Natural Compounds of Lasia spinosa (L.) Stem Potentiate Antidiabetic Actions by Regulating Diabetes and Diabetes-Related Biochemical and Cellular Indexes †
Abstract
:1. Introduction
2. Results
2.1. Phytochemical and Antioxidative Status
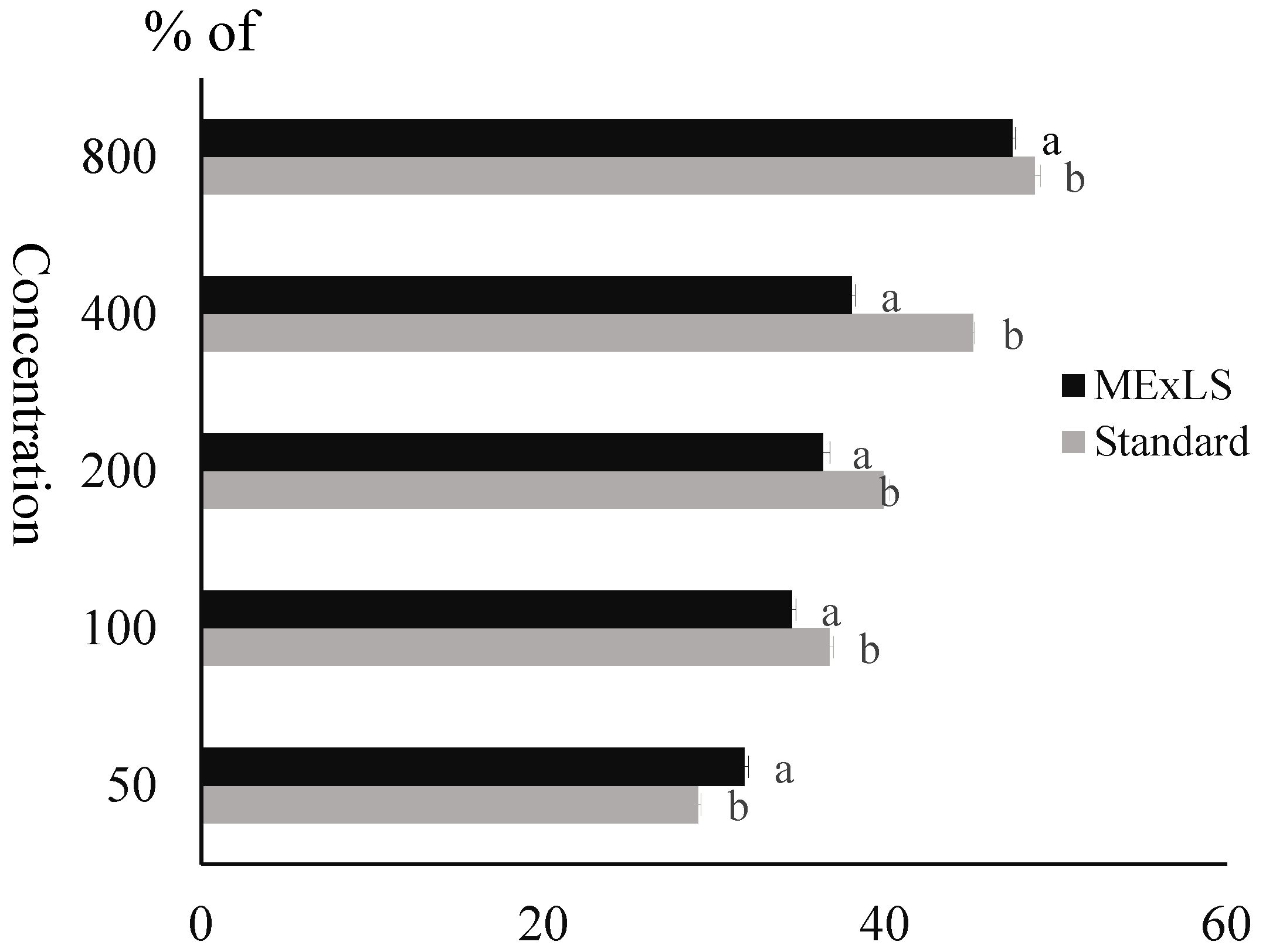
2.2. Impact of MEXLS on α-Amylase Inhibition
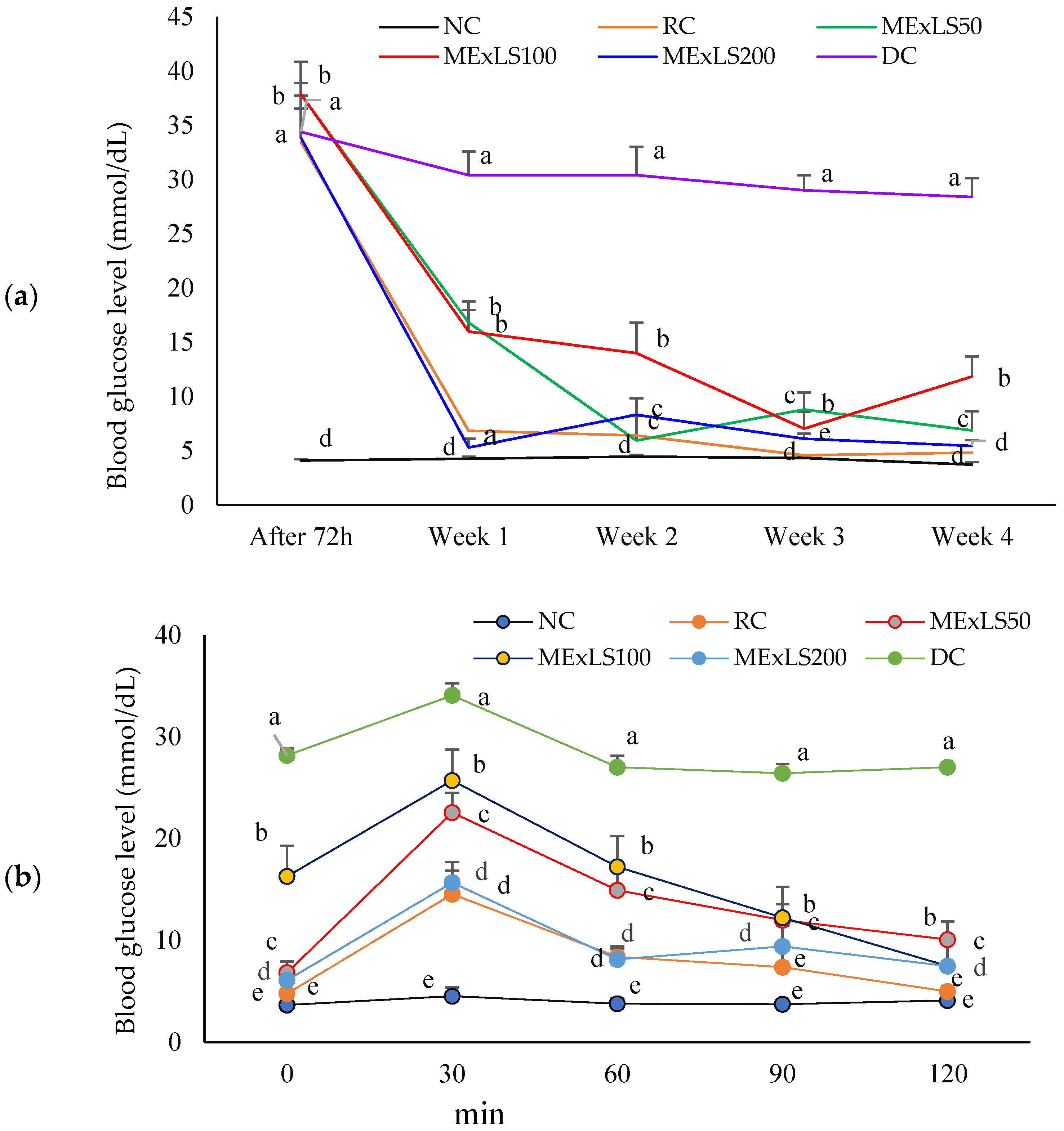
2.3. Effect of MEXLS on the In Vivo Assays
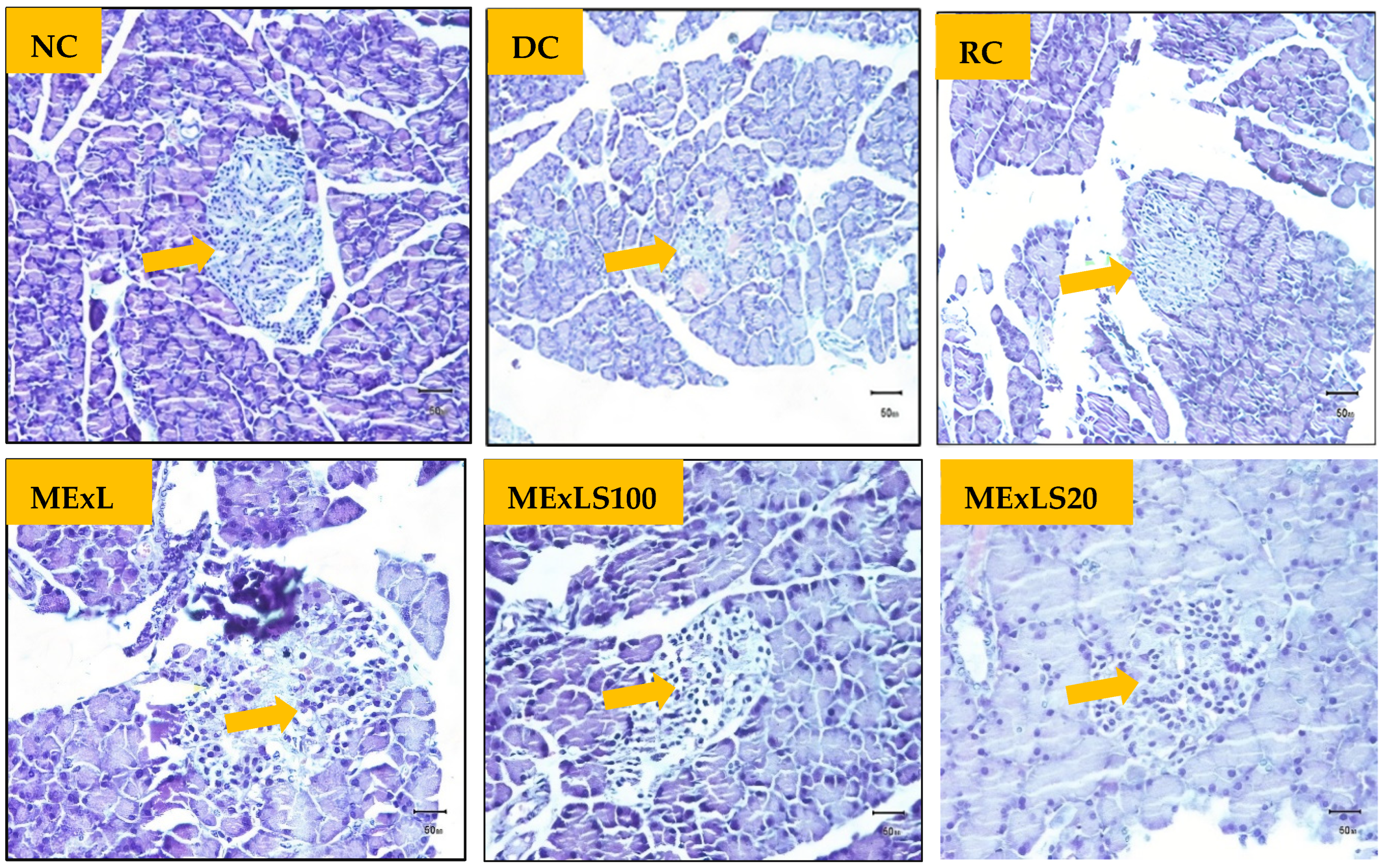
2.4. Effect of MEXLS on the Tissue’S Architectures
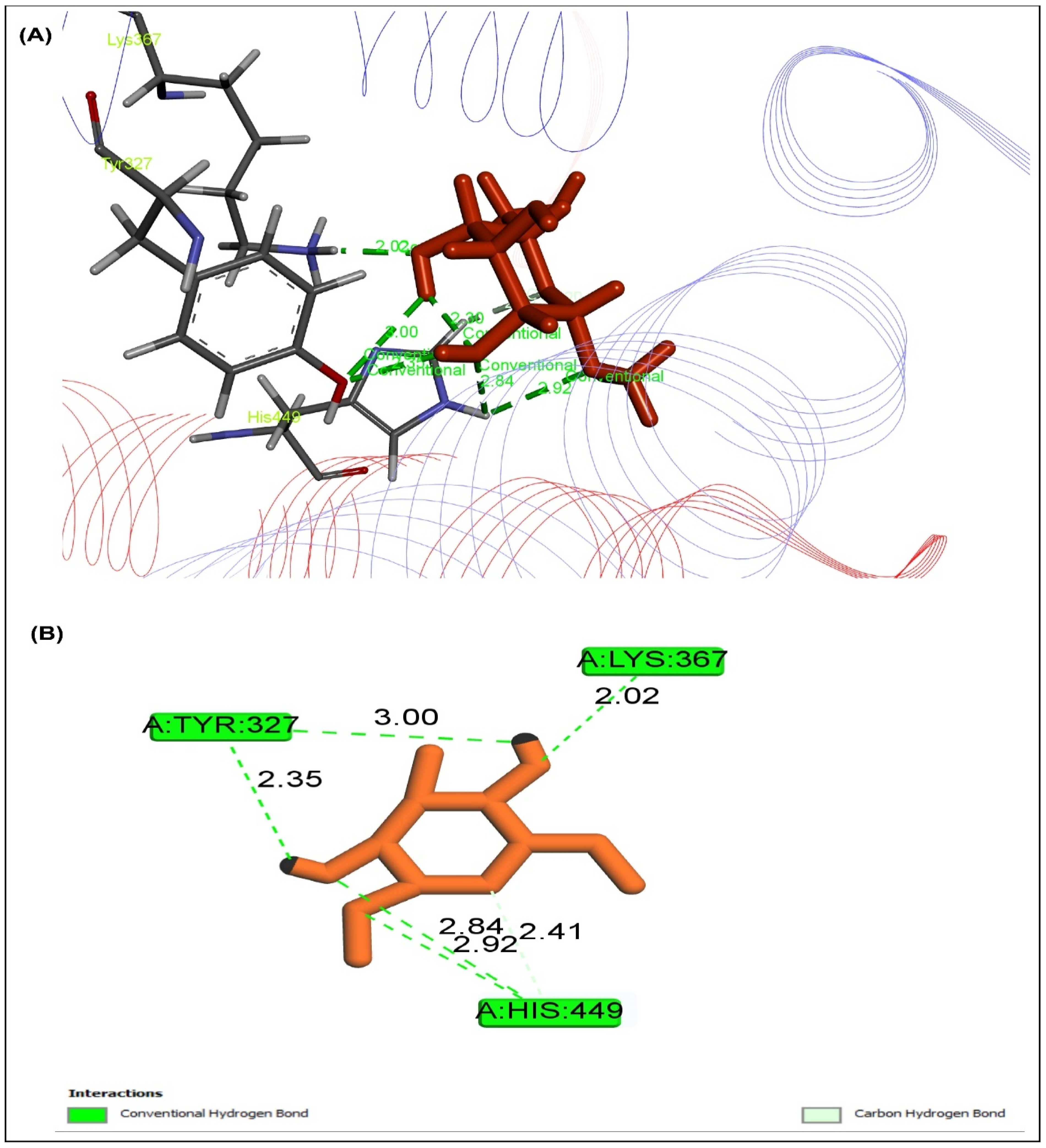
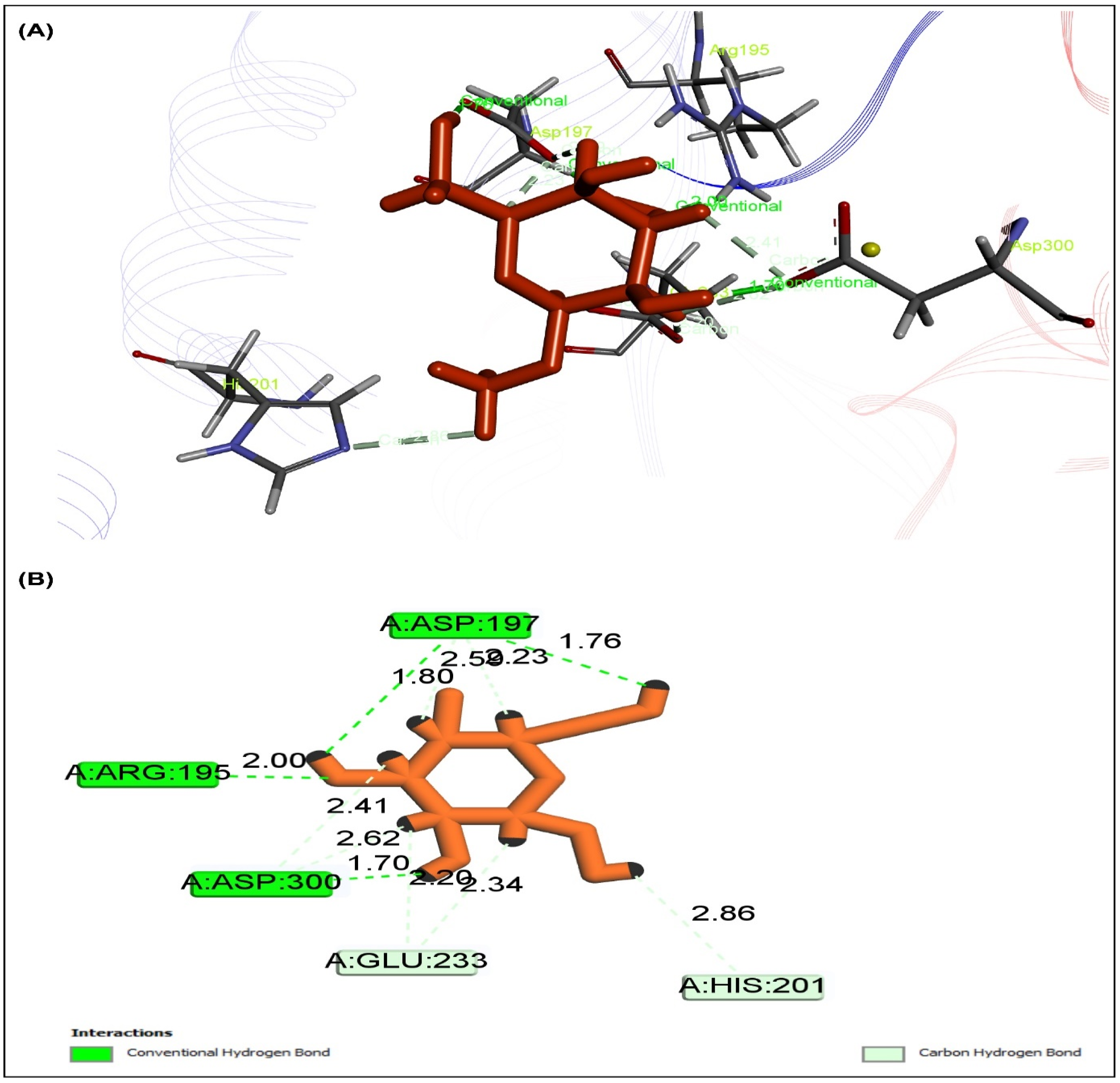
2.5. Effect of MEXLS on the Compounds-Proteins Interactions
2.6. Effect ot MEXLS on the Pharmacokinetic Properties
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Collection and Extraction of Plant Material
4.3. Assay for Phytochemical Groups Status
4.4. Gas Chromatography-Mass Spectroscopy (GC-MS) Analysis
4.5. Determination of the Antioxidative Effects of MEXLS
4.5.1. Evaluation of Total Phenolic Content (TPC), Total Flavonoid (TF), Total Antioxidative Capacity (TAC) and Total Proanthocyanidin Content (TPACC)
4.5.2. Assay of DPPH Free Radical Scavenging Effect of MEXLS
4.5.3. Assay of Iron Chelating Activity of MEXLS
4.5.4. Assay of Nitric Oxide Scavenging Activity of MEXLS
4.5.5. Assay of Hydroxyl Radical Scavenging Activity of MEXLS
4.5.6. Assay of Membrane Stabilization, Lipid Peroxidation, and Protein Denaturation Inhibition
4.5.7. Assay of α-Amylase Inhibitory Effect of MEXLS
4.6. Animal Model Experiments
4.6.1. Assay for Acute Toxicity
4.6.2. Induction of Diabetes in Animal Model
4.6.3. Animal Grouping, Intervention, and Oral Glucose Tolerance
4.6.4. Sacrifice of Animals, Collection of Blood, Tissues, and Organs for Analyses
4.6.5. Assay for Tissue Architecture
4.7. Statistical Analysis
4.8. Computational Studies
4.8.1. Molecular Docking Analysis
4.8.2. Pharmacokinetic Properties Evaluation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Petersmann, A.; Müller-Wieland, D.; Müller, U.A.; Landgraf, R.; Nauck, M.; Freckmann, G.; Heinemann, L.; Schleicher, E. Definition, classification and diagnosis of diabetes mellitus. Exp. Clin. Endocrinol. Diabetes 2019, 127, S1–S7. [Google Scholar] [CrossRef]
- Saeed, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef]
- Fowler, M.J. Microvascular and macrovascular complications of diabetes. Clin. Diabetes 2011, 29, 116–122. [Google Scholar] [CrossRef]
- Asmat, U.; Abad, K.; Ismail, K. Diabetes mellitus and oxidative stress—A concise review. Saudi Pharm. J. 2016, 24, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Prakash, O.; Kumar, R.; Srivastava, R.; Tripathi, P.; Mishra, S.; Ajeet, A. Plants explored with anti-diabetic properties: A review. Am. J. Pharmacol. Sci. 2015, 3, 55–66. [Google Scholar] [CrossRef]
- Lee, J.; Noh, S.; Lim, S.; Kim, B. Plant extracts for type 2 diabetes: From traditional medicine to modern drug discovery. Antioxidants 2021, 10, 81. [Google Scholar] [CrossRef]
- Deb, D.; Dev, S.; Das, A.K.; Khanam, D.; Banu, H.; Shahriar, M.; Ashraf, A.; Choudhuri, M.; Basher, S. Antinociceptive, anti-inflammatory and anti-diarrheal activities of the hydroalcoholic extract of Lasia spinosa Linn.(Araceae) roots. Lat. Am. J. Pharm. 2010, 29, 1269–1276. [Google Scholar]
- Yusuf, M.; Begum, J.; Hoque, M.; Chowdhury, J.U. Medicinal Plants of Bangladesh-revised and enlarged, Bangladesh Council of Scientific and Industrial Research. Dhaka Bangladesh 2009, 12, 794. [Google Scholar]
- Ngomdir, M.; Debbarma, B.; Debbarma, A.; Chanda, S.; Raha, S.; Saha, R.; Pal, S.; De, B. Antibacterial evaluation of the extracts of edible parts of few plants used by tribal people of Tripura, India. J. Pure Appl. Microbiol. 2007, 1, 65–68. [Google Scholar]
