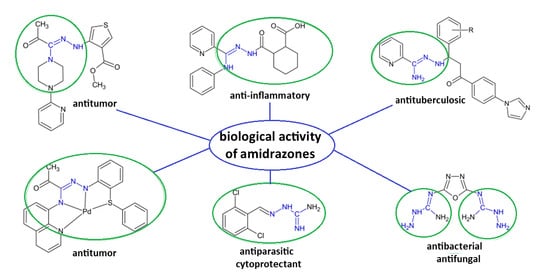
A Review of the Biological Activity of Amidrazone Derivatives
Abstract
:1. Introduction
2. Results
2.1. Antimicrobial Activity
2.1.1. Tuberculostatic Activity
2.1.2. Antibacterial Activity
2.1.3. Antifungal Activity
2.2. Antiparasitic Activity
2.3. Antiviral Activity
2.4. Anti-Inflammatory Activity
2.5. Cytoprotective Activity
2.6. Antitumor Activity
| Comp. | IC50 MCF-7 | IC50 K562 | Ref. |
|---|---|---|---|
| 69 | 2.50 µM | 3.10 µM | [69] |
| 70 | 2.70 µM | 3.50 µM | [69] |
| 71 | 7.26 µM | 9.91 µM | [70] |
| 72 | >50 µM | 1.02 µM | [71] |
| 73 | 5.18 µM | 2.89 µM | [72] |
| 74 | 5.91 µM | 5.02 µM | [73] |
| 75 | 20.20 µM | 9.30 µM | [74] |
| 76 | 4.50 µM | 1.10 µM | [75] |
| 79 | 4.30 µM | 3.00 µM | [77] |
| 81 | 0.09 µM | - | [78] |
2.7. Furin Inhibition
2.8. Acetylocholinesterase Inhibition
3. Summary
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AChE | acetylcholinesterase |
| BChE | butyrylocholinesterase |
| CNS | central nervous system |
| COX | cyclooxygenase |
| EC50 | half maximal effective concentration |
| HIV-RT | HIV reverse transcriptase |
| IC50 | half-maximal inhibitory concentration |
| IL-6 | interleukin-6 |
| LD50 | dose which causes the death of 50% of a group of test animals |
| LPS | lipopolysaccharide |
| MBC | minimal bactericidal concentration |
| MIC | minimal inhibitory concentration |
| MRSA | methicillin-resistant Staphylococcus aureus |
| MSSA | methicillin-susceptible Staphylococcus aureus |
| PBMC | peripheral blood mononuclear cell |
| TNF-α | tumor necrosis factor |
References
- Aly, A.; El-Din, A.M.N. Functionality of amidines and amidrazones. Arkivoc 2008, 1, 153–194. [Google Scholar] [CrossRef]
- Neilson, D.G.; Roger, R.; Heatlie, J.W.M.; Newlands, L.R. Chemistry of amidrazones. Chem. Rev. 1970, 70, 151–170. [Google Scholar] [CrossRef] [PubMed]
- Ben Salem, A.; Ben Salah, B.; Mhalla, D.; Trigui, M.; Mourer, M.; Regnouf-de-Vains, J.-B.; Kossentini, M. Synthesis, crystal structure and biological studies of novel amidrazones, triazoles, Thiatriazole and Triazine compounds. J. Mol. Struct. 2020, 1214, 128209. [Google Scholar] [CrossRef]
- Stepanov, A.I.; Sannikov, V.S.; Dashko, D.V.; Roslyakov, A.G.; Astrat’Ev, A.A.; Stepanova, E.V. A new preparative method and some chemical properties of 4-R-furazan-3-carboxylic acid amidrazones. Chem. Heterocycl. Compd. 2015, 51, 350–360. [Google Scholar] [CrossRef]
- Nomenclature of Organic Chemistry. IUPAC Recommendations and Preferred Names 2013. Available online: https://iupac.qmul.ac.uk/BlueBook/PDF/2013BlueBook.pdf (accessed on 18 February 2022).
- Abdel-Aziz, H.A.; Mekawey, A.A. Stereoselective synthesis and antimicrobial activity of benzofuran-based (1E)-1-(piperidin-1-yl)-N2-arylamidrazones. Eur. J. Med. Chem. 2009, 44, 4985–4997. [Google Scholar] [CrossRef]
- Modzelewska-Banachiewicz, B.; Matysiak, J.; Niewiadomy, A. Synthesis and mycological activity of the compounds obtained in the reaction of N3-substituted amidrazones with sulphinyl-bis-2,4-dihydroxybenzenethioyl. Eur. J. Med. Chem. 2001, 36, 75–80. [Google Scholar] [CrossRef]
- Gokhale, N.H.; Padhye, S.B.; Billington, D.C.; Rathbone, D.L.; Croft, S.L.; Kendrick, H.D.; Anson, C.E.; Powell, A.K. Synthesis and characterization of copper(II) complexes of pyridine-2-carboxamidrazones as potent antimalarial agents. Inorg. Chim. Acta 2003, 349, 23–29. [Google Scholar] [CrossRef]
- Modzelewska-Banachiewicz, B.; Michalec, B.; Kamińska, T.; Mazur, L.; Kozioł, A.E.; Banachiewicz, J.; Ucherek, M.; Kandefer-Szerszeń, M. Synthesis and biological activity of (Z) and (E) isomers of 3-(3,4-diaryl-1,2,4-triazole-5-yl)prop-2-enoic acid. Monatsh. Chem. 2009, 140, 439–444. [Google Scholar] [CrossRef]
- Hassan, H.Y.; El-Shorbagi, A.-N.; El-Koussi, N.A.; Abdel-Zaher, A.O. Design and synthesis of’ some new 1H-1,2,4-triazoles of potential anti-inflammatory and analgesic activities. Bull. Pharm. Sci. Assiut 1994, 17, 27–39. [Google Scholar] [CrossRef]
- Modzelewska-Banachiewicz, B.; Banachiewicz, J.; Chodkowska, A.; Jagiełło-Wójtowicz, E.; Mazur, L. Synthesis and biological activity of new derivatives of 3-(3,4-diaryl-1,2,4-triazole-5-yl)propenoic acid. Eur. J. Med. Chem. 2004, 39, 873–877. [Google Scholar] [CrossRef]
- Cocco, M.T.; Onnis, V.; Ponticelli, G.; Meier, B.; Rehder, D.; Garribba, E.; Micera, G. Synthesis, characterisation and insulin-mimetic activity of oxovanadium(IV) complexes with amidrazone derivatives. J. Inorg. Biochem. 2007, 101, 19–29. [Google Scholar] [CrossRef]
- Lee, K.; Jung, W.-H.; Park, C.W.; Park, H.D.; Lee, S.H.; Kwon, O.H. Noncovalent tripeptidic thrombin inhibitors incorporating amidrazone, amine and amidine functions at P1. Bioorg. Med. Chem. Lett. 2002, 12, 1017–1022. [Google Scholar] [CrossRef]
- Sączewski, F.; Balewski, Ł. Biological activities of guanidine compounds. Expert Opin. Ther. Patents 2009, 19, 1417–1448. [Google Scholar] [CrossRef]
- Cho, Y.L.; Jang, J. Development of Delpazolid for the Treatment of Tuberculosis. Appl. Sci. 2020, 10, 2211. [Google Scholar] [CrossRef]
- ClinicalTrials. Available online: http://www.clinicaltrials.gov/ct2/show/NCT04550832 (accessed on 22 May 2021).
