Targeting Adenosine Receptor by Polydeoxyribonucleotide: An Effective Therapeutic Strategy to Induce White-to-Brown Adipose Differentiation and to Curb Obesity
Abstract
:1. Introduction
2. Results
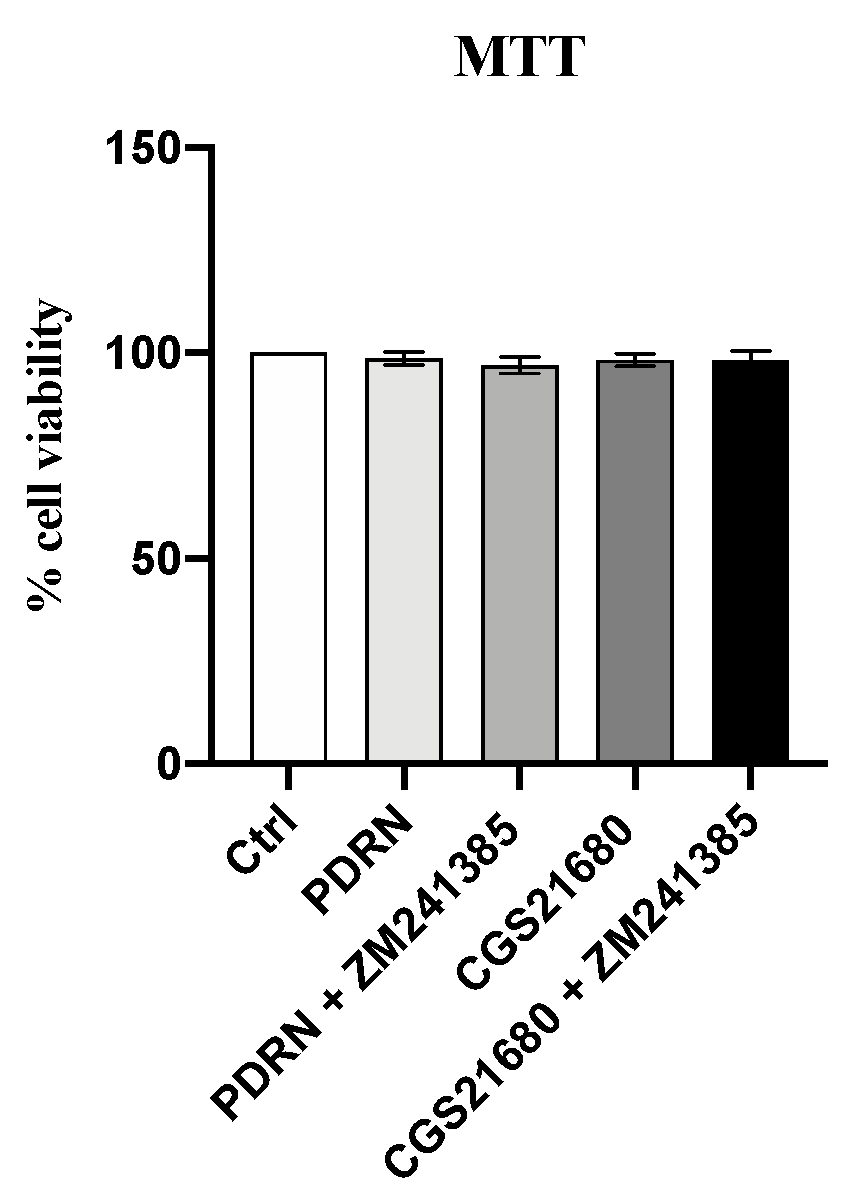
2.1. PDRN Does Not Affect Cell Viability
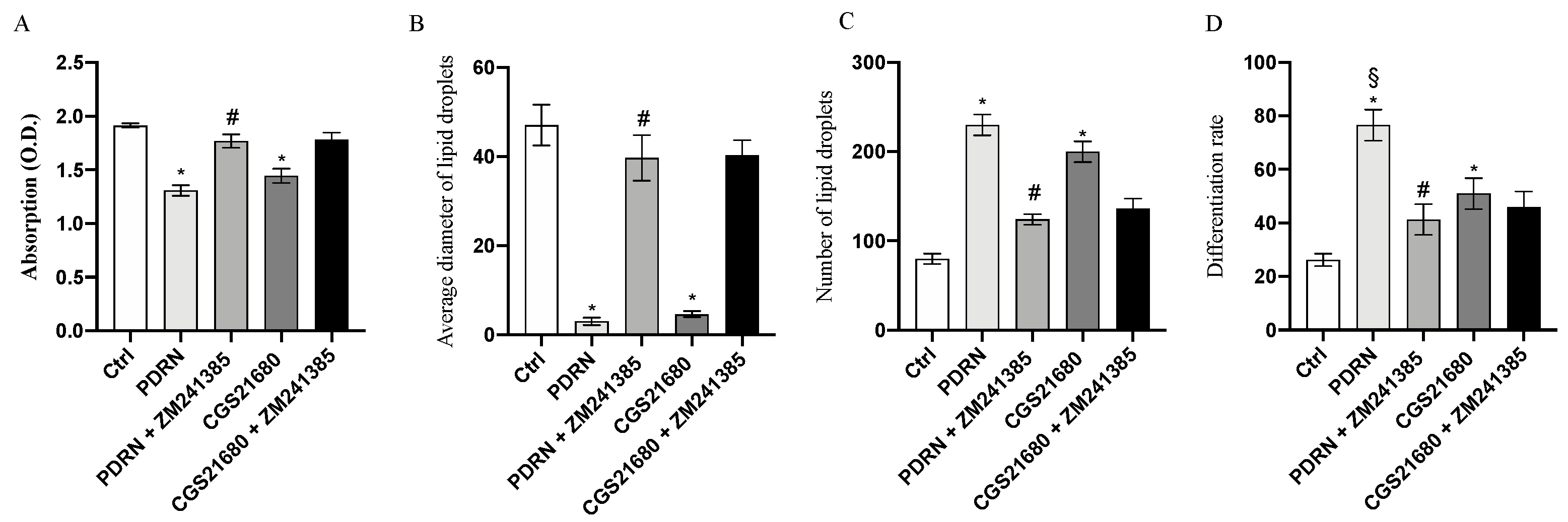
2.2. PDRN Promotes Trans-Differentiation from White Adipocytes to Beige Adipocytes
2.3. Effect of PDRN on Oxygen Consumption
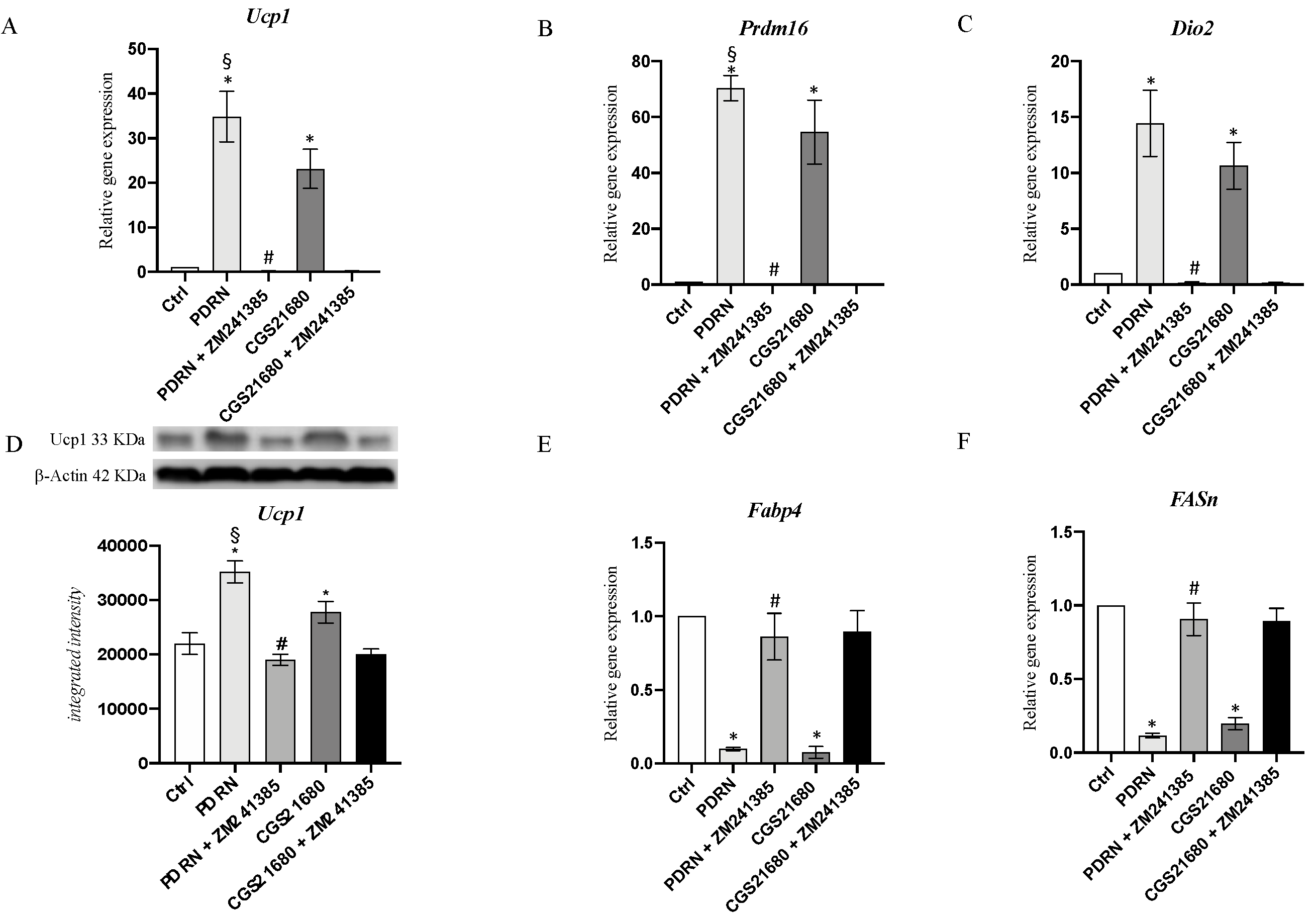
2.4. PDRN Promotes the Browning Process
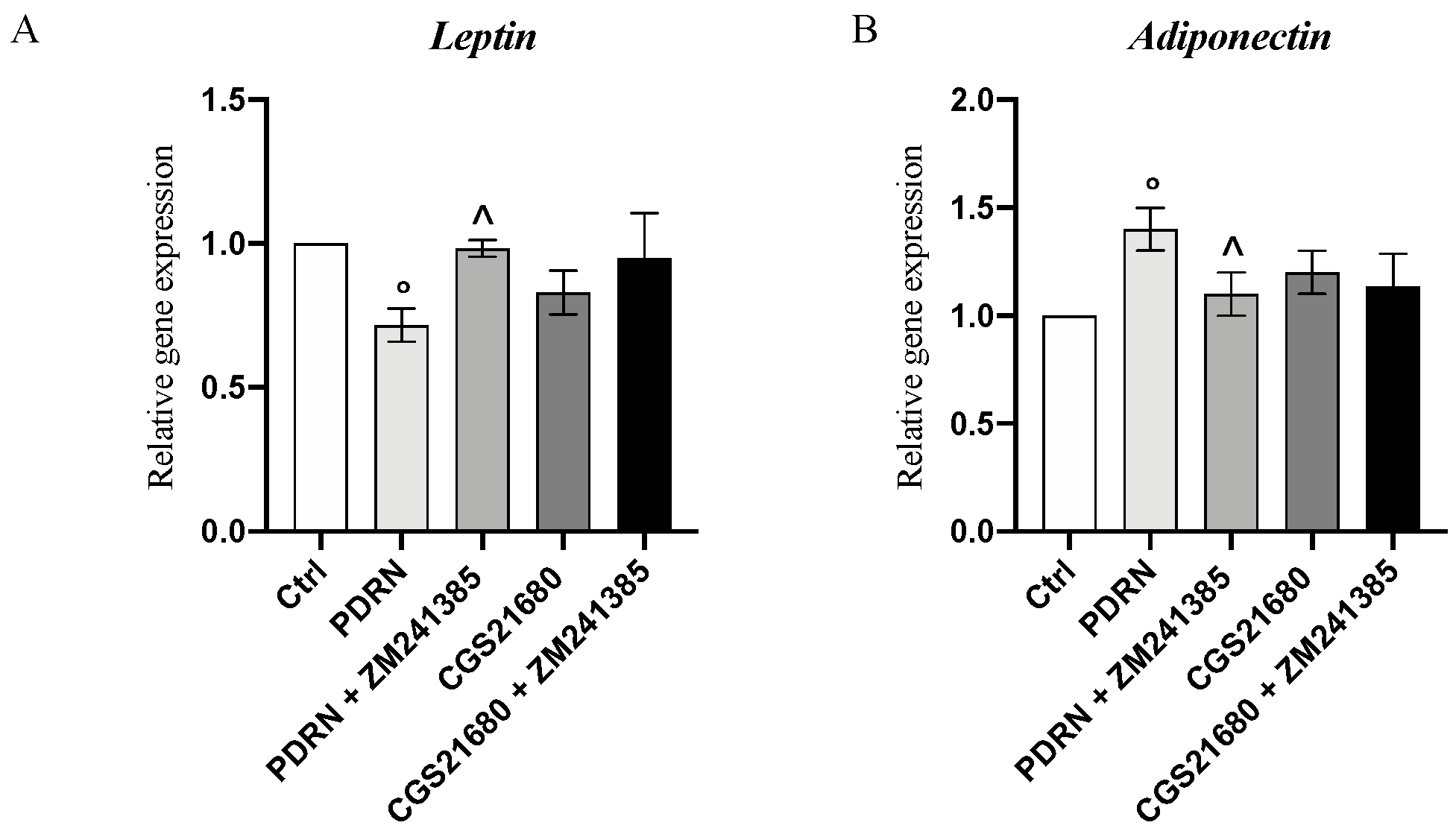
2.5. PDRN Modulates the Adipokine Network
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. T3-L1 Pre-Adipocyte Cell Culture and Differentiation
4.3. Cell Viability Assay
4.4. Oil Red O lipid staining
4.5. Lipid Accumulation
4.6. Extracellular Oxygen Consumption Assay
4.7. RNA Isolation, cDNA Synthesis and Real-Time Quantitative PCR Amplification
4.8. Western Blot Analysis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mafort, T.T.; Rufino, R.; Costa, C.H.; Lopes, A.J. Obesity: Systemic and pulmonary complications, biochemical abnormalities, and impairment of lung function. Multidiscip. Respir. Med. 2016, 11, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conway, B.; Rene, A. Obesity as a disease: No lightweight matter. Obes. Rev. 2004, 5, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.; Jain, S.K. Obesity, oxidative stress, adipose tissue dysfunction, and the associated health risks: Causes and therapeutic strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423–444. [Google Scholar] [CrossRef] [Green Version]
