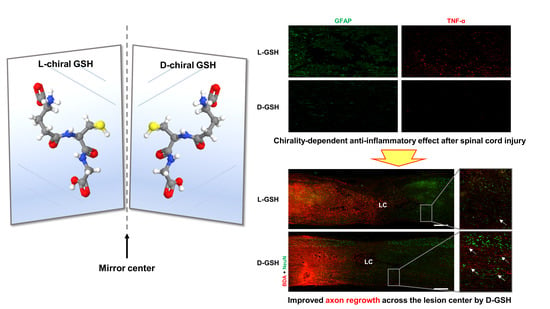
Chirality-Dependent Anti-Inflammatory Effect of Glutathione after Spinal Cord Injury in an Animal Model
Abstract
:1. Introduction
2. Results
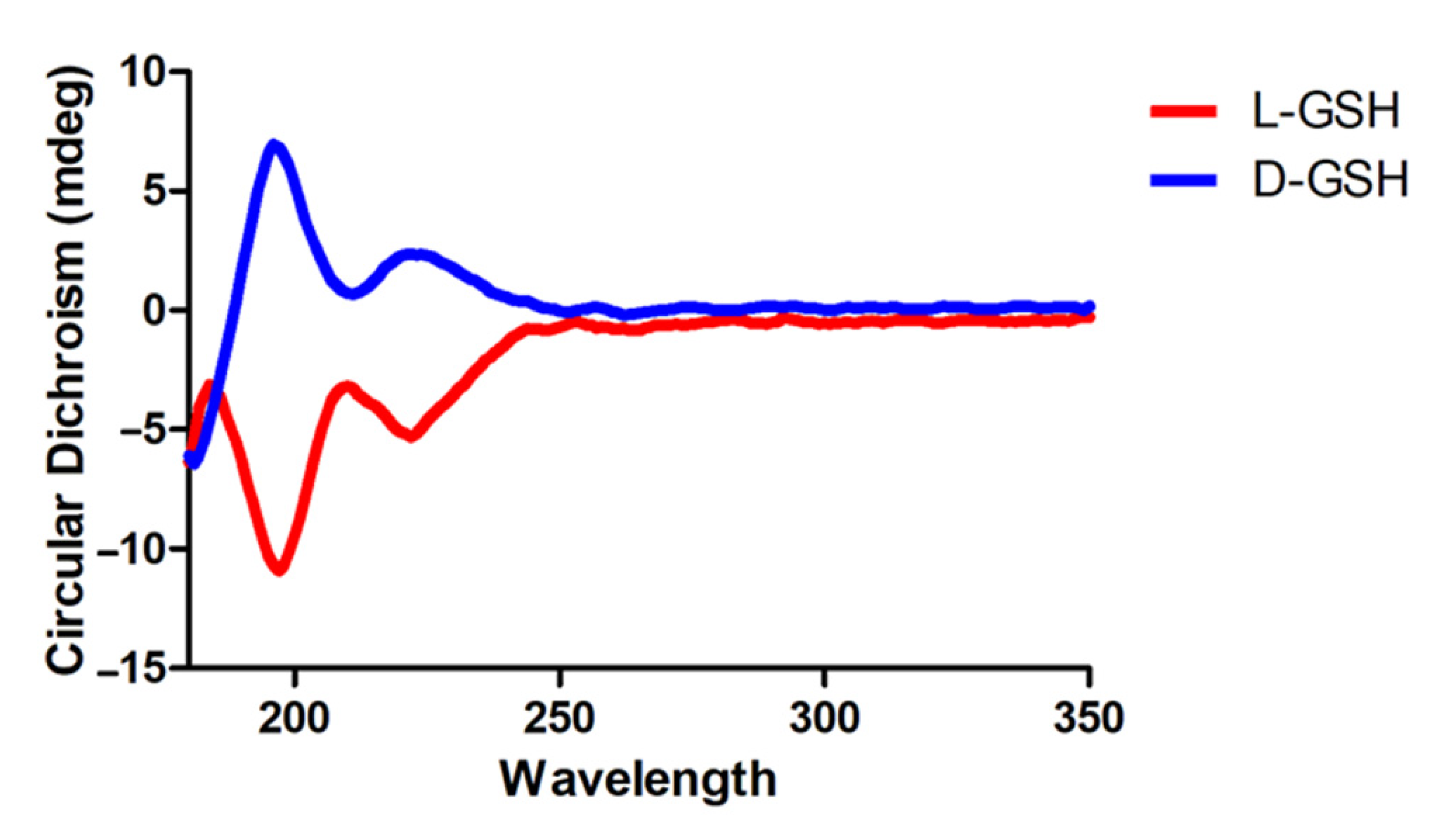
2.1. Characterization of Chiral Gshs
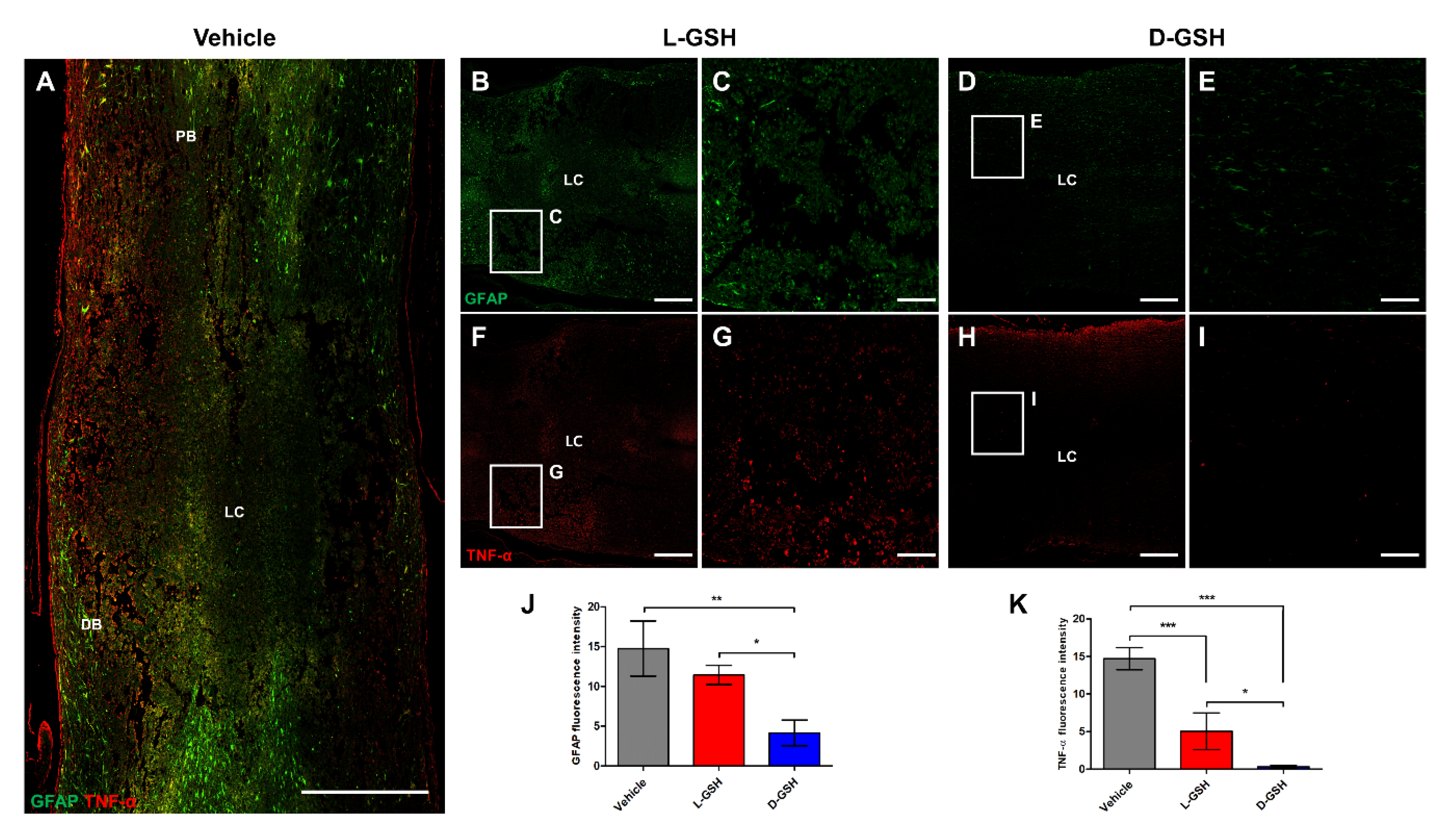
2.2. Chiral GSH Decreased the Production of Inflammatory Cytokines after SCI
2.3. D-GSH Inhibited Glial Scar Formation after SCI
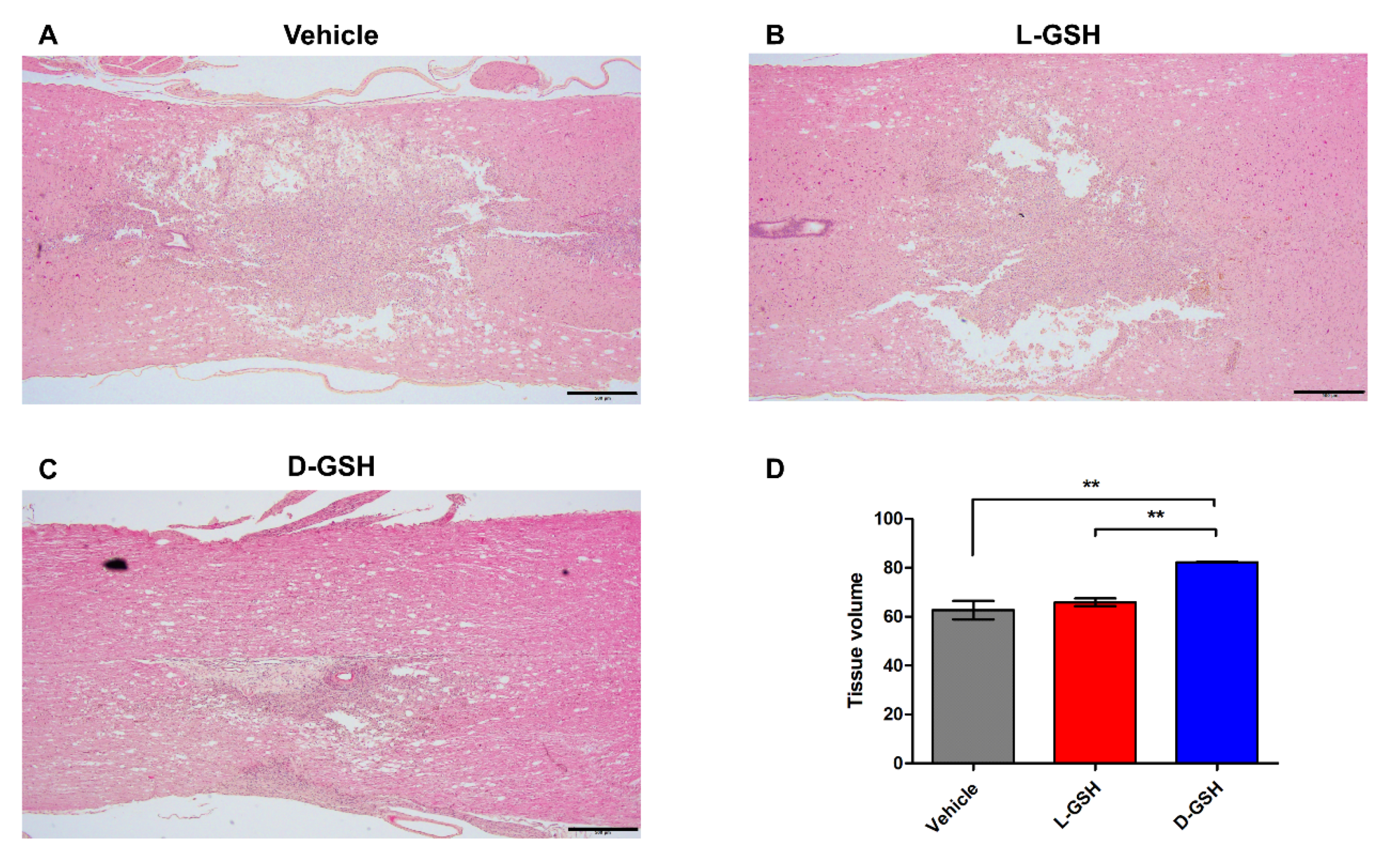
2.4. D-GSH Reduced Cystic Cavity after SCI
2.5. D-GSH-Induced Axon Regrowth across the Lesion Center after SCI
2.6. Chiral GSH Suppressed Inflammation through the Mitogen-Activated Protein Kinase (MAPK) Signaling Pathway after SCI
2.7. Different Uptake Levels of Chiral Gshs in Immune Cells
3. Discussion
4. Materials and Methods
4.1. Preparation and Characterization of Chiral Gshs
4.2. Surgical Procedures
4.3. Hindlimb Locomotor Evaluation
4.4. Histology and Immunohistochemistry
4.5. Quantification of Inflammatory Cytokines
4.6. Western Blotting
4.7. Isolation of Bone Marrow-Derived Macrophages
4.8. Calculation of Intracellular Uptake Rates
4.9. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jain, N.B.; Ayers, G.D.; Peterson, E.N.; Harris, M.B.; Morse, L.; O’Connor, K.C.; Garshick, E. Traumatic spinal cord injury in the United States, 1993–2012. JAMA 2015, 313, 2236–2243. [Google Scholar] [CrossRef]
- Rust, R.; Kaiser, J. Insights into the Dual Role of Inflammation after Spinal Cord Injury. J. Neurosci. 2017, 37, 4658–4660. [Google Scholar] [CrossRef] [Green Version]