- Zampieri, D.; Mamolo, M.G.; Vio, L.; Romano, M.; Skoko, N.; Baralle, M.; Pau, V.; De Logu, A. Antimycobacterial activity of new N1-[1-[1-aryl-3-[4-(1H-imidazol-1-yl)phenyl]-3-oxo]propyl]-pyridine-2-carboxamidrazone derivatives. Bioorg. Med. Chem. Lett. 2016, 26, 3287–3290. [Google Scholar] [CrossRef]
- Krause, M.; Foks, H.; Ziembicka, D.; Augustynowicz-Kopeć, E.; Głogowska, A.; Korona-Głowniak, I.; Bojanowski, K.; Siluk, D.; Gobis, K. 4-Substituted picolinohydrazonamides as a new class of potential antitubercular agents. Eur. J. Med. Chem. 2020, 190, 112106. [Google Scholar] [CrossRef]
- Tapera, M.; Kekeçmuhammed, H.; Sahin, K.; Krishna, V.S.; Lherbet, C.; Homberset, H.; Chebaiki, M.; Tønjum, T.; Mourey, L.; Zorlu, Y.; et al. Synthesis, characterization, anti-tuberculosis activity and molecular modeling studies of thiourea derivatives bearing aminoguanidine moiety. J. Mol. Struct. 2022, 1270, 133899. [Google Scholar] [CrossRef]
- Foks, H.; Balewski, L.; Gobis, K.; Dabrowska-Szponar, M.; Wisniewska, K. Studies on pyrazine derivatives LII: Antibacterial and antifungal activity of nitrogen heterocyclic compounds obtained by pyrazinamidrazone usage. Heteroat. Chem. 2011, 23, 49–58. [Google Scholar] [CrossRef]
- Senina, A.S.; Evdokimov, A.A.; Moskvin, A.V.; Fedorova, E.V. Synthesis, characterization and antimicrobial activity of amidrazone derivatives. J. Adv. Chem. Sci. 2016, 2, 183–187. Available online: https://www.jacsdirectory.com/journal-of-advanced-chemical-sciences/articleview.php?id=69 (accessed on 21 September 2022).
- Abdullah, A.H.; Zahra, J.A.; El-Abadelah, M.M.; Sabri, S.S.; Khanfar, M.A.; Matar, S.A.; Voelter, W. Synthesis and antibacterial activity of N1-(carbazol-3-yl)amidrazones incorporating piperazines and related congeners. Z. Naturforsch. 2016, 71, 857–867. [Google Scholar] [CrossRef]
- Saadeh, H.A.; Al-Qaoud, K.M.; Abu-Qatouseh, L.F.; Shihab, P.A.; Kaur, H.; Goyal, K.; Sehgal, R.; Mubarak, M.S. Synthesis and biological activity of novel amidrazones incorporating 5-nitroimidazole, ciprofloxacin, and 7-chloro-4-piperazinylquinoline. Med. Chem. Res. 2015, 24, 2247–2256. [Google Scholar] [CrossRef]
- Hkiri, S.; Hafidh, A.; Cavalier, J.-F.; Touil, S.; Samarat, A. Design, synthesis, antimicrobial evaluation, and molecular docking studies of novel symmetrical 2,5-difunctionalized 1,3,4-oxadiazoles. J. Heterocycl. Chem. 2019, 57, 1044–1054. [Google Scholar] [CrossRef]
- Wei, Z.-Y.; Chi, K.-Q.; Yu, Z.-K.; Liu, H.-Y.; Sun, L.-P.; Zheng, C.-J.; Piao, H.-R. Synthesis and biological evaluation of chalcone derivatives containing aminoguanidine or acylhydrazone moieties. Bioorg. Med. Chem. Lett. 2016, 26, 5920–5925. [Google Scholar] [CrossRef]
- Li, Y.-R.; Li, C.; Liu, J.-C.; Guo, M.; Zhang, T.-Y.; Sun, L.-P.; Zheng, C.-J.; Piao, H.-R. Synthesis and biological evaluation of 1,3-diaryl pyrazole derivatives as potential antibacterial and anti-inflammatory agents. Bioorg. Med. Chem. Lett. 2015, 25, 5052–5057. [Google Scholar] [CrossRef]
- Bai, X.; Zhao, L.; Liu, Z.; Li, Y.; Zhang, T.; Liu, X. Synthesis and antibacterial activity evaluation of aminoguanidine or dihydrotriazine derivatives. Indian J. Biochem. Biophys. 2019, 56, 301–308. Available online: http://op.niscair.res.in/index.php/IJBB/article/view/27359/0 (accessed on 15 September 2022).
