Abstract
Lung cancer (LC) is the leading cause of cancer-related deaths, responsible for approximately 18.4% of all cancer mortalities in both sexes combined. The use of systemic therapeutics remains one of the primary treatments for LC. However, the therapeutic efficacy of these agents is limited due to their associated severe adverse effects, systemic toxicity and poor selectivity. In contrast, pulmonary delivery of anticancer drugs can provide many advantages over conventional routes. The inhalation route allows the direct delivery of chemotherapeutic agents to the target LC cells with high local concertation that may enhance the antitumor activity and lead to lower dosing and fewer systemic toxicities. Nevertheless, this route faces by many physiological barriers and technological challenges that may significantly affect the lung deposition, retention, and efficacy of anticancer drugs. The use of lipid-based nanocarriers could potentially overcome these problems owing to their unique characteristics, such as the ability to entrap drugs with various physicochemical properties, and their enhanced permeability and retention (EPR) effect for passive targeting. Besides, they can be functionalized with different targeting moieties for active targeting. This article highlights the physiological, physicochemical, and technological considerations for efficient inhalable anticancer delivery using lipid-based nanocarriers and their cutting-edge role in LC treatment.
1. Introduction
Lung cancer (LC) is one of the major medical challenges worldwide. It is globally ranked as one of the most commonly diagnosed cancers, representing about 11.4% of all the reported cases and it is the leading cause for cancer-related deaths, responsible for approximately 18% of all cancer mortalities in both sexes combined [1,2]. In the United States alone, the American Cancer Society predicted that there will be around 235,760 new cases of LC (accounting for 12.4% of all the new diagnosed cancers) and around 131,880 deaths (accounting for 21.7% of all cancer deaths) in 2021. More persons die from LC annually than from cancer of the prostate, breast and colon combined [3,4]. Furthermore, the World Health Organization (WHO) estimates through the Global Cancer Observatory that from 2020 to 2040 the LC incidence and mortality rates for both men and women and all ages will increase by 64.4% and 67.5%, respectively [5,6].
LC may develop as a result of different environmental and genetic factors and their interactions. Tobacco smoking remains the primary cause; smokers are found to have 10- to 30-fold increased risk of developing LC in comparison to non-smokers. Other important factors include second-hand smoke, exposure to industrial and environmental hazards such as radon, asbestos, metals including chromium, cadmium and arsenic, exposure to different organic chemicals, ionizing radiation and a positive history of respiratory illnesses (e.g., bronchitis, emphysema, and tuberculosis). In families, first-degree relatives of LC probands have a 2- to 3-fold increased risk of LC and other malignancies, many of which are not smoking-related [7].
LC is classified into two main types, small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC). The latter is subdivided depending on the tumor tissue histology into three main histologic categories, including adenocarcinoma, squamous cell carcinoma, and large-cell carcinoma. NSCLC represents approximately 85% of all lung cancers, while SCLC is responsible for the remaining percentage [7,8]. The detection of LC at its early stages is crucial for best therapy outcomes, but unfortunately, the symptoms typically start to appear only at the advanced stages of the disease and sometimes they are masked by other concurrent respiratory conditions. Accordingly, the majority of patients are diagnosed with LC while the disease is at its advanced stages and turned out to be incurable with currently available treatments [9] with very poor prognosis and a 5-year survival rates of only 21% [4].
The treatment strategy depends on the type, stage of LC and the physical state of the patient. The currently available conventional treatment methods may include surgery, high doses of intravenous chemotherapeutic agents, radiation therapy, targeted therapies, immunotherapy, and photodynamic or laser therapy [7,10]. Generally, surgery is confined to the early stages of LC and is typically combined with chemotherapy and/or radiation therapy to eradicate the cancerous tissue [9,11]. The use of single chemotherapeutic agents (such as cisplatin, paclitaxel, and etoposide) or their combinations remain the main treatment method for LC. However, the therapeutic efficacies of these cytotoxic drugs are limited due to their poor selectivity, the development of multidrug resistance, and besides, their use is associated with severe adverse effects and systemic toxicity symptoms including anemia, nausea, vomiting, nephrotoxicity and neurotoxicity which in turn limit their use [12,13]. Therefore, and for a complete cure and eradication of LC, there is an immediate need to use and investigate the possible potential roles of different routes of drug administration such as the pulmonary route and novel drug delivery systems such as nanoscale materials that are highly effective with excellent targeting abilities against the LC cells and display improved toxicity profiles.
Nanotechnology represents a powerful tool in the hands of researchers today for enhancing the currently available classical therapies and developing new therapeutic strategies and diagnostic tools to combat LC. The extensive research in this field has yielded a wide range of nanosystems (including the lipid-based nanocarriers) that have the potential to dramatically change how LC is treated nowadays [13,14,15]. This is attributed to their ability to entrap drugs with different physicochemical properties, suitability for combination therapy, and enhanced permeability and retention (EPR) effect which makes them highly effective in passive targeting, besides; their surface could be functionalized with different targeting moieties for active targeting, so they can selectively target cancerous cells and neoplasms.
In this contribution, the potential role, advantages, and challenges associated with using the pulmonary route to deliver anticancer drugs via lipid-based nanocarriers are presented. The physicochemical aspects that should be considered for efficient delivery, the recent technologies, materials, and lung delivery devices used to formulate and deliver different anticancer drug-loaded lipid-based nanocarriers are discussed. Furthermore, the advances in using the inhalable lipid-based nanocarriers for combating LC and their evaluation on the in vitro, in vivo, and clinical studies are presented.
2. Methodology
The literature selection in this review was performed by manually searching the PubMed, Google Scholar, ScienceDirect, and Wiley Library databases for published literature on inhalable chemotherapy via lipid-based nanocarriers using various keywords such as (Inhaled/aerosolized/nebulized/dry powder inhalers/inhalable chemotherapy for LC, inhaled liposomes for LC, aerosolized solid lipid nanoparticles (SLNs) for LC, DPIs of nanostructured lipid carriers (NLCs) for LC, inhalable nanoemulsions (NEs) for LC, lipid-based nanoparticles for LC, etc.). For liposomes, examples of the most recent (2010–2021) studies about inhalable anticancer drug-loaded liposomal formulations that involved in vivo studies were included in this study. All the published research work for the other types of lipid-based nanocarriers (i.e., NEs, SLNs, NLCs, niosomes, and others) designed as inhalable anticancer drug-loaded formulations for the treatment and/or diagnosis of LC were reviewed in this study.
3. Inhalable Anticancer Therapy via Lipid-Based Nanocarriers: Main Advantages and Critical Challenges
Drug delivery for the treatment of LC using lipid-based nanocarriers is achieved mainly via the intravenous and pulmonary routes of administration. Regional drug delivery methods at the tumor site are also considered for certain cases.
Pulmonary delivery of anticancer drugs via lipid-based nanoparticles for LC treatment is a growing and expanding area of research. This route of drug administration is non-invasive (needle free), provides better patient compliance, and can be self-administered. It can be used to overcome the drawbacks associated with the oral or intravenous routes that may include high levels of systemic toxicity, poor aqueous solubility of the anticancer agents, low drug accumulation within the tumor, and high rates of tumor relapse [16].
The use of inhalable lipid-based nanocarriers could provide many advantages over the conventional routes for LC treatment, especially for patients with surgically unresectable LC. Pharmacokinetically, inhalation allows the delivery of chemotherapeutic agents to the target cancer cells and avoids the hepatic metabolism; thus, rapid onset of action, lower dosing and fewer systemic distribution and toxicities are expected [17]. Moreover, the alveolar region in the lungs has a large surface area of ~100 m2, extensive vasculature, and limited drug-metabolizing enzymatic activity compared to other organs such as the liver and the gastrointestinal tract. In addition, the alveolar epithelium is extremely thin (0.1–0.2 μm), which is much thinner than that in the upper bronchial tree (50–60 μm). Thus, drug absorption and bioavailability in the targeted region may be further improved [18,19]. Additionally, phospholipids, which are major constituents of many lipid-based nanoparticles, especially liposomes, are present in the lungs and constitute almost 90% of lung surfactants [20]. This favors the design of more biocompatible lipid-based formulation and enhances lung tolerability to the delivered anticancer agent. All these factors may significantly decrease treatment failures, development of drug resistance, and chemotherapy interruptions that are responsible for the repopulation of cancerous cells. Subsequently, tumors refractory to traditional systemic chemotherapy could also potentially respond to inhalational therapy.
Pulmonary drug delivery via lipid-based carriers allows anticancer drugs to target and reach various lung tumors via different pathways. After deposition in the respiratory tract, the inhaled drug can target lung tumors by directly penetrating the tumor via the achievement of elevated local concentrations and significantly high concentration gradients of therapeutic agents at the lung tumor site. Certain types of lung tumors such as squamous cell carcinomas or bronchioloalveolar cell carcinomas that are found next to or within the airways might take up the deposited drug by direct penetration. Furthermore, drugs delivered to the lung by inhalable lipid carriers can be absorbed into the local blood circulations. Due to the communication between the bronchial and pulmonary circulations, sufficient drug concentration could reach lung tumors that lack a direct connection with the main airways depending on the tumor site. The bronchial vasculature nourishes lung tumors if they are located in the conducting region, while the pulmonary circulation feeds them if they are sited at the respiratory region [21,22,23]. However, the absorption, lung clearance mechanisms, biodistribution, and tumor penetration of inhaled drug-loaded particles are subject to many factors such as the physicochemical properties of the drug/particles, the characteristics and composition of the used formulation, the site of dug deposition, the histological features of the respiratory system, the associated pathological condition. In this regard, Haque et al. evaluated how inhaled liposomal formulation affects existing lung disease by comparing the pulmonary pharmacokinetic behavior of drug-loaded 3H-labelled PEGylated liposomes after intratracheal administration to healthy rats and rats with bleomycin-induced lung inflammation by following both 3H label and drug. The results showed that liposomes were initially cleared more rapidly from inflamed lungs than from the healthy ones but exhibited similar rates of lung clearance after three days. This was interesting given that mucociliary clearance was more efficient from healthy lungs, despite evidence of higher mucus retention in inflamed lungs and reduced association of the liposomes with lung tissue. The plasma pharmacokinetics of 3H-phosphatidylcholine revealed higher liposomal bioavailability and more prolonged absorption from inflamed lungs. Concentrations of the pro-inflammatory cytokine IL-1β were increased in bronchoalveolar lavage fluid after a single pulmonary dose of liposomes to rats with inflamed lungs, but no other significant changes in inflammatory lung markers were identified in healthy or bleomycin-challenged rats [24]. Moreover, inhaled drugs are also drained by the lymphatic system; they were commonly detected in the lungs’ lymph nodes (Figure 1). Consequently, these nodes are considered as potential targets for the inhaled drug to suppress cancer metastasis to and from the lungs [25,26,27]. Videira et al. described the biodistribution of radiolabeled (99mTc) solid lipid nanoparticles (SLNs) following their pulmonary delivery to male Wistar rats. A 60 min dynamic image acquisition was performed in a gamma-camera, followed by static image collection at 30 min intervals up to 4 h post inhalation. Radiation counting was performed in organ samples collected after the animals were sacrificed. The results revealed a significant uptake of the radiolabeled SLNs into the lymphatics after inhalation and a high distribution rate in periaortic, axillar, and inguinal lymph nodes [28]. Due to all these remarkable advantages inhalation therapy is having the potential to become an effective and safe delivery method for the treatment of LC.
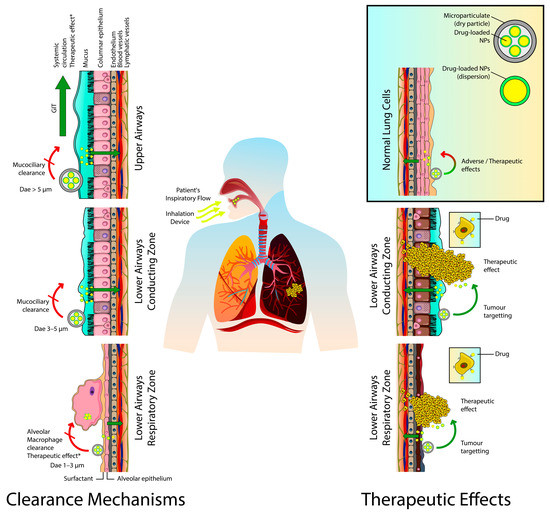
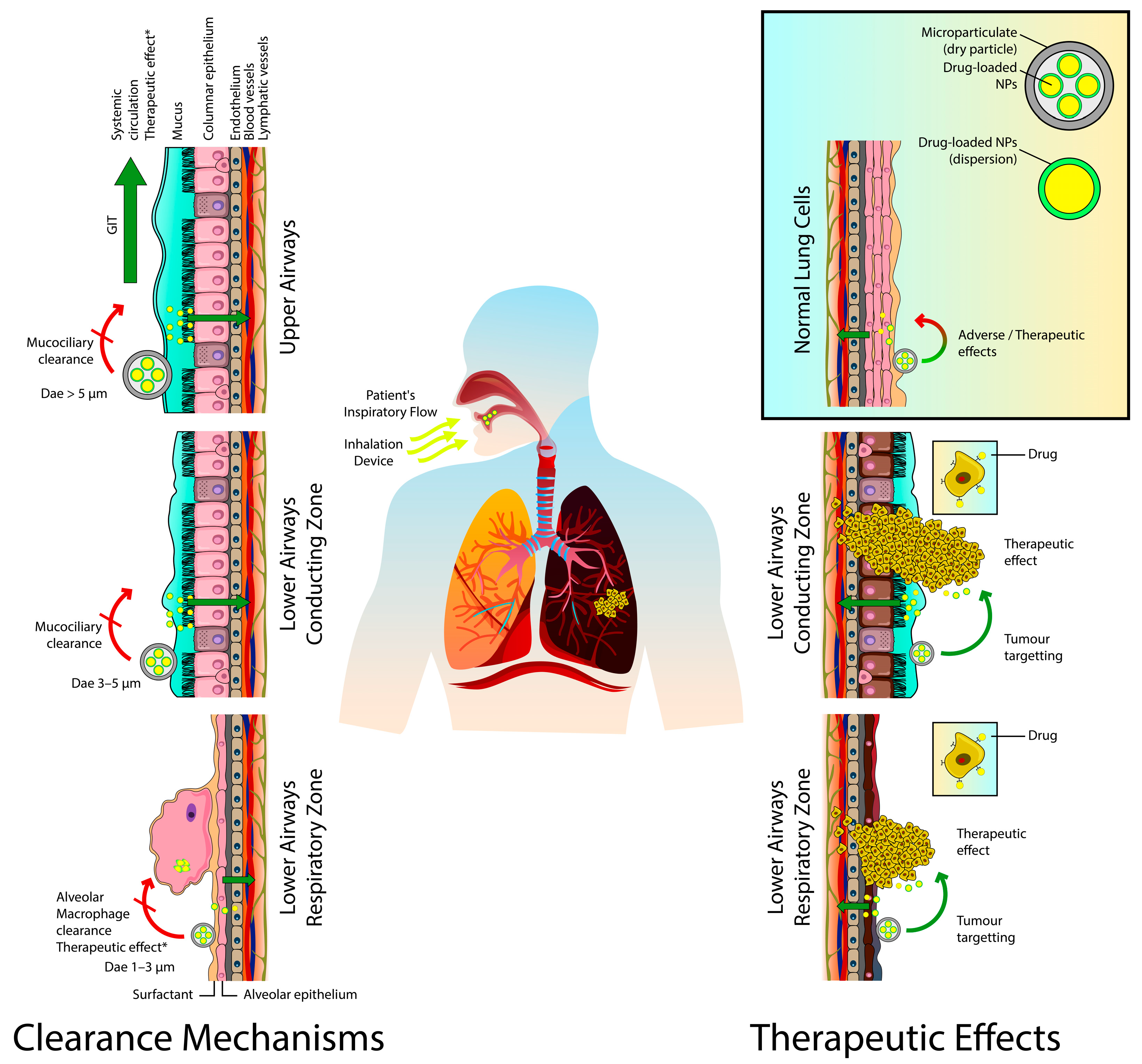
Figure 1.
The involved clearance mechanisms and therapeutic effects of the inhaled anticancer agents-loaded nanocarriers. Dae, aerodynamic diameter; GIT, gastrointestinal tract; NPs, nanoparticles. * Therapeutic effects are obtained via expression/secretion of anticancer proteins or induction of anticancer immune responses.
Despite the aforementioned advantages, we should bear in mind that the pulmonary drug delivery of anticancer drugs via lipid-based nanocarriers for LC treatment is confronted by some challenges and limitations. One of the major concerns is the lung tolerance and the potential risk of local pulmonary toxicity and adverse effects because of the cytotoxic activity of anticancer drugs themselves. Besides, the lungs’ health of LC patients is often impaired either due to LC complications or because of the presence of other concomitant lung diseases such as asthma or chronic obstructive pulmonary diseases that can significantly affect patients’ ability to tolerate the inhalable anticancer therapy.
Results from the so far conducted and published clinical trials (see Section 7) of nebulized liposomal chemotherapeutics such as 9-nitrocamptothecin (9NC) (phase I) [29], cisplatin (CIS) (phase I and Ib/IIa), (NCT00102531) [30,31,32] for the treatment of LC revealed that they have relatively safe profiles. The most reported side effects or dose-limiting toxicities (DLT) were mainly related to the respiratory tract, where grade 3 chemical pharyngitis and grade 3 bronchitis are reported as the most severe side effects [29,30]. To reduce these adverse effects, prophylactic doses of bronchodilators and/or corticosteroids before starting the anticancer inhalation therapy were used and/or recommended in clinical trials; they were found to help controlling these effects [29,33,34,35].
Furthermore, the inhaled drug-loaded particles are faced by various lung clearance mechanisms depending on various factors (Figure 1). These mechanisms can clear these particles from the lungs before reaching their targeted sites or reduce their residence time before exerting their desired therapeutic effects. The mucociliary clearance is the predominant mechanism in the conducting zone; the inhaled particles will be carried from the bronchial region to the larynx and then transferred to the gastrointestinal tract by swallowing. Almost 80 to 90 percent of the inhaled particulates can be excreted from the central and upper airways by this mechanism within 24 h. The lining mucus blanket (with thickness up to 30 μm) secreted by the goblet cells in this region represents another barrier. Additionally, particles on the alveolar epithelium (respiratory zone) may be phagocytosed by alveolar macrophages leading to lysosomal degradation, or they are taken to the upper respiratory tract by mucociliary escalator. The macrophages tend to engulf particles with geometric size of 0.5 to 5 μm. The alveolar epithelium, on the other hand, is covered by lung surfactants which can aid in drug dissolution and diffusion. If drugs are dissolved, they are either absorbed by the blood or lymphatic circulations or subjected to enzymatic degradation [36,37,38,39,40]. Lipid-based nanocarriers were employed efficiently to overcome these challenges and obtain improved therapeutic outcomes by ensuring longer drug-residence time and sustained release of therapeutic agents in the targeted sites of the lungs. Xu et al. developed a spray-dried liposomal formulation of vincristine and tested the absorption and tissue distribution of the drug after the intratracheal administration of the formulation in male SD rats. The liposomal formulation was able to enhance the pharmacokinetic behavior of the drug by decreasing drug clearance and elimination half-life by 83.2% and 81.1%, respectively, compared to the free drug solution [41]. After pulmonary delivery of paclitaxel-loaded, surface-modified solid lipid nanoparticles (SLNs) with a folate-grafted copolymer of polyethylene glycol (PEG) and chitosan, the formulation was found to significantly prolong the pulmonary exposure to the drug to up to 6 h in vivo (in female CD-1 and BALB/c mice) and limit the systemic distribution of the drug compared to inhaled Taxol-like formulation [42].