- Alam, F.; Haque, M.; Sohrab, H.; Monsur, M.A.; Hasan, C.M.; Ahmed, N. Antimicrobial and cytotoxic activity from Lasia spinosa and isolated lignan. Lat. Am. J. Pharm. 2011, 30, 550–553. [Google Scholar]
- Hossain, S.; Khatun, A.; Miajee, U. Medicinal plants used by folk medicinal practitioners in three villages of Natore and Rajshahi districts, Bangladesh. Am.-Eurasian J. Sustain. Agric. 2010, 4, 211–218. [Google Scholar]
- Hasan, M.N.; Munshi, M.; Rahman, M.H.; Alam, S.; Hirashima, A. Evaluation of antihyperglycemic activity of Lasia spinosa leaf extracts in Swiss albino mice. World J. Pharm. Pharm. Sci. 2014, 3, 118–124. [Google Scholar]
- Rahman, A.; Siddiqui, S.A.; Oke-Altuntas, F.; Okay, S.; GÜL, F.; Demirtas, I. Phenolic profile, essential oil composition and bioactivity of Lasia spinosa (L.) thwaites. Braz. Arch. Biol. Technol. 2019, 62, e19170757. [Google Scholar] [CrossRef]
- Muthukrishnan, S.; Sivakkumar, T. Physicochemical Evaluation, Preliminary Phytochemical Investigation, Fluorescence and TLC Analysis of Leaves of Schleichera oleosa (Lour.) Oken. Indian J. Pharm. Sci. 2018, 80, 525–532. [Google Scholar] [CrossRef]
- Islam, M.S.; Rashid, M.M.; Ahmed, A.A.; Reza, A.A.; Rahman, M.A.; Choudhury, T.R. The food ingredients of different extracts of Lasia spinosa (L.) Thwaites can turn it into a potential medicinal food. NFS J. 2021, 25, 56–69. [Google Scholar] [CrossRef]
- Rashid, M.M.; Rahman, M.A.; Islam, M.S.; Hossen, M.A.; Reza, A.A.; Ahmed, A.A.; Alnajeebi, A.M.; Babteen, N.A.; Khan, M.; Aboelenin, S.M. Incredible affinity of Kattosh with PPAR-γ receptors attenuates STZ-induced pancreas and kidney lesions evidenced in chemicobiological interactions. J. Cell Mol. Med. 2022, 26, 3343–3363. [Google Scholar] [CrossRef]
- Kooti, W.; Farokhipour, M.; Asadzadeh, Z.; Ashtary-Larky, D.; Asadi-Samani, M. The role of medicinal plants in the treatment of diabetes: A systematic review. Electron. Physician 2016, 8, 1832. [Google Scholar] [CrossRef]
- Huang, W.-Y.; Cai, Y.-Z.; Zhang, Y. Natural phenolic compounds from medicinal herbs and dietary plants: Potential use for cancer prevention. Nutr. Cancer 2009, 62, 1–20. [Google Scholar] [CrossRef]
- Vosough-Ghanbari, S.; Rahimi, R.; Kharabaf, S.; Zeinali, S.; Mohammadirad, A.; Amini, S.; Yasa, N.; Salehnia, A.; Toliat, T.; Nikfar, S. Effects of Satureja khuzestanica on serum glucose, lipids and markers of oxidative stress in patients with type 2 diabetes mellitus: A double-blind randomized controlled trial. Evid.-Based Complement. Altern. Med. 2010, 7, 465–470. [Google Scholar] [CrossRef]
- Duan, X.-J.; Zhang, W.-W.; Li, X.-M.; Wang, B.-G. Evaluation of antioxidant property of extract and fractions obtained from a red alga, Polysiphonia urceolata. Food Chem. 2006, 95, 37–43. [Google Scholar] [CrossRef]
- Grotto, D.; Maria, L.S.; Valentini, J.; Paniz, C.; Schmitt, G.; Garcia, S.C.; Pomblum, V.J.; Rocha, J.B.T.; Farina, M. Importance of the lipid peroxidation biomarkers and methodological aspects for malondialdehyde quantification. Quim. Nova 2009, 32, 169–174. [Google Scholar] [CrossRef]
- Davì, G.; Falco, A.; Patrono, C. Lipid peroxidation in diabetes mellitus. Antioxid. Redox Signal. 2005, 7, 256–268. [Google Scholar] [CrossRef]
- Omale, J.; Okafor, P.N. Comparative antioxidant capacity, membrane stabilization, polyphenol composition and cytotoxicity of the leaf and stem of Cissus multistriata. Afr. J. Biotechnol. 2008, 7, 3129–3133. [Google Scholar] [CrossRef]
- Mizushima, Y. Screening test for antirheumatic drugs. Lancet 1966, 288, 443. [Google Scholar] [CrossRef]