- Song, M.; Wang, S.; Wang, Z.; Fu, Z.; Zhou, S.; Cheng, H.; Liang, Z.; Deng, X. Synthesis, antimicrobial and cytotoxic activities, and molecular docking studies of N-arylsulfonylindoles containing an aminoguanidine, a semicarbazide, and a thiosemicarbazide moiety. Eur. J. Med. Chem. 2019, 166, 108–118. [Google Scholar] [CrossRef]
- Yao, X.; Hu, H.; Wang, S.; Zhao, W.; Song, M.; Zhou, Q. Synthesis, Antimicrobial Activity, and Molecular Docking Studies of Aminoguanidine Derivatives Containing an Acylhydrazone Moiety. Iran. J. Pharm. Res. 2021, 20, 536–545. [Google Scholar] [CrossRef]
- Elsebaei, M.; Mohammad, H.; Abouf, M.; Abutaleb, N.; Hegazy, Y.A.; Ghiaty, A.; Chen, L.; Zhang, J.; Malwal, S.R.; Oldfield, E.; et al. Alkynyl-containing phenylthiazoles: Systemically active antibacterial agents effective against methicillin-resistant Staphylococcus aureus (MRSA). Eur. J. Med. Chem. 2018, 148, 195–209. [Google Scholar] [CrossRef]
- Paprocka, R.; Modzelewska-Banachiewicz, B.; Pazderski, L.; Mazur, L.; Kutkowska, J.; Niedzielska, D.; Psurski, M.; Wietrzyk, J.; Sączewski, J. Synthesis, crystal structure, 1H, 13C and 15N NMR studies, and biological evaluation of a new amidrazone-derived Au(III) complex. J. Mol. Struct. 2019, 1176, 357–365. [Google Scholar] [CrossRef]
- Senina, A.S.; Gurina, S.V.; Moskvin, A.V. Antimicrobial activity of amidrazone hydrohalogenides. Farmacija 2017, 66, 41–44. [Google Scholar]
- Kumar, N.S.; Pradeep, T.; Jani, G.; Silpa, D.; Kumar, B.V. Design, synthesis, and antimicrobial screening of novel pyridyl-2-amidrazone incorporated isatin mannich bases. J. Adv. Pharm. Technol. Res. 2012, 3, 57–61. Available online: https://www.japtr.org/article.asp?issn=2231-4040;year=2012;volume=3;issue=1;spage=57;epage=61;aulast=Kumar (accessed on 21 September 2022). [PubMed]
- Ribeiro, A.I.; Gabriel, C.; Cerqueira, F.; Maia, M.; Pinto, E.; Sousa, J.C.; Medeiros, R.; Proença, M.F.; Dias, A.M. Synthesis and antimicrobial activity of novel 5-aminoimidazole-4-carboxamidrazones. Bioorg. Med. Chem. Lett. 2014, 24, 4699–4702. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, C.; Grenho, L.; Cerqueira, F.; Medeiros, R.; Dias, A.M.; Ribeiro, A.I.; Proença, M.F.; Fernandes, M.H.; Sousa, J.C.; Monteiro, F.J.; et al. Inhibitory Effect of 5-Aminoimidazole-4-Carbohydrazonamides Derivatives against Candida spp. Biofilm on Nanohydroxyapatite Substrate. Mycopathologia 2019, 184, 775–786. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, F.; Maia, M.; Gabriel, C.; Medeiros, R.; Cravo, S.; Ribeiro, A.; Dantas, D.; Dias, A.; Saraiva, L.; Raimundo, L.; et al. Mechanism of Antifungal Activity by 5-Aminoimidazole-4-Carbohydrazonamide Derivatives against Candida albicans and Candida krusei. Antibiotics 2021, 10, 183. [Google Scholar] [CrossRef]
- Douadi, K.; Chafaa, S.; Douadi, T.; Al-Noaimi, M.; Kaabi, I. Azoimine quinoline derivatives: Synthesis, classical and electrochemical evaluation of antioxidant, anti-inflammatory, antimicrobial activities and the DNA/BSA binding. J. Mol. Struct. 2020, 1217, 128305. [Google Scholar] [CrossRef]
- Babahan, I.; Çoban, E.P.; Özmen, A.; Biyik, H.; Isman, B. Synthesis, characterization and biological activity of vic-dioxime derivatives containing benzaldehydehydrazone groups and their metal complexes. Afr. J. Microbiol. Res. 2011, 5, 271–283. [Google Scholar] [CrossRef]
- Biyik, H.; Babahan, I.; Coban, E.P. Synthesis, characterisation and antimicrobial activities of vic-dioxime derivatives containing heteroaromatic hydrazone groups and their metal complexes. Maejo Int. J. Sci. Technol. 2013, 7, 26–41. [Google Scholar] [CrossRef]
- Ajdačić, V.; Senerovic, L.; Vranić, M.; Pekmezovic, M.; Arsic-Arsnijevic, V.; Veselinovic, A.; Veselinovic, J.; Šolaja, B.A.; Nikodinovic-Runic, J.; Opsenica, I.M. Synthesis and evaluation of thiophene-based guanylhydrazones (iminoguanidines) efficient against panel of voriconazole-resistant fungal isolates. Bioorg. Med. Chem. 2016, 24, 1277–1291. [Google Scholar] [CrossRef]
- Do Nascimento, M.S.; Câmara, V.R.; Da Costa, J.S.; Barbosa, J.M.; Lins, A.S.; Salomão, K.; De Castro, S.L.; Carvalho, S.A.; Da Silva, E.F.; Fraga, C.A. Identification of Novel Functionalized Carbohydrazonamides Designed as Chagas Disease Drug Candidates. Med. Chem. 2020, 16, 774–783. [Google Scholar] [CrossRef]
- Abraham, R.J.; Abraham, S.; Stevens, A.J.; Page, S.W.; McCluskey, A.; Trott, D.J.; O’Handley, R.M. Aminoguanidines: New leads for treatment of Giardia duodenalis infection. Int. J. Parasitol. Drugs Drug Resist. 2019, 10, 38–44. [Google Scholar] [CrossRef]
- Benmerzouga, I.; Checkley, L.A.; Ferdig, M.T.; Arrizabalaga, G.; Wek, R.C.; Sullivan, W.J., Jr. Guanabenz Repurposed as an Antiparasitic with Activity against Acute and Latent Toxoplasmosis. Antimicrob. Agents Chemother. 2015, 59, 6939–6945. [Google Scholar] [CrossRef]