4. Physicochemical Considerations, Passive, and Active Targeting For Efficient Pulmonary Delivery of Anticancer Drugs via Lipid-Based Nanocarriers
The physicochemical properties of the anticancer drugs, nano or microcarriers, should be well considered while designing inhalation formulations because they will affect the drug residence time within the lungs and, consequently, the therapeutic efficacy. In order to achieve the required pharmacodynamic effects, anticancer agents must be available to cancerous cells within a minimum period of time. Drugs that are readily absorbed by the lungs may then be ineffective in treating the disease. Generally, lipophilic drugs with (log P > 0) are absorbed rapidly from the lungs because of their higher ability to diffuse in the lipid membranes. In contrast, hydrophilic drugs (log P < 0) tend to have longer lung residence times [43]. As a result, formulation techniques to increase lung residence of the lipophilic drugs and prolong their exposure time to cancer must be adopted.
Aerosols of drug-loaded lipid-based nanoparticles (either as liquid dispersions or dry powders) can be deposited via various mechanisms such as inertial impaction, sedimentation, and Brownian diffusion on the respiratory epithelium. These mechanisms are governed primarily by the aerodynamic diameter (Dae) of the generated aerosol particles. The Dae is the most precise parameter for measuring the aerosol particle size. It can correlate the particles’ dynamic behavior as it is calculated based on their geometric size, density, and dynamic shape. There is a consensus in the literature that for efficient pulmonary delivery, the inhaled particles should be with Dae of (1–5 µm) to reach the lower respiratory tract and in the range of (1–3 µm) for the respiratory zone. Particles with (Dae > 5) µm will be deposited in the upper respiratory tract, while smaller particles those with (Dae < 0.5) µm are expected to be emitted out of the body via the expiratory airflow [44,45,46,47]. The lipid-based nanocarriers, can be aerosolized and delivered to the lungs as dispersions by nebulization. At the same time, due to their extremely small geometric size, they must be incorporated in secondary carriers (microparticles) to be delivered as dry powders. Various particle engineering techniques were applied for their preparation and are discussed in Section 5.
Particle shape can also substantially contribute to the developed inhaled anticancer formulation’s therapeutic efficiency because it can determine the extent of alveolar macrophage clearance. The relationship between different particles’ shapes (i.e., elliptical disks, spherical, oblate ellipsoids, rectangular disks, and worm-like shape) and the time that was taken for their clearance by phagocytosis was previously investigated [48,49]. It was found that the shape and orientation of these particles significantly affect their phagocytosis clearance time. Phagocytosis was initiated for all shapes in at least one orientation. Due to macrophages’ attachment to their principal axes, elliptical discs were engulfed in less than 6 min. Regardless of the macrophage attachment point, the spherical particles were also cleared immediately. Interestingly, macrophage attachment to the flat surfaces or minor axes of the rectangular, elliptical disks, and oblate ellipsoids failed to clear these particles even after two h [48]. Furthermore, because of their low curvature region, worm-like shaped particles resulted in significantly less phagocytosis clearance than the spherical particles [49]. By the use of particle-engineering technologies, lipid based nanocarriers could be embedded in microparticles of different morphologies for possibly enhancing their delivery.
The surface charges of the inhaled particles could also influence their cellular uptake in addition to their clearance and retention in the lungs. The positively charged particles were reported to have better penetration into tumor cells because of their higher binding tendency with tumor cells [36]. Furthermore, cationic nanoparticles were shown to be taken up quickly by the lung epithelial cells and or macrophages shortly after their administration, unlike neutral and anionic nanoparticles of the same hydrodynamic diameters. Therefore, cationic nanoparticles are retained for a longer time within lung cells, limiting their translocation to lymph nodes and bloodstream [50].
Targeting cancerous cells via drug-loaded lipid-based nanoparticles could be done by the passive and active methods. Generally, via passive targeting, nanoparticles tend to leak preferentially into cancer tissue via permeable tumor vessels and are then retained in the tumor bed due to reduced lymphatic drainage. This phenomenon is known as the enhanced permeability and retention (EPR) effect [51]. However, the EPR effect is suggested to offer less than a twofold increment in drug delivered by a nanocarrier to tumors in comparison to critical healthy organs, resulting in subtherapeutic concentrations that are not sufficient to cure most cancers [52]. On the other hand, the active targeting can enhance the therapeutic efficacy and increase the selectivity of drug delivery by attaching targeting ligands (which bind to specific receptors on the tumor cells and endothelium) to the surfaces of nanocarriers [53]. The surfaces of the lipid-based nanocarriers are highly tunable and could be functionalized using with more than one type of functional groups and surface modification techniques to provide stealth characteristics (PEGylation), and active targeting towards the cancerous cells [54,55]. The main targeting sites in LC may include the overexpressed receptors on the surfaces of the cancer cells (e.g., epidermal growth factor receptor (EGFR), folate receptors (FRs), transferrin receptors (TfRs)), cellular organelles (e.g., mitochondria, lysosomes) and the LC microenvironment (e.g., vascular cell-adhesion molecules, cluster-of-differentiation 44, matrix-metalloproteases) [56]. The strategies of developing positively charged or surface modified uni, di, or multifunctional lipid-based nanocarriers using different ligands and targeting moieties for active targeting of LC are adopted by many researchers. The results of these studies in enhancing the pulmonary delivery and therapeutic efficiency of anticancer drugs on the in vitro and in vivo levels are discussed and summarized in Section 6 of this article. Another active targeting method of malignant cells can be achieved by the development of “stimuli-responsive” nanocarriers by taking advantage of the natural physiological conditions within the target tissue, such as elevated temperature or alteration in pH, or through the application of external stimuli such as a magnetic field or ultrasonic waves [54]. However, the potential role of different types of inhaled stimuli-responsive lipid-based nanoparticles, such as the thermo-sensitive, pH-sensitive, magnetic-field, and ultrasound responsive nanocarriers in the treatments of LC, is rarely investigated. In one study, inhaled magnetic and thermo-responsive lipid vehicles were incorporated with superparamagnetic iron-oxide nanoparticles and budesonide for controlled and targeted pulmonary delivery. The formulated dry powders had a fine particle fraction (FPF) of 30%. The formulations were shown to have an accelerated drug release rate at hyperthermic temperatures (45 °C). The authors concluded that the developed lipid matrix is a good and effective drug vehicle in targeted and controlled inhalation therapy [57].
In addition to the discussed physicochemical aspects, the pathophysiological aspects of the lungs should also be considered while developing an inhalable formulation for the treatment of LC. These might include LC type and stage, concomitant diseases such as asthma, chronic obstructive pulmonary diseases, and their associated changes to normal lung physiology should also be considered while developing an inhaled formulation for the treatment of LC. These considerations are well-reviewed and discussed elsewhere [36,58].
5. Devices for the Pulmonary Drug Delivery of Anticancer Drug-Loaded Lipid-Based Nanocarriers
Drug-loaded lipid-based nanoformulations are delivered to lungs as liquid-based (i.e., solutions, dipersions) or solid-based (i.e., dry powders) aerosol systems. Nebulizers, dry powder inhalers (DPIs), pressurized metered-dose inhalers (pMDIs), and soft-mist inhalers are the main types of devices to deliver therapeutic agents into the lungs.
For effective therapeutic outcomes, higher doses (ranging from one to tens of mg) of inhaled anticancer drugs must be deposited in the lungs. The pMDIs and soft-mist inhalers can only deliver smaller drug doses of less than 1 mg; therefore, they are rarely used to deliver anticancer drugs [59,60]. On the other side, nebulizers and DPIs are suitable for delivering higher drug doses necessary for the inhaled chemotherapeutic agents to act on the cancerous cells and tumors. Therefore, these devices have the potential to be used effectively for inhalable-based anticancer therapy.
Nebulizers are liquid-based aerosol delivery devices. Different types of these devices are available, including jet, vibrating mesh, and ultrasonic nebulizers; they deliver aerosols to the lungs as finely atomized droplets with high FPF over certain periods of time using compressed gas flow, oscillating perforated membrane, or piezoelectric crystals vibrating at high frequency, respectively [61]. They are the most used delivery systems of anticancer drugs-loaded lipid-based nanoparticles in preclinical studies and the only used ones in pilot studies and clinical trials (see Section 7). Nebulizers have many potential benefits. They generate large amounts of aerosolized droplets with an aerodynamic size of <5 μm from solutions or nanoparticles dispersions to be deposited in the lungs. Also, they need minor patient collaboration and are suitable for patients with chronic pulmonary illnesses such as LC who cannot perform active inhalation or receiving mechanical ventilation [62]. However, for the delivery of therapeutic doses, the nebulization process may need to continue over a long period and for multiple cycles. Furthermore, during nebulization, large amounts of the produced aerosols are not inhaled but instead, they are lost in the nebulizer or released into the air leading to air contamination. Therefore, the nebulization of anticancer drugs needs to be performed under hospital settings only, as specific protective and safety measures should be taken to protect healthcare givers and neighbors and prevent their exposure to chemotherapeutic agents. On the other hand, factors such as pH, osmolality, and viscosity of the developed inhaled nanoformulations dispersions should be well characterized and optimized for efficient delivery and prevent coughing, lung mucosa irritation, and bronchoconstriction [27,63,64]. Furthermore, the nebulization process of lipid-based nanoparticles using the different types of nebulizers could significantly affect the size, drug loading, and the in vitro release rate of these carriers. It was reported that during nebulization by jet nebulizer, the multilamellar liposomes (with particle size (PS) of up to several microns) exhibited a decrease in PS, while unilamellar liposomes (with PS from 30 to 150 nm) have shown an increase in PS [65]. By testing the nebulization of paclitaxel-loaded lipid nanocapsules using jet, ultrasonic, and mesh nebulizers of different brands, it was revealed that vibrating mesh nebulizers were able to generate aerosols of lipid nanocapsules with good performance and stability [66]. The excipients of nanoparticles could also contribute to the stabilization of nanoparticles structure during the nebulization process. It was reported that the incorporation of cholesterol and PEGylated phospholipids in the liposomal formulations could result in an increase in liposome membrane stability in the broncho-alveolar lavage or during nebulization [67,68]. Therefore, the proper selection of nebulization technique and formulation excipients should be very well considered while developing lipid-based nanocarriers for the LC via nebulization. They could significantly contribute to the nanoparticle stability and consequently the therapeutic outcome of the developed formulation.
On the other hand, the solid-based delivery aerosol systems (i.e., DPIs) can overcome the previously mentioned drawbacks of nebulizers as they have many advantages and unique features [69]. They are easy to use, can be self-administered, portable, do not need hospitalization, cost-effective, and can efficiently deliver high doses of anticancer drugs or drug-loaded nanocarriers as dry powder to the lungs [70]. DPIs are breath-actuated using the patient’s inspiration for a short time with negligible drug exhalation, causing no air contamination during use. Furthermore, the dry powders have higher long-term stability, suitable for the formulation of lipophilic drugs [71]. Besides, DPIs can be produced as disposable devices, consequently limiting the contamination of the device and the environment [72]. Recently, many preclinical studies were published, including the development of inhaled anticancer drugs using DPIs for the treatment of LC, which reflects the growing interest in this approach [73,74,75]. However, the development of dry powders for such drugs for DPIs necessitates the need for taking extra protective and safety measures by the researchers and personnel in the industrial facilities if the formulation is to be commercialized.
Nowadays, there is a wide range of the available classical DPIs in the markets, and the number will keep increasing. The main differences among these DPIs lie in their design, airflow resistances, formulations’ type and excipients, and dry powder production techniques and dispersion methods [76,77]. These mentioned device and formulations related-variables, in addition to the patient-related variations such as the patient’s respiratory health and performance, may significantly affect the performance of these devices and lead to some variations in their drug deposition efficiencies into the lungs [78].
To ensure efficient powder aerosolization and delivery of drugs, the production of classical DPIs needs many optimization steps where the milled and micronized drug particles are usually formulated as three main particle types, namely: carrier-based, agglomerate-based (spheronized), and engineered particles. In the carrier-based type, the drug particles are attached physically to large inactive carrier particles such as lactose (if lactose was the used carrier they are called as lactose blends), while the agglomerates are composed of aggregates of the micronized drug. The engineered particles are usually composed of spray-dried particles of drug solubilized in an inert hydrophobic carrier [79].
On the other hand, nanocarriers-based DPIs also require many steps to create the inhalable drug-loaded nanocarriers dry powder beside the initial preparation and optimization of the drug-loaded nanocarriers processes. As discussed previously, the inhaled particles’ Dae must be in the range of (1–5 µm). Since the lipid-based nanoparticles possess too small Dae (due to their small particle size and or density) so they are not suitable by themselves for efficient deposition in the respiratory tract, where they may be exhaled out of the respiratory system. Besides, lipid-based nanoparticles’ high surface free energy due to their small size and enormous surface area can lead to particle aggregation, making their handling as a dry powder very difficult because of the poor flowability [44,80]. Overcoming these limitations of these nanoparticles can be done by particle engineering. One of the available potential solutions is to embed nanocarriers into microstructures (microparticles) with the required aerodynamic properties [81,82,83,84]. These nano in microparticles are also known as nanoaggregates or Trojan particles [85,86]. They must be engineered to have good dispersion properties to quickly dissolve and redisperse to release the initial nanocarriers in lung fluids upon delivery. The lipid-based nanocarriers, could be encapsulated into these microscale structures.
The excipients used in the formulation of dry powders of the nano in microparticles are typically hydrophilic excipients such as lactose, trehalose, dextran, and mannitol [87,88]. However, other additional materials were investigated such as L-leucine [89,90], hydroxypropyl β -cyclodextrin, polyvinyl alcohol, whey protein, maltodextrin, and gum Arabic [91].
Different techniques were used to produce dried lipid-based nanoparticles with or without excipients to generate stable, well-characterized, and inhalable particulates. These include spray-drying, freeze-drying (lyophilization), spray freeze-drying, milling, supercritical fluid drying, and electrohydrodynamic (electrospraying and electrospinning) methods. The pros and cons of these techniques, the critical variables that should be considered during formulation, and the properties of dry powders produced are well discussed and reviewed elsewhere [92,93].
Effervescent technology was also used to overcome the lipid-based nanoparticles’ size-related limitations and enhance their lungs’ release. It is done by embedding and co-drying of nanoparticles with an effervescent matrix, the typical excipients used in effervescent-based dry powders may include sodium carbonate, citric acid, and ammonium hydroxide.
The concept was first introduced by Ely et al. 2007 for polymer-based nanoparticles using ciprofloxacin as a drug model [94]. The technology was applied later to develop inhalational dry powders of cytotoxic drug-loaded lipid [95] or polymer-based nanoparticles [96,97] to treat LC. In one study, a comparison between inhalable effervescent-based and non-effervescent nanostructured lipid particles of 9-Bromo-noscapine was performed. The results showed that both formulations had good mean particle and aerodynamic size of 19.4 ± 6.1 nm and 3.1 ± 1.8 µm and 13.4 ± 3.2 nm and 2.3 ± 1.5 µm respectively. The cellular studies in A549 LC cells revealed that the effervescent-based formulation had enhanced cytotoxicity, apoptosis, and cellular uptake compared to the non-effervescent one. The in vivo studies were performed on Swiss albino male mice. The analysis of drug pharmacokinetics and distribution following inhalation demonstrated the superiority of effervescent-based formulation that exhibited 1.12 and 1.75-fold enhancement in drug half-life compared to non-effervescent formulation or drug powder [95].
6. Inhalable, Anticancer Drug-Loaded Lipid-Based Nanocarriers
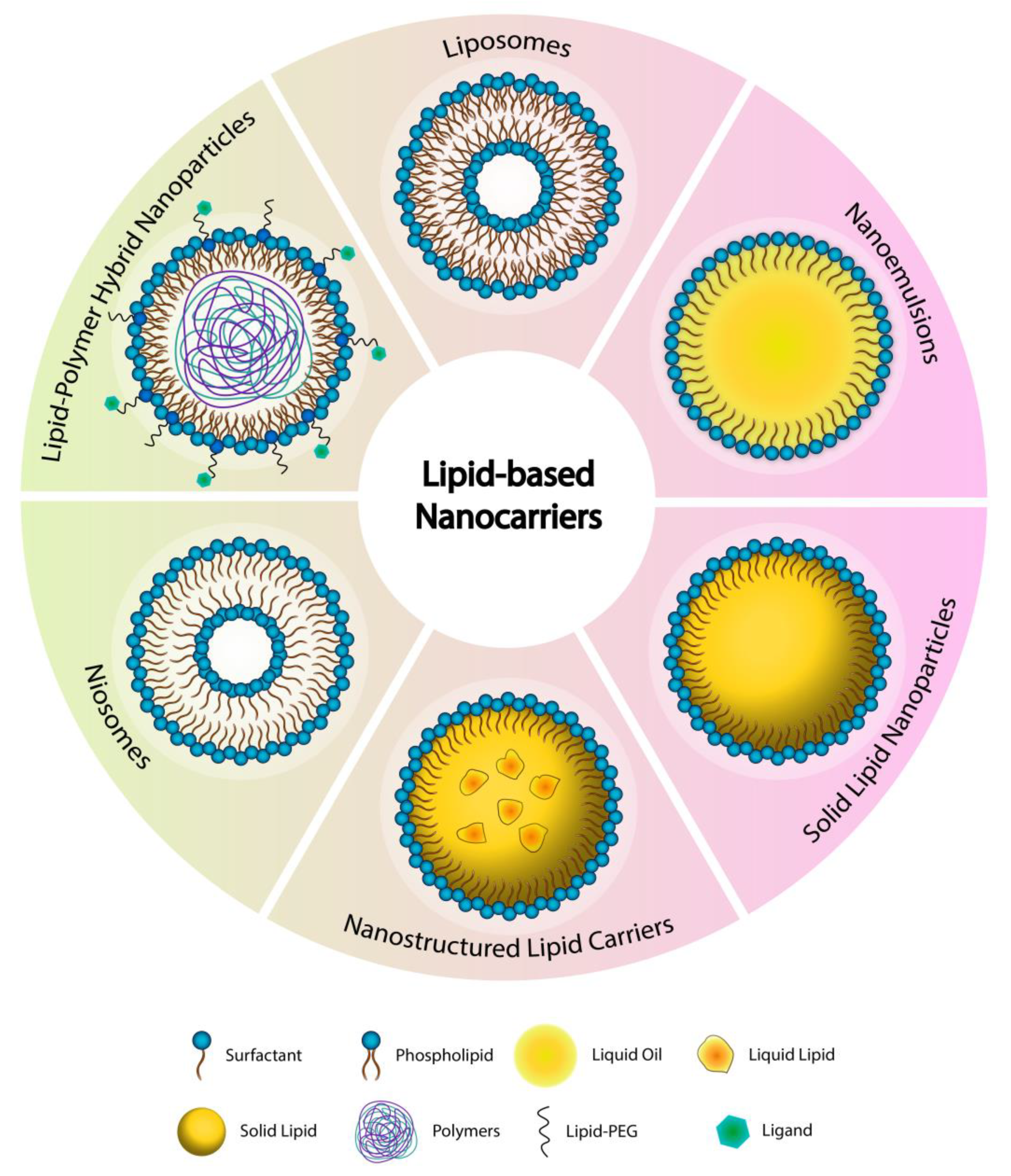
The lipid-based nanocarriers are gaining significant interest by researchers working on the development of novel formulations for the pulmonary delivery of anticancer drugs owing to their biocompatible, biodegradable, non-toxic, and non-irritant nature, the ability to entrap and deliver diverse molecules in a controlled manner with enhanced bioavailability, ability to transport across blood vessels and different membranes and barriers in addition to the ease of preparation and scale-up [98,99,100,101,102]. Furthermore, their surfaces are highly tunable and can be functionalized by different ligands to target the cancerous cells in the lungs. Taking into consideration that the majority of the newly discovered anticancer drugs belong to class II drugs according to the biopharmaceutical classification system (i.e., have poor water solubility and poor oral bioavailability) is turning lipid-based nanoparticles to be an excellent choice for researchers in this field. Lipid-based nanoparticles are the first type of drug delivery systems translated from principle to clinical application and now represent a well-developed, established, and evolving technology platform with significant clinical acceptability [103]. Each type of lipid-based carrier has a unique structure, as shown in Figure 2. In this review, the most recent studies about the inhalable anticancer drug-loaded liposomal formulation that include in vivo studies are discussed in the following section and summarized in Table 1. While all the published research work for the other types of lipid-based nanocarriers (i.e., nanoemulsions NEs, solid lipid nanoparticles SLNs, nanostructured lipid carriers NLCs, niosomes, and the others) are discussed in the following sections and summarized in Table 2.
