo, J. M., & Silva, A. C. (2021). In Vitro Studies on Nasal Formulations of Nanostructured Lipid Carriers (NLC) and Solid Lipid Nanoparticles (SLN). Pharmaceuticals, 14(8), 711. https://doi.org/10.3390/ph14080711