Ranolazine: An Old Drug with Emerging Potential; Lessons from Pre-Clinical and Clinical Investigations for Possible Repositioning
Abstract
1. Introduction
2. Ranolazine as an Antianginal and Anti-Ischemic Drug
3. Ranolazine as an Antiarrhythmic Drug
3.1. Ranolazine Has Multiple Ion Channel Effects
3.2. Clinical Evidence of Antiarrhythmic Benefits
3.3. Antiarrhythmic Mechanisms at the Ventricular Level
3.4. Antiarrhythmic Mechanisms at the Atrial Level
4. Non-Cardiac Effects of Ranolazine
4.1. Neuronal Effects
4.2. Vascular Effects of Ranolazine
4.3. Gluco-Metabolic Effects of Ranolazine
4.4. Skeletal Muscle Effects of Ranolazine
4.5. Ranolazine and Cancer
5. Adverse Effects of Ranolazine
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Balla, C.; Pavasini, R.; Ferrari, R. Treatment of Angina: Where Are We? Cardiology 2018, 140, 52–67. [Google Scholar] [CrossRef]
- Hale, S.L.; Shryock, J.C.; Belardinelli, L.; Sweeney, M.; Kloner, R.A. Late Sodium Current Inhibition as a New Cardioprotective Approach. J. Mol. Cell. Cardiol. 2008, 44, 954–967. [Google Scholar] [CrossRef]
- Nash, D.T.; Nash, S.D. Ranolazine for Chronic Stable Angina. Lancet Lond. Engl. 2008, 372, 1335–1341. [Google Scholar] [CrossRef]
- Rayner-Hartley, E.; Sedlak, T. Ranolazine: A Contemporary Review. J. Am. Heart Assoc. 2016, 5, e003196. [Google Scholar] [CrossRef]
- Anderson, J.R.; Nawarskas, J.J. Ranolazine: A Metabolic Modulator for the Treatment of Chronic Stable Angina. Cardiol. Rev. 2005, 13, 202–210. [Google Scholar] [CrossRef]
- Grynberg, A. Effectors of Fatty Acid Oxidation Reduction: Promising New Anti-Ischaemic Agents. Curr. Pharm. Des. 2005, 11, 489–509. [Google Scholar] [CrossRef]
- Schofield, R.S.; Hill, J.A. Role of Metabolically Active Drugs in the Management of Ischemic Heart Disease. Am. J. Cardiovasc. Drugs Drugs Devices Interv. 2001, 1, 23–35. [Google Scholar] [CrossRef]
- Anderson, S.E.; Murphy, E.; Steenbergen, C.; London, R.E.; Cala, P.M. Na-H Exchange in Myocardium: Effects of Hypoxia and Acidification on Na and Ca. Am. J. Physiol. 1990, 259, C940–C948. [Google Scholar] [CrossRef]
- Imahashi, K.; Pott, C.; Goldhaber, J.I.; Steenbergen, C.; Philipson, K.D.; Murphy, E. Cardiac-Specific Ablation of the Na+-Ca2+ Exchanger Confers Protection against Ischemia/Reperfusion Injury. Circ. Res. 2005, 97, 916–921. [Google Scholar] [CrossRef]
- Steenbergen, C.; Perlman, M.E.; London, R.E.; Murphy, E. Mechanism of Preconditioning. Ionic Alterations. Circ. Res. 1993, 72, 112–125. [Google Scholar] [CrossRef]
- Thireau, J.; Pasquié, J.-L.; Martel, E.; Le Guennec, J.-Y.; Richard, S. New Drugs vs. Old Concepts: A Fresh Look at Antiarrhythmics. Pharmacol. Ther. 2011, 132, 125–145. [Google Scholar] [CrossRef]
- Bing, O.H.; Keefe, J.F.; Wolk, M.J.; Finkelstein, L.J.; Levine, H.J. Tension Prolongation during Recovery from Myocardial Hypoxia. J. Clin. Investig. 1971, 50, 660–666. [Google Scholar] [CrossRef][Green Version]
- Goodwill, A.G.; Dick, G.M.; Kiel, A.M.; Tune, J.D. Regulation of Coronary Blood Flow. Compr. Physiol. 2017, 7, 321–382. [Google Scholar] [CrossRef]
- Ju, Y.K.; Saint, D.A.; Gage, P.W. Hypoxia Increases Persistent Sodium Current in Rat Ventricular Myocytes. J. Physiol. 1996, 497 Pt 2, 337–347. [Google Scholar] [CrossRef]
- Shander, G.S.; Undrovinas, A.I.; Makielski, J.C. Rapid Onset of Lysophosphatidylcholine-Induced Modification of Whole Cell Cardiac Sodium Current Kinetics. J. Mol. Cell. Cardiol. 1996, 28, 743–753. [Google Scholar] [CrossRef]
- Undrovinas, A.I.; Maltsev, V.A.; Sabbah, H.N. Repolarization Abnormalities in Cardiomyocytes of Dogs with Chronic Heart Failure: Role of Sustained Inward Current. Cell. Mol. Life Sci. 1999, 55, 494–505. [Google Scholar] [CrossRef]
- Chaitman, B.R.; Pepine, C.J.; Parker, J.O.; Skopal, J.; Chumakova, G.; Kuch, J.; Wang, W.; Skettino, S.L.; Wolff, A.A. Combination Assessment of Ranolazine In Stable Angina (CARISA) Investigators Effects of Ranolazine with Atenolol, Amlodipine, or Diltiazem on Exercise Tolerance and Angina Frequency in Patients with Severe Chronic Angina: A Randomized Controlled Trial. JAMA 2004, 291, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Antzelevitch, C.; Belardinelli, L.; Zygmunt, A.C.; Burashnikov, A.; Di Diego, J.M.; Fish, J.M.; Cordeiro, J.M.; Thomas, G. Electrophysiological Effects of Ranolazine, a Novel Antianginal Agent with Antiarrhythmic Properties. Circulation 2004, 110, 904–910. [Google Scholar] [CrossRef]
- Tavazzi, L. Ranolazine, a New Antianginal Drug. Future Cardiol. 2005, 1, 447–455. [Google Scholar] [CrossRef]
- Chaitman, B.R.; Skettino, S.L.; Parker, J.O.; Hanley, P.; Meluzin, J.; Kuch, J.; Pepine, C.J.; Wang, W.; Nelson, J.J.; Hebert, D.A.; et al. Anti-Ischemic Effects and Long-Term Survival during Ranolazine Monotherapy in Patients with Chronic Severe Angina. J. Am. Coll. Cardiol. 2004, 43, 1375–1382. [Google Scholar] [CrossRef]
- Fraser, H.; Belardinelli, L.; Wang, L.; Light, P.E.; McVeigh, J.J.; Clanachan, A.S. Ranolazine Decreases Diastolic Calcium Accumulation Caused by ATX-II or Ischemia in Rat Hearts. J. Mol. Cell. Cardiol. 2006, 41, 1031–1038. [Google Scholar] [CrossRef]
- Yu, S.; Li, G.; Huang, C.L.-H.; Lei, M.; Wu, L. Late Sodium Current Associated Cardiac Electrophysiological and Mechanical Dysfunction. Pflugers Arch. 2018, 470, 461–469. [Google Scholar] [CrossRef]
- Antzelevitch, C.; Burashnikov, A.; Sicouri, S.; Belardinelli, L. Electrophysiologic Basis for the Antiarrhythmic Actions of Ranolazine. Heart Rhythm 2011, 8, 1281–1290. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.J.; Chapman, R.A. Effects of Ranolazine on L-Type Calcium Channel Currents in Guinea-Pig Single Ventricular Myocytes. Br. J. Pharmacol. 1996, 118, 249–254. [Google Scholar] [CrossRef]
- Liu, Z.; Williams, R.B.; Rosen, B.D. The Potential Contribution of Ranolazine to Torsade de Pointe. J. Cardiovasc. Dis. Res. 2013, 4, 187–190. [Google Scholar] [CrossRef]
- Schram, G.; Zhang, L.; Derakhchan, K.; Ehrlich, J.R.; Belardinelli, L.; Nattel, S. Ranolazine: Ion-Channel-Blocking Actions and in Vivo Electrophysiological Effects. Br. J. Pharmacol. 2004, 142, 1300–1308. [Google Scholar] [CrossRef]
- Morrow, D.A.; Scirica, B.M.; Karwatowska-Prokopczuk, E.; Skene, A.; McCabe, C.H.; Braunwald, E. MERLIN-TIMI 36 Investigators Evaluation of a Novel Anti-Ischemic Agent in Acute Coronary Syndromes: Design and Rationale for the Metabolic Efficiency with Ranolazine for Less Ischemia in Non-ST-Elevation Acute Coronary Syndromes (MERLIN)-TIMI 36 Trial. Am. Heart J. 2006, 151, 1186.e1–1186.e9. [Google Scholar] [CrossRef]
- Koren, M.J.; Crager, M.R.; Sweeney, M. Long-Term Safety of a Novel Antianginal Agent in Patients with Severe Chronic Stable Angina: The Ranolazine Open Label Experience (ROLE). J. Am. Coll. Cardiol. 2007, 49, 1027–1034. [Google Scholar] [CrossRef]
- Wilson, S.R.; Scirica, B.M.; Braunwald, E.; Murphy, S.A.; Karwatowska-Prokopczuk, E.; Buros, J.L.; Chaitman, B.R.; Morrow, D.A. Efficacy of Ranolazine in Patients with Chronic Angina Observations from the Randomized, Double-Blind, Placebo-Controlled MERLIN-TIMI (Metabolic Efficiency With Ranolazine for Less Ischemia in Non-ST-Segment Elevation Acute Coronary Syndromes) 36 Trial. J. Am. Coll. Cardiol. 2009, 53, 1510–1516. [Google Scholar] [CrossRef]
