Magnetic Nanoparticles for Drug Delivery through Tapered Stenosed Artery with Blood Based Non-Newtonian Fluid
Abstract
:1. Introduction
2. Materials
3. Methods and Results
3.1. Zeroth Order System
3.2. First Order System
3.3. Second Order System
4. Discussion
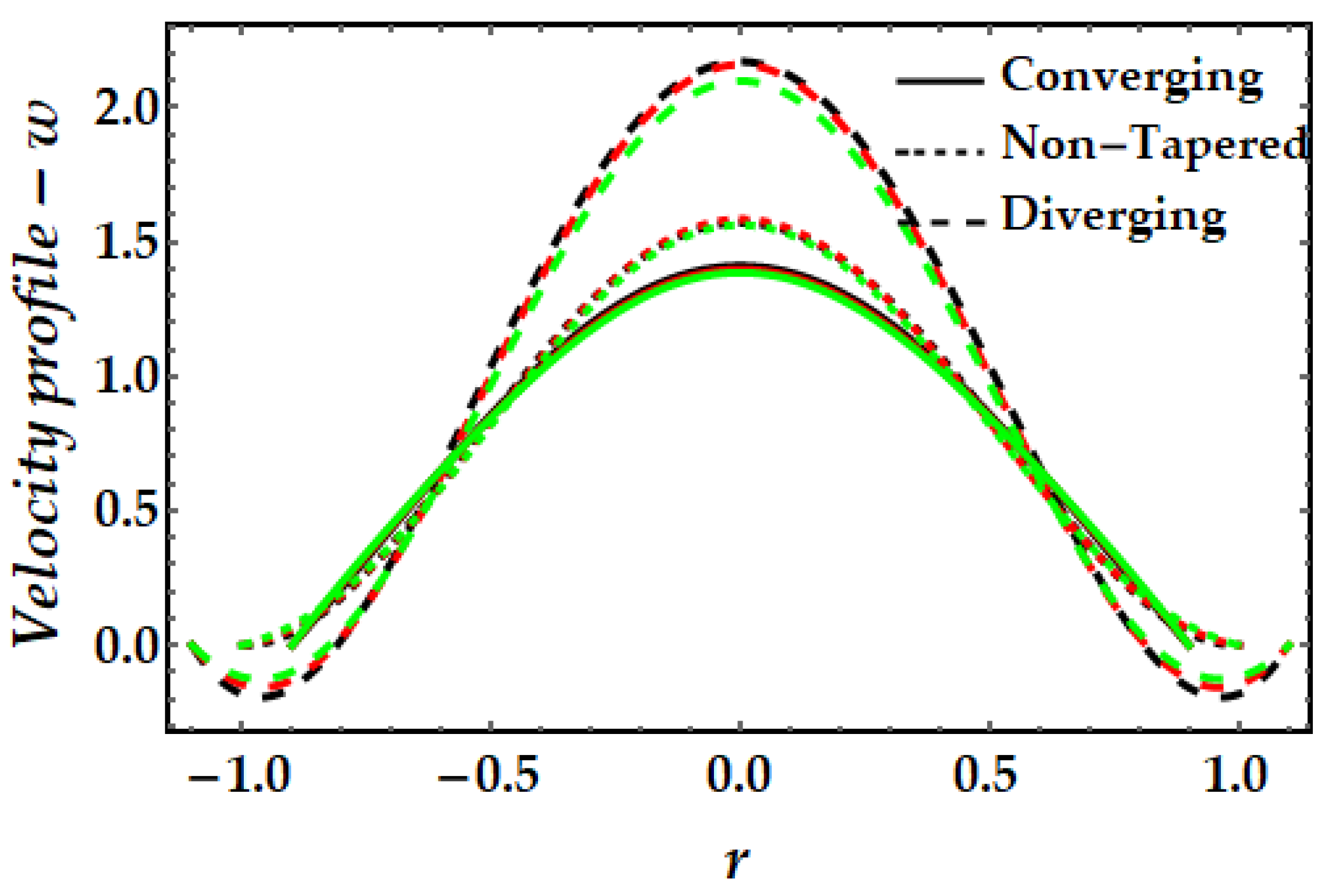
