Abstract
Devil’s claw (Harpagophytum spp., Pedaliaceae) is one of the best-documented phytomedicines. Its mode of action is largely elucidated, and its efficacy and excellent safety profile have been demonstrated in a long list of clinical investigations. The author conducted a bibliographic review which not only included peer-reviewed papers published in scientific journals but also a vast amount of grey literature, such as theses and reports initiated by governmental as well as non-governmental organizations, thus allowing for a more holistic presentation of the available evidence. Close to 700 sources published over the course of two centuries were identified, confirmed, and cataloged. The purpose of the review is three-fold: to trace the historical milestones in devil’s claw becoming a modern herbal medicine, to point out gaps in the seemingly all-encompassing body of research, and to provide the reader with a reliable and comprehensive bibliography. The review covers aspects of ethnobotany, taxonomy, history of product development and commercialization, chemistry, pharmacology, toxicology, as well as clinical efficacy and safety. It is concluded that three areas stand out in need of further investigation. The taxonomical assessment of the genus is outdated and lacking. A revision is needed to account for intra- and inter-specific, geographical, and chemo-taxonomical variation, including variation in composition. Further research is needed to conclusively elucidate the active compound(s). Confounded by early substitution, intermixture, and blending, it has yet to be demonstrated beyond a reasonable doubt that both (or all) Harpagophytum spp. are equally (and interchangeably) safe and efficacious in clinical practice.
1. Introduction
Devil’s claw is the collective name of plants from the genus Harpagophytum (Pedaliaceae). The latter includes two species, H. procumbens (Burch.) DC. ex Meisn. and H. zeyheri Decne., currently divided into five subspecies with introgression reported from overlapping habitats [1,2]. The secondary root tubers of devil’s claw are used in botanical drugs and supplements and are exported from Southern Africa, mainly Namibia. Entrepreneurial spirit, colonialism, and the absence of regulatory barriers drove the commercialization of devil’s claw in a fashion similar to that of other medicinal plants from Southern Africa, such as Umckaloabo (Pelargonium sidoides) [3], rooibos (Aspalathus linearis) and honeybush (Cyclopia spp.) [4], buchu (Agathosma betulina) [5], cape aloe (Aloe ferox) [6], uzara (Xysmalobium undulatum) [7], and to some extent, hoodia (Hoodia gordonii) [8], among others [9]. From the 1960s onward, products quickly gained popularity, initially in Germany, then France, and by the mid-1980s, all over the developed world. This led to an increase in demand and consequently harvesting pressure in the countries of origin, to the point that devil’s claw was briefly considered to be listed on CITES appendix II [10]. However, ongoing efforts to introduce good harvesting practices and cultivation attempts helped supply to become more sustainable.
Once harvested, botanical differentiation between species and subspecies is virtually impossible, and it can safely be assumed that since the 1970s, the product of commerce is one or the other and often of mixed origin [11,12,13,14]. Thus, current official compendia do not distinguish between the two botanical sources of devil’s claw but require compliance in terms of contents of the marker compound harpagoside, a cinnamoylated iridoid glucoside. The primary medicinal uses of devil’s claw are the management of arthritis, pain, and dyspepsia [15,16]. An impressive number of clinical trials, the earlier being mostly observational, the more recent randomized, placebo-controlled studies—albeit being of variable quality—indicate clinical efficacy and safety [17]. However, whether harpagoside is more than a just marker, but also the (only) active compound, remains to be demonstrated. Consequently, superiority of H. procumbens over H. zeyheri cannot be derived merely from harpagoside content [18]. Lower levels of harpagoside do not necessarily translate to lower levels of total iridoids, and phytochemically distinct extracts from H. procumbens and H. zeyheri have shown similar in vivo analgesic and anti-inflammatory properties [19].
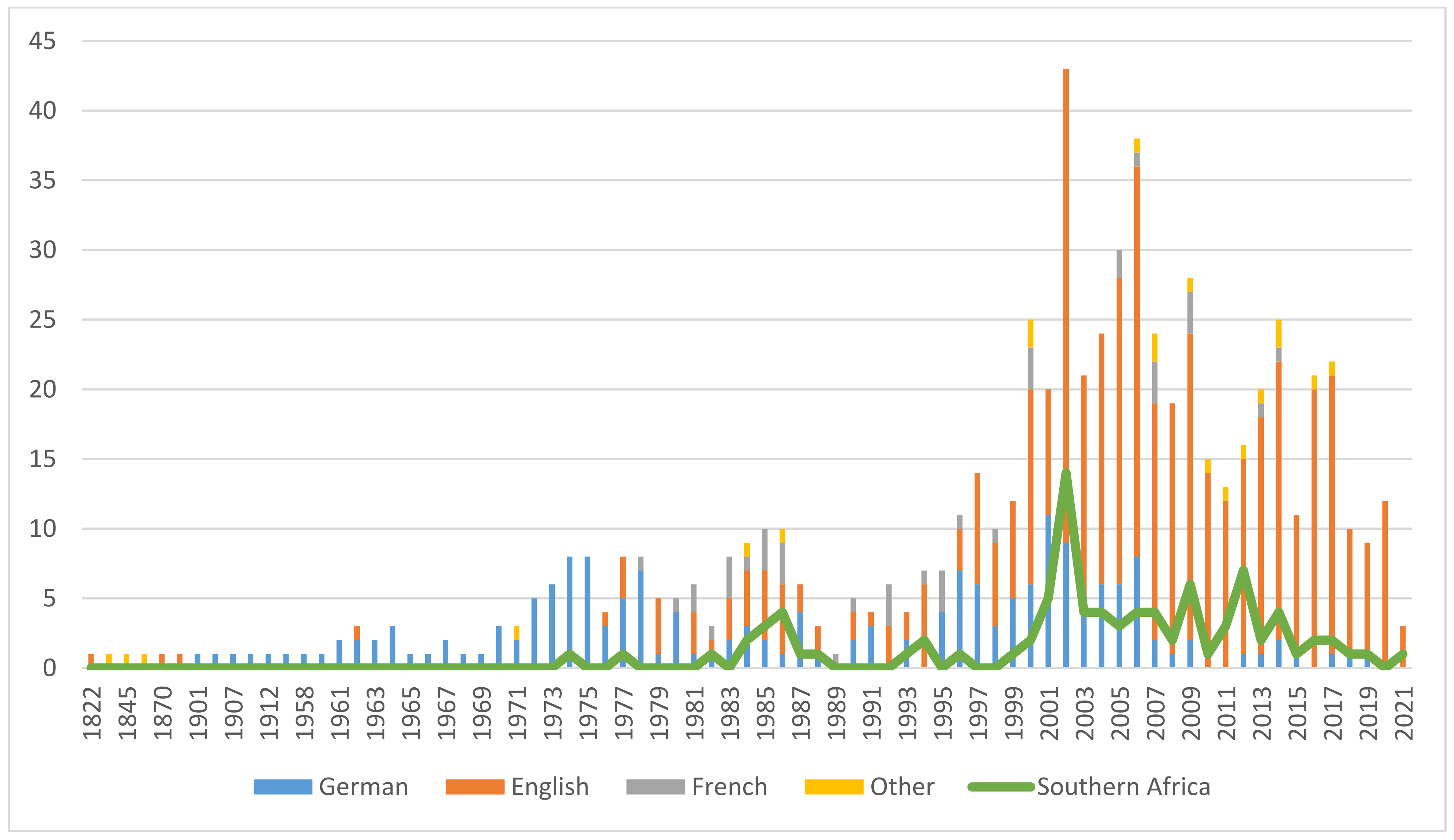
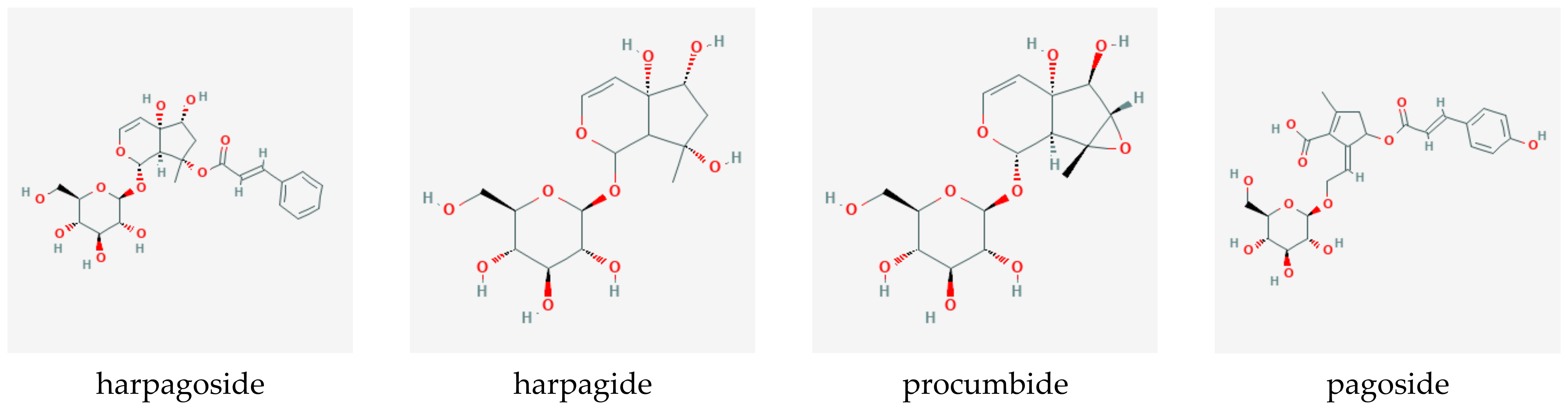
The vast body of evidence presented here—over a period of 55 years, about one general review per year was published in the scientific literature [20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80], not counting reviews specific to clinical efficacy (see Section 12.1.)—makes devil’s claw one of the best-researched botanicals. Figure 1 illustrates the growing and sustained research interest. The 694 included publications were grouped by language, which yielded a perspective on how research interest spread geographically over time. Despite English becoming the lingua franca of science toward the end of the 20th century, a trend is clearly noticeable—from Germany to France to the rest of the world—and confirmed by research, trade, and availability and popularity of pharmaceutical products. An interesting discrepancy reveals itself when comparing the total with the research output of the region of origin. Nonetheless, knowledge gaps concerning species interchangeability remain to be closed, the elucidation of which is one purpose of this review. It is hoped that the assembly of this extensive bibliography will stimulate further research of this interesting genus of medicinal plants.
Figure 1.
Publications on Harpagophytum spp., 1822–2021 (colors indicate publication language/origin of research).
2. Materials and Methods
Multiple searches were conducted in the PubMed, Scopus, and Google Scholar databases with the following keywords and combinations thereof: “Harpagophytum, harpagophyton, devil(’)s claw, Teufelskralle, grapple plant, sengaparile, garra-do-diabo, griffe du diable, (h)arpagoside, taxonomy, nomenclature, ethnobotany, traditional use, ecology, cultivation, sustainability, economy, trade, CITES, chemistry, biochemistry, compounds, pre-clinical, pharmacology, clinical, RCT, safety, toxicology, veterinary, review”. Union catalogues were also searched. The search was limited to scientific literature, and popular magazines and compendia were excluded. Also excluded were articles which only mentioned Harpagophytum without elaboration. Further excluded were reports on compounds present in Harpagophytum, that were derived from other sources (e.g., harpagoside from Scrophularia spp.).
Reference sections of selected publications were searched manually. Academic theses were retrieved primarily via the Bielefeld Academic Search Engine (BASE). Patents were retrieved from the European, US, and international (WIPO) patent office databases.
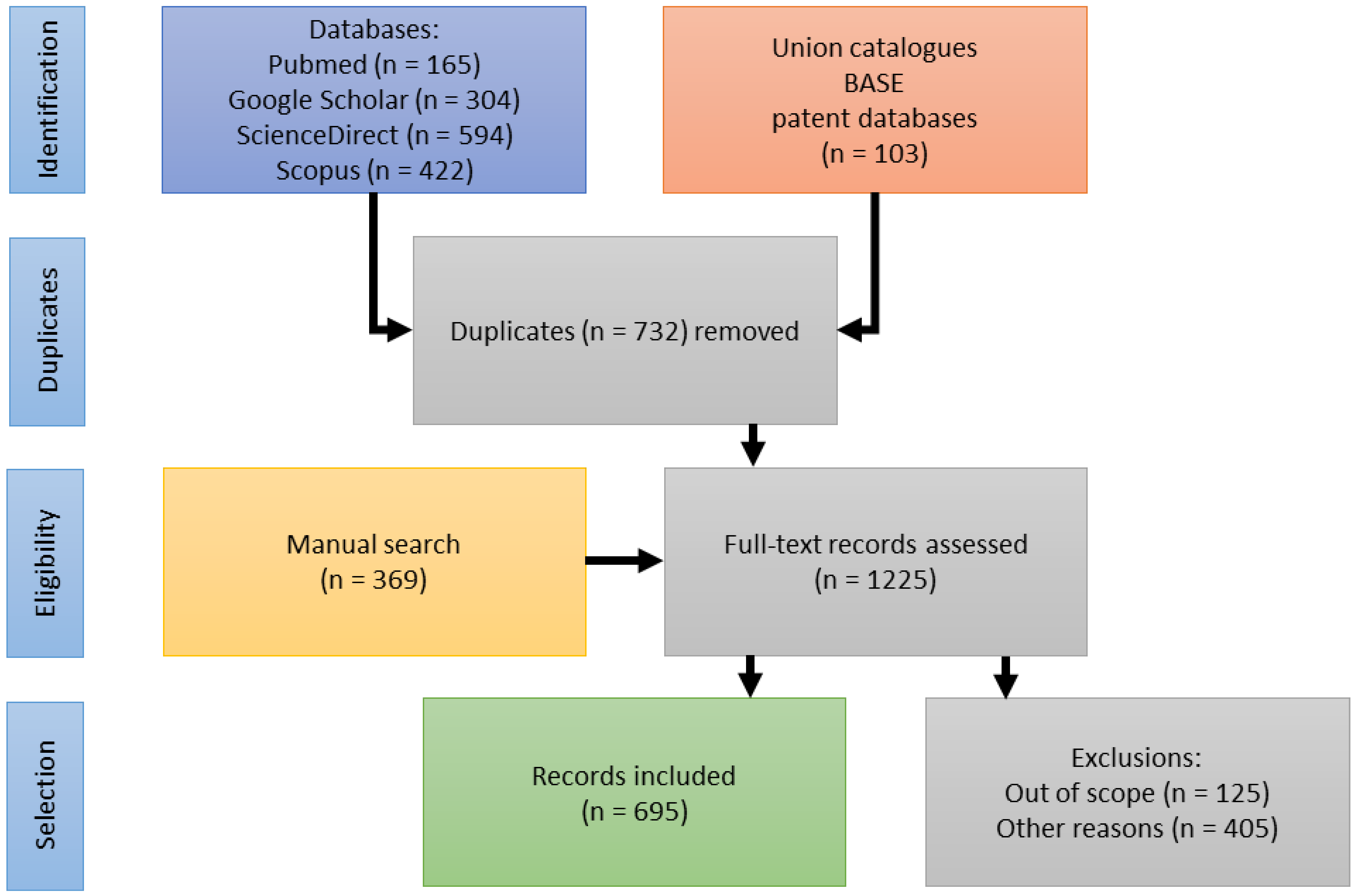
A substantial body of publications (125) was identified addressing aspects of ecology, stakeholders’ livelihoods, efforts in capacity building, as well as access-benefit-sharing (ABS) and its legislation. They are included in the publication statistics (see Figure 1). In reviewing the pharmaceutical history of devil’s claw, however, these topics appear out of scope and will be reviewed in a separate publication. Figure 2 illustrates the selection process.
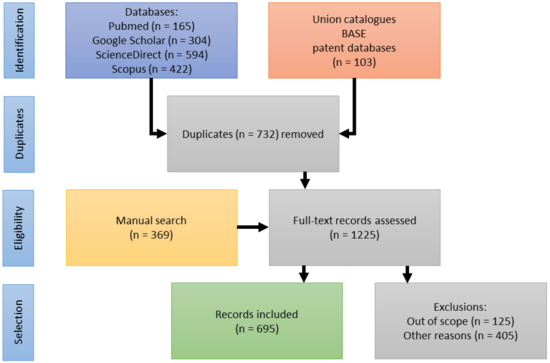
Figure 2.
Flow diagram of the reference identification, screening and inclusion.
3. Nomenclature
3.1. Taxonomy
The genus Harpagophytum was first described as Uncaria Burch. by Burchell in his Travels in the interior of southern Africa (1822) [81]. However, he was apparently unaware that Uncaria had already been used by Schreber for a genus in the Rubiaceae in 1789. Purportedly, de Candolle first noted this oversight, leading Meisner to describe the species as Harpagophytum procumbens DC [82]. However, de Candolle’s section of the Prodomus was only published in 1845 [83], making Meisner the author of the genus and creating the following complete citation as:
Harpagophytum DC. ex Meisner, PI. Vas. Gen. 1: 298 and 2: 206 (1840), syn.: Uncaria Burch., Trav. Int. S. Afr. 1:536 (1822), nom. illegit., non Schreb. 1789; type specimen: Harpagophytum procumbens (Burch.) DC. ex Meisner, PI. Vas. Gen. 2:206 (1840); basionym: Uncaria procumbens Burch., Trav. Int. S. Afr. 1: 536 (1822).
Decaisne, in his review of the Pedalineae, attributed four distinct species to the genus: H. procumbens DC., H. burchellii Decne. (= H. procumbens), and for the first time, H. zeyheri and H. leptocarpum [Uncaria leptocarpa (Decne.) Ihlenf. & Straka] [84]. The genus was last reviewed by Ihlenfeldt and Hartmann (1970) [1], who differentiated two species and five subspecies primarily based on the shape of the fruit correlated with the number of seeds. They also provide the most recent botanical descriptions for all subspecies.
Harpagophytum procumbens (Burch.) DC. ex Meisn.:
- H. procumbens (Burch.) DC. ex Meisn. ssp. procumbens—(1).
- H. procumbens (Burch.) DC. ex Meisn. ssp. transvaalense Ihlenf. & H. Hartm.—(2).
Harpagophytum zeyheri Decne.:
- H. zeyheri Decne. ssp. zeyheri—(3).
- H. zeyheri Decne. ssp. schijffii Ihlenf. & H. Hartm.—(4).
- H. zeyheri Decne. ssp. sublobatum (Engler) Ihlenf. & H. Hartm.—(5).
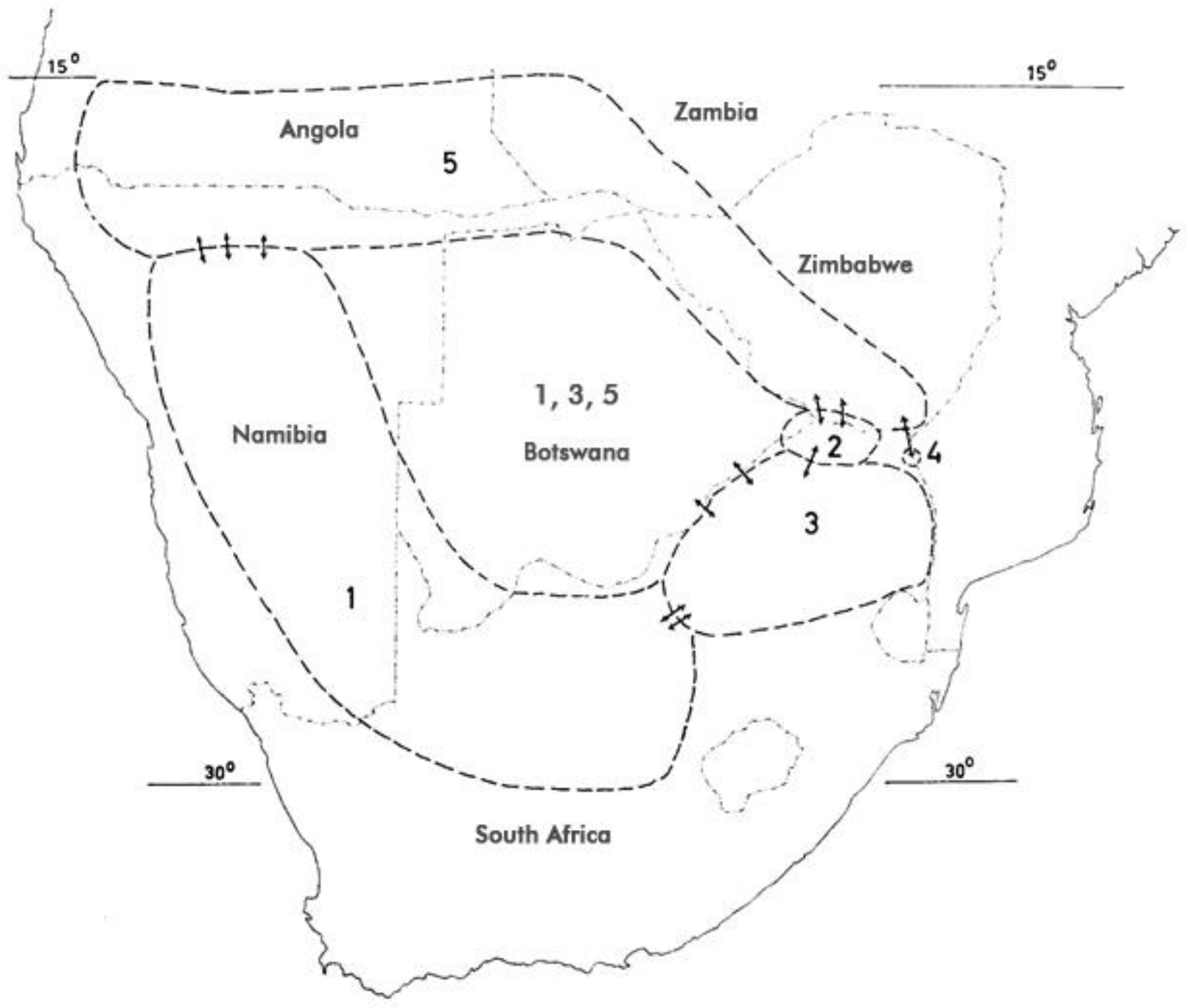
The numbers in parentheses represent the respective species in Figure 3 below.
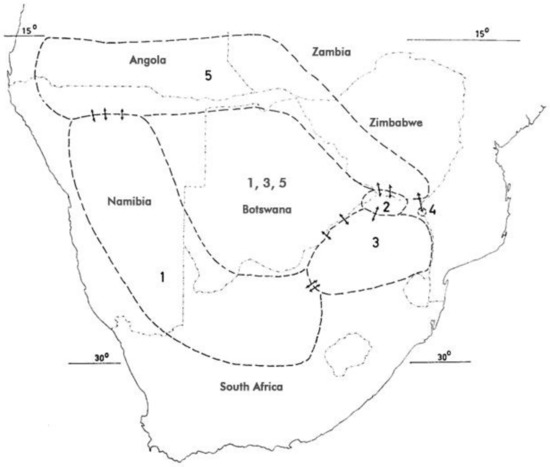
Figure 3.
Distribution of H. procumbens and H. zeyheri (after [1,64,91]). For numerical attribution of species, see Section 3.1. Arrows indicate introgression.
Synonymy:
- H. burchellii Decne. = H. procumbens ssp. procumbens DC. ex Meisn.
- H. zeyheri f. sublobatum Engl. = H. zeyheri ssp. sublobatum (Engl.) Ihlenf. & H. Hartm.
- H. procumbens var. sublobatum (Engl.) Stapf = H. zeyheri ssp. sublobatum (Engl.) Ihlenf. & H. Hartm.
- H. peglerae Stapf = H. zeyheri ssp. zeyheri Decne.
Interspecific introgression has been described [85] and shown to be reflected in morphometric measurements, and DNA profiles. Both species and all their putative hybrids also showed geographical variation in biochemical composition [2,85,86,87,88,89,90].
3.2. Vernacular Names
Teufelskralle, Trampelkette (Ger.); devil’s claw, grapple plant (Eng.); garra-do-diabo (Port.); garra del diablo (Esp.); artiglio del diavolo (It.); griffe du diable (Fr.); sengaparile (Tswana), duiwelsklou, kloudoring, duiwelsdoring, sanddoring, beesdubbeltjie, wolspinnekop (Afr.); otjihangatene (Oshiherero);//khuripe//khams, gamagu (Nama/Damara); elyata, omalyata (Oshikwanyama); ekatata, makakata (Oshindonga/Kwangali); likakata (Gciriku/Shambyu); !ao!ao,//xsamsa-//oro,//xemta≠’eisa (Kung); ||am-si-||q’oa-ka (West !Xoon), malamatwa (Silozi) [92,93,94].
4. Distribution
In the context of species interchangeability in commerce, it is noteworthy that the long-time assumption that only H. procumbens occurs in Namibia was disproved as early as the late 19th century. Ihlenfeldt discussed collections from the Etosha pan and later from the Kaokoveld and the Caprivi strip holding specimen of H. zeyheri [95]. Baum (1903) reported H. procumbens (Burch.) DC. var. sublobatum Engl. [= H. zeyheri Decne. ssp. sublobatum (Engler) Ihlenf. & H. Hartm.] from near lake Camelungo in southern Angola [96]. Cultivation has been experimented with in northern South Africa and, more recently, in Namibia, however, it has thus far neither proven very successful nor commercially viable [97,98,99].
5. Ethnobotany
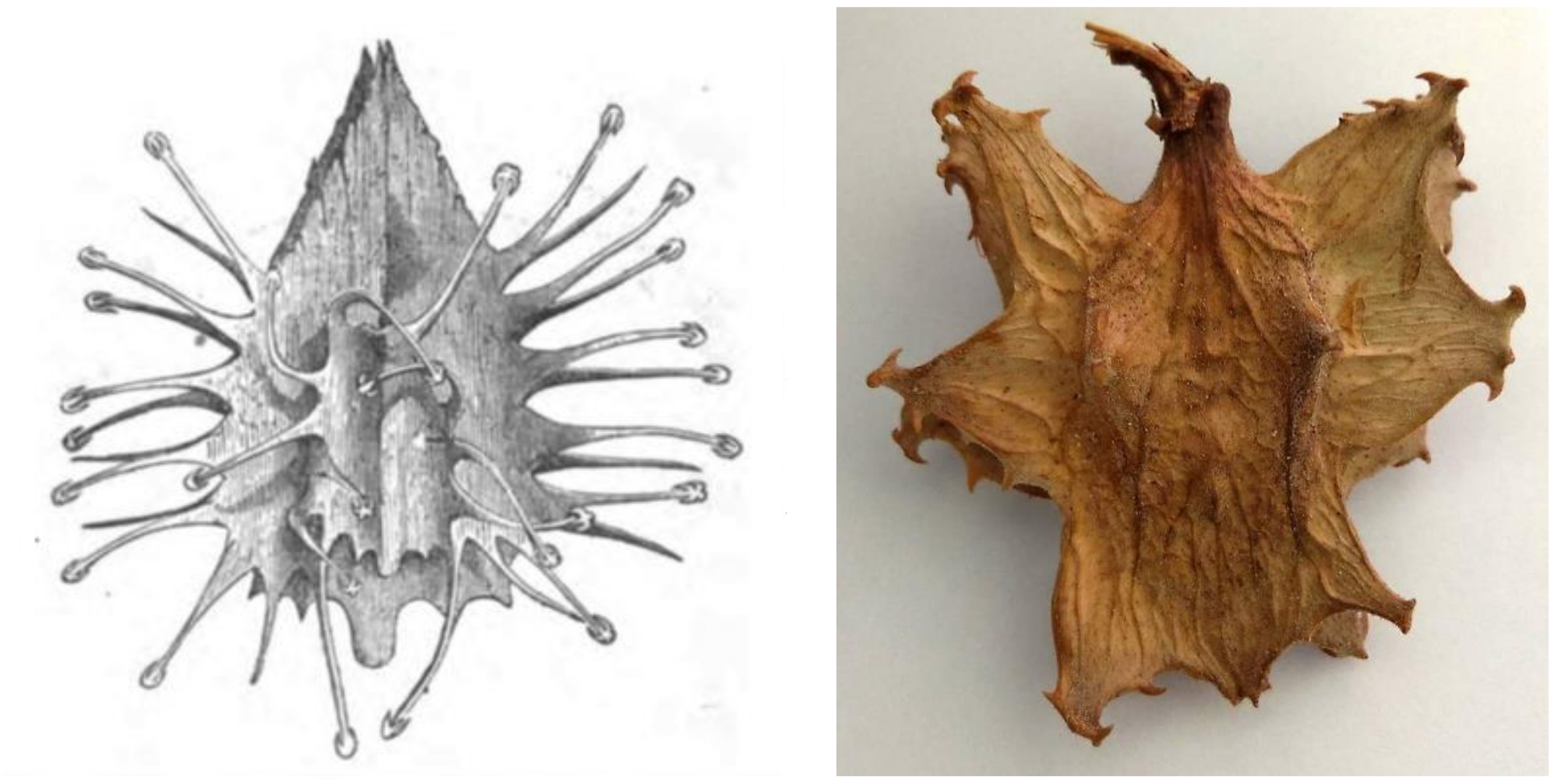
Interestingly, there are no records for indigenous use of devil’s claw until the beginning of the 20th century. Two accounts from the 19th century by Wood [100] and Cooke [101] (Figure 4) were the only ones that could be found making reference to devil’s claw (as grapple plant—Uncaria procumbens) but focus on its “devilish nature”: “The reader may easily imagine the horrors of a bush which is beset with such weapons. No one who wears clothes has a chance of escape from them. If only one hooked thorn catches but his coat-sleeve, be is a prisoner at once. […] If the reader would like to form an idea of the power of these thorns, he can do so by thrusting his arm into the middle of a thick rose-bush, and mentally multiplying the number of thorns by a hundred, and their size by fifty” [100].