- Murray, G.L.; Colombo, J. Ranolazine Improves Autonomic Balance in Heart Failure When Added to Guideline-Driven Therapy. Heart Int. 2014, 9, 59–65. [Google Scholar] [CrossRef]
- Murray, G.L. Ranolazine Is an Effective and Safe Treatment of Adults with Symptomatic Premature Ventricular Contractions Due to Triggered Ectopy. Int. J. Angiol. Off. Publ. Int. Coll. Angiol. Inc. 2016, 25, 247–251. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zeng, X.; Zhang, Y.; Lin, J.; Zheng, H.; Peng, J.; Huang, W. Efficacy and Safety of Ranolazine in Diabetic Patients: A Systematic Review and Meta-Analysis. Ann. Pharmacother. 2017, 52, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Olivotto, I.; Camici, P.G.; Merlini, P.A.; Rapezzi, C.; Patten, M.; Climent, V.; Sinagra, G.; Tomberli, B.; Marin, F.; Ehlermann, P.; et al. Efficacy of Ranolazine in Patients With Symptomatic Hypertrophic Cardiomyopathy: The RESTYLE-HCM Randomized, Double-Blind, Placebo-Controlled Study. Circ. Heart Fail. 2018, 11, e004124. [Google Scholar] [CrossRef]
- Chaitman, B.R. Ranolazine for the Treatment of Chronic Angina and Potential Use in Other Cardiovascular Conditions. Circulation 2006, 113, 2462–2472. [Google Scholar] [CrossRef]
- Szél, T.; Koncz, I.; Jost, N.; Baczkó, I.; Husti, Z.; Virág, L.; Bussek, A.; Wettwer, E.; Ravens, U.; Papp, J.G.; et al. Class I/B Antiarrhythmic Property of Ranolazine, a Novel Antianginal Agent, in Dog and Human Cardiac Preparations. Eur. J. Pharmacol. 2011, 662, 31–39. [Google Scholar] [CrossRef]
- Guns, P.-J.; Johnson, D.M.; Weltens, E.; Lissens, J. Negative Electro-Mechanical Windows Are Required for Drug-Induced Torsades de Pointes in the Anesthetized Guinea Pig. J. Pharmacol. Toxicol. Methods 2012, 66, 125–134. [Google Scholar] [CrossRef]
- Antoons, G.; Oros, A.; Beekman, J.D.M.; Engelen, M.A.; Houtman, M.J.C.; Belardinelli, L.; Stengl, M.; Vos, M.A. Late Na+ Current Inhibition by Ranolazine Reduces Torsades de Pointes in the Chronic Atrioventricular Block Dog Model. J. Am. Coll. Cardiol. 2010, 55, 801–809. [Google Scholar] [CrossRef]
- Wang, W.-Q.; Robertson, C.; Dhalla, A.K.; Belardinelli, L. Antitorsadogenic Effects of ({+/−})-N-(2,6-Dimethyl-Phenyl)-(4[2-Hydroxy-3-(2-Methoxyphenoxy)Propyl]-1-Piperazine (Ranolazine) in Anesthetized Rabbits. J. Pharmacol. Exp. Ther. 2008, 325, 875–881. [Google Scholar] [CrossRef]
- Wu, L.; Shryock, J.C.; Song, Y.; Li, Y.; Antzelevitch, C.; Belardinelli, L. Antiarrhythmic Effects of Ranolazine in a Guinea Pig in Vitro Model of Long-QT Syndrome. J. Pharmacol. Exp. Ther. 2004, 310, 599–605. [Google Scholar] [CrossRef]
- Singh, B.N.; Wadhani, N. Antiarrhythmic and Proarrhythmic Properties of QT-Prolonging Antianginal Drugs. J. Cardiovasc. Pharmacol. Ther. 2004, 9 (Suppl. S1), S85–S97. [Google Scholar] [CrossRef]
- Moss, A.J.; Zareba, W.; Schwarz, K.Q.; Rosero, S.; McNitt, S.; Robinson, J.L. Ranolazine Shortens Repolarization in Patients with Sustained Inward Sodium Current Due to Type-3 Long-QT Syndrome. J. Cardiovasc. Electrophysiol. 2008, 19, 1289–1293. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Shen, J.; Splawski, I.; Atkinson, D.; Li, Z.; Robinson, J.L.; Moss, A.J.; Towbin, J.A.; Keating, M.T. SCN5A Mutations Associated with an Inherited Cardiac Arrhythmia, Long QT Syndrome. Cell 1995, 80, 805–811. [Google Scholar] [CrossRef]
- Medeiros-Domingo, A.; Kaku, T.; Tester, D.J.; Iturralde-Torres, P.; Itty, A.; Ye, B.; Valdivia, C.; Ueda, K.; Canizales-Quinteros, S.; Tusié-Luna, M.T.; et al. SCN4B-Encoded Sodium Channel Beta4 Subunit in Congenital Long-QT Syndrome. Circulation 2007, 116, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Scirica, B.M.; Morrow, D.A.; Hod, H.; Murphy, S.A.; Belardinelli, L.; Hedgepeth, C.M.; Molhoek, P.; Verheugt, F.W.A.; Gersh, B.J.; McCabe, C.H.; et al. Effect of Ranolazine, an Antianginal Agent with Novel Electrophysiological Properties, on the Incidence of Arrhythmias in Patients with Non ST-Segment Elevation Acute Coronary Syndrome: Results from the Metabolic Efficiency With Ranolazine for Less Ischemia in Non ST-Elevation Acute Coronary Syndrome Thrombolysis in Myocardial Infarction 36 (MERLIN-TIMI 36) Randomized Controlled Trial. Circulation 2007, 116, 1647–1652. [Google Scholar] [CrossRef]
- Scirica, B.M.; Belardinelli, L.; Chaitman, B.R.; Waks, J.W.; Volo, S.; Karwatowska-Prokopczuk, E.; Murphy, S.A.; Cheng, M.L.; Braunwald, E.; Morrow, D.A. Effect of Ranolazine on Atrial Fibrillation in Patients with Non-ST Elevation Acute Coronary Syndromes: Observations from the MERLIN-TIMI 36 Trial. Eur. Eur. Pacing Arrhythm. Card. Electrophysiol. J. Work. Groups Card. Pacing Arrhythm. Card. Cell. Electrophysiol. Eur. Soc. Cardiol. 2015, 17, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Murdock, D.K.; Overton, N.; Kersten, M.; Kaliebe, J.; Devecchi, F. The Effect of Ranolazine on Maintaining Sinus Rhythm in Patients with Resistant Atrial Fibrillation. Indian Pacing Electrophysiol. J. 2008, 8, 175–181. [Google Scholar]
- Miles, R.H.; Passman, R.; Murdock, D.K. Comparison of Effectiveness and Safety of Ranolazine versus Amiodarone for Preventing Atrial Fibrillation after Coronary Artery Bypass Grafting. Am. J. Cardiol. 2011, 108, 673–676. [Google Scholar] [CrossRef] [PubMed]
- Reiffel, J.A.; Camm, A.J.; Belardinelli, L.; Zeng, D.; Karwatowska-Prokopczuk, E.; Olmsted, A.; Zareba, W.; Rosero, S.; Kowey, P.; HARMONY Investigators. The HARMONY Trial: Combined Ranolazine and Dronedarone in the Management of Paroxysmal Atrial Fibrillation: Mechanistic and Therapeutic Synergism. Circ. Arrhythm. Electrophysiol. 2015, 8, 1048–1056. [Google Scholar] [CrossRef]
- Tsanaxidis, N.; Aidonidis, I.; Hatziefthimiou, A.; Daskalopoulou, S.S.; Giamouzis, G.; Triposkiadis, F.; Skoularigis, I. Ranolazine Added to Amiodarone Facilitates Earlier Conversion of Atrial Fibrillation Compared to Amiodarone-Only Therapy. Pacing Clin. Electrophysiol. 2017, 40, 372–378. [Google Scholar] [CrossRef]
- Verrier, R.L.; Silva, A.F.G.; Bonatti, R.; Batatinha, J.A.P.; Nearing, B.D.; Liu, G.; Rajamani, S.; Zeng, D.; Belardinelli, L. Combined Actions of Ivabradine and Ranolazine Reduce Ventricular Rate during Atrial Fibrillation. J. Cardiovasc. Electrophysiol. 2015, 26, 329–335. [Google Scholar] [CrossRef]
- Hammond, D.A.; Smotherman, C.; Jankowski, C.A.; Tan, S.; Osian, O.; Kraemer, D.; DeLosSantos, M. Short-Course of Ranolazine Prevents Postoperative Atrial Fibrillation Following Coronary Artery Bypass Grafting and Valve Surgeries. Clin. Res. Cardiol. Off. J. Ger. Card. Soc. 2015, 104, 410–417. [Google Scholar] [CrossRef]
- De Ferrari, G.M.; Maier, L.S.; Mont, L.; Schwartz, P.J.; Simonis, G.; Leschke, M.; Gronda, E.; Boriani, G.; Darius, H.; Guillamón Torán, L.; et al. Ranolazine in the Treatment of Atrial Fibrillation: Results of the Dose-Ranging RAFFAELLO (Ranolazine in Atrial Fibrillation Following an ELectricaL CardiOversion) Study. Heart Rhythm 2015, 12, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Bunch, T.J.; Mahapatra, S.; Murdock, D.; Molden, J.; Weiss, J.P.; May, H.T.; Bair, T.L.; Mader, K.M.; Crandall, B.G.; Day, J.D.; et al. Ranolazine Reduces Ventricular Tachycardia Burden and ICD Shocks in Patients with Drug-Refractory ICD Shocks. Pacing Clin. Electrophysiol. 2011, 34, 1600–1606. [Google Scholar] [CrossRef] [PubMed]
