Heliciopsides A−E, Unusual Macrocyclic and Phenolic Glycosides from the Leaves of Heliciopsis terminalis and Their Stimulation of Glucose Uptake
Abstract
1. Introduction
2. Results and Discussion
2.1. Structure Elucidation
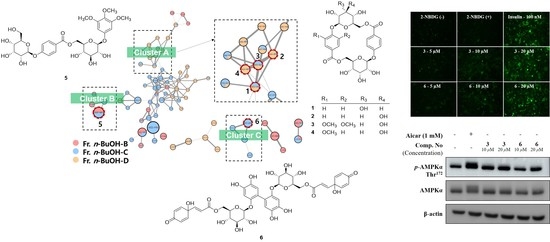
2.2. Stimulation of Glucose Uptake
2.3. Compounds 3 and 6 Increased Glucose Uptake by Activating the AMPK Signaling Pathway in Differentiated C2C12 Myoblasts
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. LC-MS/MS Analysis and Molecular Networking
3.4. Extraction and Isolation
3.5. Spectroscopic and Physical Characteristics of Compounds
3.6. Determination of the Absolute Configurations of the Sugars in Compounds 1–3, 5 and 6
3.7. Differentiation of 3T3-L1 Adipocytes
3.8. Cell Viability Assay
3.9. Measurement of Glucose Uptake Using the 2-NBDG Probe
3.10. Differentiation of C2C12 Myoblasts
3.11. Detection of p-AMPKα Thr172 by Western blotting
3.12. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, W.Y.; Dall, T.M.; Beronjia, K.; Lin, J.; Semilla, A.P.; Chakrabarti, R.; Hogan, P.F.; Assoc, A.D. Economic Costs of Diabetes in the US in 2017. Diabetes Care 2018, 41, 917–928. [Google Scholar]
- Alberti, K.G.; Zimmet, P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet. Med. 1998, 15, 539–553. [Google Scholar] [CrossRef]
- Abel, E.D.; Peroni, O.; Kim, J.K.; Kim, Y.B.; Boss, O.; Hadro, E.; Minnemann, T.; Shulman, G.I.; Kahn, B.B. Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature 2001, 409, 729–733. [Google Scholar] [CrossRef]
- Funaki, M.; Randhawa, P.; Janmey, P.A. Separation of insulin signaling into distinct GLUT4 translocation and activation steps. Mol. Cell. Biol. 2004, 24, 7567–7577. [Google Scholar] [CrossRef]
- Huang, S.; Czech, M.P. The GLUT4 glucose transporter. Cell Metab. 2007, 5, 237–252. [Google Scholar] [CrossRef]
- Leto, D.; Saltiel, A.R. Regulation of glucose transport by insulin: Traffic control of GLUT4. Nat. Rev. Mol. Cell Biol. 2012, 13, 383–396. [Google Scholar] [CrossRef]
- Krook, A.; Wallberg-Henriksson, H.; Zierath, J.R. Sending the signal: Molecular mechanisms regulating glucose uptake. Med. Sci. Sports Exerc. 2004, 36, 1212–1217. [Google Scholar] [CrossRef]
- Coughlan, K.A.; Valentine, R.J.; Ruderman, N.B.; Saha, A.K. AMPK activation: A therapeutic target for type 2 diabetes? Diabetes Metab. Syndr. Obes. 2014, 7, 241–253. [Google Scholar]
- O’Neill, H.M. AMPK and exercise: Glucose uptake and insulin sensitivity. Diabetes Metab. J. 2013, 37, 1–21. [Google Scholar] [CrossRef]
- Habegger, K.M.; Hoffman, N.J.; Ridenour, C.M.; Brozinick, J.T.; Elmendorf, J.S. AMPK enhances insulin-stimulated GLUT4 regulation via lowering membrane cholesterol. Endocrinology 2012, 153, 2130–2141. [Google Scholar] [CrossRef]
- Umezawa, S.; Higurashi, T.; Nakajima, A. AMPK: Therapeutic target for diabetes and cancer prevention. Curr. Pharm Des. 2017, 23, 3629–3644. [Google Scholar] [CrossRef]