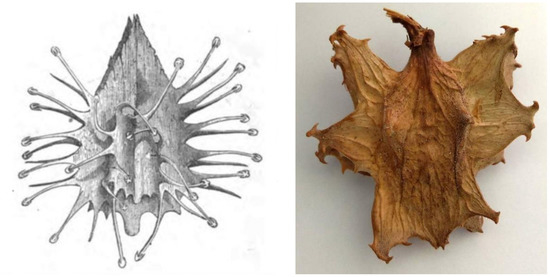
Figure 4.
Fruit of the “Grapnel” (note the misspelling!) plant from [101] vs. an actual fruit (photograph by the author).
Lübbert, in 1901, provided the first unambiguous account for the use of “Kuri-Khamiknollen” (= tubers of //khuripe//khams = Harpagophytum) in wound healing [102]. In 1907, Hellwig, medical officer of the imperial protection forces in German South-West Africa (Namibia), compiled a report on medicinal plant uses of the indigenous population, including an account of the Herero Samuel Kariko of the use of “otjihangatene” (=Harpagophytum) to treat cough, diarrhea, constipation, and venereal diseases [103]. Dinter, in 1909 and 1912 [104,105], utilized this report for his account of local food plants, but unfortunately omitted to include medicinal uses because he considered them unverified [106]. The fact that Hellwig provided an explicit source renders the colorful story of how the use of devil’s claw was “discovered” by Mehnert implausible and more likely part of a marketing strategy (see below) [107].
Later accounts corroborated these early records of traditional use of devil’s claw tubers primarily in the form of infusions and decoctions for digestive purposes, midwifery, pain relief, fever, diabetes, as a general tonic, for infectious diseases, and the dry powder topically as a wound dressing [40,92,108,109,110,111,112]. Ethnoveterinary uses in poultry have also been reported from Botswana [113]. It must be noted, however, that none of the early records clearly differentiate between species. It can only be speculated based on the origin of the records that Nama/Herero may have referred to H. procumbens, whereas reports from Botswana would concern mostly H. zeyheri.
6. Economy
6.1. History of Commercialization
The story around how a soldier of the Kaiserliche Schutztruppe (German “imperial protection forces”) and later a farmer in Mariental (Namibia) Gottreich Hubertus Mehnert came across devil’s claw is firmly anchored in the scientific literature. Sometime during the so-called Hottentot uprising from 1904 to 1908 (in fact, a brutal war and genocide of the German troops primarily against the Herero and Nama tribes, which has most recently been recognized by the German government [114]), after observing a local healer successfully improving the condition of a gravely wounded local, he questioned the healer about the magic remedy, but the healer refused to disclose the place from where he had collected it. Purportedly, access to one of the most successful botanical drugs of modern times can be attributed to Mehnert’s pointer dog [107].
This version, however, must be relegated to the world of “romance” and seen as part of an elaborate marketing campaign—it is repeated in many slightly altered versions by multiple authors. Mehnert doubtlessly experimented with the root and found it effective in a variety of ailments, but the discovery of its medicinal powers ought to be attributed first and foremost to the native tribes and secondarily to Lübbert and Hellwig (see above) with whom they shared their knowledge. It was sheer luck that nobody else developed an interest, allowing Mehnert to consolidate his “research” and to commence commercialization. He eventually shared it, while being interned at camp Andalusia during the 2nd World War, with another “collateral” prisoner, German scientist O. H. Volk, who had visited German South-West Africa at the wrong time [115]. In the camp’s botanical society, knowledge was freely exchanged, which allowed Volk to return home to Germany with likely an entire laundry list of interesting plants. The introduction of devil’s claw (and probably also rooibos) to Germany can be attributed to him [58]. He shared his knowledge with Zorn who conducted some initial pharmacological research [116] and then initiated himself a flurry of investigations elucidating devil’s claw’s basic chemistry [117,118,119,120,121,122,123,124,125,126,127,128]. Meanwhile, in the early 1950s, Mehnert trademarked “Harpago” and started exporting to Germany. Erwin Hagen trademarked “Harpago” in Germany in the early 1960s and began to market it as an infusion and later in homoeopathic preparations [129] (Figure 5). “Harpagosan” tea was registered as a botanical drug in Germany in 1977 [130].

Figure 5.
Advertisement Fa. Hagen (early 1970s).
What follows is a story of extensive biochemical, pharmacological, toxicological, and clinical investigation, and the development of multiple standardized pharmaceuticals, initially in Germany (the German drug information system AMIce alone lists a total of 434 products, most of which, however, are no longer active, see, e.g., [131]), and since the 1980s, also in France and elsewhere [132]. Demand quickly started to grow exponentially, and concerns were raised over the sustainability of harvesting practices [133,134,135]. In response to unsustainable harvesting and poor processing practices, the Namibian Devil’s Claw Exporter’s Association Trust became part of a Good Agricultural and Collection Practice (GACP) project in which it intends to ensure that Namibian devil’s claw is sustainably harvested and processed according to GACP guidelines.
6.2. Trade
Market demands impact livelihoods and policymakers alike. Trends indicate the health of an industry and inform resource assessments as well as regulatory interventions. With the following breakdown of trade and export data, I intend to address a controversy around species interchangeability, namely how the ingredient is regulated in the finished product markets. Hagen and others created a demand which local suppliers struggled to meet [133,134,135]. Sustainable collection and harvesting practices and governmental oversight were largely absent until ~1975. When originally only H. procumbens had been collected, driven by the economic boom, the collection and admixture of H. zeyheri commenced as early as the 1970s [11,12,13,14]. Furthermore, albeit on a much smaller scale than Namibia, both South Africa and Botswana [136,137,138] began to participate in the export market, also adding H. zeyheri into the supply chain (for distribution see above). Nott [14] and Taylor and Moss [138] broke down data specific to importing countries and explicitly listed importers, respectively. It is therefore safe to state that all importing markets have received either both species or mixtures thereof as early as the late 1970s. European regulators acknowledged the commercial reality by adding H. zeyheri to pharmacopeial monographs (see Section 6), while the US, for instance, remained oblivious to this practice, which stirred a controversy over the regulatory compliance and legitimacy of products containing H. zeyheri in 2015 [139]. The following overview of export volumes (Figure 6) is compiled from multiple sources [10,14,18,136,137,138,140,141,142,143,144,145,146,147,148,149,150,151] and further informed by the Namibian Ministry of Environment and Tourism (MET). The MET stopped sharing its data—based on export permits—with the public in 2015. According to one of the most prominent Namibian exporters of devil’s claw, the years 2015–2020 saw a slight increase in demand, peaking in 2019 at around 1000 metric tons, otherwise averaging around 700 metric tons annually. Materials in trade (both species) fall into four categories: conventional (lowest) quality makes up about 80% of the trade volume, GACP quality currently contributes about 10–15% to the total, though efforts are underway to dramatically increase this proportion, certified organic quality adds organic certification to GACP-compliant material and makes up about 5–10% of the total trade volume, and finally, organic and Fair for Life certified material (H. procumbens only) contributes ~1% to the trade total. Prices per kg (for full container loads, cost and freight) range from €4.00 (H. zeyheri) and €6.70 (H. procumbens) for conventional quality, via €5.40 (H. zeyheri) and €8.20 (H. procumbens) for GACP quality, and €7.20 (H. zeyheri) and €8.50 (H. procumbens) for organic quality, to €9.00 for Fair for Life certified material (pers. comm. G. Diekmann, EcoSo Dynamics cc, Namibia). While these prices and volumes make this a sizeable industry, it must be noted that most of the value is of course added during the manufacture of pharmaceuticals in the target markets. It is also noteworthy that over all this time, Namibian exports may have been bolstered by (illegal) imports from Angola and Zambia, for which—naturally—no records exist [152].
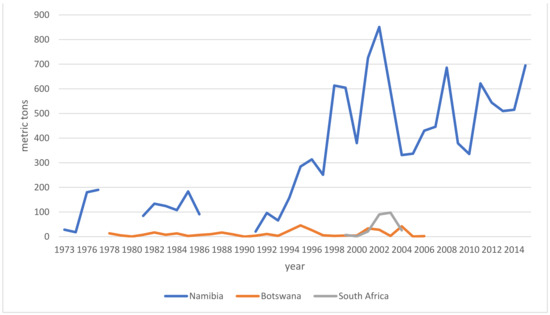
Figure 6.
Devil’s claw exports by country—gaps reflect years in which no data was reported.
7. Representation in Pharmacopeias and Authoritative Compendia
Given its presence in the European marketplace since the 1950s and in the US at least since the late 1970s, pharmacopeial standards for devil’s claw were set surprisingly late, likely due to suitable analytical methods not being available. While a qualitative assessment for the bitterness value according to the German Pharmacopoeia 7 (DAB 7) was suggested as early as 1977 [11], no specific monograph for devil’s claw was included in DAB until 1993, which, in fact, required testing for harpagoside content (see Table 1 below). The first monograph in Europe appeared in the British Herbal Pharmacopoeia in 1981. Devil’s claw first appeared in the European Pharmacopoeia in 1995, H. zeyheri, however, was not included as an allowable source species until 2003. The US Pharmacopeia, on the other hand, does not have a monograph for devil’s claw other than a draft proposal published in the Herbal Medicines Compendium in 2013 [153].

Table 1.
Representation of devil’s claw in pharmacopeias and authoritative compendia.
8. Biochemistry
After Volk’s return to Germany (see Section 6.1) and following Zorn’s first pharmacological study of devil’s claw in 1958 [116], the university of Würzburg (Germany) became a research hotspot for the elucidation of active and suitable marker compounds in devil’s claw for decades to come. The effort was largely concluded by the end of the 1980s and comparatively little has been added to this effort since. Table 2 lists all publications focused on the biochemical composition. For analytical methods and quality control, see Section 9.
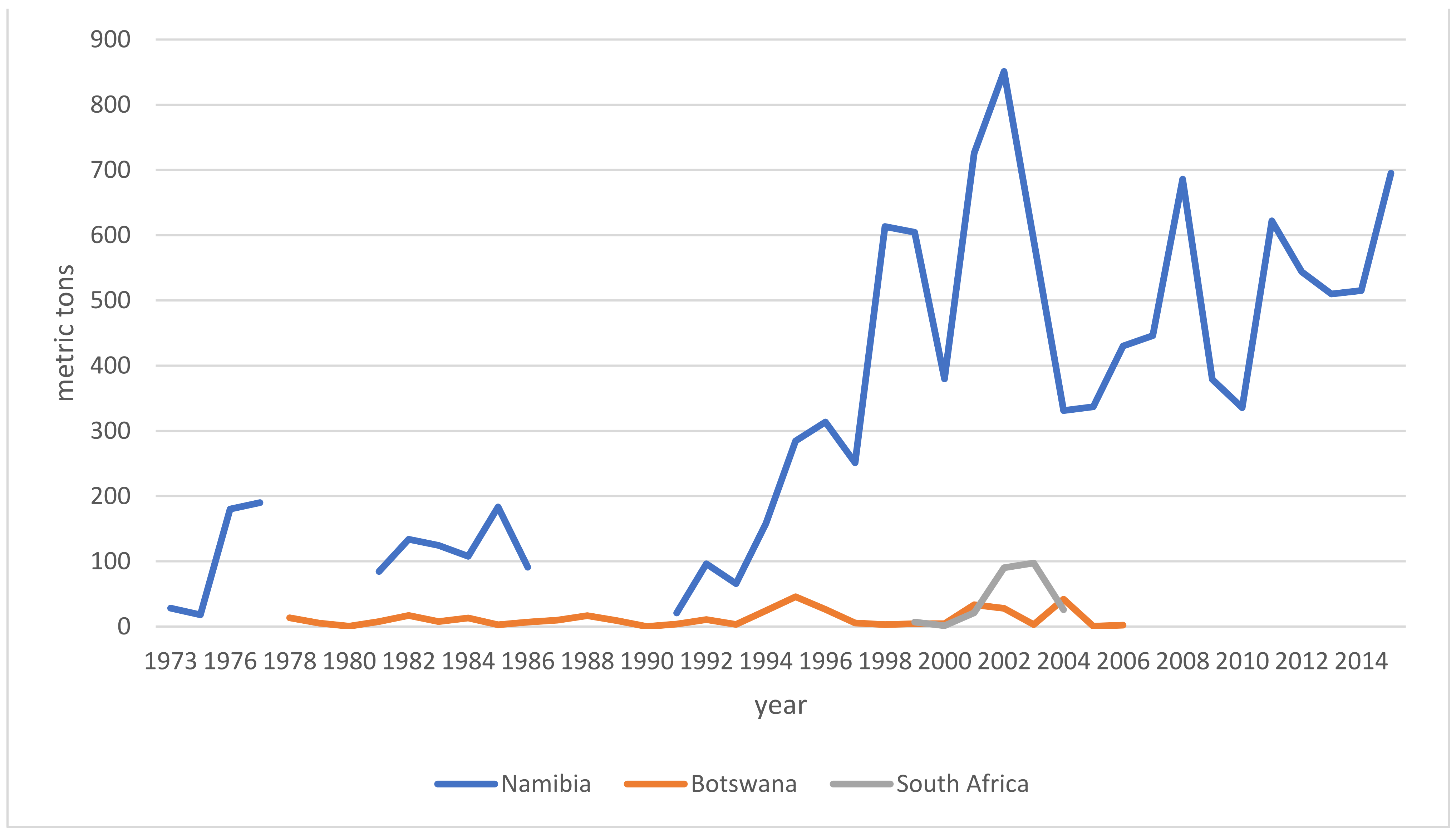
Iridoid-glycosides, primarily harpagoside, harpagide, and procumbide; phytosterols; phenylpropanoids such as verbascoside; triterpenes, such as oleanolic acid, 3β-acetyloleanolic acid, and ursolic acid; flavonoids, such as kaempferol and luteolin; unsaturated fatty acids, cinnamomic acid, chlorogenic acid, and stachyose were identified as the most prominent compounds present in the root. Figure 7 shows the chemical structures of the primary iridoid glucosides present in Harpagophytum root.
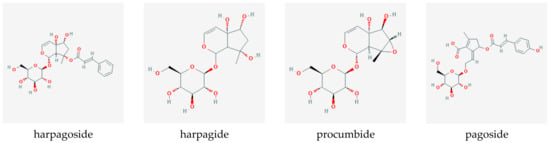
Figure 7.
Iridoid glucosides present in devil’s claw root (source PubChem).
Interestingly, the biosynthetic pathway for harpagoside is not yet well-elucidated. The first step resulting in geranyl diphosphate is still considered to be under debate [17], since while the principal steps are known, some intermediates remain hypothetical and dependent on the “chosen” pathway. Georgiev and colleagues [30] propose two different routes to the formation of geranyl diphosphate from the condensation of dimethylallyl diphosphate and isopentenyl diphosphate, the latter being supplied through either the mevalonate or the mevalonate-independent pathways. Geraniol is synthesized by geraniol diphosphate synthase and hydroxylated to form 8-hydroxygeraniol, followed by two oxidation steps and isomerization into 8-epi-iridodial. Carboxylation and glycosylation form its glycoside, which, in turn, is transformed into harpagide through decarboxylation and oxidations. Finally, harpagoside emerges as the product of cinnamoyl esterification at the 3-hydroxyl position.
Several studies have investigated differences in the quantitative composition of different Harpagophytum species, subspecies, and hybrids [19,181,182,183,184], and found the composition to be highly variable, depending on the material used, collection location, natural variation within the taxa, environmental influences, processing, and analytical methods. Content of the marker compound harpagoside is generally lower in H. zeyheri and has been found to be between 0%, 1%, and 4% in H. procumbens and between 0% and 3% in H. zeyheri. Verbascoside and isoverbascoside contents in H. procumbens varied between 0.2% and 0.4% and 0.2% and 1%, respectively. Pagoside content in H. procumbens varied between 0.06% and 0.16%. Hybrids showed the highest contents for most key compounds except harpagoside. 8-p-Coumaroylharpagide content in H. zeyheri varies between 0.7% and 1.4%, while being effectively absent in H. procumbens. The lower harpagoside content in H. zeyheri has in the past driven controversies over species equivalence in terms of clinical efficacy, however, this debate seems futile as a marker compound is not necessarily the (only) active one. Indeed, the pre-clinical research (outlined in Section 10) indicates that activities of multiple rather than single compounds may contribute to the overall effect.

Table 2.
Elucidation of the biochemical composition of devil’s claw root.
Table 2.
Elucidation of the biochemical composition of devil’s claw root.
| Topic | Year | Reference |
|---|---|---|
| Isolation and characterization of harpagoside | 1960 | [117] |
| Stachyose, raffinose, and a further glucoside in the aqueous phase | 1961 | [118] |
| Characterization of harpagoside | 1961 | [119] |
| Isolation and characterization of harpagoside and harpagide | 1962 | [120] |
| Characterization of harpagoside | 1962 | [121] |
| Characterization of harpagide | 1963 | [122] |
| Isolation of stachyose and a further glucoside | 1963 | [123] |
| Characterization of harpagoside | 1964 | [124] |
| Isolation of procumbide | 1964 | [125] |
| Structural characterization of harpagoside | 1966 | [126] |
| Characterization of procumbide and further constituents | 1967 | [127] |
| Characterization of procumbide | 1968 | [128] |
| Characterization of a chinone and other constituents | 1970 | [185] |
| Characterization of procumbide | 1971 | [186] |
| Further constituents | 1974 | [187] |
| Elucidation of triterpene esters | 1975 | [188] |
| Overview of known mono-, di-, and sesquiterpenoids with pharmacological activity | 1977 | [189] |
| Elucidation of a resin, an essential oil, and a mucilaginous fraction | 1978 | [190] |
| Structural characterization of procumbide | 1979 | [191] |
| Glucose, galactose, fructose, myo-inositol, sucrose, raffinose, and stachyose identified | 1979 | [192] |
| Preparation and structure of harpagogenine | 1981 | [193] |
| Carbohydrates and harpagoside in tissue cultures and roots of devil’s claw | 1982 | [194] |
| New iridoids: 8-O-(p-coumaryl)-harpagide and procumboside | 1983 | [195] |
| Novel iridoid and phenolic compounds | 1987 | [196] |
| Three pyridine monoterpene alkaloids from harpagoside and commercial extract | 1999 | [197] |
| Review of iridoids | 2000 | [198] |
| Review of composition (both species) | 2002 | [199] |
| Two diterpenes, (+)-8,11,13-totaratriene-12,13-diol and ferruginol | 2002 | [200] |
| New iridoid- and phenylethanoid glycosides | 2003 | [201] |
| Acetylated phenolic glycosides | 2003 | [202] |
| Pharmacological characterization of harpagoside | 2004 | [203] |
| Chinane-type tricyclic diterpenes and other minor compounds | 2006 | [204,205] |
| Review of iridoids and other compounds | 2006 | [206,207] |
| Review of chemical constituents | 2007 | [208] |
| Elucidation and characterization of compounds with specific pharmacologic profiles | 2008 | [209,210] |
| New triterpenoid glycoside, harproside, and new iridoid glycoside, pagide | 2010 | [211] |
| Kynurenic acid content | 2013 | [212] |
| New iridoid diglucoside | 2016 | [213] |
9. Analytical Methods and Quality Control
The quickly increasing popularity of devil’s claw products required an ongoing effort to develop and refine tools to identify and quantify devil’s claw in its raw, processed, and finished product states. Initially, the primary aims were identification and contaminants [214,215], later, standardization [11] and quality control [216,217], and finally identification and quantification methods to support pharmacological and clinical research. Early methods, however, did not account for species differentiation, i.e., simple pharmacy-proof methods of the 1970s would likely not have been able to differentiate between H. procumbens and H. zeyheri. In fact, methods and equipment refined enough to do so, regardless of the extent of processing, only became available in the 1990s. Analysis of retention samples retrospectively determined the presence of both species in commercial products. Table 3 provides a quick reference to publications of methods of quality control in chronological order. In current practice, the most commonly used methods for identification and assaying devil’s claw raw materials and products include TLC, HPLC, HTPLC, and LC/MS, for instance, the current edition of the European Pharmacopoeia employs microscopy and TLC for identification and LC for harpagoside quantification; more recently, chemometric modeling and hyperspectral imaging have emerged as promising methods for species differentiation.

Table 3.
Analytical methods and methods of quality control.
10. Processing, Products, Applications
The majority of data on processing and delivery systems is provided in the list of patents compiled in Section 14. EMA’s HMPC assessment report on H. procumbens and/or H. zeyheri, radix, provides an overview of extracts that are most commonly used in commercial products [167]:
- Liquid extract (1:1; 30% v/v ethanol)
- Soft extract (2.5–4.0:1; 70% v/v ethanol)
- Dry extract (1.5–2.5:1; water)
- Dry extract (5–10:1; water)
- Dry extract (2.6–4:1; 30% v/v ethanol)
- Dry extract (1.5–2.1:1; 40% v/v ethanol)
- Dry extract (3–5:1; 60% v/v ethanol)
- Dry extract (3–6:1; 80% v/v ethanol)
- Dry extract (6–12:1; 90% v/v ethanol)
- Tincture (1:5), extraction solvent ethanol 25% (v/v)
Figure 8 shows the processing from harvest to the raw material in commerce. Historically, teas [67,274], e.g., Harpagosan (see above), fluidextracts [42,67], spray-dried aqueous extract [26,67], homeopathic preparations for both oral (p.o.) and intraperitoneal (i.p.) application [26,27,67], and powder in capsules [26,67,93] were also common galenic forms. The European Pharmacopoeia stipulates a minimum of 1.2% of harpagoside in the raw material [169]. Dry extracts were standardized to contain a minimum of 1.5% m/m of harpagoside [167].

Figure 8.
Clockwise: H. procumbens, secondary tubers, drying of the sliced tubers, article of commerce (photographs by the author). The article of commerce shown here is conventional quality (see Section 6). Note the difference in color of the slices shown on the bottom right, which were harvested and processed in compliance with GACP.
More recently, Plaizier-Vercammen and Bruwier evaluated the impact of excipients on friability and hygroscopicity of direct compression of a spray-dried Harpagophytum extract [275]. Günther et al. analyzed the parameters affecting supercritical fluid extraction with CO2 of harpagoside [276]. Performance of a topical preparation with devil’s claw extract on acrylic acid polymers base compared to ketoprofen was assessed by Piechota-Urbanska and colleagues [277]. Both formulations demonstrated rheological stability and high pharmaceutical availability. Almajdoub described a freeze-dried aqueous extract of H. procumbens encapsulated in lipid vesicles by using a dry film hydration technique with and without further alginate coating for optimal (delayed) release and small intestine absorption [278]. Development of a gastro-resistant coated tablet prepared from a standardized hydroethanolic root extract for the purpose of more effective delivery and consequent dose reduction was reported by Lopes et al. [279].
11. Pre-Clinical Research
11.1. Pharmacology
Studies mainly investigated anti-inflammatory activities and were conducted with various extracts, extract fractions, or isolated compounds. Harpagophytum iridoid compounds are considered the primary actives, to which anti-inflammatory, antinociceptive, analgesic, antimicrobial, chemopreventive, hepatoprotective, neuroprotective, and immunomodulatory effects are commonly attributed [189,198,209,280,281]. As cyclooxygenase (COX)-1/2 inhibitors have emerged as important targets for treating rheumatoid arthritis, the influence on the arachidonic acid pathway has been a research focus. The most commonly used methods for measuring peripheral analgesic activity were the various forms of the writhing tests, hot-plate test, and the Randall–Selitto test in rats and mice. To demonstrate anti-inflammatory effects, different animal models of inflammation were commonly used, e.g., the carrageenan-induced mouse/rat paw edema, the 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced mouse edema, the granuloma pouch test, zymosan-induced arthritis, albumin-induced rat paw edema, adjuvant-induced arthritis in rats (M. tuberculosis; Freund adjuvant), and Adriamycin-induced rat paw edema. More advanced in vivo and a variety of in vitro and ex vivo models were developed and employed over time (see Table 4, Table 5, Table 6 and Table 7 below).

Table 4.
In Vitro experiments regarding analgesic/antinociceptive and anti-inflammatory effects of devil’s claw preparations and compounds.

Table 5.
In Vivo experiments regarding analgesic/antinociceptive and anti-inflammatory effects of devil’s claw preparations and compounds.

Table 6.
Ex vivo experiments regarding analgesic/antinociceptive and anti-inflammatory effects of devil’s claw preparations and compounds.

Table 7.
Mixed experiments regarding analgesic/antinociceptive and anti-inflammatory effects of devil’s claw preparations and compounds.
Investigated targets for anti-inflammatory effects and their respective IC50 (significant inhibitions, primary sources only) are summarized in Table 8.

Table 8.
Anti-inflammatory targets of Harpagophytum preparations and compounds.
Table 9 summarizes the results of pre-clinical experiments which studied other effects of Harpagophytum and its compounds.

Table 9.
Experiments regarding other effects of devil’s claw preparations and compounds.
Primary—anti-inflammatory, analgesic/antinociceptive, and antioxidant—effects have been demonstrated in multiple in vitro, in vivo, and ex vivo assays with crude extracts, fractions, and isolated compounds of Harpagophytum. However, experiments show some inconsistencies, likely caused by deviations in experimental models and insufficient characterization of the purportedly active compounds, as well as variation in solvent systems [394,395,396]. Further, the consolidated data show that efficacy cannot be clearly attributed to any one of the compounds present in Harpagophytum. Focus on harpagoside—albeit serving as a convenient marker—cannot be substantiated in an efficacy context. On the other hand, the presence and effect of verbascoside in Harpagophytum, a compound with well-documented anti-inflammatory properties, has not been adequately studied.
11.2. Pharmacokinetics
Most of the available pharmacokinetics data were created as a byproduct or in the context of pharmacological experiments with Harpagophytum preparations or its compounds. Vanhaelen [52] experimented with harpagoside and harpagide under conditions mimicking those found in the stomach and concluded by suggesting enteric-coated preparations for harpagoside to slow down acid hydrolysis. Chrubasik [217] investigated release and stability of harpagoside in gastric and intestinal fluids and stability for 3 and 6 h, respectively. The author also found harpagoside to be of low bioavailability, a daily dose of 100 mg could not be detected in serum or urine. Chrubasik et al. (2000) [238] established an octanol–water distribution coefficient of approximately 4 that is not dependent on pH or temperature.
Neither Harpagophytum ethanolic extract nor harpagoside had a relevant effect on cytochrome P (CYP) 450 3A4 in vitro [397]. An investigation of different Harpagophytum extracts elucidated maximum levels of plasma harpagoside after 1.3 to 2.5 h and suggested a correlation between serum harpagoside levels and inhibition of leukotriene biosynthesis in vitro and ex vivo [285,398]. In human liver microsomes and subtype-specific CYP substrates, Harpagophytum at a dose derived from [157] activated CYP 2E1 and inhibited CYP 2C19 [399]. Inhibition of CYP 450 was shown for methanolic extracts of H. procumbens, and while inhibition of CYP 1A2 and 2D6 was relatively low, moderate inhibition of CYP 2C8/9/19 and 3A4 was noted (IC50 between 100 and 350 μg/mL) [400]. However, the impact on drugs metabolized via those enzymes is merely theoretical. Romiti et al. [401] found Harpagophytum to interact with the multidrug transporter ABCB1/P-glycoprotein, unrelated to relative harpagoside content. Modarai et al. [402] found Harpagophytum preparations, but not harpagoside or harpagide, to weakly inhibit CYP 3A4, but deemed clinical relevance unlikely.
11.3. Toxicology
Acute and chronic toxicity have been investigated for the herbal substance, its preparations, and compounds isolated from Harpagophytum. Multiple publications cite an unpublished experiment by Albus (1958) in which an LD50 in mice was established for a liquid extract (not specified) at 34 mL/kg i.v. and 220 mL/kg p.o. [22,42,51,120]. An LD50 in rats was given at 10 g/kg for a spray extract and in mice at 1 g/kg for harpagoside [403]. Vollmann [379] established an LD50 of 23 and 10 mL/kg for an infusion and a chloroform/butanolic extract (4:1), respectively. Möse [404], in an unpublished report (cited in [27,44,67,405]) conducted toxicity tests with a Harpagophytum infusion in primate and chicken tissue cultures, and no effect on cell development was found, nor did the infusion promote growth of Ehrlich ascites carcinoma in mice. Eichler and Koch referenced toxicity at above 0.5 g/kg without citation [305]. Erdös and colleagues [308] demonstrated Harpagophytum aqueous, methanolic, and butanolic extracts to be effectively non-toxic (LD0 at 4640 mg/kg p.o. and >1000 mg/kg i.v.), and for harpagoside, a LD0 of 395 mg/kg and a LD50 of 511 mg/kg. Marzin (1978, cited in [67]) confirmed these results. The same author investigated the toxicity of an extract (2.7% total iridoids), p.o. or i.p., in rats and mice. Administration p.o. was effectively non-toxic, while i.p., some toxicity was observed with a calculated LD50 of 10 g/kg (Marzin, 1981, cited in [274]). Vanhalen and colleagues tested toxicity of harpagoside and harpagide in mice and established an LD50 of 1 and 3.2 g/kg, respectively [224]. Schmidt [44] elaborated unpublished toxicological investigations with Harpagophytum D2 and Harpagosan (DER 2:1) [406,407], establishing an LD50 of 20 mL/kg and >30 mg/kg, respectively. Whitehouse et al. [341] established an LD50 at 13.5 g/kg p.o. for a Harpagophytum root extract (not specified) in mice and no toxicity at 7.5 g/kg over three weeks in rats, while 2 g/kg over one week showed no impact on liver parameters. Ibrahim et al. [408] conducted a battery of toxicity studies in mice (acute, sub-acute, and chronic) with a commercial product (Boiron, France—composition not declared) and found no clinically relevant changes in any of the tested outcomes, attributing a slight increase in liver enzymes to the anti-inflammatory effect. Al-Harbi and colleagues [409] found no oral acute toxicity in mice at 1 and 3 g/kg Harpagophytum powder. In a 90-day chronic toxicity study (test substance not characterized), no clinically relevant changes in tested parameters were established, except for a significant decrease in blood sugar and uric acid levels. Both chronic assessments, however, must be considered inadequate due to the insufficiently characterized test material. Allard et al. [410] discussed herb-induced nephrotoxicity, and in that context, called for further investigation of whether a theoretical impact of Harpagophytum on major renal transport processes is of clinical relevance. Joshi et al. [411] investigated the toxicology of a H. procumbens aqueous-ethanolic extract (1 g/kg/day, equivalent to 7.5–10× the human recommended dose) in male and female Sprague Dawley rats over 4 and 12 weeks. While no significant histopathological effects were found, the study yielded significant—albeit not clinically relevant—sex-related differences in blood chemistry. All these results stand in stark contrast to those of Zorn [116], casting considerable doubt over the authenticity of the plant material used in his experiments.