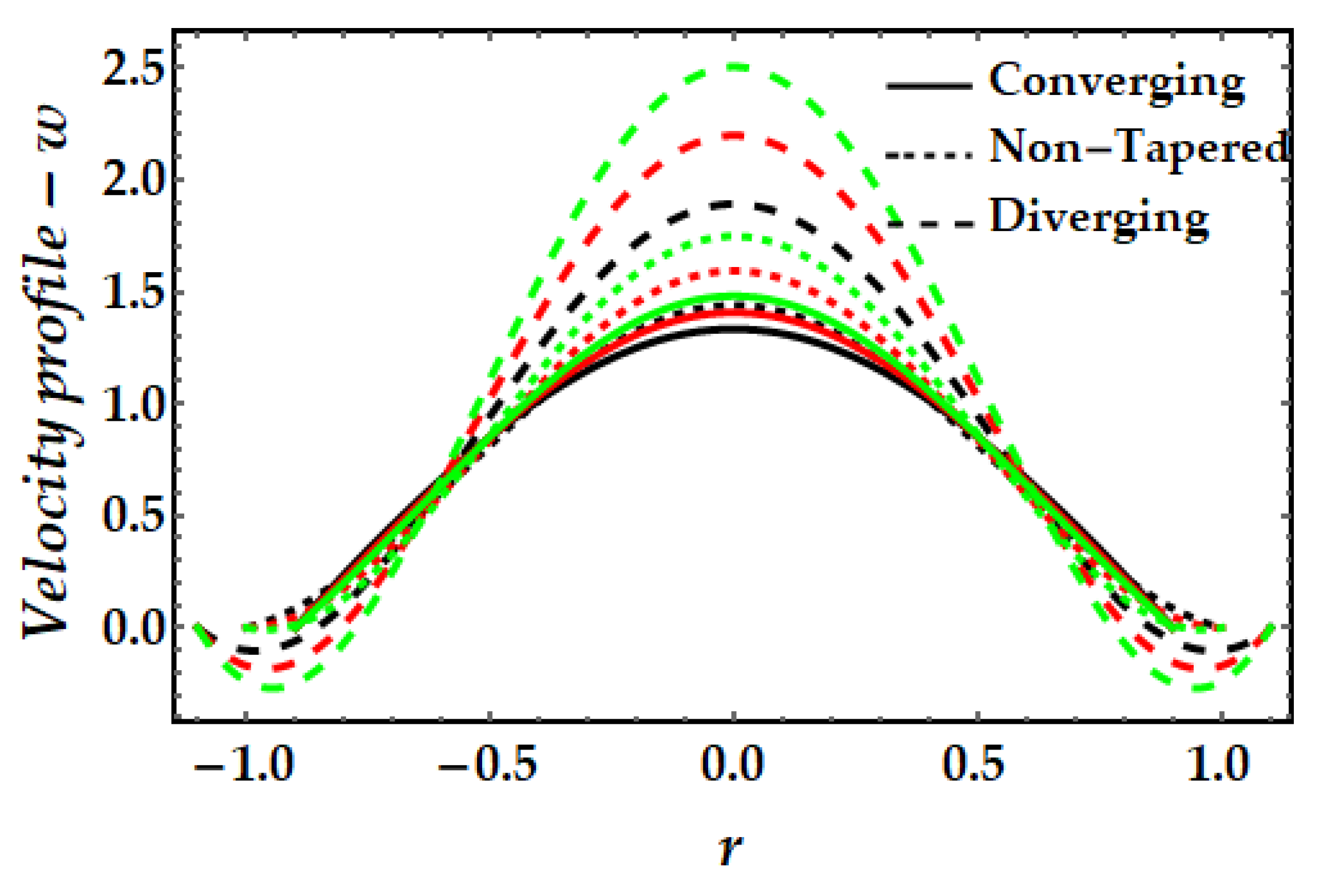
4.1. Velocity Mechanism−w
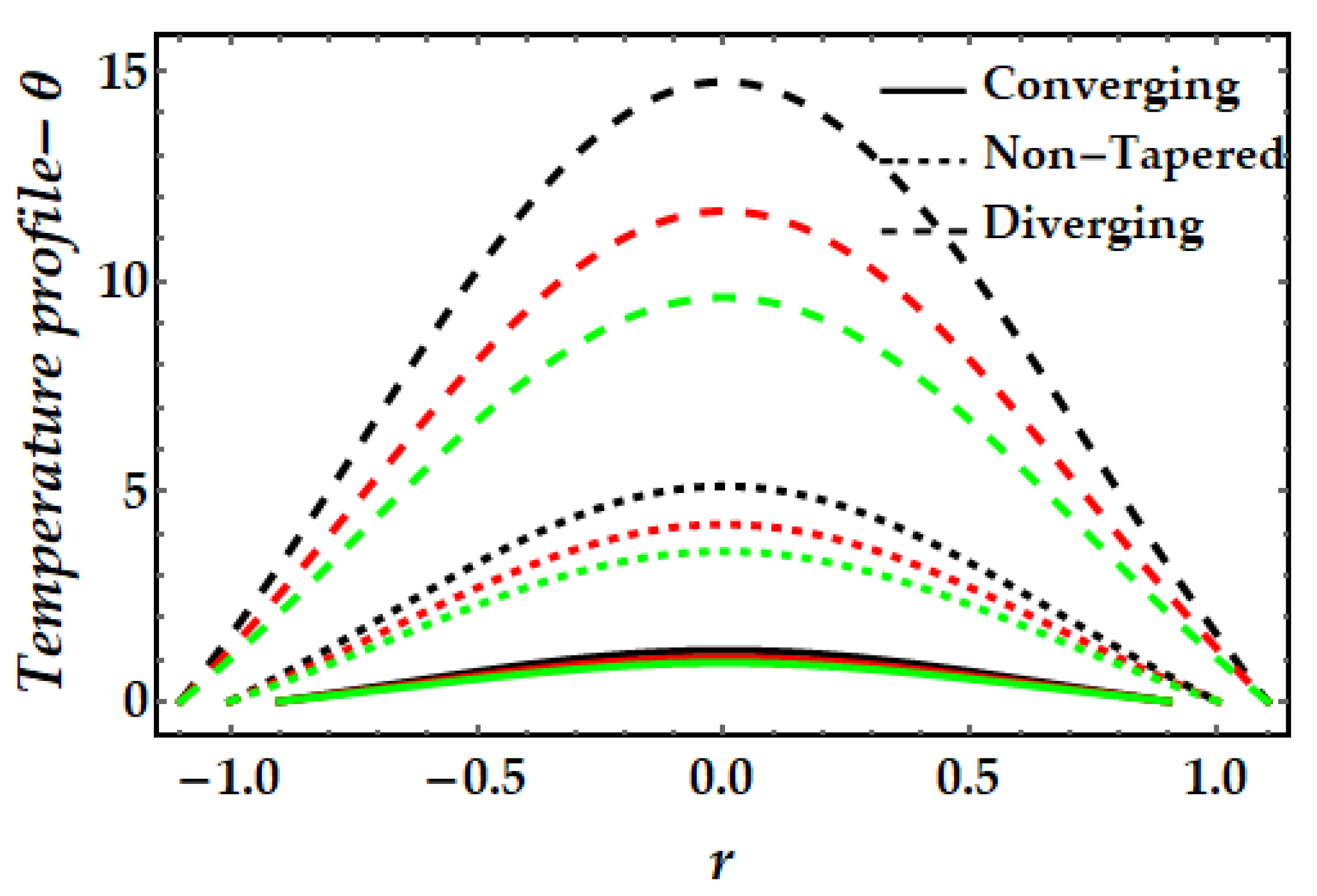