- Lee, H.J.; Cho, H.M.; Park, E.J.; Lee, B.W.; Nghiem, D.T.; Pham, H.T.; Pan, C.H.; Oh, W.K. Triterpenoid saponins from the leaves and stems of Pericampylus glaucus and their insulin mimetic activities. Bioorg. Chem. 2021, 117, 105445. [Google Scholar] [CrossRef]
- Kim, H.W.; Park, E.J.; Cho, H.M.; An, J.P.; Chin, Y.W.; Kim, J.; Sung, S.H.; Oh, W.K. Glucose uptake-stimulating galloyl ester triterpenoids from Castanopsis sieboldii. J. Nat. Prod. 2020, 83, 3093–3101. [Google Scholar] [CrossRef]
- An, J.P.; Park, E.J.; Ryu, B.; Lee, B.W.; Cho, H.M.; Doan, T.P.; Pham, H.T.T.; Oh, W.K. Oleanane triterpenoids from the leaves of Gymnema inodorum and their insulin mimetic activities. J. Nat. Prod. 2020, 83, 1265–1274. [Google Scholar] [CrossRef]
- Lee, B.W.; Ha, T.K.Q.; Pham, H.T.T.; Hoang, Q.H.; Tran, V.O.; Oh, W.K. Hydroxyoleoside-type seco-iridoids from Symplocos cochinchinensis and their insulin mimetic activity. Sci. Rep. 2019, 9, 2270. [Google Scholar] [CrossRef]
- Pham, H.T.T.; Ha, T.K.Q.; Cho, H.M.; Lee, B.W.; An, J.P.; Tran, V.O.; Oh, W.K. Insulin mimetic activity of 3,4-seco and hexanordammarane triterpenoids isolated from Gynostemma longipes. J. Nat. Prod. 2018, 81, 2470–2482. [Google Scholar] [CrossRef]
- Kubitzki, K.; Bayer, C.; Stevens, P.F. Flowering Plants: Eudicots; Berberidopsidales, Buxales, Crossosomatales, Fabales p.p., Geraniales, Gunnerales, Myrtales p.p., Proteales, Saxifragales, Vitales, Zygophyllales, Clusiaceae Alliance, Passifloraceae Alliance, Dilleniaceae, Huaceae, Picramniaceae, Sabiaceae; Springer: Berlin, Germany; New York, NY, USA, 2007. [Google Scholar]
- Giang, P.M.; Thao, D.T.; Nga, N.T.; Trung, B.V.; Anh, D.H.; Viet, P.H. Evaluation of the antioxidant, hepatoprotective, and anti-Inflammatory activities of bisresorcinol isolated from the trunk of Heliciopsis terminalis. Pharm. Chem. J. 2019, 53, 628–634. [Google Scholar] [CrossRef]
- Prommee, N.; Itharat, A.; Panthong, S.; Makchuchit, S.; Ooraikul, B. Ethnopharmacological analysis from Thai traditional medicine called prasachandaeng remedy as a potential antipyretic drug. J. Ethnopharmacol. 2021, 268, 113520. [Google Scholar] [CrossRef]
- Saechan, C.; Nguyen, U.H.; Wang, Z.; Sugimoto, S.; Yamano, Y.; Matsunami, K.; Otsuka, H.; Phan, G.M.; Pham, V.H.; Tipmanee, V.; et al. Potency of bisresorcinol from Heliciopsis terminalis on skin aging: In vitro bioactivities and molecular interactions. PeerJ 2021, 9, e11618. [Google Scholar] [CrossRef]
- Nguyen, P.H.; Choi, H.S.; Ha, T.K.Q.; Seo, J.Y.; Yang, J.L.; Jung, D.W.; Williams, D.R.; Oh, W.K. Anthraquinones from Morinda longissima and their insulin mimetic activities via AMP-activated protein kinase (AMPK) activation. Bioorg. Med. Chem. Lett. 2017, 27, 40–44. [Google Scholar] [CrossRef]
- Qiu, L.; Yuan, H.M.; Liang, J.M.; Cheng, X.L.; Wang, P.; Du, Y.F.; Fu, Q. Clemochinenosides C and D, two new macrocyclic glucosides from Clematis chinensis, J. Asian Nat. Prod. Res. 2018, 20(11), 1038–1044. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.S.; Nakamura, N.; Meselhy, M.R.; Makhboul, M.A.; El-Emary, N.; Hattori, M. Phenolic constituents from Grevillea robusta. Phytochemistry 2000, 53, 149–154. [Google Scholar] [CrossRef]