Mahomed and Ojewole [412,413] conducted experiments in vitro suggesting spasmogenic and uterotonic actions for an H. procumbens aqueous extract (10–1000 µg/mL). Whether these results are of clinical relevance in vivo remains to be established (see Section 11.2). Pearson [414] studied the reproductive toxicity of a combination product containing Harpagophytum (exact composition not disclosed) for veterinary use in pregnant female Sprague Dawley rats and showed no signs of toxicity. The study, however, is poorly reported and of limited relevance given the unknown composition of the test substance. Contrarily, Davari and colleagues [415] reported teratogenic effects and histopathological changes in fetal tissues (but no significant structural malformations or abnormalities) from an experiment with H. procumbens (200, 400, 600 mg/kg) in pregnant Balb/C mice.
12. Clinical Research
12.1. Efficacy
The efficacy of devil’s claw has been investigated in more than 50 human studies, and case reports and observational studies are summarized in Table 10, while randomized, controlled trials (RCTs) are summarized in Table 11. Indications were primarily degenerative joint diseases as well as low back pain. Trials utilized a variety of methodological designs, with different preparations of devil’s claw and daily doses of harpagoside, varying from <30 to >100 mg. While harpagoside is considered to contribute to the overall activity of devil’s claw preparations, it is not yet fully understood which other compounds may also be of relevance. Furthermore, an investigation into the harpagoside content of commercially available devil’s claw preparations revealed substantial variation, with contents often below the recommended daily dose of 4.5–9 g crude drug (equivalent >50 mg harpagoside) [173,232,233,234,237,416].
Trials have been reviewed systematically with regards to their quality and results concerning safety and efficacy of Harpagophytum preparations in publications between 1973 and 2019 [17,23,139,156,417,418,419,420,421,422,423,424,425,426,427,428,429,430,431,432,433]. Another set of reviews considered the efficacy of devil’s claw preparations or its active compounds in specific need states [23,130,434,435,436,437,438,439,440,441,442,443,444,445,446,447,448,449,450,451,452,453,454,455,456,457,458,459,460,461,462,463,464,465,466,467,468,469,470,471,472,473,474,475,476,477,478,479,480,481,482,483,484,485,486,487,488]. All trials observed improvement of the outcome criteria under treatment (some significant), however, significant superiority of the Harpagophytum preparations vs. conventional NSAIDs was not reported. This is partly because most trials were observational and/or comparative, while the outcomes of placebo-controlled trials were often inconclusive or overshadowed by methodological deficiencies. Many trials allowed for conventional emergency or co-medication, which further limits the value of the data collected. Despite some studies providing evidence for the effectiveness of certain preparations, the overall quality of evidence is not sufficient. Furthermore, the relevance of early studies with homeopathic dilutions—while included here for completeness’ sake—is limited from a perspective of rational phytotherapy.

Table 10.
Case reports and observational studies conducted between 1971 and 2021.
Table 10.
Case reports and observational studies conducted between 1971 and 2021.
| Indication | Trial Type, Size | Results | Year | Reference |
|---|---|---|---|---|
| Chemosis | CR 1 | Initial treatment with multiple preparations that did not lead to improvement, then with 300 mg Harpagophytum extract (not specified) 3 times daily, orally, for 6 months, leading to drastic improvement. | 1983 | Belaiche [489] |
| Familial Mediterranean fever | CR 17 | Harpagophytum extracts characterized as aqueous (DER 1:2.4, 2.5% harpagoside)—this characterization may also apply to previous trials by Belaiche and Dahout (see above)—6–9 g single dose, duration not provided; significantly decreased recurrence in 80% of patients. | 1983 | Belaiche [490] |
| Cancer | CR 2 | Tumor regression after taking Harpagophytum extract (500 mg daily) and/or Essiac respectively, without cytotoxic therapy. | 2009 | Wilson [491] |
| DJD | O ~120 | Harpagophytum D4–D6, IA, and D1 orally; 1–6 months; substantial improvement of symptoms in most cases. | 1971 | Beham [492] |
| CP | O 60 | Harpagophytum D2, IA, plus tea (2–3 tsp per 1 L water) or 3 × 2 tablets orally, duration not provided; dose-dependent response; 60% substantial improvement of symptoms, 20% improvement, 20% no change. | 1972 | Schmidt [43] |
| CP, DJD | O 146 | Harpagophytum D2, IA, duration not provided; improvement in 134 patients. | 1972 | Zimmermann, cited in [130] |
| DJD | O 25 | Harpagophytum D2–D3, IA, and SC, 1–2 mL, pain-free after 6 injections, or tea (1 tsp per 300 mL) daily for 3–6 weeks. | 1972 | Brantner [493] |
| DJD | O 70 | Harpagophytum D2, IA, some + tea, some + indometacin, duration not provided; improvement in 90% of patients. | 1976 | Wilhelmer, cited in [44] |
| CP, DJD | O 21+ | Harpagophytum D1–D3, IA, SC, and i.v., tea, orally, duration not provided; significant improvement in 30% of patients. | 1977 | Zimmermann [494] |
| DJD | O 84 | 250 or 500 mg Harpagophytum extract (not specified) 3 times daily orally for 2–6 months, improvement in 72% of patients. | 1979 | Dahout, cited in [495] |
| CP, DJD | O 600 | Harpagosan tea (2 tea bags in 500 mL water daily) plus D2 SC for up to 6 months. Symptoms disappeared in 200 patients; 400 patients improved after having received additional conventional medication for the first 3–4 weeks. | 1983 | Warning cited in Schmidt [44] |
| Rheumatoid arthritis | O 1 | Improvement after treatment with low-potency Harpagophytum i.v. and orally, duration not provided. | 1987 | Stübler [496,497] |
| DJD | O 553 | Patients treated with 2–6 capsules of 400 mg Harpagophytum extract (1.5–2.5:1) for 8 to 180 days. Outcomes confirmed RCT results in terms of efficacy and safety. | 2000 | Müller et al. [498] |
| DJD | O 255 | Post-marketing surveillance study of biopsychosocial determinants and treatment response. Patients treated with Harpagophytum extract (60 mg harpagoside/day) for 2 months. Outcome parameters were significantly worse in non-responders. | 2009 | Thanner et al. [499] |
| CP, DJD, dyspepsia, hypercholesterolemia, detoxication | O, CR 700+ | Harpagophytum tea, up to 12 weeks, D2, SC, 20 injections, further improvement with additional D2 i.v. and tea. | 1978 | Schmidt [130] |
| Diabetes mellitus with lipometabolic disorder | OT 10 | 4 patients 3 weeks, 6 patients 4 and 3 weeks, over a total of 6 months; Harpagophytum tea, amount not specified; cholesterol, lipid, and blood sugar levels normalized. | 1974 | Hoppe [500] |
| Hypercholesterolemia and hyperuricemia | OT 100 | Harpagophytum tea, 2 tea bags per ½ L water, 3× daily before meals 1/3 of the tea; 20–21 days; lowered cholesterol levels in 80%, normal levels in 45%, 66% improvement in hyperuricemia. | 1978 | Grünewald [405] |
| DJD | OT 13 | Harpagophytum extract (<30 mg harpagoside/day), for 6 weeks, followed up for another six weeks; no overall statistically significant improvements in the conditions. | 1981 | Grahame and Robinson [501] |
| DJD | OT 630 | 42% to 85% of the patients (depending on grouping) showed improvements after 6 months with Harpagophytum extract (>90 mg harpagoside/day). | 1982 | Belaiche [502] |
| DJD | OT 38 | Comparison of Formica rufa D6 with Harpagophytum D4, for 3 months; improvement in pain severity and mobility with both, Formica rufa slightly superior. | 1991 | Kröner [503] |
| Effect on eicosanoid biosynthesis | OT 34 (25/8) healthy volunteers | Harpagophytum, 4 capsules (500 mg powder, 3% of total glucoiridoids) daily for 21 days. No effect vs. control. | 1992 | Moussard et al. [504] |
| MSD | OT 102 (51,51) | Patients treated with Harpagophytum extract (30 mg harpagoside/day) or conventional therapy (mainly oral NSAIDs). Number of pain-free patients and changes in Arhus scores after 4 and 6 weeks of treatment was comparable between the groups. | 1997 | Chrubasik et al. [505] |
| DJD | OT 43 | Harpagophytum powder 3 g daily for 60 days. Reduction of pain intensity in 89%, increased mobility in 83%. | 1997 | Pinget and Lecomte [506] |
| MSD | OT 2053 | Patients treated with Harpagophytum extract (30 mg harpagoside/day) for 6 weeks. Symptoms improved over time. | 1999 | Schwarz et al. [507] |
| DJD | OT 45 | Patients treated with Harpagophytum extract (30 mg harpagoside/day) for two weeks plus NSAID treatment, and devil’s claw alone, for four weeks. No worsening of scores was observed during treatment with devil’s claw alone. | 2000 | Szczepanski et al. [508] |
| MSD | OT 1026 | Patients treated with Harpagophytum extract (30 mg harpagoside/day) for 6 weeks. Symptoms improved. | 2000 | Usbeck [509,510] |
| MSD | OT 130 | Patients treated with Harpagophytum extract (~30 mg harpagoside/day) for 8 weeks. Arhus back pain index decreased significantly during treatment. Other measures also improved significantly. | 2001 | Laudahn et al. [511,512,513] |
| DJD | OT 583 | Patients treated with Harpagophytum extract (~30 mg harpagoside/day) for 8 weeks. Symptoms improved and the dose of co-medication (NSAIDs) could be reduced. | 2001 | Schendel [514] |
| DJD | OT 675 | Patients treated with Harpagophytum extract (~30 mg harpagoside/day) for 8 weeks. Efficacy rated good or very good in 82% of cases. The symptom scores decreased, and co-medication was successfully reduced or even discontinued. | 2001 | Ribbat and Schakau [515] |
| MSD | OT 250 | Patients treated with Harpagophytum extract (60 mg harpagoside/day) for 8 weeks. Both generic and disease-specific outcome measures improved. | 2002 | Chrubasik et al. [516] |
| DJD | OT 614 | Patients treated with Harpagophytum extract (480 mg twice daily) for 8 weeks. Symptoms improved in the majority of patients; treatment was well-tolerated. | 2003 | Kloker and Flammersfeld [517,518] |
| DJD | OT 75 | Patients treated with Harpagophytum extract (50 mg harpagoside/day) for 12 weeks. WOMAC index and 10 cm VAS pain scale improved notably. | 2003 | Wegener and Lüpke [519,520] |
| MSD | OT 99 | Patients treated with Harpagophytum extract (~30 mg harpagoside/day) for 6 weeks. Symptoms improved. | 2005 | Rütten and Kuhn [521] |
| MSD | OT 102 (29/22/51) | Patients treated with Harpagophytum extract (~30 mg harpagoside/day) and/or conventional therapy for 6 weeks. Efficacy was found in all groups, advantages for devil’s claw were not statistically significant. | 2005 | Schmidt et al. [522,523] |
| DJD | OT 65 | Patients treated with combination of Harpagophytum procumbens, Zingiber officinale, and Urtica sp. (ratio not disclosed) for 8 weeks. Improvements in all efficacy parameters were observed. | 2005 | Sohail et al. [524] |
| Endometriosis | OT 6, 12 | Patients treated with Harpagophytum extract (1600 mg daily) for 12 weeks. Reduction of symptoms in 4 (6) patients after 4 weeks, in all patients after 12 weeks. | 2005, 2006 | Arndt et al. [525,526] |
| DJD | OT 259 | Patients treated with Harpagophytum extract (1.5–3:1, 960 mg daily) and NSAIDs for 8 weeks. At the end of the treatment, 44.8% could decrease NSAID dosage. All parameters improved significantly. | 2006 | Suter et al. [527,528] |
| MSD | OT 114 | Patients treated with Harpagophytum extract (60 mg harpagoside/day) for up to 54 weeks. Most outcome scores improved significantly over time. | 2007 | Chrubasik et al. [529] |
| DJD | OT 42 | Patients treated with combination of Harpagophytum (1800 mg), Curcuma longa (1200 mg), and bromelain (900 mg) daily, plus conventional therapies for 2 weeks. Clinically relevant improvement of joint pain scores in all patients. | 2014 | Conrozier et al. [530] |
| DJD | OT 20 | Patients treated with combination of 500 mg glucosamine sulfate, 400 mg chondroitin sulfate, 10 mg collagen type II, and 40 mg Harpagophytum per day for 12 months. Femoral hyaline cartilage thickness significantly improved and radiographic progression of knee osteoarthritis delayed. | 2019 | Vreju et al. [531] |
| MSD | OT 39/40/16 | Otherwise healthy subjects with mild/moderate neck/shoulder pain related to sport; cream containing a combination of ingredients, including H. procumbens root extract + standard treatment, standard treatment, diclofenac patch + standard treatment respectively, for 2 weeks; significant improvement in pain, stiffness, mobility, and working capacity, compared to non-cream groups. | 2021 | Hu et al. [532] |
CP = chronic polyarthritis; IA = intra-articular; SC = subcutaneous; DJD = degenerative joint diseases (osteoarthritis); MSD = musculo-skeletal disorders (low back pain); OT = observational trial; O = observation; CR = case report; NSAID = non-steroidal anti-inflammatory drug; WOMAC = Western Ontario and McMaster Universities.

Table 11.
RCTs conducted between 1980 and 2017.
Table 11.
RCTs conducted between 1980 and 2017.
| DJD | RCT 39 | 400 mg Harpagophytum extract (not specified), and 25 mg diclofenac, or placebo 3× daily for 6 months. Overall confirmation of anti-inflammatory effects without side effects. | ~1980 | Chaouat, cited in [66,67] |
| DJD | RCT 50 (25/25) | Harpagophytum extract (<30 mg harpagoside/day) and phenybutazone (300 mg per day for the first four days, then 200 mg) respectively, for 28 days. Devil’s claw found equally effective to phenybutazone. | 1980 | Schrüffler [533] |
| DJD | RCT 50 (25/25) | Patients treated with Harpagophytum extract (<20 mg harpagoside/day) or placebo for three weeks showed a significant decrease in pain severity vs. placebo. | 1984 | Guyader [534] |
| DJD | RCT 100 (50/50) | Patients treated with Harpagophytum extract (60 mg harpagoside/day) or placebo for 30 days. Only 6 patients in the verum group still experienced moderate pain vs. 32 in the placebo group. | 1990 | Pinget and Lecomte [535] |
| DJD | RCT 89 (45/44) | Patients treated with Harpagophytum extract (60 mg harpagoside/day) or placebo for two months. Significant decrease in severity of pain and significant increase in spinal and cofexomoral mobility vs. placebo. | 1992 | Lecomte and Costa [536] |
| MSD | RCT 118 (59,59) | Patients treated with Harpagophytum extract (50 mg harpagoside/day) or placebo for 4 weeks. Treatment group used less analgesics, had greater improvement in median Arhus scores (20% vs. 8%; p < 0.059), and had more patients pain-free at the end (9/51 vs. 1/54; p = 0.008). | 1996 | Chrubasik et al. [537,538,539] |
| MSD | RCT 109 (54/55) | Patients treated with Harpagophytum extract (50 mg harpagoside/day) or placebo for 4 weeks. Rescue medication: tramadol. Significant improvement in Arhus index and pain index, and co-medication reduced vs. placebo. | 1997 | Chrubasik et al. [540] |
| DJD | RCT 100 (50/50) | Patients treated with Harpagophytum extract (30 mg harpagoside/day) or placebo for 30 days. Favorable effects were evident after 10 days vs. placebo. | 1997 | Schmelz and Hämmerle [541] |
| MSD | RCT 197 (65/66/66) | Patients treated with Harpagophytum extract (50 mg (1), 100 mg (2) harpagoside/day) or placebo (3) for four weeks. 6, 10, and 3 patients were pain-free in groups 1, 2 and 3, respectively. Arhus index score decreased but not statistically significant. Dose-related effect not confirmed. | 1999 | Chrubasik et al. [542] |
| DJD | RCT 122 (62/60) | Patients treated with Harpagophytum extract (57 mg harpagoside/day) or diacerhein at 100 mg daily for four months. Results showed significant improvement in both groups at a similar rate. | 2000 | Chantre et al. [543,544] |
| MSD | RCT 63 (31/32) | Patients treated with Harpagophytum extract (~30 mg harpagoside/day) or placebo for 4 weeks. Significant efficacy for visual analogue scale, pressure algometer test, muscle stiffness test, and muscular ischemia test. No differences to placebo in anti-nociceptive muscular reflexes or electromyogram activity. | 2000 | Göbel et al. [512,513,545,546] |
| DJD | RCT 46 (24/22) | Patients treated with ibuprofen (800 mg) and Harpagophytum extract (~30 mg harpagoside/day) or placebo for 20 weeks. WOMAC scores decreased similarly, but during an ibuprofen-free period, symptoms worsened less than 20% for 71% of devil’s claw patients vs. 41% of placebo patients. | 2001 | Frerick et al. [547] |
| DJD | RCT 78 (39/39) | Patients treated with Harpagophytum extract (~30 mg harpagoside/day) or placebo for 20 weeks. Co-medication ibuprofen. Symptoms improved similarly for both groups. | 2002 | Biller [548] |
| MSD | RCT 88 (44/44) | Patients treated with Harpagophytum extract (60 mg harpagoside/day) for 6 weeks or 12.5 mg/day of rofecoxib. Outcome scores improved similarly for both groups. Follow-up confirmed the results of the pilot study. | 2003 | Chrubasik et al. [538,539,549,550,551,552] |
| MSD | RCT 97 (36/31/30) | Patients treated with Harpagophytum extract (~30 mg harpagoside/day) or NSAID (Voltaren 150 mg or Vioxx 12.5 mg), duration not provided; outcomes show equality of treatment. | 2005 | Lienert et al. [553,554] |
| DJD | RCT 60 (30/30) | Patients treated with combination of Harpagophytum and Apium graveolens extract (cream, 1.5 cm, twice daily) or placebo for 2 weeks. Treatment group showed significant improvement in algometer, flexion, and extension readings. | 2006 | Pillay [555] |
| Sore throat after tracheal intubation | RCT 60 (30/30) | Patients treated with Harpagophytum extract (480 mg one hour before intubation) or placebo plus premedication (fentanyl, midazolam, propofol). No significant difference was observed between groups. | 2016 | Anvari et al. [556] |
| DJD | RCT 92 (46/46) | Patients treated with combination of Rosa canina, Urtica sp., Harpagophytum procumbens, and vitamin D (20.0 g puree and 4.0 g juice concentrate, 160 mg dry extract, 108 mg dry extract, 5 µg, respectively) or placebo for 12 weeks. WOMAC and quality of life scores significantly improved vs. placebo. | 2017 | Moré et al. [557] |
DJD = degenerative joint diseases (osteoarthritis); MSD = musculo-skeletal disorders (low back pain); RCT = randomized controlled trial; NSAID = non-steroidal anti-inflammatory drug; WOMAC = Western Ontario and McMaster Universities.
12.2. Safety
A broad spectrum of claims regarding the safety of Harpagophytum in clinical practice can be found in the literature, ranging from unsubstantiated cautioning against its use altogether [215,558] to overly optimistic perspectives in the lay press. The truth, as it often does, lies somewhere in between.
12.2.1. Clinical Safety
Short- and long-term use (on average 30–60 days, in several long-term studies up to 54 weeks) have been described as safe and well-tolerated, and the most reported adverse events in clinical investigations were of mild gastrointestinal nature [559]. These may be related to its anticholinesterase effect in vitro [318,366]. A review of the safety of Harpagophytum preparations [560] concluded that they are likely to be safe with only few and no serious adverse events observed, however, it was also established that further, more rigorous safety investigations are required [561], especially considering that the dosage in most studies was found at the lower limit, and for the recommended long-term use.
12.2.2. Interaction Potential
Harpagophytum was found to be a weak inhibitor of CYP 1A2 and CYP 2D6, and a moderate inhibitor of CYP 2C8, CYP 2C9, CYP 2C19, and CYP 3A4 in vitro [397,399,400,562], however, clinical relevance is unlikely [402]. Increased anticoagulant effects have been reported with concurrent anticoagulant use [563,564,565,566,567]. While an interaction is possible, evidence is inconclusive [568] and has only been demonstrated in vitro. Herb–drug interactions and interference with anticoagulants are hypothetical and have not been conclusively demonstrated.
12.2.3. Adverse Event Reports
A case of hyponatremia in a patient with systemic hypertension has been associated with Harpagophytum (co-medications were losartan, clonidine, omeprazole, and simvastatin) [569]. Another case report suggests development of grade 2 symptomatic hypertension in a normotensive woman during self-administration of Harpagophytum [570]. However, available data do not suggest interaction potential with conventional antihypertensives at recommended doses (animal studies demonstrating a hypotensive effect used much higher doses). A case-controlled surveillance study has associated Harpagophytum with a pancreatoxic potential [571]. One early case report points at a potential allergic reaction after professional exposure to Harpagophytum [572]. Rahman and colleagues [573] included Harpagophytum in a review of botanicals with drug-interaction potential in the elderly with inflammatory bowel disease, however, did not present any causality that would justify concern.
12.2.4. Side Effects
Considering the size of the total patient collective from all clinical investigations listed in Section 11.1. (>11,000), and the most common side effects being mild gastrointestinal complaints (nausea, abdominal pain, diarrhea), CNS disorders (dizziness, headache), and allergic skin reactions, the aforementioned case reports should be further investigated, but, until corroborated by new data, their clinical relevance can be deemed as limited.
12.2.5. Pregnancy and Lactation
In Vitro data suggest spasmogenic and uterotonic effects in mammalian uterine muscles [412,413]. In the absence of adequate in vivo data [408,409], use during pregnancy and lactation should be cautioned.
13. Veterinary Applications
Veterinary applications of devil’s claw have received increased attention and gained popularity over the last 15 years, with focus on equines and canines. Colas and colleagues [250,255,256,574] provided methods for detection and control of iridoid glucosides from Harpagophytum in horse urine. Torfs et al. [575] discussed the potential benefits of devil’s claw products in veterinary practice and cited one study conducted by Montavon [576] in which ten horses with tarsal osteoarthritis were treated with an herbal powder mix containing Harpagophytum (20 g total) and smaller quantities of Ribes nigrum, Equisetum arvense, and Salix alba for 10 days a month over three consecutive months. The control group received 2 g of phenylbutazone daily. Locomotor scores improved significantly with the test medication vs. conventional NSAID. However, study results are of limited reliability due to size, lack of blinding, and subjective assessment. Axmann and colleagues [577,578] investigated pharmacokinetics and clinical efficacy of a Harpagophytum extract in horses. They provided a method with which they were able to detect harpagoside in plasma for up to 9 h after administration. Efficacy was investigated in a RCT design with 40 horses (20/20), the study medication was 10 g daily of an aqueous Harpagophytum extract (25.3% harpagoside) or placebo for 8 weeks, and a follow-up after 16 weeks. Locomotor abnormalities were assessed on a treadmill with an optoelectronic motion capture system, and follow-up was conducted via questionnaires. While the objective motion assessment did not yield significant differences between baseline and the end of the study, evaluation of the questionnaires reflected significant improvements and a “lingering” effect in the subjective assessment.
Moreau and colleagues [579] investigated the efficacy of Harpagophytum (harpagoside > 2.7%) as part of a complex mixture of ingredients for improving symptoms of canine osteoarthritis in a RCT with 32 dogs (16 per group) over 8 weeks. The primary endpoint, peak vertical force, was significantly higher in treated dogs vs. placebo after 4 and 8 weeks, and clinical signs overall improved with treatment.
Ethnoveterinary uses of devil’s claw have also been recorded. Moreki [113] reports on ethnoveterinary practices in Botswana to include the use of a decoction of Harpagophytum in poultry.
A reliable body of clinical data confirming the efficacy of Harpagophytum in veterinary applications is clearly lacking but is needed to better exploit the potential benefits. In this context, it must be noted that the use of devil’s claw—just like other analgesics—is highly restricted in equestrian sport. Harpagoside is included in the “Equine Prohibited Substance List” of the Federation Equestre Internationale as a “controlled medication”, the use of which is prohibited during training and competitions. Curiously, harpagoside is not included in the very same organization’s “List of Detection Times”, leaving horse owners in the dark as to when to discontinue use prior to a tournament. This lack of clarity may further hamper more prolific use in veterinary practice.
14. Patents
As mentioned in Section 10, the majority of patents refer to processing methods, specifically extraction and dosage forms, which constitute the only legitimately patentable intellectual property for the pharmaceutical industry, except in cases where new effects or combinations, not previously described in ethnobotanical use accounts, were elucidated. It is noteworthy that most of the earlier patents listed below in Table 12 (pre-2000) have expired or been withdrawn. Pending patents have been excluded.

Table 12.
Patents pertaining to Harpagophytum and its preparations.
15. Discussion and Conclusions
Devil’s claw is a well-established phytopharmaceutical. A large body of data exists in which composition, pharmacological activities, and clinical effects are elucidated, and in turn support and affirm traditional use applications. Nonetheless, several aspects requiring further investigation were highlighted by this review.
Revision of the genus to account for introgression, geographical, and biochemical variation, and geo-authenticity is needed.
In view of the interchangeable use of both Harpagophytum species and mixtures thereof in clinical practice, further comparative examination of the composition of both species is needed. Verbascoside as an anti-inflammatory compound present in Harpagophytum could be an interesting target of future research.
Despite some inconsistent outcomes and contradictory results, pharmacological evidence appears to be overall sufficient to support clinical use. Sufficient pharmacological differentiation between Harpagophytum species, however, is lacking.
Toxicological evaluations of Harpagophytum indicate a low toxicity in animal models. While genotoxicity testing is part of the regulatory requirements for the market authorization of herbal medicinal products in Europe, results are proprietary (product-related) and have not been published. Adequate tests on reproductive toxicity, genotoxicity, and carcinogenicity, performed according to currently valid OECD guidelines, need to be made publicly available.
While there may be strong clinical evidence that devil’s claw preparations are effective in the treatment of degenerative joint diseases and musculoskeletal disorders in principle, this conclusion cannot be extended to specific preparations, because of the varying pharmaceutical quality of individual preparations.
Further investigations are required (a) to identify the therapeutically active substances or fractions and thus enable tests which (b) use accordingly standardized and sufficiently dosed preparations with a carefully designed setup and methodology in order to obtain quantifiable results for the efficacy of devil’s claw preparations. These need to be conducted with both Harpagophytum spp. individually but prepared identically. Trial designs should be guided by the recommendations of the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH). Specifically, both species could be compared in a two-arm cross-over design. Conventional medication could be added as a third arm to assess comparative efficacy. Studies should be of adequate power, randomized, placebo-controlled, and double-blinded. Problematic in an ethical sense is the denial of “first aid” medication in placebo-controlled studies, permission of which would confound outcomes. Outcomes should be objective or at least a combination of objective and subjective measures.
Further research is also warranted in the area of clinical safety, specifically with regard to the drug interaction potential of devil’s claw preparations. Until then, safety considerations as expressed in current compendia, e.g., [15], should be considered appropriate.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data has been presented in the main text.
Acknowledgments
Ernst Schneider, Mathias Schmidt, Sigrun Chrubasik, Margret Moré, Dave Cole, Josef Brinckmann, Wolfram Hartmann, Cyril Lombard, Ben-Erik van Wyk, and Karen Nott kindly assisted with the procurement of some illusive publications.
Conflicts of Interest
The author declares no conflict of interest.
References
- Ihlenfeldt, H.-D.; Hartmann, H. Die Gattung Harpagophytum (Burch.) DC. Ex Meissn. (Monographie der afrikanischen Pedaliaceae II). Hambg. Staatsinst. Allg. Bot. Mitt. 1970, 13, 15–69. [Google Scholar]
- Muzila, M.; Werlemark, G.; Ortiz, R.; Sehic, J.; Fatih, M.; Setshogo, M.; Mpoloka, W.; Nybom, H. Assessment of diversity in Harpagophytum with RAPD and ISSR markers provides evidence of introgression. Hereditas 2014, 151, 91–101. [Google Scholar] [CrossRef]
- Brendler, T.; van Wyk, B.E. A historical, scientific and commercial perspective on the medicinal use of Pelargonium sidoides (Geraniaceae). J. Ethnopharmacol. 2008, 119, 420–433. [Google Scholar] [CrossRef]
- Stander, M.A.; Brendler, T.; Redelinghuys, H.; Van Wyk, B.E. The commercial history of Cape herbal teas and the analysis of phenolic compounds in historic teas from a depository of 1933. J. Food Compos. Anal. 2019, 76, 66–73. [Google Scholar] [CrossRef]
- Low, C.H. Different histories of buchu: Euro-American appropriation of San and Khoekhoe knowledge of buchu plants. Environ. Hist. 2007, 13, 333–361. [Google Scholar] [CrossRef]
- Brendler, T.; Cock, I.E. A short history of Cape aloe bitters. S. Afr. J. Bot. 2021. under review. [Google Scholar]
- Helmstädter, A. Xysmalobium undulatum (Uzara) research—How everything began. J. Ethnopharmacol. 2015, 164, 385–387. [Google Scholar] [CrossRef] [PubMed]
- Brendler, T. The rise and fall of Hoodia: A lesson on the art and science of natural product commercialization. In African Natural Plant Products, Volume III: Discoveries and Innovations in Chemistry, Bioactivity, and Applications; ACS Publications: Washington, DC, USA, 2020; pp. 313–324. ISBN 1947-5918. [Google Scholar]
- Van Wyk, B.E. A review of commercially important African medicinal plants. J. Ethnopharmacol. 2015, 176, 118–134. [Google Scholar] [CrossRef]
- CITES. Inclusion of Harpagophytum Procumbens in Appendix II in Accordance with Article II 2(a) and Inclusion of Harpagophytum Zeyheri in Appendix II in Accordance with Article II 2(b) for Reasons of Look-Alike Problems. 2000, pp. 1–9. Available online: https://cites.org/sites/default/files/eng/cop/11/prop/60.pdf (accessed on 12 April 2021).