- Jeremic, N.; Chaturvedi, P.; Tyagi, S.C. Browning of white fat: Novel insight into factors, mechanisms, and therapeutics. J. Cell. Physiol. 2017, 232, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Fenzl, A.; Kiefer, F.W. Brown adipose tissue and thermogenesis. Horm. Mol. Biol. Clin. Investig. 2014, 19, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Murano, I.; Barbatelli, G.; Giordano, A.; Cinti, S. Noradrenergic parenchymal nerve fiber branching after cold acclimatisation correlates with brown adipocyte density in mouse adipose organ. J. Anat. 2009, 214, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Braun, K.; Oeckl, J.; Westermeier, J.; Li, Y.; Klingenspor, M. Non-adrenergic control of lipolysis and thermogenesis in adipose tissues. J. Exp. Biol. 2018, 7, 221. [Google Scholar] [CrossRef] [Green Version]
- Busiello, R.A.; Savarese, S.; Lombardi, A. Mitochondrial uncoupling proteins and energy metabolism. Front. Physiol. 2015, 6, 36. [Google Scholar] [CrossRef] [Green Version]
- Vitali, A.; Murano, I.; Zingaretti, M.C.; Frontini, A.; Ricquier, D.; Cinti, S. The adipose organ of obesity-prone C57BL/6J mice is composed of mixed white and brown adipocytes. J. Lipid Res. 2012, 53, 619–629. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Cohen, P.; Spiegelman, B.M. Adaptive thermogenesis in adipocytes: Is beige the new brown? Genes Dev. 2013, 27, 234–250. [Google Scholar] [CrossRef] [Green Version]
- Abbracchio, M.; Burnstock, G.; Verkhratsky, A.; Zimmermann, H. Purinergic signalling in the nervous system: An overview. Trends Neurosci. 2009, 32, 19–29. [Google Scholar] [CrossRef]