- Pollack, R.M.; Donath, M.Y.; LeRoith, D.; Leibowitz, G. Anti-inflammatory agents in the treatment of diabetes and its vascular complications. Diabetes Care 2016, 39, S244–S252. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo-González, M.; Grosso, C.; Valentão, P.; Andrade, P.B. α-Glucosidase and α-amylase inhibitors from Myrcia spp.: A stronger alternative to acarbose? J. Pharm. Biomed. Anal. 2016, 118, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Joshi, T.; Joshi, T.; Chandra, S.; Tamta, S. Molecular dynamics simulation for screening phytochemicals as α-amylase inhibitors from medicinal plants. J. Biomol. Struct. Dyn. 2021, 39, 6524–6538. [Google Scholar] [CrossRef]
- Saha, S.; Verma, R. Inhibitory potential of traditional herbs on α-amylase activity. Pharm. Biol. 2012, 50, 326–331. [Google Scholar] [CrossRef]
- Prashanth, D.; Padmaja, R.; Samiulla, D. Effect of certain plant extracts on α-amylase activity. Fitoterapia 2001, 72, 179–181. [Google Scholar] [CrossRef]
- Ibrahim, H.; Osilesi, O.; Adebawo, O.; Onajobi, F.; Karigidi, K.; Muhammad, L. Antidiabetic and haematological effects of Chrysophyllum albidum supplemented diet on streptozotocin induced diabetic rats. J. Appl. Life Sci. Int. 2019, 20, 1–17. [Google Scholar] [CrossRef]
- Reaven, G.M.; Brand, R.J.; Ida Chen, Y.-D.; Mathur, A.K.; Goldfine, I. Insulin resistance and insulin secretion are determinants of oral glucose tolerance in normal individuals. Diabetes 1993, 42, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Haffner, D.; Grund, A.; Leifheit-Nestler, M. Renal effects of growth hormone in health and in kidney disease. Pediatr. Nephrol. 2021, 36, 2511–2530. [Google Scholar] [CrossRef] [PubMed]
- Yagihashi, S. Diabetes and pancreas size, does it matter? J. Diabetes Investig. 2017, 8, 413–415. [Google Scholar] [CrossRef]
- Merzouk, H.; Madani, S.; Chabane Sari, D.; Prost, J.; Bouchenak, M.; Belleville, J. Time course of changes in serum glucose, insulin, lipids and tissue lipase activities in macrosomic offspring of rats with streptozotocin-induced diabetes. Clin. Sci. 2000, 98, 21–30. [Google Scholar] [CrossRef]
- Bhanudas, K.S.; Gopal, P.K. Estimation of liver glycogen in normal control, diabetic control and Tinospora cordifolia extract treated albino rats. Glob. J. Sci. Front. Res. C Biol. Sci. 2016, 16, 17–20. [Google Scholar]
- Ohaeri, O. Effect of garlic oil on the levels of various enzymes in the serum and tissue of streptozotocin diabetic rats. Biosci. Rep. 2001, 21, 19–24. [Google Scholar] [CrossRef]
- Gulsin, G.S.; Athithan, L.; McCann, G.P. Diabetic cardiomyopathy: Prevalence, determinants and potential treatments. Ther. Adv. Endocrinol. Metab. 2019, 10, 2042018819834869. [Google Scholar] [CrossRef]
- Huang, E.-J.; Kuo, W.-W.; Chen, Y.-J.; Chen, T.-H.; Chang, M.-H.; Lu, M.-C.; Tzang, B.-S.; Hsu, H.-H.; Huang, C.-Y.; Lee, S.-D. Homocysteine and other biochemical parameters in type 2 diabetes mellitus with different diabetic duration or diabetic retinopathy. Clin. Chim. Acta 2006, 366, 293–298. [Google Scholar] [CrossRef]
- Mironova, M.A.; Klein, R.L.; Virella, G.T.; Lopes-Virella, M.F. Anti-modified LDL antibodies, LDL-containing immune complexes, and susceptibility of LDL to in vitro oxidation in patients with type 2 diabetes. Diabetes 2000, 49, 1033–1041. [Google Scholar] [CrossRef]
- Goldberg, I.J. Diabetic dyslipidemia: Causes and consequences. J. Clin. Endocrinol. Metab. 2001, 86, 965–971. [Google Scholar] [CrossRef]