- Czygan, F.-C.; Krüger, A.; Schier, W.; Volk, O.H. Pharmazeutisch-biologische Untersuchungen der Gattung Harpagophytum (Bruch.) DC ex Meissn. 1. Mitteilung: Phytochemische Standardisierung von Tubera Harpagophyti. Dtsch. Apoth. Ztg. 1977, 117, 1431–1434. [Google Scholar]
- Eich, J.; Schmidt, M.; Betti, G.J.R. HPLC analysis of iridoid compounds of Harpagophytum taxa: Quality control of pharmaceutical drug material. Pharm. Pharmacol. Lett. 1998, 8, 75–78. [Google Scholar]
- Feistel, B.; Gaedcke, F. Analytical identification of Radix Harpagophyti procumbentis and zeyheri. Z. Phytother. 2000, 21, 246–251. [Google Scholar]
- Nott, K. A Survey of the Harvesting and Export of Harpagophytum procumbens and Harpagophytum Zeyheri in SWA/Namibia; Etosha Ecological Institute: Okaukuejo, Namibia, 1986. [Google Scholar]
- EMA. European Union Herbal Monograph on Harpagophytum procumbens DC. and/or Harpagophytum zeyheri Decne., Radix. EMA/HMPC/627057/2015; Committee on Herbal Medicinal Products (HMPC): London, UK, 2016. [Google Scholar]
- European Scientific Cooperative on Phytotherapy. Harpagophyti radix. In ESCOP Monographs, 2nd ed.; Thieme: Stuttgart, Germany, 2003; pp. 233–240. [Google Scholar]
- Menghini, L.; Recinella, L.; Leone, S.; Chiavaroli, A.; Cicala, C.; Brunetti, L.; Vladimir-Knezevic, S.; Orlando, G.; Ferrante, C. Devil’s claw (Harpagophytum procumbens) and chronic inflammatory diseases: A concise overview on preclinical and clinical data. Phytother. Res. 2019, 33, 2152–2162. [Google Scholar] [CrossRef] [PubMed]
- Stewart, K.M.; Cole, D. The commercial harvest of devil’s claw (Harpagophytum spp.) in southern Africa: The devil’s in the details. J. Ethnopharmacol. 2005, 100, 225–236. [Google Scholar] [CrossRef]
- Baghdikian, B.; Lanhers, M.C.; Fleurentin, J.; Ollivier, E.; Maillard, C.; Balansard, G.; Mortier, F. An analytical study, anti-inflammatory and analgesic effects of Harpagophytum procumbens and Harpagophytum zeyheri. Planta Med. 1997, 63, 171–176. [Google Scholar] [CrossRef]
- Anonymous. Harpagophytum procumbens (devil’s claw). Altern. Med. Rev. 2008, 13, 248–252. [Google Scholar]
- Barnes, J. Charms & harms: Devil’s claw. J. Prim. Health Care 2009, 1, 238–239. [Google Scholar] [CrossRef]
- Caprasse, M. Description, identification et usages thérapeutiques de la «griffe du diable»: Harpagophytum procumbens DC. J. Pharm. Belg. 1980, 35, 143–149. [Google Scholar]
- Chrubasik, S. Wirksamkeit pflanzlicher Schmerzmittel am Beispiel des Teufelskrallenwurzelextrakts. Orthopäde 2004, 33, 804–808. [Google Scholar] [CrossRef] [PubMed]
- Czygan, F.-C. Harpago- oder Teufelskrallentee, das Auf und Ab einer Modedroge. Z. Phytother. 1984, 5, 922–925. [Google Scholar]
- Czygan, F.-C. Nochmals Harpagophytum. Z. Phytother. 1984, 5, 972. [Google Scholar]
- Czygan, F.-C. Portrait einer Arzneipflanze: Harpagophytum—Teufelskralle. Z. Phytother. 1987, 8, 17–20. [Google Scholar]
- Dittrich, C. Harpagophytum procumbens DC. Österr. Apoth. Ztg. 1974, 28, 53–54. [Google Scholar]
- Esdorn, I. Afrikanische Reiseeindrücke in pharmazeutischer und kultureller Hinsicht. Dtsch. Apoth. Ztg. 1963, 103, 785–789. [Google Scholar]
- Faivre, C.; Ghedira, K.; Goetz, P.; Lejeune, R. Harpagophytum procumbens (Pedaliaceae). Phytothérapie 2007, 5, 150–153. [Google Scholar] [CrossRef]
- Georgiev, M.; Ivanovska, N.; Alipieva, K.; Dimitrova, P.; Verpoorte, R. Harpagoside: From Kalahari Desert to pharmacy shelf. Phytochemistry 2013, 92, 8–15. [Google Scholar] [CrossRef]
- Hansen, C. Arzneistoff Porträt—Die Afrikanische Teufelskralle—Voodoo oder wirksames Arzneimittel? Dtsch. Apoth. Ztg. 2000, 140, 85–89. [Google Scholar]
- Jaspersen-Schib, R. Harpagophyti radix—Wirklich eine Wunderdroge. Dtsch. Apoth. Ztg. 1990, 130, 71. [Google Scholar]
- Kampffmeyer, H. Teufelskralle—Gibt es eine therapeutische Wirkung? ZFA 1980, 56, 618. [Google Scholar]
- Kannacher, M. Harpagophytum procumbens—Die Teufelskralle. Tubera harpagophyti, die Speicherknollen. Volksheilkunde 1993, 45, 44. [Google Scholar]
- Lis, K. Diabelska moc czarciego pazura. Reumatologia 2010, 48, 128–132. [Google Scholar]
- McGregor, G.; Fiebich, B.; Wartenberg, A.; Brien, S.; Lewith, G.; Wegener, T. Devil’s claw (Harpagophytum procumbens): An anti-inflammatory herb with therapeutic potential. Phytochem. Rev. 2005, 4, 47–53. [Google Scholar] [CrossRef]
- McGregor, G.P. Harpagophytum procumbens—Traditional anti-inflammatory herbal drug with broad therapeutic potential. In Herbal Drugs: Ethnomedicine to Modern Medicine; Springer: New York, NY, USA, 2009; pp. 81–95. [Google Scholar]
- Miraldi, E.; Biagi, M.; Giachetti, D. A comprehensive systematic pharmacological review on Harpagophytum procumbens DC. (Devil’s claw). Biol. Sci. PJSIR 2008, 51, 165–176. [Google Scholar]
- Mncwangi, N.; Chen, W.; Vermaak, I.; Viljoen, A.; Gericke, N. Devil’s claw—A review of the ethnobotany, phytochemistry and biological activity of Harpagophytum procumbens. J. Ethnopharmacol. 2012, 143, 755–771. [Google Scholar] [CrossRef] [PubMed]
- Olivier, D.K. The Ethnobotany and Chemistry of South African Traditional Tonic Plants. Ph.D. Thesis, University of Johannesburg, Johannesburg, South Africa, 2012; p. 481. [Google Scholar]
- Richter, T. Gut beraten mit Teufelskralle? Z. Phytother. 2001, 22, 43. [Google Scholar]
- Schmidt, S. Die antiarthritische Wirkung der Harpagophytum-Wurzel. Österr. Apoth. Ztg. 1971, 25, 829. [Google Scholar]
- Schmidt, S. Rheumatherapie mit Harpagophytum. Therapiewoche 1972, 22, 1072–1074. [Google Scholar]
- Schmidt, S. Teufelskralle und Rheuma. Österr. Apoth. Ztg. 1983, 37, 111–113. [Google Scholar]
- Scholz, H. Die Wurzel aus dem roten Sand. Kosmos 1977, 73, 122–124. [Google Scholar]
- Schwabe, W. Übersicht über neuere Arzneipflanzen, die sich in den letzten 20 Jahren in der Homöopathie und der Phytotherapie bewährt haben. Allg. Homöopath. Ztg. 1980, 225, 217–229. [Google Scholar] [CrossRef]
- Seeger, P.G. Harpagophytum, ein wirksames Phytotherapeutikum. Erfahrungsheilkunde 1973, 8, 255–256. [Google Scholar]
- Seeger, P.G. Harpagophytum—Ein wirksames Phytotherapeutikum. Naturheilpraxis 1973, 10, 488–492. [Google Scholar]
- Sprecher, E. Problems with modern drugs: Ginseng-taiga root—Devil’s claw. Schr. Bundesapothekerkamm. Wiss. Fortbild. Gelbe Reihe 1977, 5, 71–95. [Google Scholar]
- Sticher, O. Die aktuelle Droge: Harpagophytum procumbens. Dtsch. Apoth. Ztg. 1977, 117, 1279–1284. [Google Scholar]
- Vanhaelen, M. La biochimie et l’activite de Harpagophytum procumbens et de Glycyrrhiza glabra. Toxicite de Symphytum consolida. J. Pharm. Belg. 1986, 41, 172–182. [Google Scholar]
- Vanhaelen, M.; Vanhaelen-Fastré, R.; Samaey-Fontaine, J.; Elchamid, A.; Niebes, P.; Matagne, D. Aspects botaniques, constitution chimique et activite pharmacologique d’Harpagophytum procumbens. Phytotherapy 1983, 5, 7–13. [Google Scholar]
- Vogel, A.; Vogel, R. Die Teufelskralle (Harpagophytum). A. Vogel Gesundh. Nachr. 1988, 45, 54–55. [Google Scholar]
- Vogel, A.; Vogel, S. Teufelskralle, Harpago. A. Vogel Gesundh. Nachr. 1973, 30, 102–104. [Google Scholar]
- Vogel, A.; Vogel, S. Harpago, Teufelskralle. A. Vogel Gesundh. Nachr. 1978, 35, 42–43. [Google Scholar]
- Vogel, A.; Vogel, S. Die Teufelskralle. A. Vogel Gesundh. Nachr. 1978, 35, 153–154. [Google Scholar]
- Vogel, G. Wissenschaftliche Erkenntnisse zu Wirksamkeit und Unbedenklichkeit pflanzlicher Arzneimittel. Therapiewoche 1984, 34, 4078–4086. [Google Scholar]
- Volk, O.H. Zur Kenntnis von Harpagophytum procumbens DC. Dtsch. Apoth. Ztg. 1964, 104, 573–576. [Google Scholar]
- Voloshyn, O.I.; Smiyan, S.I.; Voloshyna, L.O.; Horevych, S.S. Испoльзoвание мартинии душистoй (Harpagophytum procumbens) в ревматoлoгии: взгляд сквoзь призму кoмoрбиднoсти (Обзoр литературы). Semejnaâ Med. 2020, 3, 88–97. [Google Scholar] [CrossRef]
- Wegener, T. Die Teufelskralle (Harpagophytum procumbens DC.) in der Therapie rheumatischer Erkrankungen. Z. Phytother. 1998, 19, 284–294. [Google Scholar]
- Wegener, T. Wissenschaftliches Erkenntnismaterial zu Harpagophyti radix (Südafrikanische Teufelskralle) ab 1990—Unter Berücksichtigung Relevanter Früherer Studien; Kooperation Phytopharmaka: Bonn, Germany, 1998; p. 25. [Google Scholar]
- Wegener, T. Devil’s claw: From African traditional remedy to modern analgesic and antiinflammatory. HerbalGram 2000, 50, 47–54. [Google Scholar]
- Wegener, T.; Winterhoff, H. Zubereitungen aus der südafrikanischen Teufelskralle. Dtsch. Apoth. Ztg. 2001, 141, 5613–5621. [Google Scholar]
- Wiss, H.-J. Was Wissen Wir über die Teufelskralle? Library of the Namibia Scientific Society: Windhoek, Namibia, 1974; p. 13. [Google Scholar]
- Graner, G.; Lautenbacher, L. Harpagophytum procumbens DC (Teufelskralle); Kooperation Phytopharmaka: Bonn, Germany; p. 36.
- Brossier, Y. Harpagophytum procumbens DC: Apport Bénéfique de la Phytothérapie dans le Traitement de la Maladie Inflammatoire Chronique. Ph.D. Thesis, Université Paul Sabatier, Toulouse, France, 1986; p. 163. [Google Scholar]
- Mattern, B. L’Harpagophytum procumbens DC: Une Recente Acquisition de la Phytotherapie. Ph.D. Thesis, Universite de Bordeaux II, Bordeaux, France, 1983; p. 76. [Google Scholar]
- Mundy, P.J.; Ncube, S.F. Devil’s claw—A natural substitute for diclofenac? Vulture News 2014, 67, 43–47. [Google Scholar] [CrossRef]
- Anonymous. Teufelskralle als pflanzliche Alternative. Ärztez. Nat. 1999, 40, 500. [Google Scholar]
- Bonnefoy-Cudraz, Q. Le Droguier de la Faculté de Pharmacie de Montpellier (Sauvegarde du Patrimoine et Intérêt d’une des Plantes, l Harpagophytum procumbens Species). Ph.D. Thesis, Université de Montpellier I, Montpellier, France, 2013; p. 145. [Google Scholar]
- Camponovo, F. Mise au Point de Procédés pour l’Analyse Phytochimique et Étude Comparative de Quelques Médicaments à Base de Ginkgo Biloba, Panax Ginseng et Harpagophytum procumbens. Ph.D. Thesis, Université de Lausanne, Lausanne, Switzerland, 1996; p. 289. [Google Scholar]
- Couplan, F.; Danton, P. L’Harpagophytum. Un cadeau d’Afrique à menager. Rev. Monde Végétal 2000, 50, 16–19. [Google Scholar]
- Ferrara, L.; Borrelli, F.; Borbone, N. Harpagophytum procumbens: New scientific evidences. In Proceedings of the 3rd International Symposium on Natural Drugs, Napoli, Italy, 2–4 October 2003. [Google Scholar]
- Fontanel, D. L’Harpagophytum. Lett. Phytothér. Rev. Inf. Pharm. Méd. 2005, 5, 1–6. [Google Scholar]
- Franchi, G.G. Harpagophytum procumbens DC.: Una pianta africana entrata a far parte della medicina europea. Alcune osservazioni a carattere botanico, ecologico e farmacognostico. Piante Med. 2006, 5, 5–10. [Google Scholar]
- Hadolt, H. Harpagophytum procumbens: Teufelskralle, Trampelklette. PhD of Thesis, Universität Wien, Wien, Austria, 1987; p. 57. [Google Scholar]
- Kämpf, R. Harpagophytum procumbens DC, devil’s claw. Schweiz. Apoth. 1976, 114, 337–342. [Google Scholar]
- Quer, J.-C. Harpagophytum procumbens, Aspects Récents. PhD of Thesis, Université Descartes, Paris, France, 2007; p. 84. [Google Scholar]
- Schmidt, T. Harpagophytum procumbens DC. Inf. Biol. Prax. 1972, 8, 21–23. [Google Scholar]
- Smithies, S.J. Harpagophytum procumbens (Burch.) DC. Ex Meisn. Subsp. procumbens and Subsp. Transvaalense Ihlenf. & HEK Hartmann (Pedaliaceae). 2006. Available online: http://opus.sanbi.org/bitstream/20.500.12143/3477/1/Harpagophytumprocumbens_PlantzAfrica.pdf (accessed on 15 April 2021).
- Burchell, W.J. Travels in the Interior of Southern Africa; Printed for Longman, Hurst, Rees, Orme, and Brown: London, UK, 1822; Volume 1, pp. 529–537. [Google Scholar]
- Meisner, C.D.F. Plantarum Vascularium Genera: Secundum Ordines Naturales Digesta Eorumque Differentiae et Affinitates Tabulis Diagnostacis Expositae; Libraria Weidmannia: Leipzig, Germany, 1836–1843; Volume 1,2. [Google Scholar]
- De Candolle, A. Prodromus Systematis Naturalis Regni Vegetabilis, Sive, Enumeratio Contracta Ordinum Generum Specierumque Plantarum Huc Usque Cognitarium, Juxta Methodi Naturalis, Normas Digesta: Pedalineae; Fortin, Masson et Sociorum: Paris, France, 1845; Volume 9, pp. 253–257. [Google Scholar]
- Decaisne, M.J. Revue du groupe des pédalinées: Harpagophytum DC. Ann. Sci. Nat. Bot. 1865, 5, 321–336. [Google Scholar]
- Mncwangi, N.; Vermaak, I.; Viljoen, A. Mid-infrared spectroscopy and short wave infrared hyperspectral imaging—A novel approach in the qualitative assessment of Harpagophytum procumbens and H. zeyheri (Devil’s claw). Phytochem. Lett. 2014, 7, 143–149. [Google Scholar] [CrossRef]
- Muzila, M. Genetic, Morphological and Chemical Variation in the Genus Harpagophytum. Ph.D. Thesis, Swedish University of Agricultural Sciences, Alnarp, Sweden, 2016; p. 76. [Google Scholar]
- Muzila, M.; Setshogo, M.P.; Mpoloka, S.W. Multivariate analysis of Harpagophytum DC. Ex Meisn (Pedaliaceae) based on fruit characters. Int. J. Biodivers. Conserv. 2011, 3, 101–109. [Google Scholar]
- Mncwangi, N.; Viljoen, A.; Vermaak, I.; Chen, W.; Zhang, J.; Khan, I.A. Metabolomic profiling and quality control of Harpagophytum spp. (Devil’s claw). Planta Med. 2014, 80, CL1. [Google Scholar] [CrossRef]
- Mosoabisane, M.F.T. Variation in chemical composition of Harpagophytum species as function of age and locality. Master’s Thesis, University of the Free State, Bloemfontein, South Africa, 2009; p. 181. [Google Scholar]
- Steenkamp, P.A.; Steenkamp, L.H. UPLC-MS profiling, identification of major peaks and comparison of Harpagophytum procumbens extracts from different locations. S. Afr. J. Bot. 2019, 124, 138–143. [Google Scholar] [CrossRef]
- Hargreaves, B.J. The sesame family in Botswana. Botsw. Notes Rec. 1993, 25, 141–159. [Google Scholar]
- Van Wyk, B.E.; Gericke, N. People’s Plants—A Guide to Useful Plants of Southern Africa, 2nd, revised, and expanded ed.; Briza Publications: Queenswood, South Africa, 2018; pp. 174, 224. [Google Scholar]
- Von Koenen, E. Heil-, Gift- und Essbare Pflanzen in Namibia; Klaus Hess Verlag: Göttingen, Germany, 1996; p. 336. ISBN 3980451828. [Google Scholar]
- Smith, C.A. Common Names of South African Plants; Government Printer: Pretoria, South Africa, 1966; p. 642.
- Ihlenfeldt, H.-D. Bemerkungen zur Taxonomie der süDwestafrikanischen Pedaliaceae; Mitteilungen der Botanischen Staatssammlung München Band VI.: München, Germany, 1967; pp. 593–612. [Google Scholar]
- Baum, H. Kunene-Sambesi-Expedition; Otto Warburg, Verlag des Kolonial-Wirtschaftlichen Komitees: Berlin, Germany, 1903; p. 604. [Google Scholar]
- Blank, R.J. Voraussetzungen und Möglichkeiten für Einen Feldmäßigen Anbau der Wildpflanze Harpagophytum procumbens (Teufelskralle). Master Thesis, Universität Hohenhein, Stuttgart-Hohenheim, Germany, 1973; p. 63. [Google Scholar]
- Blank, R.J. Arbeiten und Berichte 19. Versuche zur Vermehrung von Harpagophytum procumbens DC. (Teufelskralle); Universität Hohenheim, Abteilung Pflanzenbau in den Tropen und Subtropen: Stuttgart-Hohenheim, Germany, 1976. [Google Scholar]
- Von Willert, D.J.; Schneider, E. Teufelskralle: Anbau und Wildsammlung—Ein Beitrag zur pharmakognostischen Ökologie. Dtsch. Apoth. Ztg. 2001, 141, 683–688. [Google Scholar]
- Wood, J.G. The Uncivilized Races, or Natural History of Man; American Publishing Company: Hartford, CT, USA, 1870; Volume 1, p. 783. [Google Scholar]
- Cooke, M.C. Freaks and Marvels of Plant Life: Or Curiosities of Vegetation; Society for Promoting Christian Knowledge: London, UK, 1882; p. 463. [Google Scholar]
- Lübbert, A. Aus dem deutsch-südwestafrikanischen Schutzgebiete. Ueber die Heilmethoden und Heilmittel der Eingeborenen in Deutsch-Südwestafrika. Mitth. Forsch. Gelehrt. Dtsch. Schutzgeb. 1901, 14, 77–90. [Google Scholar]
- Hellwig, M. Angaben von Eingeborenen über die Feldkost und die Arzneipflanzen der Herrero und Hottentotten; Reichskolonialamt (Bundesarchiv R 1001/5989, fol. 78–81): Berlin, Germany, 1907. [Google Scholar]
- Dinter, K. Die Vegetabilische Veldkost Deutsch-Südwest-Afrikas; Selbstverlag: Okahandja, Namibia, 1912; p. 24. [Google Scholar]
- Dinter, K. Deutsch-Südwest-Afrika. Flora, Forst- und Landwirtschaftliche Fragmente; Weigel: Leipzig, Germany, 1909; p. 212. [Google Scholar]
- Schön, A. Vom Pfeilgift zur Arznei: Untersuchungen von Arzneidrogen und Giften aus den Ehemaligen Deutschen Kolonien West- und Südwestafrikas, Vornehmlich an Berliner Instituten (1884–1918): Ein Beitrag zur Kolonialpharmazie; Wissenschaftliche Verlagsgesellschaft mbH: Stuttgart, Germany, 2017; p. 593. ISBN 3804737684. [Google Scholar]
- Kroemer, B. Mit Schwert & Pflugschar in Sachsen und Südwestafrika. Anekdoten und Geschichten Eines Südwester Pioniers: Gottreich Hubertus Mehnert; “Glanz & Gloria” Verlag: Windhoek, Namibia, 2007; p. 128. ISBN 9789991668970. [Google Scholar]
- Anderson, S.; Staugard, F. Traditional Midwives (Traditional Medicine in Botswana); Ipelegeng Publishers: Gaborone, Botswana, 1986; p. 264. ISBN 978-9178103973. [Google Scholar]
- Bieg, S. Beiträge Zur Kenntnis Einiger Heilpflanzen aus Deutsch-Südwestafrika mit Einer Liste der Dort Vorkommenden Medizinisch Verwendeten Pflanzen. Ph.D. Thesis, Technische Hochschule, Stuttgart, Germany, 1939; p. 49. [Google Scholar]
- Van Damme, P.; van den Eynden, V.; Vernemmen, P. Plant uses by the Topnaar of the Sesfontein area (Namib desert). Afr. Focus 1992, 8. [Google Scholar] [CrossRef]
- Watt, J.M.; Breyer-Brandwijk, M.G. The Medicinal and Poisonous Plants of Southern and Eastern Africa, 2nd ed.; Livingstone: London, UK, 1962; p. 1457. [Google Scholar]
- Staugard, F. Traditional midwives in Botswana. Botsw. Natl. Health Bull. 1985, 1, 42–60. [Google Scholar] [PubMed]
- Moreki, J.C. Documentation of ethnoveterinary practices used in family poultry in Botswana. Vet. World 2013, 6, 18–21. [Google Scholar] [CrossRef]
- Maas, H. Außenminister Maas zum Abschluss der Verhandlungen mit Namibia. 2021. Available online: https://www.auswaertiges-amt.de/de/newsroom/-/2463396 (accessed on 10 June 2021).
- Kock, R. Erinnerungen an die Internierungszeit (1939–1946) und Zeitgeschichtliche Ergänzungen; Selbstverlag “Andalusia”: Windhoek, Namibia, 1975; p. 209. [Google Scholar]
- Zorn, B. Über die antiarthritische Wirkung der Harpagophytum-Wurzel. Dtsch. Rheumaforsch. 1958, 17, 134–138. [Google Scholar]
- Lux, R.E. Über ein Glukosid der Wurzel von Harpagophytum procumbens. Ph.D. Thesis, Julius-Maximilians-Universität, Würzburg, Germany, 1960; p. 75. [Google Scholar]
- Stierstorfer, N. Ein Beitrag zur Kenntnis der Inhaltsstoffe von Harpagophytum procumbens DC. Ph.D. of Thesis, Julius-Maximilians-Universität, Würzburg, Germany, 1961; p. 54. [Google Scholar]
- Tunmann, P.; Lux, R.E. Zur chemischen Konstitution des Harpagosids. Pharm. Ztg. 1961, 106, 1357. [Google Scholar]
- Tunmann, P.; Lux, R.E. Zur Kenntnis der Inhaltsstoffe aus der Wurzel von Harpagophytum procumbens DC. 1. Mitteilung: Isolierung und Eigenschaften der Glukoside Harpagosid und Harpagid. Dtsch. Apoth. Ztg. 1962, 102, 1274–1275. [Google Scholar]
- Tunmann, P.; Lux, R.E. Zur chemischen Konstitution des Harpagosids. Dtsch. Apoth. Ztg. 1962, 101, 1383. [Google Scholar]
- Fickentscher, K. Beitrag zur Chemischen Konstitution des Harpagids. Ph.D. Thesis, Julius-Maximilians-Universität, Würzburg, Germany, 1963; p. 63. [Google Scholar]
- Tunmann, P.; Stierstorfer, N. Zur Kenntnis der Inhaltsstoffe aus der Wurzel von Harpagophytum procumbens DC. 2. Mitteilung. Dtsch. Apoth. Ztg. 1963, 103, 395–397. [Google Scholar]
- Lichti, H.; von Wartburg, A. Zur Konstitution von Harpagosid. Tetrahedron Lett. 1964, 5, 835–843. [Google Scholar] [CrossRef]
- Tunmann, P.; Stierstorfer, N. Zur Kenntnis der Inhaltsstoffe aus der Wurzel von Harpagophytum procumbens DC. Tetrahedron Lett. 1964, 5, 1697–1699. [Google Scholar] [CrossRef]
- Lichti, H.; von Wartburg, A. Die Struktur des Harpagosids. 2. Mitteilung über Iridoide. Helv. Chim. Acta 1966, 49, 1552–1580. [Google Scholar] [CrossRef]
- Hammer, H.-E. Konstitution des Procumbids und ein Beitrag zur Kenntnis Weiterer Inhaltsstoffe aus der Wurzel von Harpagophytum procumbens DC. Ph.D. Thesis, Julius-Maximilians-Universität, Würzburg, Germany, 1967; p. 65. [Google Scholar]
- Tunmann, P.; Hammer, H.-E. Inhaltsstoffe aus der Wurzel von Harpagophytum procumbens DC., IV. Konstitution des Procumbids. Justus Liebigs Ann. Chem. 1968, 712, 138–145. [Google Scholar] [CrossRef]
- Hagen, E. Correspondence Concerning the Trademarking of “Harpago”; Library of the Namibia Scientific Society: Windhoek, Namibia, 1961–1976; p. 10. [Google Scholar]
- Schmidt, S. Phytotherapie beim rheumatischen Formenkreis. Arch. Arzneither. 1978, 3, 266–271. [Google Scholar]
- Beck, H.; Sand, J.-M.; Kuhlmann, J. Arzneimittelmarkt aktuell. Dtsch. Apoth. Ztg. 1981, 121, 2884–2889. [Google Scholar]
- Marandet, E. Harpagophytum procumbens DC. De l’Utilisation Traditionnelle à la Réglementation Européenne. Ph.D. Thesis, Université de Reims Champagne-Ardenne, Reims, France, 2009; p. 97. [Google Scholar]
- Hagen, E. Harpagophytum. Letter to H.-J. Wiss Concerning Sustainability of Devil’s Claw Exports to Germany; Library of the Namibia Scientific Society: Windhoek, Namibia, 1975; p. 2. [Google Scholar]
- Achtnich, W. Harpagophytum. Letter to the South African Embassy in Germany (Cologne) Concerning Sustainability of Devil’s Claw Exports to Germany; Library of the Namibia Scientific Society: Windhoek, Namibia, 1975; p. 2. [Google Scholar]
- De Bruine, J.R.; Clark, D.L. A Short Revue of the Harpagophytum procumbens Problem; Department of Nature Conservation: Windhoek, Namibia, 1976.
- Kgathi, D.L. The Grapple Plant Project. Seventh Progress Report: Aspects of Grapple Trade; University of Botswana: Gaborone, Botswana, 1987. [Google Scholar]
- Kgathi, D.L. The grapple trade in Botswana. Botsw. Notes Rec. 1988, 20, 119–124. [Google Scholar]
- Taylor, F.W.; Moss, H. Final Report on the Potential for Commercial Utilization of Veld Products. The Resource and its Management; Ministry of Commerce & Industry: Gaborone, Botswana, 1982; Volume 1, p. 205.
- Engels, G.; Brinckmann, J.A. Devil’s claw—Harpagophytum procumbens, H. zeyheri. HerbalGram 2018, 118, 1–14. [Google Scholar]
- Cunningham, A.B. African Medicinal Plants: Setting Priorities at the Interface Between Conservation and Primary Health Care; UNESCO: Paris, France, 1993. [Google Scholar]
- Hachfeld, B. Analysis of the Trade Potential and Possible Over-Exploitation of a Southern African Medicinal Plant: Harpagophytum Procumbens; Bundesamt für Naturschutz: Bonn, Germany, 1999. [Google Scholar]
- CITES. Biological And Trade Status of Harpagophytum. 2002, pp. 1–15. Available online: https://cites.org/sites/default/files/eng/cop/12/doc/E12-46.pdf (accessed on 12 April 2021).