- Zareba, W.; Daubert, J.P.; Beck, C.A.; Huang, D.T.; Alexis, J.D.; Brown, M.W.; Pyykkonen, K.; McNitt, S.; Oakes, D.; Feng, C.; et al. Ranolazine in High-Risk Patients With Implanted Cardioverter-Defibrillators: The RAID Trial. J. Am. Coll. Cardiol. 2018, 72, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Rajamani, S.; El-Bizri, N.; Shryock, J.C.; Makielski, J.C.; Belardinelli, L. Use-Dependent Block of Cardiac Late Na+ Current by Ranolazine. Heart Rhythm 2009, 6, 1625–1631. [Google Scholar] [CrossRef] [PubMed]
- Maier, L.S.; Sossalla, S. The Late Na Current as a Therapeutic Target: Where Are We? J. Mol. Cell. Cardiol. 2013, 61, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Undrovinas, A.; Maltsev, V.A. Late Sodium Current Is a New Therapeutic Target to Improve Contractility and Rhythm in Failing Heart. Cardiovasc. Hematol. Agents Med. Chem. 2008, 6, 348–359. [Google Scholar] [CrossRef]
- Chambers, J.C.; Zhao, J.; Terracciano, C.M.N.; Bezzina, C.R.; Zhang, W.; Kaba, R.; Navaratnarajah, M.; Lotlikar, A.; Sehmi, J.S.; Kooner, M.K.; et al. Genetic Variation in SCN10A Influences Cardiac Conduction. Nat. Genet. 2010, 42, 149–152. [Google Scholar] [CrossRef]
- Sotoodehnia, N.; Isaacs, A.; de Bakker, P.I.W.; Dörr, M.; Newton-Cheh, C.; Nolte, I.M.; van der Harst, P.; Müller, M.; Eijgelsheim, M.; Alonso, A.; et al. Common Variants in 22 Loci Are Associated with QRS Duration and Cardiac Ventricular Conduction. Nat. Genet. 2010, 42, 1068–1076. [Google Scholar] [CrossRef]
- Stroud, D.M.; Yang, T.; Bersell, K.; Kryshtal, D.O.; Nagao, S.; Shaffer, C.; Short, L.; Hall, L.; Atack, T.C.; Zhang, W.; et al. Contrasting Nav1.8 Activity in Scn10a−/− Ventricular Myocytes and the Intact Heart. J. Am. Heart Assoc. 2016, 5, e002946. [Google Scholar] [CrossRef]
- Yang, T.; Atack, T.C.; Stroud, D.M.; Zhang, W.; Hall, L.; Roden, D.M. Blocking Scn10a Channels in Heart Reduces Late Sodium Current and Is Antiarrhythmic. Circ. Res. 2012, 111, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Saint, D.A.; Ju, Y.K.; Gage, P.W. A Persistent Sodium Current in Rat Ventricular Myocytes. J. Physiol. 1992, 453, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Shryock, J.C.; Song, Y.; Rajamani, S.; Antzelevitch, C.; Belardinelli, L. The Arrhythmogenic Consequences of Increasing Late INa in the Cardiomyocyte. Cardiovasc. Res. 2013, 99, 600–611. [Google Scholar] [CrossRef] [PubMed]
- Gupta, T.; Khera, S.; Kolte, D.; Aronow, W.S.; Iwai, S. Antiarrhythmic Properties of Ranolazine: A Review of the Current Evidence. Int. J. Cardiol. 2015, 187, 66–74. [Google Scholar] [CrossRef]
- Moreno, J.D.; Clancy, C.E. Pathophysiology of the Cardiac Late Na Current and Its Potential as a Drug Target. J. Mol. Cell. Cardiol. 2012, 52, 608–619. [Google Scholar] [CrossRef]
- Noble, D.; Noble, P.J. Late Sodium Current in the Pathophysiology of Cardiovascular Disease: Consequences of Sodium-Calcium Overload. Heart Br. Card. Soc. 2006, 92 (Suppl. S4), iv1–iv5. [Google Scholar] [CrossRef]
- Li, P.; Rudy, Y. A Model of Canine Purkinje Cell Electrophysiology and Ca(2+) Cycling: Rate Dependence, Triggered Activity, and Comparison to Ventricular Myocytes. Circ. Res. 2011, 109, 71–79. [Google Scholar] [CrossRef]
- Belardinelli, L.; Giles, W.R.; Rajamani, S.; Karagueuzian, H.S.; Shryock, J.C. Cardiac Late Na+ Current: Proarrhythmic Effects, Roles in Long QT Syndromes, and Pathological Relationship to CaMKII and Oxidative Stress. Heart Rhythm 2015, 12, 440–448. [Google Scholar] [CrossRef]
- Bossu, A.; Houtman, M.J.C.; Meijborg, V.M.F.; Varkevisser, R.; Beekman, H.D.M.; Dunnink, A.; de Bakker, J.M.T.; Mollova, N.; Rajamani, S.; Belardinelli, L.; et al. Selective Late Sodium Current Inhibitor GS-458967 Suppresses Torsades de Pointes by Mostly Affecting Perpetuation but Not Initiation of the Arrhythmia. Br. J. Pharmacol. 2018, 175, 2470–2482. [Google Scholar] [CrossRef]
- Fredj, S.; Lindegger, N.; Sampson, K.J.; Carmeliet, P.; Kass, R.S. Altered Na+ Channels Promote Pause-Induced Spontaneous Diastolic Activity in Long QT Syndrome Type 3 Myocytes. Circ. Res. 2006, 99, 1225–1232. [Google Scholar] [CrossRef]
- Wu, L.; Ma, J.; Li, H.; Wang, C.; Grandi, E.; Zhang, P.; Luo, A.; Bers, D.M.; Shryock, J.C.; Belardinelli, L. Late Sodium Current Contributes to the Reverse Rate-Dependent Effect of IKr Inhibition on Ventricular Repolarization. Circulation 2011, 123, 1713–1720. [Google Scholar] [CrossRef]
- Carlsson, L.; Almgren, O.; Duker, G. QTU-Prolongation and Torsades de Pointes Induced by Putative Class III Antiarrhythmic Agents in the Rabbit: Etiology and Interventions. J. Cardiovasc. Pharmacol. 1990, 16, 276–285. [Google Scholar] [CrossRef]
- Sossalla, S.; Wallisch, N.; Toischer, K.; Sohns, C.; Vollmann, D.; Seegers, J.; Lüthje, L.; Maier, L.S.; Zabel, M. Effects of Ranolazine on Torsades de Pointes Tachycardias in a Healthy Isolated Rabbit Heart Model. Cardiovasc. Ther. 2014, 32, 170–177. [Google Scholar] [CrossRef]
- Fredj, S.; Sampson, K.J.; Liu, H.; Kass, R.S. Molecular Basis of Ranolazine Block of LQT-3 Mutant Sodium Channels: Evidence for Site of Action. Br. J. Pharmacol. 2006, 148, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Burashnikov, A.; Di Diego, J.M.; Zygmunt, A.C.; Belardinelli, L.; Antzelevitch, C. Atrium-Selective Sodium Channel Block as a Strategy for Suppression of Atrial Fibrillation: Differences in Sodium Channel Inactivation between Atria and Ventricles and the Role of Ranolazine. Circulation 2007, 116, 1449–1457. [Google Scholar] [CrossRef]
- Undrovinas, A.I.; Belardinelli, L.; Undrovinas, N.A.; Sabbah, H.N. Ranolazine Improves Abnormal Repolarization and Contraction in Left Ventricular Myocytes of Dogs with Heart Failure by Inhibiting Late Sodium Current. J. Cardiovasc. Electrophysiol. 2006, 17 (Suppl. S1), S169–S177. [Google Scholar] [CrossRef] [PubMed]
- Antzelevitch, C.; Belardinelli, L. The Role of Sodium Channel Current in Modulating Transmural Dispersion of Repolarization and Arrhythmogenesis. J. Cardiovasc. Electrophysiol. 2006, 17 (Suppl. S1), S79–S85. [Google Scholar] [CrossRef] [PubMed]
- Qi, D.; Yang, Z.; Robinson, V.M.; Li, J.; Gao, C.; Guo, D.; Kowey, P.R.; Yan, G.-X. Heterogeneous Distribution of INa-L Determines Interregional Differences in Rate Adaptation of Repolarization. Heart Rhythm 2015, 12, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- Zygmunt, A.C.; Eddlestone, G.T.; Thomas, G.P.; Nesterenko, V.V.; Antzelevitch, C. Larger Late Sodium Conductance in M Cells Contributes to Electrical Heterogeneity in Canine Ventricle. Am. J. Physiol. Heart Circ. Physiol. 2001, 281, H689–H697. [Google Scholar] [CrossRef]
- Kumar, K.; Nearing, B.D.; Bartoli, C.R.; Kwaku, K.F.; Belardinelli, L.; Verrier, R.L. Effect of Ranolazine on Ventricular Vulnerability and Defibrillation Threshold in the Intact Porcine Heart. J. Cardiovasc. Electrophysiol. 2008, 19, 1073–1079. [Google Scholar] [CrossRef]
- Zhao, Z.; Fefelova, N.; Shanmugam, M.; Bishara, P.; Babu, G.J.; Xie, L.-H. Angiotensin II Induces Afterdepolarizations via Reactive Oxygen Species and Calmodulin Kinase II Signaling. J. Mol. Cell. Cardiol. 2011, 50, 128–136. [Google Scholar] [CrossRef]