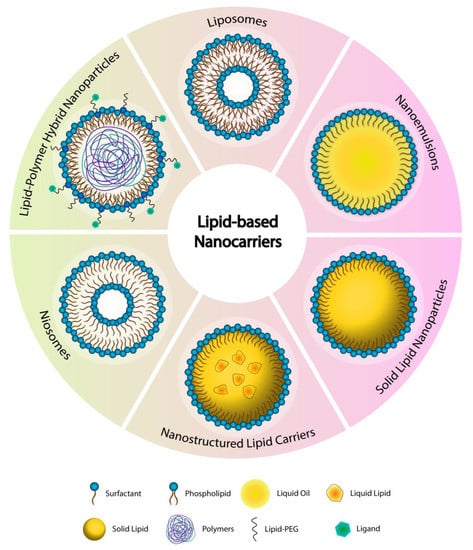
Figure 2.
The various types of the lipid-based nanocarriers.

Table 1.
Summary of the recently published in vivo studies about the inhalable anticancer drugs-loaded liposomal formulations for the treatment of LC.

Table 2.
Summary of all the published preclinical studies on the developed anticancer drugs-loaded lipid-based nanocarriers for the treatment of LC.
6.1. Liposomes
Liposomes are the primary and the most widely studied systems of the lipid-based nanocarriers for the delivery of anticancer agents using different targeting strategies for the treatment of various tumors, including LC. They are first reported and described by Bangham and his colleagues in 1960 [136]. In the subsequent years, several phospholipid bilayer structures were defined, originally called bangosomes and then liposomes, as a result of combining two Greek words, “lipos” meaning fat, and “soma” signifying “body” [137]. Liposomes are self-assembled unilamellar or multilamellar spherical vesicular systems typically composed of one or more phospholipids bilayers surrounding an aqueous core (Figure 2). Liposomal properties vary considerably depending on their lipid composition, preparation method, size, surface charge and functionalization moiety. Liposomes are typically prepared using phospholipids of various origins (natural sources such as egg yolk and soybean oil, or synthetic), cholesterol and surfactants. Generally, liposomal constituents are mimicking the biological membranes and naturally present in the pulmonary surfactants that make them non-immunogenic, biodegradable and biocompatible. The size range of liposomal systems varies between 30 nm up to several micrometers [138,139]. The surface of the liposomes is highly tunable and could be functionalized using various formulation and targeting moieties. Furthermore, because of their unique structure and composition, liposomes are able to incorporate and deliver anticancer agents (such as chemotherapeutics, genes, and peptides) of highly diverse physicochemical properties and lipophilicities, where they can enhance the therapeutic efficacy by passive or active lung targeting, reduce toxicity and improve the pharmacokinetic profile of the incorporated drugs/agents [140]. All these properties turned liposomes to be excellent candidates and active area of research for pulmonary delivery and LC therapy.
Recently, inhaled hydroxycamptothecin-loaded cationic liposomes were used with concomitant intratracheally delivered sonosensitizer (5-aminolevulinic acid) for the combined chemo-sonodynamic (Chemo-STD) therapy for metastatic LC. Liposomes were prepared using the thin film method and composed of soybean phosphatidylcholine, cholesterol, and octadecylamine. The in vivo cytotoxicity studies showed that the combined Chemo-STD therapy had better cytotoxicity effects than using the hydroxycamptothecin-loaded cationic liposomes or the SDT only. The in vivo studies on metastatic LC-bearing mice showed that the highest anticancer activity was obtained using the inhaled combined Chemo-SDT than the single therapy via either inhaled or intravenously administered hydroxycamptothecin-loaded cationic liposomes or the SDT alone. The authors suggested that the synergistic effect of the inhaled chemotherapy and STD led to improved apoptosis of cancer cells and the enhanced production of reactive oxygen species [104].
Inhalable cationic liposomal formulations loaded with unmethylated oligodeoxynucleotides containing CpG motifs (CpG) and polyinosinic-polycytidylic acid (poly I:C) double-stranded RNA were also prepared recently as locally delivered immunotherapy against LC where liposomes could increase the uptake of the loaded nucleic acids by the lung phagocytes thereby the activation of toll-like receptors within endosomes. Dioleoyltrimethylammoniumpropane (DOTAP) and dipalmitoylphosphatidylcholine (DPPC) were used in the preparation of liposomes. The formulations were tested in vivo using murine B16F10 model of metastatic LC. Delayed tumor growth was observed via both agents (i.e., poly I:C and CpG). However, increased pulmonary levels of interferon-γ were observed with CpG only. Inhalation of the CpG was superior to its intraperitoneal injection to slow the growth of lung metastases and to induce the production of granzyme B, a pro-apoptotic protein, and interferon-γ, monokine induced by the gamma interferon (MIG) and the (regulated upon activation, normal T cell expressed and presumably secreted) (RANTES), T helper type 1 cytokines and chemokines, in the lungs. These antitumor activities of CpG were efficiently enhanced by CpG loading in liposomal formulations [105].
Functionalized inhalable dry powder of folic acid-conjugated liposomal formulation of docetaxel was developed for the treatment of LC [75]. The folic acid-conjugated liposomes were prepared by the thin-film hydration method and were composed of phosphatidylcholine, cholesterol, DSPE-PEG2000-FA, and DESP-PEG2000-COOH. The prepared liposomal dispersions were then co-spray dried with mannitol and leucine at different concentrations. The particle size (PS), dispersity (Đ), zeta potential (ZP), and entrapment efficiency (EE%) of the re-dispersed liposomes were 346.8 ± 4.7 nm, 0.401, −29.3 ± 1.8 mV, and 99.5 ± 0.3%, respectively. While the liposomal dry powder had Dae, FPF, spray drying production yield (PY), angle of repose (θ), Carr’s index and Hausner ratio of 3.10 ± 0.005 μm, 10.0 ± 0.1%, 61.9% ± 0.5%, 36.8 ± 0.4 °, 32.1 ± 1.86 and 1.47 ± 0.04, respectively. The morphological studies of re-dispersed liposomes showed that they were spherical as before; instead, they had irregular shapes attributed to the effects of the spray drying process. The results of in vivo studies on Sprague Dawley rats showed a 45-fold higher concentration of docetaxel in the lungs of the studied rats at 30 min after the tracheal administration compared with the intravenously administered formulation. Higher drug exposure at the tumor site was obtained by the tracheal administration of the dry powder without exposure increment to other organs. The authors concluded that the inhaled dry powders might be clinically effective for the treatment of LC [75].
Liposomal dry powder formulation of curcumin was developed as an inhalable treatment for primary LC to overcome the drug-associated drawbacks such as low water solubility, poor bioavailability, and rapid metabolism that significantly limits clinical applications. The liposomes were initially prepared using the thin film method and were composed of soybean lecithin and cholesterol. The resulted liposomes were then lyophilized in the presence of mannitol as a cryoprotectant to obtain the final liposomal curcumin dry powder. The rehydrated curcumin-loaded liposomes had PS and Đ of (94.65 ± 22.01 nm) and (0.26 ± 0.01), respectively. While the liposomal power had Dae of 5.81 μm with FPF of 46.71%, rendering the powder suitable for pulmonary delivery. The in vitro cell culture studies showed significantly greater and faster cellular uptake of curcumin-loaded liposomes by human LC A549 cells than free curcumin. Furthermore, the high cytotoxicity of curcumin-loaded liposomes on A549 cells and their low cytotoxic activity against normal human bronchial BEAS-2B epithelial cells produced a high selection index partly due to increased cell apoptosis. The in vivo studies were performed by directly spraying curcumin liposomal powder, curcumin powder, and gemcitabine into the lungs of male Sprague–Dawley (SD)rats with LC through the trachea. Higher anticancer effects were obtained by developed liposomal curcumin powder than the other two tested medications in terms of pathology and the expression of various cancer-related markers such as VEGF, malondialdehyde, TNF-α, caspase-3, and BCL-2. Accordingly, the developed curcumin liposomal dry powder formulation has the potential to be used as inhalation therapy for LC [108].
The use of bacterial therapy is an emerging treatment technique for various cancers and may represent a promising strategy to combat LC when locally delivered by inhalation. Recently, inhaled live carriers (paclitaxel-in-liposomes-in-bacteria) were prepared and evaluated for the treatment of primary LC. The paclitaxel-load liposomes were prepared using the thin film method and composed of soy phosphatidylcholine and cholesterol. The drug-loaded liposomes were then internalized by electroporation into bacteria (Escherichia coli or Lactobacillus casei) to get LP-in-E. Coli (LPE) or LP-in-L. Casei (LPL). The PS, Đ, ZP and EE% of the developed paclitaxel-load liposomes were 64.3 ± 2.4 nm, 0.35 ± 0.08, −9.96 ± 0.48 mV and 97.2 ± 0.5% respectively. In vitro cytotoxicity studies on the A549 cell line revealed that LPE caused the highest inhibition of cellular proliferation compared to LPL, paclitaxel-loaded liposomes, a mixture of paclitaxel-load liposomes and bacteria. Paclitaxel-in-liposomes-in-bacteria delivered the cargos into the cells quicker than the other tested samples. The results of the in vivo studies on primary LC animal model using male Sprague–Dawley (SD) showed that among all the studied formulations, LPE had the highest anticancer effect with the downregulation of vascular endothelial growth factor (VEGF) and hypoxia-inducible factor 1-alpha (HIF-1α) and the enhancement of malignant cell apoptosis following the intratracheal administration. Furthermore, the live bacterial carriers significantly improved the expressions of (tumor necrosis factor- α, interleukin 4, and interferon-γ) immune markers and (leukocytes and neutrophils) immune cells [106].
6.2. Nanoemulsions
The first record in the history of nanoemulsions (NEs) began in 1943 with Hoar and Schulman [141]. However, it was not until 1993 that the term “nanoemulsions” or “ultrafine emulsion” was first reported, reflecting a formulation with a nanoscale droplet size (PS) of 20 nm to 200 nm, a transparent and semi-translucent appearance, and long-term thermodynamic stability against sedimentation by preventing flocculation, aggregation, coalescence, and Ostwald ripening [142,143]. Generally, the International Union of Pure and Applied Chemistry (IUPAC) does not yet have a fixed PS range for NEs [144], while the US FDA is considering NEs PS in the nanoscale range (approximately 1–100 nm) [145]. However, NEs prepared for pulmonary delivery must comply with the PS parameter set for this route.
NEs have gained popularity from the fact that they can be formulated from natural or synthetic excipients that are generally recognized as safe (GRAS) or approved by the US FDA [143,146,147]. Their structure is illustrated in Figure 2. In chemotherapy delivery, NEs superiority over conventional delivery systems originates from their ability to achieve the required therapeutic effect by enhancing the solubility and bioavailability of poorly soluble drugs, which may significantly contribute to decreasing drugs’ dosing and frequency as the drug is released in a sustained release manner over longer times [148,149]. Since vascularized tissues surround the cancer cells, NEs can easily accumulate in these tissues because of the small PS that gives them the advantage to pass through such barriers [150] via direct transcellular or paracellular transport. By proper selection of formulation excipients, they could have the ability to inhibit the P-gp efflux, thus enhancing cellular and mucosal permeability of the incorporated anticancer drug [118]. Besides, NEs lipophilic core is augmenting the nanosystem’s stability by protecting the drug/compound against the enzymatic hydrolysis allowing better drug delivery [151].
NEs can be categorized as simple or multiple emulsions depending on whether the core is either water or oil and the complexity of the carrier [147]. As far as pulmonary drug delivery is concerned, NEs can be classified into three generations; first-generation are prepared by spontaneous emulsification and composed of oil, surfactants, co-solvents, and a selected aqueous phase such as deionized water or saline solution [151,152]. The second-generation NEs contain the same materials as the first, but their droplets are additionally decorated with specific polymers (chitosan, hyaluronan, hydroxypropyl methylcellulose (HPMC)) to enhance mucoadhesive properties, while the third generation droplets are decorated with ligands and/or polymers for targeted drug delivery [59]. As a nonequilibrium system and a spontaneous formation is unfeasible, high energy input is applied to form NEs. This can be achieved by homogenizing the aqueous phase with an immiscible oil phase using low-and/or high-energy emulsification techniques. The size of the droplets will depend heavily on the hydrophilic-lipophilic balance (HLB) values of the NEs’ excipients [153], the type of instruments used, and their process parameters, such as time, stirring speed, temperature, and sample composition [147].
Although NEs may be constructed using long, medium, short-chain fatty acids or any mixture of them, however, it is noted that the inhaled NEs prepared for the delivery of conventional (non-cytotoxic) drugs were mostly composed of either medium [154,155,156] or long-chain fatty acids separately [152,157]. In contrast, inhaled NEs for anticancer delivery are usually prepared using a mixture of both (i.e., the long and medium-chain fatty acids), as illustrated in the following sections. Future studies could focus on comparing the impact of the fatty acid chain lengths on the suitability, efficiency, and biocompatibility of the inhaled anticancer NEs for lung delivery [158,159].
In addition to the oil phase, selecting a proper surfactant system is essential for the proper development of NEs for pulmonary delivery. The use of non-ionic surfactants is more prevalent than ionic surfactants in the formulation of NEs, due to the suggested deterioration of the biological membrane by their use. The superiority of non-ionic surfactants also comes from their ability to enhance poorly soluble drug dissolution, particle size, shape, and stability [160]. NEs safety is another concern that is primarily associated with the use of synthetic emulsifiers and is a key issue that needs to be addressed in particular to the adverse negative interactions between lipids and surfactants of the lung alveoli [161]. Most synthetic emulsifiers may trigger toxic symptoms with prolonged administration, including the potential binding of anionic emulsifiers to proteins, enzymes, and phospholipid membranes in the human body, resulting in various adverse reactions, such as enzyme dysfunction, protein structure modification, and membrane cell phospholipid [162]. Consequently, replacing synthetic emulsifiers and excipients with natural substitutes is one of the novelties on-demand in the construction of the NEs. Co-solvents may also be included in the formulation of NEs. Glycerol is used as the preferred co-solvent in almost every inhaled NEs for the lung delivery of anticancer drugs. This could be due to its ability to modify the aerodynamic distribution of the PS of the emitted aerosol droplets and to produce a slower dissolution rate, with the potential to modify the cell permeability of the loaded drugs, which can significantly impact their lung absorption and distribution [163,164].
Since NEs behave similarly to solutions, these formulations tend to exhibit significant improvements in their in vitro aerosolization performance when nebulized compared to other suspended nanoformulations’ types [165,166]. Although there are various solidification techniques for the production of NEs as dry powders, no dry powder of anticancer drug-loaded NEs were produced. All the developed NEs were aerosolized using nebulizers only.
NEs of docetaxel were recently formulated using biocompatible excipients for the drug pulmonary delivery to overcome the drug’s low solubility and improve its bioavailability and efficacy. A mixture of medium (lauric fatty acids and palm kernel oil esters) and long-chain fatty acid (myristic fatty acids) were used as the oil phase in these NEs. The surfactants system was composed of non-ionic (Tween 80® and Span 80®) and amphipathic (lecithin) surfactants as they are known to be non-toxic, biocompatible, and unaffected by pH. The optimized docetaxel-loaded NEs formulation had a spherical shape with PS, ZP, and entrapment efficacy (EE%) of 94.35 ± 0.77 nm, −38.64 ± 1.43 mV, and 100%, respectively. Besides, the optimized NEs were also shown to have neutral pH, with an osmolality of (301 ± 1.00 mOsm/kg) and viscosity of (1.92 ± 0.08 cP) that are suitable for the pulmonary delivery. The optimized NEs were aerosolized using OMRON MicroAIR nebulizer and were evaluated using the Andersen cascade impactor method. The nebulized NEs showed desirable aerosolization properties for pulmonary delivery where the Dae and the FPF were 3.02 ± 0.26 and 92.76 ± 0.63, respectively. The in vitro cell culture studies found that the final formulation is more selective on human lung carcinoma cells (A549) than the normal cell (MRC-5). It was concluded that the developed NEs are potential carriers for docetaxel in targeting LC via the inhalation route [118].
Aerosolization of NEs for pulmonary delivery for LC using docetaxel and curcumin were also reported by the same group. The NEs for both drugs (separately) were designed with a mixture of medium (palm kernel oil ester) and long (safflower seed oil) chain fatty acids and a set of non-ionic (Tween 85® and Span 85®) and amphipathic (lecithin) surfactants, as well as glycerol as a co-solvent. Both formulations were characterized and found to have the required physicochemical and aerosolization properties suitable for inhalation [119].