- Effendi, W.I.; Nagano, T.; Kobayashi, K.; Nishimura, Y. Focusing on adenosine receptors as a potential targeted therapy in human diseases. Cells. 2020, 9, 785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gnad, T.; Scheibler, S.; von Kügelgen, I.; Scheele, C.; Kilić, A.; Glöde, A.; Hoffmann, L.S.; Reverte-Salisa, L.; Horn, P.; Mutlu, S.; et al. Adenosine activates brown adipose tissue and recruits beige adipocytes via A2A receptors. Nature 2014, 516, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Lahesmaa, M.; Oikonen, V.; Helin, S.; Luoto, P.; Din, M.U.; Pfeifer, A.; Nuutila, P.; Virtanen, K.A. Regulation of human brown adipose tissue by adenosine and A2A receptors—Studies with [15O]H2O and [11C]TMSX PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 743–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irrera, N.; Arcoraci, V.; Mannino, F.; Vermiglio, G.; Pallio, G.; Minutoli, L.; Bagnato, G.; Anastasi, G.P.; Mazzon, E.; Bramanti, P.; et al. Activation of A2A receptor by PDRN reduces neuronal damage and stimulates WNT/β-CATENIN driven neurogenesis in spinal cord injury. Front. Pharmacol. 2018, 9, 506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irrera, N.; Bitto, A.; Vaccaro, M.; Mannino, F.; Squadrito, V.; Pallio, G.; Arcoraci, V.; Minutoli, L.; Ieni, A.; Lentini, M.; et al. PDRN, a bioactive natural compound, ameliorates imiquimod-induced psoriasis through NF-κB pathway inhibition and Wnt/β-Catenin signaling modulation. Int. J. Mol. Sci. 2020, 21, 1215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pallio, G.; Bitto, A.; Ieni, A.; Irrera, N.; Mannino, F.; Pallio, S.; Altavilla, D.; Squadrito, F.; Scarpignato, C.; Minutoli, L. Combined treatment with polynucleotides and hyaluronic acid improves tissue repair in experimental colitis. Biomedicines 2020, 8, 438. [Google Scholar] [CrossRef]