- Wu, L.; Rajamani, S.; Li, H.; January, C.T.; Shryock, J.C.; Belardinelli, L. Reduction of Repolarization Reserve Unmasks the Proarrhythmic Role of Endogenous Late Na+ Current in the Heart. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1048–H1057. [Google Scholar] [CrossRef] [PubMed]
- Zareba, W.; Moss, A.J.; le Cessie, S. Dispersion of Ventricular Repolarization and Arrhythmic Cardiac Death in Coronary Artery Disease. Am. J. Cardiol. 1994, 74, 550–553. [Google Scholar] [CrossRef]
- Weiss, J.N.; Garfinkel, A.; Karagueuzian, H.S.; Chen, P.-S.; Qu, Z. Early Afterdepolarizations and Cardiac Arrhythmias. Heart Rhythm 2010, 7, 1891–1899. [Google Scholar] [CrossRef] [PubMed]
- Thireau, J.; Karam, S.; Roberge, S.; Roussel, J.; Aimond, F.; Cassan, C.; Gac, A.; Babuty, D.; Le Guennec, J.-Y.; Lacampagne, A.; et al. Β-Adrenergic Blockade Combined with Subcutaneous B-Type Natriuretic Peptide: A Promising Approach to Reduce Ventricular Arrhythmia in Heart Failure? Heart Br. Card. Soc. 2014, 100, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Fischer, T.H.; Herting, J.; Mason, F.E.; Hartmann, N.; Watanabe, S.; Nikolaev, V.O.; Sprenger, J.U.; Fan, P.; Yao, L.; Popov, A.-F.; et al. Late INa Increases Diastolic SR-Ca2+-Leak in Atrial Myocardium by Activating PKA and CaMKII. Cardiovasc. Res. 2015, 107, 184–196. [Google Scholar] [CrossRef]
- Wu, J.; Corr, P.B. Palmitoyl Carnitine Modifies Sodium Currents and Induces Transient Inward Current in Ventricular Myocytes. Am. J. Physiol. 1994, 266, H1034–H1046. [Google Scholar] [CrossRef]
- Viatchenko-Karpinski, S.; Kornyeyev, D.; El-Bizri, N.; Budas, G.; Fan, P.; Jiang, Z.; Yang, J.; Anderson, M.E.; Shryock, J.C.; Chang, C.-P.; et al. Intracellular Na+ Overload Causes Oxidation of CaMKII and Leads to Ca2+ Mishandling in Isolated Ventricular Myocytes. J. Mol. Cell. Cardiol. 2014, 76, 247–256. [Google Scholar] [CrossRef]
- Sag, C.M.; Mallwitz, A.; Wagner, S.; Hartmann, N.; Schotola, H.; Fischer, T.H.; Ungeheuer, N.; Herting, J.; Shah, A.M.; Maier, L.S.; et al. Enhanced Late INa Induces Proarrhythmogenic SR Ca Leak in a CaMKII-Dependent Manner. J. Mol. Cell. Cardiol. 2014, 76, 94–105. [Google Scholar] [CrossRef]
- Undrovinas, N.A.; Maltsev, V.A.; Belardinelli, L.; Sabbah, H.N.; Undrovinas, A. Late Sodium Current Contributes to Diastolic Cell Ca2+ Accumulation in Chronic Heart Failure. J. Physiol. Sci. 2010, 60, 245–257. [Google Scholar] [CrossRef]
- Wasserstrom, J.A.; Sharma, R.; O’Toole, M.J.; Zheng, J.; Kelly, J.E.; Shryock, J.; Belardinelli, L.; Aistrup, G.L. Ranolazine Antagonizes the Effects of Increased Late Sodium Current on Intracellular Calcium Cycling in Rat Isolated Intact Heart. J. Pharmacol. Exp. Ther. 2009, 331, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Guerra, F.; Romandini, A.; Barbarossa, A.; Belardinelli, L.; Capucci, A. Ranolazine for Rhythm Control in Atrial Fibrillation: A Systematic Review and Meta-Analysis. Int. J. Cardiol. 2017, 227, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Burashnikov, A.; Antzelevitch, C. Atrial-Selective Sodium Channel Block for the Treatment of Atrial Fibrillation. Expert Opin. Emerg. Drugs 2009, 14, 233–249. [Google Scholar] [CrossRef]
- Ratte, A.; Wiedmann, F.; Kraft, M.; Katus, H.A.; Schmidt, C. Antiarrhythmic Properties of Ranolazine: Inhibition of Atrial Fibrillation Associated TASK-1 Potassium Channels. Front. Pharmacol. 2019, 10, 1367. [Google Scholar] [CrossRef] [PubMed]
- Caves, R.E.; Cheng, H.; Choisy, S.C.; Gadeberg, H.C.; Bryant, S.M.; Hancox, J.C.; James, A.F. Atrial-Ventricular Differences in Rabbit Cardiac Voltage-Gated Na+ Currents: Basis for Atrial-Selective Block by Ranolazine. Heart Rhythm 2017, 14, 1657–1664. [Google Scholar] [CrossRef] [PubMed]
- Sossalla, S.; Kallmeyer, B.; Wagner, S.; Mazur, M.; Maurer, U.; Toischer, K.; Schmitto, J.D.; Seipelt, R.; Schöndube, F.A.; Hasenfuss, G.; et al. Altered Na+ Currents in Atrial Fibrillation Effects of Ranolazine on Arrhythmias and Contractility in Human Atrial Myocardium. J. Am. Coll. Cardiol. 2010, 55, 2330–2342. [Google Scholar] [CrossRef]
- Nesterenko, V.V.; Zygmunt, A.C.; Rajamani, S.; Belardinelli, L.; Antzelevitch, C. Mechanisms of Atrial-Selective Block of Na+ Channels by Ranolazine: II. Insights from a Mathematical Model. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H1615–H1624. [Google Scholar] [CrossRef]
- Guo, D.; Young, L.; Wu, Y.; Belardinelli, L.; Kowey, P.R.; Yan, G.-X. Increased Late Sodium Current in Left Atrial Myocytes of Rabbits with Left Ventricular Hypertrophy: Its Role in the Genesis of Atrial Arrhythmias. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H1375–H1381. [Google Scholar] [CrossRef]
- Song, Y.; Shryock, J.C.; Belardinelli, L. An Increase of Late Sodium Current Induces Delayed Afterdepolarizations and Sustained Triggered Activity in Atrial Myocytes. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H2031–H2039. [Google Scholar] [CrossRef]
- Li, D.; Melnyk, P.; Feng, J.; Wang, Z.; Petrecca, K.; Shrier, A.; Nattel, S. Effects of Experimental Heart Failure on Atrial Cellular and Ionic Electrophysiology. Circulation 2000, 101, 2631–2638. [Google Scholar] [CrossRef]
- Fischer, T.H.; Herting, J.; Tirilomis, T.; Renner, A.; Neef, S.; Toischer, K.; Ellenberger, D.; Förster, A.; Schmitto, J.D.; Gummert, J.; et al. Ca2+/Calmodulin-Dependent Protein Kinase II and Protein Kinase A Differentially Regulate Sarcoplasmic Reticulum Ca2+ Leak in Human Cardiac Pathology. Circulation 2013, 128, 970–981. [Google Scholar] [CrossRef]
- Kirchhof, P.; Eckardt, L.; Franz, M.R.; Mönnig, G.; Loh, P.; Wedekind, H.; Schulze-Bahr, E.; Breithardt, G.; Haverkamp, W. Prolonged Atrial Action Potential Durations and Polymorphic Atrial Tachyarrhythmias in Patients with Long QT Syndrome. J. Cardiovasc. Electrophysiol. 2003, 14, 1027–1033. [Google Scholar] [CrossRef] [PubMed]
- Verheule, S.; Wilson, E.; Everett, T.; Shanbhag, S.; Golden, C.; Olgin, J. Alterations in Atrial Electrophysiology and Tissue Structure in a Canine Model of Chronic Atrial Dilatation Due to Mitral Regurgitation. Circulation 2003, 107, 2615–2622. [Google Scholar] [CrossRef] [PubMed]
- Lip, G.Y.H.; Fauchier, L.; Freedman, S.B.; Van Gelder, I.; Natale, A.; Gianni, C.; Nattel, S.; Potpara, T.; Rienstra, M.; Tse, H.-F.; et al. Atrial Fibrillation. Nat. Rev. Dis. Primer 2016, 2, 16016. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Nearing, B.D.; Carvas, M.; Nascimento, B.C.G.; Acar, M.; Belardinelli, L.; Verrier, R.L. Ranolazine Exerts Potent Effects on Atrial Electrical Properties and Abbreviates Atrial Fibrillation Duration in the Intact Porcine Heart. J. Cardiovasc. Electrophysiol. 2009, 20, 796–802. [Google Scholar] [CrossRef]
- Sicouri, S.; Belardinelli, L.; Antzelevitch, C. Effect of Autonomic Influences to Induce Triggered Activity in Muscular Sleeves Extending into the Coronary Sinus of the Canine Heart and Its Suppression by Ranolazine. J. Cardiovasc. Electrophysiol. 2019, 30, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Aidonidis, I.; Doulas, K.; Hatziefthimiou, A.; Tagarakis, G.; Simopoulos, V.; Rizos, I.; Tsilimingas, N.; Molyvdas, P.-A. Ranolazine-Induced Postrepolarization Refractoriness Suppresses Induction of Atrial Flutter and Fibrillation in Anesthetized Rabbits. J. Cardiovasc. Pharmacol. Ther. 2013, 18, 94–101. [Google Scholar] [CrossRef]