- Baxmann, A.C.; Ahmed, M.S.; Marques, N.C.; Menon, V.B.; Pereira, A.B.; Kirsztajn, G.M.; Heilberg, I.P. Influence of muscle mass and physical activity on serum and urinary creatinine and serum cystatin C. Clin. J. Am. Soc. Nephrol. 2008, 3, 348–354. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, V.; Prakash, O. Antidiabetic, hypolipidemic and histopathological analysis of Dillenia indica (L.) leaves extract on alloxan induced diabetic rats. Asian Pac. J. Trop. Med. 2011, 4, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Attique, S.A.; Hassan, M.; Usman, M.; Atif, R.M.; Mahboob, S.; Al-Ghanim, K.A.; Bilal, M.; Nawaz, M.Z. A Molecular Docking Approach to Evaluate the Pharmacological Properties of Natural and Synthetic Treatment Candidates for Use against Hypertension. Int. J. Environ. Res. Public Health 2019, 16, 923. [Google Scholar] [CrossRef] [PubMed]
- Mani, R.; Natesan, V. Chrysin: Sources, beneficial pharmacological activities, and molecular mechanism of action. Phytochemistry 2018, 145, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, M.; Eroglu, Z.; Solmaz, S.; Ganidagli, S.; Tetik, A.; Canataroglu, A. The Relationship of the Peroxisome Proliferator Activated Receptor–Gamma 2 Exon 2 and Exon 6 Gene Polymorphism in Type 2 Diabetic Patients with and without Diabetic Foot Ulcers. MedScience 2014, 3, 1582–1594. [Google Scholar] [CrossRef]
- Hughes, T.S.; Giri, P.K.; de Vera, I.M.S.; Marciano, D.P.; Kuruvilla, D.S.; Shin, Y.; Blayo, A.; Kamenecka, T.M.; Burris, T.P.; Griffin, R.P.; et al. An alternate binding site for PPARγ ligands. Nat. Commun. 2014, 5, 3571. [Google Scholar] [CrossRef]
- Coughlan, K.A.; Valentine, R.J.; Ruderman, N.B.; Saha, A.K. AMPK activation: A therapeutic target for type 2 diabetes? Diabetes Metab. Syndr. Obes. 2014, 7, 241. [Google Scholar] [CrossRef]
- Hauner, H. The mode of action of thiazolidinediones. Diabetes Metab. Res. Rev. 2002, 18, S10–S15. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, Isolation, and Identification of Bioactive Compounds from Plant Extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef]
- Sofowora, A. Medicinal Plants and Traditional Medicine in Africa; Spectrum Books Ltd.: Ibadan, Nigeria, 1993; pp. 191–289. [Google Scholar]
- Trease, G.; Evans, W. Pharmacognsy, 11th ed.; Brailliar Tiridel Can; Cassell and Collier Macmillan Publishers Ltd.: London, UK, 1989. [Google Scholar]
- Harborne, J.B. Phenolic compounds. In Phytochemical Methods; Springer: Berlin/Heidelberg, Germany; Chapmann and Hall: London, UK, 1973; pp. 33–88. [Google Scholar]
- Jung, M.; Park, M.; Lee, H.C.; Kang, Y.-H.; Kang, E.S.; Kim, S.K. Antidiabetic agents from medicinal plants. Curr. Med. Chem. 2006, 13, 1203–1218. [Google Scholar] [CrossRef]
- Kaur, C.; Kapoor, H. Anti-Oxidant Activity and Total Phenolic Content of Some Asian Vegetables. Int. J. Food Sci. Technol. 2002, 37, 153–161. [Google Scholar] [CrossRef]
- Kumaran, A.; Karunakaran, R.J. In vitro antioxidant activities of methanol extracts of five Phyllanthus species from India. LWT-Food Sci. Technol. 2007, 40, 344–352. [Google Scholar] [CrossRef]
- Saha, M.; Hasan, S.; Akter, R.; Hossain, M.; Alam, M.; Alam, M.; Mazumder, M. In vitro free radical scavenging activity of methanol extract of the leaves of Mimusops elengi Linn. Bangladesh J. Vet. Med. 2008, 6, 197–202. [Google Scholar] [CrossRef]
- Broadhurst, R.B.; Jones, W.T. Analysis of condensed tannins using acidified vanillin. J. Sci. Food Agric. 1978, 29, 788–794. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Marcocci, L.; Maguire, J.J.; Droylefaix, M.T.; Packer, L. The nitric oxide-scavenging properties of Ginkgo biloba extract EGb 761. Biochem. Biophys. Res. Commun. 1994, 201, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Saeed, N.; Khan, M.R.; Shabbir, M. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complement. Altern. Med. 2012, 12, 221. [Google Scholar] [CrossRef] [PubMed]