The in-vitro aerosolization and toxicity of curcuminoids NEs for LC were investigated by Al Ayoub et al.; the formulated NEs were composed of medium (limonene) and long (oleic acid) fatty acids as oil phases, Tween 80® as the surfactant, and ethanol as the co-surfactant. Based on the loaded amount of curcumin (100–500 µg/mL), the developed NEs had the PS of (13–39 nm) and Đ of (0.1–0.2) as well as osmolality, pH, and viscosity in the range of (336 to 600 mOsm/kg), (6–7), and (1.1–1.7 mPas) respectively. The nebulized NEs prepared with limonene oil had FPF and Dae ranged from 50% and 4.6 μm to 45% and 5.6 μm, respectively; whereas the FPF and Dae of the nebulized NEs prepared with oleic acid oil ranged from 46% and 4.9 μm to 44% and 5.6 μm, respectively. Genotoxicity using Comet assay showed that the developed NEs are nontoxic at the tested curcuminoid doses suggesting the safety and suitability of the developed NEs. The authors recommend further pre-clinical and clinical studies [120]. However, additional cytotoxicity evaluation and in vitro release study are also essential in such formulations.
Quercetin is a flavonoid phytochemical that is suggested to treat LC via its antiproliferative and antimetastatic effect on A549 cells through the impact on the cytoskeleton and repressing the metastatic capacity of LC via suppressing, as well as promoting apoptosis in LC [167]. NEs of quercetin were employed to enhance the lung delivery of this poorly soluble flavonoid for the treatment of LC. The in vitro cytotoxicity studies showed some selectivity of the quercetin-loaded NEs towards the A549 cells line without affecting the normal cells [121,122].
Although the used excipients in all these studies are considered safe, but the long-term safety studies due to possible adverse interactions with lung surfactants and efficacy of developed formulations against LC are strongly encouraged at the in vitro and preclinical level before reaching to clinical trials.
Like other lipid-based formulations, NEs that are working through passive targeting, are facing limitations in recognizing cancer or normal cells. Active targeting of the nanoemulsions could be approached by modifying the surface of these carriers, where the attached ligand (monoclonal antibodies, transferrin, folic acid, hyaluronic acid, aptamer, or antibody fragments) aids in recognition of the target tumor cells [146]. The development of anticancer-loaded NEs for active targeting decorated with ligands such as the folate-targeted NEs loaded with docetaxel [168] and transferrin-targeted docetaxel NEs [169] for ovarian cancer are already developed but currently limited for intravenous delivery. Inhaled NEs with active targeting moieties for the treatment of LC as far as we are aware, are not explored yet.
6.3. Solid-Lipid Nanoparticles (SLNs)
Solid lipid nanocarriers (SLNs) were introduced in 1991 as an upgrade to the traditional colloidal drug delivery systems. They are best represented as a mixture of liposomes and niosomes containing phospholipids and surfactant molecules, with a submicron PS ranging from 40 to 1000 nm [170,171]; they are derived from oil-in-water (O/W) emulsions by replacing liquid lipids with a lipid matrix that is solid at room and body temperatures [172], as illustrated in Figure 2. The use of solid lipids instead of liquid oils can result in controlled release of drugs as the mobility of the drug in a solid lipid matrix is significantly lower than that of liquid oil [173]. SLNs are composed of physiologically tolerated and safe lipids such as fatty acids (e.g., stearic acid), monoglycerides (e.g., glycerol monostearate), diglycerides (e.g., glycerol behenate), triglycerides (e.g., tripalmitin, tristearin, trilaurin), waxes (e.g., cetyl palmitate), or steroids (e.g., cholesterol) that are dispersed with an appropriate surfactant phase [174]. Next to the design of the inhalation devices, drug carrier’s selection is equally important in assuring the sufficient stability and appropriate size delivery of the loaded drug, thus, lipids and surfactants selection is an essential factor for SLNs characteristics [175]. Generally, high-pressure homogenization and microemulsion methods are being the most commonly used for the preparation of SLNs.
Pharmacokinetically, SLNs, and liposomes have been reported to be eliminated from the lungs at comparable rates, even though SLNs are deposited after intratracheal instillation in the upper respiratory tract and, in particular, through the mucociliary escalator and do not stimulate significant inflammatory reactions [176]. The inhaled radiolabelled SLNs biodistribution showed significant uptake in lymphatics, with a high rate of distribution in periaortic, axillary, and inguinal lymph nodes and these findings indicate that SLNs may have the potential to be efficient carriers for lymphoscintigraphy or pulmonary therapy [28]. Besides, some SLNs may remain mostly intact in the pulmonary area, which may lead to longer lung retention times [177].
As safety and lung tolerability are of the essential parameters to be considered while developing formulations for pulmonary delivery, some studies preferred to assess the toxicity of the inhaled blank SLNs before deciding to load them with active materials. For instance, a blank SLNs formulation was designed using a lipid matrices mixture of triglycerides (Softisan®) and phosphatidylcholine (Phospholipon® 90G), Solutol®HS15 as a surfactant, and double-distilled water. The high-pressure homogenization method was used for the preparation. The produced SLNs had PS, Đ, and ZP of 98.4 nm, 0.148, and −14.6 mV, respectively. The MTT assay and neutral red uptake assay (NRU) on the A549 cell line for 24 h showed the blank SLNs ability to reduce this cell line viability with calculated half-maximal effective concentration (EC50) of 3090 µg/mL and 2090 µg/mL, respectively. The organotypic cultures of lung tissue showed that the SLNs reduced the metabolic activity of the used murine precision-cut lung slices after incubating it with SLNs for 24 h and using WST-1 assay at EC50 of 575 µg/mL. The SLNs were nebulized by a jet-driven aerosol generator system. The in vivo cytotoxicity study on female BALB/c mice for 16 days showed no significant changes or upregulation in lactate dehydrogenase levels as an exponent of low levels of damage to the cell membrane, as well as bronchoalveolar lavage fluid protein as an indicator of cytotoxicity in lung tissues, and different inflammation indicators (TNF-a, IL-8 (A549), IL-6, and chemokine KC) [123]. Interestingly, this system was reported without loading it with an active ingredient; moreover, such evaluation should be conducted using both LC and normal cell lines to get a complete understanding of the developed SLNs’ cytotoxicity and selectivity.
Inhaled SLNs were also used to get rapid drug deposition in lungs, less systemic side effects, and improved drug therapeutic efficiency of erlotinib (a quinazoline derivative with antineoplastic properties). The SLNs were synthesized from Compritol 888 ATO® (solid lipid), Tween 80® (surfactant), and Poloxamer 407® (an aqueous phase surfactant) using the hot homogenization method [124]. The erlotinib-loaded SLNs owned a PS (< 100 nm), Đ (0.367), DL% (4.17%), and EE% of (78.21%). For aerosolization of the developed NLCs, they were further spray dried in the absence and presence of mannitol (as an inert bulking agent). The dry powder of aerosolized erlotinib-loaded SLNs in the presence of mannitol had Dae (3.93), emitted dose (ED)% (94.91), FPF% (30.98), and geometric standard deviation (GSD) of (4.339). The TEM and scanning electron microscopy (SEM) micrographs of both liquid and powder SLNs indicated a regular and spheroidal shape with smooth surfaces. The in vitro release studies using the dialysis membrane method showed that here was no burst release from the formulated SLNs and cumulative drug release occurred with a steady rate to reach approximately 12% at 8 h, as compared to ~18% with free drug. Besides, the MTT assay revealed significantly higher anticancer activity of the erlotinib-loaded SLNs against A549 cells in comparison to the free drug and after 18 h of incubation. However, no in vivo studies were performed for the elevation of organs distribution and anticancer activity were performed in this study.
Epirubicin which is an anthracycline and a stereoisomer of doxorubicin that has shown activity against various types of tumors including LC, but its use is associated with major side effects including hematological and cardiac toxicity, thus specific targeting through simple, safe and stable formulations is highly recommended. Accordingly, Epirubicin-loaded SLNs were prepared. The SLNs were composed of soy lecithin, compritol 888 ATO®, and poloxamer 188®. The produced SLNs had the characterization of PS, ZP and EE% of 223.7 nm, −30.6mV, 78.9% respectively. The formulation was nebulized using (Pari Inhalierboy, Starnberg, Germany). No significant changes in PS ZP or EE% were observed after nebulization [125]. The nebulized formulations were evaluated for their in vitro deposition by a Twin Stage Impinges (TSI). The blank SLNs, epirubicin-loaded SLNs and pure epirubicin solution showed respirable fractions (RF) of 77.03%, 78.46%, and 59.51%, respectively indicating the decrease in drug loss, besides the SLN possible ability to deliver the drug into the deep lung. The cytotoxicity on A549 cells using 0.1% crystal violet after incubation for 24 h revealed the improved cytotoxic effects of the developed SLNs in comparison to the free drug. Pharmacokinetically, and upon analyzing plasma and lung samples via HPLC, aerosolized epirubicin-loaded SLNs showed excellent lung deposition characteristics compared to epirubicin solution in male Sprague–Dawley rats, while the plasma area under the curve values for epirubicin-loaded SLNs was 2.07-fold higher than that after epirubicin solution suggesting the potential suitability of the developed inhaled SLNs for pulmonary delivery to treat LC.
SLNs were employed also for the co-delivery of afatinib and paclitaxel for the treatment of epidermal growth factor receptor tyrosine kinase resistant NSCLC. In this study, afatinib was first loaded in SLNs composed of stearic acid and poloxamer 188 and had PS, Đ, and EE% of 358.3 nm, 0.167, and 87.9% respectively. Furthermore, these SLNs were lyophilized using trehalose (as a cryoprotectant) and loaded with paclitaxel into poly-lactide-co-glycolide-based porous microspheres. These inhaled microspheres systems are characterized with Dae, FPF, fine particle dose (FPD), and GSD of 3.26 and 3.25 µm, 23.04 and 24.07%, 41.01 and 59.66 µg, 2.26 and 2.32, as well as EE% 53–70.85% of afatinib and paclitaxel, respectively. These final formulations showed an initial in vitro drug release for paclitaxel (20%) and afatinib (30%), with extremely high retention (more than 65%) in the induction port (17.21 ± 0.22% for afatinib and 16.00 ± 1.52% for paclitaxel), and no interaction between drugs and carriers when characterized by FTIR and NMR spectroscopy [126]. On the cellular level, there was a significant synergistic effect between afatinib and paclitaxel and superior treatment capability of the final loaded microspheres for drug-resistant NSCLC on H1975 and PC9/G cells. The pharmacokinetics and tissue distribution results demonstrated that afatinib and paclitaxel in the microspheres exhibited 96 h of a two-stage release and high lung concentration. The final loaded microspheres did not distribute to other critical organs. These results revealed that the drug combination therapy using these nanocarriers is highly promising for treating drug-resistant LC.
6.4. Nanostructured Lipid Carriers (NLCs)
The nanostructured lipid carriers (NLCs) represent an advanced type of the SLNs. These carriers can overcome the SLNs-related disadvantages, such as the drug loading capacity and formulation stability challenges by creating a less structured solid lipid matrix via mixing fluid lipid with solid lipid (as shown in Figure 2), resulting in less drug expulsion during storage [173,178,179]. NLCs are the products of o/w emulsion process, hence the available surfactants typically have a high HLB range, and ideally dissolved in the external aqueous phase of the emulsion [160]. Besides, these nanocarriers can be used to circumvent the limitations associated with conventional cancer chemotherapy such as poor drug solubility, and multiple drug-resistance by enhancing chemotherapy’s targeting and selectivity index [180,181]. Additionally, NLCs are suitable to carry drugs with different physicochemical properties, natural compounds and small interfering RNA (e.g., siRNA), where the latter is currently trending as an NLCs conjugate due to its proved ability in recognizing a homologous mRNA sequence in the cancer cell and induce its degradation [182]. In this regard, and chemistry wise, a smooth conjugation between thiol-modified DNA or RNA molecules (e.g., siRNA) and the NLCs surface occurs by biodegradable disulfide (S–S) bonds. Further conjugations with NLC include polymers conjugation (e.g., PEG) with targeting fractions (e.g., luteinizing-hormone releasing hormone (LHRH) peptide) [183,184]. However, the key drawback of NLCs is the need to use organic solvents to initially solubilize the hydrophobic drugs before loading [185], as well as the short-term stability of the liquid NLCs compared to the solid ones [186,187]. Like the previously discussed lipid-based nanocarriers, the use of NLCs as localized inhaled dosage forms is still under investigation mainly as active carriers for anti-tuberculosis [188], genetic disorders such as lung cystic fibrosis [189], antibiotics lung delivery [190,191], in addition to LC therapies. In LC, NLCs are often used to resolve p-glycoprotein (P-gp) efflux, and drug resistance which is generally associated with over-expression of MRP1 protein (responsible for cancer cell drug efflux) and BCL2 protein (responsible for anti-apoptotic cellular defense) [192,193,194,195].
Inhaled NLCs were used for the pulmonary delivery of various drugs and approaches for LC treatment. The cyclooxygenase-2 enzyme, which is responsible for the progression and growth of NSCLC and found to be up-regulated among different cancers [196,197]. Thus, the efficacy of inhaled celecoxib-loaded NLCs in NSCLC in combination with IV administered docetaxel was evaluated using a metastatic A549 tumor model in Nu/Nu mice. The NLCs were initially prepared using a hot melt homogenization technique via mixing compritol (solid lipid), miglyol (liquid lipid), and sodium taurocholate (surfactant). The PS, Đ, ZP, drug content, DL, EE% of the NLC produced were 211 nm, 0.22, 25.30 mV, 1.8 mg/mL, 4 w/w%, and 95.6%, respectively. The celecoxib-loaded NLCs were nebulized using Inexpose™ (SCIREQ Scientific Respiratory Equipment Inc, Montreal, QC, Canada). The aerosolized NLCs had Dae and FPF were 1.58 μm and 76.2%, respectively. The isobologram of the interaction between docetaxel and celecoxib-NLC in the A549 NSCLC cell line suggests moderate synergistic activity. While the analysis of the 28-days in vivo studies showed that treatment with inhaled celecoxib-NLC, IV docetaxel, and the combination of both treatments decreased tumor volume by 25%, 37%, and 67%, respectively, without a substantial decrease in mice weight compared to control group. Besides, the inhaled celecoxib-NLCs, IV docetaxel, and combined therapy have also decreased vascular endothelial growth factor expressions in regressive tumors by 0.27, 0.44, and 0.65 times, respectively, compared to control. The quantitative proteomic analysis shows a significant reduction in the regulation of multiple proteins demonstrating enhanced anticancer activity in combination therapy compared to docetaxel treatment alone [128].
A comparison in lung deposition was evaluated in vivo using Wistar rats between pulmonary delivered paclitaxel loaded-NLCs (as a dry powder delivered using insufflators (Penny Century, PA, USA)) and orally administered methanolic PBS suspension of the drug [129]. The NLCs were prepared by the emulsification and ultrasonication method using various surfactants. The solid and liquid lipids phase consisted of stearic acid (or glyceryl monostearate) and oleic acid at different concentrations, while the aqueous phase was composed of different amounts of Tween 80®, Tween 20®, or Tween 40®. The statistical analysis showed that the low lipid ratio, the high levels of surfactant concentration and, the medium homogenization speed provided favorable ranges of PS, Đ, and ZP values for Tween 20® (178.7 nm, 0.158, −15.22 mV), Tween 80® (243.1 nm, 0.225, −16.12 mV), and Tween 60® (298.2 nm, 0.281, −22.23 mV). The NLCs formulated with Tween 20® showed the highest uptake of Caco-2 cells, which could be attributed to Tween 20® ability to inhibit P-gp efflux [198]. As a result, the Tween 20®-based NLCs were further spray-dried using leucine as anti-adherent to produce NLCs powder with PS, Đ, ZP, and an in vitro release of 283.4 nm, 0.226, −25.12 mV, 64.9%, respectively. The dried NLCs had good powder and flow properties with a Dae of 3.53 μm. Lungs’ uptake of the drug from the powdered NLCs was higher than the plain drug suspension. This could be attributed to the less clearance of the drug from the lungs due to the slow release of the drug from the NLCs and the retention of the drug in lipid nanoparticles. This indicates the superiority of local delivery via the pulmonary route [129].
The concept of multifunctional NLCs-based delivery systems substantially enhanced the efficiency of NSCLC therapy with suggested abilities to limit the adverse side effects of the treatments, primarily when targeting strategies are used and administered via inhalation. In this regard, multifunctional anticancer (doxorubicin or paclitaxel) and siRNA-loaded NLCs for pulmonary delivery via nebulization were developed for the treatment of LC. The NLCs were functionalized with a modified synthetic analog of luteinizing hormone-releasing hormone (LHRH) as a targeting moiety. In addition, they were conjugated with (1,2-Distearoyl-sn-glycero-3-phosphoethanolamine-poly(ethylene glycol) (DSPE- PEG). The developed doxorubicin-NLCs were primarily used to evaluate cellular uptake and the intracellular localization due to the intrinsic fluorescence of doxorubicin, while the paclitaxel-NLCs were used to assess the anticancer efficacy of the formulation. After the preparation process, the final NLC was purified via dialysis (MWC 10,000) and lyophilized with mannitol (5%) as a cryoprotectant. In vivo orthotopic model of human LC in nu/nu mice was used to evaluate the anticancer activity and tissue distribution. After inhalation, the developed NLCs efficiently delivered their payload into LC cells, leaving healthy lung tissues unaffected compared with IV injection. The tumor size decreased from 117 mm3 to 20.8 mm3 and 2.6 mm3 upon treatment with LHRH-NLC- paclitaxel, and LHRH-NLC- paclitaxel -siRNAs, respectively. The obtained results showed the high efficiency of the inhaled NLCs for tumor-targeted local delivery, specifically LC cells. As a result, effective suppression of tumor growth and prevention of adverse side effects on healthy organs [130].
The same concept in the latter study was used recently to developed paclitaxel tumor-targeted NLCs using the melted ultrasonic method after successfully mixing Precirol ATO 5® (solid lipid), squalene (liquid lipid), and soybean phosphatidylcholine (emulsifier) with the aqueous phase, which was composed of Tween 80® (surfactant) and (N-[1-(2,3-dioleoyloxy)propyl]-N,N,N-trimethylammonium) “DOTAP” (a cationic lipid which grants positive charge to NLC) in deionized distilled water, while paclitaxel was dissolved in dimethyl sulfoxide (DMSO). PEG2000 was the most suitable choice for the linkage of LHRH peptide with the NLCs, and later it was further conjugated with siRNA. The LHRH-NLC-siRNAs-paclitaxel-loaded nanoparticles had distinct spherical shape with PS, ZP, and loading efficiency of 113 nm, +45 mV, and 98%, respectively. On the cellular level, the toxicity of the developed formulation was superior to the traditionally available epidermal growth factor inhibitor, gefitinib, in three types of cells, including H1781, H3255, and A549 cells lines, as such sensitivity was linked to the presence of LHRH. The in vivo study was performed using an orthotopic NSCLC mouse model. The NLCs formulations were administered via IV and inhalation (using a Collison nebulizer (BGI, Inc., Waltham, MA, USA) methods. The results showed that developed multifunctional NLCs had a suggested efficient accumulation and retention in the lungs when inhaled compared to the IV route. The immunoperoxidase assay indicated that the formulation did not induce an immune response in human peripheral blood lymphocytes. Besides, no signs of toxicity were observed (in vivo) in the (liver, kidney, spleen, heart, lung, brain) of nude mice following inhalation or IV administration [131].