- Ko, W.K.; Kim, S.J.; Jo, M.J.; Choi, H.; Lee, D.; Kwon, I.K.; Lee, S.H.; Han, I.B.; Sohn, S. Ursodeoxycholic Acid Inhibits Inflammatory Responses and Promotes Functional Recovery After Spinal Cord Injury in Rats. Mol. Neurobiol. 2019, 56, 267–277. [Google Scholar] [CrossRef]
- Kim, J.W.; Mahapatra, C.; Hong, J.Y.; Kim, M.S.; Leong, K.W.; Kim, H.W.; Hyun, J.K. Functional Recovery of Contused Spinal Cord in Rat with the Injection of Optimal-Dosed Cerium Oxide Nanoparticles. Adv. Sci. 2017, 4, 1700034. [Google Scholar] [CrossRef]
- Qian, T.; Guo, X.; Levi, A.D.; Vanni, S.; Shebert, R.T.; Sipski, M.L. High-dose methylprednisolone may cause myopathy in acute spinal cord injury patients. Spinal Cord 2005, 43, 199–203. [Google Scholar] [CrossRef] [Green Version]
- Sambrook, P.N.; Hassall, J.E.; York, J.R. Osteonecrosis after high dosage, short term corticosteroid therapy. J. Rheumatol. 1984, 11, 514–516. [Google Scholar]
- Topal, F.; Ozaslan, E.; Akbulut, S.; Kucukazman, M.; Yuksel, O.; Altiparmak, E. Methylprednisolone-induced toxic hepatitis. Ann. Pharmacother. 2006, 40, 1868–1871. [Google Scholar] [CrossRef]
- Hu, Z.; Meng, D.; Lin, F.; Zhu, X.; Fang, Z.; Wu, X. Plasmonic circular dichroism of gold nanoparticle based nanostructures. Adv. Opt. Mater. 2019, 7, 1801590. [Google Scholar] [CrossRef]
- Nguyen, L.A.; He, H.; Pham-Huy, C. Chiral drugs: An overview. Int J. Biomed. Sci 2006, 2, 85–100. [Google Scholar] [PubMed]
- Green, D.W.; Lee, J.M.; Kim, E.J.; Lee, D.J.; Jung, H.S. Chiral biomaterials: From molecular design to regenerative medicine. Adv. Mater. Interfaces 2016, 3, 1500411. [Google Scholar] [CrossRef]
- Yeom, J.; Guimaraes, P.P.G.; Ahn, H.M.; Jung, B.K.; Hu, Q.; McHugh, K.; Mitchell, M.J.; Yun, C.O.; Langer, R.; Jaklenec, A. Chiral Supraparticles for Controllable Nanomedicine. Adv. Mater. 2020, 32, e1903878. [Google Scholar] [CrossRef]