- Sadique, J.; Al-Rqobahs, W.; Bughaith, E.; Gindi, A. The bioactivity of certain medicinal plants on the stabilization of RBC membrane system. Fitoterapia 1989, 60, 525–532. [Google Scholar]
- Oyedapo, O.; Akinpelu, B.; Akinwunmi, K.; Adeyinka, M.; Sipeolu, F. Red blood cell membrane stabilizing potentials of extracts of Lantana camara and its fractions. Int. J. Plant Physiol. Biochem. 2010, 2, 46–51. [Google Scholar]
- Badmus, J.A.; Adedosu, T.O.; Fatoki, J.O.; Adegbite, V.A.; Adaramoye, O.A.; Odunola, O.A. Lipid peroxidation inhibition and antiradical activities of some leaf fractions of Mangifera indica. Acta Pol. Pharm. 2011, 68, 23–29. [Google Scholar]
- Williams, L.; O’Connar, A.; Latore, L.; Dennis, O.; Ringer, S.; Whittaker, J.; Conrad, J.; Vogler, B.; Rosner, H.; Kraus, W. The in vitro anti-denaturation effects induced by natural products and non-steroidal compounds in heat treated (immunogenic) bovine serum albumin is proposed as a screening assay for the detection of anti-inflammatory compounds, without the use of animals, in the early stages of the drug discovery process. West Indian Med. J. 2008, 57, 327–331. [Google Scholar] [PubMed]
- Hossain, S.; El-Sayed, M.; Aoshima, H. Antioxidative and anti-α-amylase activities of four wild plants consumed by pastoral nomads in Egypt. Orient. Pharm. Exp. Med. 2009, 9, 217–224. [Google Scholar] [CrossRef]
- Zaoui, A.; Cherrah, Y.; Mahassini, N.; Alaoui, K.; Amarouch, H.; Hassar, M. Acute and chronic toxicity of Nigella sativa fixed oil. Phytomedicine 2002, 9, 69–74. [Google Scholar] [CrossRef]
- Mostafavinia, A.; Amini, A.; Ghorishi, S.K.; Pouriran, R.; Bayat, M. The effects of dosage and the routes of administrations of streptozotocin and alloxan on induction rate of type 1 diabetes mellitus and mortality rate in rats. Lab. Anim. Res. 2016, 32, 160–165. [Google Scholar] [CrossRef]
- Mawa, J.; Rahman, M.A.; Hashem, M.; Hosen, M.J. Leea macrophylla root extract upregulates the mRNA expression for antioxidative enzymes and repairs the necrosis of pancreatic β-cell and kidney tissues in fructose-fed Type 2 diabetic rats. Biomed. Pharmacother. 2019, 110, 74–84. [Google Scholar] [CrossRef]
- Birru, E.M.; Abdelwuhab, M.; Shewamene, Z. Effect of hydroalcoholic leaves extract of Indigofera spicata Forssk. on blood glucose level of normal, glucose loaded and diabetic rodents. BMC Complement. Altern. Med. 2015, 15, 321. [Google Scholar] [CrossRef]
- Al-Araby, S.; Rahman, M.A.; Chowdhury, M.A.; Das, R.; Chowdhury, T.; Hasan, C.M.M.; Afroze, M.; Hashem, M.; Hajjar, D.; Alelwani, W. Padina tenuis (marine alga) attenuates oxidative stress and streptozotocin-induced type 2 diabetic indices in Wistar albino rats. S. Afr. J. Bot. 2020, 128, 87–100. [Google Scholar] [CrossRef]
- Lo, S.; Russell, J.; Taylor, A. Determination of glycogen in small tissue samples. J. App. Physiol. 1970, 28, 234–236. [Google Scholar] [CrossRef] [PubMed]
- Hussein, H.A.; Borrel, A.; Geneix, C.; Petitjean, M.; Regad, L.; Camproux, A.-C. PockDrug-Server: A new web server for predicting pocket druggability on holo and apo proteins. Nucleic Acids Res. 2015, 43, W436–W442. [Google Scholar] [CrossRef]
- Hossen, M.A.; Ali Reza, A.; Amin, M.B.; Nasrin, M.S.; Khan, T.A.; Rajib, M.H.R.; Tareq, A.M.; Haque, M.A.; Rahman, M.A.; Haque, M.A. Bioactive metabolites of Blumea lacera attenuate anxiety and depression in rodents and computer-aided model. Food Sci. Nutr. 2021, 9, 3836–3851. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, A.; Sugumar, S.; Bitragunta, S.; Balasubramanyan, N. Molecular docking studies of (4Z, 12Z)-cyclopentadeca-4, 12-dienone from Grewia hirsuta with some targets related to type 2 diabetes. BMC Complement. Altern. Med. 2015, 15, 73. [Google Scholar] [CrossRef] [PubMed]




