Adherent Bacteria and Parasiticidal Secretion Products of Human Cervicovaginal Microbiota-Associated Lactobacillus gasseri Confer Non-Identical Cell Protection against Trichomonas vaginalis-Induced Cell Detachment
Abstract
1. Introduction
2. Results
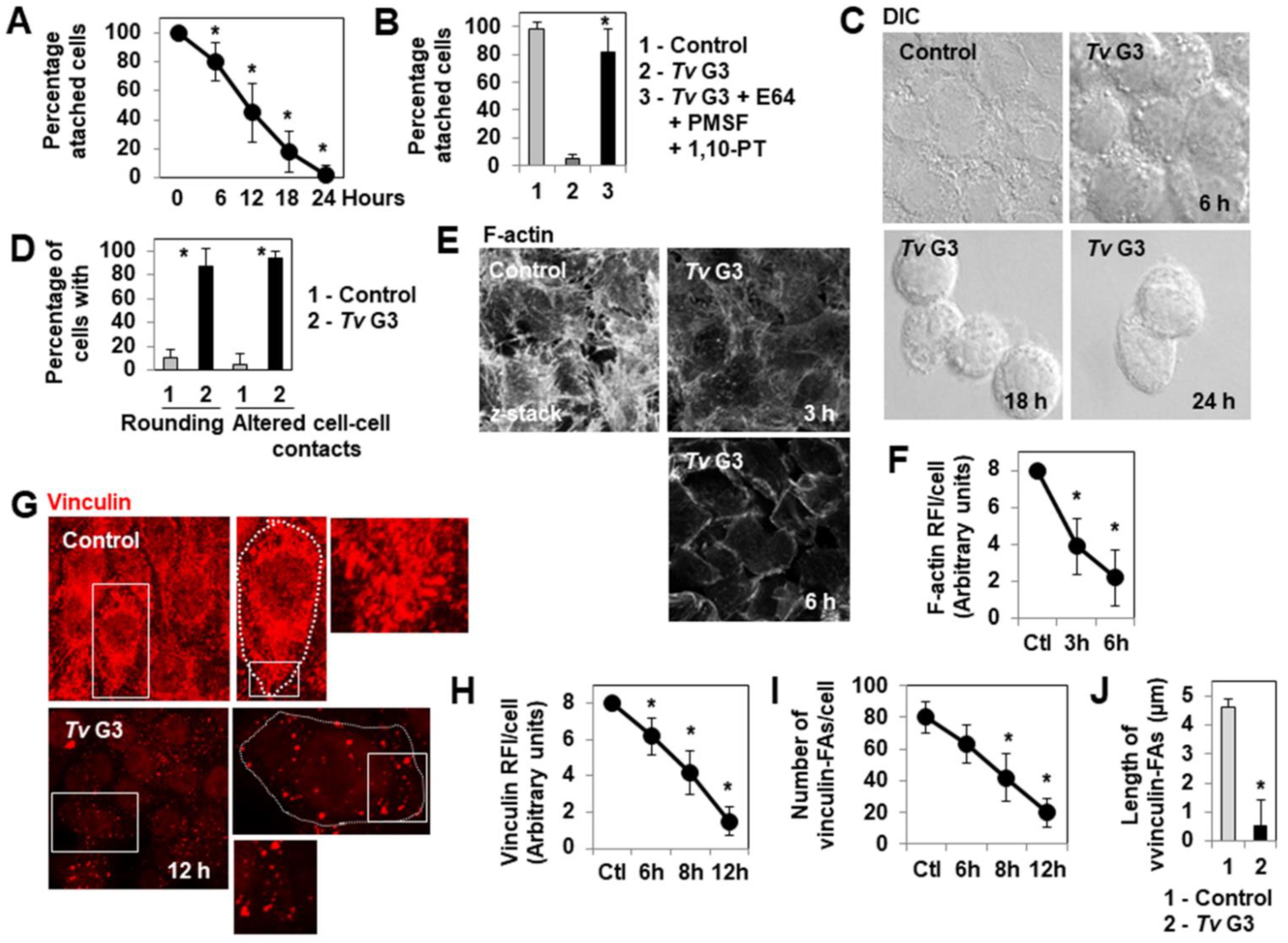
2.1. Mechanism of Tv G3-Induced Cell Detachment in Human Cervicovaginal Epithelial HeLa Cells
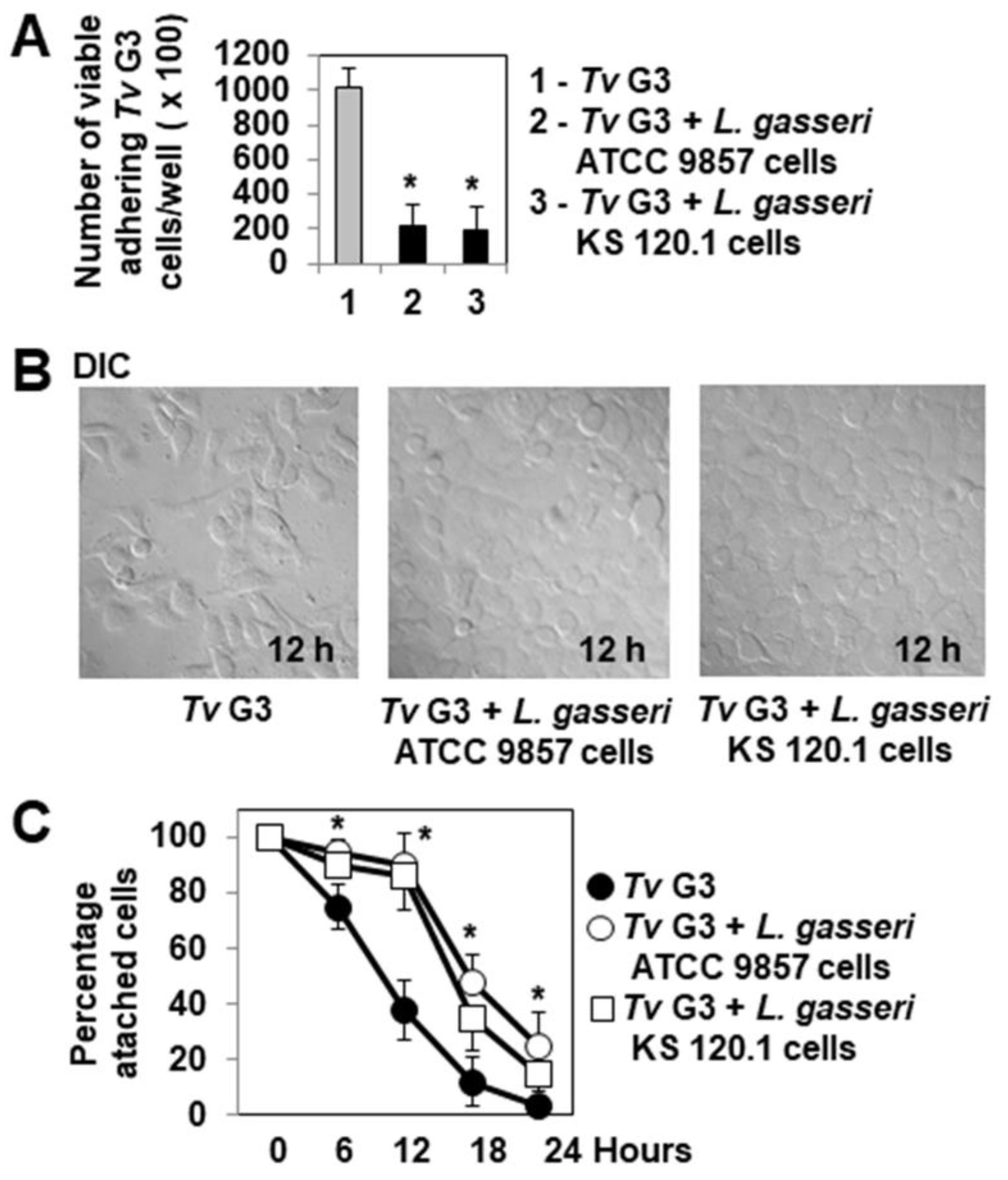
2.2. Pre-Adherent L. gasseri Bacterial Cells Delay Tv G3-Induced Cell Detachment
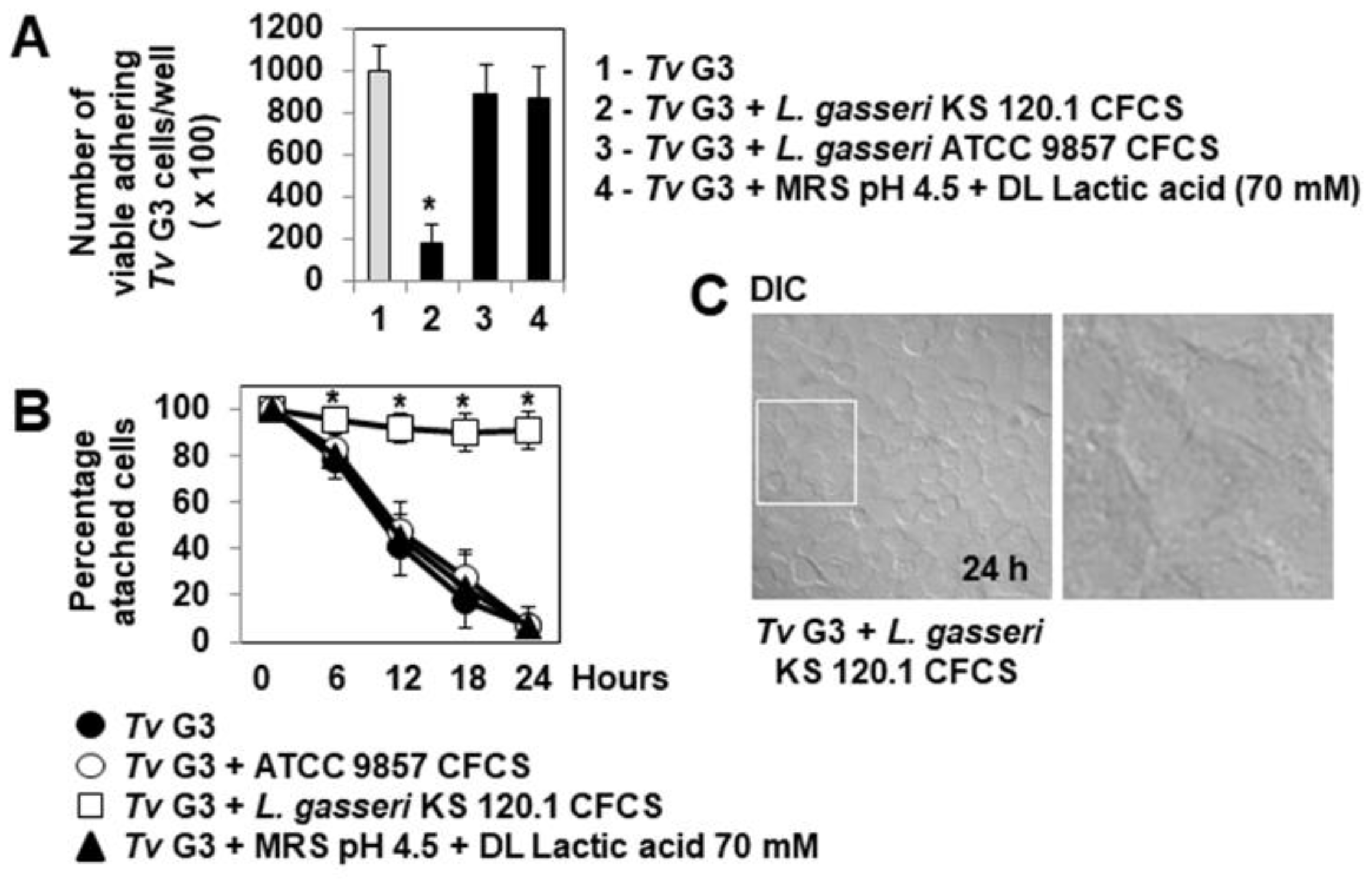
2.3. Anti-Trichomonadal Activity of Secreted Products of L. gasseri
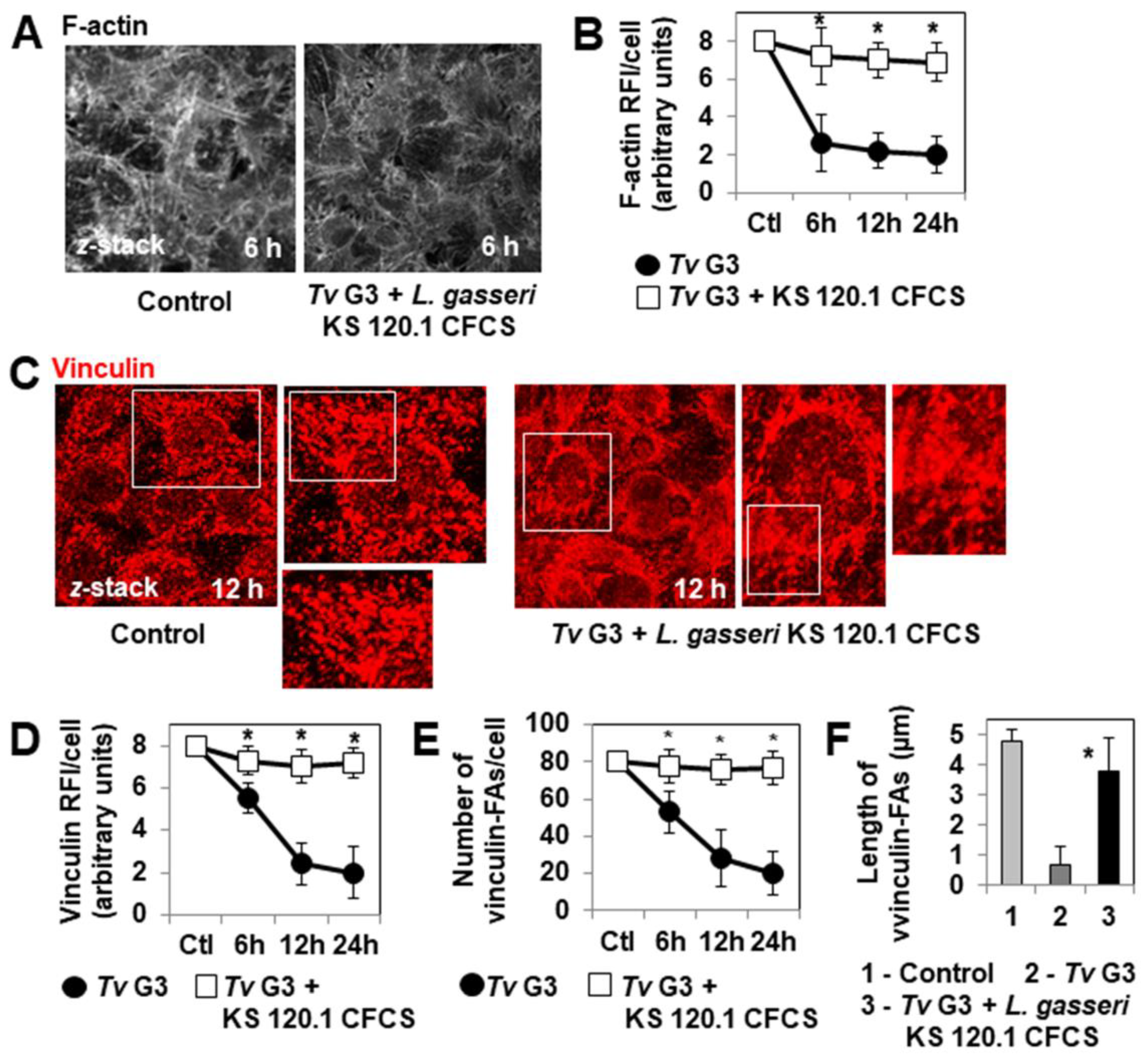
2.4. The Anti-Trichomonadal Activity of L. gasseri Secretion Products Abolishes the Cell-Detachment Effect in Cells Pre-Exposed to Tv G3 Parasites
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. T. vaginalis Strain G3
4.3. Lactobacillus Strains
4.4. Determination of Anti-Trichomonadal Activity
4.5. Culture of Human Cervicovaginal Epithelial HeLa Cells
4.6. Cytoadhesion Assay
4.7. Cell-Detachment Assay
4.8. Fluorescence Labeling
4.9. Confocal Laser Scanning Microscopy (CLMS) and Imaging Analysis
4.10. Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Gerwen, O.T.; Muzny, C.A. Recent advances in the epidemiology, diagnosis, and management of Trichomonas vaginalis infection. F1000Research 2019, 8, 1666. [Google Scholar] [CrossRef] [PubMed]
- Balkus, J.E.; Richardson, B.A.; Rabe, L.K.; Taha, T.E.; Mgodi, N.; Kasaro, M.P.; Ramjee, G.; Hoffman, I.F.; Abdool Karim, S.S. Bacterial vaginosis and the risk of Trichomonas vaginalis acquisition among hiv-1-negative women. Sex. Transm. Dis. 2014, 41, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Brotman, R.M.; Bradford, L.L.; Conrad, M.; Gajer, P.; Ault, K.; Peralta, L.; Forney, L.J.; Carlton, J.M.; Abdo, Z.; Ravel, J. Association between Trichomonas vaginalis and vaginal bacterial community composition among reproductive-age women. Sex. Transm. Dis. 2012, 39, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Fichorova, R.N.; Buck, O.R.; Yamamoto, H.S.; Fashemi, T.; Dawood, H.Y.; Fashemi, B.; Hayes, G.R.; Beach, D.H.; Takagi, Y.; Delaney, M.L.; et al. The villain team-up or how Trichomonas vaginalis and bacterial vaginosis alter innate immunity in concert. Sex. Transm. Infect. 2013, 89, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Swidsinski, A.; Guschin, A.; Tang, Q.; Dorffel, Y.; Verstraelen, H.; Tertychnyy, A.; Khayrullina, G.; Luo, X.; Sobel, J.D.; Jiang, X. Vulvovaginal candidiasis: Histologic lesions are primarily polymicrobial and invasive and do not contain biofilms. Am. J. Obstet. Gynecol. 2019, 220, 91.e1–91.e8. [Google Scholar] [CrossRef]