- Konrad, C.; Queener, S.F.; Wek, R.C.; Sullivan, W.J. Inhibitors of eIF2α Dephosphorylation Slow Replication and Stabilize Latency in Toxoplasma gondii. Antimicrob. Agents Chemother. 2013, 57, 1815–1822. [Google Scholar] [CrossRef]
- De Aquino, T.M.; França, P.H.B.; Rodrigues, E.E.S.; Nascimento, I.J.; Santos-Júnior, P.F.S.; Aquino, P.G.V.; Santos, M.S.; Queiroz, A.C.; Araújo, M.V.; Alexandre-Moreira, M.S.; et al. Synthesis, Antileishmanial Activity and in silico Studies of Aminoguanidine Hydrazones (AGH) and Thiosemicarbazones (TSC) Against Leishmania chagasi Amastigotes. Med. Chem. 2022, 18, 151–169. [Google Scholar] [CrossRef]
- Paprocka, R.; Kołodziej, P.; Wiese-Szadkowska, M.; Helmin-Basa, A.; Bogucka-Kocka, A. Evaluation of Anthelmintic and Anti-Inflammatory Activity of 1,2,4-Triazole Derivatives. Molecules 2022, 27, 4488. [Google Scholar] [CrossRef]
- Abdel-Aziza, H.A.; Abdel-Wahab, B.F.; Badria, F.A. Stereoselective Synthesis and Antiviral Activity of (1E,2Z,3E)-1-(Piperidin-1-yl)-1-(arylhydrazono)-2-[(benzoyl/benzothiazol- 2-oyl)hydrazono]-4-(aryl1)but-3-enes. Arch. Pharm. 2010, 343, 152–159. [Google Scholar] [CrossRef]
- Gomha, S.M.; Badrey, M.G.; Abdalla, M.M.; Arafa, R.K. Novel anti-HIV-1 NNRTIs based on a pyrazolo[4,3-d]isoxazole backbone scaffold: Design, synthesis and insights into the molecular basis of action. MedChemComm 2014, 5, 1685–1692. [Google Scholar] [CrossRef]
- Mazur, L.; Modzelewska-Banachiewicz, B.; Paprocka, R.; Zimecki, M.; Wawrzyniak, U.E.; Kutkowska, J.; Ziółkowska, G. Synthesis, crystal structure and biological activities of a novel amidrazone derivative and its copper(II) complex—A potential antitumor drug. J. Inorg. Biochem. 2012, 114, 55–64. [Google Scholar] [CrossRef]
- Modzelewska-Banachiewicz, B.; Ucherek, M.; Zimecki, M.; Kutkowska, J.; Kaminska, T.; Morak-Młodawska, B.; Paprocka, R.; Szulc, M.; Lewandowski, G.; Marciniak, J.; et al. Reactions of N3-Substituted Amidrazones with cis-1,2-Cyclohexanedicarboxylic Anhydride and Biological Activities of the Products. Arch. Pharm. 2012, 345, 486–494. [Google Scholar] [CrossRef]
- Paprocka, R.; Modzelewska-Banachiewicz, B.; Wiese, M.; Eljaszewicz, A.; Michałkiewicz, J. Synthesis and anti-inflammatory activity of hydrazide derivatives of 2-methylidene-1,4-dicarboxybutanoic acid. Acta Pol. Pharm. 2012, 69, 1390–1394. Available online: https://www.ptfarm.pl/pub/File/Acta_Poloniae/2012/6/1390.pdf (accessed on 22 November 2012).
- Paprocka, R.; Wiese-Szadkowska, M.; Helmin-Basa, A.; Mazur, L.; Kutkowska, J.; Michałkiewicz, J.; Modzelewska-Banachiewicz, B.; Pazderski, L. Synthesis and evaluation of new amidrazone-derived hydrazides as a potential anti-inflammatory agents. Monatsh. Chem. 2018, 149, 1493–1500. [Google Scholar] [CrossRef]
- Paprocka, R.; Wiese, M.; Eljaszewicz, A.; Helmin-Basa, A.; Gzella, A.; Modzelewska-Banachiewicz, B.; Michalkiewicz, J. Synthesis and anti-inflammatory activity of new 1,2,4-triazole derivatives. Bioorg. Med. Chem. Lett. 2015, 25, 2664–2667. [Google Scholar] [CrossRef]
- Paprocka, R.; Pazderski, L.; Mazur, L.; Wiese-Szadkowska, M.; Kutkowska, J.; Nowak, M.; Helmin-Basa, A. Synthesis and Structural Study of Amidrazone Derived Pyrrole-2,5-Dione Derivatives: Potential Anti-Inflammatory Agents. Molecules 2022, 27, 2891. [Google Scholar] [CrossRef]
- El-Din, N.; Barseem, A. Synthesis, bioactivity and Docking Study of Some New Indole-hydrazone Derivatives. J. Appl. Pharm. Sci. 2016, 6, 075–083. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, Z.S.; Liu, X.H.; Chen, F.H. Novel amidrazone derivatives: Design, synthesis and activity evaluation. Bioorg. Med. Chem. 2018, 26, 3158–3165. [Google Scholar] [CrossRef]
- Pasten, C.; Lozano, M.; Rocco, J.; Carrión, F.; Alvarado, C.; Liberona, J.; Michea, L.; Irarrázabal, C.E. Aminoguanidine Prevents the Oxidative Stress, Inhibiting Elements of Inflammation, Endothelial Activation, Mesenchymal Markers, and Confers a Renoprotective Effect in Renal Ischemia and Reperfusion Injury. Antioxidants 2021, 10, 1724. [Google Scholar] [CrossRef]
- Heimfarth, L.; Carvalho, A.M.S.; Quintans, J.D.S.S.; Pereira, E.W.M.; Teles Lima, N.; Carvalho, M.T.B.; Barreto, R.D.S.S.; Moreira, J.C.F.; Da Silva-Júnior, E.F.; Schmitt, M.; et al. Indole-3-guanylhydrazone hydrochloride mitigates long-term cognitive impairment in a neonatal sepsis model with involvement of MAPK and NFκB pathways. Neurochem. Int. 2020, 134, 104647. [Google Scholar] [CrossRef]
- Luh, L.M.; Bertolotti, A. Potential benefit of manipulating protein quality control systems in neurodegenerative diseases. Curr. Opin. Neurobiol. 2020, 61, 125–132. [Google Scholar] [CrossRef]
- ClinicalTrials. Available online: https://clinicaltrials.gov/ct2/show/NCT02423083 (accessed on 21 February 2022).