| S. N | Compounds Name | Molecular Formula | Molecular Weight (g/mol) | Running Time | Area |
|---|---|---|---|---|---|
| 1 | Methyl alpha-d-Galactopyranoside | C7H14O6 | 194.1825 | 9.675 | 4616107 |
| 2 | Methyl alpha-d-Glucopyranoside | C7H14O6 | 194.1825 | 9.675 | 4616107 |
| 3 | 2-Penten-1-ol, (Z)-, TMS derivative | C8H18OSi | 158.3134 | 10.140 | 74145 |
| 4 | 2-Butene-1,4-diol, TMS derivative | C7H16O2Si | 160.29 | 10.140 | 74145 |
| 5 | Silane, [[4-[1,2-bis[(trimethylsilyl)oxy]ethyl]-1,2-phenylene]bis(oxy)]bis[trimethyl-] | C20H42O4Si4 | 458.90 | 10.144 | 2431169 |
| 6 | 2-Buten-1-ol, (E), TBDMS derivative | C10H22OSi | 186.3666 | 10.140 | 74145 |
| 7 | 2-[(2,4,4,6,6,8,8-Heptamethyl-1,3,5,7,2,4,6,8-tetroxatetrasilocan-2-yl)oxy]-2,4,4,6,6,8,8,10,10-nonamethyl-1,3,5,7,9,2,4,6,8,10-pentoxapentasilecane | C16H48O10Si9 | 653.316 | 12.169 | 30800 |
| 8 | Phenethylamine, N-methyl-.β.,3,4-tris(trimethylsiloxy)- | C18H37NO3Si3 | 399.74 | 12.169 | 30800 |
| 9 | Ethyl tridecanoate | C15H30O2 | 242.3975 | 14.205 | 244709 |
| 10 | Undecanoic acid, ethyl ester | C13H26O2 | 214.3443 | 14.205 | 244709 |
| 11 | Hexadecanoic acid, ethyl ester | C18H36O2 | 284.4772 | 14.205 | 244709 |
| 12 | Decanoic acid, ethyl ester | C12H24O2 | 200.3178 | 14.205 | 244709 |
| 13 | Tridecanoic acid, 12-methyl-, methyl ester | C15H30O2 | 242.3975 | 15.534 | 580270 |
| 14 | Methyl stearate | C19H38O2 | 298.5038 | 15.534 | 580270 |
| 15 | Docosanoic acid, ethyl ester | C24H48O2 | 368.6367 | 16.144 | 10315 |
| 16 | Hexadecanoic acid, ethyl ester | C18H36O2 | 284.4772 | 16.144 | 10315 |
| 17 | Trisiloxane, 1,1,1,5,5,5-hexamethyl-3,3-bis[(trimethylsilyl)oxy]- | C12H36O4Si5 | 384.8393 | 16.989 | 77570 |
| 18 | Phloroglucitol | C6H6O3 | 126.11 | 16.989 | 77570 |
| 19 | 3-Trifluoroacetoxydodecane | C14H25F3O2 | 282.34 | 17.245 | 40356 |
| 20 | Cyclononasiloxane, octadecamethyl- | C18H54O9Si9 | 667.3855 | 17.701 | 570902 |
| 21 | Hexadecanoic acid, 2-hydroxy-1-(hydroxymethyl) ethyl ester | C19H38O4 | 330.5026 | 20.223 | 402651 |
| 22 | Glycerol 1-palmitate | C19H38O4 | 330.5026 | 20.223 | 402651 |
| 23 | Pentadecanoic acid, 2-hydroxy-1-(hydroxymet | 20.223 | 402651 | ||
| 24 | 2-Hydroxy-1-(hydroxymethyl) ethyl icosanoate | C23H46O4 | 386.60 | 20.223 | 402651 |
| 25 | Hexadecanoic acid,1,1′-[(1S)-1-(hydroxymethyl)-1,2-ethanediyl] ester | C35H68O5 | 568.91 | 20.223 | 402651 |
| 26 | Octadecanoic acid, 2-hydroxy-1,3-propanediyl ester | C39H76O5 | 625.032 | 20.223 | 402651 |
| 27 | 10-Undecenoic acid, octyl ester | C19H36O2 | 296.4879 | 20.605 | 200047 |
| 28 | Octadecanoic acid, 2-hydroxy-1,3-propanediyl ester | C39H76O5 | 625.032 | 20.223 | 159887 |
| 29 | 2-Hydroxy-1-(hydroxymethyl) ethyl icosanoate | C23H46O4 | 386.60 | 23.863 | 159887 |
| 30 | Heptadecanoic acid, heptadecyl ester | C34H68O2 | 508.90 | 23.863 | 159887 |
| 31 | 13-Docosenamide, (Z)- | C22H43NO | 337.5829 | 24.618 | 13399817 |
| Sample Name | TFC (mg Rutin Equivalent/g Dry Extract | TPC (mg GAE/g Dry Extract) | TAC (mg AAE/g Extract) | TPACC (mg Cat/g Dry Extract) | DPPH Free Radical Scavenging Activity (IC50 μg/mL) | Iron Chelating Activity (IC50 μg/mL) | Nitric Oxide Scavenging Activity (IC50 μg/mL) | Hydroxyl Radical Scavenging Activity (IC50 μg/mL) | Lipid Peroxidation Inhibition Capacity (IC50 μg/mL) | Protein Denaturation Inhibition Effect (IC50 μg/mL) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MEXLS | 154.06 ± 0.62 | 277.50 ± 2.25 | 157.70 ± 2.60 | 337.50 ± 29.92 | STD (AA) | MELS | STD (AA) | MEXLS | STD (Quer) | MEXLS | STD (Cat) | MEXLS | STD (Cat) | MEXLS | STD (Asp) | MEXLS |
