Vasorelaxant Effects of Syzygium samarangense (Blume) Merr. and L.M.Perry Extract Are Mediated by NO/cGMP Pathway in Isolated Rat Thoracic Aorta
Abstract
:1. Introduction
2. Results
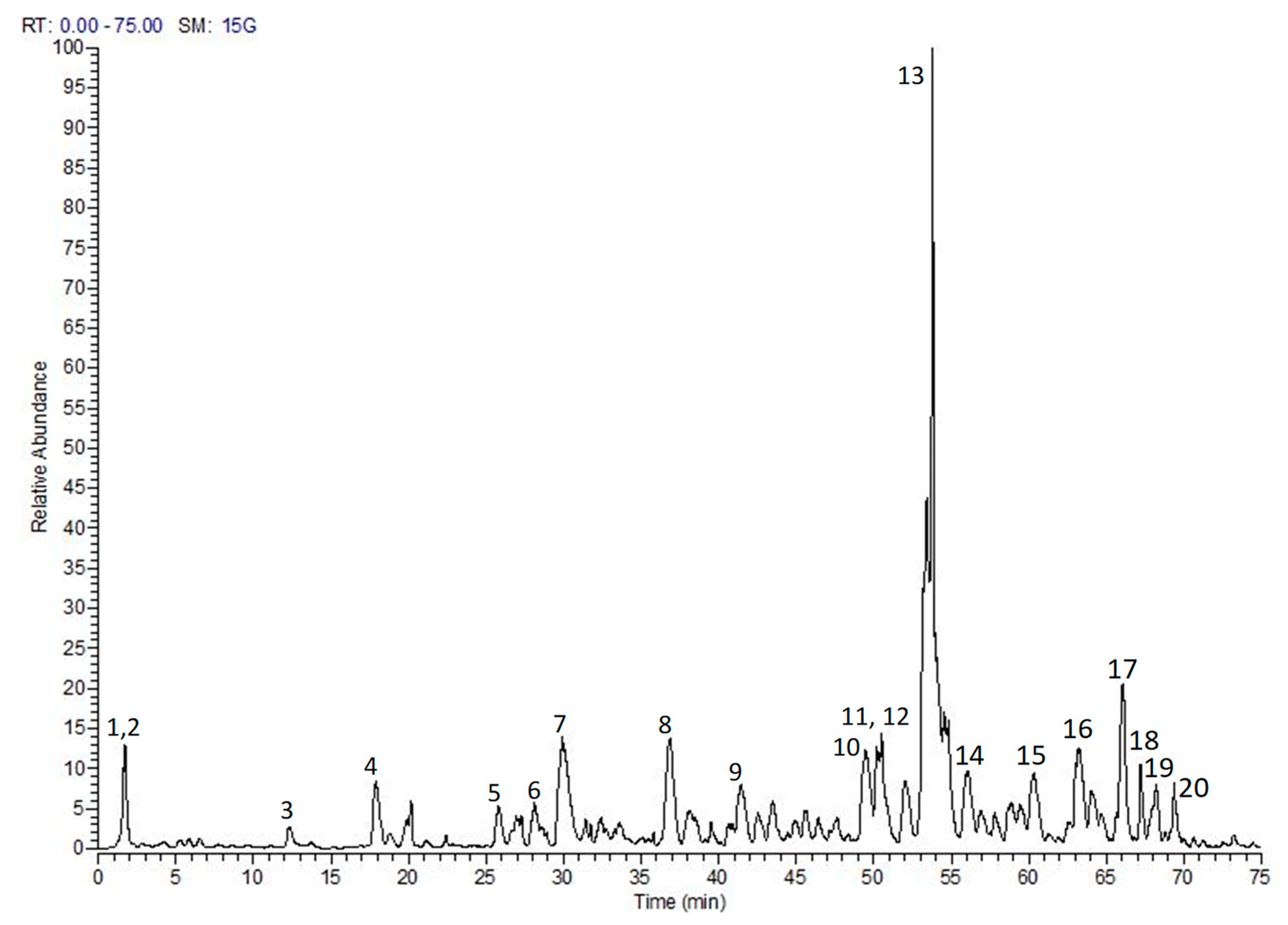
2.1. Chemical Composition
2.2. Vasodilatation Effect of S. samarangense Extract in the Endothelium-Intact Aorta Sections
2.3. Role of Endothelium Denudation in the Observed Vasodilation Effect of S. samarangense Extract
2.4. Role of Endothelial NO/cGMP Pathway in the Observed Vasodilation Effect of S. samarangense Extract
2.5. Receptors Related to the Vasodilation Effect of S. samarangense Extract
2.6. Investigation of the Potential Role of Endothelium-Dependent Hyperpolarization
2.7. Investigation of the Potential Role of Prostacyclin (PGI2)/cAMP Pathway in S. samarangense Extract-Induced Vascular Relaxation
2.8. Role of Different K+ Channels in the Observed Vasodilation Effect of S. samarangense Extract
2.9. Role of Ca2+ Influx and Mobilization in S. samarangense Extract Induced Vasodilation
2.10. Molecular Docking
3. Discussion
4. Materials and Methods
4.1. Extraction, Drugs and Chemicals
4.2. Animals
4.3. Aortae Isolation
4.4. Studying the Vasodilation Effect of S. samarangense Extract
4.5. Molecular Docking
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huo, L.; Zhang, J.; Qu, Z.; Chen, H.; Li, Y.; Gao, W. Vasorelaxant effects of Shunaoxin pill are mediated by NO/cGMP pathway, HO/CO pathway and calcium channel blockade in isolated rat thoracic aorta. J. Ethnopharmacol. 2015, 173, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Behrendt, D.; Ganz, P. Endothelial function: From vascular biology to clinical applications. Am. J. Cardiol. 2002, 90, L40–L48. [Google Scholar] [CrossRef]
- Williams, I.L.; Wheatcroft, S.B.; Shah, A.M.; Kearney, M.T. Obesity, atherosclerosis and the vascular endothelium: Mechanisms of reduced nitric oxide bioavailability in obese humans. Int. J. Obes. 2002, 26, 754–764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuo, H.; Okamoto, R.; Zaima, K.; Hirasawa, Y.; Ismail, I.S.; Lajis, N.H.; Morita, H. New vasorelaxant indole alkaloids, villocarines A–D from Uncaria villosa. Bioorg. Med. Chem. 2011, 19, 4075–4079. [Google Scholar] [CrossRef] [PubMed]
- Morita, H.; Zaima, K.; Koga, I.; Saito, A.; Tamamoto, H.; Okazaki, H.; Kaneda, T.; Hashimoto, T.; Asakawa, Y. Vasorelaxant effects of macrocyclic bis (bibenzyls) from liverworts. Bioorg. Med. Chem. 2011, 19, 4051–4056. [Google Scholar] [CrossRef] [PubMed]