- Folkers, K.; Dahmen, J.; Ohta, M.; Stepien, H.; Leban, J.; Sakura, N.; Lundanes, E.; Rampold, G.; Patt, Y.; Goldman, R. Isolation of glutathione from bovine thymus and its significance to research relevant to immune systems. Biochem. Biophys. Res. Commun. 1980, 97, 590–594. [Google Scholar] [CrossRef]
- Utsugi, M.; Dobashi, K.; Koga, Y.; Shimizu, Y.; Ishizuka, T.; Iizuka, K.; Hamuro, J.; Nakazawa, T.; Mori, M. Glutathione redox regulates lipopolysaccharide-induced IL-12 production through p38 mitogen-activated protein kinase activation in human monocytes: Role of glutathione redox in IFN-gamma priming of IL-12 production. J. Leukoc. Biol. 2002, 71, 339–347. [Google Scholar]
- Lowry, T.M. The Cotton Effect. Nature 1933, 132, 552. [Google Scholar] [CrossRef]
- Litman, B.J.; Schellman, J.A. The n—π* Cotton Effect of the Peptide Linkage. J. Phys. Chem. 1965, 69, 978–983. [Google Scholar] [CrossRef]
- Basso, D.M.; Beattie, M.S.; Bresnahan, J.C. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma 1995, 12, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Ko, W.K.; Lee, S.H.; Kim, S.J.; Jo, M.J.; Kumar, H.; Han, I.B.; Sohn, S. Anti-inflammatory effects of ursodeoxycholic acid by lipopolysaccharide-stimulated inflammatory responses in RAW 264.7 macrophages. PLoS ONE 2017, 12, e0180673. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.J.; Ko, W.K.; Jo, M.J.; Arai, Y.; Choi, H.; Kumar, H.; Han, I.B.; Sohn, S. Anti-inflammatory effect of Tauroursodeoxycholic acid in RAW 264.7 macrophages, Bone marrow-derived macrophages, BV2 microglial cells, and spinal cord injury. Sci. Rep. 2018, 8, 3176. [Google Scholar] [CrossRef] [PubMed]