- Youn, J.-Y.; Zhang, J.; Zhang, Y.; Chen, H.; Liu, D.; Ping, P.; Weiss, J.N.; Cai, H. Oxidative Stress in Atrial Fibrillation: An Emerging Role of NADPH Oxidase. J. Mol. Cell. Cardiol. 2013, 62, 72–79. [Google Scholar] [CrossRef]
- Song, Y.; Shryock, J.C.; Belardinelli, L. A Slowly Inactivating Sodium Current Contributes to Spontaneous Diastolic Depolarization of Atrial Myocytes. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1254–H1262. [Google Scholar] [CrossRef]
- Bhimani, A.A.; Yasuda, T.; Sadrpour, S.A.; Khrestian, C.M.; Lee, S.; Zeng, D.; Belardinelli, L.; Waldo, A.L. Ranolazine Terminates Atrial Flutter and Fibrillation in a Canine Model. Heart Rhythm 2014, 11, 1592–1599. [Google Scholar] [CrossRef]
- Sicouri, S.; Glass, A.; Belardinelli, L.; Antzelevitch, C. Antiarrhythmic Effects of Ranolazine in Canine Pulmonary Vein Sleeve Preparations. Heart Rhythm 2008, 5, 1019–1026. [Google Scholar] [CrossRef] [PubMed]
- Burashnikov, A.; Sicouri, S.; Di Diego, J.M.; Belardinelli, L.; Antzelevitch, C. Synergistic Effect of the Combination of Ranolazine and Dronedarone to Suppress Atrial Fibrillation. J. Am. Coll. Cardiol. 2010, 56, 1216–1224. [Google Scholar] [CrossRef] [PubMed]
- Carstensen, H.; Kjær, L.; Haugaard, M.M.; Flethøj, M.; Hesselkilde, E.Z.; Kanters, J.K.; Pehrson, S.; Buhl, R.; Jespersen, T. Antiarrhythmic Effects of Combining Dofetilide and Ranolazine in a Model of Acutely Induced Atrial Fibrillation in Horses. J. Cardiovasc. Pharmacol. 2018, 71, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Jarecki, B.W.; Sheets, P.L.; Jackson, J.O.; Cummins, T.R. Paroxysmal Extreme Pain Disorder Mutations within the D3/S4-S5 Linker of Nav1.7 Cause Moderate Destabilization of Fast Inactivation. J. Physiol. 2008, 586, 4137–4153. [Google Scholar] [CrossRef]
- Theile, J.W.; Cummins, T.R. Recent Developments Regarding Voltage-Gated Sodium Channel Blockers for the Treatment of Inherited and Acquired Neuropathic Pain Syndromes. Front. Pharmacol. 2011, 2, 54. [Google Scholar] [CrossRef]
- Kahlig, K.M.; Lepist, I.; Leung, K.; Rajamani, S.; George, A.L. Ranolazine Selectively Blocks Persistent Current Evoked by Epilepsy-Associated Naν1.1 Mutations. Br. J. Pharmacol. 2010, 161, 1414–1426. [Google Scholar] [CrossRef]
- Peters, C.H.; Sokolov, S.; Rajamani, S.; Ruben, P.C. Effects of the Antianginal Drug, Ranolazine, on the Brain Sodium Channel Na(V)1.2 and Its Modulation by Extracellular Protons. Br. J. Pharmacol. 2013, 169, 704–716. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.J.; Lee, Y.S.; Kang, D.W.; Koo, J.S.; Yoon, B.W.; Roh, J.K.; Gu, J.S. Neuroprotective Effect of Low Dose Riluzole in Gerbil Model of Transient Global Ischemia. Neurosci. Lett. 2000, 294, 29–32. [Google Scholar] [CrossRef]
- Baptiste, D.C.; Fehlings, M.G. Pharmacological Approaches to Repair the Injured Spinal Cord. J. Neurotrauma 2006, 23, 318–334. [Google Scholar] [CrossRef]
- Schwartz, G.; Fehlings, M.G. Evaluation of the Neuroprotective Effects of Sodium Channel Blockers after Spinal Cord Injury: Improved Behavioral and Neuroanatomical Recovery with Riluzole. J. Neurosurg. 2001, 94, 245–256. [Google Scholar] [CrossRef]
- Anderson, L.L.; Thompson, C.H.; Hawkins, N.A.; Nath, R.D.; Petersohn, A.A.; Rajamani, S.; Bush, W.S.; Frankel, W.N.; Vanoye, C.G.; Kearney, J.A.; et al. Antiepileptic Activity of Preferential Inhibitors of Persistent Sodium Current. Epilepsia 2014, 55, 1274–1283. [Google Scholar] [CrossRef] [PubMed]
- Belardinelli, L.; Liu, G.; Smith-Maxwell, C.; Wang, W.-Q.; El-Bizri, N.; Hirakawa, R.; Karpinski, S.; Li, C.H.; Hu, L.; Li, X.-J.; et al. A Novel, Potent, and Selective Inhibitor of Cardiac Late Sodium Current Suppresses Experimental Arrhythmias. J. Pharmacol. Exp. Ther. 2013, 344, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Sicouri, S.; Belardinelli, L.; Antzelevitch, C. Antiarrhythmic Effects of the Highly Selective Late Sodium Channel Current Blocker GS-458967. Heart Rhythm 2013, 10, 1036–1043. [Google Scholar] [CrossRef]
- Zipes, D.P. Heart-Brain Interactions in Cardiac Arrhythmias: Role of the Autonomic Nervous System. Cleve. Clin. J. Med. 2008, 75 Suppl 2, S94–S96. [Google Scholar] [CrossRef]
- Estacion, M.; Waxman, S.G.; Dib-Hajj, S.D. Effects of Ranolazine on Wild-Type and Mutant HNav1.7 Channels and on DRG Neuron Excitability. Mol. Pain 2010, 6, 35. [Google Scholar] [CrossRef]
- Nodera, H.; Rutkove, S.B. Changes of the Peripheral Nerve Excitability in Vivo Induced by the Persistent Na+ Current Blocker Ranolazine. Neurosci. Lett. 2012, 518, 36–40. [Google Scholar] [CrossRef]
- Gould, H.J.; Garrett, C.; Donahue, R.R.; Paul, D.; Diamond, I.; Taylor, B.K. Ranolazine Attenuates Behavioral Signs of Neuropathic Pain. Behav. Pharmacol. 2009, 20, 755–758. [Google Scholar] [CrossRef]
- Wang, G.K.; Calderon, J.; Wang, S.-Y. State- and Use-Dependent Block of Muscle Nav1.4 and Neuronal Nav1.7 Voltage-Gated Na+ Channel Isoforms by Ranolazine. Mol. Pharmacol. 2008, 73, 940–948. [Google Scholar] [CrossRef]
- Aman, T.K.; Grieco-Calub, T.M.; Chen, C.; Rusconi, R.; Slat, E.A.; Isom, L.L.; Raman, I.M. Regulation of Persistent Na Current by Interactions between Beta Subunits of Voltage-Gated Na Channels. J. Neurosci. Off. J. Soc. Neurosci. 2009, 29, 2027–2042. [Google Scholar] [CrossRef]
- Mishra, S.; Reznikov, V.; Maltsev, V.A.; Undrovinas, N.A.; Sabbah, H.N.; Undrovinas, A. Contribution of Sodium Channel Neuronal Isoform Nav1.1 to Late Sodium Current in Ventricular Myocytes from Failing Hearts. J. Physiol. 2015, 593, 1409–1427. [Google Scholar] [CrossRef] [PubMed]
- Welch, N.C.; Lin, W.; Juranka, P.F.; Morris, C.E.; Stys, P.K. Traditional AMPA Receptor Antagonists Partially Block Na v1.6-Mediated Persistent Current. Neuropharmacology 2008, 55, 1165–1171. [Google Scholar] [CrossRef] [PubMed]
- Murray, G.L.; Colombo, J. Ranolazine Preserves and Improves Left Ventricular Ejection Fraction and Autonomic Measures When Added to Guideline-Driven Therapy in Chronic Heart Failure. Heart Int. 2014, 9, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Teng, S.; Ren, Z.; Zhao, K. Vagal Stimulation Facilitates Improving Effects of Ranolazine on Cardiac Function in Rats with Chronic Ischemic Heart Failure. Curr. Mol. Med. 2018, 18, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Yang, Y.; Chen, J.; Wu, Z.; Zheng, Y.; Li, W.; Dai, W.; Guan, P.; Zhong, C. Ranolazine Attenuated Heightened Plasma Norepinephrine and B-Type Natriuretic Peptide-45 in Improving Cardiac Function in Rats with Chronic Ischemic Heart Failure. Am. J. Transl. Res. 2016, 8, 1295–1301. [Google Scholar]
- Aldakkak, M.; Camara, A.K.S.; Heisner, J.S.; Yang, M.; Stowe, D.F. Ranolazine Reduces Ca2+ Overload and Oxidative Stress and Improves Mitochondrial Integrity to Protect against Ischemia Reperfusion Injury in Isolated Hearts. Pharmacol. Res. 2011, 64, 381–392. [Google Scholar] [CrossRef]
- Aldasoro, M.; Guerra-Ojeda, S.; Aguirre-Rueda, D.; Mauricio, M.D.; Vila, J.M.; Marchio, P.; Iradi, A.; Aldasoro, C.; Jorda, A.; Obrador, E.; et al. Effects of Ranolazine on Astrocytes and Neurons in Primary Culture. PLoS ONE 2016, 11, e0150619. [Google Scholar] [CrossRef]