- Bella, E.D.; Bersano, E.; Antonini, G.; Borghero, G.; Capasso, M.; Caponnetto, C.; Chiò, A.; Corbo, M.; Filosto, M.; Giannini, F.; et al. The unfolded protein response in amyotrophic later sclerosis: Results of a phase 2 trial. Brain 2021, 144, 2635–2647. [Google Scholar] [CrossRef]
- Wang, L.; Popko, B.; Tixier, E.; Roos, R.P. Guanabenz, which enhances the unfolded protein response, ameliorates mutant SOD1-induced amyotrophic lateral sclerosis. Neurobiol. Dis. 2014, 71, 317–324. [Google Scholar] [CrossRef]
- Martynowicz, J.; Augusto, L.; Wek, R.C.; Boehm, S.L.; Sullivan, W.J. Guanabenz Reverses a Key Behavioral Change Caused by Latent Toxoplasmosis in Mice by Reducing Neuroinflammation. mBio 2019, 10, e00381-19. [Google Scholar] [CrossRef]
- ClinicalTrials. Available online: https://clinicaltrials.gov/ct2/show/NCT03124459 (accessed on 21 February 2022).
- Chen, Y.; Podojil, J.R.; Kunjamma, R.B.; Jones, J.; Weiner, M.; Lin, W.; Miller, S.D.; Popko, B. Sephin1, which prolongs the integrated stress response, is a promising therapeutic for multiple sclerosis. Brain 2019, 142, 344–361. [Google Scholar] [CrossRef] [PubMed]
- Krzyzosiak, A.; Sigurdardottir, A.; Luh, L.; Carrara, M.; Das, I.; Schneider, K.; Bertolotti, A. Target-Based Discovery of an Inhibitor of the Regulatory Phosphatase PPP1R15B. Cell 2018, 174, 1216–1228.e19. [Google Scholar] [CrossRef] [PubMed]
- Claes, Z.; Jonkhout, M.; Crespillo-Casado, A.; Bollen, M. The antibiotic robenidine exhibits guanabenz-like cytoprotective properties by a mechanism independent of protein phosphatase PP1:PPP1R15A. J. Biol. Chem. 2019, 294, 13478–13486. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Jalil, R.J.; El Momani, E.Q.; Hamad, M.; Voelter, W.; Mubarak, M.S.; Smith, B.H.; Peters, D.G. Synthesis, antitumor activity, and electrochemical behavior of some piperazinyl amidrazones. Monatsh. Chem. 2010, 141, 251–258. [Google Scholar] [CrossRef]
- Habashneh, A.Y.; El-Abadelah, M.M.; Bardaweel, S.K.; Taha, M. Synthesis and Structure-Activity Relationship; Exploration of some Potent Anti-Cancer Phenyl Amidrazone Derivatives. Med. Chem. 2018, 14, 468–477. [Google Scholar] [CrossRef]
- Abadleh, M.M.; El-Abadelah, M.M.; Sabri, S.S.; Mohammed, H.H.; Zihlif, M.A.; Voelter, W. Synthesis and Antitumor Activity of Some N2-(Thien-3-yl)amidrazones. Z. Naturforsch. 2014, 69, 811–816. [Google Scholar] [CrossRef]
- Almansour, A.M.; Zahra, J.A.; Sabri, S.S.; El-Abadelah, M.M.; Zihlif, M.A.; Taha, M.O. Synthesis and Anticancer Properties of Methyl N1-(thien-4-yl)amidrazone- 3-carboxylates. Lett. Drug Des. Discov. 2018, 15, 1268–1275. [Google Scholar] [CrossRef]
- Habashneh, A.Y.; Zihlif, M.A.; Imraish, A.; Taha, M.O.; El-Abadelah, M.M. Synthesis and Antitumor Activities of Some New N1-(Flavon-6-yl)amidrazone Derivatives. Arch. Pharm. 2014, 347, 415–422. [Google Scholar] [CrossRef]
- Abu-Aisheh, M.N.; Mustafa, M.S.; El-Abadelah, M.M.; Naffa, R.G.; Ismail, S.I.; Zihlif, M.A.; Taha, M.O.; Mubarak, M.S. Synthesis and biological activity assays of some new N1-(flavon-7-yl)amidrazone derivatives and related congeners. Eur. J. Med. Chem. 2012, 54, 65–74. [Google Scholar] [CrossRef]
- Mustafa, M.S.; El-Abadelah, M.M.; Zihlif, M.A.; Naffa, R.; Mubarak, M.S. Synthesis, and Antitumor Activity of Some N1-(Coumarin-7-yl) Amidrazones and Related Congeners. Molecules 2011, 16, 4305–4317. [Google Scholar] [CrossRef]
- Sweidan, K.; Zalloum, H.; Sabbah, D.A.; Idris, G.; Abudosh, K.; Mubarak, M.S. Synthesis, characterization, and anticancer evaluation of some new N1-(anthraquinon-2-yl) amidrazone derivatives. Can. J. Chem. 2018, 96, 1123–1128. [Google Scholar] [CrossRef]
- Sabbah, D.A.; Hajjo, R.; Sweidan, K.; Zhong, H.A. An Integrative Informatics Approach to Explain the Mechanism of Action of N1-(Anthraquinon-2-yl) Amidrazones as BCR/ABL Inhibitors. Curr. Comput. Aided-Drug Des. 2020, 17, 817–830. [Google Scholar] [CrossRef]
- Al-Qtaitat, M.A.; El-Abadelah, M.M.; Sabbah, D.A.; Bardaweel, S.; Sweidan, K.; Sabri, S.S.; Mubarak, M.S. Synthesis, characterization, and bioactivity of new bisamidrazone derivatives as possible anticancer agents. Med. Chem. Res. 2018, 27, 1419–1431. [Google Scholar] [CrossRef]