- Grote, K. The Increased Harvest and Trade of Devil’s Claw (Harpagophytum procumbens) and Its Impacts on the Peoples and Environment of Namibia, Botswana and South Africa; Global Facilitation Unit for Underutilized Species: Maccarese, Italy, 2003; p. 30. [Google Scholar]
- Hachfeld, B. Ecology and Utilisation of the Medicinal Plant Harpagophytum procumbens (Burch.) DC. ex Meissn. (Pedaliaceae) in Southern Africa; Bundesamt für Naturschutz: Bonn, Germany, 2004. [Google Scholar]
- Hachfeld, B. Ecology and Utilisation of the Medicinal Plant Harpagophytum procumbens (Burch.) DC. ex Meissn. (Pedaliaceae) in Southern Africa. Ph.D. Thesis, Universität Hamburg, Hamburg, Germany, 2004; p. 305. [Google Scholar]
- Cole, D.; Bennett, B. Trade, Poverty and Natural Products: Lessons Learned from Namibian Organic Devil’S Claw. 2007. Available online: http://searchworks.stanford.edu/view/7838432 (accessed on 12 April 2021).
- Hachfeld, B.; Schippmann, U. Conservation data sheet 2: Exploitation, trade and population status of Harpagophytum procumbens in southern Africa. Med. Plant Conserv. 2000, 6, 4–9. [Google Scholar]
- Kathe, W.; Barsch, F.; Honnef, S. Trade in Devil’s Claw (Harpagophytum spp.) in Germany—Status, Trends and Certification. 2003, pp. 1–40. Available online: http://foris.fao.org/static/pdf/NWFP/Germany_devils_claw.pdf (accessed on 13 April 2021).
- Schippmann, U. Imports of Harpagophytum in Germany. In Proceedings of the First Regional Devil’s Claw Conference, Windhoek, Namibia, 26–28 February 2002; p. 3. [Google Scholar]
- Suckert, B. Successful marketing strategies as a tool for development. In Proceedings of the First Regional Devil’s Claw Conference, Windhoek, Namibia, 26–28 February 2002; p. 2. [Google Scholar]
- Censkowsky, U.; Helberg, U.; Nowack, A.; Steidle, M. Overview of World Production and Marketing of Organic Wild Collected Products; ITC: Geneva, Switzerland, 2007; p. 91. [Google Scholar]
- Nott, K.; Nott, A.; Newton, D. A Critical Assessment of the Economic and Environmental Sustainability of the Namibian Indigenous Forest/Timber Industry with Reference to Zambia and Angola; TRAFFIC: Pretoria, South Africa, 2020; p. 101. [Google Scholar]
- United States Pharmacopeial Convention. Harpagophytum Species Root. Proposed For Development Version 0.1. Herbal Medicines Compendium; USP: Rockville, MD, USA, 2013. [Google Scholar]
- British Herbal Medicine Association. Harpagophytum. In British Herbal Pharmacopoeia, Part Three; BHMA: Cowling, UK, 1981; p. 49. [Google Scholar]
- ANSM. Harpagophyton. Harpagophytum procumbens. In Pharmacopée Française, 10th ed.; Maisonneuve: Sainte-Ruffine, France, 1989; pp. 183–184. [Google Scholar]
- Koch, H.P.; Hadold, H. Harpagophytum procumbens. Teufelskralle, Trampelklette; Kooperation Phytopharmaka: Bonn, Germany, 1988; p. 75. [Google Scholar]
- Kommission, E. Harpagophyti radix (Berichtigung). Bundesanzeiger 1990, 164, 1. [Google Scholar]
- ANSM. Extrait d’Harpagophyton (Sec). Harpagophyti extractum siccum. In Pharmacopée Française, 10th ed.; Maisonneuve: Sainte-Ruffine, France, 1992; pp. 1–4. [Google Scholar]
- Bundesministerium für Gesundheit und Soziale Sicherung. Teufelskrallenwurzel. Harpagophyti radix. In Deutsches Arzneibuch (DAB) 10. 2. Nachtrag; Deutscher Apotheker Verlag: Stuttgart, Germany, 1993. [Google Scholar]
- EDQM. Devil’s claw root, Harpagophyti radix, 1997:1095. In European Pharmacopoeia, 3rd ed.; published June 1996, replaces the 2nd ed on 1 January 1997; Council of Europe: Strasbourg, France, 1997; p. 1821. [Google Scholar]
- EDQM. Devil’s claw root, Harpagophyti radix, 01/2003:1095. In European Pharmacopoeia, Supplement 4.3 to the Fourth Edition, published 20 June 2002; Council of Europe: Strasbourg, France, 2003; p. 359. [Google Scholar]
- ANSM. Devil’s claw root for homoeopathic preparations. Harpagophytum for homoeopathic preparations. Harpagophytum ad praeparationes homoeopathicas. In Pharmacopée Française, 10th ed.; Maisonneuve: Sainte-Ruffine, France, 2007; pp. 1–3. [Google Scholar]
- EDQM. Devil’s claw dry extract, Harpagophyti extractum siccum, 01/2008:1871. In European Pharmacopoeia, 7th ed.; Council of Europe: Strasbourg, France, 2008. [Google Scholar]
- Health Canada. Devil’s Claw—Harpagophytum. Nat. Health Prod. Ingred. Database 2021. Available online: http://webprod.hc-sc.gc.ca/nhpid-bdipsn/ingredReq.do?id=6188&lang=eng (accessed on 12 April 2021).
- EDQM. Devil’s claw root, Harpagophyti radix, 01/2011:1095. In European Pharmacopoeia, 7th ed.; Council of Europe: Strasbourg, France, 2011. [Google Scholar]
- Bacler-Żbikowska, B.; Drobnik, J. Komentarz botaniczny do roślin leczniczych i surowców roślinnych wymienionych w Farmakopei polskiej VIII. Część, I. Ann. Acad Med. Silesiensis 2011, 1–2, 48–60. [Google Scholar]
- EMA. Assessment Report on Harpagophytum procumbens DC. and/or Harpagophytum zeyheri Decne., Radix. EMA/HMPC/627058/2015; Committee on Herbal Medicinal Products (HMPC): London, UK, 2016. [Google Scholar]
- EMA. List of References Supporting the Assessment of Harpagophytum procumbens DC. and/or Harpagophytum zeyheri Decne., radix. EMA/HMPC/627059/2015; Committee on Herbal Medicinal Products (HMPC): London, UK, 2016. [Google Scholar]
- EDQM. Devil’s claw root, Harpagophyti radix, 01/2011:1095 corrected 9.6. In European Pharmacopoeia, 9th ed.; 6th supplement, Council of Europe: Strasbourg, France, 2018. [Google Scholar]
- Kriukova, A.; Vladymyrova, I.; Gubar, S.; Kotov, A.; Kotova, E. Question introduction to the State Pharmacopoeia of Ukraine monograph «Devil’s claw root». Manag. Econ. Qual. Assur. Pharm. 2018, 6–12. [Google Scholar] [CrossRef]
- European Scientific Cooperative on Phytotherapy. Harpagophyti radix. In ESCOP Monographs. Fascicule 2; ESCOP: Exeter, UK, 1996; pp. 1–7. [Google Scholar]
- Bradley, P.R. Addendum to the ESCOP monograph on Harpagophytum procumbens: Reply from ESCOP. Phytomedicine 2004, 11, 696. [Google Scholar]
- Chrubasik, S. Addendum to the ESCOP monograph on Harpagophytum procumbens. Phytomedicine 2004, 11, 696. [Google Scholar] [CrossRef]
- World Health Organization. Radix Harpagophyti. In WHO Monographs on Selected Medicinal Plants; World Health Organization: Geneva, Switzerland, 2007; Volume 3, pp. 182–193. ISBN 9241545372. [Google Scholar]
- Anonymous. Harpagophytum procumbens. In African Herbal Pharmacopoeia; Brendler, T., Eloff, J.N., Gurib-Fakim, A., Phillips, L.D., Eds.; Association for African Medicinal Plants Standards Port Louis: Port Louis, Mauritius, 2010; pp. 127–134. ISBN 9990389098. [Google Scholar]
- Devil’s claw root. In Martindale: The Complete Drug Reference; Alison, B., Ed.; The Pharmaceutical Press: London, UK, 2017; ISBN 978-0857113092. [Google Scholar]
- Kemper, K.J.; Devil’s Claw (Harpagophytum procumbens). Longwood Herb. Task Force Cent. Holist. Pediatr. Educ. Res. 1999, pp. 1–11. Available online: http://ineldea.com.ua/images/devilsclaw.pdf (accessed on 13 April 2021).
- Devil’s claw. In Herbal Medicines; Barnes, J., Baxter, I.A., Smith, M., Veitch, N.C., Eds.; Pharmaceutical Press: London, UK, 2013; pp. 238–245. ISBN 9780857110350. [Google Scholar]
- Edwards, S.E.; da Costa Rocha, I.; Williamson, E.M.; Heinrich, M. Devil’s Claw Harpagophytum procumbens (Burch.) DC. Ex Meissner, H. zeyheri Decne. In Phytopharmacy: An Evidence-Based Guide to Herbal Medicinal Products; Willey Blackwell: Hoboken, NJ, USA, 2015; p. 131. ISBN 9781118543436. [Google Scholar]
- Kooperation Phytopharmaka. Harpagophytum procumbens DC (Teufelskralle). Arzneipflanzenlexikon. 2020. Available online: https://arzneipflanzenlexikon.info/teufelskralle.php (accessed on 12 April 2021).
- Karioti, A.; Fani, E.; Vincieri, F.F.; Bilia, A.R. Analysis and stability of the constituents of Curcuma longa and Harpagophytum procumbens tinctures by HPLC-DAD and HPLC-ESI-MS. J. Pharm. Biomed. Anal. 2011, 55, 479–486. [Google Scholar] [CrossRef]
- Mncwangi, N.; Viljoen, A.; Zhao, J.; Vermaak, I.; Chen, W.; Khan, I.A. What the devil is in your phytomedicine? Exploring species substitution in Harpagophytum through chemometric modeling of 1H-NMR and UHPLC-MS datasets. Phytochemistry 2014, 106, 104–115. [Google Scholar] [CrossRef]
- Mncwangi, N.; Viljoen, A.M.; Vermaak, I.; Chen, W. Variation of the biologically active constituent harpagoside in Harpagophytum procumbens and H. zeyheri. Planta Med. 2013, 79, P58. [Google Scholar] [CrossRef]
- Muzila, M.; Ekholm, A.; Nybom, H.; Widén, C.; Rumpunen, K. Harpagophytum germplasm varies in tuber peel and pulp content of important phenylpropanoids and iridoids. S. Afr. J. Bot. 2018, 115, 153–160. [Google Scholar] [CrossRef]
- Koch, J.-K.-H. Über ein Chinon und Weitere Inhaltsstoffe aus der Wurzel von Harpagophytum procumbens DC. Ph.D. Thesis, Julius-Maximilians-Universität, Würzburg, Germany, 1970; p. 60. [Google Scholar]
- Bianco, A.; Esposito, P.; Guiso, M.; Scarpati, M.L. Iridoids. 10. Procumbide, a diastereoisomer of antirrinoside. Gazz. Chim. Ital. 1971, 101, 764. [Google Scholar]
- Bauersfeld, H.-J. Über Weitere Inhaltsstoffe aus der Wurzel von Harpagophytum procumbens DC. Ph.D. Thesis, Julius-Maximilians-Universität, Würzburg, Germany, 1974; p. 64. [Google Scholar]
- Tunmann, P.; Bauersfeld, H.J. Über weitere Inhaltsstoffe der Wurzel von Harpagophytum procumbens DC. Arch. Pharm. 1975, 308, 655–657. [Google Scholar] [CrossRef] [PubMed]
- Sticher, O. Plant mono-, di- and sesquiterpenoids with pharmacological or therapeutical activity. In New Natural Products and Plant Drugs with Pharmacological, Biological or Therapeutical Activity. In Proceedings of the First International Congress on Medicinal Plant Research, Section A, Munich, Germany, 6–10 September 1976; Wagner, H., Wolff, P., Eds.; Springer: Berlin/Heidelberg, Germany, 1977; pp. 137–176, ISBN 978-3-642-66684-1. [Google Scholar]
- Kwasniewski, V. Beitrag zur Kenntnis der Inhaltsstoffe der Sekundärwurzeln von Harpagophytum procumbens DC. und zur Frage ihres eventuellen Ersatzes durch einheimische Drogen. Dtsch. Apoth. Ztg. 1978, 118, 49–50. [Google Scholar]
- Bendall, M.R.; Ford, C.W.; Thomas, D.M. The structure of procumbide. Aust. J. Chem. 1979, 32, 2085–2091. [Google Scholar] [CrossRef]
- Ziller, K.H.; Franz, G. Analysis of the water soluble fraction from the roots of Harpagophytum procumbens. Planta Med. 1979, 37, 340–348. [Google Scholar] [CrossRef]
- Vanhaelen, M.; Vanhaelen Fastré, R.; Elchami, A.; Fontaine, J. Activité biologique d’Harpagophytum procumbens DC. I. Préparation et structure de l’harpagogénine. J. Pharm. Belg. 1981, 36, 38–42. [Google Scholar]
- Franz, G.; Czygan, F.C.; Abou-Mandour, A.A. Untersuchungen der Gattung Harpagophytum. 4. Mitteilung: Gehalt an freien Zuckern und Harpagosid in Kalluskulturen und genuinen Wurzelgeweben von Harpagophytum procumbens. Planta Med. 1982, 44, 218–220. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, T.; Matsuda, S.; Kubo, Y.; Namba, T. New iridoid glucosides from Harpagophytum procumbens DC. Chem. Pharm. Bull. 1983, 31, 2296–2301. [Google Scholar] [CrossRef]
- Burger, J.F.W.; Brandt, E.V.; Ferreira, D. Iridoid and phenolic glycosides from Harpagophytum procumbens. Phytochemistry 1987, 26, 1453–1457. [Google Scholar] [CrossRef]
- Baghdikian, B.; Ollivier, E.; Faure, R.; Debrauwer, L.; Rathelot, P.; Balansard, G. Two new pyridine monoterpene alkaloids by chemical conversion of a commercial extract of Harpagophytum procumbens. J. Nat. Prod. 1999, 62, 211–213. [Google Scholar] [CrossRef]
- Picavet, S. Iridoides d’Harpagophytum procumbens DC (Plante a Propriete Anti-Rhumatismale). Ph.D. Thesis, Université de Reims Champagne-Ardenne, Reims, France, 2000; p. 31. [Google Scholar]
- Boje, K. Phytochemische und Biopharmazeutische Untersuchungen an Harpagophytum procumbens DC. Ph.D. Thesis, Westfälische Wilhelms-Universität Münster, Münster, Germany, 2002; p. 286. [Google Scholar]
- Clarkson, C.; Campbell, W.E.; Smith, P. In Vitro antiplasmodial activity of abietane and totarane diterpenes isolated from Harpagophytum procumbens (Devil’s claw). Planta Med. 2003, 69, 720–724. [Google Scholar] [CrossRef] [PubMed]
- Boje, K.; Lechtenberg, M.; Nahrstedt, A. New and known iridoid-and phenylethanoid glycosides from Harpagophytum procumbens and their in vitro inhibition of human leukocyte elastase. Planta Med. 2003, 69, 820–825. [Google Scholar] [CrossRef]
- Munkombwe, N.M. Acetylated phenolic glycosides from Harpagophytum procumbens. Phytochemistry 2003, 62, 1231–1234. [Google Scholar] [CrossRef]
- Goroll, K.A. Pharmakologische Charakterisierung von Harpagosid, Einem Wirkstoff der Teufelskralle. Ph.D. Thesis, Universität Ulm, Ulm, Germany, 2004; p. 97. [Google Scholar]
- Clarkson, C.; Staerk, D.; Hansen, S.H.; Smith, P.J.; Jaroszewski, J.W. Discovering new natural products directly from crude extracts by HPLC-SPE-NMR: Chinane diterpenes in Harpagophytum procumbens. J. Nat. Prod. 2006, 69, 527–530. [Google Scholar] [CrossRef]
- Clarkson, C.; Staerk, D.; Hansen, S.H.; Smith, P.J.; Jaroszewski, J.W. Identification of major and minor constituents of Harpagophytum procumbens (Devil’s claw) using HPLC-SPE-NMR and HPLC-ESIMS/APCIMS. J. Nat. Prod. 2006, 69, 1280–1288. [Google Scholar] [CrossRef]
- Qi, J.; Chen, J.J.; Cheng, Z.H.; Zhou, J.H.; Yu, B.Y.; Qiu, S.X. Iridoid glycosides from Harpagophytum procumbens DC (Devil’s claw). Phytochemistry 2006, 67, 1372–1377. [Google Scholar] [CrossRef]
- Qi, J.; Zhou, J.; Chen, L.; Chen, J.J.; Yu, B.; Qiu, S. Study on chemical constituents in tuber of Harpagophytum procumbens DC. Chin. Pharm. J. 2006, 41, 1613–1615. [Google Scholar]
- Qi, J.; Chen, J.; Tu, Y.; Chen, L.; Yu, B. Chemical constituents of African plant Harpagophytum procumbens. Chin. J. Nat. Med. 2007, 5, 105–107. [Google Scholar]
- Wilken, D.; Röhnert, P.; Appel, K. Erschließung und Charakterisierung Therapeutisch Neuroprotektiver and Anti-Inflammatorischer Wirkstoffe aus Harpagophytum procumbens (Teufelskralle)—TheraTek. Abschlußbericht; BioPlanta GmbH: Leipzig, Germany, 2008; p. 41. [Google Scholar]
- Appel, K.; Rose, T.; Fiebich, B.; Röhnert, P.; Claus, D.; Gerth, A.; Wilken, D. Neuroprotective and antiinflammatory effects of extracts from in vitro cultivated Harpagophytum procumbens (Devil’s claw). Z. Phytother. 2009, 30, P03. [Google Scholar] [CrossRef]
- Qi, J.; Li, N.; Zhou, J.H.; Yu, B.Y.; Qiu, S.X. Isolation and anti-inflammatory activity evaluation of triterpenoids and a monoterpenoid glycoside from Harpagophytum procumbens. Planta Med. 2010, 76, 1892–1896. [Google Scholar] [CrossRef] [PubMed]
- Zgrajka, W.; Turska, M.; Rajtar, G.; Majdan, M.; Parada-Turska, J. Kynurenic acid content in anti-rheumatic herbs. Ann. Agric. Environ. Med. 2013, 20, 800–802. [Google Scholar] [PubMed]
- Tomassini, L.; Serafini, M.; Foddai, S.; Ventrone, A.; Nicoletti, M. A new iridoid diglucoside from Harpagophytum procumbens. Nat. Prod. Res. 2016, 30, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S. “Teufelskralle” vielfach bakteriell bedenklich. Dtsch. Apoth. Ztg. 1978, 118, 1808–1809. [Google Scholar]
- Abramowicz, M. Toxic reactions to plant products sold in health food stores. Med. Lett. Drugs Ther. 1979, 21, 29–31. [Google Scholar]
- Loew, D.; Schuster, O.; Möllerfeld, J. Stabilität und biopharmazeutische Qualität. Voraussetzung für Bioverfügbarkeit und Wirksamkeit von Harpagophytum procumbens. In Phytopharmaka II, Forschung und Klinische Anwendung; Loew, D., Rietbrock, N., Eds.; Steinkopff: Darmstadt, Germany, 1996; pp. 83–93. [Google Scholar]
- Chrubasik, S. Biopharmazeutische Qualität und klinische Wirksamkeit von Zubereitungen aus Harpagophytum Extrakt. In Rheumatherapie mit Phytopharmaka; Chrubasik, S., Wink, M., Eds.; Hippokrates: Stuttgart, Germany, 1997; pp. 77–85. [Google Scholar]
- Schier, W.; Bauersfeld, H.-J. Handelssorten von Harpagophytum procumbens DC. 1. Mitteilung. Dtsch. Apoth. Ztg. 1973, 113, 795–796. [Google Scholar]
- Schier, W. Handelssorten von Harpagophytum procumbens DC. 2. Mitteilung. Dtsch. Apoth. Ztg. 1974, 114, 1800–1801. [Google Scholar]
- Becker, H.; Richter, S. Eine einfache dünnschichtchromatographische Untersuchung von Harpagophytum procumbens für das Apotheken-Labor. Pharm. Ztg. 1975, 120, 441–442. [Google Scholar]
- Czygan, F.-C.; Krüger, A. Pharmazeutisch-biologische Untersuchungen der Gattung Harpagophytum—3. Mitteilung: Zur Verteilung des Iridoid-Glycosids Harpagosid in den einzelnen Organen von Harpagophytum procumbens DC und Harpagophytum zeyheri Decne. Planta Med. 1977, 31, 305–307. [Google Scholar] [CrossRef]
- Haag-Berrurier, M.; Kuballa, B.; Anton, R. Dosage des glucoiridoïdes totaux dans la racine d’Harpagophytum procumbens DC. Plantes Méd. Phytothér. 1978, 12, 197–206. [Google Scholar]
- Sticher, O.; Meier, B. Quantitative Bestimmung von Harpagosid in Wurzeln von Harpagophytum procumbens mit Hochleistungsflüssigkeitschromatographie (HPLC). Dtsch. Apoth. Ztg. 1980, 120, 1592–1594. [Google Scholar]
- Vanhaelen, M.; Vanhaelen-Fastré, R.; Elchami, A.A. Gas-liquid chromatographic determination of the iridoid content in Harpagophytum procumbens DC. J. Chromatogr. A 1981, 209, 476–478. [Google Scholar] [CrossRef]
- Ragusa, S.; Circosta, C.; Galati, E.M.; Tumino, G. A drug used in traditional medicine. Harpagophytum procumbens DC I. Scanning electron microscope observations. J. Ethnopharmacol. 1984, 11, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Pourrat, H.; Texier, O.; Vennat, B.; Pourrat, A.; Galliard, J. Etude de la stabilité des iridoïdes d’Harpagophytum procumbens DC au cours de la préparation de poudres et d’atomisats. Ann. Pharm. Fr. 1985, 43, 601–606. [Google Scholar] [PubMed]
- Ficarra, P.; Ficarra, R.; Tommasini, A.; De Pasquale Costa, R.; Guarniera Fenech, C.; Ragusa, S. Analisi mediante HPLC di una droga della medicina tradizionale: Harpagophytum procumbens DC. Nota I. Boll. Chim. Farm. 1986, 125, 250–253. [Google Scholar] [PubMed]
- Franke, A.; Rimpler, H. GC/MS-Analyse methylierter Iridoidglykoside. Planta Med. 1986, 52, 89–95. [Google Scholar] [CrossRef]
- Guillerault, L.; Ollivier, E.; Elias, R.; Balansard, G. Determination of harpagide, 8-para-coumaroyl harpagide, and harpagoside by high performance liquid chromatography in Harpagophytum procumbens drugs and in a commercial extract. J. Liq. Chromatogr. 1994, 17, 2951–2960. [Google Scholar] [CrossRef]
- Mestdagh, O.; Torck, M. Etude de la qualité de gélules d’Harpagophyton. Ann. Pharm. Fr. 1995, 53, 135–137. [Google Scholar] [PubMed]
- Wolf, J. Mikro-Dünnschichtchromatographie Teufelskrallenwurzel. Pharm. Ztg. 1995, 140, 28. [Google Scholar]
- Chrubasik, S.; Sporer, F.; Wink, M. Zum Harpagosidgehalt verschiedener Trockenextraktpulver aus Harpagophytum procumbens. Complement. Med. Res. 1996, 3, 6–11. [Google Scholar] [CrossRef]
- Chrubasik, S.; Sporer, F.; Wink, M. Zum Wirkstoffgehalt in Teezubereitungen aus Harpagophytum procumbens. Complement. Med. Res. 1996, 3, 116–119. [Google Scholar] [CrossRef]
- Chrubasik, S.; Sporer, F.; Wink, M. Zum Wirkstoffgehalt in Arzneimitteln aus Harpagophytum procumbens. Complement. Med. Res. 1996, 3, 57–63. [Google Scholar] [CrossRef]
- Poukens-Renwart, P.; Tits, M.; Angenot, L. Quantitative densitometric evaluation of harpagoside in the secondary roots of Harpagophytum procumbens DC. J. Planar Chromatogr. 1996, 9, 199–202. [Google Scholar]
- Marotta, M.; Addabbo, I.; Kosasi, S. The quality control and stability testing of homeopathic preparations. Boll. Chim. Farm. 1998, 137, 439–441. [Google Scholar]
- Sporer, F.; Chrubasik, S. Präparate aus der Teufelskralle (Harpagophytum procumbens). Z. Phytother. 1999, 20, 335–336. [Google Scholar]
- Chrubasik, S.; Sporer, F.; Dillmann-Marschner, R.; Friedmann, A.; Wink, M. Physicochemical properties of harpagoside and its in vitro release from Harpagophytum procumbens extract tablets. Phytomedicine 2000, 6, 469–473. [Google Scholar] [CrossRef]
- Schneider, E.; Sanders, J.; Von Willert, D.J. Vermeidung von Verfälschungen der Teufelskralle Harpagophytum procumbens: Ein Beitrag zur pharmakognostischen Ökologie. Drogenreport 2001, 14, 12–16. [Google Scholar]
- Chrubasik, S.; Pollak, S.; Fiebich, B. Harpagophytum extracts. Clin. Pharm. 2002, 71, 104–105. [Google Scholar] [CrossRef] [PubMed]
- Gray, B.R. Retention of the Harpagoside Content in Dried Harpagophytum procumbens (Devil’s Claw) Root Through Controlled Drying and the Application of Near Infrared Spectroscopy (NIRS) as Rapid Method of Determination. Master’s Thesis, University of Stellenbosch, Stellenbosch, South Africa, 2003; p. 138. [Google Scholar]
- Baranska, M.; Schulz, H.; Siuda, R.; Strehle, M.A.; Rosch, P.; Popp, J.; Joubert, E.; Manley, M. Quality control of Harpagophytum procumbens and its related phytopharmaceutical products by means of NIR-FT-Raman spectroscopy. Biopolymers 2005, 77, 1–8. [Google Scholar] [CrossRef]
- Günther, M.; Schmidt, P.C. Comparison between HPLC and HPTLC-densitometry for the determination of harpagoside from Harpagophytum procumbens CO(2)-extracts. J. Pharm. Biomed. Anal. 2005, 37, 817–821. [Google Scholar] [CrossRef]
- Joubert, E.; Manley, M.; Gray, B.R.; Schulz, H. Rapid measurement and evaluation of the effect of drying conditions on harpagoside content in Harpagophytum procumbens (devil’s claw) root. J. Agric. Food Chem. 2005, 53, 3493–3502. [Google Scholar] [CrossRef]
- Schmidt, A.H. Fast HPLC for quality control of Harpagophytum procumbens by using a monolithic silica column: Method transfer from conventional particle-based silica column. J. Chromatogr. A 2005, 1073, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.H. Validation of a fast-HPLC method for the separation of iridoid glycosides to distinguish between the Harpagophytum species. J. Liq. Chromatogr. Relat. Technol. 2007, 28, 2339–2347. [Google Scholar] [CrossRef]
- Seger, C.; Godejohann, M.; Tseng, L.H.; Spraul, M.; Girtler, A.; Sturm, S.; Stuppner, H. LC-DAD-MS/SPE-NMR hyphenation. A tool for the analysis of pharmaceutically used plant extracts: Identification of isobaric iridoid glycoside regioisomers from Harpagophytum procumbens. Anal. Chem. 2005, 77, 878–885. [Google Scholar] [CrossRef]
- Zucchi, O.L.; Moreira, S.; de Jesus, E.F.; Neto, H.S.; Salvador, M.J. Characterization of hypoglycemiant plants by total reflection X-ray fluorescence spectrometry. Biol. Trace Elem. Res. 2005, 103, 277–290. [Google Scholar] [CrossRef]
- Arranz, I.; Sizoo, E.; van Egmond, H.; Kroeger, K.; Legarda, T.M.; Burdaspal, P.; Reif, K.; Stroka, J. Determination of aflatoxin B1 in medical herbs: Interlaboratory study. J. AOAC Int. 2006, 89, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Colas, C.; Garcia, P.; Popot, M.A.; Bonnaire, Y.; Bouchonnet, S. Liquid chromatography/electrospray ionization mass spectrometric characterization of Harpagophytum in equine urine and plasma. Rapid Commun. Mass Spectrom. 2006, 20, 3257–3266. [Google Scholar] [CrossRef] [PubMed]
- Colas, C.; Bouchonnet, S.; Rogalewicz-Gilard, F.; Popot, M.A.; Ohanessian, G. Proton and sodium cation affinities of harpagide: A computational study. J. Phys. Chem. A 2006, 110, 7503–7508. [Google Scholar] [CrossRef]
- Schmidt, A.H.; Schiemann, U. Teufelskralle aus dem Drogeriemarkt oder aus der Apotheke? Dtsch. Apoth. Ztg. 2006, 146, 50–55. [Google Scholar]
- Spriano, D.; Krasniqi, B.; Strompen, T.; Tobler, M.; Meier, B. The drug-extract-ratio of aqueous/ethanolic Harpagophyti radix extracts has to be revised. Planta Med. 2006, 72, P_287. [Google Scholar] [CrossRef]
- Street, R.A.; Southway, C.; Stirk, W.A.; Van Staden, J. Determination of mineral elements and heavy metals in indigenous medicinal plants of KwaZulu-Natal. S. Afr. J. Bot. 2007, 73, 315. [Google Scholar] [CrossRef]
- Colas, C.; Popot, M.-A.; Garcia, P.; Bonnaire, Y.; Bouchonnet, S. Analysis of iridoids from Harpagophytum and eleutherosides from Eleutherococcus senticosus in horse urine. Biomed. Chromatogr. 2008, 22, 912–917. [Google Scholar] [CrossRef]
- Colas, C.; Garcia, P.; Popot, M.-A.; Bonnaire, Y.; Bouchonnet, S. Optimization of solid-phase extraction for the liquid chromatography—Mass spectrometry analysis of harpagoside, 8-para-coumaroyl harpagide, and harpagide in equine plasma and urine. J. Chromatogr. Sci. 2008, 46, 174–183. [Google Scholar] [CrossRef][Green Version]
- Wagner, S.; Urena, A.; Reich, E.; Merfort, I. Validated HPTLC methods for the determination of salicin in Salix sp. and of harpagoside in Harpagophytum procumbens. J. Pharm. Biomed. Anal. 2008, 48, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Chigome, S.; Nindi, M.; Munkombwe, N. Quality control of Harpagophytum procumbens products using High Pressure Liquid Chromatography-Diode Array Detection (HPLC-DAD). Niger. J. Nat. Prod. Med. 2009, 13, 26–29. [Google Scholar] [CrossRef]
- Babili, F.E.; Fouraste, I.; Rougaignon, C.; Moulis, C.; Chatelain, C. Anatomical study of secondary tuberized roots of Harpagophytum procumbens DC and quantification of harpagoside by high-performance liquid chromatography method. Pharm. Mag. 2012, 8, 175–180. [Google Scholar] [CrossRef]
- Newmaster, S.G.; Grguric, M.; Shanmughanandhan, D.; Ramalingam, S.; Ragupathy, S. DNA barcoding detects contamination and substitution in North American herbal products. BMC Med. 2013, 11, 222. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, Y.; Heneidak, S.; Bhatt, A.; Kasim, N.; Naidoo, G. Morphology, histochemistry, and ultrastructure of foliar mucilage-producing trichomes of Harpagophytum procumbens (Pedaliaceae). Turk. J. Bot. 2014, 38, 60–67. [Google Scholar] [CrossRef]
- Zhao, J.; Mncwangi, N.; Viljoen, A.; Wang, M.; Khan, I.A. Differentiation of Harpagophytum procumbens and H. zeyheri through NMR-based chemometric approach. Planta Med. 2014, 80, PPL17. [Google Scholar] [CrossRef]
- Kondamudi, N.; Turner, M.W.; McDougal, O.M. Harpagoside content in devil’s claw extracts. Nat. Prod. Commun. 2016, 11, 1215–1216. [Google Scholar] [CrossRef] [PubMed]
- Kriukova, A.; Vladymyrova, I. The definition of numeric indicators for the root of Harpagophytum procumbens. In Proceedings of the Topical Issues of New Drugs Development: XXIII International Scientific and Practical Conference of Young Scientists and Students, Kharkiv, Ukraine, 21 April 2016; p. 188. [Google Scholar]
- Baghdikian, B.; Filly, A.; Fabiano-Tixier, A.-S.; Petitcolas, E.; Mabrouki, F.; Chemat, F.; Ollivier, É. Extraction by solvent using microwave and ultrasound-assisted techniques followed by HPLC analysis of Harpagoside from Harpagophytum procumbens and comparison with conventional solvent extraction methods. C. R. Chim. 2016, 19, 692–698. [Google Scholar] [CrossRef]
- Diuzheva, A.; Carradori, S.; Andruch, V.; Locatelli, M.; De Luca, E.; Tiecco, M.; Germani, R.; Menghini, L.; Nocentini, A.; Gratteri, P.; et al. Use of innovative (micro)extraction techniques to characterise Harpagophytum procumbens root and its commercial food supplements. Phytochem. Anal. 2018, 29, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Kriukova, A.; Vladymyrova, I.; Tishakova, T. Rationale for choosing of extraction solvent for obtaining liquid extract from the roots of Harpagophytum procumbens DC. Scr. Sci. Pharm. 2017, 4, 37. [Google Scholar]