- Stoll, G.; Jander, S.; Schroeter, M. Inflammation and glial responses in ischemic brain lesions. Prog. Neurobiol. 1998, 56, 149–171. [Google Scholar] [CrossRef]
- Macaya, D.; Spector, M. Injectable hydrogel materials for spinal cord regeneration: A review. Biomed. Mater. 2012, 7, 012001. [Google Scholar] [CrossRef]
- Hong, L.T.A.; Kim, Y.M.; Park, H.H.; Hwang, D.H.; Cui, Y.; Lee, E.M.; Yahn, S.; Lee, J.K.; Song, S.C.; Kim, B.G. An injectable hydrogel enhances tissue repair after spinal cord injury by promoting extracellular matrix remodeling. Nat. Commun. 2017, 8, 533. [Google Scholar] [CrossRef]
- Kyriakis, J.M.; Avruch, J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol. Rev. 2001, 81, 807–869. [Google Scholar] [CrossRef] [Green Version]
- Qu, W.S.; Tian, D.S.; Guo, Z.B.; Fang, J.; Zhang, Q.; Yu, Z.Y.; Xie, M.J.; Zhang, H.Q.; Lu, J.G.; Wang, W. Inhibition of EGFR/MAPK signaling reduces microglial inflammatory response and the associated secondary damage in rats after spinal cord injury. J. Neuroinflammation 2012, 9, 178. [Google Scholar] [CrossRef] [Green Version]
- Cargnello, M.; Roux, P.P. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol. Mol. Biol. Rev. 2011, 75, 50–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sunderkotter, C.; Steinbrink, K.; Goebeler, M.; Bhardwaj, R.; Sorg, C. Macrophages and angiogenesis. J. Leukoc. Biol. 1994, 55, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Rabchevsky, A.G.; Hall, E.D. Role of peroxynitrite in secondary oxidative damage after spinal cord injury. J. Neurochem. 2007, 100, 639–649. [Google Scholar] [CrossRef]