In summary, NLCs are potential carriers for the pulmonary delivery of anticancer drugs, they were successfully developed for this purpose using GRAS materials. They have the advantage to be efficiently functionalized using different ligands for active targeting. Besides, they can be aerosolized using nebulization or converted to dry powders to be used in DPIs. The results from the in vitro and in vivo studies are highly promising in the treatment of LC. However, these lipid-based nanocarriers were not tested in any clinical trial yet.
6.5. Miscellaneous Inhaled Lipid-Based Nanocarriers
A number of certain types of lipid-based nanocarriers were addressed for the delivery of anticancer for the treatment of LC via inhalation is available but at a very limited scale. Among these are the lipid-polymer hybrid nanoparticles (LPHNs), these nanocarriers incorporate the advantages of both liposomes and polymeric nanoparticles into one novel drug delivery platform [199]. LPHNs are typically consist of a biodegradable polymeric hydrophobic core and an outer shell made of lipid or lipid-ligand [200] (Figure 2). LPHNs may offer some benefits, such as physical stability and biocompatibility; their surfaces are highly tunable so they are suitable for the passive and active drug targeting, they also provide controlled release of drugs [201]; reduced systemic toxicity; and therefor they can potentially enhance efficacy of anticancer drugs [202]. However, despite all these potential advantages, these nanocarriers are not well explored for the pulmonary delivery.
In one study, LPHNs were used in the downregulation of genes involved in the pathogenesis of severe lung diseases such as LC through the local siRNA delivery. The developed LPHNs were composed of poly(lactic-co-glycolic) acid and dipalmitoylphosphatidylcholine as siRNA inhalation formulation and prepared using the emulsion/solvent diffusion method. The optimized formulation was found to have PS and ZP in the range of (135 to 169 nm) and (−16 to −30 mV), respectively, with Đ < 0.130 and EE% of 75%. The formulation possessed a peculiar triphasic release profile, characterized by an initial burst, with more than 50% of siRNA released in the first hours, followed by a slow-release phase lasting a couple of days and a final fast release time period after 4–5 days. The nebulized formulation was having Dae < 5.39 µm. Before each experiment, freeze-dried HLPNs were dispersed in 0.5 mM sodium chloride. The stability of the developed siRNA-loaded LPHNs was confirmed by TEM analysis of freeze-dried formulation in the presence of mannitol before and after nebulization in the Vitrocell Cloud system (Vitrocell Systems GmbH, Waldkirch, Germany). On the cellular level, these LPHNs were able to penetrate into cells effectively and are localized intracellularly on the TCCC cell line leading to an effective in vitro gene silencing (on A549 cells line) in the form of knocking down both aENaC and bENaC subunit proteins up to 72 h. The developed nanosystem was muco-inert and stable inside artificial mucus with no cytotoxic or acute proinflammatory effect toward any of the cell components of the co-culture model. The results demonstrated the high potential of using HLPNs as carriers for pulmonary delivery of siRNA [132].
These hybrid nanocarriers are having excellent potential for the pulmonary delivery of drugs, and their possible role in delivering inhaled anticancer drugs is not well investigated and could be considered for future studies in this field.
Niosomes are also among the systems that are rarely investigated for inhaled anticancer therapy to treat LC. Niosomes are also known as non-ionic surfactant-based vesicles (Figure 2). These carriers gained much interest in the pharmaceutical field due to their excellent abilities to encapsulate and efficiently deliver drugs/agents of different physicochemical properties via different routes of drug administration. Furthermore, their production is easy to scale up at low costs. Besides, these nanoparticles demonstrated to be more stable than liposomes during the formulation phase or upon storage. The required pharmacokinetic properties can be achieved by optimizing the components or modifying the surface of niosomes [203]. Particle size and zeta potential are essential to the pharmacokinetics, bio-distribution, toxicity, and stability of niosomes and should be well considered [204,205].
Inhalable cationic niosomes of curcumin were developed for effective and local delivery to LC cells [134], to circumvent the poor physicochemical and biopharmaceutical limitations of curcumin associated with its oral and parenteral administration, such as the poor and unpredictable bioavailability at the site of action and the extensive first-pass metabolism and irregular bio-distribution [206,207]. The developed niosomes were prepared using the reverse-phase evaporation method and composed of span 80®, diethyl ether, and chloroform with or without cholesterol. The prepared formulations were further freeze-dried using mannitol as a cryoprotectant. The resulted curcumin-loaded niosomes (containing cholesterol) (Cur-C-SUNS) were cationic and unilamellar with PS (97.4 nm), ZP (+28.5 mV), and %EE of (83.3%). While the freeze-dried niosomes prepared without cholesterol (Cur-SUNS) had a smaller PS (83.8 nm), and ZP value of (−3.02 mV), and EE% of (78.8%). The in vitro release of the powdered niosomes using dialysis membrane technique was enhanced by (30.1%), which could be due to the amorphization of nanovesicles that ultimately enhanced the solubility and release rate of the drug [208]. The optimized formulation (Cur-C-SUNS) was able to inhibit the A549 cells proliferation at the IC50 of 3.1 μM, which is significantly lower than 7.5 μM for Cur-SUNS and curcumin dispersion (< 32 μM). The in vitro cellular uptake results illustrated higher endocytosis of Cur-C-SUNS as compared to Cur-SUNS due to electrostatic interaction between cationic nanovesicles and negatively charged plasma membrane of A549 cells [134]. Although the obtained in vitro results were promising, no further in vivo studies were performed to investigate the potential roles of inhaled niosomes for the delivery of anticancer drugs for LC treatment.
Sterosomes, are new and promising non-phospholipid type of liposomes drug delivery nanoparticles, typically they composed of stearylamine and cholesterol. They are named as ‘sterosomes’ owing to their high sterol content. These carriers are highly tunable and suggested to have better stability and longer circulation and residence time than the classical liposomes [209,210].
Sterosomes were recently reported to deliver the widely used antidiabetic drug (metformin) as an inhalation dosage form because it was shown to have anticancer activities via inhibition of cellular proliferation of many cancers, including LC. The safety, tolerability and pharmacokinetics of inhaled metformin sterosomal formulation or solution. In this study, cholesterol was mixed with stearylamine or myristic acid followed by dissolving accurately weighed quantities of the solid chemicals in a mixture of benzene/methanol. The PS, %EE and ZP of the developed formulation have ranged approximately from 288.7 to 578 nm, 71% to 89% and + 16.2 to + 63.2 mV, respectively. The Đ values were generally <0.4. The measured Dae, GSD and FPF values for aerosolized metformin-loaded sterosomes by jet nebulizer were 3.3 µm, 2.114, 62.36 respectively. The MTT assay on A549 cell lines (for 48 h) showed that survival rate after exposure to metformin-containing sterosomes was very low <50%. The clinical study in this work (3 females: 3 males) at average age of 32 years old showed that the volunteers noted the greasy and ammonia-like smell of metformin sterosomal preparation. The entire process of aerosol administration of the prepared metformin-loaded sterosomes and metformin solution was generally feasible and well tolerated. The metformin-loaded sterosomes enhanced the half-life, area under the curve, and mean residence time of metformin in all healthy volunteers after inhalation of a single dose of 750 mg of metformin sterosomal formulation [135]. Authors have addressed some limitations of this study such as the relatively small number of subjects which could lead to improper variability in the results, and the lack of comparison between multiple and different doses. More extensive clinical trials with long-term follow-up are needed to confirm the safety and efficacy of the developed formulation.
7. Inhalable Anticancer Drug-Loaded Lipid-Based Nanocarriers in Clinical Trials
Despite the proven advantages offered by inhalable anticancer therapy via lipid-based nanocarriers in preclinical studies, the number of conducted clinical trials is still limited (Table 3). In addition, the most advanced development of inhaled anticancer therapy was performed up to phase II only; consequently, no inhaled lipid-based nanocarrier product reached the market yet. This could be attributed to the associated challenges of using this route of administration, as discussed in Section 3 of this review.

Table 3.
Summary of clinical trials that have been conducted on the inhalable anticancer drug-loaded lipid-based nanocarriers for the treatment of LC.
Among the various types of inhalable lipid-based nanocarriers, only liposomes were evaluated in clinical trials. The safety and pharmacokinetics of aerosolized sustained-release lipid inhalation targeting (SLIT) of cisplatin in patients with lung carcinoma were investigated. Seventeen patients and one tracheostomy patient on compassionate use received treatment. The results showed that the aerosolized liposomal cisplatin was well tolerated. In addition, no DLT was observed at the maximum delivered dose. Safety data showed that no hematologic toxicity, nephrotoxicity, ototoxicity, or neurotoxicity was observed. Pharmacokinetically, very low plasma platinum levels were obtained only with the longest repeated inhalations. The aerosolized cisplatin-loaded liposomal formulation was found to be feasible and safe [30].
To evaluate the safety and efficacy of inhaled lipid cisplatin (ILC) in patients with recurrent osteosarcoma who only had pulmonary metastases, an open-label, phase Ib/IIa study was performed (NCT00102531). The study involved nineteen patients. The results showed that no patients experienced hematologic toxicity, nephrotoxicity, or ototoxicity. The inhaled liposomal cisplatin was well tolerated in heavily treated osteosarcoma patients. In addition, the typical toxicities associated with intravenous cisplatin did not appear with the inhaled therapy [31,32]. A phase II clinical trial to establish whether treatment with inhaled liposomal cisplatin (ILC) formulation is effective in delaying/preventing pulmonary relapse in osteosarcoma patients in complete surgical remission following one or two prior pulmonary relapses was completed in 2018 (NCT01650090). However, no data have been published yet [211].
Aerosolized 9-nitro-20(S)-camptothecin (9NC)-loaded liposomal formulation was evaluated clinically for safety and feasibility in a group of 25 patients with primary or metastatic LC. The patients received the aerosolized liposomal formulation for five consecutive days/week for 1, 2, 4, or 6 weeks followed by two weeks of rest to determine feasibility. As mentioned previously, chemical pharyngitis was the DLT at 26 mg/kg/day. After inhalation, 9NC was absorbed in a rapid and sustained manner through the lung parenchyma to the bloodstream circulation. Stabilization occurred in 3 patients with primary LC, and partial remissions were observed in 2 patients with uterine cancer. The results revealed that the pulmonary administration of 13.3 mg/kg/day of the 9NC-loaded liposomal formulation was feasible and safe. Furthermore, the researchers recommended a dose of 13.3 mg/kg/day of the liposomal formulation for phase II of the study [29].
There is other six clinical trials of aerosolized 9NC-loaded liposomes have been completed but no results were released or published to the author’s best knowledge, and they are namely: (NCT00492141) for determining the effectiveness of L9NC given by aerosol in combination with temozolomide in patients with solid tumors involving the lungs (Phase II, completed in September 2009) [212], (NCT00249990) for determining efficacy and toxicity profile in metastatic or recurrent endometrial cancer (Phase II, completed in September 2007) [213], (NCT00250016) to determine the amount of aerosolized drug in patients’ blood and tumor (completed in August 2007) [214], (NCT00250068) to determine the overall response rate to 9NC administered by aerosolization in patients with NSCLC any stage (Phase II, completed in December 2007) [215], (NCT00277082) to determine the concentration of the drug in the alveolar fluid over time (completed in June 2005) [216], (NCT00250120) to determine the overall response rate to the inhaled liposomal drug in patients with NSCLC at any stage (withdrawn, Phase II, completed in August 2007) [217].
With the new advancements in the fields of lipid-based nanocarriers, drug targeting, and pulmonary delivery devices, clinical studies are currently needed to reveal the promising potentials of inhalation chemotherapy.
8. Conclusions
Inhalable anticancer therapy via lipid-based nanocarriers is an exciting and growing research area. It is a promising treatment strategy to combat LC and lung metastases. Due to the unique properties of the lipid-based nanocarriers of great biocompatibility, high drug loading, and tunable surfaces for active targeting and controlled drug-release behavior, they are gaining much interest. Results from the recent studies on preclinical levels revealed that the drugs loaded in these inhalable nanocarriers will be concentrated in the lungs and then diffuse gradually into the blood circulation and the lymphatic system to target the cancerous cells. Among the currently available pulmonary devices, only nebulizers and DPIs are potentially suitable for the efficient delivery of these nanoparticles. The use of DPIs as devices for inhaled anticancer drugs loaded in lipid-based nanoparticles is quite promising as they have many advantages and could overcome the challenges associated with this route. Combining the use of lipid-based nanocarriers, DPIs devices, particle engineering, and formulation sciences opens the door for new advancements and possibilities. However, the research in this field is still in its infancy, particularly at the in vivo and clinical studies levels. The general formulation strategy should concentrate on developing uni- or multifunctional lipid-based nanocarriers for active targeting, with good drug loading and sustained release properties, embedded in well-engineered microparticles composed of safe and well-tolerated excipients of high FPF for efficient lung deposition, drug delivery, and antitumor activity.
Author Contributions
Conceptualization, I.M.A., H.A.W., Y.D.; writing—original draft preparation, I.M.A., R.A.A.; writing—review and editing, I.M.A., R.A.A., H.A.W., A.Y., Y.D.; visualization, F.G.S. and I.M.A.; supervision, H.A.W., A.Y, Y.D and T.P.; project administration, H.A.W., N.M., T.P.; funding acquisition, H.A.W., N.M., T.P., I.M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Malaysian ministry of higher education, fundamental research grant scheme (FRGS) grant number FRGS/1/2020/SKK0/USM/01/4 (account no: 203.PFARMASI.6711891).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Acknowledgments
Ibrahim M. Abdulbaqi and Reem Abou Assi gratefully acknowledge Universiti Sains Malaysia (USM), the Institute of Postgraduate Studies (IPS), Penang, Malaysia for awarding of USM Fellowship.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- World Health Organization. The Global Cancer Observatory. Cancer Fact Sheets. Available online: http://gco.iarc.fr/today/data/factsheets/cancers/15-Lung-fact-sheet.pdf (accessed on 23 April 2021).
- American Cancer Society. Key Statistics for Lung Cancer. Available online: https://www.cancer.org/content/cancer/en/cancer/lung-cancer/about/key-statistics.html (accessed on 23 April 2021).
- National Cancer Institute. The Surveillance, Epidemiology, and End Results (SEER) Program. Cancer Stat Facts: Lung and Bronchus Cancer. Available online: https://seer.cancer.gov/statfacts/html/lungb.html (accessed on 23 April 2021).
- World Health Organization. International Agency for Research on Cancer. The Global Cancer Observatory (GCO). Cancer Tomorrow (Incidence). Available online: http://gco.iarc.fr/tomorrow/graphic-line?type=0&population=900&mode=population&sex=0&cancer=39&age_group=value&apc_male=0&apc_female=0#collapse-group-1-1 (accessed on 23 April 2021).
- World Health Organization. International Agency for Research on Cancer. The Global Cancer Observatory (GCO). Cancer Tomorrow (Mortality). Available online: http://gco.iarc.fr/tomorrow/graphic-line?type=1&population=900&mode=population&sex=0&cancer=39&age_group=value&apc_male=0&apc_female=0#collapse-group-1-1 (accessed on 23 April 2021).
- Lovly, L.H.C.M. Neoplasms of the lung. In Harrison’s Principles of Internal Medicine, 20th ed.; Jameson, J.L., Dennis, A.S.F., Kasper, L., Hauser, S.L., Longo, D.L., Loscalzo, J., Eds.; McGraw-Hill Education: New York, NY, USA, 2018. [Google Scholar]
- Butler, S.K. Lung cancer. In Applied Therapeutics: The Clinical Use of Drugs, 11th ed.; Zeind, C.S., Carvalho, M.G., Eds.; Wolters Kluwer: Philadelphia, PA, USA, 2018. [Google Scholar]
- Postmus, P.E.; Kerr, K.M.; Oudkerk, M.; Senan, S.; Waller, D.A.; Vansteenkiste, J.; Escriu, C.; Peters, S.; on behalf of the ESMO Guidelines Committee. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv1–iv21. [Google Scholar] [CrossRef]
- American Cancer Society. Treating Non-Small Cell Lung Cancer. Available online: https://www.cancer.org/cancer/lung-cancer/treating-non-small-cell.html (accessed on 23 April 2021).
- American Cancer Society. Surgery for Small Cell Lung Cancer. Available online: https://www.cancer.org/cancer/lung-cancer/treating-small-cell/surgery.html (accessed on 23 April 2021).
- Nurgali, K.; Jagoe, R.T.; Abalo, R. Editorial: Adverse effects of cancer chemotherapy: Anything new to improve tolerance and reduce sequelae? Front. Pharm. 2018, 9, 245. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.Y.; Cheng, R.; Yang, Z.; Tian, Z.M. Nanotechnology for cancer therapy based on chemotherapy. Molecules 2018, 23, 826. [Google Scholar] [CrossRef] [PubMed]
- Gonciar, D.; Mocan, T.; Matea, C.T.; Zdrehus, C.; Mosteanu, O.; Mocan, L.; Pop, T. Nanotechnology in metastatic cancer treatment: Current achievements and future research trends. J. Cancer 2019, 10, 1358–1369. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Cancer and Nanotechnology: Treatment and Therapy. Available online: https://www.cancer.gov/nano/cancer-nanotechnology/treatment (accessed on 22 December 2019).