- Chang, C.-J.; Cheng, C.-C.; Yang, T.-F.; Chen, Y.-C.; Lin, Y.-K.; Chen, S.-A.; Chen, Y.-J. Selective and Non-Selective Non-Steroidal Anti-Inflammatory Drugs Differentially Regulate Pulmonary Vein and Atrial Arrhythmogenesis. Int. J. Cardiol. 2015, 184, 559–567. [Google Scholar] [CrossRef]
- Tocchetti, C.G.; Carpi, A.; Coppola, C.; Quintavalle, C.; Rea, D.; Campesan, M.; Arcari, A.; Piscopo, G.; Cipresso, C.; Monti, M.G.; et al. Ranolazine Protects from Doxorubicin-Induced Oxidative Stress and Cardiac Dysfunction. Eur. J. Heart Fail. 2014, 16, 358–366. [Google Scholar] [CrossRef]
- Wang, G.-T.; Li, H.; Yu, Z.-Q.; He, X.-N. Effects of Ranolazine on Cardiac Function in Rats with Heart Failure. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 9625–9632. [Google Scholar] [CrossRef]
- Clerc, O.F.; Haaf, P.; Buechel, R.R.; Gaemperli, O.; Zellweger, M.J. New Therapies to Modulate Post-Infarction Inflammatory Alterations in the Myocardium: State of the Art and Forthcoming Applications. Curr. Radiopharm. 2021, 14, 273–299. [Google Scholar] [CrossRef]
- Paredes-Carbajal, M.C.; Monsalvo, I.; Hernández-Díaz, C.; Regla, I.; Demare, P.; Mascher, D. Effects of Ranolazine on Vasomotor Responses of Rat Aortic Rings. Arch. Med. Res. 2013, 44, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Virsolvy, A.; Farah, C.; Pertuit, N.; Kong, L.; Lacampagne, A.; Reboul, C.; Aimond, F.; Richard, S. Antagonism of Nav Channels and A1-Adrenergic Receptors Contributes to Vascular Smooth Muscle Effects of Ranolazine. Sci. Rep. 2015, 5, 17969. [Google Scholar] [CrossRef]
- Khazraei, H.; Mirkhani, H.; Purkhosrow, A. Vasorelaxant Effect of Ranolazine on Isolated Normal and Diabetic Rat Aorta: A Study of Possible Mechanisms. Acta Physiol. Hung. 2013, 100, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Stone, P.H.; Chaitman, B.R.; Stocke, K.; Sano, J.; DeVault, A.; Koch, G.G. The Anti-Ischemic Mechanism of Action of Ranolazine in Stable Ischemic Heart Disease. J. Am. Coll. Cardiol. 2010, 56, 934–942. [Google Scholar] [CrossRef]
- Malavaki, C.; Hatziefthimiou, A.; Daskalopoulou, S.S.; Stefanidis, I.; Karatzaferi, C.; Aidonidis, I. Ranolazine Enhances Nicardipine-Induced Relaxation of Alpha1-Adrenoceptor-Mediated Contraction on Isolated Rabbit Aorta. Acta Cardiol. 2015, 70, 157–162. [Google Scholar] [CrossRef]
- Ahmed, B.; Mondragon, J.; Sheldon, M.; Clegg, S. Impact of Ranolazine on Coronary Microvascular Dysfunction (MICRO) Study. Cardiovasc. Revasculariz. Med. Mol. Interv. 2017, 18, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Rambarat, C.A.; Elgendy, I.Y.; Handberg, E.M.; Bairey Merz, C.N.; Wei, J.; Minissian, M.B.; Nelson, M.D.; Thomson, L.E.J.; Berman, D.S.; Shaw, L.J.; et al. Late Sodium Channel Blockade Improves Angina and Myocardial Perfusion in Patients with Severe Coronary Microvascular Dysfunction: Women’s Ischemia Syndrome Evaluation-Coronary Vascular Dysfunction Ancillary Study. Int. J. Cardiol. 2019, 276, 8–13. [Google Scholar] [CrossRef]
- Zhu, H.; Xu, X.; Fang, X.; Zheng, J.; Zhao, Q.; Chen, T.; Huang, J. Effects of the Antianginal Drugs Ranolazine, Nicorandil, and Ivabradine on Coronary Microvascular Function in Patients With Nonobstructive Coronary Artery Disease: A Meta-Analysis of Randomized Controlled Trials. Clin. Ther. 2019, 41, 2137–2152. [Google Scholar] [CrossRef]
- Lamendola, P.; Nerla, R.; Pitocco, D.; Villano, A.; Scavone, G.; Stazi, A.; Russo, G.; Di Franco, A.; Sestito, A.; Ghirlanda, G.; et al. Effect of Ranolazine on Arterial Endothelial Function in Patients with Type 2 Diabetes Mellitus. Atherosclerosis 2013, 226, 157–160. [Google Scholar] [CrossRef]
- Rehberger-Likozar, A.; Šebeštjen, M. Influence of Trimetazidine and Ranolazine on Endothelial Function in Patients with Ischemic Heart Disease. Coron. Artery Dis. 2015, 26, 651–656. [Google Scholar] [CrossRef]
- Fort, A.; Cordaillat, M.; Thollon, C.; Salazar, G.; Mechaly, I.; Villeneuve, N.; Vilaine, J.-P.; Richard, S.; Virsolvy, A. New Insights in the Contribution of Voltage-Gated Na(v) Channels to Rat Aorta Contraction. PLoS ONE 2009, 4, e7360. [Google Scholar] [CrossRef] [PubMed]
- Nieminen, T.; Tavares, C.A.M.; Pegler, J.R.M.; Belardinelli, L.; Verrier, R.L. Ranolazine Injection into Coronary or Femoral Arteries Exerts Marked, Transient Regional Vasodilation without Systemic Hypotension in an Intact Porcine Model. Circ. Cardiovasc. Interv. 2011, 4, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.-Y.; Kuang, S.-J.; Rao, F.; Yang, H.; Fang, X.-H.; Shan, Z.-X.; Li, X.-H.; Zhou, Z.-L.; Lin, Q.-X.; Yang, M.; et al. Effect of Ranolazine on Rat Intrarenal Arteries in Vitro. Eur. J. Pharmacol. 2012, 683, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Marchio, P.; Guerra-Ojeda, S.; Aldasoro, M.; Valles, S.L.; Martín-Gonzalez, I.; Martínez-León, J.B.; Mauricio, M.D.; Vila, J.M. Relaxant and Antiadrenergic Effects of Ranolazine in Human Saphenous Vein. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2020, 58, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Kim, K.C.; Choe, S.Y.; Hong, Y.M. Reduced Immunoreactivities of B-Type Natriuretic Peptide in Pulmonary Arterial Hypertension Rats after Ranolazine Treatment. Anat. Cell Biol. 2016, 49, 7–14. [Google Scholar] [CrossRef]
- Liles, J.T.; Hoyer, K.; Oliver, J.; Chi, L.; Dhalla, A.K.; Belardinelli, L. Ranolazine Reduces Remodeling of the Right Ventricle and Provoked Arrhythmias in Rats with Pulmonary Hypertension. J. Pharmacol. Exp. Ther. 2015, 353, 480–489. [Google Scholar] [CrossRef]
- Khan, S.S.; Cuttica, M.J.; Beussink-Nelson, L.; Kozyleva, A.; Sanchez, C.; Mkrdichian, H.; Selvaraj, S.; Dematte, J.E.; Lee, D.C.; Shah, S.J. Effects of Ranolazine on Exercise Capacity, Right Ventricular Indices, and Hemodynamic Characteristics in Pulmonary Arterial Hypertension: A Pilot Study. Pulm. Circ. 2015, 5, 547–556. [Google Scholar] [CrossRef]
- Gomberg-Maitland, M.; Schilz, R.; Mediratta, A.; Addetia, K.; Coslet, S.; Thomeas, V.; Gillies, H.; Oudiz, R.J. Phase I Safety Study of Ranolazine in Pulmonary Arterial Hypertension. Pulm. Circ. 2015, 5, 691–700. [Google Scholar] [CrossRef]
- Han, Y.; Forfia, P.R.; Vaidya, A.; Mazurek, J.A.; Park, M.H.; Ramani, G.; Chan, S.Y.; Waxman, A.B. Rationale and Design of the Ranolazine PH-RV Study: A Multicentred Randomised and Placebo-Controlled Study of Ranolazine to Improve RV Function in Patients with Non-Group 2 Pulmonary Hypertension. Open Heart 2018, 5, e000736. [Google Scholar] [CrossRef]
- Han, Y.; Forfia, P.; Vaidya, A.; Mazurek, J.A.; Park, M.H.; Ramani, G.; Chan, S.Y.; Waxman, A.B. Ranolazine Improves Right Ventricular Function in Patients With Precapillary Pulmonary Hypertension: Results From a Double-Blind, Randomized, Placebo-Controlled Trial. J. Card. Fail. 2021, 27, 253–257. [Google Scholar] [CrossRef]
- Quignard, J.F.; Ryckwaert, F.; Albat, B.; Nargeot, J.; Richard, S. A Novel Tetrodotoxin-Sensitive Na+ Current in Cultured Human Coronary Myocytes. Circ. Res. 1997, 80, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Boccara, G.; Choby, C.; Frapier, J.M.; Quignard, J.F.; Nargeot, J.; Dayanithi, G.; Richard, S. Regulation of Ca2+ Homeostasis by Atypical Na+ Currents in Cultured Human Coronary Myocytes. Circ. Res. 1999, 85, 606–613. [Google Scholar] [CrossRef]
- Kuriyama, H.; Kitamura, K.; Nabata, H. Pharmacological and Physiological Significance of Ion Channels and Factors That Modulate Them in Vascular Tissues. Pharmacol. Rev. 1995, 47, 387–573. [Google Scholar] [PubMed]