- Rashied, R.M.; Abdelfattah, M.A.; El-Beshbishy, H.A.; ElShazly, A.M.; Mahmoud, M.F.; Sobeh, M. Syzygium samarangense leaf extract exhibits distinct antidiabetic activities: Evidences from in silico and in vivo studies. Arab. J. Chem. 2022, 15, 103822. [Google Scholar] [CrossRef]
- Mahmoud, M.F.; Nabil, M.; Abdo, W.; Abdelfattah, M.A.; El-Shazly, A.M.; El Kharrassi, Y.; Sobeh, M. Syzygium samarangense leaf extract mitigates indomethacin-induced gastropathy via the NF-κB signaling pathway in rats. Biomed. Pharmacother. 2021, 139, 111675. [Google Scholar] [CrossRef]
- Abdelfattah, M.A.; Ibrahim, M.A.; Abdullahi, H.L.; Aminu, R.; Saad, S.B.; Krstin, S.; Wink, M.; Sobeh, M. Eugenia uniflora and Syzygium samarangense extracts exhibit anti-trypanosomal activity: Evidence from in-silico molecular modelling, in vitro, and in vivo studies. Biomed. Pharmacother. 2021, 138, 111508. [Google Scholar] [CrossRef] [PubMed]
- Sobeh, M.; Petruk, G.; Osman, S.; El Raey, M.A.; Imbimbo, P.; Monti, D.M.; Wink, M. Isolation of myricitrin and 3, 5-di-O-methyl gossypetin from Syzygium samarangense and evaluation of their involvement in protecting keratinocytes against oxidative stress via activation of the Nrf-2 pathway. Molecules 2019, 24, 1839. [Google Scholar] [CrossRef] [Green Version]
- Sobeh, M.; Youssef, F.S.; Esmat, A.; Petruk, G.; El-Khatib, A.H.; Monti, D.M.; Ashour, M.L.; Wink, M. High resolution UPLC-MS/MS profiling of polyphenolics in the methanol extract of Syzygium samarangense leaves and its hepatoprotective activity in rats with CCl4-induced hepatic damage. Food Chem. Toxicol. 2018, 113, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Ayele, Y.; Urga, K.; Engidawork, E. Evaluation of in vivo antihypertensive and in vitro vasodepressor activities of the leaf extract of syzygium guineense (willd) DC. Phytother. Res. 2010, 24, 1457–1462. [Google Scholar] [CrossRef] [PubMed]
- Pakdeechote, P.; Meephat, S.; Sakonsinsiri, C.; Phetcharaburanin, J.; Bunbupha, S.; Maneesai, P. Syzygium gratum extract alleviates vascular alterations in hypertensive rats. Medicina 2020, 56, 509. [Google Scholar] [CrossRef]
- Ismail, A.; Ahmad, W.A.N.W. Vasorelaxation effect of Syzygium polyanthum (wight) walp. Leaves extract on isolated thoracic aorta rings of normal and hypertensive rats. IIUM Med. J. Malays. 2016, 15, 102. [Google Scholar] [CrossRef]
- Knox, M.; Vinet, R.; Fuentes, L.; Morales, B.; Martínez, J.L. A review of endothelium-dependent and-independent vasodilation induced by phytochemicals in isolated rat aorta. Animals 2019, 9, 623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sailaja, G.R.; Sriramavaratharajan, V.; Murugan, R.; Mallavarapu, G.R.; Chellappan, D.R. Vasorelaxant property of Plectranthus vettiveroides root essential oil and its possible mechanism. J. Ethnopharmacol. 2021, 274, 114048. [Google Scholar] [CrossRef]