- Achenbach, H.; Benirschke, G. Joannesialactone and other compounds from Joannesia princeps. Phytochemistry 1997, 45, 149–157. [Google Scholar] [CrossRef]
- Tsao-Jun Su, H.-S.C.; Peng, C.-F.; Lee, S.-J.; Chen, I.-S. Antitubercular resorcinols and cytotoxic alkyl benzoquinones from Ardisia kusukuensis. Taiwan Pharm. J. 2009, 61, 89–105. [Google Scholar]
- Shi, S.P.; Dong, C.X.; Jiang, D.; Tu, P.F. Macrocyclic glycosides from Clematis hexapetala. Helv. Chim. Acta 2006, 89, 3002–3006. [Google Scholar] [CrossRef]
- Wang, X.Y.; Xu, M.; Yang, C.R.; Zhang, Y.J. Phenylpropanoid glycosides from the seeds of Michelia hedyosperma. Food Chem. 2011, 126, 1039–1043. [Google Scholar] [CrossRef]
- Yan, L.H.; Yang, S.L.; Zou, Z.M.; Luo, X.Z.; Xu, L.Z. Two new macrocyclic compounds from the stems of Clematis armandii. Heterocycles 2006, 68, 1917–1924. [Google Scholar] [CrossRef]
- Tanaka, T.; Nakashima, T.; Ueda, T.; Tomii, K.; Kouno, I. Facile discrimination of aldose enantiomers by reversed-phase HPLC. Chem. Pharm. Bull. 2007, 55, 899–901. [Google Scholar] [CrossRef]
- Peng, D.; Lin, X.L.; Jiang, L.; Huang, J.; Zeng, G.Y.; Deng, X.; Zhou, Y.J. Five macrocyclic glycosides from Schoenoplectus tabernaemontani. Nat. Prod. Res. 2019, 33, 427–434. [Google Scholar] [CrossRef]
- Richter, E.A.; Ruderman, N.B. AMPK and the biochemistry of exercise: Implications for human health and disease. Biochem. J. 2009, 418, 261–275. [Google Scholar] [CrossRef]
- Joshi, T.; Singh, A.K.; Haratipour, P.; Sah, A.N.; Pandey, A.K.; Naseri, R.; Juyal, V.; Farzaei, M.H. Targeting AMPK signaling pathway by natural products for treatment of diabetes mellitus and its complications. J. Cell. Physiol. 2019, 234, 17212–17231. [Google Scholar] [CrossRef]
- Uddin, M.N.; Choi, H.S.; Lim, S.-I.; Oh, W.K. AMPK activators from natural products: A patent review. Nat. Prod. Sci. 2013, 19, 1–17. [Google Scholar]
- Yin, J.; Gao, Z.G.; Liu, D.; Liu, Z.J.; Ye, J.P. Berberine improves glucose metabolism through induction of glycolysis. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E148–E156. [Google Scholar] [CrossRef]
- Nguyen, P.H.; Le, T.V.T.; Kang, H.W.; Chae, J.; Kim, S.K.; Kwon, K.I.; Seo, D.B.; Lee, S.J.; Oh, W.K. AMP-activated protein kinase (AMPK) activators from Myristica fragrans (nutmeg) and their anti-obesity effect. Bioorg. Med. Chem. Lett. 2010, 20, 4128–4131. [Google Scholar] [CrossRef]
- Wang, J.; Ha, T.K.Q.; Shi, Y.P.; Oh, W.K.; Yang, J.L. Hypoglycemic triterpenes from Gynostemma pentaphyllum. Phytochemistry 2018, 155, 171–181. [Google Scholar] [CrossRef]
- Pu, P.; Wang, X.A.; Salim, M.; Zhu, L.H.; Wang, L.; Chen, K.J.; Xiao, J.F.; Deng, W.; Shi, H.W.; Jiang, H.; et al. Baicalein, a natural product, selectively activating AMPKα(2) and ameliorates metabolic disorder in diet-induced mice. Mol. Cell. Endocrinol. 2012, 362, 128–138. [Google Scholar] [CrossRef]