- Dheilly, N.M.; Ewald, P.W.; Brindley, P.J.; Fichorova, R.N.; Thomas, F. Parasite-microbe-host interactions and cancer risk. PLoS Pathog. 2019, 15, e1007912. [Google Scholar] [CrossRef]
- Workowski, K.A.; Bachmann, L.H.; Chan, P.A.; Johnston, C.M.; Muzny, C.A.; Park, I.; Reno, H.; Zenilman, J.M.; Bolan, G.A. Sexually transmitted infections treatment guidelines. MMWR Recomm. Rep. Morb. Mortal. Wkly. Rep. 2021, 70, 1–187. [Google Scholar]
- Arroyo, R.; Gonzalez-Robles, A.; Martinez-Palomo, A.; Alderete, J.F. Signalling of Trichomonas vaginalis for amoeboid transformation and adhesion synthesis follows cytoadherence. Mol. Microbiol. 1993, 7, 299–309. [Google Scholar] [CrossRef]
- Kusdian, G.; Woehle, C.; Martin, W.F.; Gould, S.B. The actin-based machinery of Trichomonas vaginalis mediates flagellate-amoeboid transition and migration across host tissue. Cell. Microbiol. 2013, 15, 1707–1721. [Google Scholar]
- Carlton, J.M.; Hirt, R.P.; Silva, J.C.; Delcher, A.L.; Schatz, M.; Zhao, Q.; Wortman, J.R.; Bidwell, S.L.; Alsmark, U.C.; Besteiro, S.; et al. Draft genome sequence of the sexually transmitted pathogen Trichomonas vaginalis. Science 2007, 315, 207–212. [Google Scholar] [CrossRef]
- Gould, S.B.; Woehle, C.; Kusdian, G.; Landan, G.; Tachezy, J.; Zimorski, V.; Martin, W.F. Deep sequencing of Trichomonas vaginalis during the early infection of vaginal epithelial cells and amoeboid transition. Intern. J. Parasitol. 2013, 43, 707–719. [Google Scholar] [CrossRef]
- Alderete, J.F.; O’Brien, J.L.; Arroyo, R.; Engbring, J.A.; Musatovova, O.; Lopez, O.; Lauriano, C.; Nguyen, J. Cloning and molecular characterization of two genes encoding adhesion proteins involved in Trichomonas vaginalis cytoadherence. Mol. Microbiol. 1995, 17, 69–83. [Google Scholar] [CrossRef]
- Arroyo, R.; Engbring, J.; Nguyen, J.; Musatovova, O.; Lopez, O.; Lauriano, C.; Alderete, J.F. Characterization of cdnas encoding adhesin proteins involved in Trichomonas vaginalis cytoadherence. Arch. Med. Res. 1995, 26, 361–369. [Google Scholar]
- Hernandez, H.; Sariego, I.; Garber, G.; Delgado, R.; Lopez, O.; Sarracent, J. Monoclonal antibodies against a 62 kda proteinase of Trichomonas vaginalis decrease parasite cytoadherence to epithelial cells and confer protection in mice. Parasite Immunol. 2004, 26, 119–125. [Google Scholar] [CrossRef]
- de Miguel, N.; Lustig, G.; Twu, O.; Chattopadhyay, A.; Wohlschlegel, J.A.; Johnson, P.J. Proteome analysis of the surface of Trichomonas vaginalis reveals novel proteins strain-dependent differential expression. Mol. Cell. Proteom. 2010, 9, 1554–1566. [Google Scholar] [CrossRef]