| 9.22 ± 0.80 | 337.50 ± 29.92 | 48.39 ± 1.87 | 118.48 ± 2.84 | 3.06 ± 0.64 | 14.4 ± 0.17 | 163.87 ± 3.35 | 184.40 ± 0.71 | 43.82 ± 3.13 | 60.71 ± 4.24 | 110.56 ± 5.23 | 386.44 ± 6.32 | |||||
| Groups | Liver Weight ± SD (mg) | Kidney Weight ± SD (mg) | Pancreatic Weight ± SD (mg) |
|---|---|---|---|
| NC | 9.32 ± 0.05 *** | 1.68 ± 0.02 ** | 0.67 ± 0.01 *** |
| RC | 8.53 ± 0.09 *** | 1.71 ± 0.02 * | 0.47 ± 0.01 ** |
| MEXLS50 | 9.52 ± 0.02 ** | 1.51 ± 0.03 *** | 0.47 ± 0.00 ** |
| MEXLS100 | 8.28 ± 0.07 *** | 1.62 ± 0.02 *** | 0.45 ± 0.00 * |
| MEXLS200 | 9.88 ± 0.04 * | 1.67 ± 0.02 ** | 0.49 ± 0.00 *** |
| DC | 7.34 ± 0.13 | 1.81 ± 0.02 | 0.40 ± 0.01 |
| Parameters | NC | RC | MEXLS50 | MEXLS100 | MEXLS200 | DC |
|---|---|---|---|---|---|---|
| ALT (U/L) | 73.50 ± 1.36 *** | 48.30 ± 1.66 *** | 60.60 ± 2.18 *** | 83.50 ± 2.01 *** | 87.70 ± 1.50 *** | 97.30 ± 1.86 |
| AST (U/L) | 75.20 1.85 ** | 85.30 ± 1.44 *** | 100.60 ± 2.14 *** | 122.00 ± 3.48 *** | 142.00 ± 2.80 *** | 170.00 ± 2.24 |
| Creatinine (mg/dL) | 0.52 ± 0.02 *** | 0.55 ± 0.02 *** | 0.60 ± 0.01 *** | 0.45 ± 0.01 *** | 0.59 ± 0.01 *** | 0.90 ± 0.02 |
| CKMB (U/L) | 171.20 ± 4.12 *** | 87.60 ± 3.91 *** | 101.20 ± 2.23 *** | 152.00 ± 4.84 *** | 172.40 ± 3.14 *** | 220.80 ± 2.40 |
| HDL (mg/dL) | 13.80 ± 0.37 * | 16.60 ± 0.50 *** | 13.60 ± 0.47 * | 14.00 ± 0.71 * | 17.40 ± 0.51 *** | 11.60 ± 0.51 |
| LDL (mg/dL) | 47.60 ± 1.47 ** | 37.60 ± 1.03 *** | 38.80 ± 0.86 *** | 44.00 ± 1.01 *** | 57.80 ± 0.86 *** | 52.00 ± 1.02 |
| LDH (U/L) | 547.60 ± 5.23 *** | 539.00 ± 0.41 *** | 865.00 ± 8.82 *** | 331.40 ± 6.01 *** | 273.40 ± 3.08 *** | 782.60 ± 5.09 |
| Liver glycogen (mg/g) | 0.25 ± 0.00 *** | 0.30 ± 0.00 ** | 0.03 ± 0.00 ** | 0.60 ± 0.00 *** | 0.054 ± 0.00 * | 0.41 ± 0.01 |
| Total cholesterol (mg/dL) | 62.40 ± 2.11 *** | 69.60 ± 1.08 * | 54.80 ± 2.22 *** | 60.80 ± 1.50 *** | 67.20 ± 1.57 ** | 76.00 ± 0.70 |
| TG (mg/dL) | 111.00 ± 4.14 *** | 42.20 ± 1.88 *** | 63.80 ± 2.42 *** | 117.60 ± 1.91 *** | 94.40 ± 3.54 *** | 167.80 ± 2.96 |
| Urea (mg/dL) | 47.00 ± 1.20 * | 45.20 ± 1.30 ** | 37.60 ± 1.40 *** | 42.10 ± 2.80 *** | 45.00 ± 1.90 ** | 56.20 ± 2.70 |
| Uric acid (mg/dL) | 6.17 ± 0.05 ** | 5.88 ± 0.13 *** | 5.93 ± 0.07 *** | 5.05 ± 0.07 *** | 5.19 ± 0.06 *** | 6.46 ± 0.03 |
| Serum Insulin level (mL U/mL) | 0.12 ± 0.05 * | 0.21 ± 0.06 ** | 0.20 ± 0.04 ** | 0.15 ± 0.09 * | 0.11 ± 0.06 * | 0.06 ± 0.02 |
| Changes in Pancreatic Tissues | ||||||
|---|---|---|---|---|---|---|
| NC | DC | RC | MEXLS50 | MEXLS100 | MEXLS200 | |
| Diameter of islet of Langerhans (μm) | 173 ± 47 | ND | 125 ± 28 | 220 ± 18 | 205 ± 13 | 200 ± 57 |
| Area occupied by β-cell/islet of ± Langerhans (μm2) | 20,703 ± 4730 | ND | 11,227 ± 2309 | 46,400 ± 1861 | 30,450 ± 1366 | 43,700 ± 5773 |
| Necrotic cells | − | +++ | + | + | − | − |
| Degenerated Cells | − | +++ | + | + | + | + |
| Predisposing markers | NC | DC | RC | MEXLS50 | MEXLS100 | MEXLS200 |
|---|---|---|---|---|---|---|
| Tubular epithelial cell degeneration | − | + | − | − | − | − |
| Tubular epithelial cell necrosis | − | + | − | + | − | − |
| Increased fibrous tissue | − | + | − | + | + | − |
| Interstitial mononuclear cell titration | − | + | + | − | + | − |
| Hyperemic vessels in the interstitium | − | + | − | + | + | − |
| Eosinophilic secretion in the tubules lumen | − | + | − | − | − | − |
| Atrophic glomerulus and tubules | − | ++ | − | + | − | − |