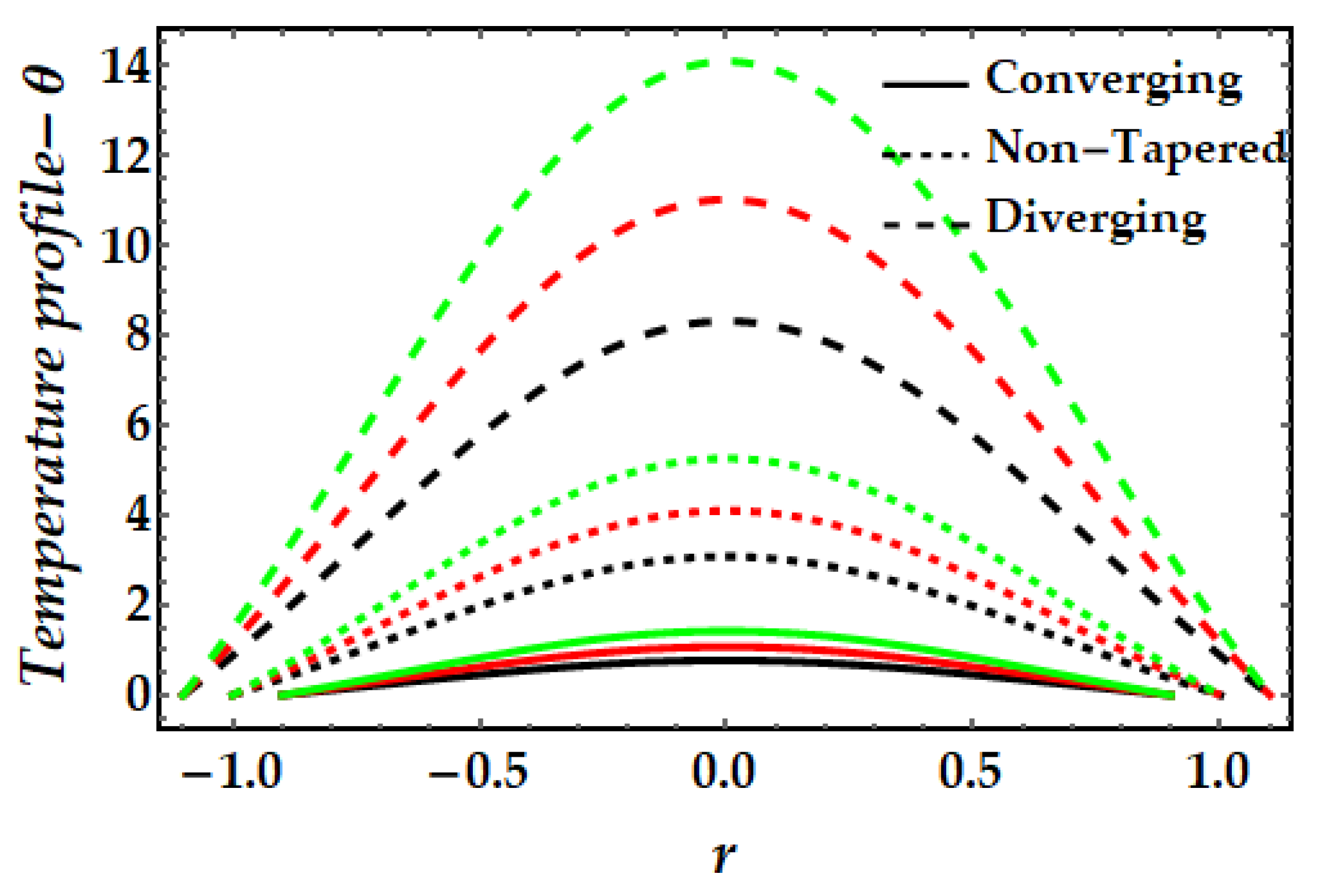
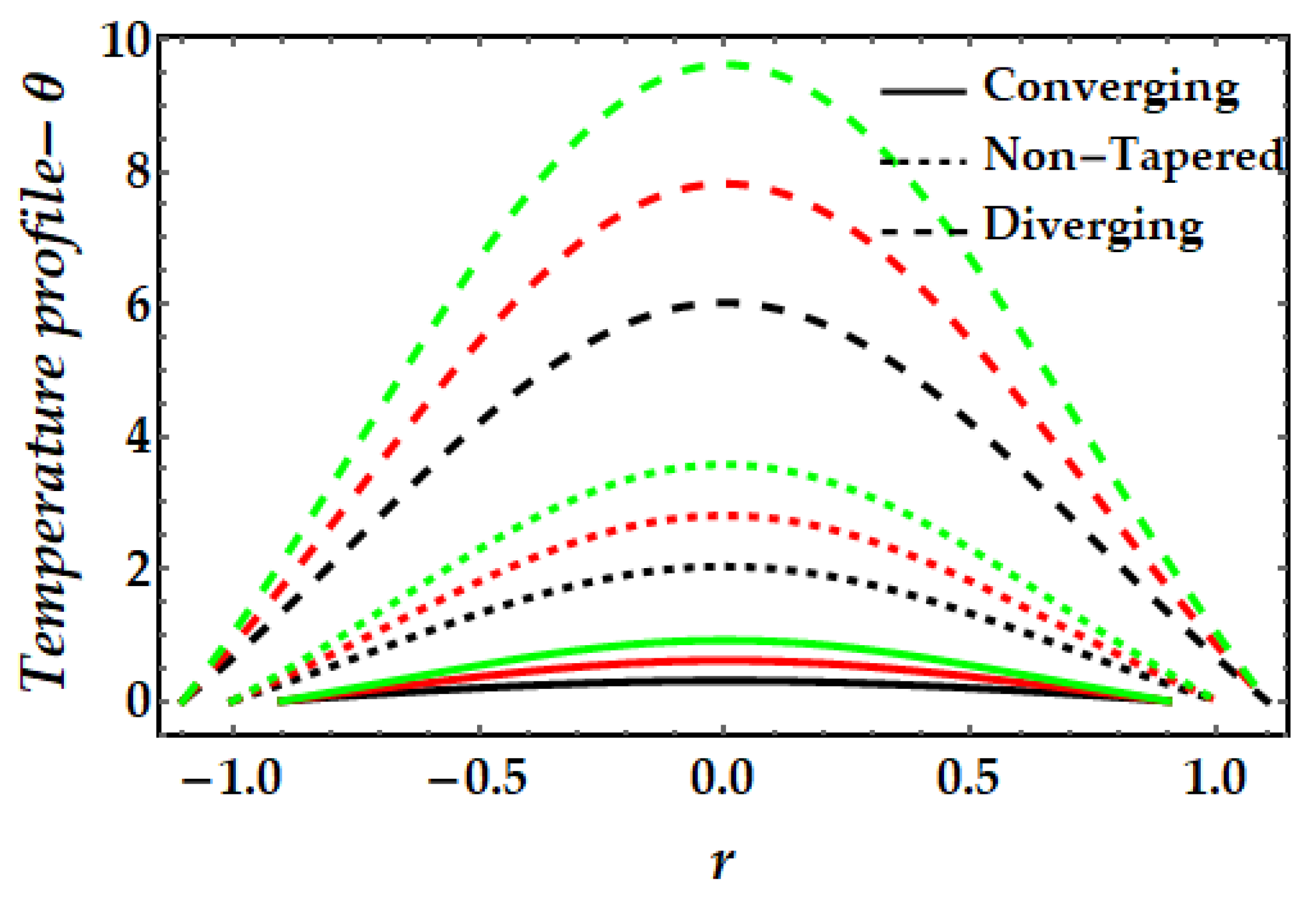