- Bastida-Corcuera, F.D.; Okumura, C.Y.; Colocoussi, A.; Johnson, P.J. Trichomonas vaginalis lipophosphoglycan mutants have reduced adherence and cytotoxicity to human ectocervical cells. Eukaryotic Cell 2005, 4, 1951–1958. [Google Scholar] [CrossRef]
- Munoz, C.; Perez, M.; Orrego, P.R.; Osorio, L.; Gutierrez, B.; Sagua, H.; Castillo, J.L.; Martinez-Oyanedel, J.; Arroyo, R.; Meza-Cervantez, P.; et al. A protein phosphatase 1 gamma (PP1gamma) of the human protozoan parasite Trichomonas vaginalis is involved in proliferation and cell attachment to the host cell. Intern. J. Parasitol. 2012, 42, 715–727. [Google Scholar] [CrossRef]
- Chen, Y.P.; Riestra, A.M.; Rai, A.K.; Johnson, P.J. A novel cadherin-like protein mediates adherence to and killing of host cells by the parasite Trichomonas vaginalis. MBio 2019, 10, e00720-19. [Google Scholar] [CrossRef]
- Kucknoor, A.S.; Mundodi, V.; Alderete, J.F. The proteins secreted by Trichomonas vaginalis and vaginal epithelial cell response to secreted and episomally expressed AP65. Cell. Microbiol. 2007, 9, 2586–2597. [Google Scholar] [CrossRef]
- Alderete, J.F.; Pearlman, E. Pathogenic Trichomonas vaginalis cytotoxicity to cell culture monolayers. Br. J. Ven. Dis. 1984, 60, 99–105. [Google Scholar] [CrossRef]
- Alvarez-Sanchez, M.E.; Avila-Gonzalez, L.; Becerril-Garcia, C.; Fattel-Facenda, L.V.; Ortega-Lopez, J.; Arroyo, R. A novel cysteine proteinase (CP65) of Trichomonas vaginalis involved in cytotoxicity. Microb. Pathog. 2000, 28, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Jesus, J.B.; Vannier-Santos, M.A.; Britto, C.; Godefroy, P.; Silva-Filho, F.C.; Pinheiro, A.A.; Rocha-Azevedo, B.; Lopes, A.H.; Meyer-Fernandes, J.R. Trichomonas vaginalis virulence against epithelial cells and morphological variability: The comparison between a well-established strain and a fresh isolate. Parasitol. Res. 2004, 93, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.C.; Chang, W.T.; Chang, T.Y.; Shin, J.W. The pathogenesis of human cervical epithelium cells induced by interacting with Trichomonas vaginalis. PLoS ONE 2015, 10, e0124087. [Google Scholar] [CrossRef] [PubMed]
- Lustig, G.; Ryan, C.M.; Secor, W.E.; Johnson, P.J. Trichomonas vaginalis contact-dependent cytolysis of epithelial cells. Infect. Immun. 2013, 81, 1411–1419. [Google Scholar] [CrossRef] [PubMed]
- Riestra, A.M.; Gandhi, S.; Sweredoski, M.J.; Moradian, A.; Hess, S.; Urban, S.; Johnson, P.J. A Trichomonas vaginalis rhomboid protease and its substrate modulate parasite attachment and cytolysis of host cells. PLoS Pathog. 2015, 11, e1005294. [Google Scholar] [CrossRef]