- Deans, B.J.; Kilah, N.L.; Jordan, G.J.; Bissember, A.C.; Smith, J.A. Arbutin derivatives isolated from ancient Proteaceae: Potential phytochemical markers present in Bellendena, Cenarrhenes, and Persoonia Genera. J. Nat. Prod. 2018, 81, 1241–1251. [Google Scholar] [CrossRef]
- Qi, W.Y.; Ou, N.; Wu, X.D.; Xu, H.M. New arbutin derivatives from the leaves of Heliciopsis lobata with cytotoxicity. Chin. J. Nat. Med. 2016, 14, 789–793. [Google Scholar] [CrossRef]
- Yamashita-Higuchi, Y.; Sugimoto, S.; Matsunami, K.; Otsuka, H.; Nakai, T. Grevillosides J−Q, arbutin derivatives from the leaves of Grevillea robusta and their melanogenesis inhibitory activity. Chem. Pharm. Bull. 2014, 62, 364–372. [Google Scholar] [CrossRef]
- Mukhtar, N.; Malik, A.; Riaz, N.; Iqbal, K.; Tareen, R.B.; Khan, S.N.; Nawaz, S.A.; Siddiqui, J. Pakistolides A and B, novel rnzyme inhibitory and antioxidant dimeric 4-(glucosyloxy)benzoates from Berchemia pakistanica. Helv. Chim. Acta 2004, 87, 416. [Google Scholar] [CrossRef]
- Song, C.-Q.; Xu, R.-S. Clemochinenoside A, a macrocyclic compound from Clamatis chinensis. Chin. Chem. Lett. 1992, 3, 119. [Google Scholar]
- Sakurai, N.; Kobayashi, M.; Shigihara, A.; Inoue, T. Berchemolide, a novel dimeric vanillic acid glucoside from Berchemia racemose. Chem. Pharm. Bull. 1992, 40, 851–853. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, J.; Gong, Y.; Yu, B. Synthesis of oligomeric 4-(glycosyloxy)benzoate macrocyclic glycosides. J. Org. Chem. 2011, 76, 3654–3663. [Google Scholar] [CrossRef] [PubMed]





| No. | 1 a | 2 a | 3 a | 5 b | ||||
|---|---|---|---|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | |
| 1 | 122.4 | - | 122.6 | - | 124.6 | - | 153.6 | - |
| 2 | 131.3 | 8.02, d (8.8) | 131.2 | 7.99, d (8.8) | 107.0 | 7.09, d (1.6) | 94.3 | 6.32, s |
| 3 | 115.8 | 7.19, d (8.8) | 115.8 | 7.19, d (8.8) | 153.1 | - | 153.1 | - |
| 4 | 160.6 | - | 160.4 | - | 137.7 | - | 132.6 | - |
| 5 | 115.8 | 7.19, d (8.8) | 115.8 | 7.19, d (8.8) | 152.0 | - | 153.1 | - |
| 6 | 131.3 | 8.02, d (8.8) | 131.2 | 7.99, d (8.8) | 106.7 | 7.52, d (1.6) | 94.3 | 6.32, s |
| 7 | 165.1 | - | 165.0 | - | 164.9 | - | - | |
| d-allo | d-glc | d-glc | d-glc | |||||
| 1′ | 96.8 | 5.39, d (8.0) | 98.4 | 5.22, d (7.2) | 100.9 | 5.36, d (7.2) | 100.2 | 4.98, d (8.0) |
| 2′ | 69.9 | 3.56, dd (8.0, 2.4) | 72.9 | 3.34, m | 73.9 | 3.30, overlap | 73.1 | 3.26, overlap |
| 3′ | 71.5 | 3.99, dd (2.4, 2.4) | 76.7 | 3.38, m | 76.7 | 3.30, overlap | 76.3 | 3.32, overlap |
| 4′ | 68.3 | 3.45, dd (9.6, 2.4) | 70.7 | 3.17, m | 71.1 | 3.12, dd (9.6, 8.8) | 70.0 | 3.26, overlap |
| 5′ | 71.2 | 4.29, ddd (10.4, 9.6, 2.4) | 73.5 | 3.97, m | 73.8 | 3.49, dd (10.4, 2.4) | 73.7 | 3.80, m |
| 6a′ 6b′ | 65.4 | 4.37, dd (11.2, 1.6) 4.11, dd (11.2, 10.4) | 65.0 | 4.40, dd (11.2, 2.4) 4.07, br d (11.2) | 64.9 | 4.40, br d (11.2) 3.89, dd (11.2, 10.4) | 64.2 | 4.58, br d (11.5) 4.23, dd (11.5, 7.0) |
| 1″ | - | - | 122.5 | - | 122.5 | - | 122.9 | - |
| 2″ | - | - | 131.2 | 7.98, d (8.8) | 130.7 | 7.45, d (8.8) | 131.1 | 7.86, d (8.5) |
| 3″ | - | - | 115.8 | 7.18, d (8.8) | 115.9 | 6.98, d (8.8) | 155.9 | 7.09, d (8.5) |