- Villa, L.M.; Salas, E.; Darley-Usmar, V.M.; Radomski, M.W.; Moncada, S. Peroxynitrite induces both vasodilatation and impaired vascular relaxation in the isolated perfused rat heart. Proc. Natl. Acad. Sci. USA 1994, 91, 12383–12387. [Google Scholar] [CrossRef] [Green Version]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef] [Green Version]
- Jeong, E.M.; Yoon, J.H.; Lim, J.; Shin, J.W.; Cho, A.Y.; Heo, J.; Lee, K.B.; Lee, J.H.; Lee, W.J.; Kim, H.J.; et al. Real-Time Monitoring of Glutathione in Living Cells Reveals that High Glutathione Levels Are Required to Maintain Stem Cell Function. Stem Cell Rep. 2018, 10, 600–614. [Google Scholar] [CrossRef] [Green Version]
- Han, G.H.; Kim, S.J.; Ko, W.K.; Lee, D.; Han, I.B.; Sheen, S.H.; Hong, J.B.; Sohn, S. Transplantation of tauroursodeoxycholic acid-inducing M2-phenotype macrophages promotes an anti-neuroinflammatory effect and functional recovery after spinal cord injury in rats. Cell Prolif. 2021, 54, e13050. [Google Scholar] [CrossRef]
- Kim, S.J.; Ko, W.-K.; Heo, D.N.; Lee, S.J.; Lee, D.; Heo, M.; Han, I.-B.; Kwon, I.K.; Sohn, S. Anti-neuroinflammatory gold nanocomplex loading ursodeoxycholic acid following spinal cord injury. Chem. Eng. J. 2019, 375, 122088. [Google Scholar] [CrossRef]
- Trouplin, V.; Boucherit, N.; Gorvel, L.; Conti, F.; Mottola, G.; Ghigo, E. Bone marrow-derived macrophage production. J. Vis. Exp. 2013, e50966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guizar-Sahagun, G.; Ibarra, A.; Espitia, A.; Martinez, A.; Madrazo, I.; Franco-Bourland, R.E. Glutathione monoethyl ester improves