- Ramon-Luing, L.A.; Rendon-Gandarilla, F.J.; Puente-Rivera, J.; Avila-Gonzalez, L.; Arroyo, R. Identification and characterization of the immunogenic cytotoxic TvCP39 proteinase gene of Trichomonas vaginalis. Int. J. Biochem. Cell Biol. 2011, 43, 1500–1511. [Google Scholar] [CrossRef]
- Alderete, J.F.; Garza, G.E. Specific nature of Trichomonas vaginalis parasitism of host cell surfaces. Infect. Immun. 1985, 50, 701–708. [Google Scholar] [CrossRef]
- Ma, L.; Meng, Q.; Cheng, W.; Sung, Y.; Tang, P.; Hu, S.; Yu, J. Involvement of the GP63 protease in infection of Trichomonas vaginalis. Parasitol. Res. 2011, 109, 71–79. [Google Scholar] [CrossRef]
- Arroyo, R.; Alderete, J.F. Trichomonas vaginalis surface proteinase activity is necessary for parasite adherence to epithelial cells. Infect. Immun. 1989, 57, 2991–2997. [Google Scholar] [CrossRef]
- Quan, J.H.; Choi, I.W.; Yang, J.B.; Zhou, W.; Cha, G.H.; Zhou, Y.; Ryu, J.S.; Lee, Y.H. Trichomonas vaginalis metalloproteinase induces mtor cleavage of siha cells. Korean J. Parasitol. 2014, 52, 595–603. [Google Scholar] [CrossRef]
- Sommer, U.; Costello, C.E.; Hayes, G.R.; Beach, D.H.; Gilbert, R.O.; Lucas, J.J.; Singh, B.N. Identification of Trichomonas vaginalis cysteine proteases that induce apoptosis in human vaginal epithelial cells. J. Biol. Chem. 2005, 280, 23853–23860. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhao, L.; Brittingham, A.; Bai, Q.; Wakefield, M.R.; Fang, Y. Trichomonas vaginalis inhibits hela cell growth through modulation of critical molecules for cell proliferation and apoptosis. Anticancer Res. 2018, 38, 5079–5086. [Google Scholar] [CrossRef]
- Gao, F.F.; Quan, J.H.; Lee, M.A.; Ye, W.; Yuk, J.M.; Cha, G.H.; Choi, I.W.; Lee, Y.H. Trichomonas vaginalis induces apoptosis via ROS and ER stress response through ER-mitochondria crosstalk in SiHa cells. Parasites Vectors 2021, 14, 603. [Google Scholar] [CrossRef]
- Miranda-Ozuna, J.F.T.; Rivera-Rivas, L.A.; Cardenas-Guerra, R.E.; Hernandez-Garcia, M.S.; Rodriguez-Cruz, S.; Gonzalez-Robles, A.; Chavez-Munguia, B.; Arroyo, R. Glucose-restriction increases Trichomonas vaginalis cellular damage towards hela cells and proteolytic activity of cysteine proteinases (CPS), such as TvCP2. Parasitology 2019, 146, 1156–1166. [Google Scholar] [CrossRef]
- Pindak, F.F.; Gardner, W.A., Jr.; Mora de Pindak, M. Growth and cytopathogenicity of Trichomonas vaginalis in tissue cultures. J. Clin. Microbiol. 1986, 23, 672–678. [Google Scholar] [CrossRef]