- Qian, Y.; Zhang, H.-J.; Lv, P.-C.; Zhu, H.-L. Synthesis, molecular modeling and biological evaluation of guanidine derivatives as novel antitubulin agents. Bioorg. Med. Chem. 2010, 18, 8218–8225. [Google Scholar] [CrossRef]
- Andreani, A.; Granaiola, M.; Leoni, A.; Locatelli, A.; Morigi, R.; Rambaldi, M.; Varoli, L.; Lannigan, D.; Smith, J.; Scudiero, D.; et al. Imidazo[2,1-b]thiazole guanylhydrazones as RSK2 inhibitors. Eur. J. Med. Chem. 2011, 46, 4311–4323. [Google Scholar] [CrossRef]
- Basu, A.; Sinha, B.N.; Saiko, P.; Graser, G.; Szekeres, T. N-Hydroxy-N′-aminoguanidines as anti-cancer lead molecule: QSAR, synthesis and biological evaluation. Bioorg. Med. Chem. Lett. 2011, 21, 3324–3328. [Google Scholar] [CrossRef]
- Silva, F.P.L.; Dantas, B.B.; Martins, G.V.F.; De Araújo, D.A.M.; Vasconcellos, M.L.A.D.A. Synthesis and Anticancer Activities of Novel Guanylhydrazone and Aminoguanidine Tetrahydropyran Derivatives. Molecules 2016, 21, 671. [Google Scholar] [CrossRef]
- Liu, D.C.; Gao, M.J.; Huo, Q.; Ma, T.; Wang, Y.; Wu, C.Z. Design, synthesis, and apoptosis-promoting effect evaluation of novel pyrazole with benzo[d]thiazole derivatives containing aminoguanidine units. J. Enzym. Inhib. Med. Chem. 2019, 34, 829–837. [Google Scholar] [CrossRef]
- Al-Noaimi, M.; Awwadi, F.F.; Mansi, I.A.; Sawwan, M.; Abu-Irmaileh, B.; Dege, N. Polymorphism, spectroscopic, DFT and anticancer activity of a palladium(II) complex with a thiophenyl azoimine-quinoline SNN’N” ligand. Polyhedron 2022, 211, 115541. [Google Scholar] [CrossRef]
- Al-Noaimi, M.; Awwadi, F.F.; Talib, W.; Atia, S.; Hammud, H.H. Cis and trans- palladium (II) complexes derived from SNN amidrazone pincer ligand: Synthesis, crystal structures and biological evaluation. J. Mol. Struct. 2019, 1197, 282–291. [Google Scholar] [CrossRef]
- Lapasam, A.; Pinder, E.; Phillips, R.M.; Kaminsky, W.; Kollipara, M.R. Synthesis, structure and bonding modes of pyrazine based ligands of Cp*Rh and Cp*Ir complexes: The study of in-vitro cytotoxicity against human cell lines. J. Organomet. Chem. 2019, 899, 120887. [Google Scholar] [CrossRef]
- Dömötör, O.; May, N.V.; Gál, G.T.; Spengler, G.; Dobrova, A.; Arion, V.B.; Enyedy, É.A. Solution Equilibrium Studies on Salicylidene Aminoguanidine Schiff Base Metal Complexes: Impact of the Hybridization with L-Proline on Stability, Redox Activity and Cytotoxicity. Molecules 2022, 27, 2044. [Google Scholar] [CrossRef]
- Climova, A.; Pivovarova, E.; Rogalewicz, B.; Raducka, A.; Szczesio, M.; Korona-Głowniak, I.; Korga-Plewko, A.; Iwan, M.; Gobis, K.; Czylkowska, A. New Coordination Compounds Based on a Pyrazine Derivative: Design, Characterization, and Biological Study. Molecules 2022, 27, 3467. [Google Scholar] [CrossRef]
- Czylkowska, A.; Rogalewicz, B.; Szczesio, M.; Raducka, A.; Gobis, K.; Szymański, P.; Czarnecka, K.; Camargo, B.C.; Szczytko, J.; Babich, A.; et al. Antitumor Activity against A549 Cancer Cells of Three Novel Complexes Supported by Coating with Silver Nanoparticles. Int. J. Mol. Sci. 2022, 23, 2980. [Google Scholar] [CrossRef]
- Sielaff, F.; Than, M.E.; Bevec, D.; Lindberg, I.; Steinmetzer, T. New furin inhibitors based on weakly basic amidinohydrazones. Bioorg. Med. Chem. Lett. 2011, 21, 836–840. [Google Scholar] [CrossRef]
- Cheng, Y.-W.; Chao, T.-L.; Li, C.-L.; Chiu, M.-F.; Kao, H.-C.; Wang, S.-H.; Pang, Y.-H.; Lin, C.-H.; Tsai, Y.-M.; Lee, W.-H.; et al. Furin Inhibitors Block SARS-CoV-2 Spike Protein Cleavage to Suppress Virus Production and Cytopathic Effects. Cell Rep. 2020, 33, 108254. [Google Scholar] [CrossRef]
- Abu-Aisheh, M.N.; Al-Aboudi, A.; Mustafa, M.S.; El-Abadelah, M.M.; Ali, S.Y.; Ul-Haq, Z.; Mubarak, M.S. Coumarin derivatives as acetyl- and butyrylcholinestrase inhibitors: An in vitro, molecular docking, and molecular dynamics simulations study. Heliyon 2019, 5, e01552. [Google Scholar] [CrossRef]