- Okabe, K.; Kitamura, K.; Kuriyama, H. The Existence of a Highly Tetrodotoxin Sensitive Na Channel in Freshly Dispersed Smooth Muscle Cells of the Rabbit Main Pulmonary Artery. Pflugers Arch. 1988, 411, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Cox, R.H.; Zhou, Z.; Tulenko, T.N. Voltage-Gated Sodium Channels in Human Aortic Smooth Muscle Cells. J. Vasc. Res. 1998, 35, 310–317. [Google Scholar] [CrossRef]
- Choby, C.; Mangoni, M.E.; Boccara, G.; Nargeot, J.; Richard, S. Evidence for Tetrodotoxin-Sensitive Sodium Currents in Primary Cultured Myocytes from Human, Pig and Rabbit Arteries. Pflugers Arch. 2000, 440, 149–152. [Google Scholar] [CrossRef]
- Meguro, K.; Iida, H.; Takano, H.; Morita, T.; Sata, M.; Nagai, R.; Nakajima, T. Function and Role of Voltage-Gated Sodium Channel NaV1.7 Expressed in Aortic Smooth Muscle Cells. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H211–H219. [Google Scholar] [CrossRef]
- Jo, T.; Nagata, T.; Iida, H.; Imuta, H.; Iwasawa, K.; Ma, J.; Hara, K.; Omata, M.; Nagai, R.; Takizawa, H.; et al. Voltage-Gated Sodium Channel Expressed in Cultured Human Smooth Muscle Cells: Involvement of SCN9A. FEBS Lett. 2004, 567, 339–343. [Google Scholar] [CrossRef]
- Rocchetti, M.; Sala, L.; Rizzetto, R.; Staszewsky, L.I.; Alemanni, M.; Zambelli, V.; Russo, I.; Barile, L.; Cornaghi, L.; Altomare, C.; et al. Ranolazine Prevents INaL Enhancement and Blunts Myocardial Remodelling in a Model of Pulmonary Hypertension. Cardiovasc. Res. 2014, 104, 37–48. [Google Scholar] [CrossRef]
- Platoshyn, O.; Remillard, C.V.; Fantozzi, I.; Sison, T.; Yuan, J.X.-J. Identification of Functional Voltage-Gated Na(+) Channels in Cultured Human Pulmonary Artery Smooth Muscle Cells. Pflugers Arch. 2005, 451, 380–387. [Google Scholar] [CrossRef]
- Meyer, N.; Tran, O.; Hartsfield, C.; Nguyen, L.; Kazi, D.S.; Koch, B. Revascularization Rates and Associated Costs in Patients With Stable Ischemic Heart Disease Initiating Ranolazine Versus Traditional Antianginals as Add-on Therapy. Am. J. Cardiol. 2019, 123, 1602–1609. [Google Scholar] [CrossRef]
- Lopaschuk, G.D. Metabolic Modulators in Heart Disease: Past, Present, and Future. Can. J. Cardiol. 2017, 33, 838–849. [Google Scholar] [CrossRef]
- McCormack, J.G.; Stanley, W.C.; Wolff, A.A. Ranolazine: A Novel Metabolic Modulator for the Treatment of Angina. Gen. Pharmacol. 1998, 30, 639–645. [Google Scholar] [CrossRef]
- McCormack, J.G.; Barr, R.L.; Wolff, A.A.; Lopaschuk, G.D. Ranolazine Stimulates Glucose Oxidation in Normoxic, Ischemic, and Reperfused Ischemic Rat Hearts. Circulation 1996, 93, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Gralinski, M.R.; Black, S.C.; Kilgore, K.S.; Chou, A.Y.; McCormack, J.G.; Lucchesi, B.R. Cardioprotective Effects of Ranolazine (RS-43285) in the Isolated Perfused Rabbit Heart. Cardiovasc. Res. 1994, 28, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Arnold, S.V.; McGuire, D.K.; Spertus, J.A.; Li, Y.; Yue, P.; Ben-Yehuda, O.; Belardinelli, L.; Jones, P.G.; Olmsted, A.; Chaitman, B.R.; et al. Effectiveness of Ranolazine in Patients with Type 2 Diabetes Mellitus and Chronic Stable Angina According to Baseline Hemoglobin A1c. Am. Heart J. 2014, 168, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Greiner, L.; Hurren, K.; Brenner, M. Ranolazine and Its Effects on Hemoglobin A1C. Ann. Pharmacother. 2016, 50, 410–415. [Google Scholar] [CrossRef]
- Teoh, I.H.; Banerjee, M. Effect of Ranolazine on Glycaemia in Adults with and without Diabetes: A Meta-Analysis of Randomised Controlled Trials. Open Heart 2018, 5, e000706. [Google Scholar] [CrossRef]
- Rosano, G.M.C.; Vitale, C.; Volterrani, M. Pharmacological Management of Chronic Stable Angina: Focus on Ranolazine. Cardiovasc. Drugs Ther. 2016, 30, 393–398. [Google Scholar] [CrossRef]
- Gilbert, B.W.; Sherard, M.; Little, L.; Branstetter, J.; Meister, A.; Huffman, J. Antihyperglycemic and Metabolic Effects of Ranolazine in Patients With Diabetes Mellitus. Am. J. Cardiol. 2018, 121, 509–512. [Google Scholar] [CrossRef]
- Rousseau, M.F.; Pouleur, H.; Cocco, G.; Wolff, A.A. Comparative Efficacy of Ranolazine versus Atenolol for Chronic Angina Pectoris. Am. J. Cardiol. 2005, 95, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Jain, D.; Dasgupta, P.; Hughes, L.O.; Lahiri, A.; Raftery, E.B. Ranolazine (RS-43285): A Preliminary Study of a New Anti-Anginal Agent with Selective Effect on Ischaemic Myocardium. Eur. J. Clin. Pharmacol. 1990, 38, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Cocco, G.; Rousseau, M.F.; Bouvy, T.; Cheron, P.; Williams, G.; Detry, J.M.; Pouleur, H. Effects of a New Metabolic Modulator, Ranolazine, on Exercise Tolerance in Angina Pectoris Patients Treated with Beta-Blocker or Diltiazem. J. Cardiovasc. Pharmacol. 1992, 20, 131–138. [Google Scholar] [PubMed]
- Hawash, A.A.; Voss, A.A.; Rich, M.M. Inhibiting Persistent Inward Sodium Currents Prevents Myotonia. Ann. Neurol. 2017, 82, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.H.; Denman, K.; DuPont, C.; Hawash, A.A.; Novak, K.R.; Koesters, A.; Grabner, M.; Dayal, A.; Voss, A.A.; Rich, M.M. The Mechanism Underlying Transient Weakness in Myotonia Congenita. eLife 2021, 10, e65691. [Google Scholar] [CrossRef] [PubMed]
- Novak, K.R.; Norman, J.; Mitchell, J.R.; Pinter, M.J.; Rich, M.M. Sodium Channel Slow Inactivation as a Therapeutic Target for Myotonia Congenita. Ann. Neurol. 2015, 77, 320–332. [Google Scholar] [CrossRef]
- Lorusso, S.; Kline, D.; Bartlett, A.; Freimer, M.; Agriesti, J.; Hawash, A.A.; Rich, M.M.; Kissel, J.T.; David Arnold, W. Open-Label Trial of Ranolazine for the Treatment of Paramyotonia Congenita. Muscle Nerve 2019, 59, 240–243. [Google Scholar] [CrossRef]
- Arnold, W.D.; Kline, D.; Sanderson, A.; Hawash, A.A.; Bartlett, A.; Novak, K.R.; Rich, M.M.; Kissel, J.T. Open-Label Trial of Ranolazine for the Treatment of Myotonia Congenita. Neurology 2017, 89, 710–713. [Google Scholar] [CrossRef]
- El-Bizri, N.; Kahlig, K.M.; Shyrock, J.C.; George, A.L.; Belardinelli, L.; Rajamani, S. Ranolazine Block of Human Na v 1.4 Sodium Channels and Paramyotonia Congenita Mutants. Channels Austin Tex. 2011, 5, 161–172. [Google Scholar] [CrossRef][Green Version]
- Koltai, T. Voltage-Gated Sodium Channel as a Target for Metastatic Risk Reduction with Re-Purposed Drugs. F1000Research 2015, 4, 297. [Google Scholar] [CrossRef]
- Roger, S.; Potier, M.; Vandier, C.; Besson, P.; Le Guennec, J.-Y. Voltage-Gated Sodium Channels: New Targets in Cancer Therapy? Curr. Pharm. Des. 2006, 12, 3681–3695. [Google Scholar] [CrossRef] [PubMed]
- Fraser, S.P.; Onkal, R.; Theys, M.; Bosmans, F.; Djamgoz, M.B.A. Neonatal NaV 1.5 Channels: Pharmacological Distinctiveness of a Cancer-Related Voltage-Gated Sodium Channel Splice Variant. Br. J. Pharmacol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, E.; Messerer, D.A.C.; Karpel-Massler, G.; Fauler, M.; Zimmer, T.; Jungwirth, B.; Föhr, K.J. Block of Voltage-Gated Sodium Channels as a Potential Novel Anti-Cancer Mechanism of TIC10. Front. Pharmacol. 2021, 12, 737637. [Google Scholar] [CrossRef]
- Brackenbury, W.J. Voltage-Gated Sodium Channels and Metastatic Disease. Channels Austin Tex. 2012, 6, 352–361. [Google Scholar] [CrossRef]