- Kriukova, A.; Vladymyrova, I. The GC-MS determination of chemical constituents from Harpagophytum procumbens DC roots. Technol. Transf. Innov. Solut. Med. 2017, 52–54. [Google Scholar] [CrossRef]
- Pretorius, E.; Van der Bank, M.; Viljoen, A.M. DNA Barcoding detects contamination and substitution in herbal products containing Harpagophytum spp. S. Afr. J. Bot. 2017, 109, 364–365. [Google Scholar] [CrossRef]
- Avato, P.; Argentieri, M.P. Quality assessment of commercial spagyric tinctures of Harpagophytum procumbens and their antioxidant properties. Molecules 2019, 24, 2251. [Google Scholar] [CrossRef] [PubMed]
- Nalluri, B.N.; Sujithkumar, S. Characterization and estimation of harpagoside in dried root extract and oral powder formulations of Harpagophytum procumbens by validated RP-HPLC-PDA method. J. Drug Deliv. Ther. 2019, 9, 38–46. [Google Scholar] [CrossRef]
- De Aragao Tannus, C.; de Souza Dias, F.; Santana, F.B.; Dos Santos, D.; Magalhaes, H.I.F.; de Souza Dias, F.; de Freitas Santos Junior, A. Multielement determination in medicinal plants and herbal medicines containing Cynara scolymus L., Harpagophytum procumbens DC, and Maytenus ilifolia (Mart.) ex Reiss from Brazil using ICP OES. Biol. Trace Elem. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Ribeiro, G.; de Assis Carneiro, A.; Martins, D.H.N.; Simeoni, L.A.; Silveira, D.; Magalhães, P.O.; Fonseca-Bazzo, Y.M. Determination of harpagoside in Harpagophytum procumbens DC tablet’s using analytical method by high performance liquid chromatography. Eclét. Quím. J. 2020, 45, 47–55. [Google Scholar] [CrossRef]
- Duband, F. Harpagophytum procumbens DC: Recherche d’une Activité Anti-Inflammatoire Aiguë. Ph.D. Thesis, UFR de Pharmacie, Clermont-Ferrand, France, 1986; p. 76. [Google Scholar]
- Plaizier-Vercammen, J.A.; Bruwier, C. Evaluation of excipients for direct compression of the spray-dried extract of Harpagophytum procumbens. Soc. Fr. Sci. Tech. Pharm. 1986, 2, 525–530. [Google Scholar]
- Günther, M.; Maus, M.; Wagner, K.G.; Schmidt, P.C. Hydrophilic solutes in modified carbon dioxide extraction-prediction of the extractability using molecular dynamic simulation. Eur. J. Pharm. Sci. 2005, 25, 321–329. [Google Scholar] [CrossRef]
- Piechota-Urbanska, M.; Kolodziejska, J.; Berner-Strzelczyk, A. Zastosowanie wyciągu z czarciego pazura w przeciwzapalnych preparatach aplikowanych na skórę, wytworzonych na bazie polimerów kwasu akrylowego. Polim. Med. 2009, 39, 9–15. [Google Scholar]
- Almajdoub, S.S. Polymer Coating of an Optimized Nano Lipid Carrier System of Harpagophytum procumbens Extract for Oral Delivery. Master’s Thesis, University of the Western Cape, Cape Town, South Africa, 2017; p. 182. [Google Scholar]
- Lopes, A.P.; Bagatela, B.S.; Lopes, I.P.; Gregorio, L.E.; Maistro, E.L.; Fonseca, F.L.A.; Perazzo, F.F. Production of gastro-resistant coated tablets prepared from the hydroethanolic standardized roots extract of Harpagophytum procumbens DC. Afr. J. Pharm. Pharmacol. 2017, 11, 491–500. [Google Scholar] [CrossRef]
- Villasenor, I.M. Bioactivities of iridoids. Anti. Inflamm. Anti. Allergy Agents Med. Chem. (Former. Curr. Med. Chem. Anti Inflamm. Anti Allergy Agents) 2007, 6, 307–314. [Google Scholar] [CrossRef]
- Serrano, A.; Ros, G.; Nieto, G. Bioactive compounds and extracts from traditional herbs and their potential anti-inflammatory health effects. Medicines 2018, 5, 76. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, J.; Elchami, A.; Vanhaelen, M.; Vanhaelen Fastré, R. Activité biologique d’Harpagophytum procumbens DC. 2. Analyse pharmacologique des effets de l’harpagoside, l’harpagide et l’harpagogenine sur l’ileon isole de cobaye. J. Pharm. Belg. 1981, 36, 321–324. [Google Scholar]
- Benito, P.B.; Lanza, A.M.D.; Sen, A.M.S.; De Santos Galindez, J.; Matellano, L.F.; Gómez, A.S.; Martínez, M.J.A. Effects of some iridoids from plant origin on arachidonic acid metabolism in cellular systems. Planta Med. 2000, 66, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Fiebich, B.L.; Heinrich, M.; Hiller, K.O.; Kammerer, N. Inhibition of TNF-alpha synthesis in LPS-stimulated primary human monocytes by Harpagophytum extract SteiHap 69. Phytomedicine 2001, 8, 28–30. [Google Scholar] [CrossRef]
- Loew, D.; Mollerfeld, J.; Schrodter, A.; Puttkammer, S.; Kaszkin, M. Investigations on the pharmacokinetic properties of Harpagophytum extracts and their effects on eicosanoid biosynthesis in vitro and ex vivo. Clin. Pharm. 2001, 69, 356–364. [Google Scholar] [CrossRef]
- Wahrendorf, M.S.; Sporer, F.; Wink, M. Anti-inflammation assays on Harpagophytum procumbens. Des Sources du Savoir aux Médicaments du Futur: Actes du 4e Congrès Européen d’Ethnopharmacologie. In Proceedings of the 4th European Congress on Ethnopharmocology, Metz, France, 11–13 May 2000; Fleurentin, J., Pelt, J., Mazars, G., Lejosne, J.C., Cabalion, P., Eds.; IRD: Metz, France, 2002; pp. 399–401, ISBN 2709915049. [Google Scholar]
- Jang, M.H.; Lim, S.; Han, S.M.; Park, H.J.; Shin, I.; Kim, J.W.; Kim, N.J.; Lee, J.S.; Kim, K.A.; Kim, C.J. Harpagophytum procumbens suppresses lipopolysaccharide-stimulated expressions of cyclooxygenase-2 and inducible nitric oxide synthase in fibroblast cell line L929. J. Pharm. Sci. 2003, 93, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Schulze-Tanzil, G.; Hansen, C.; Shakibaei, M. Wirkung des Extraktes aus Harpagophytum procumbens DC auf Matrix-Metalloproteinasen in menschlichen Knorpelzellen in vitro. Arzneimittelforschung 2004, 54, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Chrubasik, J.E. Zur Knorpelprotektion von Harpagophytum procumbens DC: Histologische, Zellbiologische und Molekularbiologische Untersuchungen. Ph.D. Thesis, Justus-Liebig-Universität Gießen, Gießen, Germany, 2006; p. 135. [Google Scholar]
- Chrubasik, J.E.; Neumann, E.; Lindhorst, E.; Chrubasik, S.; Muller-Ladner, U. Evaluation of the chondroprotective effect of Harpagophytum procumbens. Med. Klin. 2006, 101, A90. [Google Scholar]
- Hadzhiyski, H.; Torda, T.; Chrubasik, S.; Lindhorst, E.; Raif, W. Impact of Harpagophytum procumbens on the urinary pyridinoline deoxypyridinoline ratio in experimental osteoarthritis. Focus Altern. Complement. Ther. 2006, 11, 13. [Google Scholar] [CrossRef]
- Günther, M.; Laufer, S.; Schmidt, P.C. High anti-inflammatory activity of harpagoside-enriched extracts obtained from solvent-modified super- and subcritical carbon dioxide extractions of the roots of Harpagophytum procumbens. Phytochem. Anal. 2006, 17, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.H.; Tran, V.H.; Duke, R.K.; Tan, S.; Chrubasik, S.; Roufogalis, B.D.; Duke, C.C. Harpagoside suppresses lipopolysaccharide-induced iNOS and COX-2 expression through inhibition of NF-kappa B activation. J. Ethnopharmacol. 2006, 104, 149–155. [Google Scholar] [CrossRef]
- Balthazar, L.v.; Eggenschwiler, J.; Rohrer, J.; Suter, A. Investigations on the antiinflammatory way of action of a Harpagophytum extract using microarray technology. Planta Med. 2009, 75, PJ158. [Google Scholar] [CrossRef]
- Ebrahim, N.; Uebel, R.A. Direct inhibition of cyclooxygenase-2 enzyme by an extract of Harpagophytum procumbens, harpagoside and harpagide. Afr. J. Pharm. Pharmacol. 2011, 5, 2209–2212. [Google Scholar] [CrossRef]
- Gyurkovska, V.; Alipieva, K.; Maciuk, A.; Dimitrova, P.; Ivanovska, N.; Haas, C.; Bley, T.; Georgiev, M. Anti-inflammatory activity of Devil’s claw in vitro systems and their active constituents. Food Chem. 2011, 125, 171–178. [Google Scholar] [CrossRef]
- Fiebich, B.L.; Munoz, E.; Rose, T.; Weiss, G.; McGregor, G.P. Molecular targets of the antiinflammatory Harpagophytum procumbens (Devil’s claw): Inhibition of TNFalpha and COX-2 gene expression by preventing activation of AP-1. Phytother. Res. 2012, 26, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Ebrahim, N. Development and Characterization of a Transdermal Formula for an Extract of the Medicinal Plant Harpagophytum procumbens. Ph.D. Thesis, University of the Western Cape, Belville, South Africa, 2013; p. 199. [Google Scholar]
- Hostanska, K.; Melzer, J.; Rostock, M.; Suter, A.; Saller, R. Alteration of anti-inflammatory activity of Harpagophytum procumbens (Devil’s claw) extract after external metabolic activation with S9 mix. J. Pharm. Pharmacol. 2014, 66, 1606–1614. [Google Scholar] [CrossRef]
- Haseeb, A.; Leigh, D.; Haqqi, T.M. A small molecule harpagoside inhibits IL-1beta-induced expression of IL-6 by blocking the expression of C-FOS in primary human osteoarthritis chondrocytes. Osteoarthr. Cartil. 2015, 23, A155–A156. [Google Scholar] [CrossRef][Green Version]
- Haseeb, A.; Ansari, M.Y.; Haqqi, T.M. Harpagoside suppresses IL-6 expression in primary human osteoarthritis chondrocytes. J. Orthop. Res. 2017, 35, 311–320. [Google Scholar] [CrossRef]
- Kim, T.K.; Park, K.S. Inhibitory effects of harpagoside on TNF-alpha-induced pro-inflammatory adipokine expression through PPAR-gamma activation in 3T3-L1 adipocytes. Cytokine 2015, 76, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Schopohl, P.; Gruneberg, P.; Melzig, M.F. The influence of harpagoside and harpagide on TNFalpha-secretion and cell adhesion molecule mRNA-expression in IFNgamma/LPS-stimulated THP-1 cells. Fitoterapia 2016, 110, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Mariano, A.; Di Sotto, A.; Leopizzi, M.; Garzoli, S.; Di Maio, V.; Gulli, M.; Dalla Vedova, P.; Ammendola, S.; Scotto d’Abusco, A. Antiarthritic effects of a root extract from Harpagophytum procumbens DC: Novel insights into the molecular mechanisms and possible bioactive phytochemicals. Nutrients 2020, 12, 2545. [Google Scholar] [CrossRef]
- Eichler, O.; Koch, C. Über die antiphlogistische, analgetische und spasmolytische Wirksamkeit von Harpagosid, einem Glykosid aus der Wurzel von Harpagophytum procumbens DC. Drug Res. 1970, 20, 107–109. [Google Scholar]
- Anonymous. Prüfung von “Harpagophytum procumbens DC” auf Antiphlogistische Wirksamkeit am Rattenpfotenödem; International Bio Research, Mitteilung an Fa. Hagen: Freilassing, Germany, 1974. [Google Scholar]
- Anonymous. Subakute Studie an der Ratte zur Prüfung des Einflusses von Harpagosan-Extrakt auf Verschiedene Blutkomponente; International Bio Research, Mitteilung an Fa. Hagen: Freilassing, Germany, 1974. [Google Scholar]
- Erdös, A.; Fontaine, R.; Friehe, H.; Durand, R.; Pöppinghaus, T. Beitrag zur Pharmakologie und Toxikologie verschiedener Extrakte, sowie des Harpagosids aus Harpagophytum procumbens DC. Planta Med. 1978, 34, 97–108. [Google Scholar] [CrossRef]
- McLeod, D.W.; Revell, P.; Robinson, B.V. Investigations of Harpagophytum procumbens (Devil’s claw) in the treatment of experimental inflammation and arthritis in the rat. Br. J. Pharmacol. 1979, 66, 140P–141P. [Google Scholar]
- Manez, S.; Alcaraz, M.; Paya, M.; Rios, J.; Hancke, J. Selected extracts from medicinal plants as anti-inflammatory agents. Planta Med. 1990, 56, 656. [Google Scholar] [CrossRef]
- Lanhers, M.C.; Fleurentin, J.; Mortier, F.; Vinche, A.; Younos, C. Anti-inflammatory and analgesic effects of an aqueous extract of Harpagophytum procumbens. Planta Med. 1992, 58, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Jadot, G.; Lecomte, A. Activité anti-inflammatoire d’Harpagophytum procumbens DC. Lyon Méditerr. Méd. Méd. Sud. Est. 1992, 28, 833–835. [Google Scholar]
- Del Carmen Recio, M.; Giner, R.M.; Máñez, S.; Ríos, J.L. Structural considerations on the iridoids as anti-inflammatory agents. Planta Med. 1994, 60, 232–234. [Google Scholar] [CrossRef] [PubMed]
- Soulimani, R.; Younos, C.; Mortier, F.; Derrieu, C. The role of stomachal digestion on the pharmacological activity of plant extracts, using as an example extracts of Harpagophytum procumbens. Can. J. Physiol. Pharmacol. 1994, 72, 1532–1536. [Google Scholar] [CrossRef] [PubMed]
- Abe, S.; Ishibashi, H.; Masuo, K.; Tanaka, S.-I.; Yamaguchi, H. Suppression of carrageenan-induced edema by oral administration of extracts of Uncaria tomentosa and/or Harpagophytum procumbens. Oyo Yakuri Pharmacomet. 2002, 62, 27–32. [Google Scholar]
- Andersen, M.L.; Santos, E.H.; Seabra Mde, L.; da Silva, A.A.; Tufik, S. Evaluation of acute and chronic treatments with Harpagophytum procumbens on Freund’s adjuvant-induced arthritis in rats. J. Ethnopharmacol. 2004, 91, 325–330. [Google Scholar] [CrossRef]
- Mahomed, I.M.; Ojewole, J.A.O. Analgesic, antiinflammatory and antidiabetic properties of Harpagophytum procumbens DC (Pedaliaceae) secondary root aqueous extract. Phytother. Res. 2004, 18, 982–989. [Google Scholar] [CrossRef]
- Mahomed, I.M. Some Pharmacological Properties of Harpagophytum procumbens DC [Pedaliaceae] Secondary Root Extract. Ph.D. Thesis, University of KwaZulu-Natal, Durban, South Africa, 2004; p. 172. [Google Scholar]
- Ahmed, M.I.; Afifi, M.I.; Younos, I.H. Harpagophytum procumbens (Devil’s claw): A possible natural anti-inflammatory agent (an experimental study). IJPT 2005, 4, 54–63. [Google Scholar]
- Kundu, J.K.; Mossanda, K.S.; Na, H.K.; Surh, Y.J. Inhibitory effects of the extracts of Sutherlandia frutescens (L.) R. Br. and Harpagophytum procumbens DC. on phorbol ester-induced COX-2 expression in mouse skin: AP-1 and CREB as potential upstream targets. Cancer Lett. 2005, 218, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Catelan, S.C.; Belentani, R.M.; Marques, L.C.; Silva, E.R.; Silva, M.A.; Caparroz-Assef, S.M.; Cuman, R.K.; Bersani-Amado, C.A. The role of adrenal corticosteroids in the anti-inflammatory effect of the whole extract of Harpagophytum procumbens in rats. Phytomedicine 2006, 13, 446–451. [Google Scholar] [CrossRef]
- Chrubasik, J.E.; Lindhorst, E.; Neumann, E.; Gerlach, U.; Faller-Marquardt, M.; Torda, T.; Muller-Ladner, U.; Chrubasik, S. Potential molecular basis of the chondroprotective effect of Harpagophytum procumbens. Phytomedicine 2006, 13, 598–600. [Google Scholar] [CrossRef] [PubMed]
- Uchida, S.; Hirai, K.; Hatanaka, J.; Hanato, J.; Umegaki, K.; Yamada, S. Antinociceptive effects of St. John’s wort, Harpagophytum procumbens extract and Grape seed proanthocyanidins extract in mice. Biol. Pharm. Bull. 2008, 31, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Wachsmuth, L.; Lindhorst, E.; Wrubel, S.; Hadzhiyski, H.; Hudelmaier, M.; Eckstein, F.; Chrubasik, S. Micro-morphometrical assessment of the effect of Harpagophytum procumbens extract on articular cartilage in rabbits with experimental osteoarthritis using magnetic resonance imaging. Phytother. Res. 2011, 25, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Wrubel, S. Untersuchungen zur Wirkung von Harpagophytum procumbens auf Kniegelenksarthrose im Kaninchen unter Verwendung der Magentresonanztomographie. Ph.D. Thesis, Ludwig-Maximilians-Universität, München, Germany, 2014; p. 65. [Google Scholar]
- Bisinotto, R. Efeito Anti-Inflamatório do Extrato Etanólico da Harpagophytum procumbens Durante a Inflamação Intestinal De Camundongos Infectados Com Salmonella Enteritidis (ATCC13076). Master’s Thesis, Universidade Federal de São Carlos, São Carlos, Brazil, 2014; p. 100. [Google Scholar]
- Lim, D.W.; Kim, J.G.; Han, D.; Kim, Y.T. Analgesic effect of Harpagophytum procumbens on postoperative and neuropathic pain in rats. Molecules 2014, 19, 1060–1068. [Google Scholar] [CrossRef] [PubMed]
- Parenti, C.; Arico, G.; Chiechio, S.; Di Benedetto, G.; Parenti, R.; Scoto, G.M. Involvement of the heme-oxygenase pathway in the antiallodynic and antihyperalgesic activity of Harpagophytum procumbens in Rats. Molecules 2015, 20, 16758–16769. [Google Scholar] [CrossRef]
- Ucuncu, Y.; Celik, N.; Ozturk, C.; Turkoglu, M.; Cetin, N.; Kockara, N.; Sener, E.; Dundar, C.; Arslan, A.; Dogan, H.; et al. Chondroprotective effects of a new glucosamine combination in rats: Gene expression, biochemical and histopathological evaluation. Life Sci. 2015, 130, 31–37. [Google Scholar] [CrossRef]
- Parenti, C.; Arico, G.; Pennisi, M.; Venditti, A.; Scoto, G.M. Harpagophytum procumbens extract potentiates morphine antinociception in neuropathic rats. Nat. Prod. Res. 2016, 30, 1248–1255. [Google Scholar] [CrossRef]
- Radomska-Lesniewska, D.M.; Skopinska-Rozewska, E.; Demkow, U.; Jozwiak, J.; Sobiecka, M.; Balan, B.J. A natural herbal remedy modulates angiogenic activity of bronchoalveolar lavage cells from sarcoidosis patients. Cent. Eur. J. Immunol. 2016, 41, 25–34. [Google Scholar] [CrossRef]
- Tippler, B.; Syrovets, T.; Plaza, N.; Loew, D.; Simmet, T.H. Harpagophytum procumbens DC used in traditional medicine inhibits eicosanoid biosynthesis in human whole blood. Int. J. Tissue React. 1997, 19, 101. [Google Scholar]
- Tippler, B.; Syrovets, T.; Loew, D.; Simmet, T.H. Harpagophytum procumbens: Wirkung von Extrakten auf die Eicosanoidbiosynthese in lonophor A23187-stimuliertem menschlichem Vollblut. In Phytopharmaka II, Forschung und Klinische Anwendung; Loew, D., Rietbrock, N., Eds.; Steinkopff: Darmstadt, Germany, 1996; pp. 95–100. [Google Scholar]
- Anauate, M.C. Efeito Dos Extratos De Harpagohytum procumbens (Garra-do-Diabo) E Suas Frações Na Atividade da COX-1 e COX-2 e Na Produção De NO Em Sangue Total. Ph.D. Thesis, Universidade de São Paulo, São Paulo, Brazil, 2007; p. 98. [Google Scholar]
- Abdelouahab, N.; Heard, C.M. Effect of the major glycosides of Harpagophytum procumbens (Devil’s claw) on epidermal cyclooxygenase-2 (COX-2) in vitro. J. Nat. Prod. 2008, 71, 746–749. [Google Scholar] [CrossRef] [PubMed]
- Abdelouahab, N.; Heard, C.M. Dermal and transcutaneous delivery of the major glycoside constituents of Harpagophytum procumbens (Devil’s claw) in vitro. Planta Med. 2008, 74, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Ouitas, N.A.; Heard, C.M. Topical Delivery and Effects of Harpagophytum procumbens. Ph.D. Thesis, Cardiff University, Cardiff, UK, 2009; p. 298. [Google Scholar]
- Ouitas, N.A.; Heard, C.M. A novel ex vivo skin model for the assessment of the potential transcutaneous anti-inflammatory effect of topically applied Harpagophytum procumbens extract. Int. J. Pharm. 2009, 376, 63–68. [Google Scholar] [CrossRef]
- Ouitas, N.A.; Heard, C.M. Estimation of the relative antiinflammatory efficacies of six commercial preparations of Harpagophytum procumbens (Devil’s claw). Phytother. Res. 2010, 24, 333–338. [Google Scholar] [CrossRef]
- Anauate, M.C.; Torres, L.M.; de Mello, S.B. Effect of isolated fractions of Harpagophytum procumbens DC (Devil’s claw) on COX-1, COX-2 activity and nitric oxide production on whole-blood assay. Phytother. Res. 2010, 24, 1365–1369. [Google Scholar] [CrossRef] [PubMed]
- Whitehouse, L.W.; Znamirowska, M.; Paul, C.J. Devil’s claw (Harpagophytum procumbens): No evidence for anti-inflammatory activity in the treatment of arthritic disease. Can. Med. Assoc. J. 1983, 129, 249–251. [Google Scholar]
- Na, H.K.; Mossanda, K.S.; Lee, J.Y.; Surh, Y.J. Inhibition of phorbol ester-induced COX-2 expression by some edible African plants. Biofactors 2004, 21, 149–153. [Google Scholar] [CrossRef]
- Inaba, K.; Murata, K.; Naruto, S.; Matsuda, H. Inhibitory effects of devil’s claw (secondary root of Harpagophytum procumbens) extract and harpagoside on cytokine production in mouse macrophages. J. Nat. Med. 2010, 64, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, A.; Razmkhah, K.; Mehrnia, M.; Mohamadnia, A.; Sahebjamee, H.; Salehi, S.; Asl, E.A.; Tahmasebi, H.; Shandiz, S.A.S.; Davouodbeglou, F.; et al. Molecular docking and binding study of harpagoside and harpagide as novel anti-inflammatory and anti-analgesic compound from Harpagophytum procumbens based on their interactions with COX-2 enzyme. Asian Pac. J. Trop. Dis. 2016, 6, 227–231. [Google Scholar] [CrossRef]
- Locatelli, M.; Ferrante, C.; Carradori, S.; Secci, D.; Leporini, L.; Chiavaroli, A.; Leone, S.; Recinella, L.; Orlando, G.; Martinotti, S.; et al. Optimization of aqueous extraction and biological activity of Harpagophytum procumbens root on ex vivo rat colon inflammatory model. Phytother. Res. 2017, 31, 937–944. [Google Scholar] [CrossRef]
- Leporini, L.; Ferrante, C.; Recinella, L.; Orlando, G.; Chiavaroli, A.; Martinotti, S.; Carradori, S.; Locatelli, M.; Vecchiotti, G.; Menghini, L. Evaluation of protective effect of Harpagophytum procumbens DC. Ex Meisn. Root water extraction by microwave, biological activity on ex vivo rat colon inflammatory model and microscopic investigation. In Proceedings of the 112° Congresso della Società Botanica Italiana, IV International Plant Science Conference (IPSC), Parma, Italy, 20–23 September 2017; p. 1. [Google Scholar]
- Cholet, J.; Decombat, C.; Vareille-Delarbre, M.; Gainche, M.; Berry, A.; Ogéron, C.; Ripoche, I.; Delort, L.; Vermerie, M.; Fraisse, D. Comparison of the anti-inflammatory and immunomodulatory mechanisms of two medicinal herbs: Meadowsweet (Filipendula ulmaria) and Harpagophytum (Harpagophytum procumbens). Int. J. Plant Anim. Environ. Sci. 2019, 9, 145–163. [Google Scholar]
- Recinella, L.; Chiavaroli, A.; Ronci, M.; Menghini, L.; Brunetti, L.; Leone, S.; Tirillini, B.; Angelini, P.; Covino, S.; Venanzoni, R.; et al. Multidirectional pharma-toxicological study on Harpagophytum procumbens DC. Ex Meisn.: An IBD-focused investigation. Antioxidants 2020, 9, 168. [Google Scholar] [CrossRef] [PubMed]
- Grant, L.; McBean, D.E.; Fyfe, L.; Warnock, A.M. The inhibition of free radical generation by preparations of Harpagophytum procumbens in vitro. Phytother. Res. 2009, 23, 104–110. [Google Scholar] [CrossRef]
- Kaszkin, M.; Beck, K.F.; Koch, E.; Erdelmeier, C.; Kusch, S.; Pfeilschifter, J.; Loew, D. Downregulation of iNOS expression in rat mesangial cells by special extracts of Harpagophytum procumbens derives from harpagoside-dependent and independent effects. Phytomedicine 2004, 11, 585–595. [Google Scholar] [CrossRef]
- Bae, Y.H.; Cuong, T.D.; Hung, T.M.; Kim, J.A.; Woo, M.H.; Byeon, J.S.; Choi, J.S.; Min, B.S. Cholinesterase inhibitors from the roots of Harpagophytum procumbens. Arch. Pharm. Res. 2013, 37, 1124–1129. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Bhattcharya, S.K. Anti-oxidant activity of Harpagophytum procumbens (Devil’s claw). Br. J. Phytother. 1998, 5, 68–71. [Google Scholar]
- Langmead, L.; Dawson, C.; Hawkins, C.; Banna, N.; Loo, S.; Rampton, D.S. Antioxidant effects of herbal therapies used by patients with inflammatory bowel disease: An in vitro study. Aliment Pharm. Ther. 2002, 16, 197–205. [Google Scholar] [CrossRef]
- Betancor-Fernandez, A.; Perez-Galvez, A.; Sies, H.; Stahl, W. Screening pharmaceutical preparations containing extracts of turmeric rhizome, artichoke leaf, devil’s claw root and garlic or salmon oil for antioxidant capacity. J. Pharm. Pharmacol. 2003, 55, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.C.; Soares, S.F.; Abreu, P.R.; Jesus, L.M.; Brito, L.C.; Bernardo-Filho, M. Protective effect of an aqueous extract of Harpagophytum procumbens upon Escherichia coli strains submitted to the lethal action of stannous chloride. Cell. Mol. Biol. 2007, 53, OL923-927. [Google Scholar]
- Grant, L. The Putative Anti-Inflammatory and Analgesic Properties of Harpagophytum procumbens. Ph.D. Thesis, Queen Margaret University College, Edinburgh, UK, 2005; p. 350. [Google Scholar]
- Georgiev, M.; Alipieva, K.; Pashova, S.; Denev, P.; Angelova, M.; Kerns, G.; Bley, T. Antioxidant activity of devil’s claw cell biomass and its active constituents. Food Chem. 2010, 121, 967–972. [Google Scholar] [CrossRef]
- Georgiev, M.; Alipieva, K.; Orhan, I.E. Cholinesterases inhibitory and antioxidant activities of Harpagophytum procumbens from in vitro systems. Phytother. Res. 2011, 26, 313–316. [Google Scholar] [CrossRef]
- Schaffer, L.F.; Peroza, L.R.; Boligon, A.A.; Athayde, M.L.; Alves, S.H.; Fachinetto, R.; Wagner, C. Harpagophytum procumbens prevents oxidative stress and loss of cell viability in vitro. Neurochem. Res. 2013, 38, 2256–2267. [Google Scholar] [CrossRef]
- Schaffer, L.F. Efeito Do Harpagophytum procumbens Sobre Parâmetros De Estresse Oxidativo E a Viabilidade Celular In Vitro. Master’s Thesis, Universidade Federal De Santa Maria, Santa Maria, Brazil, 2013; p. 62. [Google Scholar]
- Muzila, M.; Rumpunen, K.; Wright, H.; Roberts, H.; Grant, M.; Nybom, H.; Sehic, J.; Ekholm, A.; Widen, C. Alteration of neutrophil reactive oxygen species production by extracts of Devil’s claw (Harpagophytum). Oxid. Med. Cell. Longev. 2016, 2016, 3841803. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, L.F.; de Freitas, C.M.; Chiapinotto Ceretta, A.P.; Peroza, L.R.; de Moraes Reis, E.; Krum, B.N.; Busanello, A.; Boligon, A.A.; Sudati, J.H.; Fachinetto, R.; et al. Harpagophytum procumbens ethyl acetate fraction reduces fluphenazine-induced vacuous chewing movements and oxidative stress in rat brain. Neurochem. Res. 2016, 41, 1170–1184. [Google Scholar] [CrossRef]
- Mosca, F.; Marruchella, G.; Mariani, F.; Tiscar, P.G. Effetto dell’arpagoside sul burst respiratorio dei neutrofili di suino. In Proceedings of the XLIII Meeting Annuale SIPAS, Reggio Emilia, Italy, 16–17 March 2017; pp. 191–195. [Google Scholar]
- Ungerer, G.; Cui, J.; Ndam, T.; Bekemeier, M.; Song, H.; Li, R.; Siedhoff, H.R.; Yang, B.; Appenteng, M.K.; Greenlief, C.M.; et al. Harpagophytum procumbens extract ameliorates allodynia and modulates oxidative and antioxidant stress pathways in a rat model of spinal cord injury. Neuromol. Med. 2020, 22, 278–292. [Google Scholar] [CrossRef]
- Ncube, S.F.; McGaw, L.J.; Njoya, E.M.; Ndagurwa, H.G.T.; Mundy, P.G.; Sibanda, S. In Vitro antioxidant activity of crude extracts of Harpagophytum zeyheri and their anti-inflammatory and cytotoxicity activity compared with diclofenac. BMC Complement. Altern. Med. 2021, in press. [Google Scholar] [CrossRef]
- Mahomed, I.M.; Nsabimana, A.M.; Ojewole, J.A.O. Pharmacological effects of Harpagophytum procumbens DC [Pedaliaceae] secondary root aqueous extract on isolated gastro-intestinal tract muscles of the chick, guinea-pig and rabbit. Afr. J. Pharm. Pharmacol. 2005, 2, 31–45. [Google Scholar] [CrossRef]
- Guérin, J.C.; Réveillère, H.P. Activité antifongique d’extraits végétaux à usage thérapeutique. II. Étude de 40 extraits sur 9 souches fongiques. Ann. Pharm. Fr. 1985, 43, 77–81. [Google Scholar] [PubMed]
- Weckesser, S.; Engel, K.; Simon-Haarhaus, B.; Wittmer, A.; Pelz, K.; Schempp, C.M. Screening of plant extracts for antimicrobial activity against bacteria and yeasts with dermatological relevance. Phytomedicine 2007, 14, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, S.R.P. Avaliação do efeito do extrato etanólico bruto de Harpagophytum procumbens em camundongos infectados com Toxocara canis. Ropositório Inst. UFSCar. 2012. Available online: https://repositorio.ufscar.br/handle/ufscar/7011 (accessed on 13 April 2021).