- Krátký, M.; Štěpánková, Š.; Konečná, K.; Svrčková, K.; Maixnerová, J.; Švarcová, M.; Janďourek, O.; Trejtnar, F.; Vinšová, J. Novel Aminoguanidine Hydrazone Analogues: From Potential Antimicrobial Agents to Potent Cholinesterase Inhibitors. Pharmaceuticals 2021, 14, 1229. [Google Scholar] [CrossRef]











| Comp. | IC50 AChE [µM] | IC50 BChE [µM] | Ref. |
|---|---|---|---|
| 75 | 24.25 ± 2.97 | 0.002 ± 0.0014 | [91] |
| 94 | 17.95 ± 0.90 | 17.51 ± 0.21 | [92] |
| 95 | 28.16 ± 0.98 | 1.69 ± 0.17 | [92] |
| 96 | 24.75 ± 0.17 | >500 | [92] |
| tacrine | 0.124 ± 0.02 | 7.8 ± 0.06 | [91] |
| rivastigmine | 56.10 ± 1.41 | 38.40 ± 1.97 | [92] |
| Comp. | Activity | Animal Model | Dose | Effect | Reference Drug | Ref. |
|---|---|---|---|---|---|---|
| 23 | anti-inflammatory | xylene-induced ear edema test in mice | 100 mg/kg | 92.45% edema reduction | indomethacin 89.38% reduction, ibuprofen 87.36% reduction | [25] |
| 25 | anti-inflammatory | xylene-induced ear edema test in mice | 50 mg/kg | 93.56% edema reduction | indomethacin 45.23% reduction, ibuprofen 29.56% reduction | [26] |
| 26 | 50 mg/kg | 81.65% edema reduction | ||||
| 30 | antibacterial | MRSA-infected C. elegans | 20 mg/mL | reduction in the MRSA burden by ~90% | vancomycin ~90% reduction | [30] |
| MRSA murine skin infection | 2% suspension | 73% reduction in MRSA burden | fusidic acid 78% reduction | |||
| MRSA-infected mice | 20 mg/kg | 77% reduction in MRSA burden | vancomycin 66% reduction | |||
| 31 | MRSA-infected C. elegans | 20 mg/mL | reduction in the MRSA burden by ~90% | vancomycin ~90% reduction | ||
| MRSA murine skin infection | 2% suspension | 71% reduction in MRSA burden | fusidic acid 78% reduction | |||
| 55 | anti-inflammatory | carrageenan-induced rat hind paw edema | 21 mg/kg | 65–73% edema reduction (0.5–2 h) | diclofenac 50–58% edema reduction (0.5–8 h) | [50] |
| 42 mg/kg | 38–60% edema reduction (0.5–2 h) | |||||
| antinociceptive | hot-plate test in mice | 21 mg/kg | analgesic effect (0.5–2 h) | morphine analgesic effect(0.5–1 h) | ||
| 42 mg/kg | analgesic effect (0.5–2 h) | |||||
| 60 | anti-inflammatory | carrageenan-induced rat hind paw edema | 65 mg/kg | 89.3% edema reduction | indomethacin 46% edema reduction | [55] |
| 61 | 65 mg/kg | 87.7% edema reduction | ||||
| 62 | 61 mg/kg | 80.7% edema reduction | ||||
| 63 | 61 mg/kg | 79.5% edema reduction | ||||
| 65 | anti-inflammatory neonatal sepsis treatment | LPS-induced sepsis in neonatal mice | 50 mg/kg | reduction in anxiety-like behavior and cognitive disorders in adult life | - | [58] |
| Comp. | Activity | Mechanism | Ref. |
|---|---|---|---|
| AG | anti-inflammatory | suppression of oxidative stress, inhibition of IL-1β, IL-6, and Foxp3 mRNA upregulation | [57] |
| 1 | antituberculosic | inhibiting protein synthesis via direct binding to the bacterial ribosomal subunit | [15] |
| 8 | antibacterial | inhA inhibition | [19] |
| 27 | antibacterial | inhibition of DHFR protein | [27] |
| 28 | antibacterial | interaction with E. coli FabH-CoA receptor. | [28] |
| 29 | antibacterial | interaction with β-ketoacyl-ACP synthase III (FabH) | [29] |
| 30 | antibacterial | inhibitor of undecaprenyl diphosphate phosphatase and undecaprenyl diphosphate | [30] |
| 38–39 | antifungal | interaction with DNA (intercalation) | [37] |
| 43 | antifungal | inhibition of 14-α-demethylase (CYP51) | [40] |
| 46 | antigiardial | inhibition of adherence of trophozoides | [42] |
| 47 | cytoprotective | inhibition of R15A, inhibition of dephosphorylation of enzyme eIF2α | [59] |
| 48 | cytoprotective | inhibition of R15B, inhibition of dephosphorylation of enzyme eIF2α | [66] |
| 48–50 | antiparasitic | binding trypanothione reductase enzyme | [45] |
| 53 | antiviral | inhibition of HIV-RT | [48] |
| 54 | anti-inflammatory | decreasing production of TNF-α | [49] |
| 55 | anti-inflammatory | decreasing production of IL-6 | [50] |
| 56 | anti-inflammatory | decreasing production of TNF-α | [51] |
| 57 | anti-inflammatory | G1 phase arrest | [52] |
| 58 | anti-inflammatory | decreasing production of IL-6 | [54] |
| 60–63 | anti-inflammatory | inhibition of COX-1 and COX-2 | [55] |
| 64 | antarthritic | inhibition expression of ASIC1a protein | [56] |