- Jiang, J.; Zheng, J.P.; Li, Y.; Gan, Z.; Jiang, Y.; Huang, D.; Li, H.; Liu, Z.; Ke, Y. Differential contribution of endothelium-derived relaxing factors to vascular reactivity in conduit and resistance arteries from normotensive and hypertensive rats. Clin. Exp. Hypertens. 2016, 38, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Liu, R.; Wu, J.X.; Chen, L. Structural insights into the mechanism of human soluble guanylate cyclase. Nature 2019, 574, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Kang, Y.; Chen, L. Activation mechanism of human soluble guanylate cyclase by stimulators and activators. Nat. Commun. 2021, 12, 1–10. [Google Scholar] [CrossRef]
- Hort, M.A.; Brighente, I.M.C.; Pizzolatti, M.G.; Ribeiro-do-Valle, R.M. Mechanisms involved in the endothelium-dependent vasodilatory effect of an ethyl acetate fraction of Cyathea phalerata Mart. in isolated rats’ aorta rings. J. Tradit. Complementary Med. 2020, 10, 360–365. [Google Scholar] [CrossRef]
- Vanhoutte, P.M.; Zhao, Y.; Xu, A.; Leung, S.W. Thirty years of saying NO: Sources, fate, actions, and misfortunes of the endothelium-derived vasodilator mediator. Circ. Res. 2016, 119, 375–396. [Google Scholar] [CrossRef]
- Ciccarelli, M.; Cipolletta, E.; Santulli, G.; Campanile, A.; Pumiglia, K.; Cervero, P.; Pastore, L.; Astone, D.; Trimarco, B.; Iaccarino, G. Endothelial β2 adrenergic signaling to AKT: Role of Gi and SRC. Cell. Signal. 2007, 19, 1949–1955. [Google Scholar] [CrossRef] [PubMed]
- McGuire, J.J.; Ding, H.; Triggle, C.R. Endothelium-derived relaxing factors: A focus on endothelium-derived hyperpolarizing factor(s). Can. J. Physiol. Pharmacol. 2001, 79, 443–470. [Google Scholar] [CrossRef]
- Adeagbo, A.; Triggle, C.R. Varying extracellular [K+]: A functional approach to separating EDHF-and EDNO-related mechanisms in perfused rat mesenteric arterial bed. J. Cardiovasc. Pharmacol. 1993, 21, 423–429. [Google Scholar] [CrossRef]
- Busse, R.; Edwards, G.; Félétou, M.; Fleming, I.; Vanhoutte, P.M.; Weston, A.H. EDHF: Bringing the concepts together. Trends Pharmacol. Sci. 2002, 23, 374–380. [Google Scholar] [CrossRef]