4.2. Temperature Mechanism−θ
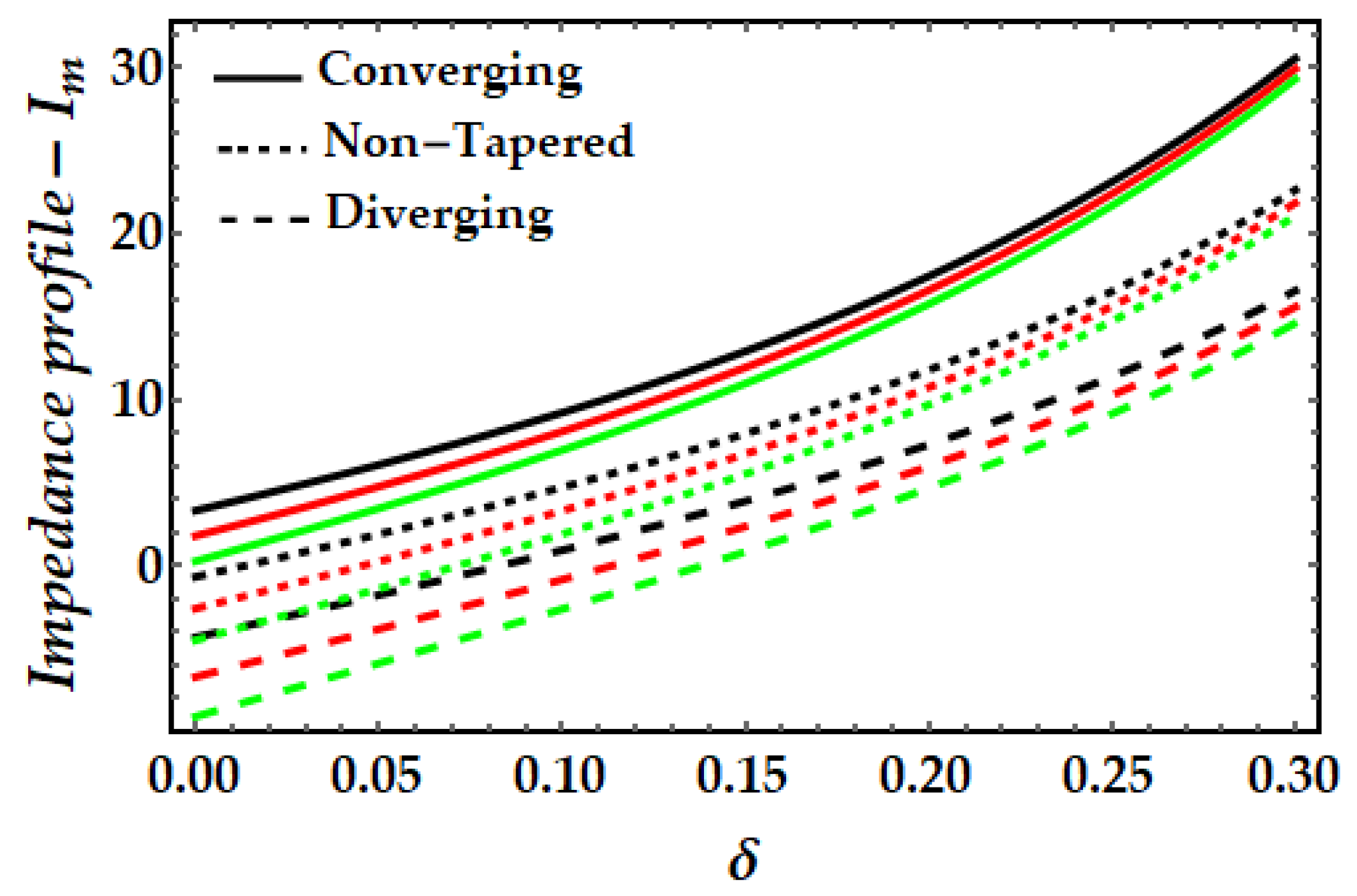
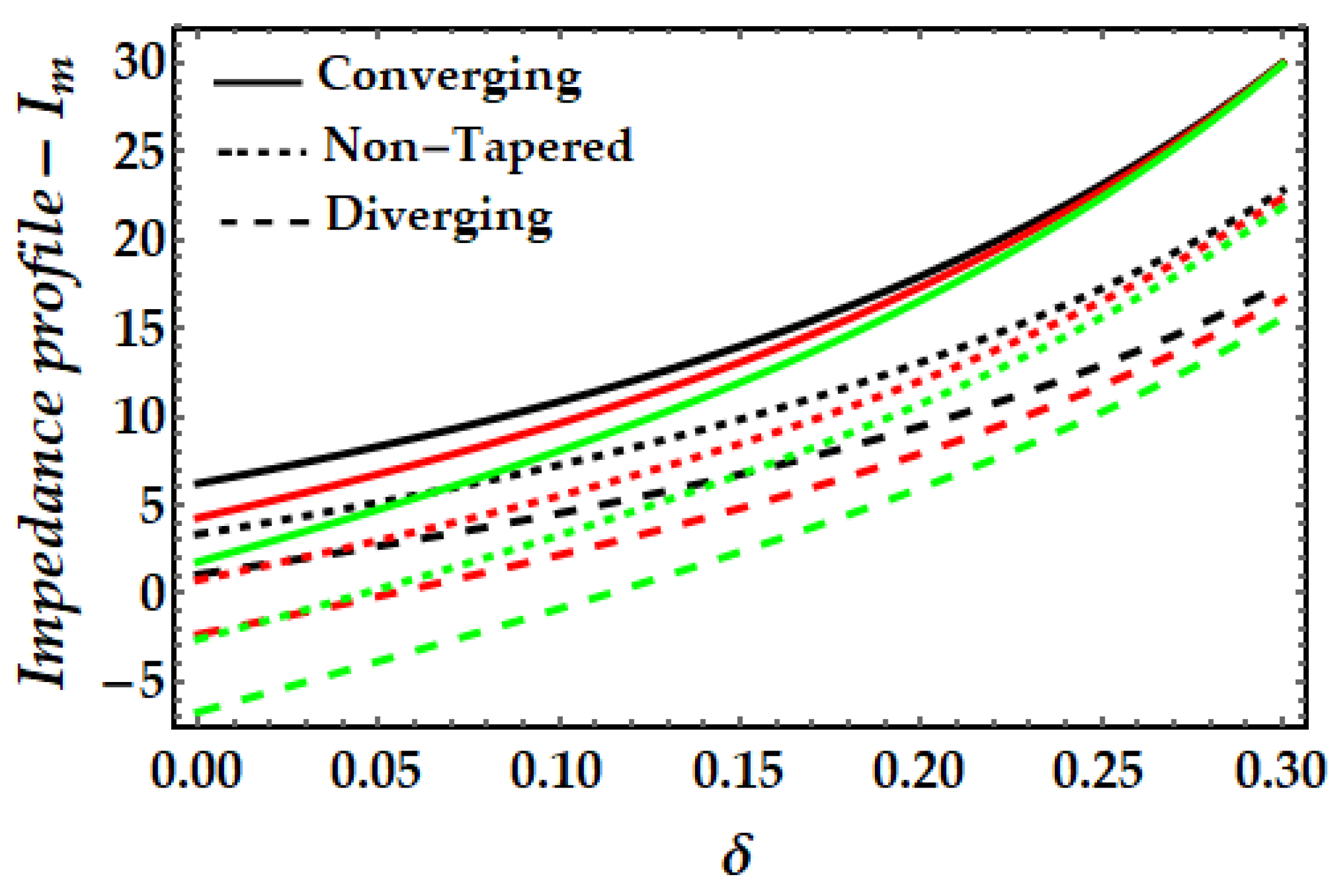