functional recovery, enhances neuron survival, and stabilizes spinal cord blood flow after spinal cord injury in rats. Neuroscience 2005, 130, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, P. Role of glutathione in immunity and inflammation in the lung. Int. J. Gen. Med. 2011, 4, 105–113. [Google Scholar] [CrossRef] [Green Version]
- Villa, P.; Saccani, A.; Sica, A.; Ghezzi, P. Glutathione protects mice from lethal sepsis by limiting inflammation and potentiating host defense. J. Infect. Dis. 2002, 185, 1115–1120. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-J.; Ko, W.-K.; Han, G.-H.; Lee, D.; Lee, Y.; Sheen, S.-H.; Hong, J.-B.; Sohn, S. Chirality-Dependent Anti-Inflammatory Effect of Glutathione after Spinal Cord Injury in an Animal Model. Pharmaceuticals 2021, 14, 792. https://doi.org/10.3390/ph14080792
Kim S-J, Ko W-K, Han G-H, Lee D, Lee Y, Sheen S-H, Hong J-B, Sohn S. Chirality-Dependent Anti-Inflammatory Effect of Glutathione after Spinal Cord Injury in an Animal Model. Pharmaceuticals. 2021; 14(8):792. https://doi.org/10.3390/ph14080792
Chicago/Turabian StyleKim, Seong-Jun, Wan-Kyu Ko, Gong-Ho Han, Daye Lee, Yuhan Lee, Seung-Hun Sheen, Je-Beom Hong, and Seil Sohn. 2021. "Chirality-Dependent Anti-Inflammatory Effect of Glutathione after Spinal Cord Injury in an Animal Model" Pharmaceuticals 14, no. 8: 792. https://doi.org/10.3390/ph14080792
APA StyleKim, S.-J., Ko, W.-K., Han, G.-H., Lee, D., Lee, Y., Sheen, S.-H., Hong, J.-B., & Sohn, S. (2021). Chirality-Dependent Anti-Inflammatory Effect of Glutathione after Spinal Cord Injury in an Animal Model. Pharmaceuticals, 14(8), 792. https://doi.org/10.3390/ph14080792