- Quan, J.H.; Kang, B.H.; Cha, G.H.; Zhou, W.; Koh, Y.B.; Yang, J.B.; Yoo, H.J.; Lee, M.A.; Ryu, J.S.; Noh, H.T.; et al. Trichonomas vaginalis metalloproteinase induces apoptosis of SiHa cells through disrupting the Mcl-1/Bim and Bcl-xL/Bim complexes. PLoS ONE 2014, 9, e110659. [Google Scholar]
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. 1), 4680–4687. [Google Scholar] [CrossRef]
- France, M.; Alizadeh, M.; Brown, S.; Ma, B.; Ravel, J. Towards a deeper understanding of the vaginal microbiota. Nat. Microbiol. 2022, 7, 367–378. [Google Scholar] [CrossRef]
- Torok, M.R.; Miller, W.C.; Hobbs, M.M.; Macdonald, P.D.; Leone, P.A.; Schwebke, J.R.; Sena, A.C. The association between Trichomonas vaginalis infection and level of vaginal lactobacilli, in nonpregnant women. J. Infect. Dis. 2007, 196, 1102–1107. [Google Scholar] [CrossRef]
- Muzny, C.A.; Laniewski, P.; Schwebke, J.R.; Herbst-Kralovetz, M.M. Host-vaginal microbiota interactions in the pathogenesis of bacterial vaginosis. Curr. Opin. Infect. Dis. 2020, 33, 59–65. [Google Scholar] [CrossRef]
- Whiteside, S.A.; Razvi, H.; Dave, S.; Reid, G.; Burton, J.P. The microbiome of the urinary tract—A role beyond infection. Nat. Rev. Urol. 2015, 12, 81–90. [Google Scholar] [CrossRef]
- Martinotti, M.G.; Martinetto, P.; Savoia, D. Adherence of Trichomonas vaginalis to cell culture monolayers. Eur. J. Clin. Microbiol. 1986, 5, 320–323. [Google Scholar] [CrossRef] [PubMed]
- Phukan, N.; Brooks, A.E.S.; Simoes-Barbosa, A. A cell surface aggregation-promoting factor from Lactobacillus gasseri contributes to inhibition of Trichomonas vaginalis adhesion to human vaginal ectocervical vells. Infect. Immun. 2018, 86, e00907-17. [Google Scholar] [CrossRef] [PubMed]
- Phukan, N.; Parsamand, T.; Brooks, A.E.; Nguyen, T.N.; Simoes-Barbosa, A. The adherence of Trichomonas vaginalis to host ectocervical cells is influenced by lactobacilli. Sex. Transm. Infect. 2013, 89, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Suissa, R.; Oved, R.; Jankelowitz, G.; Turjeman, S.; Koren, O.; Kolodkin-Gal, I. Molecular genetics for probiotic engineering: Dissecting lactic acid bacteria. Trends Microbiol. 2022, 30, 293–306. [Google Scholar] [CrossRef]
- Atassi, F.; Brassart, D.; Grob, P.; Graf, F.; Servin, A.L. Lactobacillus strains isolated from the vaginal microbiota of healthy women inhibit Prevotella bivia and Gardnerella vaginalis in coculture and cell culture. FEMS Immunol. Med. Microbiol. 2006, 48, 424–432. [Google Scholar] [CrossRef]