- Amararathna, M.; Goralski, K.; Hoskin, D.W.; Rupasinghe, H.P.V. Pulmonary nano-drug delivery systems for lung cancer: Current knowledge and prospects. J. Lung Health Dis. 2019, 3, 11–28. [Google Scholar] [CrossRef]
- Dolovich, M.B.; Dhand, R. Aerosol drug delivery: Developments in device design and clinical use. Lancet 2011, 377, 1032–1045. [Google Scholar] [CrossRef]
- Patton, J.S.; Byron, P.R. Inhaling medicines: Delivering drugs to the body through the lungs. Nat. Rev. Drug Discov. 2007, 6, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-H.; Loo, C.-Y.; Traini, D.; Young, P.M. Nano- and micro-based inhaled drug delivery systems for targeting alveolar macrophages. Expert Opin. Drug Deliv. 2015, 12, 1009–1026. [Google Scholar] [CrossRef]
- Garmany, T.H.; Moxley, M.A.; White, F.V.; Dean, M.; Hull, W.M.; Whitsett, J.A.; Nogee, L.M.; Hamvas, A. Surfactant Composition and Function in Patients with ABCA3 Mutations. Pediatric Res. 2006, 59, 801–805. [Google Scholar] [CrossRef]
- Kwok, P.C.L.; Chan, H.-K. Advances in Pulmonary Drug Delivery, 1st ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar] [CrossRef]
- Williams, R.O.; Taft, D.R.; McConville, J.T. Advanced Drug Formulation Design to Optimize Therapeutic Outcomes, 1st ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar] [CrossRef]
- Mangal, S.; Gao, W.; Li, T.; Zhou, Q. Pulmonary delivery of nanoparticle chemotherapy for the treatment of lung cancers: Challenges and opportunities. Acta Pharmacol. Sin. 2017, 38, 782. [Google Scholar] [CrossRef]
- Haque, S.; Feeney, O.; Meeusen, E.; Boyd, B.J.; McIntosh, M.P.; Pouton, C.W.; Whittaker, M.; Kaminskas, L.M. Local inflammation alters the lung disposition of a drug loaded pegylated liposome after pulmonary dosing to rats. J. Control. Release 2019, 307, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; White, D.; Imondi, A.R.; Placke, M.E.; Vail, D.M.; Kris, M.G. Development of inhalational agents for oncologic use. J. Clin. Oncol. 2001, 19, 1839–1847. [Google Scholar] [CrossRef]
- Tatsumura, T.; Koyama, S.; Tsujimoto, M.; Kitagawa, M.; Kagamimori, S. Further study of nebulisation chemotherapy, a new chemotherapeutic method in the treatment of lung carcinomas: Fundamental and clinical. Br. J. Cancer 1993, 68, 1146–1149. [Google Scholar] [CrossRef] [PubMed]
- Zarogoulidis, P.; Chatzaki, E.; Porpodis, K.; Domvri, K.; Hohenforst-Schmidt, W.; Goldberg, E.P.; Karamanos, N.; Zarogoulidis, K. Inhaled chemotherapy in lung cancer: Future concept of nanomedicine. Int J. Nanomed. 2012, 7, 1551–1572. [Google Scholar] [CrossRef]
- Videira, M.A.; Botelho, M.F.; Santos, A.C.; Gouveia, L.F.; Pedroso de Lima, J.J.; Almeida, A.J. lymphatic uptake of pulmonary delivered radiolabelled solid lipid nanoparticles. J. Drug Target. 2002, 10, 607–613. [Google Scholar] [CrossRef]
- Verschraegen, C.F.; Gilbert, B.E.; Loyer, E.; Huaringa, A.; Walsh, G.; Newman, R.A.; Knight, V. Clinical evaluation of the delivery and safety of aerosolized liposomal 9-nitro-20(s)-camptothecin in patients with advanced pulmonary malignancies. Clin. Cancer Res. 2004, 10, 2319–2326. [Google Scholar] [CrossRef]
- Wittgen, B.P.; Kunst, P.W.; van der Born, K.; van Wijk, A.W.; Perkins, W.; Pilkiewicz, F.G.; Perez-Soler, R.; Nicholson, S.; Peters, G.J.; Postmus, P.E. Phase I study of aerosolized SLIT cisplatin in the treatment of patients with carcinoma of the lung. Clin. Cancer Res. 2007, 13, 2414–2421. [Google Scholar] [CrossRef] [PubMed]
- Chou, A.J.; Gupta, R.; Bell, M.D.; Riewe, K.O.; Meyers, P.A.; Gorlick, R. Inhaled lipid cisplatin (ILC) in the treatment of patients with relapsed/progressive osteosarcoma metastatic to the lung. Pediatr. Blood Cancer 2013, 60, 580–586. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Inhalation SLIT Cisplatin (Liposomal) for the Treatment of Osteosarcoma Metastatic to the Lung. Available online: https://clinicaltrials.gov/ct2/show/NCT00102531 (accessed on 25 June 2021).
- Lemarie, E.; Vecellio, L.; Hureaux, J.; Prunier, C.; Valat, C.; Grimbert, D.; Boidron-Celle, M.; Giraudeau, B.; le Pape, A.; Pichon, E.; et al. Aerosolized gemcitabine in patients with carcinoma of the lung: Feasibility and safety study. J. Aerosol Med. Pulm. Drug Deliv. 2011, 24, 261–270. [Google Scholar] [CrossRef]
- Zarogoulidis, P.; Eleftheriadou, E.; Sapardanis, I.; Zarogoulidou, V.; Lithoxopoulou, H.; Kontakiotis, T.; Karamanos, N.; Zachariadis, G.; Mabroudi, M.; Zisimopoulos, A.; et al. Feasibility and effectiveness of inhaled carboplatin in NSCLC patients. Investig. New Drugs 2012, 30, 1628–1640. [Google Scholar] [CrossRef] [PubMed]
- Kosmidis, C.; Sapalidis, K.; Zarogoulidis, P.; Sardeli, C.; Koulouris, C.; Giannakidis, D.; Pavlidis, E.; Katsaounis, A.; Michalopoulos, N.; Mantalobas, S.; et al. Inhaled cisplatin for NSCLC: Facts and results. Int. J. Mol. Sci. 2019, 20, 2005. [Google Scholar] [CrossRef]
- Dabbagh, A.; Abu Kasim, N.H.; Yeong, C.H.; Wong, T.W.; Abdul Rahman, N. Critical parameters for particle-based pulmonary delivery of chemotherapeutics. J. Aerosol Med. Pulm. Drug Deliv. 2018, 31, 139–154. [Google Scholar] [CrossRef] [PubMed]
- Ruge, C.A.; Kirch, J.; Lehr, C.-M. Pulmonary drug delivery: From generating aerosols to overcoming biological barriers—Therapeutic possibilities and technological challenges. Lancet Respir. Med. 2013, 1, 402–413. [Google Scholar] [CrossRef]
- Olsson, B.; Bondesson, E.; Borgström, L.; Edsbäcker, S.; Eirefelt, S.; Ekelund, K.; Gustavsson, L.; Hegelund-Myrbäck, T. Pulmonary drug metabolism, clearance, and absorption. In Controlled Pulmonary Drug Delivery; Smyth, H.D.C., Hickey, A.J., Eds.; Springer: New York, NY, USA, 2011; pp. 21–50. [Google Scholar] [CrossRef]
- Hidalgo, A.; Cruz, A.; Pérez-Gil, J. Barrier or carrier? Pulmonary surfactant and drug delivery. Eur. J. Pharm. Biopharm. 2015, 95, 117–127. [Google Scholar] [CrossRef]
- Lee, W.-H.; Loo, C.-Y.; Traini, D.; Young, P.M. Inhalation of nanoparticle-based drug for lung cancer treatment: Advantages and challenges. Asian J. Pharm. Sci. 2015, 10, 481–489. [Google Scholar] [CrossRef]
- Xu, J.; Lu, X.; Zhu, X.; Yang, Y.; Liu, Q.; Zhao, D.; Lu, Y.; Wen, J.; Chen, X.; Li, N. Formulation and characterization of spray-dried powders containing vincristine-liposomes for pulmonary delivery and its pharmacokinetic evaluation from in vitro and in vivo. J. Pharm. Sci. 2019, 108, 3348–3358. [Google Scholar] [CrossRef]
- Rosière, R.; Van Woensel, M.; Gelbcke, M.; Mathieu, V.; Hecq, J.; Mathivet, T.; Vermeersch, M.; Van Antwerpen, P.; Amighi, K.; Wauthoz, N. New folate-grafted chitosan derivative to improve delivery of paclitaxel-loaded solid lipid nanoparticles for lung tumor therapy by inhalation. Mol. Pharm. 2018, 15, 899–910. [Google Scholar] [CrossRef]
- Carvalho, T.C.; Carvalho, S.R.; McConville, J.T. Formulations for pulmonary administration of anticancer agents to treat lung malignancies. J. Aerosol Med. Pulm. Drug Deliv. 2011, 24, 61–80. [Google Scholar] [CrossRef]
- Yang, W.; Peters, J.I.; Williams, R.O. Inhaled nanoparticles—A current review. Int. J. Pharm. 2008, 356, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Smola, M.; Vandamme, T.; Sokolowski, A. Nanocarriers as pulmonary drug delivery systems to treat and to diagnose respiratory and non respiratory diseases. Int J. Nanomed. 2008, 3, 1–19. [Google Scholar]
- El-Sherbiny, I.M.; Villanueva, D.G.; Herrera, D.; Smyth, H.D.C. Overcoming lung clearance mechanisms for controlled release drug delivery. In Controlled Pulmonary Drug Delivery; Smyth, H.D.C., Hickey, A.J., Eds.; Springer: New York, NY, USA, 2011; pp. 101–126. [Google Scholar] [CrossRef]
- Carvalho, T.C.; Peters, J.I.; Williams, R.O., 3rd. Influence of particle size on regional lung deposition—What evidence is there? Int. J. Pharm. 2011, 406, 1–10. [Google Scholar] [CrossRef]
- Champion, J.A.; Mitragotri, S. Role of target geometry in phagocytosis. Proc. Natl. Acad. Sci. USA 2006, 103, 4930–4934. [Google Scholar] [CrossRef]
- Champion, J.A.; Mitragotri, S. Shape induced inhibition of phagocytosis of polymer particles. Pharm. Res. 2009, 26, 244–249. [Google Scholar] [CrossRef]
- Choi, H.S.; Ashitate, Y.; Lee, J.H.; Kim, S.H.; Matsui, A.; Insin, N.; Bawendi, M.G.; Semmler-Behnke, M.; Frangioni, J.V.; Tsuda, A. Rapid translocation of nanoparticles from the lung airspaces to the body. Nat. Biotechnol. 2010, 28, 1300–1303. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar] [PubMed]
- Nakamura, Y.; Mochida, A.; Choyke, P.L.; Kobayashi, H. Nanodrug delivery: Is the enhanced permeability and retention effect sufficient for curing cancer? Bioconjug. Chem. 2016, 27, 2225–2238. [Google Scholar] [CrossRef] [PubMed]
- Hirsjärvi, S.; Passirani, C.; Benoit, J.P. Passive and active tumour targeting with nanocarriers. Curr. Drug Discov. Technol. 2011, 8, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Lee, S.G.; Kang, M.J.; Lee, S.; Choi, Y.W. Surface modification of lipid-based nanocarriers for cancer cell-specific drug targeting. J. Pharm. Investig. 2017, 47, 203–227. [Google Scholar] [CrossRef]
- Yan, W.; Leung, S.S.; To, K.K. Updates on the use of liposomes for active tumor targeting in cancer therapy. Nanomedicine 2020, 15, 303–318. [Google Scholar] [CrossRef]
- Riaz, M.K.; Riaz, M.A.; Zhang, X.; Lin, C.; Wong, K.H.; Chen, X.; Zhang, G.; Lu, A.; Yang, Z. Surface functionalization and targeting strategies of liposomes in solid tumor therapy: A review. Int. J. Mol. Sci. 2018, 19, 195. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, D.; Scalia, S.; Vogel, R.; Wheate, N.; Salama, R.O.; Young, P.M.; Traini, D.; Chrzanowski, W. Magnetised thermo responsive lipid vehicles for targeted and controlled lung drug delivery. Pharm. Res. 2012, 29, 2456–2467. [Google Scholar] [CrossRef] [PubMed]
- Wauthoz, N.; Rosière, R.; Amighi, K. Inhaled cytotoxic chemotherapy: Clinical challenges, recent developments, and future prospects. Expert Opin. Drug Deliv. 2020, 1–22. [Google Scholar] [CrossRef]
- Rosière, R.; Amighi, K.; Wauthoz, N. Nanomedicine-based inhalation treatments for lung cancer. In Nanotechnology-Based Targeted Drug Delivery Systems for Lung Cancer; Academic Press: Cambridge, MA, USA, 2019; pp. 249–268. [Google Scholar] [CrossRef]
- Zhou, Q.T.; Tang, P.; Leung, S.S.; Chan, J.G.; Chan, H.K. Emerging inhalation aerosol devices and strategies: Where are we headed? Adv. Drug Deliv. Rev. 2014, 75, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Garrastazu Pereira, G.; Lawson, A.J.; Buttini, F.; Sonvico, F. Loco-regional administration of nanomedicines for the treatment of lung cancer. Drug Deliv. 2016, 23, 2881–2896. [Google Scholar] [CrossRef] [PubMed]
- Longest, W.; Spence, B.; Hindle, M. Devices for improved delivery of nebulized pharmaceutical aerosols to the lungs. J. Aerosol Med. Pulm. Drug Deliv. 2019, 32, 317–339. [Google Scholar] [CrossRef] [PubMed]
- Labiris, N.R.; Dolovich, M.B. Pulmonary drug delivery. Part II: The role of inhalant delivery devices and drug formulations in therapeutic effectiveness of aerosolized medications. Br. J. Clin. Pharmacol. 2003, 56, 600–612. [Google Scholar] [CrossRef]
- Labiris, N.R.; Dolovich, M.B. Pulmonary drug delivery. Part I: Physiological factors affecting therapeutic effectiveness of aerosolized medications. Br. J. Clin. Pharmacol. 2003, 56, 588–599. [Google Scholar] [CrossRef]
- Cipolla, D.; Gonda, I.; Chan, H.K. Liposomal formulations for inhalation. Ther. Deliv. 2013, 4, 1047–1072. [Google Scholar] [CrossRef]
- Hureaux, J.; Lagarce, F.; Gagnadoux, F.; Vecellio, L.; Clavreul, A.; Roger, E.; Kempf, M.; Racineux, J.L.; Diot, P.; Benoit, J.P.; et al. Lipid nanocapsules: Ready-to-use nanovectors for the aerosol delivery of paclitaxel. Eur. J. Pharm. Biopharm. 2009, 73, 239–246. [Google Scholar] [CrossRef]
- Anabousi, S.; Kleemann, E.; Bakowsky, U.; Kissel, T.; Schmehl, T.; Gessler, T.; Seeger, W.; Lehr, C.M.; Ehrhardt, C. Effect of PEGylation on the stability of liposomes during nebulisation and in lung surfactant. J. Nanosci. Nanotechnol. 2006, 6, 3010–3016. [Google Scholar] [CrossRef]
- Wauthoz, N.; Amighi, K. Phospholipids in pulmonary drug delivery. Eur. J. Lipid Sci. Technol. 2014, 116, 1114–1128. [Google Scholar] [CrossRef]
- ElKasabgy, N.A.; Adel, I.M.; Elmeligy, M.F. Respiratory tract: Structure and attractions for drug delivery using dry powder inhalers. AAPS PharmSciTech 2020, 21, 238. [Google Scholar] [CrossRef] [PubMed]
- Gamal, A.; Saeed, H.; Sayed, O.M.; Kharshoum, R.M.; Salem, H.F. Proniosomal microcarriers: Impact of constituents on the physicochemical properties of proniosomes as a new approach to enhance inhalation efficiency of dry powder inhalers. AAPS PharmSciTech 2020, 21, 156. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-H.; Loo, C.-Y.; Traini, D.; Young, P.M. Development and evaluation of paclitaxel and curcumin dry powder for inhalation lung cancer treatment. Pharmaceutics 2021, 13, 9. [Google Scholar] [CrossRef] [PubMed]
- Party, P.; Bartos, C.; Farkas, Á.; Szabó-Révész, P.; Ambrus, R. Formulation and in vitro and in silico characterization of “nano-in-micro” dry powder inhalers containing meloxicam. Pharmaceutics 2021, 13, 211. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Bothiraja, C.; Mali, A.; Kamble, R. Investigation of sorafenib tosylate loaded liposomal dry powder inhaler for the treatment of non-small cell lung cancer. Part. Sci. Technol. 2021, 1–10. [Google Scholar] [CrossRef]
- Satari, N.; Taymouri, S.; Varshosaz, J.; Rostami, M.; Mirian, M. Preparation and evaluation of inhalable dry powder containing glucosamine-conjugated gefitinib SLNs for lung cancer therapy. Drug Dev. Ind. Pharm. 2020, 46, 1265–1277. [Google Scholar] [CrossRef]
- Zhu, X.; Kong, Y.; Liu, Q.; Lu, Y.; Xing, H.; Lu, X.; Yang, Y.; Xu, J.; Li, N.; Zhao, D.; et al. Inhalable dry powder prepared from folic acid-conjugated docetaxel liposomes alters pharmacodynamic and pharmacokinetic properties relevant to lung cancer chemotherapy. Pulm. Pharm. Ther. 2019, 55, 50–61. [Google Scholar] [CrossRef]
- Malamatari, M.; Charisi, A.; Malamataris, S.; Kachrimanis, K.; Nikolakakis, I. Spray drying for the preparation of nanoparticle-based drug formulations as dry powders for inhalation. Processes 2020, 8, 788. [Google Scholar] [CrossRef]
- Yang, M.Y.; Chan, J.G.Y.; Chan, H.-K. Pulmonary drug delivery by powder aerosols. J. Control. Release 2014, 193, 228–240. [Google Scholar] [CrossRef]
- Weers, J.; Clark, A. The impact of inspiratory flow rate on drug delivery to the lungs with dry powder inhalers. Pharm. Res. 2017, 34, 507–528. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.R.; Weers, J.G.; Dhand, R. The confusing world of dry powder inhalers: It is all about inspiratory pressures, not inspiratory flow rates. J. Aerosol Med. Pulm. Drug Deliv. 2019, 33, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, L.; Chan, H.-K.; Watanabe, W. Formation, characterization, and fate of inhaled drug nanoparticles. Adv. Drug Deliv. Rev. 2011, 63, 441–455. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Kłodzińska, S.N.; Wan, F.; Nielsen, H.M. Nanoparticle-mediated pulmonary drug delivery: State of the art towards efficient treatment of recalcitrant respiratory tract bacterial infections. Drug Deliv. Transl. Res. 2021, 1–21. [Google Scholar] [CrossRef]
- Bohr, A.; Water, J.; Beck-Broichsitter, M.; Yang, M. Nanoembedded microparticles for stabilization and delivery of drug-loaded nanoparticles. Curr. Pharm. Des. 2015, 21, 5829–5844. [Google Scholar] [CrossRef] [PubMed]
- Umerska, A.; Mugheirbi, N.A.; Kasprzak, A.; Saulnier, P.; Tajber, L. Carbohydrate-based Trojan microparticles as carriers for pulmonary delivery of lipid nanocapsules using dry powder inhalation. Powder Technol. 2020, 364, 507–521. [Google Scholar] [CrossRef]
- Assadpour, E.; Jafari, S.M. Advances in spray-drying encapsulation of food bioactive ingredients: From microcapsules to nanocapsules. Annu. Rev. Food Sci. Technol. 2019, 10, 103–131. [Google Scholar] [CrossRef]