- Lowell, B.B.; Spiegelman, B.M. Towards a molecular understanding of adaptive thermogenesis. Nature 2000, 404, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Fain, J.N.; Pointer, R.H.; Ward, W.F. Effects of adenosine nucleosides on adenylate cyclase, phosphodiesterase, cyclic adenosine monophosphate accumulation, and lipolysis in fat cells. J. Biol. Chem. 1972, 247, 6866–6872. [Google Scholar] [CrossRef]
- DeOliveira, C.C.; Paiva Caria, C.R.; Ferreira Gotardo, E.M.; Ribeiro, M.L.; Gambero, A. Role of A1 and A2A adenosine receptor agonists in adipose tissue inflammation induced by obesity in mice. Eur. J. Pharmacol. 2017, 799, 154–159. [Google Scholar] [CrossRef]
- Tozzi, M.; Novak, I. Purinergic receptors in adipose tissue as potential targets in metabolic disorders. Front. Pharmacol. 2017, 8, 878. [Google Scholar] [CrossRef]
- Fantuzzi, G. Adipose tissue, adipokines, and inflammation. J. Allergy Clin. Immunol. 2005, 115, 911–919. [Google Scholar] [CrossRef]
- Stern, J.H.; Rutkowski, J.M.; Scherer, P.E. Adiponectin, leptin, and fatty acids in the maintenance of metabolic homeostasis through adipose tissue crosstalk. Cell Metab. 2016, 23, 770–784. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.; Luo, N.; Klein, R.L.; Garvey, W.T. Adiponectin promotes adipocyte differentiation, insulin sensitivity, and lipid accumulation. J. Lipid Res. 2005, 4, 1369–1379. [Google Scholar] [CrossRef] [Green Version]
- William, W.N., Jr.; Ceddia, R.B.; Curi, R. Leptin controls the fate of fatty acids in isolated rat white adipocytes. J. Endocrinol. 2002, 17, 735–744. [Google Scholar] [CrossRef] [Green Version]