- Meyrelles, S.S.; Peotta, V.A.; Pereira, T.; Vasquez, E.C. Endothelial dysfunction in the apolipoprotein E-deficient mouse: Insights into the influence of diet, gender and aging. Lipids Health Dis. 2011, 10, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baranowska, M.; Kozłowska, H.; Korbut, A.; Malinowska, B. Kanały potasowe w naczyniach krwionośnych ich znaczenie w fizjologii i patologii Potassium channels in blood vessels: Their role in health and disease. Post. Hig. Med. Doœw 2007, 61, 596–605. [Google Scholar]
- Abdallah, H.M.; El-Bassossy, H.M.; Mohamed, G.A.; El-Halawany, A.M.; Alshali, K.Z.; Banjar, Z.M. Phenolics from Garcinia mangostana alleviate exaggerated vasoconstriction in metabolic syndrome through direct vasodilatation and nitric oxide generation. BMC Complement. Altern. Med. 2016, 16, 359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, D.K.; Ashcroft, F.M. Glimepiride block of cloned β-cell, cardiac and smooth muscle KATP channels. Br. J. Pharmacol. 2001, 133, 193–199. [Google Scholar] [CrossRef] [Green Version]
- Schneider, J.C.; El Kebir, D.; Chéreau, C.; Lanone, S.; Huang, X.L.; De Buys Roessingh, A.S.; Mercier, J.C.; Dall’Ava-Santucci, J.; Dinh-Xuan, A.T. Involvement of Ca2+/calmodulin-dependent protein kinase II in endothelial NO production and endothelium-dependent relaxation. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, H2311–H2319. [Google Scholar] [CrossRef] [Green Version]
- Horowitz, A.; Menice, C.B.; Laporte, R.; Morgan, K.G. Mechanisms of smooth muscle contraction. Physiol. Rev. 1996, 76, 967–1003. [Google Scholar] [CrossRef]
- Laban, A.I.; El-Bassossy, H.M.; Hassan, N.A. Hinokitiol produces vasodilation in aortae from normal and angiotensin II-induced hypertensive rats via endothelial-dependent and independent pathways. Vasc. Pharmacol. 2022, 146, 107092. [Google Scholar] [CrossRef] [PubMed]
- de Alencar Silva, A.; Pereira-de-Morais, L.; da Silva, R.E.R.; de Menezes Dantas, D.; Milfont, C.G.B.; Gomes, M.F.; Araújo, I.M.; Kerntopf, M.R.; de Menezes, I.R.A.; Barbosa, R. Pharmacological screening of the phenolic compound caffeic acid using rat aorta, uterus and ileum smooth muscle. Chem. Biol. Interact. 2020, 332, 109269. [Google Scholar] [CrossRef] [PubMed]
- Deliorman, D.; Çalış, İ.; Ergun, F.; Doğan, B.S.U.; Buharalıoğlu, C.K.; Kanzık, I. Studies on the vascular effects of the fractions and phenolic compounds isolated from Viscum album ssp. album. J. Ethnopharmacol. 2000, 72, 323–329. [Google Scholar] [CrossRef]