- Kaur, P.; Mishra, V.; Shunmugaperumal, T.; Goyal, A.K.; Ghosh, G.; Rath, G. Inhalable spray dried lipidnanoparticles for the co-delivery of paclitaxel and doxorubicin in lung cancer. J. Drug Deliv. Sci. Technol. 2020, 56, 101502. [Google Scholar] [CrossRef]
- Anton, N.; Jakhmola, A.; Vandamme, T.F. Trojan microparticles for drug delivery. Pharmaceutics 2012, 4, 1–25. [Google Scholar] [CrossRef]
- Abdelaziz, H.M.; Freag, M.S.; Elzoghby, A.O. Solid lipid nanoparticle-based drug delivery for lung cancer. In Nanotechnology-Based Targeted Drug Delivery Systems for Lung Cancer; Academic Press: Cambridge, MA, USA, 2019; pp. 95–121. [Google Scholar] [CrossRef]
- Lechanteur, A.; Evrard, B. Influence of composition and spray-drying process parameters on carrier-free dpi properties and behaviors in the lung: A review. Pharmaceutics 2020, 12, 55. [Google Scholar] [CrossRef]
- El-Gendy, N.; Berkland, C. Combination chemotherapeutic dry powder aerosols via controlled nanoparticle agglomeration. Pharm. Res. 2009, 26, 1752–1763. [Google Scholar] [CrossRef]
- Varshosaz, J.; Hassanzadeh, F.; Mardani, A.; Rostami, M. Feasibility of haloperidol-anchored albumin nanoparticles loaded with doxorubicin as dry powder inhaler for pulmonary delivery. Pharm. Dev. Technol. 2015, 20, 183–196. [Google Scholar] [CrossRef]
- Li, X.; Anton, N.; Ta, T.M.C.; Zhao, M.; Messaddeq, N.; Vandamme, T.F. Microencapsulation of nanoemulsions: Novel Trojan particles for bioactive lipid molecule delivery. Int. J. Nanomed. 2011, 6, 1313–1325. [Google Scholar] [CrossRef][Green Version]
- Chaurasiya, B.; Zhao, Y.Y. Dry powder for pulmonary delivery: A comprehensive review. Pharmaceutics 2020, 13, 31. [Google Scholar] [CrossRef] [PubMed]
- Nikolaou, M.; Krasia-Christoforou, T. Electrohydrodynamic methods for the development of pulmonary drug delivery systems. Eur. J. Pharm. Sci. 2018, 113, 29–40. [Google Scholar] [CrossRef]
- Ely, L.; Roa, W.; Finlay, W.H.; Löbenberg, R. Effervescent dry powder for respiratory drug delivery. Eur. J. Pharm. Biopharm. 2007, 65, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Jyoti, K.; Kaur, K.; Pandey, R.S.; Jain, U.K.; Chandra, R.; Madan, J. Inhalable nanostructured lipid particles of 9-bromo-noscapine, a tubulin-binding cytotoxic agent: In vitro and in vivo studies. J. Colloid Interface Sci. 2015, 445, 219–230. [Google Scholar] [CrossRef]
- Roa, W.H.; Azarmi, S.; Al-Hallak, M.H.; Finlay, W.H.; Magliocco, A.M.; Löbenberg, R. Inhalable nanoparticles, a non-invasive approach to treat lung cancer in a mouse model. J. Control. Release 2011, 150, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Al-Hallak, M.H.; Sarfraz, M.K.; Azarmi, S.; Roa, W.H.; Finlay, W.H.; Rouleau, C.; Löbenberg, R. Distribution of effervescent inhalable nanoparticles after pulmonary delivery: An in vivo study. Ther. Deliv. 2012, 3, 725–734. [Google Scholar] [CrossRef]
- Kumar, R. Lipid-based nanoparticles for drug-delivery systems. In Nanocarriers for Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2019; pp. 249–284. [Google Scholar] [CrossRef]
- Muller, R.H.; Keck, C.M. Challenges and solutions for the delivery of biotech drugs--a review of drug nanocrystal technology and lipid nanoparticles. J. Biotechnol. 2004, 113, 151–170. [Google Scholar] [CrossRef] [PubMed]
- Narvekar, M.; Xue, H.Y.; Eoh, J.Y.; Wong, H.L. Nanocarrier for poorly water-soluble anticancer drugs—Barriers of translation and solutions. AAPS PharmSciTech 2014, 15, 822–833. [Google Scholar] [CrossRef] [PubMed]
- Mohanta, B.C.; Palei, N.N.; Surendran, V.; Dinda, S.C.; Rajangam, J.; Deb, J.; Sahoo, B.M. Lipid based nanoparticles: Current strategies for brain tumor targeting. Curr. Nanomater. 2019, 4, 84–100. [Google Scholar] [CrossRef]
- Talluri, S.V.; Kuppusamy, G.; Karri, V.V.; Tummala, S.; Madhunapantula, S.V. Lipid-based nanocarriers for breast cancer treatment—Comprehensive review. Drug Deliv. 2016, 23, 1291–1305. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhuang, B.; Zhang, G.; Li, M.; Jin, Y. Pulmonary delivery of cationic liposomal hydroxycamptothecin and 5-aminolevulinic acid for chemo-sonodynamic therapy of metastatic lung cancer. Int. J. Pharm. 2021, 601, 120572. [Google Scholar] [CrossRef] [PubMed]
- Loira-Pastoriza, C.; Vanvarenberg, K.; Ucakar, B.; Machado Franco, M.; Staub, A.; Lemaire, M.; Renauld, J.-C.; Vanbever, R. Encapsulation of a CpG oligonucleotide in cationic liposomes enhances its local antitumor activity following pulmonary delivery in a murine model of metastatic lung cancer. Int. J. Pharm. 2021, 600, 120504. [Google Scholar] [CrossRef]
- Zhang, M.; Li, M.; Du, L.; Zeng, J.; Yao, T.; Jin, Y. Paclitaxel-in-liposome-in-bacteria for inhalation treatment of primary lung cancer. Int. J. Pharm. 2020, 578, 119177. [Google Scholar] [CrossRef]
- Adel, I.M.; ElMeligy, M.F.; Abdelrahim, M.E.A.; Maged, A.; Abdelkhalek, A.A.; Abdelmoteleb, A.M.M.; Elkasabgy, N.A. Design and characterization of spray-dried proliposomes for the pulmonary delivery of curcumin. Int. J. Nanomed. 2021, 16, 2667–2687. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, Y.; Ge, Y.; Hu, Y.; Li, M.; Jin, Y. Inhalation treatment of primary lung cancer using liposomal curcumin dry powder inhalers. Acta Pharm. Sin. B 2018, 8, 440–448. [Google Scholar] [CrossRef]
- Yuan, N.; Zhang, X.; Cao, Y.; Jiang, X.; Zhao, S.; Feng, Y.; Fan, Y.; Lu, Z.; Gao, H. Contrast-enhanced computerized tomography combined with a targeted nanoparticle contrast agent for screening for early-phase non-small cell lung cancer. Exp. Ther. Med. 2017, 14, 5063–5068. [Google Scholar] [CrossRef][Green Version]
- Tagami, T.; Kubota, M.; Ozeki, T. Effective remote loading of doxorubicin into DPPC/poloxamer 188 hybrid liposome to retain thermosensitive property and the assessment of carrier-based acute cytotoxicity for pulmonary administration. J. Pharm. Sci. 2015, 104, 3824–3832. [Google Scholar] [CrossRef]
- Mainelis, G.; Seshadri, S.; Garbuzenko, O.B.; Han, T.; Wang, Z.; Minko, T. Characterization and application of a nose-only exposure chamber for inhalation delivery of liposomal drugs and nucleic acids to mice. J. Aerosol Med. Pulm. Drug Deliv. 2013, 26, 345–354. [Google Scholar] [CrossRef]
- Gaspar, M.M.; Radomska, A.; Gobbo, O.L.; Bakowsky, U.; Radomski, M.W.; Ehrhardt, C. Targeted delivery of transferrin-conjugated liposomes to an orthotopic model of lung cancer in nude rats. J. Aerosol Med. Pulm. Drug Deliv. 2012, 25, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Riaz, M.K.; Zhang, X.; Wong, K.H.; Chen, H.; Liu, Q.; Chen, X.; Zhang, G.; Lu, A.; Yang, Z. Pulmonary delivery of transferrin receptors targeting peptide surface-functionalized liposomes augments the chemotherapeutic effect of quercetin in lung cancer therapy. Int. J. Nanomed. 2019, 14, 2879–2902. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Zhang, X.; Chen, H.; Bian, Z.; Zhang, G.; Riaz, M.K.; Tyagi, D.; Lin, G.; Zhang, Y.; Wang, J.; et al. Dual-ligand modified liposomes provide effective local targeted delivery of lung-cancer drug by antibody and tumor lineage-homing cell-penetrating peptide. Drug Deliv. 2018, 25, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Hamzawy, M.A.; Abo-Youssef, A.M.; Salem, H.F.; Mohammed, S.A. Antitumor activity of intratracheal inhalation of temozolomide (TMZ) loaded into gold nanoparticles and/or liposomes against urethane-induced lung cancer in BALB/c mice. Drug Deliv. 2017, 24, 599–607. [Google Scholar] [CrossRef]
- Gandhi, M.; Pandya, T.; Gandhi, R.; Patel, S.; Mashru, R.; Misra, A.; Tandel, H. Inhalable liposomal dry powder of gemcitabine-HCl: Formulation, in vitro characterization and in vivo studies. Int. J. Pharm. 2015, 496, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, R.; Li, M.; Bao, J.; Chen, Y.; Ge, Y.; Jin, Y. Comparative study of intratracheal and oral gefitinib for the treatment of primary lung cancer. Eur. J. Pharm. Sci. 2020, 149, 105352. [Google Scholar] [CrossRef] [PubMed]
- Asmawi, A.A.; Salim, N.; Ngan, C.L.; Ahmad, H.; Abdulmalek, E.; Masarudin, M.J.; Abdul Rahman, M.B. Excipient selection and aerodynamic characterization of nebulized lipid-based nanoemulsion loaded with docetaxel for lung cancer treatment. Drug Deliv. Transl. Res. 2019, 9, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Asmawi, A.A.; Salim, N.; Abdulmalek, E.; Abdul Rahman, M.B. Modeling the effect of composition on formation of aerosolized nanoemulsion system encapsulating docetaxel and curcumin using D-optimal mixture experimental design. Int. J. Mol. Sci. 2020, 21, 4357. [Google Scholar] [CrossRef] [PubMed]
- Al Ayoub, Y.; Gopalan, R.C.; Najafzadeh, M.; Mohammad, M.A.; Anderson, D.; Paradkar, A.; Assi, K.H. Development and evaluation of nanoemulsion and microsuspension formulations of curcuminoids for lung delivery with a novel approach to understanding the aerosol performance of nanoparticles. Int. J. Pharm. 2019, 557, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Arbain, N.H.; Basri, M.; Salim, N.; Wui, W.T.; Abdul Rahman, M.B. Development and characterization of aerosol nanoemulsion system encapsulating low water soluble quercetin for lung cancer treatment. Mater. Today Proc. 2018, 5, S137–S142. [Google Scholar] [CrossRef]
- Arbain, N.H.; Salim, N.; Masoumi, H.R.F.; Wong, T.W.; Basri, M.; Abdul Rahman, M.B. In vitro evaluation of the inhalable quercetin loaded nanoemulsion for pulmonary delivery. Drug Deliv. Transl. Res. 2019, 9, 497–507. [Google Scholar] [CrossRef]
- Nassimi, M.; Schleh, C.; Lauenstein, H.; Hussein, R.; Hoymann, H.; Koch, W.; Pohlmann, G.A.; Krug, N.; Sewald, K.; Rittinghausen, S. A toxicological evaluation of inhaled solid lipid nanoparticles used as a potential drug delivery system for the lung. Eur. J. Pharm. Biopharm. 2010, 75, 107–116. [Google Scholar] [CrossRef]
- Bakhtiary, Z.; Barar, J.; Aghanejad, A.; Saei, A.A.; Nemati, E.; Ezzati Nazhad Dolatabadi, J.; Omidi, Y. Microparticles containing erlotinib-loaded solid lipid nanoparticles for treatment of non-small cell lung cancer. Drug Dev. Ind. Pharm. 2017, 43, 1244–1253. [Google Scholar] [CrossRef]
- Hu, L.; Jia, Y.; Ding, W. Preparation and characterization of solid lipid nanoparticles loaded with epirubicin for pulmonary delivery. Pharmazie 2010, 65, 585–587. [Google Scholar] [PubMed]
- Yang, Y.; Huang, Z.; Li, J.; Mo, Z.; Huang, Y.; Ma, C.; Wang, W.; Pan, X.; Wu, C. PLGA Porous microspheres dry powders for codelivery of afatinib-loaded solid lipid nanoparticles and paclitaxel: Novel therapy for EGFR tyrosine kinase inhibitors resistant nonsmall cell lung cancer. Adv. Healthc. Mater. 2019, 8, 1900965. [Google Scholar] [CrossRef] [PubMed]
- Nafee, N.; Gaber, D.M.; Elzoghby, A.O.; Helmy, M.W.; Abdallah, O.Y. Promoted antitumor activity of myricetin against lung carcinoma via nanoencapsulated phospholipid complex in respirable microparticles. Pharm. Res. 2020, 37, 82. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.R.; Chougule, M.B.; Patlolla, R.; Wang, G.; Singh, M. Efficacy of aerosolized celecoxib encapsulated nanostructured lipid carrier in non-small cell lung cancer in combination with docetaxel. Pharm. Res. 2013, 30, 1435–1446. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Garg, T.; Rath, G.; Murthy, R.R.; Goyal, A.K. Development, optimization and evaluation of surfactant-based pulmonary nanolipid carrier system of paclitaxel for the management of drug resistance lung cancer using Box-Behnken design. Drug Deliv. 2016, 23, 1912–1925. [Google Scholar] [CrossRef] [PubMed]
- Taratula, O.; Kuzmov, A.; Shah, M.; Garbuzenko, O.B.; Minko, T. Nanostructured lipid carriers as multifunctional nanomedicine platform for pulmonary co-delivery of anticancer drugs and siRNA. J. Control. Release 2013, 171, 349–357. [Google Scholar] [CrossRef]
- Garbuzenko, O.B.; Kuzmov, A.; Taratula, O.; Pine, S.R.; Minko, T. Strategy to enhance lung cancer treatment by five essential elements: Inhalation delivery, nanotechnology, tumor-receptor targeting, chemo- and gene therapy. Theranostics 2019, 9, 8362–8376. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, I.; Costabile, G.; Durantie, E.; Brocca, P.; Rondelli, V.; Russo, A.; Russo, G.; Miro, A.; Quaglia, F.; Petri-Fink, A.; et al. Hybrid lipid/polymer nanoparticles for pulmonary delivery of siRNA: Development and fate upon in vitro deposition on the human epithelial airway barrier. J. Aerosol Med. Pulm. Drug Deliv. 2018, 31, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Saimi, M.N.I.; Salim, N.; Ahmad, N.; Abdulmalek, E.; Abdul Rahman, M.B. Aerosolized niosome formulation containing gemcitabine and cisplatin for lung cancer treatment: Optimization, characterization and in vitro evaluation. Pharmaceutics 2021, 13, 59. [Google Scholar] [CrossRef] [PubMed]
- Jyoti, K.; Pandey, R.S.; Madan, J.; Jain, U.K. Inhalable cationic niosomes of curcumin enhanced drug delivery and apoptosis in lung cancer cells. Indian J. Pharm. Educ. Res. 2016, 50. [Google Scholar] [CrossRef]
- Osama, H.; Sayed, O.M.; Hussein, R.R.S.; Abdelrahim, M.; Elberry, A. Design, optimization, characterization, and in vivo evaluation of sterosomes as a carrier of metformin for treatment of lung cancer. J. Liposome Res. 2020, 30, 150–162. [Google Scholar] [CrossRef] [PubMed]
- Bangham, A.D.; Standish, M.M.; Watkins, J.C. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 1965, 13, IN26–IN27. [Google Scholar] [CrossRef]
- Çağdaş, M.; Sezer, A.D.; Bucak, S. Liposomes as potential drug carrier systems for drug delivery. In Application of Nanotechnology in Drug Delivery; IntechOpen: London, UK, 2014. [Google Scholar] [CrossRef]
- Paranjpe, M.; Muller-Goymann, C.C. Nanoparticle-mediated pulmonary drug delivery: A review. Int. J. Mol. Sci. 2014, 15, 5852–5873. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef]
- Noble, G.T.; Stefanick, J.F.; Ashley, J.D.; Kiziltepe, T.; Bilgicer, B. Ligand-targeted liposome design: Challenges and fundamental considerations. Trends Biotechnol. 2014, 32, 32–45. [Google Scholar] [CrossRef]
- Choudhury, H.; Gorain, B.; Chatterjee, B.; Mandal, U.K.; Sengupta, P.; Tekade, R.K. Pharmacokinetic and pharmacodynamic features of nanoemulsion following oral, intravenous, topical and nasal route. Curr. Pharm. Des. 2017, 23, 2504–2531. [Google Scholar] [CrossRef] [PubMed]
- Jideani, Y.M.a.V.A. Factors Affecting the Stability of Emulsions Stabilised by Biopolymers, Science and Technology Behind Nanoemulsions; IntechOpen: London, UK, 2018. [Google Scholar]
- Nguyen, T.T.L.; Anton, N.; Vandamme, T.F. Oral pellets loaded with nanoemulsions. In Nanostructures for Oral Medicine; Andronescu, E., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 203–230. [Google Scholar] [CrossRef]
- Kale, S.N.; Deore, S.L. Emulsion micro emulsion and nano emulsion: A review. Syst. Rev. Pharm. 2017, 8, 39. [Google Scholar] [CrossRef]
- Gutiérrez, J.; González, C.; Maestro, A.; Solè, I.; Pey, C.; Nolla, J. Nano-emulsions: New applications and optimization of their preparation. Curr. Opin. Colloid Interface Sci. 2008, 13, 245–251. [Google Scholar] [CrossRef]
- Choudhury, H.; Pandey, M.; Gorain, B.; Chatterjee, B.; Madheswaran, T.; Md, S.; Mak, K.K.; Tambuwala, M.; Chourasia, M.K.; Kesharwani, P. Nanoemulsions as effective carriers for the treatment of lung cancer. In Nanotechnology-Based Targeted Drug Delivery Systems for Lung Cancer; Elsevier: Amsterdam, The Netherlands, 2019; pp. 217–247. [Google Scholar] [CrossRef]
- Ngan, C.L.; Asmawi, A.A. Lipid-based pulmonary delivery system: A review and future considerations of formulation strategies and limitations. Drug Deliv. Transl. Res. 2018, 8, 1527–1544. [Google Scholar] [CrossRef] [PubMed]
- Ganta, S.; Amiji, M. Coadministration of Paclitaxel and curcumin in nanoemulsion formulations to overcome multidrug resistance in tumor cells. Mol. Pharm. 2009, 6, 928–939. [Google Scholar] [CrossRef]
- Agrawal, N.; Maddikeri, G.L.; Pandit, A.B. Sustained release formulations of citronella oil nanoemulsion using cavitational techniques. Ultrason. Sonochem. 2017, 36, 367–374. [Google Scholar] [CrossRef]
- Tiwari, S.; Tan, Y.-M.; Amiji, M. Preparation and in vitro characterization of multifunctional nanoemulsions for simultaneous MR imaging and targeted drug delivery. J. Biomed. Nanotechnol. 2006, 2, 217–224. [Google Scholar] [CrossRef]