- Camillo, L.; Oliveira, S.R.P.; Ribeiro, R.D.; Rodolpho, J.M.A.; Caroccia, G.H.G.; Albuquerque, S.; Anibal, F.F. Efeito de bioterápico na eosinofilia durante a SLMV experimental. Rev. Ciênc. Farm. Básica Apl. 2014, 35, 701–708. [Google Scholar]
- Oliveira, S.R.P.; Rodolpho, J.M.A.; Dejani, N.D.; Souza, L.C.; Correia, R.O.; Neris, D.M.; Galvão, A.; de Matos, A.P.K.; Vieira, P.C.; Afonso, A.; et al. Harpagophytum procumbens modulates eosinophilic response during infection by Toxocara canis. Int. J. Recent Sci. Res. 2014, 5, 2008–2013. [Google Scholar]
- Correia, R. Ação Do Tratamento Com Mentha piperita L. e Harpagophytum procumbens Contra Schistosoma mansoni—In Vitro: Análise Proteômica. Ph.D. Thesis, Universidade Federal de São Carlos, São Carlos, Brazil, 2014; p. 122. [Google Scholar]
- Cock, I.E.; Bromley, A. Inhibition of bacterial triggers of selected autoimmune inflammatory diseases by Harpagophytum procumbens Burch. DC. Ex Meisn. fruit extracts. S. Afr. J. Bot. 2017, 100, 329. [Google Scholar] [CrossRef]
- Luigi, M. Etude des Propriétés Antimutagènes de l’Harpagophytum procumbens et de l’Harpagoside: Généralisation aux Plantes Anti-Inflammatoires. Ph.D. Thesis, Ecole Doctorale Sciences de l’Environnement, Aix-Marseille, France, 2014; p. 340. [Google Scholar]
- Luigi, M.; Baghdikian, B.; Orsière, T.; Pompili, J.; Mabrouki, F.; Ollivier, E.; Botta, A. Antimutagenic potential of harpagoside and Harpagophytum procumbens against 1-nitropyrene. Pharmacogn. Mag. 2015, 11, S29–S36. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, S.H.; Baek, J.M.; Erkhembaatar, M.; Kim, M.S.; Yoon, K.H.; Oh, J.; Lee, M.S. Harpagoside inhibits RANKL-induced osteoclastogenesis via Syk-Btk-PLCgamma2-Ca(2+) signaling pathway and prevents inflammation-mediated bone loss. J. Nat. Prod. 2015, 78, 2167–2174. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.J.; Kyung Kim, W.; Joo Park, H.; Cho, L.; Kim, M.R.; Kim, M.J.; Shin, J.S.; Ho Lee, J.; Ha, I.H.; Kook Lee, S. Anti-osteoporotic activity of harpagide by regulation of bone formation in osteoblast cell culture and ovariectomy-induced bone loss mouse models. J. Ethnopharmacol. 2016, 179, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.J.; Kim, W.K.; Oh, J.; Kim, M.R.; Shin, J.S.; Lee, J.; Ha, I.H.; Lee, S.K. Anti-osteoporotic activity of Harpagoside by upregulation of the BMP2 and Wnt signaling pathways in osteoblasts and suppression of differentiation in osteoclasts. J. Nat. Prod. 2017, 80, 434–442. [Google Scholar] [CrossRef]
- Vollmann, D. Zu Einigen Pharmakologischen Wirkungen von Zubereitungen des Harpagophytum procumbens DC. Ph.D. Thesis, Karl-Marx-Universität, Leipzig, Germany, 1965; p. 34. [Google Scholar]
- Circosta, C.; Occhiuto, F.; Ragusa, S.; Trovato, A.; Tumino, G.; Briguglio, F.; De Pasquale, A. A drug used in traditional medicine: Harpagophytum procumbens DC II. Cardiovascular activity. J. Ethnopharmacol. 1984, 11, 259–274. [Google Scholar] [CrossRef]
- De Pasquale, R.; Circosta, C.; Iauk, L.; Ragusa, S.; Busa, G. Effetti dell’ Harpagophytum procumbens DC e dell’arpagoside sulle aritmie sperimentali “in vitro”. Pharm. Mediterr. 1984, 15, 153. [Google Scholar]
- De Pasquale, R.; Busa, G.; Circosta, C.; Iauk, L.; Ragusa, S.; Ficarra, P.; Occhiuto, F. A drug used in traditional medicine: Harpagophytum procumbens DC. III. Effects on hyperkinetic ventricular arrhythmias by reperfusion. J. Ethnopharmacol. 1985, 13, 193–199. [Google Scholar] [CrossRef]
- Occhiuto, F.; Circosta, C.; Ragusa, S.; Ficarra, P.; Costa De Pasquale, R. A drug used in traditional medicine: Harpagophytum procumbens DC. IV. effects on some isolated muscle preparations. J. Ethnopharmacol. 1985, 13, 201–208. [Google Scholar] [CrossRef]
- Occhiuto, F.; De Pasquale, A. Electrophysiological and haemodynamic effects of some active principles of Harpagophytum procumbens DC. in the dog. Pharm. Res. 1990, 22, 72–73. [Google Scholar] [CrossRef]
- Mahomed, I.M.; Ojewole, J.A.O. Cardiovascular effects of Harpagophytum procumbens DC. Afr. J. Tradit. Complement. Altern. Med. 2004, 1, 30–44. [Google Scholar]
- Mahomed, I.M.; Ojewole, J.A.O. Anticonvulsant activity of Harpagophytum procumbens DC [Pedaliaceae] secondary root aqueous extract in mice. Brain Res. Bull. 2006, 69, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, C.; Recinella, L.; Locatelli, M.; Guglielmi, P.; Secci, D.; Leporini, L.; Chiavaroli, A.; Leone, S.; Martinotti, S.; Brunetti, L.; et al. Protective effects induced by microwave-assisted aqueous Harpagophytum extract on rat cortex synaptosomes challenged with amyloid beta-peptide. Phytother. Res. 2017, 31, 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhang, H.; Xu, H.; Xue, R.; Zheng, Y.; Wu, T.; Lian, Y. Harpagoside rescues the memory impairments in chronic cerebral hypoperfusion rats by inhibiting PTEN activity. J. Alzheimers Dis. 2018, 63, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Peruru, R.; Usha Rani, R.; Thatiparthi, J.; Sampathi, S.; Dodoala, S.; Prasad, K. Devil’s claw (Harpagophytum procumbens) ameliorates the neurobehavioral changes and neurotoxicity in female rats exposed to arsenic. Heliyon 2020, 6, e03921. [Google Scholar] [CrossRef]
- Prosinska, J.; Sawicka, T.; Drozd, J. Investigation of the thymomimetic activity of a selected phytopharmaceutical preparation-Reumaherb tablets by flow cytometry. Acta Pol. Pharm. 2002, 59, 265–274. [Google Scholar]
- Torres-Fuentes, C.; Theeuwes, W.F.; McMullen, M.K.; McMullen, A.K.; Dinan, T.G.; Cryan, J.F.; Schellekens, H. Devil’s claw to suppress appetite—Ghrelin receptor modulation potential of a Harpagophytum procumbens root extract. PLoS ONE 2014, 9, e103118. [Google Scholar] [CrossRef] [PubMed]
- Saleh, S.; Jahromi, H.K.; Sarikhani, Y.; Jahromi, Z.K.; Dowlatkhah, H. The effects of hydroalcoholic extract of devil’s claw on serum levels of obestatin and body weight in male rats. J. Glob. Pharma Technol. 2016, 12, 40–43. [Google Scholar]
- Anonymous. Der Einfluß von Harpagosan (Harpagophytum procumbens DC) auf die Blei-Einlagerung in Rattenorganen; International Bio Research, Mitteilung an Fa. Hagen: Freilassing, Germany, 1975. [Google Scholar]
- Chrubasik, S.; Wink, M. Zur pharmakologischen Wirkung der Teufelskralle (Harpagophytum procumbens). Complement. Med. Res. 1995, 2, 323–325. [Google Scholar] [CrossRef]
- Loew, D.; Kaszkin, M. Experimentell- und klinisch-pharmakologische Modelle zum Nachweis der analgetischen und antiphlogistischen Wirkungen von Teufelskralle. Ärztez. Nat. 2002, 43, 576–585. [Google Scholar]
- Fleurentin, J.; Mortier, F. Entzündungshemmende und analgetische Wirkungen von Harpagophytum procumbens und H. zeyheri. In Rheumatherapie mit Phytopharmaka; Chrubasik, S., Wink, M., Eds.; Hippokrates: Stuttgart, Germany, 1997; pp. 68–76. [Google Scholar]
- Budzinski, J.W.; Foster, B.C.; Vandenhoek, S.; Arnason, J.T. An in vitro evaluation of human cytochrome P450 3A4 inhibition by selected commercial herbal extracts and tinctures. Phytomedicine 2000, 7, 273–282. [Google Scholar] [CrossRef]
- Puttkammer, S. Untersuchungen zur Pharmakokinetik des Harpagophytum-Spezialextraktes HF 8858 und Einfluß auf Leukotrien C4-Biosynthese Sowie die Thromboxan B2-Freisetzung. Ph.D. Thesis, Johann Wolfgang Goethe-Universität Frankfurt/M., Frankfurt, Germany, 2000; p. 45. [Google Scholar]
- Hilgendorf, C.; Döppenschmitt, S. Popular herbal drugs: Screening of potential CYP inhibition/activation based metabolic interactions. Int. J. Clin. Pharm. 2003, 41, 537. [Google Scholar]
- Unger, M.; Frank, A. Simultaneous determination of the inhibitory potency of herbal extracts on the activity of six major cytochrome P450 enzymes using liquid chromatography/mass spectrometry and automated online extraction. Rapid Commun. Mass Spectrom. 2004, 18, 2273–2281. [Google Scholar] [CrossRef]
- Romiti, N.; Tramonti, G.; Corti, A.; Chieli, E. Effects of Devil’s claw (Harpagophytum procumbens) on the multidrug transporter ABCB1/P-glycoprotein. Phytomedicine 2009, 16, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- Modarai, M.; Suter, A.; Kortenkamp, A.; Heinrich, M. The interaction potential of herbal medicinal products: A luminescence-based screening platform assessing effects on cytochrome P450 and its use with devil’s claw (Harpagophyti radix) preparations. J. Pharm. Pharmacol. 2011, 63, 429–438. [Google Scholar] [CrossRef]
- Anton, R. Réflexions sur quelques nouvelles acquisitions en phytothérapie. J. Pharm. Belg. 1987, 42, 138–151. [Google Scholar]
- Möse, J.R. Untersuchung der Toxischen Wirkung von Harpago-Tee auf Zellen und Dessen Verhalten in Einem Orientierenden Versuch am Tumor-Tier; Hygiene-Institut, Universität Graz. Mitteilung an Fa. Hagen: Freilassing, Germany, 1969. [Google Scholar]
- Grünewald, K. Erfahrungen mit Harpagosan bei Hypercholesterinämie und Hyperurikämie. Arch. Arzneither. 1978, 1, 73–76. [Google Scholar]
- Anonymous. Akute Toxizitätsprüfung von Harpagophytum D2 nach Intravenöser Applikation an der Ratte; International Bio Research, Mitteilung an Fa. Hagen: Freilassing, Germany, 1974. [Google Scholar]
- Anonymous. Akute Toxizitätsprüfung von Harpagophytum procumbens DC nach Oraler Applikation bei der Ratte; International Bio Research, Mitteilung an Fa. Hagen: Freilassing, Germany, 1975. [Google Scholar]
- Ibrahim, K.E.; Al–Ashban, R.M.; El–Sammani, S.A. Toxicity studies on devil’s claw herbal medicine. Res. J. Pharmacol. 2010, 4, 69–73. [Google Scholar] [CrossRef]
- Al-Harbi, N.O.; Al-Ashban, R.M.; Shah, A.H. Toxicity studies on Harpagophytum procumbens (Devil’s claw) capsules in mice. J. Med. Plants Res. 2013, 7, 3089–3097. [Google Scholar] [CrossRef]
- Allard, T.; Wenner, T.; Greten, H.J.; Efferth, T. Mechanisms of herb-induced nephrotoxicity. Curr. Med. Chem. 2013, 20, 2812–2819. [Google Scholar] [CrossRef] [PubMed]
- Joshi, K.; Parrish, A.; Grunz-Borgmann, E.A.; Gerkovich, M.; Folk, W.R. Toxicology studies of aqueous-alcohol extracts of Harpagophytum procumbens subsp. procumbens (Burch.) DC. Ex Meisn. (Pedaliaceae) in female and male rats. BMC Complement. Altern. Med. 2020, 20, 9. [Google Scholar] [CrossRef]
- Mahomed, I.M.; Ojewole, J.A.O. Oxytocin-like effect of Harpagophytum procumbens DC [Pedaliaceae] secondary root aqueous extract on rat isolated uterus. Afr. J. Tradit. Complement. Altern. Med. 2006, 3, 82–89. [Google Scholar]
- Mahomed, I.M.; Ojewole, J.A. Uterotonic effect of Harpagophytum procumbens DC (Pedaliaceae) secondary root aqueous extract on rat isolated uterine horns. J. Smooth Muscle Res. 2009, 45, 231–239. [Google Scholar] [CrossRef]
- Pearson, W. Reproductive Toxicity Study of an Herbal Product (Nasprin) in Sprague Dawley Rats; Nutraceutical Alliance: Burlington, ON, Canada, 2012. [Google Scholar]
- Davari, S.A. Teratogenic effects of Harpagophytum procumbens ethanolic extract in mice and fetuses. Zahedan J. Res. Med. Sci. 2016, 18, e3481. [Google Scholar] [CrossRef]
- Chrubasik, S.; Ziegler, R. Wirkstoffgehalt in Arzneimitteln aus Harpagophytum procumbens und klinische Wirksamkeit von Harpagophytum-Trockenextrakt. In Phytopharmaka II, Forschung und Klinische Anwendung; Loew, D., Rietbrock, N., Eds.; Steinkopff: Darmstadt, Germany, 1996; pp. 101–114. [Google Scholar]
- Brendler, T.; Grünwald, J.; Ulbricht, C.; Basch, E. Devil’s claw (Harpagophytum procumbens DC): An evidence-based systematic review by the Natural Standard Research Collaboration. J. Herb. Pharm. 2006, 6, 89–126. [Google Scholar] [CrossRef]
- Büechi, S.; Wegener, T. Harpagophyti radix (Teufelskrallenwurzel). Phytotherapie 2002, 4, 28–33. [Google Scholar]
- Chrubasik, S.; Conradt, C. Audiatur et al.tera pars: Teufelskralle in der Diskussion. Z. Phytother. 2002, 23, 84–86. [Google Scholar]
- Chrubasik, S.; Conradt, C.; Black, A. The quality of clinical trials with Harpagophytum procumbens. Phytomedicine 2003, 10, 613–623. [Google Scholar] [CrossRef][Green Version]
- Chrubasik, S.; Conradt, C.; Roufogalis, B.D. Effectiveness of Harpagophytum extracts and clinical efficacy. Phytother. Res. 2004, 18, 187–189. [Google Scholar] [CrossRef] [PubMed]
- Chrubasik, S.; Eisenberg, E. Treatment of rheumatic pain with kampo medicine in Europe. Part 1. Harpagophytum procumbens. Pain Clin. 1999, 11, 171–178. [Google Scholar]
- Chrubasik, S.; Pollak, S. Teufelskrallenwurzelextrakt bei schmerzhafter Arthrose und Rückenschmerzen. Z. Phytother. 2002, 23, 210–215. [Google Scholar]
- Chrubasik, S.; Shvartzman, P. Rheumatic pain treatment with devil’s claw (Harpagophyti radix). 1999, 1/99. Available online: http://www.iaam.nl/coherence/msaima/199-3.html (accessed on 14 April 2021).