- Altavilla, D.; Bitto, A.; Polito, F.; Marini, H.; Minutoli, L.; Di Stefano, V.; Irrera, N.; Cattarini, G.; Squadrito, F. Polydeoxyribonucleotide (PDRN): A safe approach to induce therapeutic angiogenesis in peripheral artery occlusive disease and in diabetic foot ulcers. Cardiovasc. Hematol. Agents Med. Chem. 2009, 7, 313–321. [Google Scholar] [CrossRef]
- Squadrito, F.; Calapai, G.; Altavilla, D.; Cucinotta, D.; Zingarelli, B.; Arcoraci, V.; Campo, G.M.; Caputi, A.P. Central serotoninergic system involvement in the anorexia induced by NG-nitro-L-arginine, an inhibitor of nitric oxide synthase. Eur. J. Pharmacol. 1994, 255, 51–55. [Google Scholar] [CrossRef]
- Kim, J.K.; Chung, J.Y. Effectiveness of polydeoxyribonucleotide injection versus normal saline injection for treatment of chronic plantar fasciitis: A prospective randomised clinical trial. Int. Orthop. 2015, 39, 1329–1334. [Google Scholar] [CrossRef]
- Lee, D.O.; Yoo, J.H.; Cho, H.I.; Cho, S.; Cho, H.R. Comparing effectiveness of polydeoxyribonucleotide injection and corticosteroid injection in plantar fasciitis treatment: A prospective randomized clinical study. Foot Ankle Surg. 2020, 26, 657–661. [Google Scholar] [CrossRef]
- Kim, M.S.; Cho, R.K.; In, Y. The efficacy and safety of polydeoxyribonucleotide for the treatment of knee osteoarthritis: Systematic review and meta-analysis of randomized controlled trials. Medicine 2019, 98, e17386. [Google Scholar] [CrossRef]
- Picciolo, G.; Mannino, F.; Irrera, N.; Altavilla, D.; Minutoli, L.; Vaccaro, V.; Arcoraci, V.; Squadrito, V.; Picciolo, G.; Squadrito, F.; et al. PDRN, a natural bioactive compound, blunts inflammation and positively reprograms healing genes in an “in vitro” model of oral mucositis. Biomed. Pharmacother. 2021, 138, 111538. [Google Scholar] [CrossRef]