- Atassi, F.; Pho Viet Ahn, D.L.; Lievin-Le Moal, V. Diverse expression of antimicrobial activities against bacterial vaginosis and urinary tract infection pathogens by cervicovaginal microbiota strains of Lactobacillus gasseri and Lactobacillus crispatus. Front. Microbiol. 2019, 10, 2900. [Google Scholar] [CrossRef]
- Spurbeck, R.R.; Arvidson, C.G. Inhibition of Neisseria gonorrhoeae epithelial cell interactions by vaginal Lactobacillus species. Infect. Immun. 2008, 76, 3124–3130. [Google Scholar] [CrossRef]
- Hirt, R.P.; de Miguel, N.; Nakjang, S.; Dessi, D.; Liu, Y.C.; Diaz, N.; Rappelli, P.; Acosta-Serrano, A.; Fiori, P.L.; Mottram, J.C. Trichomonas vaginalis pathobiology new insights from the genome sequence. Adv. Parasitol. 2011, 77, 87–140. [Google Scholar]
- Figueroa-Angulo, E.E.; Rendon-Gandarilla, F.J.; Puente-Rivera, J.; Calla-Choque, J.S.; Cardenas-Guerra, R.E.; Ortega-Lopez, J.; Quintas-Granados, L.I.; Alvarez-Sanchez, M.E.; Arroyo, R. The effects of environmental factors on the virulence of Trichomonas vaginalis. Microbes Infect. 2012, 14, 1411–1427. [Google Scholar] [CrossRef]
- Mercer, F.; Johnson, P.J. Trichomonas vaginalis: Pathogenesis, symbiont interactions, and host cell immune responses. Trends Parasitol. 2018, 34, 683–693. [Google Scholar] [CrossRef]
- Svitkina, T.M. Ultrastructure of the actin cytoskeleton. Curr. Opin. Cell. Biol. 2018, 54, 1–8. [Google Scholar] [CrossRef]
- Bays, J.L.; DeMali, K.A. Vinculin in cell-cell and cell-matrix adhesions. CMLS 2017, 74, 2999–3009. [Google Scholar] [CrossRef]
- Stoner, K.A.; Rabe, L.K.; Meyn, L.A.; Hillier, S.L. Survival of Trichomonas vaginalis in wet preparation and on wet mount. Sex. Transm. Infect. 2013, 89, 485–488. [Google Scholar] [CrossRef]
- Haase, K.; Pelling, A.E. The role of the actin cortex in maintaining cell shape. Comm. Integr. Biol. 2013, 6, e26714. [Google Scholar] [CrossRef]
- Lesay, A.; Hickman, J.A.; Gibson, R.M. Disruption of focal adhesions mediates detachment during neuronal apoptosis. Neuroreport 2001, 12, 2111–2115. [Google Scholar] [CrossRef]
- Levkau, B.; Herren, B.; Koyama, H.; Ross, R.; Raines, E.W. Caspase-mediated cleavage of focal adhesion kinase pp125FAK and disassembly of focal adhesions in human endothelial cell apoptosis. J. Exp. Med. 1998, 187, 579–586. [Google Scholar] [CrossRef]
- van de Water, B.; Nagelkerke, J.F.; Stevens, J.L. Dephosphorylation of focal adhesion kinase (FAK) and loss of focal contacts precede caspase-mediated cleavage of FAK during apoptosis in renal epithelial cells. J. Biol. Chem. 1999, 274, 13328–13337. [Google Scholar] [CrossRef]
- Lievin-Le Moal, V.; Comenge, Y.; Ruby, V.; Amsellem, R.; Nicolas, V.; Servin, A.L. Secreted autotransporter toxin (Sat) triggers autophagy in epithelial cells that relies on cell detachment. Cell. Microbiol. 2011, 13, 992–1013. [Google Scholar] [CrossRef]