- Denner, S.S. A review of the efficacy and safety of devil’s claw for pain associated with degenerative musculoskeletal diseases, rheumatoid, and osteoarthritis. Holist. Nurse Pr. 2007, 21, 203–207. [Google Scholar] [CrossRef]
- Gäbler, H. Harpagophytum procumbens. Homöopath. Mon. 1972, 97, 123–126. [Google Scholar]
- Gagnier, J.J.; Chrubasik, S.; Manheimer, E. Harpgophytum procumbens for osteoarthritis and low back pain: A systematic review. BMC Complement. Altern. Med. 2004, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- Gagnier, J.J.; Chrubasik, S.; Manheimer, E. Correction: Harpagophytum procumbens for osteoarthritis and low back pain: A systematic review. BMC Complement. Altern. Med. 2005, 5, 1. [Google Scholar] [CrossRef]
- Grant, L.; McBean, D.E.; Fyfe, L.; Warnock, A.M. A review of the biological and potential therapeutic actions of Harpagophytum procumbens. Phytother. Res. 2007, 21, 199–209. [Google Scholar] [CrossRef]
- Loew, D. Harpagophytum procumbens DC. Eine Übersicht zur Pharmakologie und WIrksamkeit. Erfahrungsheilkunde 1995, 2, 74–79. [Google Scholar]
- Savustyanenko, A.V. Эффективнoсть экстракта мартинии душистoй (сустамар) при oстеoартритах, пoясничнoй бoли и фибрoмиалгии: Обзoр исследoваний. Pain Jt. Spine 2014, 3, 45–53. [Google Scholar] [CrossRef]
- Tarleton, A. Devil’s claw for low back pain. Herbalgram 1997, 41, 20. [Google Scholar]
- Wegener, T. Degenerative Erkrankungen des Bewegungsapparates—Übersicht zu aktuellen klinischen Studien mit Extrakten aus der Teufelskralle (Harpagophyti radix). Wien. Med. Wochenschr. 2002, 152, 389–392. [Google Scholar] [CrossRef]
- Ameye, L.G.; Chee, W.S. Osteoarthritis and nutrition. From nutraceuticals to functional foods: A systematic review of the scientific evidence. Arthritis Res. 2006, 8, R127. [Google Scholar] [CrossRef]
- Amling, R. Phytotherapeutika in der Neurologie. Z. Phytother. 1991, 12, 9–14. [Google Scholar]
- Arndt, D.; Bobermien, K.; Heyer, H.; Hinken, B. Alternative Therapieoptionen bei Endometriose. Gynäkologe 2007, 40, 553–558. [Google Scholar] [CrossRef]
- Belova, K.Y.; Nazarova, A.V. Стратегия лечения oстеoартрита у мультимoрбидных пациентoв: баланс эффективнoсти и безoпаснoсти при выбoре лекарственнoй терапии. Med. Counc. 2020, 11, 164–176. [Google Scholar] [CrossRef]
- Brien, S.; Lewith, G.T.; McGregor, G. Devil’s claw (Harpagophytum procumbens) as a treatment for osteoarthritis: A review of efficacy and safety. J. Altern. Complement. Med. 2006, 12, 981–993. [Google Scholar] [CrossRef]
- Chrubasik, C.; Black, A.; Muller-Ladner, U.; Chrubasik, S. Impact of herbal medicines on physical impairment. Phytomedicine 2008, 15, 536–539. [Google Scholar] [CrossRef]
- Chrubasik, J.E.; Roufogalis, B.D.; Chrubasik, S. Evidence of effectiveness of herbal antiinflammatory drugs in the treatment of painful osteoarthritis and chronic low back pain. Phytother. Res. 2007, 21, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Chrubasik, S. Osteoarthritis: Pharmacology and clinical indications of selected botanicals. Pain Clin. 2004, 16, 1–16. [Google Scholar] [CrossRef]
- Chrubasik, S.; Neumann, E.; Müller-Ladner, U. Zur antientzündlichen Wirksamkeit von Arzneimitteln aus der Teufelskralle. Z. Phytother. 2009, 30, 222–226. [Google Scholar] [CrossRef]
- Chrubasik, S.; Pollak, S. Schmerzbehandlung mit pflanzlichen Antirheumatika. Wien. Med. Wochenschr. 2002, 152, 198–203. [Google Scholar] [CrossRef]
- Chrubasik, S.; Wink, M. Traditional herbal therapy for the treatment of rheumatic pain: Preparations from devil’s claw and stinging nettle. Pain Dig. 1998, 8, 94–101. [Google Scholar]
- Chrubasik, S.; Wink, M. Treatment of osteoarthritic pain with herbal drugs. In Proceedings of the 8th World Congress: The Pain Clinic, Tenerife, Spain, 6–10 May 1998; pp. 507–514. [Google Scholar]
- Chrubasik-Hausmann, S. Phytotherapie bei Arthrose. Erfahrungsheilkunde 2015, 64, 22–27. [Google Scholar] [CrossRef]
- Corciova, A.; Matei, D.; Ivanescu, B. Medicinal herbs as possible sources of anti-inflammatory products. Balneo Res. J. 2017, 8, 231–241. [Google Scholar] [CrossRef]
- Corp, N.; Pendry, B. The role of Western herbal medicine in the treatment of gout. J. Herb. Med. 2013, 3, 157–170. [Google Scholar] [CrossRef]
- Darshan, S.; Doreswamy, R. Patented antiinflammatory plant drug development from traditional medicine. Phytother. Res. 2004, 18, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Del Grossi Moura, M.; Lopes, L.C.; Biavatti, M.W.; Kennedy, S.A.; de Oliveira, E.S.M.C.; Silva, M.T.; de Cassia Bergamaschi, C. Oral herbal medicines marketed in Brazil for the treatment of osteoarthritis: A systematic review and meta-analysis. Phytother. Res. 2017, 31, 1676–1685. [Google Scholar] [CrossRef]
- Deyo, R.A. Conservative therapy for low back pain. Distinguishing useful from useless therapy. JAMA 1983, 250, 1057–1062. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, C.; dell’Agli, M.; Badea, M.; Dima, L.; Colombo, E.; Sangiovanni, E.; Restani, P.; Bosisio, E. Plant food supplements with anti-inflammatory properties: A systematic review (II). Crit. Rev. Food Sci. Nutr. 2013, 53, 507–516. [Google Scholar] [CrossRef]
- Dragos, D.; Gilca, M.; Gaman, L.; Vlad, A.; Iosif, L.; Stoian, I.; Lupescu, O. Phytomedicine in joint disorders. Nutrients 2017, 9, 70. [Google Scholar] [CrossRef]
- Ernst, E.; Chrubasik, S. Phyto-anti-inflammatories. A systematic review of randomized, placebo-controlled, double-blind trials. Rheum. Dis. Clin. N. Am. 2000, 26, 13–27. [Google Scholar] [CrossRef]
- Gagnier, J.J. Evidence based review of natural health products for non-specific low back pain. Open Pain J. 2010, 3. [Google Scholar] [CrossRef]
- Gagnier, J.J.; van Tulder, M.; Berman, B.; Bombardier, C. Herbal medicine for low back pain. Cochrane Database Syst. Rev. 2006, CD004504. [Google Scholar] [CrossRef]
- Gagnier, J.J.; van Tulder, M.W.; Berman, B.; Bombardier, C. Herbal medicine for low back pain: A Cochrane review. Spine 2007, 32, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Gregory, P.J.; Sperry, M.; Wilson, A.F. Dietary supplements for osteoarthritis. Am. Fam. Physician 2008, 77, 177–184. [Google Scholar] [PubMed]
- Halliwell, B. Drug antioxidant effects. A basis for drug selection? Drugs 1991, 42, 569–605. [Google Scholar] [CrossRef]
- Long, L.; Soeken, K.; Ernst, E. Herbal medicines for the treatment of osteoarthritis: A systematic review. Rheumatology 2001, 40, 779–793. [Google Scholar] [CrossRef]
- Lopez, H.L. Nutritional interventions to prevent and treat osteoarthritis. Part II: Focus on micronutrients and supportive nutraceuticals. PMR 2012, 4, S155–S168. [Google Scholar] [CrossRef]
- Marlowe, D. Complementary and alternative medicine treatments for low back pain. Prim. Care 2012, 39, 533–546. [Google Scholar] [CrossRef]
- Oltean, H.; Robbins, C.; van Tulder, M.W.; Berman, B.M.; Bombardier, C.; Gagnier, J.J. Herbal medicine for low-back pain. Cochrane Database Syst. Rev. 2014, CD004504. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S. A systematic review on anti-inflammatory activity of harpagoside. J. Biochem. Mol. Biol. Res. 2016, 2, 166–169. [Google Scholar] [CrossRef]
- Roth-Utzschneider, S. Erfolgreiche Rheumatherapie mit Teufelskralle und Weidenrinde. Ärztez. Nat. 2006, 47, 488. [Google Scholar]
- Rubinstein, S.M.; van Middelkoop, M.; Kuijpers, T.; Ostelo, R.; Verhagen, A.P.; de Boer, M.R.; Koes, B.W.; van Tulder, M.W. A systematic review on the effectiveness of complementary and alternative medicine for chronic non-specific low-back pain. Eur. Spine J. 2010, 19, 1213–1228. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.; Grundmann, O. The use of glucosamine, devil’s claw (Harpagophytum procumbens), and acupuncture as complementary and alternative treatments for osteoarthritis. Altern. Med. Rev. 2011, 16, 228. [Google Scholar]
- Schmidt, S. Phytotherapie beim rheumatischen Formenkreis. Heilkunst 1973, 86, 18–20. [Google Scholar]
- Setty, A.R.; Sigal, L.H. Herbal medications commonly used in the practice of rheumatology: Mechanisms of action, efficacy, and side effects. Semin. Arthritis Rheum. 2005, 34, 773–784. [Google Scholar] [CrossRef]
- Soeken, K.L. Selected CAM therapies for arthritis-related pain: The evidence from systematic reviews. Clin. J. Pain. 2004, 20, 13–18. [Google Scholar] [CrossRef]
- Spelman, K.; Burns, J.; Nichols, D.; Winters, N.; Ottersberg, S.; Tenborg, M. Modulation of cytokine expression by traditional medicines: A review of herbal immunomodulators. Altern. Med. Rev. 2006, 11, 128–150. [Google Scholar]
- Teut, M.; Warning, A. Knochenmetastasen bei Mamma-Karzinom. Komplementmed. 2006, 13, 46–48. [Google Scholar] [CrossRef]
- Viljoen, A.; Mncwangi, N.; Vermaak, I. Anti-inflammatory iridoids of botanical origin. Curr. Med. Chem. 2012, 19, 2104–2127. [Google Scholar] [CrossRef]
- Zimmermann, W. Pflanzliche Bitterstoffe in der Gastroenterologie. ZFA 1976, 54, 1178–1184. [Google Scholar]
- Zimmermann, W. Interne Phytotherapie der rheumatischen und arthrotischen Erkrankungen. Herba Pol. 1979, 4, 333–342. [Google Scholar]
- Zimmermann, W. Bitterstoffe. Kassenarzt 1985, 8, 44, 47–48. [Google Scholar]
- Zimmermann, W. Der obere Dünndarm. Eine Phytotherapiestudie. Therapiewoche 1985, 35, 1592–1602. [Google Scholar]
- Gagnier, J.J.; Oltean, H.; van Tulder, M.W.; Berman, B.M.; Bombardier, C.; Robbins, C.B. Herbal medicine for low back pain: A cochrane review. Spine 2016, 41, 116–133. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. Teufelskralle zur Therapie der Osteoarthritis. Dtsch. Apoth. Ztg. 2001, 141, 1–2. [Google Scholar]
- Baillard, O. Intérêt de l’Harpagophytum procumbens dans les Affections Rhumatismales. Ph.D. Thesis, Université François Rabelais, Tours, France, 2009; p. 86. [Google Scholar]
- Beer, A.M. Teufelskralle und Gymnastik für Knie und Schulter. MMW Med. 2014, 156, 24. [Google Scholar]
- Chrubasik, S. Efficacy and safety of Harpagophytum and Salix extract preparations. In Des Sources du Savoir aux Médicaments du Futur; Fleurentin, J., Pelt, J., Mazars, G., Eds.; IRD Éditions: Marseille, France, 2002; ISBN 2709915049. [Google Scholar]
- Chrubasik, S. Weidenrinde und Teufelskralle bei chronischen Gelenk- und Rückenschmerzen: Evidenzlage favorisiert pflanzliche Präparate. Schweiz. Z. Ganzheitsmed. Swiss J. Integr. Med. 2004, 16, 355–359. [Google Scholar]
- Chrubasik, S. Zur Evidenz der Wirksamkeit Pflanzlicher Entzündungshemmer bei Arthrose und Rückenschmerzen. Ph.D. Thesis, Klinikum der Albert-Ludwigs-Universität, Freiburg im Breisgau, Germany, 2013; p. 47. [Google Scholar]
- Schmidt, S. Therapeutische Wirkung der Teufelskralle. ZFA 1983, 56, 1442. [Google Scholar]
- Vanhaelen, M. L’activite de Harpagophytum procumbens et de Glycyrrhiza glabra; Toxicite de drogues contenant des alcaloides derives de la pyrrolizidine. Farm. Tijdschr. Belg. 1986, 63, 31–43. [Google Scholar]
- Wenzel, P.; Wegener, T. Teufelskralle-ein pflanzliches Antirheumatikum. Dtsch. Apoth. Ztg. 1995, 135, 15–27. [Google Scholar]
- Zimmermann, W. Harpagophyturn Ampullen D2 der Fa. Hagen. Klinisches Gutachten; Schriftliche Mitteilung an Fa. Hagen: Freilassing, Germany, 1972. [Google Scholar]
- Belaiche, P. Un cas de chemosis grave traité avec succès par le nébulisat aqueux de radix Harpagophytum procumbens. Phytotherapy 1983, 5, 15–16. [Google Scholar]
- Belaiche, P. Action du nebulisat aqueux de radix Harpagophytum procumbens dans 17 cas de fièvre familiale méditerranéenne (maladie périodique). Phytotherapy 1983, 8, 5–11. [Google Scholar]
- Wilson, K.S. Regression of follicular lymphoma with Devil’s claw: Coincidence or causation? Curr. Oncol. 2009, 16, 67–70. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Beham, A. Die Anwendung der Harpagophytum-Wurzel bei rheumatischen Erkrankungen. Allg. Homöopath. Ztg. 1971, 216, 204–208. [Google Scholar] [CrossRef]
- Brantner, F. Praktische Phytotherapie. Phys. Med. Rehabil. 1972, 13, 87–88. [Google Scholar]
- Zimmermann, W. Erfahrungen mit Harpagophytum. Phys. Med. Rehabil. 1977, 18, 317–319. [Google Scholar]
- Vanhaelen, M. Mise a jour des recherches relatives a harpagophvtum procumbens. Phytotherapy 1985, 16, 19–24. [Google Scholar]
- Stübler, M. Die Behandlung chronischer Gelenkerkrankungen mit Harpagophytum. Allg. Homöopath. Ztg. 1987, 232, 60–62. [Google Scholar] [CrossRef]
- Stübler, M. Harpagophytum procumbens in rheumatoid arthritis. Br. Homoeopath. J. 1987, 76, 161. [Google Scholar] [CrossRef]
- Müller, B.; Deitelhoff, P.; Petrowicz, O. Harpagophytum procumbens ist effizient bei degenerativen Erkrankungen des Bewegungsapparates. Nat. Med. 2000, 21–29. [Google Scholar]
- Thanner, J.; Kohlmann, T.; Kunzel, O.; Chrubasik, S. Retrospective evaluation of biopsychosocial determinants and treatment response in patients receiving devil’s claw extract (doloteffin). Phytother. Res. 2009, 23, 742–744. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, H. Einfluß der Droge Harpagophytum procumbens DC (auch Teufelskralle genannt) auf Diabetes mellitus mit Fettstoffwechselstörungen. Erfahrungsheilkunde 1974, 7, 230–233. [Google Scholar]
- Grahame, R.; Robinson, B.V. Devils’s claw (Harpagophytum procumbens): Pharmacological and clinical studies. Ann. Rheum. Dis. 1981, 40, 632. [Google Scholar] [CrossRef]
- Belaiche, P. Etude clinique de 630 cas d’artrose traites par le nebulisat aqueux d’Harpagophytum procumbens (Radix). Phytotherapy 1982, 1, 22–28. [Google Scholar]
- Kröner, W. Wirksamkeit der Homöopathischen Arzneimittel “Formica rufa” und “Harpagophytum procumbens” bei der Gonarthrose: Eine Kontrollierte Studie bei Niedergelassenen Ärzten. Ph.D. Thesis, Universität Ulm, Ulm, Germany, 1991; p. 82. [Google Scholar]
- Moussard, C.; Alber, D.; Toubin, M.M.; Thevenon, N.; Henry, J.C. A drug used in traditional medicine, Harpagophytum procumbens: No evidence for NSAID-like effect on whole blood eicosanoid production in human. Prostaglandins Leukot. Essent. Fat. Acids 1992, 46, 283–286. [Google Scholar] [CrossRef]
- Chrubasik, S.; Schmidt, A.; Junck, H.; Pfisterer, M. Wirksamkeit und Wirtschaftlichkeit von Teufelskrallenwurzelextrakt bei Rückenschmerzen: Erste Ergebnisse einer therapeutischen Kohortenstudie. Complement. Med. Res. 1997, 4, 332–336. [Google Scholar] [CrossRef]
- Pinget, M.; Lecomte, A. Die Wirkung der “Harpagophytum Arkocaps” bei degenerativem Rheuma. Naturheilpraxis 1997, 50, 267–269. [Google Scholar]
- Schwarz, G.; Hämmerle, H.D.; Benvenuti, E. Teufelskralle hilft sanft bei Rückenschmerzen. Allgemeinarzt 1999, 12, 1036. [Google Scholar]
- Szczepański, L.; Chudzik, D.; Mazurek, M.; Soroka, P. Badania skuteczności i tolerancji preparatu Pagosid (wyciąg z korzenia Harpagophytum procumbens) w leczeniu reumatoidalnego zapalenia stawów i choroby zwyrodnieniowej stawów. Reumatologia 2000, 38, 67–73. [Google Scholar]
- Usbeck, C. Teufelskralle: Behandlung chronischer Schmerzen. Arzneim. Forum 2000, 3, 23–25. [Google Scholar]
- Engel, S. Rivoltan (Li 174) zur Behandlung von Patienten mit degenerativen Erkrankungen des Bewegungsapparates. Dtsch. Apoth. Ztg. 2000, 140, 1369. [Google Scholar]
- Laudahn, D.; Walper, A. Efficacy and tolerance of Harpagophytum extract LI 174 in patients with chronic non-radicular back pain. Phytother. Res. 2001, 15, 621–624. [Google Scholar] [CrossRef]
- Richter, T. Teufelskrallenextrakt bei chronischen Rückenschmerzen. Dtsch. Apoth. Ztg. 2002, 142, 131–133. [Google Scholar]
- Ullmann, M. Therapie chronischer Rückenschmerzen: Teufelskrallen-Extrakt erzielt hochsignifikante Schmerzreduktion. Allgemeinarzt 1999, 11, 1018–1019. [Google Scholar]
- Schendel, U.M. Arthrose-therapie: Verträglich geht es auch. Kassenarzt 2001, 29, 36–39. [Google Scholar]
- Ribbat, J.M.; Schakau, D. Behandlung chronisch aktivierter Schmerzen am Bewegungsapparat. Nat. Med. 2001, 16, 23–32. [Google Scholar]
- Chrubasik, S.; Thanner, J.; Künzel, O.; Conradt, C.; Black, A.; Pollak, S. Comparison of outcome measures during treatment with the proprietary Harpagophytum extract doloteffin in patients with pain in the lower back, knee or hip. Phytomedicine 2002, 9, 181–194. [Google Scholar] [CrossRef]
- Flammersfeld, L.; Weinmann, B. Traitement du rhumatisme par l’extrait sec de racine d’Harpagophytum. Une étude multicentrique en cabinet médical. Phytotherapie 2005, 3, 104–106. [Google Scholar] [CrossRef]
- Kloker, B.; Flammersfeld, L. Rheumatherapie mit Teufelskrallenwurzel-Trockenextrakt. Eine multizentrische Praxisstudie. Ärztez. Nat. 2003, 44, 108–111. [Google Scholar]
- Wegener, T.; Lüpke, N.P. Treatment of patients with arthrosis of hip or knee with an aqueous extract of devil’s claw (Harpagophytum procumbens DC.). Phytother. Res. 2003, 17, 1165–1172. [Google Scholar] [CrossRef]
- Wegener, T. Zur Klinischen Wirksamkeit der Südafrikanischen Teufelskrallenwurzel (Harpagophyti radix) bei Patienten mit Cox-und Gonarthrose: Ergebnisse und Bewertung Einer Klinischen Studie der Phase IV. Ph.D. Thesis, Universität Osnabrück, Osnabrück, Germany, 2005; p. 115. [Google Scholar]
- Rütten, S.; Kuhn, M. Spezialextrakt aus Teufelskrallenwurzel. Klinische Vergleichsstudie bei Patienten mit unspezifischen Schmerzen im Lumbalbereich. Ärztez. Nat. 2005, 46, 114–116. [Google Scholar]
- Schmidt, A.; Berghof, U.; Schmidt, E. Therapie der unspezifischen Lumbalgie mit Teufelskrallenwurzelextrakt—Ergebnisse einer klinischen Studie. Phys. Med. Rehabil. Kurortmed. 2005, 15, 317–321. [Google Scholar] [CrossRef]
- Schmidt, A.P.; Schmidt, E.G. Über die Wirksamkeit von Teufelskrallenwurzelextrakt zur Therapie Unspezifischer Lumbaler Rückenschmerzen; Cuvillier Verlag: Göttingen, Germany, 2005; p. 101. ISBN 3865376118. [Google Scholar]
- Sohail, M.T.; Chaudhry, M.I.; Usman, M.K.; Mian, T.; Ishaq, M.N. Efficacy and tolerance of atrisin in degenerative and inflammatory joint disorders. Phytother. Res. 2005, 19, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Arndt, D.; Bobermien, K.; Heyer, H.; Braun, D.; Köhler, G. Harpagophytum procumbens—Erste Erfahrungen in der Endometriosetherapie. Zent. Gynakol. 2005, 127, A1. [Google Scholar] [CrossRef]
- Arndt, D.; Bobermien, K.; Heyer, H.; Köhler, G. Hormonfreie Endometriosetherapie mit Harpagophytum procumbens—Ein neuer Weg in der Endometriosetherapie. Geburtshilfe Frauenheilkd. 2006, 66, PO_E_03_10. [Google Scholar] [CrossRef]
- Suter, A.; Whittaker, P.; Dickson, S.; McIntyre, L.; Tan, J. Positive influence of a Harpagophytum procumbens preparation on different rheumatic complaints—Results from clinical trial. Planta Med. 2006, 72, P_200. [Google Scholar] [CrossRef]
- Warnock, M.; McBean, D.; Suter, A.; Tan, J.; Whittaker, P. Effectiveness and safety of Devil’s claw tablets in patients with general rheumatic disorders. Phytother. Res. 2007, 21, 1228–1233. [Google Scholar] [CrossRef] [PubMed]
- Chrubasik, S.; Chrubasik, C.; Kunzel, O.; Black, A. Patient-perceived benefit during one year of treatment with Doloteffin. Phytomedicine 2007, 14, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Conrozier, T.; Mathieu, P.; Bonjean, M.; Marc, J.F.; Renevier, J.L.; Balblanc, J.C. A complex of three natural anti-inflammatory agents provides relief of osteoarthritis pain. Altern. Health Med. 2014, 20, 32–37. [Google Scholar]
- Vreju, F.A.; Ciurea, P.L.; Rosu, A.; Chisalau, B.A.; Parvanescu, C.D.; Firulescu, S.C.; Turcu Stiolica, A.; Barbulescu, A.L.; Dinescu, S.C.; Dumitrescu, C.I. The effect of glucosamine, chondroitin and Harpagophytum procumbens on femoral hyaline cartilage thickness in patients with knee osteoarthritis—An MRI versus ultrasonography study. J. Mind Med. Sci. 2019, 6, 162–168. [Google Scholar] [CrossRef]
- Hu, S.; Belcaro, G.; Cesarone, M.R.; Feragalli, B.; Cotellese, R.; Dugall, M.; Scipione, C.; Scipione, V.; Maione, C.; Maramaldi, G.; et al. A sport cream (Harpago-Boswellia-ginger-escin) for localized neck/shoulder pain. Minerva Med. 2021, 112, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Schrüffler, H. Ein Fortschritt in der nichtsteroidalen antirheumatischen Therapie. Med. Publ. 1980, 1, 1–8. [Google Scholar]
- Guyader, M. Les Plantes Anti-Rhumatismales. Etude Historique et Pharmacologique. Et Etude Clinique du Nebulisat d’Harpagophytum procumbens DC chez 50 Patients Arthrosioues Suivis en Service Hospitalier. Ph.D. Thesis, Université Pierre et Marie Curie, Paris, France, 1984; p. 151. [Google Scholar]
- Pinget, M.; Lecomte, A. Etude des effets de l’Harpagophytum en rhumatologie dégénérative. 37°2 Le Mag. 1990, 10, 23–25. [Google Scholar]
- Lecomte, A.; Costa, J.P. Harpagophytum dans l’arthrose: Etude en double insu contre placebo. 37°2 Le Mag. 1992, 15, 27–30. [Google Scholar]
- Chrubasik, S.; Zimpfer, C.; Schütt, U.; Ziegler, R. Effectiveness of Harpagophytum procumbens in treatment of acute low back pain. Phytomedicine 1996, 3, 1–10. [Google Scholar] [CrossRef]
- Chrubasik, S.; Pollak, S.; Black, A. Effectiveness of devil’s claw for osteoarthritis. Rheumatology 2002, 41, 1332–1333, author reply 1333. [Google Scholar] [CrossRef][Green Version]
- Chrubasik, S.; Fiebich, B.; Black, A.; Pollak, S. Treating low back pain with an extract of Harpagophytum that inhibits cytokine release. Eur. J. Anaesthesiol. 2002, 19, 209. [Google Scholar] [CrossRef]
- Chrubasik, S.; Zimpfer, C.; Schütt, U.; Ziegler, R. The arhus low back rating scale: A useful tool to evaluate the success of low back pain treatment. Reg. Anesth. J. Neural Blockade Obstet. Surg. Pain Control 1997, 22, 100. [Google Scholar]
- Schmelz, H.; Hämmerle, H.D.; Springorum, H.W. Analgetische Wirkung eines Teufelskrappenwurzel-Extraktes bei verschiedenen chronisch-degenrativen Gelenkerkrankungen. In Rheumatherapie mit Phytopharmaka; Chrubasik, S., Wink, M., Eds.; Hippokrates: Stuttgart, Germany, 1997; pp. 86–89. [Google Scholar]
- Chrubasik, S.; Junck, H.; Breitschwerdt, H.; Conradt, C.; Zappe, H. Effectiveness of Harpagophytum extract WS 1531 in the treatment of exacerbation of low back pain: A randomized, placebo-controlled, double-blind study. Eur. J. Anaesthesiol. 1999, 16, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Chantre, P.; Cappelaere, A.; Leblan, D.; Guedon, D.; Vandermander, J.; Fournie, B. Efficacy and tolerance of Harpagophytum procumbens versus diacerhein in treatment of osteoarthritis. Phytomedicine 2000, 7, 177–183. [Google Scholar] [CrossRef]
- Leblan, D.; Chantre, P.; Fournié, B. L’harpagophyton dans le traitement de la gonarthrose et de la coxarthrose. Résultats à quatre mois d’une étude prospective multicentrique, contrôlée en double aveugle, versus diacerhéine. Rev. Rhum. 2000, 67, 634–640. [Google Scholar] [CrossRef]
- Göbel, H.; Heinze, A.; Ingwersen, M.; Niederberger, U.; Gerber, D. Wirkmechanismus von Harpagophytum-procumbens-Extrakt L1 174 bei der Behandlung von unspezifischen Rückenschmerzen. In Phytopharmaka VI, Forschung und klinische Anwendung; Rietbrock, N., Ed.; Steinkopff: Darmstadt, Germany, 2000; pp. 99–115. [Google Scholar]
- Göbel, H.; Heinze, A.; Ingwersen, M.; Niederberger, U.; Gerber, D. Harpagophytum-Extrakt LI 174 (Teufelskralle) bei der Behandlung unspezifischer Ruckenschmerzen—Effekte auf die sensible, motorische und vaskulare Muskelreagibilitat. Schmerz 2001, 15, 10–18. [Google Scholar] [CrossRef]
- Frerick, H.; Biller, A.; Schmidt, U. Stufenschema bei Coxarthrose. Kassenarzt 2001, 5, 34–41. [Google Scholar]
- Biller, A. Ergebnisse zweier randomisierter kontrollierter Studien und einer Anwendungsbeobachtung mit Teufelskrallenextrakt. In Phytopharmaka VII, Forschung und klinische Anwendung; Schulz, V., Rietbrock, N., Roots, I., Loew, D., Eds.; Steinkopff: Darmstadt, Germany, 2002; pp. 81–92. ISBN 978-3-642-57528-0. [Google Scholar]
- Chrubasik, S.; Model, A.; Black, A.; Pollak, S. A randomized double-blind pilot study comparing Doloteffin and Vioxx in the treatment of low back pain. Rheumatology 2003, 42, 141–148. [Google Scholar] [CrossRef]
- Chrubasik, S.; Künzel, O.; Thanner, J.; Conradt, C.; Black, A. A 1-year follow-up after a pilot study with Doloteffin for low back pain. Phytomedicine 2005, 12, 1–9. [Google Scholar] [CrossRef]
- Chrubasik, S.; Künzel, O.; Thanner, J.; Conradt, C. A short-term follow-up after a randomised double-blind pilot study comparing Doloteffin® vs. rofecoxib for low back pain. Focus Altern. Complement. Ther. 2003, 8, 133. [Google Scholar] [CrossRef]
- Ullmann, M.; Model, A.; Chrubasik, S. Treatment of acute low back pain with the Harpagophytum extract Doloteffin and the COX-2 inhibitor VIOXX: A pilot study. In Proceedings of the 3rd International Congress on Phytomedicine, Munich, Germany, 11–13 October 2000. [Google Scholar]
- Lienert, A.; Rütten, S.; Kuhn, M.; Wartenberg-Demand, A. A randomised, active-controlled, mono-centric study of the herbal drug, Devil’s claw (Harpagophytum procumbens) (ALLYA® tablets), Voltaren® and Vioxx® indicates equal efficacy in the treatment of patients with unspecific lumbar pain. 54. Jahrestag. Norddtsch. Orthopädenver. Eingetrag. Ver. Hambg. 2005, 16, Doc05novEP26. [Google Scholar]
- Lienert, A.; Rütten, S.; Kuhn, M.; Wartenberg-Demand, A. Efecto analgésico de un extracto estandarizado de harpagofito. Rev. Fitoter. 2007, 7, 15–20. [Google Scholar]
- Pillay, D. A Double-Blinded, Placebo Controlled Clinicaltrial Evaluating the Efficacy of the Harpago and Celery Seed Cream in Mild to Moderate Degenerative Joint Disease of the Knee. Ph.D. Thesis, Durban Institute of Technology, Durban, South Africa, 2006; p. 138. [Google Scholar]
- Anvari, H.M.P.; Panahi, J.R.; Ansari, M.; Iraji, M.; Ghorbanian, N.; Dehghani, A. The effect of single dose of Harpagophytum capsule (teltonal) on post tracheal intubation sore throat after general anesthesia. Arch. Anesthesiol. Crit. Care 2016, 2, 239–242. [Google Scholar]
- Moré, M.; Grünwald, J.; Pohl, U.; Uebelhack, R. A Rosa canina—Urtica dioica—Harpagophytum procumbens/zeyheri combination significantly reduces gonarthritis symptoms in a randomized, placebo-controlled double-blind study. Planta Med. 2017, 83, 1384–1391. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. Devil’s claw root: Ulcers and gastrointestinal bleeding? Prescrire Int. 2013, 22, 296. [Google Scholar]
- Gallo, E.; Lucenteforte, E.; Firenzuoli, F.; Menniti-Ippolito, F.; Maggini, V.; Pugi, A.; Mascherini, V.; Gori, L.; Mugelli, A.; Vannacci, A. Herbalists’ perception of risks involving commonly sold medicinal plants in Italy. Complement. Med. 2014, 22, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Vlachojannis, J.; Roufogalis, B.D.; Chrubasik, S. Systematic review on the safety of Harpagophytum preparations for osteoarthritic and low back pain. Phytother. Res. 2008, 22, 149–152. [Google Scholar] [CrossRef]
- Posadzki, P.; Watson, L.K.; Ernst, E. Adverse effects of herbal medicines: An overview of systematic reviews. Clin. Med. 2013, 13, 7–12. [Google Scholar] [CrossRef]
- Biazi, B.I.; D’Epiro, G.F.; Zanetti, T.A.; de Oliveira, M.T.; Ribeiro, L.R.; Mantovani, M.S. Risk assessment via metabolism and cell growth inhibition in a HepG2/C3A cell line upon treatment with arpadol and its active component harpagoside. Phytother. Res. 2017, 31, 387–394. [Google Scholar] [CrossRef]
- Cordier, W.; Steenkamp, V. Drug interactions in African herbal remedies. Drug Metab. Drug Interact. 2011, 26, 53–63. [Google Scholar] [CrossRef]
- Shaw, D.; Leon, C.; Kolev, S.; Murray, V. Traditional remedies and food supplements. A 5-year toxicological study (1991–1995). Drug Saf. 1997, 17, 342–356. [Google Scholar] [CrossRef] [PubMed]
- Argento, A.; Tiraferri, E.; Marzaloni, M. Anticoagulanti orali e piante medicinali. Una interazione emergente. Ann. Ital. Med. Int. 2000, 15, 139–143. [Google Scholar]
- Heck, A.M.; DeWitt, B.A.; Lukes, A.L. Potential interactions between alternative therapies and warfarin. Am. J. Health Syst. Pharm. 2000, 57, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, N.A.; Kenny, M.W.; Davies, G.; Patel, J.P. Complimentary and alternative medicine use among patients starting warfarin. Br. J. Haematol. 2005, 130, 777–780. [Google Scholar] [CrossRef]
- Izzo, A.A.; Di Carlo, G.; Borrelli, F.; Ernst, E. Cardiovascular pharmacotherapy and herbal medicines: The risk of drug interaction. Int. J. Cardiol. 2005, 98, 1–14. [Google Scholar] [CrossRef]
- Carvalho, R.R.; Donadel, C.D.; Cortez, A.F.; Valviesse, V.R.; Vianna, P.F.; Correa, B.B. Syndrome of inappropriate antidiuretic hormone secretion induced by the phytotherapy Harpagophytum procumbers: Case report. J. Bras. Nefrol. 2017, 39, 79–81. [Google Scholar] [CrossRef] [PubMed]
- Cuspidi, C.; Sala, C.; Tadic, M.; Grassi, G.; Mancia, G. Systemic Hypertension Induced by Harpagophytum procumbens (Devil’s claw): A case report. J. Clin. Hypertens. 2015, 17, 908–910. [Google Scholar] [CrossRef] [PubMed]
- Douros, A.; Bronder, E.; Andersohn, F.; Klimpel, A.; Thomae, M.; Ockenga, J.; Kreutz, R.; Garbe, E. Drug-induced acute pancreatitis: Results from the hospital-based Berlin case-control surveillance study of 102 cases. Aliment. Pharmacol. Ther. 2013, 38, 825–834. [Google Scholar] [CrossRef]
- Altmeyer, N.; Garnier, R.; Rosenberg, N.; Geerolf, A.M.; Ghaem, A. Conjonctivite, rhinite et asthme rythmes par l’exposition professionnelle a l’Harpagophytum. Arch. Mal. Prof. Méd. Trav. Sécurité Soc. 1991, 53, 289–291. [Google Scholar]
- Rahman, H.; Kim, M.; Leung, G.; Green, J.A.; Katz, S. Drug-herb interactions in the elderly patient with IBD: A growing concern. Curr. Treat. Options Gastroenterol. 2017, 15, 618–636. [Google Scholar] [CrossRef]
- Colas, C. Développement de Méthodes Physico-Chimiques pour le Contrôle de la Médicationpar l’Harpagophytum et l’Eleutherococcus, Principes Actifs Utilisés en Phytothérapie Équine. Ph.D. Thesis, École Polytechnique, Université Paris-Saclay, Paris, France, 2006; p. 313. [Google Scholar]
- Torfs, S.; Delesalle, C.; Vanschandevijl, K.; de Clercq, D.; Van Loon, G.; Nollet, H.; Deprez, P. Anti-inflammatory phytotherapeutics: A valuable alternative to NSAID treatment in horses. Vlaams Diergeneeskd. Tijdschr. 2008, 78, 161–170. [Google Scholar]
- Montavon, S. Efficacité d’une préparation phytothérapique à base d’Harpagophytum procumbens dans les cas d’éparvin chez le cheval adulte. Prat. Vét. Equine 1994, 26, 49–53. [Google Scholar]
- Axmann, S. Studien zur Pharmakokinetik und zur Klinischen Wirksamkeit von Harpagosid aus Einem Extrakt der Teufelskralle (“Harpagophytum procumbens” DC ex Meisn.) bei Pferden. Ph.D. Thesis, Veterinärmedizinische Universität, Wien, Austria, 2019; p. 106. [Google Scholar]
- Axmann, S.; Hummel, K.; Nöbauer, K.; Razzazi-Fazeli, E.; Zitterl-Eglseer, K. Pharmacokinetics of harpagoside in horses after intragastric administration of a Devil’s claw (Harpagophytum procumbens) extract. J. Vet. Pharmacol. Ther. 2019, 42, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Moreau, M.; Lussier, B.; Pelletier, J.P.; Martel-Pelletier, J.; Bedard, C.; Gauvin, D.; Troncy, E. A medicinal herb-based natural health product improves the condition of a canine natural osteoarthritis model: A randomized placebo-controlled trial. Res. Vet. Sci. 2014, 97, 574–581. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).