- Fan, Q.; Xi, P.; Tian, D.; Jia, L.; Cao, Y.; Zhan, K.; Sun, T.; Zhang, Y.; Wang, Q. Ginsenoside Rb1 facilitates browning by repressing Wnt/β-Catenin signaling in 3T3-L1 adipocytes. Med. Sci. Monit. 2021, 7, 27. [Google Scholar]
- Irrera, N.; D'Ascola, A.; Pallio, G.; Bitto, A.; Mazzon, E.; Mannino, F.; Squadrito, V.; Arcoraci, V.; Minutoli, L.; Campo, G.M.; et al. β-Caryophyllene Mitigates Collagen Antibody Induced Arthritis (CAIA) in Mice Through a Cross-Talk between CB2 and PPAR-γ Receptors. Biomolecules. 2019, 9, 326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pallio, G.; Micali, A.; Benvenga, S.; Antonelli, A.; Marini, H.R.; Puzzolo, D.; Macaione, V.; Trichilo, V.; Santoro, G.; Irrera, N.; et al. Myo-inositol in the protection from cadmium-induced toxicity in mice kidney: An emerging nutraceutical challenge. Food Chem. Toxicol. 2019, 132, 110675. [Google Scholar] [CrossRef]
- Minutoli, L.; Marini, H.; Rinaldi, M.; Bitto, A.; Irrera, N.; Pizzino, G.; Pallio, G.; Calò, M.; Adamo, E.B.; Trichilo, V.; et al. A dual inhibitor of cyclooxygenase and 5-lipoxygenase protects against kainic acid-induced brain injury. Neuromol. Med. 2015, 17, 192–201. [Google Scholar] [CrossRef] [PubMed]


| Gene | Forward | Reverse |
|---|---|---|
| GAPDH | 5′ GAGTCAACGGATTTGGTCGT3′ | 5′ TTGATTTTGGAGGGATCTCG3′ |
| FASn | 5′ TTGCTGGCACTACAGAATGC3′ | 5′ AACAGCCTCAGAGCGACAAT3′ |
| FABP4 | 5′ TCACCTGGAAGACAGCTCCT3′ | 5′ AATCCCCATTTACGCTGATG3′ |
| Adiponectin | 5′ GTTGCAAGCTCTCCTGTTCC3′ | 5′ TCTCCAGGAGTGCCATCTCT3′ |
| UCP1 | 5′ ATGGTGAACCCGACAACTTC3′ | 5′ CAGCGGGAAGGTGATGATA3′ |
| DIO2 | 5′ ATGGGACTCCTCAGCGTAGA3′ | 5′ GGAGGAAGCTGTTCCAGACA3′ |
| PRDM16 | 5′ CGAGGAGGAGACCGAAGAC3′ | 5′ GAAGTCTGGTGGGATTGGAA3′ |