- Navarro-Garcia, F.; Serapio-Palacios, A.; Vidal, J.E.; Salazar, M.I.; Tapia-Pastrana, G. Espc promotes epithelial cell detachment by enteropathogenic Escherichia coli via sequential cleavages of a cytoskeletal protein and then focal adhesion proteins. Infect. Immun. 2014, 82, 2255–2265. [Google Scholar] [CrossRef]
- Bhatt, A.; Kaverina, I.; Otey, C.; Huttenlocher, A. Regulation of focal complex composition and disassembly by the calcium-dependent protease calpain. J. Cell. Sci. 2002, 115, 3415–3425. [Google Scholar] [CrossRef] [PubMed]
- Cuevas, B.D.; Abell, A.N.; Witowsky, J.A.; Yujiri, T.; Johnson, N.L.; Kesavan, K.; Ware, M.; Jones, P.L.; Weed, S.A.; DeBiasi, R.L.; et al. Mekk1 regulates calpain-dependent proteolysis of focal adhesion proteins for rear-end detachment of migrating fibroblasts. Embo. J. 2003, 22, 3346–3355. [Google Scholar] [CrossRef] [PubMed]
- Muzny, C.A.; Taylor, C.M.; Swords, W.E.; Tamhane, A.; Chattopadhyay, D.; Cerca, N.; Schwebke, J.R. An updated conceptual model on the pathogenesis of bacterial vaginosis. J. Infect. Dis. 2019, 220, 1399–1405. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, R.O.; Elia, G.; Beach, D.H.; Klaessig, S.; Singh, B.N. Cytopathogenic effect of Trichomonas vaginalis on human vaginal epithelial cells cultured in vitro. Infect. Immun. 2000, 68, 4200–4206. [Google Scholar] [CrossRef] [PubMed]
- Lievin-Le Moal, V.; Servin, A.L. Anti-infective activities of Lactobacillus strains in the human intestinal microbiota: From probiotics to gastrointestinal anti-infectious biotherapeutic agents. Clin. Microbiol. Rev. 2014, 27, 167–199. [Google Scholar] [CrossRef]
- Spurbeck, R.R.; Arvidson, C.G. Lactobacilli at the front line of defense against vaginally acquired infections. Future Microbiol. 2011, 6, 567–582. [Google Scholar] [CrossRef]
- FAO/WHO. Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid bacteria. Cordoba, Argentina 1 to 4 October 2001. Available online: https://www.fao.org/3/a0512e/a0512e.pdf (accessed on 1 January 2006).
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Nader-Macias, M.E.; Juarez Tomas, M.S. Profiles and technological requirements of urogenital probiotics. Adv. Drug Deliv. Rev. 2015, 92, 84–104. [Google Scholar] [CrossRef]
- van de Wijgert, J.; Verwijs, M.C. Lactobacilli-containing vaginal probiotics to cure or prevent bacterial or fungal vaginal dysbiosis: A systematic review and recommendations for future trial designs. BJOG 2020, 127, 287–299. [Google Scholar] [CrossRef]