| Compounds | PubChem ID | 3G9E | 4CFH | 1PPI |
|---|---|---|---|---|
| Methyl alpha-d-galactopyranoside | 76935 | −5.218 | −5.764 | −5.615 |
| Undecanoic acid ethyl ester | 12327 | −1.831 | −0.509 | −1.073 |
| Hexadecanoic acid, 2-hydroxy-1-(hydroxymethyl) ethyl ester | 123409 | −5.562 | −4.791 | −3.766 |
| Glycerol 1-palmitate | 14900 | −5.783 | −1.474 | −3.476 |
| Pentadecanoic acid, 2-hydroxy-1-(hydroxymethyl) | 537297 | −5.683 | −4.824 | −4.264 |
| 2-Hydroxy-1-(hydroxymethyl) ethyl icosanoate | 537294 | −5.372 | −4.906 | −3.429 |
| Hexadecanoic acid, 1,1′-[(1S)-1-(hydroxymethyl)-1,2-ethanediyl] ester | 644078 | −4.975 | −2.53 | −4.975 |
| 13-Docosenamide. (Z)- | 5365371 | −6.785 | −5.451 | −3.726 |
| 9-Octadecenamide. (Z)- | 5283387 | −3.059 | −2.018 | 0.104 |
| Decanoic acid, ethyl ester | 8048 | −1.372 | −0.3 | −1.041 |
| Compounds | Lipinski Rules | Lipinski’s Violations | Veber Rules | ||||
|---|---|---|---|---|---|---|---|
| MW (g/mol) | HBA | HBD | Log P | nRB | TPSA | ||
| Methyl α-d-Galactopyranoside | 194.1825 | 6 | 4 | −2.40 | 0 | 2 | 99.38 Å2 |
| Ethyl tridecanoate | 242.3975 | 2 | 0 | 3.94 | 0 | 13 | 26.30 Å2 |
| Undecanoic acid. ethyl ester | 214.3443 | 2 | 9 | 3.42 | 0 | 11 | 26.30 Å2 |
| Decanoic acid. ethyl ester | 200.3178 | 2 | 0 | 3.15 | 0 | 10 | 26.30 Å2 |
| Hexadecanoic acid. 2-hydroxy-1-(hydroxymethyl) ethyl ester | 330.5026 | 4 | 2 | 3.18 | 0 | 18 | 66.76 Å2 |
| Glycerol 1-palmitate | 330.5026 | 4 | 2 | 3.18 | 0 | 18 | 66.76 Å2 |
| Pentadecanoic acid. 2-hydroxy-1-(hydroxymethyl) | 316.48 | 4 | 2 | 2.94 | 0 | 17 | 66.76 Å2 |
| 2-Hydroxy-1-(hydroxymethyl) ethyl icosanoate | 386.60 | 4 | 2 | 4.06 | 0 | 22 | 66.76 Å2 |
| Hexadecanoic acid.1.1′-[(1S)-1-(hydroxymethyl)-1.2-ethanediyl] ester | 568.91 | 5 | 1 | 6.27 | 2 | 34 | 72.83 Å2 |
| 13-Docosenamide. (Z)- | 337.5829 | 1 | 1 | 5.06 | 1 | 19 | 43.09 Å2 |
| 9-Octadecenamide. (Z)- | 281.4766 | 1 | 1 | 4.16 | 1 | 15 | 43.09 Å2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rashid, M.M.; Rahman, M.A.; Islam, M.S.; Hossen, M.A.; Ahmed, A.M.A.; Afroze, M.; Habib, A.H.; Mansoury, M.M.S.; Alharbi, H.F.; Algheshairy, R.M.; et al. Natural Compounds of Lasia spinosa (L.) Stem Potentiate Antidiabetic Actions by Regulating Diabetes and Diabetes-Related Biochemical and Cellular Indexes. Pharmaceuticals 2022, 15, 1466. https://doi.org/10.3390/ph15121466
Rashid MM, Rahman MA, Islam MS, Hossen MA, Ahmed AMA, Afroze M, Habib AH, Mansoury MMS, Alharbi HF, Algheshairy RM, et al. Natural Compounds of Lasia spinosa (L.) Stem Potentiate Antidiabetic Actions by Regulating Diabetes and Diabetes-Related Biochemical and Cellular Indexes. Pharmaceuticals. 2022; 15(12):1466. https://doi.org/10.3390/ph15121466
Chicago/Turabian StyleRashid, Md. Mamunur, Md. Atiar Rahman, Md. Shahidul Islam, Md. Amjad Hossen, A. M. Abu Ahmed, Mirola Afroze, Alaa H. Habib, Manal M. S. Mansoury, Hend F. Alharbi, Reham M. Algheshairy, and et al. 2022. "Natural Compounds of Lasia spinosa (L.) Stem Potentiate Antidiabetic Actions by Regulating Diabetes and Diabetes-Related Biochemical and Cellular Indexes" Pharmaceuticals 15, no. 12: 1466. https://doi.org/10.3390/ph15121466
APA StyleRashid, M. M., Rahman, M. A., Islam, M. S., Hossen, M. A., Ahmed, A. M. A., Afroze, M., Habib, A. H., Mansoury, M. M. S., Alharbi, H. F., Algheshairy, R. M., Alelwani, W., Alnajeebi, A. M., Tangpong, J., Saha, S., Qadhi, A., & Azhar, W. (2022). Natural Compounds of Lasia spinosa (L.) Stem Potentiate Antidiabetic Actions by Regulating Diabetes and Diabetes-Related Biochemical and Cellular Indexes. Pharmaceuticals, 15(12), 1466. https://doi.org/10.3390/ph15121466