- Gupta, S.; Kesarla, R.; Omri, A. Formulation strategies to improve the bioavailability of poorly absorbed drugs with special emphasis on self-emulsifying systems. ISRN Pharm. 2013, 2013, 848043. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Chan, L.W.; Wong, T.W. Critical physicochemical and biological attributes of nanoemulsions for pulmonary delivery of rifampicin by nebulization technique in tuberculosis treatment. Drug Deliv. 2017, 24, 1631–1647. [Google Scholar] [CrossRef] [PubMed]
- Lovelyn, C.; Attama, A.A. Current state of nanoemulsions in drug delivery. J. Biomater. Nanobiotechnol. 2011, 02, 626–639. [Google Scholar] [CrossRef]
- Nesamony, J.; Shah, I.S.; Kalra, A.; Jung, R. Nebulized oil-in-water nanoemulsion mists for pulmonary delivery: Development, physico-chemical characterization and in vitro evaluation. Drug Dev. Ind. Pharm. 2014, 40, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Krahn, C.L.; Raffin, R.P.; Santos, G.S.; Queiroga, L.B.; Cavalcanti, R.L.; Serpa, P.; Dallegrave, E.; Mayorga, P.E.; Pohlmann, A.R.; Natalini, C.C.; et al. Isoflurane-loaded nanoemulsion prepared by high-pressure homogenization: Investigation of stability and dose reduction in general anesthesia. J. Biomed. Nanotechnol. 2012, 8, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Amani, A.; York, P.; Chrystyn, H.; Clark, B.J. Evaluation of a nanoemulsion-based formulation for respiratory delivery of budesonide by nebulizers. AAPS PharmSciTech 2010, 11, 1147–1151. [Google Scholar] [CrossRef]
- Li, M.; Zhu, L.; Liu, B.; Du, L.; Jia, X.; Han, L.; Jin, Y. Tea tree oil nanoemulsions for inhalation therapies of bacterial and fungal pneumonia. Colloids Surf. B Biointerfaces 2016, 141, 408–416. [Google Scholar] [CrossRef]
- Mason, T.G.; Wilking, J.N.; Meleson, K.; Chang, C.B.; Graves, S.M. Nanoemulsions: Formation, structure, and physical properties. J. Phys. Condens. Matter 2006, 18, R635–R666. [Google Scholar] [CrossRef]
- Qi, K.; Al-Haideri, M.; Seo, T.; Carpentier, Y.A.; Deckelbaum, R.J. Effects of particle size on blood clearance and tissue uptake of lipid emulsions with different triglyceride compositions. JPEN J. Parenter. Enter. Nutr. 2003, 27, 58–64. [Google Scholar] [CrossRef]
- Kaur, P.; Garg, T.; Rath, G.; Murthy, R.S.R.; Goyal, A.K. Surfactant-based drug delivery systems for treating drug-resistant lung cancer. Drug Deliv. 2016, 23, 717–728. [Google Scholar] [CrossRef]
- Mansour, H.M.; Rhee, Y.S.; Wu, X. Nanomedicine in pulmonary delivery. Int. J. Nanomed. 2009, 4, 299–319. [Google Scholar] [CrossRef]
- Dammak, I.; Sobral, P.J.d.A.; Aquino, A.; Neves, M.A.d.; Conte-Junior, C.A. Nanoemulsions: Using emulsifiers from natural sources replacing synthetic ones—A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2721–2746. [Google Scholar] [CrossRef]
- Renne, R.A.; Wehner, A.P.; Greenspan, B.; Deford, H.; Ragan, H.A.; Westerberg, R.; Buschbom, R.; Burger, G.; Hayes, A.W.; Suber, R.; et al. 2-week and 13-week inhalation studies of aerosolized glycerol in rats. Inhal. Toxicol. 2008, 4, 95–111. [Google Scholar] [CrossRef]
- Terakosolphan, W. Pharmacokinetic-Modifying Effects of Glycerol in Inhaled Medicines. Ph.D. Thesis, King’s College, London, UK, 2019. [Google Scholar]
- Darquenne, C. Aerosol deposition in the human lung in reduced gravity. J. Aerosol Med. Pulm. Drug Deliv. 2014, 27, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Kamali, H.; Abbasi, S.; Amini, M.A.; Amani, A. Investigation of factors affecting aerodynamic performance of nebulized nanoemulsion. Iran. J. Pharm Res. 2016, 15, 687–693. [Google Scholar] [PubMed]
- Vafadar, A.; Shabaninejad, Z.; Movahedpour, A.; Fallahi, F.; Taghavipour, M.; Ghasemi, Y.; Akbari, M.; Shafiee, A.; Hajighadimi, S.; Moradizarmehri, S.; et al. Quercetin and cancer: New insights into its therapeutic effects on ovarian cancer cells. Cell Biosci. 2020, 10, 32. [Google Scholar] [CrossRef]
- Ganta, S.; Singh, A.; Rawal, Y.; Cacaccio, J.; Patel, N.R.; Kulkarni, P.; Ferris, C.F.; Amiji, M.M.; Coleman, T.P. Formulation development of a novel targeted theranostic nanoemulsion of docetaxel to overcome multidrug resistance in ovarian cancer. Drug Deliv. 2016, 23, 968–980. [Google Scholar] [CrossRef]
- Afzal, S.M.; Shareef, M.Z.; Kishan, V. Transferrin tagged lipid nanoemulsion of docetaxel for enhanced tumor targeting. J. Drug Deliv. Sci. Technol. 2016, 36, 175–182. [Google Scholar] [CrossRef]
- Sajid, M.; Cameotra, S.S.; Khan, M.S.A.; Ahmad, I. Chapter 23—Nanoparticle-based delivery of phytomedicines: Challenges and opportunities. In New Look to Phytomedicine; Khan, M.S.A., Ahmad, I., Chattopadhyay, D., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 597–623. [Google Scholar] [CrossRef]
- Borges, A.; de Freitas, V.; Mateus, N.; Fernandes, I.; Oliveira, J. Solid lipid nanoparticles as carriers of natural phenolic compounds. Antioxidants 2020, 9, 998. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Eldridge, D.; Palombo, E.; Harding, I. Lipid Nanoparticles: Production, Characterization and Stability; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Mehnert, W.; Mäder, K. Solid lipid nanoparticles: Production, characterization and applications. Adv. Drug Deliv. Rev. 2001, 47, 165–196. [Google Scholar] [CrossRef]
- Shah, R.; Eldridge, D.; Palombo, E.; Harding, I. Composition and structure. In Lipid Nanoparticles: Production, Characterization and Stability; Springer: Berlin/Heidelberg, Germany, 2015; pp. 11–22. [Google Scholar]
- Müller, R.H.; Rühl, D.; Runge, S.A. Biodegradation of solid lipid nanoparticles as a function of lipase incubation time. Int. J. Pharm. 1996, 144, 115–121. [Google Scholar] [CrossRef]
- Haque, S.; Whittaker, M.; McIntosh, M.P.; Pouton, C.W.; Phipps, S.; Kaminskas, L.M. A comparison of the lung clearance kinetics of solid lipid nanoparticles and liposomes by following the 3H-labelled structural lipids after pulmonary delivery in rats. Eur. J. Pharm. Biopharm. 2018, 125, 1–12. [Google Scholar] [CrossRef]
- Huang, Z.; Huang, Y.; Wang, W.; Fu, F.; Wang, W.; Dang, S.; Li, C.; Ma, C.; Zhang, X.; Zhao, Z.; et al. Relationship between particle size and lung retention time of intact solid lipid nanoparticle suspensions after pulmonary delivery. J. Control. Release 2020, 325, 206–222. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.H.; Radtke, M.; Wissing, S.A. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv. Drug Deliv. Rev. 2002, 54 (Suppl. S1), S131–S155. [Google Scholar] [CrossRef]
- Müller, R.H.; Radtke, M.; Wissing, S.A. Nanostructured lipid matrices for improved microencapsulation of drugs. Int. J. Pharm. 2002, 242, 121–128. [Google Scholar] [CrossRef]
- Ahmad, J.; Akhter, S.; Rizwanullah, M.; Amin, S.; Rahman, M.; Ahmad, M.Z.; Rizvi, M.A.; Kamal, M.A.; Ahmad, F.J. Nanotechnology-based inhalation treatments for lung cancer: State of the art. Nanotechnol. Sci. Appl. 2015, 8, 55–66. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Hao, J.; Li, B.; Li, M.; Xiuwen, W. Lung cancer combination therapy: Co-delivery of paclitaxel and doxorubicin by nanostructured lipid carriers for synergistic effect. Drug Deliv. 2016, 23, 1398–1403. [Google Scholar] [CrossRef] [PubMed]
- Chernikov, I.V.; Vlassov, V.V.; Chernolovskaya, E.L. Current development of siRNA bioconjugates: From research to the clinic. Front. Pharmacol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Kuzmov, A.; Minko, T. Nanotechnology approaches for inhalation treatment of lung diseases. J. Control. Release 2015, 219, 500–518. [Google Scholar] [CrossRef]
- Anderson, C.F.; Grimmett, M.E.; Domalewski, C.J.; Cui, H. Inhalable nanotherapeutics to improve treatment efficacy for common lung diseases. WIREs Nanomed. Nanobiotechnol. 2020, 12, e1586. [Google Scholar] [CrossRef]
- Jaiswal, P.; Gidwani, B.; Vyas, A. Nanostructured lipid carriers and their current application in targeted drug delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 27–40. [Google Scholar] [CrossRef]
- Gerhardt, A.; Voigt, E.; Archer, M.; Reed, S.; Larson, E.; Van Hoeven, N.; Kramer, R.; Fox, C.; Casper, C. A Thermostable, flexible RNA vaccine delivery platform for pandemic response. bioRxiv 2021, 1–26. [Google Scholar] [CrossRef]
- Emami, J.; Rezazadeh, M.; Varshosaz, J.; Tabbakhian, M.; Aslani, A. Formulation of LDL targeted nanostructured lipid carriers loaded with paclitaxel: A detailed study of preparation, freeze drying condition, and in vitro cytotoxicity. J. Nanomater. 2012, 2012, 358782. [Google Scholar] [CrossRef]
- Magalhães, J.; Pinheiro, M.; Drasler, B.; Septiadi, D.; Petri-Fink, A.; Santos, S.G.; Rothen-Rutishauser, B.; Reis, S. Lipid nanoparticles biocompatibility and cellular uptake in a 3D human lung model. Nanomedicine 2020, 15, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Garbuzenko, O.B.; Kbah, N.; Kuzmov, A.; Pogrebnyak, N.; Pozharov, V.; Minko, T. Inhalation treatment of cystic fibrosis with lumacaftor and ivacaftor co-delivered by nanostructured lipid carriers. J. Control. Release 2019, 296, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Sastre, M.; Pastor, M.; Esquisabel, A.; Sans, E.; Vinas, M.; Fleischer, A.; Palomino Lago, E.; Bachiller, D.; Pedraz, J. Pulmonary delivery of tobramycin-loaded nanostructured lipid carriers for Pseudomonas aeruginosa infections associated with cystic fibrosis. Int. J. Pharm. 2016, 498, 263–273. [Google Scholar] [CrossRef]
- Sans-Serramitjana, E.; Jorba, M.; Fusté, E.; Pedraz, J.L.; Vinuesa, T.; Viñas, M. Free and nanoencapsulated tobramycin: Effects on planktonic and biofilm forms of pseudomonas. Microorganisms 2017, 5, 35. [Google Scholar] [CrossRef] [PubMed]
- Pakunlu, R.I.; Wang, Y.; Tsao, W.; Pozharov, V.; Cook, T.J.; Minko, T. Enhancement of the Efficacy of chemotherapy for lung cancer by simultaneous suppression of multidrug resistance and antiapoptotic cellular defense. Nov. Multicompon. Deliv. Syst. 2004, 64, 6214–6224. [Google Scholar] [CrossRef] [PubMed]
- Saad, M.; Garbuzenko, O.B.; Minko, T. Co-delivery of siRNA and an anticancer drug for treatment of multidrug-resistant cancer. Nanomedicine 2008, 3, 761–776. [Google Scholar] [CrossRef]
- Taratula, O.; Garbuzenko, O.B.; Chen, A.M.; Minko, T. Innovative strategy for treatment of lung cancer: Targeted nanotechnology-based inhalation co-delivery of anticancer drugs and siRNA. J. Drug Target. 2011, 19, 900–914. [Google Scholar] [CrossRef]
- Garbuzenko, O.B.; Saad, M.; Pozharov, V.P.; Reuhl, K.R.; Mainelis, G.; Minko, T. Inhibition of lung tumor growth by complex pulmonary delivery of drugs with oligonucleotides as suppressors of cellular resistance. Proc. Natl. Acad. Sci. USA 2010, 107, 10737–10742. [Google Scholar] [CrossRef] [PubMed]
- Hida, T.; Kozaki, K.-i.; Muramatsu, H.; Masuda, A.; Shimizu, S.; Mitsudomi, T.; Sugiura, T.; Ogawa, M.; Takahashi, T. Cyclooxygenase-2 inhibitor induces apoptosis and enhances cytotoxicity of various anticancer agents in non-small cell lung cancer cell lines. Clin. Cancer Res. 2000, 6, 2006–2011. [Google Scholar]
- Shaik, M.S.; Chatterjee, A.; Jackson, T.; Singh, M. Enhancement of antitumor activity of docetaxel by celecoxib in lung tumors. Int. J. Cancer 2006, 118, 396–404. [Google Scholar] [CrossRef][Green Version]
- Abou Assi, R.; Abdulbaqi, I.M.; Seok Ming, T.; Siok Yee, C.; Wahab, H.A.; Asif, S.M.; Darwis, Y. Liquid and solid self-emulsifying drug delivery systems (SEDDs) as carriers for the oral delivery of azithromycin: Optimization, in vitro characterization and stability assessment. Pharmaceutics 2020, 12, 1052. [Google Scholar] [CrossRef]
- Liu, J.; Cheng, H.; Le Han, Z.Q.; Zhang, X.; Gao, W.; Zhao, K.; Song, Y. Synergistic combination therapy of lung cancer using paclitaxel-and triptolide-coloaded lipid–polymer hybrid nanoparticles. Drug Des. Dev. Ther. 2018, 12, 3199. [Google Scholar] [CrossRef]
- Yugui, F.; Wang, H.; Sun, D.; Zhang, X. Nasopharyngeal cancer combination chemoradiation therapy based on folic acid modified, gefitinib and yttrium 90 co-loaded, core-shell structured lipid-polymer hybrid nanoparticles. Biomed. Pharmacother. 2019, 114, 108820. [Google Scholar] [CrossRef]
- Mukherjee, A.; Waters, A.K.; Kalyan, P.; Achrol, A.S.; Kesari, S.; Yenugonda, V.M. Lipid-polymer hybrid nanoparticles as a next-generation drug delivery platform: State of the art, emerging technologies, and perspectives. Int. J. Nanomed. 2019, 14, 1937–1952. [Google Scholar] [CrossRef]
- Wang, G.; Wang, Z.; Li, C.; Duan, G.; Wang, K.; Li, Q.; Tao, T. RGD peptide-modified, paclitaxel prodrug-based, dual-drugs loaded, and redox-sensitive lipid-polymer nanoparticles for the enhanced lung cancer therapy. Biomed. Pharmacother. 2018, 106, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Wei, M.; He, S.; Yuan, W.-E. Advances of non-ionic surfactant vesicles (niosomes) and their application in drug delivery. Pharmaceutics 2019, 11, 55. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; Fang, C.; Pei, Y. Stealth PEG-PHDCA niosomes: Effects of chain length of PEG and particle size on niosomes surface properties, in vitro drug release, phagocytic uptake, in vivo pharmacokinetics and antitumor activity. J. Pharm. Sci. 2006, 95, 1873–1887. [Google Scholar] [CrossRef]
- Tangri, P.; Khurana, S. Niosomes: Formulation and evaluation. Int. J. Biopharm. 2011, 2229, 7499. [Google Scholar]
- Heger, M.; van Golen, R.F.; Broekgaarden, M.; Michel, M.C. The molecular basis for the pharmacokinetics and pharmacodynamics of curcumin and its metabolites in relation to cancer. Pharm. Rev. 2014, 66, 222–307. [Google Scholar] [CrossRef]
- Merisko-Liversidge, E.; Liversidge, G.G. Nanosizing for oral and parenteral drug delivery: A perspective on formulating poorly-water soluble compounds using wet media milling technology. Adv. Drug Deliv. Rev. 2011, 63, 427–440. [Google Scholar] [CrossRef]
- Gajra, B.; Dalwadi, C.; Patel, R. Formulation and optimization of itraconazole polymeric lipid hybrid nanoparticles (Lipomer) using Box Behnken design. DARU J. Pharm. Sci. 2015, 23, 3. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.K.; Fan, J.; Kim, S.; Bezouglaia, O.; Fartash, A.; Wu, B.M.; Aghaloo, T.; Lee, M. Delivery of siRNA via cationic Sterosomes to enhance osteogenic differentiation of mesenchymal stem cells. J. Control. Release 2015, 217, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.K.; Kim, S.; Baljon, J.J.; Doroudgar, M.; Lafleur, M.; Wu, B.M.; Aghaloo, T.; Lee, M. Design and characterization of a therapeutic non-phospholipid liposomal nanocarrier with osteoinductive characteristics to promote bone formation. ACS Nano 2017, 11, 8055–8063. [Google Scholar] [CrossRef] [PubMed]
- ClinicaTtrials.gov. Phase 2 Study of Inhaled Lipid Cisplatin in Pulmonary Recurrent Osteosarcoma. Available online: https://clinicaltrials.gov/ct2/show/NCT01650090 (accessed on 26 June 2021).
- ClinicalTrials.gov. Aerosol L9-NC and Temozolomide in Ewing’s Sarcoma. Available online: https://clinicaltrials.gov/ct2/show/NCT00492141 (accessed on 26 June 2021).
- ClinicalTrials.gov. Phase II Study of Aerosolized Liposomal 9-Nitro-20 (S)- Camptothecin (L9NC). Available online: https://clinicaltrials.gov/ct2/show/record/NCT00249990 (accessed on 26 June 2021).
- ClinicalTrials.gov. Pharmacology Study of Aerosolized Liposomal 9-Nitro-20 (S)-Camptothecin (L9NC). Available online: https://clinicaltrials.gov/ct2/show/NCT00250016 (accessed on 25 June 2021).
- ClinicalTrials.gov. Study of Aerosolized Liposomal 9-Nitro-20 (S)- Camptothecin (L9NC). Available online: https://clinicaltrials.gov/ct2/show/NCT00250068 (accessed on 26 June 2021).
- ClinicalTrials.gov. Aerosolized Liposomal Camptothecin in Patients with Metastatic or Recurrent Cancer of the Endometrium or the Lung. Available online: https://clinicaltrials.gov/ct2/show/record/NCT00277082 (accessed on 26 June 2021).
- ClinicalTrials.gov. Pharmacology Study of Aerosolized Liposomal. Available online: https://clinicaltrials.gov/ct2/show/NCT00250120 (accessed on 26 June 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).