| 4″ | - | - | 160.7 | - | 160.7 | - | 161.4 | - |
| 5″ | - | - | 115.8 | 7.18, d (8.8) | 115.9 | 6.98, d (8.8) | 155.9 | 7.09, d (8.5) |
| 6″ | - | - | 131.2 | 7.98, d (8.8) | 130.7 | 7.45, d (8.8) | 131.1 | 7.86, d (8.5) |
| 7″ | - | - | 165.1 | - | 164.87 | - | 165.3 | - |
| d-allo | d-allo | d-allo | ||||||
| 1‴ | - | - | 96.8 | 5.37, d (8.0) | 97.6 | 5.17, d (8.0) | 98.1 | 5.23, d (7.5) |
| 2‴ | - | - | 69.9 | 3.54, m | 70.0 | 3.53, dd (8.0, 2.4) | 70.2 | 3.46, overlap |
| 3‴ | - | - | 71.5 | 3.97, m | 71.5 | 3.99, m | 71.5 | 3.94, m |
| 4‴ | - | - | 68.2 | 3.43, m | 68.3 | 3.45, m | 66.9 | 3.46, overlap |
| 5‴ | - | - | 71.2 | 4.26, ddd (10.4, 10.4, 1.6) | 71.6 | 4.28, ddd (10.4, 10.4, 1.6) | 74.7 | 3.73, m |
| 6a‴ 6b‴ | - | - | 65.4 | 4.36, dd (11.2, 1.6) 4.09, br d (11.2) | 64.8 | 4.50, dd (11.2, 11.2) 4.40, br d (11.2) | 60.8 | 3.68, m 3.46, overlap |
| 3-OMe | - | - | - | - | 56.0 | 3.61, s | 55.8 | 3.65, s |
| 4-OMe | - | - | - | - | - | - | 60.2 | 3.57, s |
| 5-OMe | - | - | - | - | 56.5 | 3.98, s | 55.8 | 3.65, s |
| No. | δC | δH (J in Hz) | No. | δC | δH (J in Hz) | No. | δC | δH (J in Hz) |
|---|---|---|---|---|---|---|---|---|
| 1 | 148.8 | 1′ | 103.3 | 4.63, d (7.7) | 1″ | 70.4 | - | |
| 2 | 106.7 | 6.66, s | 2′ | 74.8 | 3.27, m | 2″,6″ | 151.2 | 6.89, m |
| 3 | 141.5 | 3′ | 77.7 | 3.33, overlap | 3″,5″ | 128.7 | 6.22, m | |
| 4 | 145.9 | 4′ | 71.4 | 3.33, overlap | 4″ | 187.2 | - | |
| 5 | 119.1 | 6.62, s | 5′ | 75.3 | 3.49, m | 7″ | 148.1 | 6.71, d (16.2) |
| 6 | 121.4 | 6′a 6′b | 64.8 | 4.44, dd (11.2, 2.4) 4.29, dd (11.2, 6.4) | 8″ | 122.9 | 6.30, d (16.2) | |
| 9″ | 167.4 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryu, B.; Park, E.-J.; Doan, T.-P.; Cho, H.-M.; An, J.-P.; Pham, T.-L.-G.; Pham, H.-T.-T.; Oh, W.-K. Heliciopsides A−E, Unusual Macrocyclic and Phenolic Glycosides from the Leaves of Heliciopsis terminalis and Their Stimulation of Glucose Uptake. Pharmaceuticals 2022, 15, 1315. https://doi.org/10.3390/ph15111315
Ryu B, Park E-J, Doan T-P, Cho H-M, An J-P, Pham T-L-G, Pham H-T-T, Oh W-K. Heliciopsides A−E, Unusual Macrocyclic and Phenolic Glycosides from the Leaves of Heliciopsis terminalis and Their Stimulation of Glucose Uptake. Pharmaceuticals. 2022; 15(11):1315. https://doi.org/10.3390/ph15111315
Chicago/Turabian StyleRyu, Byeol, Eun-Jin Park, Thi-Phuong Doan, Hyo-Moon Cho, Jin-Pyo An, Thi-Linh-Giang Pham, Ha-Thanh-Tung Pham, and Won-Keun Oh. 2022. "Heliciopsides A−E, Unusual Macrocyclic and Phenolic Glycosides from the Leaves of Heliciopsis terminalis and Their Stimulation of Glucose Uptake" Pharmaceuticals 15, no. 11: 1315. https://doi.org/10.3390/ph15111315
APA StyleRyu, B., Park, E.-J., Doan, T.-P., Cho, H.-M., An, J.-P., Pham, T.-L.-G., Pham, H.-T.-T., & Oh, W.-K. (2022). Heliciopsides A−E, Unusual Macrocyclic and Phenolic Glycosides from the Leaves of Heliciopsis terminalis and Their Stimulation of Glucose Uptake. Pharmaceuticals, 15(11), 1315. https://doi.org/10.3390/ph15111315