- Sgibnev, A.; Kremleva, E. Probiotics in addition to metronidazole for treatment Trichomonas vaginalis in the presence of BV: A randomized, placebo-controlled, double-blind study. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 345–351. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef] [PubMed]
- Palmeira-de-Oliveira, R.; Palmeira-de-Oliveira, A.; Martinez-de-Oliveira, J. New strategies for local treatment of vaginal infections. Adv. Drug Deliv. Rev. 2015, 92, 105–122. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.G.; Diamond, L.S. Methods for cultivation of luminal parasitic protists of clinical importance. Clin. Microbiol. Rev. 2002, 15, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Lievin-Le Moal, V.; Amsellem, R.; Servin, A.L. Impairment of swimming motility by antidiarrheic Lactobacillus acidophilus strain lb retards internalization of Salmonella enterica serovar typhimurium within human enterocyte-like cells. Antimicrob. Agents Chemother. 2011, 55, 4810–4820. [Google Scholar] [CrossRef]
- Burgess, D.E.; Knoblock, K.F.; Daugherty, T.; Robertson, N.P. Cytotoxic and hemolytic effects of Trichomonas foetus on mammalian cells. Infect. Immun. 1990, 58, 3627–3632. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pradines, B.; Domenichini, S.; Lievin-Le Moal, V. Adherent Bacteria and Parasiticidal Secretion Products of Human Cervicovaginal Microbiota-Associated Lactobacillus gasseri Confer Non-Identical Cell Protection against Trichomonas vaginalis-Induced Cell Detachment. Pharmaceuticals 2022, 15, 1350. https://doi.org/10.3390/ph15111350
Pradines B, Domenichini S, Lievin-Le Moal V. Adherent Bacteria and Parasiticidal Secretion Products of Human Cervicovaginal Microbiota-Associated Lactobacillus gasseri Confer Non-Identical Cell Protection against Trichomonas vaginalis-Induced Cell Detachment. Pharmaceuticals. 2022; 15(11):1350. https://doi.org/10.3390/ph15111350
Chicago/Turabian StylePradines, Bénédicte, Séverine Domenichini, and Vanessa Lievin-Le Moal. 2022. "Adherent Bacteria and Parasiticidal Secretion Products of Human Cervicovaginal Microbiota-Associated Lactobacillus gasseri Confer Non-Identical Cell Protection against Trichomonas vaginalis-Induced Cell Detachment" Pharmaceuticals 15, no. 11: 1350. https://doi.org/10.3390/ph15111350
APA StylePradines, B., Domenichini, S., & Lievin-Le Moal, V. (2022). Adherent Bacteria and Parasiticidal Secretion Products of Human Cervicovaginal Microbiota-Associated Lactobacillus gasseri Confer Non-Identical Cell Protection against Trichomonas vaginalis-Induced Cell Detachment. Pharmaceuticals, 15(11), 1350. https://doi.org/10.3390/ph15111350