| 65 | anti-inflammatory | inhibition of NFκB activation | [58] |
| 66 | cytoprotective | inhibition of R15A, inhibition of dephosphorylation of enzyme eIF2α | [59] |
| 72 | antitumor | tyrosine kinase brc-abl inhibitor | [71] |
| 73 | antitumor | tyrosine kinase brc-abl inhibitor | [72] |
| 74 | antitumor | tyrosine kinase brc-abl inhibitor | [73] |
| 75 | antitumor | tyrosine kinase brc-abl inhibitor | [74] |
| 76 | antitumor | tyrosine kinase brc-abl inhibitor | [75] |
| 79 | antitumor | phosphatidylinositol 3-kinase inhibitor | [77] |
| 81 | antitumor | inhibition of tubulin polymerization, colchicine binding | [78] |
| 82 | antitumor | inhibition of ribosomal kinase RSK2 | [79] |
| 92 | enzyme inhibition | furin inhibitor, trypsin inhibitor | [89] |
| 93 | enzyme inhibition | furin inhibitor, thrombin inhibitor | [89] |
| 75 | enzyme inhibition | BChE inhibitor | [91] |
| 94 | enzyme inhibition | AChE and BChE inhibitor | [92] |
| 95–96 | enzyme inhibition | BChE inhibitor | [92] |
| Comp. | Animal Model | Time | Toxicity | Ref. |
| 18 | brine shrimp | 24 h | IC50 > 50 µg/mL | [23] |
| 19 | brine shrimp | 24 h | IC50 > 50 µg/mL | [23] |
| 20 | brine shrimp | 24 h | IC50 > 12.5 µg/mL | [23] |
| 21 | brine shrimp | 24 h | IC50 > 12.5 µg/mL | [23] |
| 43 | zebrafish embryos | 96 h | LC50 = 8.2 µg/mL | [40] |
| 55 | Swiss mice | - | LD50 = 417 mg/kg | [50] |
| 78 | brine shrimp | 24 h | IC50 > 50 µg/mL | [23] |
| Comp. | Studied cells | Origin | Toxicity | Ref. |
| 2 | Vero | monkey | IC50 = 28.7 µM | [17] |
| 3 | Vero | monkey | IC50 = 23.1 µM | [17] |
| 4 | Vero | monkey | IC50 = 27.8 µM | [17] |
| 5 | Vero | monkey | IC50 = 298 µM | [17] |
| 6 | fibroblasts | human | IC50 = 10.39 µg/mL | [18] |
| 7 | fibroblasts | human | IC50 = 3.29 µg/mL | [18] |
| 28 | HEK 293T | human | IC50 = 56.39 µmol/L | [28] |
| 32 | fibroblasts | mice | IC50 = 41.8 µg/mL | [31] |
| 43 | MRC-5 | human | IC50 = 2.5 µg/mL | [40] |
| 23 | LO2 | human | IC50 = 18.1 µg/mL | [25] |
| 30–31 | HRT-18 | human | IC50 > 32 µg/mL | [30] |
| 44 | macrophages | mice | IC50 = 79.59 µM | [41] |
| 45 | macrophages | mice | IC50 = 423.33 µM | [41] |
| 46 | RAW264.7 | mice | IC50 = 17.1 µM | [42] |
| 48–50 | J774.A1 | mice | IC50 > 10 μM | [45] |
| 51 | PBMC | human | IC50 > 100 µg/mL | [46] |
| 54 | PBMC | human | IC50 > 100 µg/mL | [49] |
| 56 | PBMC | human | IC50 > 10 µg/mL | [51] |
| 57 | PBMC | human | IC50 > 50 µg/mL | [52] |
| 58–59 | PBMC | human | IC50 > 100 µg/mL | [54] |
| 64 | chondrocytes | rat | IC50 > 25 µM | [56] |
| 72 | fibroblasts | human | IC50 > 50 µM | [71] |
| 76 | fibroblasts | human | IC50 = 15 µM | [75] |
| 83 | Vero | monkey | IC50 > 100 µM | [80] |
| 87 | Vero | monkey | IC50 > 611.09 µM | [84] |
| 88 | ARPE-19 | human | IC50 = 38.82 µM | [85] |
| 89 | ARPE-19 | human | IC50 = 41.23 µM | [85] |
| 90 | MRC-5 | human | IC50 = 58.9 µM | [86] |
| 91 | PBMC | human | IC50 > 25 µg/mL | [49] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paprocka, R.; Wiese-Szadkowska, M.; Kosmalski, T.; Frisch, D.; Ratajczak, M.; Modzelewska-Banachiewicz, B.; Studzińska, R. A Review of the Biological Activity of Amidrazone Derivatives. Pharmaceuticals 2022, 15, 1219. https://doi.org/10.3390/ph15101219
Paprocka R, Wiese-Szadkowska M, Kosmalski T, Frisch D, Ratajczak M, Modzelewska-Banachiewicz B, Studzińska R. A Review of the Biological Activity of Amidrazone Derivatives. Pharmaceuticals. 2022; 15(10):1219. https://doi.org/10.3390/ph15101219
Chicago/Turabian StylePaprocka, Renata, Małgorzata Wiese-Szadkowska, Tomasz Kosmalski, Daria Frisch, Magdalena Ratajczak, Bożena Modzelewska-Banachiewicz, and Renata Studzińska. 2022. "A Review of the Biological Activity of Amidrazone Derivatives" Pharmaceuticals 15, no. 10: 1219. https://doi.org/10.3390/ph15101219
APA StylePaprocka, R., Wiese-Szadkowska, M., Kosmalski, T., Frisch, D., Ratajczak, M., Modzelewska-Banachiewicz, B., & Studzińska, R. (2022). A Review of the Biological Activity of Amidrazone Derivatives. Pharmaceuticals, 15(10), 1219. https://doi.org/10.3390/ph15101219