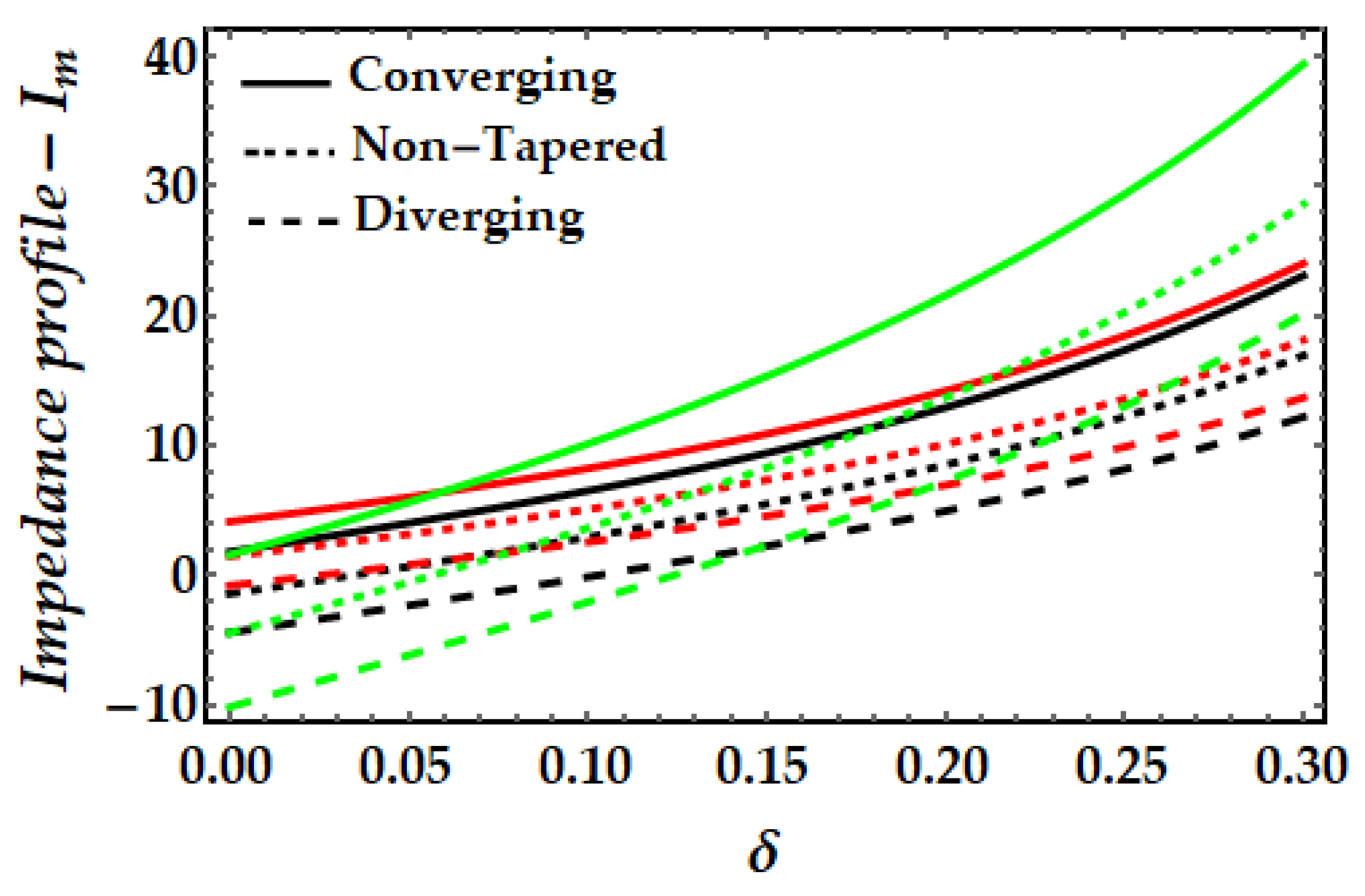
4.3. Impedance Profile−Im