- Djamgoz, M.B.A.; Onkal, R. Persistent Current Blockers of Voltage-Gated Sodium Channels: A Clinical Opportunity for Controlling Metastatic Disease. Recent Patents Anticancer Drug Discov. 2013, 8, 66–84. [Google Scholar] [CrossRef]
- Wang, J.; Lu, Z.; Wu, C.; Li, Y.; Kong, Y.; Zhou, R.; Shi, K.; Guo, J.; Li, N.; Liu, J.; et al. Evaluation of the Anticancer and Anti-Metastasis Effects of Novel Synthetic Sodium Channel Blockers in Prostate Cancer Cells in Vitro and in Vivo. Prostate 2019, 79, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Driffort, V.; Gillet, L.; Bon, E.; Marionneau-Lambot, S.; Oullier, T.; Joulin, V.; Collin, C.; Pagès, J.-C.; Jourdan, M.-L.; Chevalier, S.; et al. Ranolazine Inhibits NaV1.5-Mediated Breast Cancer Cell Invasiveness and Lung Colonization. Mol. Cancer 2014, 13, 264. [Google Scholar] [CrossRef]
- Bugan, I.; Kucuk, S.; Karagoz, Z.; Fraser, S.P.; Kaya, H.; Dodson, A.; Foster, C.S.; Altun, S.; Djamgoz, M.B.A. Anti-Metastatic Effect of Ranolazine in an in Vivo Rat Model of Prostate Cancer, and Expression of Voltage-Gated Sodium Channel Protein in Human Prostate. Prostate Cancer Prostatic Dis. 2019, 22, 569–579. [Google Scholar] [CrossRef]
- Guth, A.; Monk, E.; Agarwal, R.; Bergman, B.C.; Zemski-Berry, K.A.; Minic, A.; Jordan, K.; Schlaepfer, I.R. Targeting Fat Oxidation in Mouse Prostate Cancer Decreases Tumor Growth and Stimulates Anti-Cancer Immunity. Int. J. Mol. Sci. 2020, 21, 9660. [Google Scholar] [CrossRef]
- Suckow, M.A.; Gutierrez, L.S.; Risatti, C.A.; Wolter, W.R.; Taylor, R.E.; Pollard, M.; Navari, R.M.; Castellino, F.J.; Paoni, N.F. The Anti-Ischemia Agent Ranolazine Promotes the Development of Intestinal Tumors in APC(−/+) Mice. Cancer Lett. 2004, 209, 165–169. [Google Scholar] [CrossRef]
- Guzel, R.M.; Ogmen, K.; Ilieva, K.M.; Fraser, S.P.; Djamgoz, M.B.A. Colorectal Cancer Invasiveness in Vitro: Predominant Contribution of Neonatal Nav1.5 under Normoxia and Hypoxia. J. Cell. Physiol. 2019, 234, 6582–6593. [Google Scholar] [CrossRef] [PubMed]
- Cappetta, D.; Esposito, G.; Coppini, R.; Piegari, E.; Russo, R.; Ciuffreda, L.P.; Rivellino, A.; Santini, L.; Rafaniello, C.; Scavone, C.; et al. Effects of Ranolazine in a Model of Doxorubicin-Induced Left Ventricle Diastolic Dysfunction. Br. J. Pharmacol. 2017, 174, 3696–3712. [Google Scholar] [CrossRef] [PubMed]
- Riccio, G.; Antonucci, S.; Coppola, C.; D’Avino, C.; Piscopo, G.; Fiore, D.; Maurea, C.; Russo, M.; Rea, D.; Arra, C.; et al. Ranolazine Attenuates Trastuzumab-Induced Heart Dysfunction by Modulating ROS Production. Front. Physiol. 2018, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Minotti, G.; Menna, P.; Calabrese, V.; Greco, C.; Armento, G.; Annibali, O.; Marchesi, F.; Salvatorelli, E.; Reggiardo, G. Pharmacology of Ranolazine versus Common Cardiovascular Drugs in Patients with Early Diastolic Dysfunction Induced by Anthracyclines or Nonanthracycline Chemotherapeutics: A Phase 2b Minitrial. J. Pharmacol. Exp. Ther. 2019, 370, 197–205. [Google Scholar] [CrossRef]
- Aldakkak, M.; Stowe, D.F.; Camara, A.K.S. Safety and Efficacy of Ranolazine for the Treatment of Chronic Angina Pectoris. Clin. Med. Insights Ther. 2013, 2013, 1–14. [Google Scholar] [CrossRef]
- Caron, J.; Libersa, C. Adverse Effects of Class I Antiarrhythmic Drugs. Drug Saf. 1997, 17, 8–36. [Google Scholar] [CrossRef]
- MacNeil, D.J. The Side Effect Profile of Class III Antiarrhythmic Drugs: Focus on d,l-Sotalol. Am. J. Cardiol. 1997, 80, 90G–98G. [Google Scholar] [CrossRef]
- Park, H.-S.; Kim, Y.-N. Adverse Effects of Long-Term Amiodarone Therapy. Korean J. Intern. Med. 2014, 29, 571–573. [Google Scholar] [CrossRef]
- Southard, R.A.; Blum, R.M.; Bui, A.H.; Blankstein, R. Neurologic Adverse Effects of Ranolazine in an Elderly Patient with Renal Impairment. Pharmacotherapy 2013, 33, e9–e13. [Google Scholar] [CrossRef]
- Eworuke, E.; Welch, E.C.; Tobenkin, A.; Maro, J.C. Use of FDA’s Sentinel System to Quantify Seizure Risk Immediately Following New Ranolazine Exposure. Drug Saf. 2019, 42, 897–906. [Google Scholar] [CrossRef]
- Zagelbaum, N.K.; Mondal, P.; Frishman, W.H.; Yandrapalli, S. Ranolazine Induced Delirium as a Rare Side Effect. Am. J. Ther. 2018, 25, e700–e701. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, H.; Jerling, M. Effect of Hepatic Impairment on the Multiple-Dose Pharmacokinetics of Ranolazine Sustained-Release Tablets. J. Clin. Pharmacol. 2005, 45, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Pham, D.Q.; Mehta, M. Ranolazine: A Novel Agent That Improves Dysfunctional Sodium Channels. Int. J. Clin. Pract. 2007, 61, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Reddy, B.M.; Weintraub, H.S.; Schwartzbard, A.Z. Ranolazine: A New Approach to Treating an Old Problem. Tex. Heart Inst. J. 2010, 37, 641–647. [Google Scholar]
- Fihn, S.D.; Gardin, J.M.; Abrams, J.; Berra, K.; Blankenship, J.C.; Dallas, A.P.; Douglas, P.S.; Foody, J.M.; Gerber, T.C.; Hinderliter, A.L.; et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients with Stable Ischemic Heart Disease: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation 2012, 126, e354–e471. [Google Scholar] [CrossRef]
- Masters, J.C.; Shah, M.M.; Feist, A.A. Drug Interaction between Sirolimus and Ranolazine in a Kidney Transplant Patient. Case Rep. Transplant. 2014, 2014, 548243. [Google Scholar] [CrossRef]
- Zack, J.; Berg, J.; Juan, A.; Pannacciulli, N.; Allard, M.; Gottwald, M.; Zhang, H.; Shao, Y.; Ben-Yehuda, O.; Jochelson, P. Pharmacokinetic Drug-Drug Interaction Study of Ranolazine and Metformin in Subjects with Type 2 Diabetes Mellitus. Clin. Pharmacol. Drug Dev. 2015, 4, 121–129. [Google Scholar] [CrossRef]
- Jerling, M.; Huan, B.-L.; Leung, K.; Chu, N.; Abdallah, H.; Hussein, Z. Studies to Investigate the Pharmacokinetic Interactions between Ranolazine and Ketoconazole, Diltiazem, or Simvastatin during Combined Administration in Healthy Subjects. J. Clin. Pharmacol. 2005, 45, 422–433. [Google Scholar] [CrossRef]

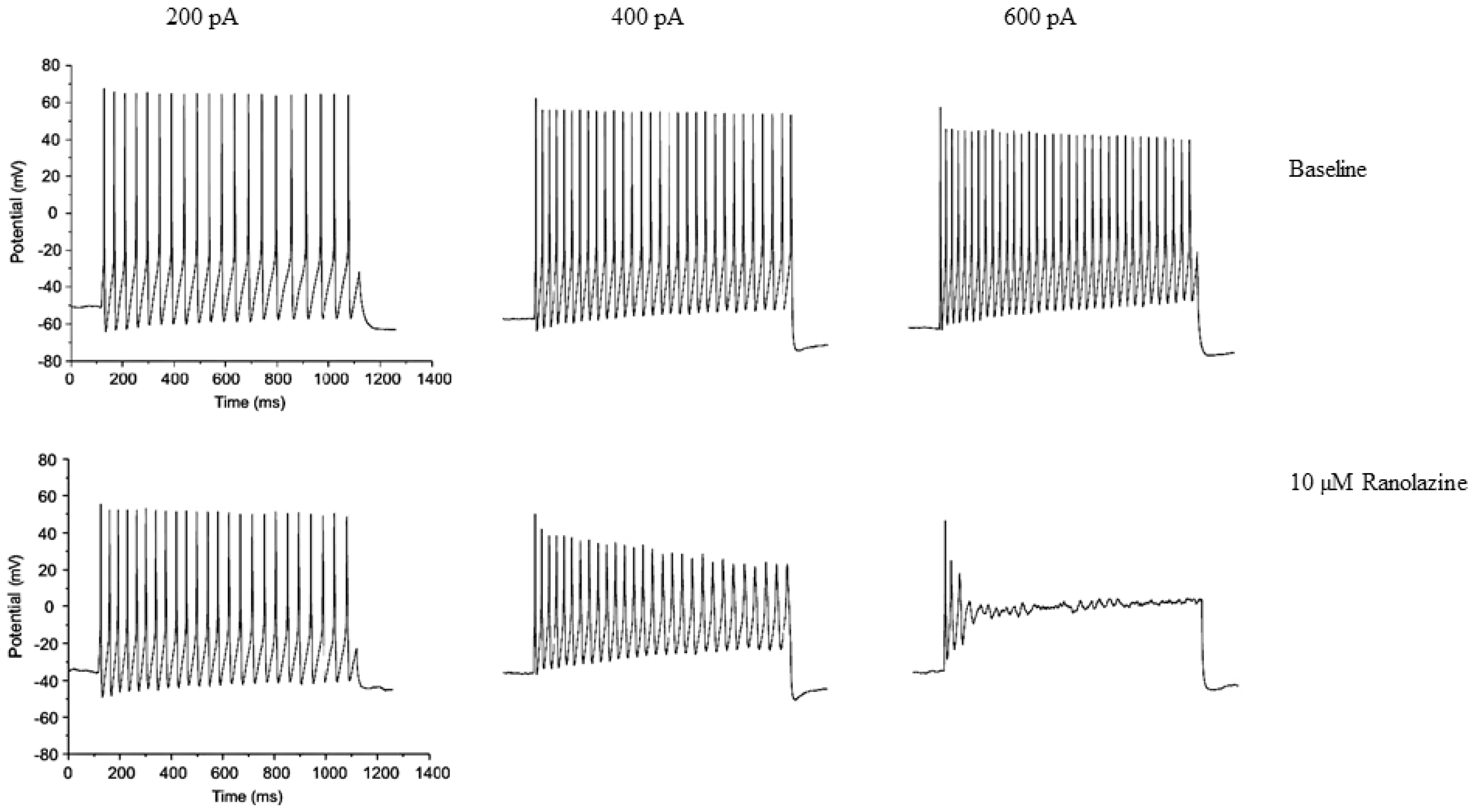
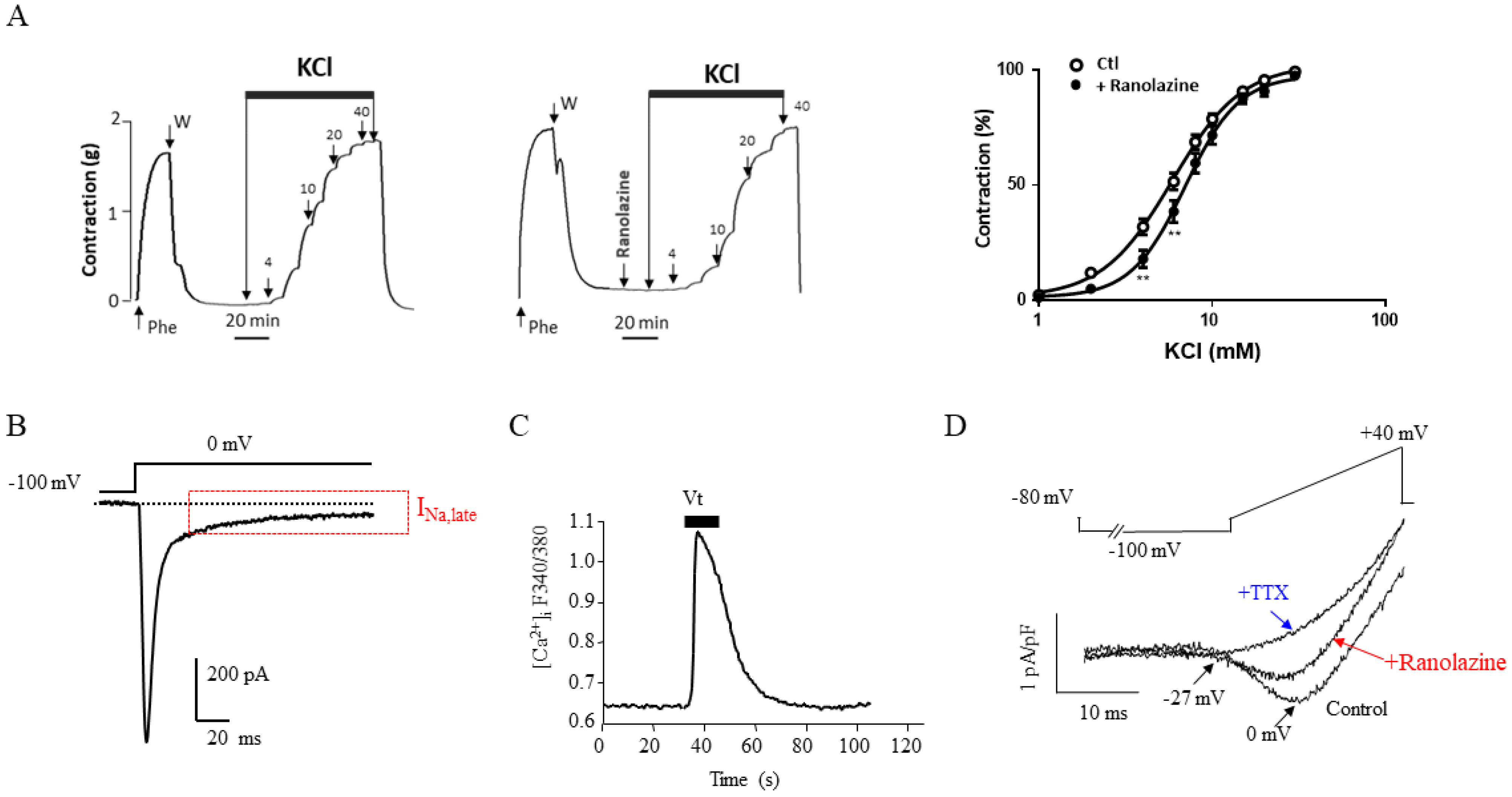
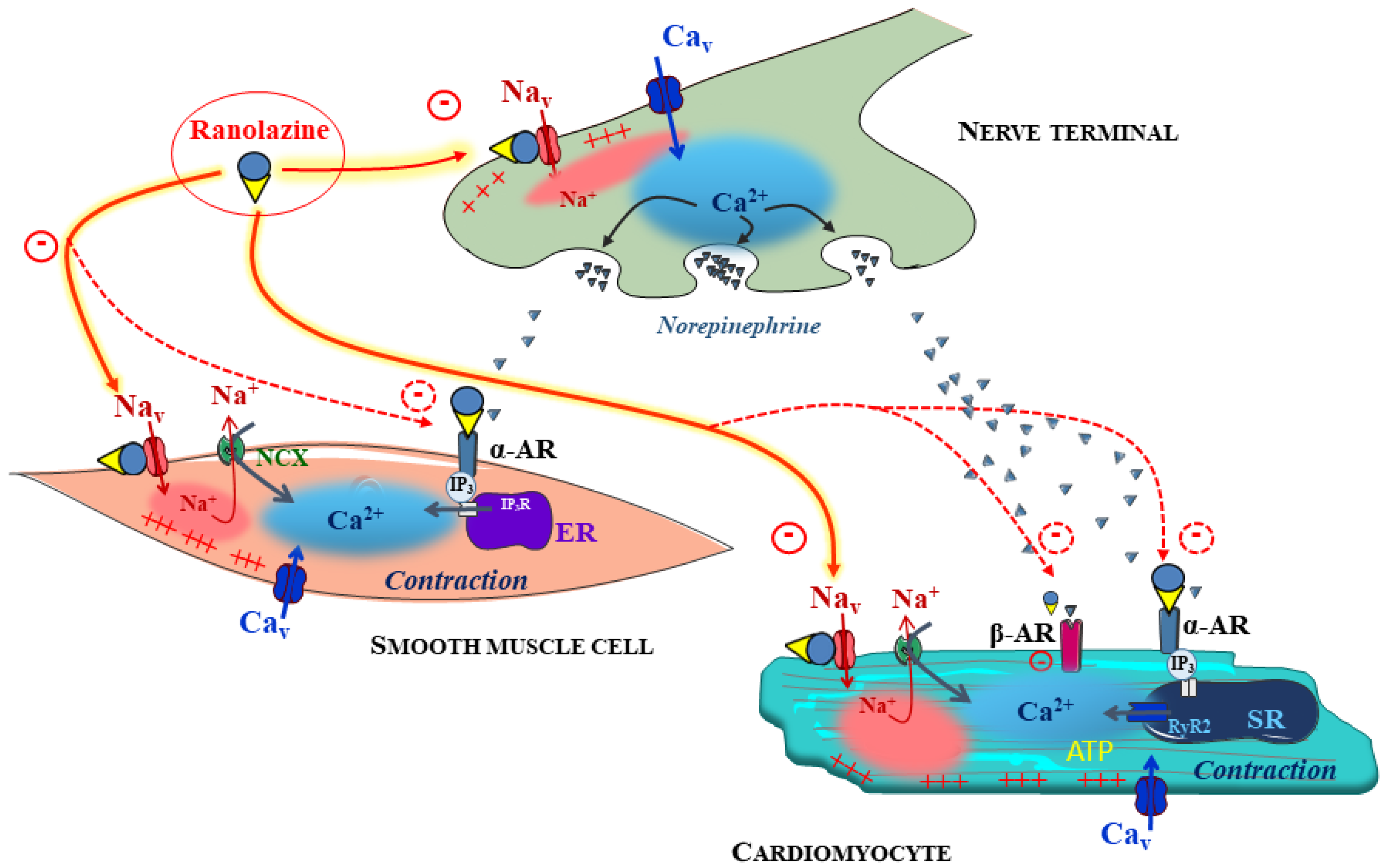
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rouhana, S.; Virsolvy, A.; Fares, N.; Richard, S.; Thireau, J. Ranolazine: An Old Drug with Emerging Potential; Lessons from Pre-Clinical and Clinical Investigations for Possible Repositioning. Pharmaceuticals 2022, 15, 31. https://doi.org/10.3390/ph15010031
Rouhana S, Virsolvy A, Fares N, Richard S, Thireau J. Ranolazine: An Old Drug with Emerging Potential; Lessons from Pre-Clinical and Clinical Investigations for Possible Repositioning. Pharmaceuticals. 2022; 15(1):31. https://doi.org/10.3390/ph15010031
Chicago/Turabian StyleRouhana, Sarah, Anne Virsolvy, Nassim Fares, Sylvain Richard, and Jérôme Thireau. 2022. "Ranolazine: An Old Drug with Emerging Potential; Lessons from Pre-Clinical and Clinical Investigations for Possible Repositioning" Pharmaceuticals 15, no. 1: 31. https://doi.org/10.3390/ph15010031
APA StyleRouhana, S., Virsolvy, A., Fares, N., Richard, S., & Thireau, J. (2022). Ranolazine: An Old Drug with Emerging Potential; Lessons from Pre-Clinical and Clinical Investigations for Possible Repositioning. Pharmaceuticals, 15(1), 31. https://doi.org/10.3390/ph15010031