- Abdallah, H.M.; Hassan, N.A.; El-Halawany, A.M.; Mohamed, G.A.; Safo, M.K.; El-Bassossy, H.M. Major flavonoids from Psiadia punctulata produce vasodilation via activation of endothelial dependent NO signaling. J. Adv. Res. 2020, 24, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Hassan, N.A.; El Bassossy, H.M.; Fahmy, A.; Mahmoud, M.F. Limonin alleviates macro-and micro-vascular complications of metabolic syndrome in rats: A comparative study with azelnidipine. Phytomedicine 2018, 43, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Hassan, N.A.; El-Bassossy, H.M.; Mahmoud, M.F.; Fahmy, A. Caffeic acid phenethyl ester, a 5-lipoxygenase enzyme inhibitor, alleviates diabetic atherosclerotic manifestations: Effect on vascular reactivity and stiffness. Chem. Biol. Interact. 2014, 213, 28–36. [Google Scholar] [CrossRef]
- Mahmoud, M.F.; Hassan, N.A.; El Bassossy, H.M.; Fahmy, A. Quercetin protects against diabetes-induced exaggerated vasoconstriction in rats: Effect on low grade inflammation. PLoS ONE 2013, 8, e63784. [Google Scholar] [CrossRef]
- El-Bassossy, H.M.; Mahmoud, M.F.; Eid, B.G. The vasodilatory effect of allopurinol mediates its antihypertensive effect: Effects on calcium movement and cardiac hemodynamics. Biomed. Pharmacother. 2018, 100, 381–387. [Google Scholar] [CrossRef]
- Rees, D.D.; Palmer, R.M.; Schulz, R.; Hodson, H.F.; Moncada, S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br. J. Pharmacol. 1990, 101, 746–752. [Google Scholar] [CrossRef] [Green Version]
- Green, B.D.; Hand, K.V.; Dougan, J.E.; McDonnell, B.M.; Cassidy, R.S.; Grieve, D.J. GLP-1 and related peptides cause concentration-dependent relaxation of rat aorta through a pathway involving KATP and cAMP. Arch. Biochem. Biophys. 2008, 478, 136–142. [Google Scholar] [CrossRef] [Green Version]
- Schrammel, A.; Behrends, S.; Schmidt, K.; Koesling, D.; Mayer, B. Characterization of 1H-[1,2,4] oxadiazolo [4, 3-a] quinoxalin-1-one as a heme-site inhibitor of nitric oxide-sensitive guanylyl cyclase. Mol. Pharmacol. 1996, 50, 1–5. [Google Scholar] [PubMed]
- Hunt, N.H.; Evans, T. RMI 12330A, an inhibitor of cyclic nucleotide phosphodiesterases and adenylate cyclase in kidney preparations. Biochim. Biophys. Acta (BBA) Enzymol. 1980, 613, 499–506. [Google Scholar] [CrossRef]
- Vane, J.R. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat. New Biol. 1971, 231, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Arsyad, A.; Dobson, G.P. Adenosine relaxation in isolated rat aortic rings and possible roles of smooth muscle K v channels, K ATP channels and A 2a receptors. BMC Pharmacol. Toxicol. 2016, 17, 1–11. [Google Scholar] [CrossRef] [Green Version]
- El-Bassossy, H.M.; Elberry, A.A.; Ghareib, S.A. Geraniol improves the impaired vascular reactivity in diabetes and metabolic syndrome through calcium channel blocking effect. J. Diabetes Complicat. 2016, 30, 1008–1016. [Google Scholar] [CrossRef]