| LEPTIN | 5′ TCTTTCCGGAACATTTGGAG3′ | 5′ TGTGAGATCAACCCTGGACA3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mannino, F.; Pallio, G.; Bitto, A.; Altavilla, D.; Minutoli, L.; Squadrito, V.; Arcoraci, V.; Giorgi, D.A.; Pirrotta, I.; Squadrito, F.; et al. Targeting Adenosine Receptor by Polydeoxyribonucleotide: An Effective Therapeutic Strategy to Induce White-to-Brown Adipose Differentiation and to Curb Obesity. Pharmaceuticals 2021, 14, 728. https://doi.org/10.3390/ph14080728
Mannino F, Pallio G, Bitto A, Altavilla D, Minutoli L, Squadrito V, Arcoraci V, Giorgi DA, Pirrotta I, Squadrito F, et al. Targeting Adenosine Receptor by Polydeoxyribonucleotide: An Effective Therapeutic Strategy to Induce White-to-Brown Adipose Differentiation and to Curb Obesity. Pharmaceuticals. 2021; 14(8):728. https://doi.org/10.3390/ph14080728
Chicago/Turabian StyleMannino, Federica, Giovanni Pallio, Alessandra Bitto, Domenica Altavilla, Letteria Minutoli, Violetta Squadrito, Vincenzo Arcoraci, Domenico Antonio Giorgi, Igor Pirrotta, Francesco Squadrito, and et al. 2021. "Targeting Adenosine Receptor by Polydeoxyribonucleotide: An Effective Therapeutic Strategy to Induce White-to-Brown Adipose Differentiation and to Curb Obesity" Pharmaceuticals 14, no. 8: 728. https://doi.org/10.3390/ph14080728
APA StyleMannino, F., Pallio, G., Bitto, A., Altavilla, D., Minutoli, L., Squadrito, V., Arcoraci, V., Giorgi, D. A., Pirrotta, I., Squadrito, F., & Irrera, N. (2021). Targeting Adenosine Receptor by Polydeoxyribonucleotide: An Effective Therapeutic Strategy to Induce White-to-Brown Adipose Differentiation and to Curb Obesity. Pharmaceuticals, 14(8), 728. https://doi.org/10.3390/ph14080728