| Compound | Docking Score (kcal/mol) | |
|---|---|---|
| sGC Activated State (7D9R) | sGC Inactivated State (7D9T) | |
| Myricitrin | −14.77 | −11.74 |
| Malaferin B | −13.32 | −11.92 |
| Xanthohumol | −13.30 | Fail |
| Sinapic acid | −12.12 | −10.50 |
| 4-Hydroxybenzoic acid glucoside | −11.48 | −9.83 |
| Sinensetin | −11.28 | −11.09 |
| Jaceosidin | −10.99 | −8.63 |
| Oleacein | −10.97 | Fail |
| Rosmarinic acid | −10.67 | −12.53 |
| Myrigalon B | −10.63 | −9.33 |
| Oleuropein aglycone | −10.61 | −10.27 |
| Tangeretin | −10.53 | −10.32 |
| Gardenin B | −10.50 | −10.10 |
| Curcumin | −10.40 | Fail |
| Tricin | −10.39 | −9.35 |
| Pinocembrin | −9.36 | Fail |
| Syringaresinol | −9.36 | Fail |
| Phloretin | −9.34 | Fail |
| Cryptostrobin | −9.15 | −7.92 |
| Caffeic acid | −8.96 | Fail |
| Umbelliferone | −8.62 | Fail |
| Isorhamnetin | −8.26 | −9.78 |
| Myrigalone B | −8.08 | Fail |
| 2-Methoxy-2-phenylacetic acid | Fail | −6.83 |
| Resveratrol | Fail | −7.03 |
| Galangin | Fail | −8.65 |
| Geraldone | Fail | −8.56 |
| Myricetin | Fail | −9.47 |
| Quercetin | Fail | −8.67 |
| Riociguat (reference stimulator) | −11.45 | - |
| Cinaciguat (reference activator) | - | −14.97 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, N.A.; Abdelfattah, M.A.O.; Mandour, Y.M.; El-Shazly, A.M.; Sobeh, M.; Mahmoud, M.F. Vasorelaxant Effects of Syzygium samarangense (Blume) Merr. and L.M.Perry Extract Are Mediated by NO/cGMP Pathway in Isolated Rat Thoracic Aorta. Pharmaceuticals 2022, 15, 1349. https://doi.org/10.3390/ph15111349
Hassan NA, Abdelfattah MAO, Mandour YM, El-Shazly AM, Sobeh M, Mahmoud MF. Vasorelaxant Effects of Syzygium samarangense (Blume) Merr. and L.M.Perry Extract Are Mediated by NO/cGMP Pathway in Isolated Rat Thoracic Aorta. Pharmaceuticals. 2022; 15(11):1349. https://doi.org/10.3390/ph15111349
Chicago/Turabian StyleHassan, Noura A., Mohamed A. O. Abdelfattah, Yasmine M. Mandour, Assem M. El-Shazly, Mansour Sobeh, and Mona F. Mahmoud. 2022. "Vasorelaxant Effects of Syzygium samarangense (Blume) Merr. and L.M.Perry Extract Are Mediated by NO/cGMP Pathway in Isolated Rat Thoracic Aorta" Pharmaceuticals 15, no. 11: 1349. https://doi.org/10.3390/ph15111349
APA StyleHassan, N. A., Abdelfattah, M. A. O., Mandour, Y. M., El-Shazly, A. M., Sobeh, M., & Mahmoud, M. F. (2022). Vasorelaxant Effects of Syzygium samarangense (Blume) Merr. and L.M.Perry Extract Are Mediated by NO/cGMP Pathway in Isolated Rat Thoracic Aorta. Pharmaceuticals, 15(11), 1349. https://doi.org/10.3390/ph15111349