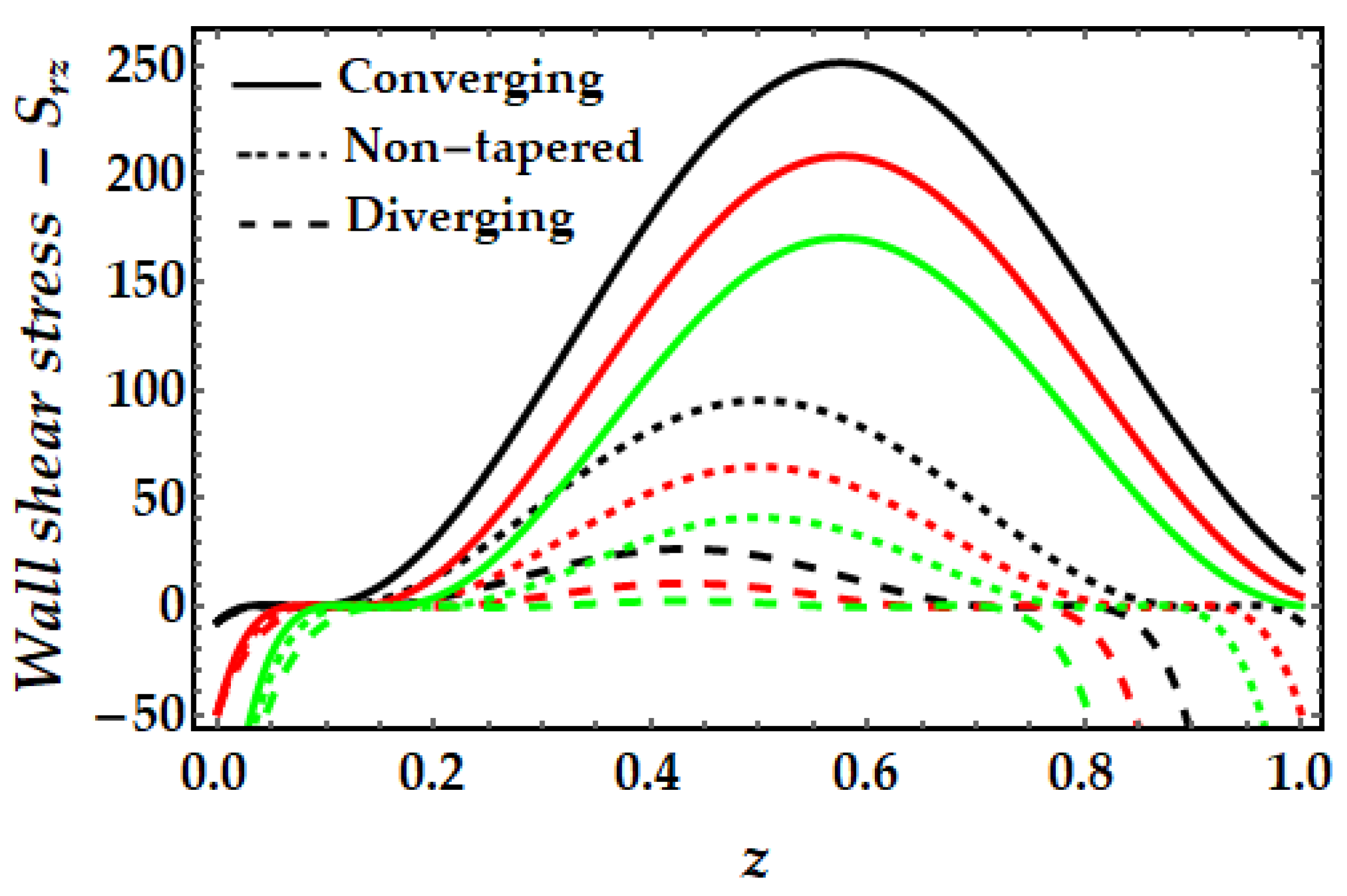
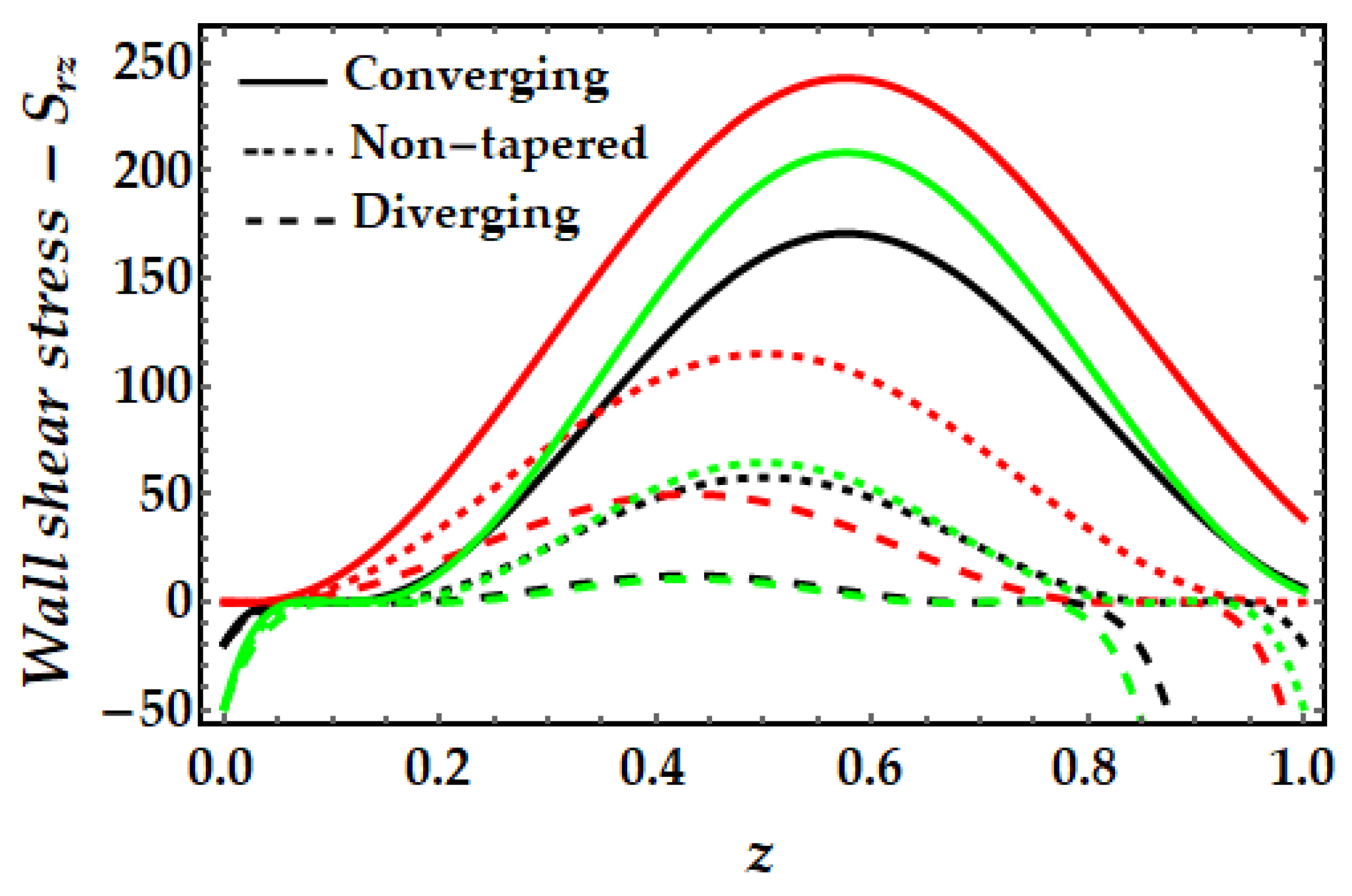
4.4. Wall Shear Stress−Srz
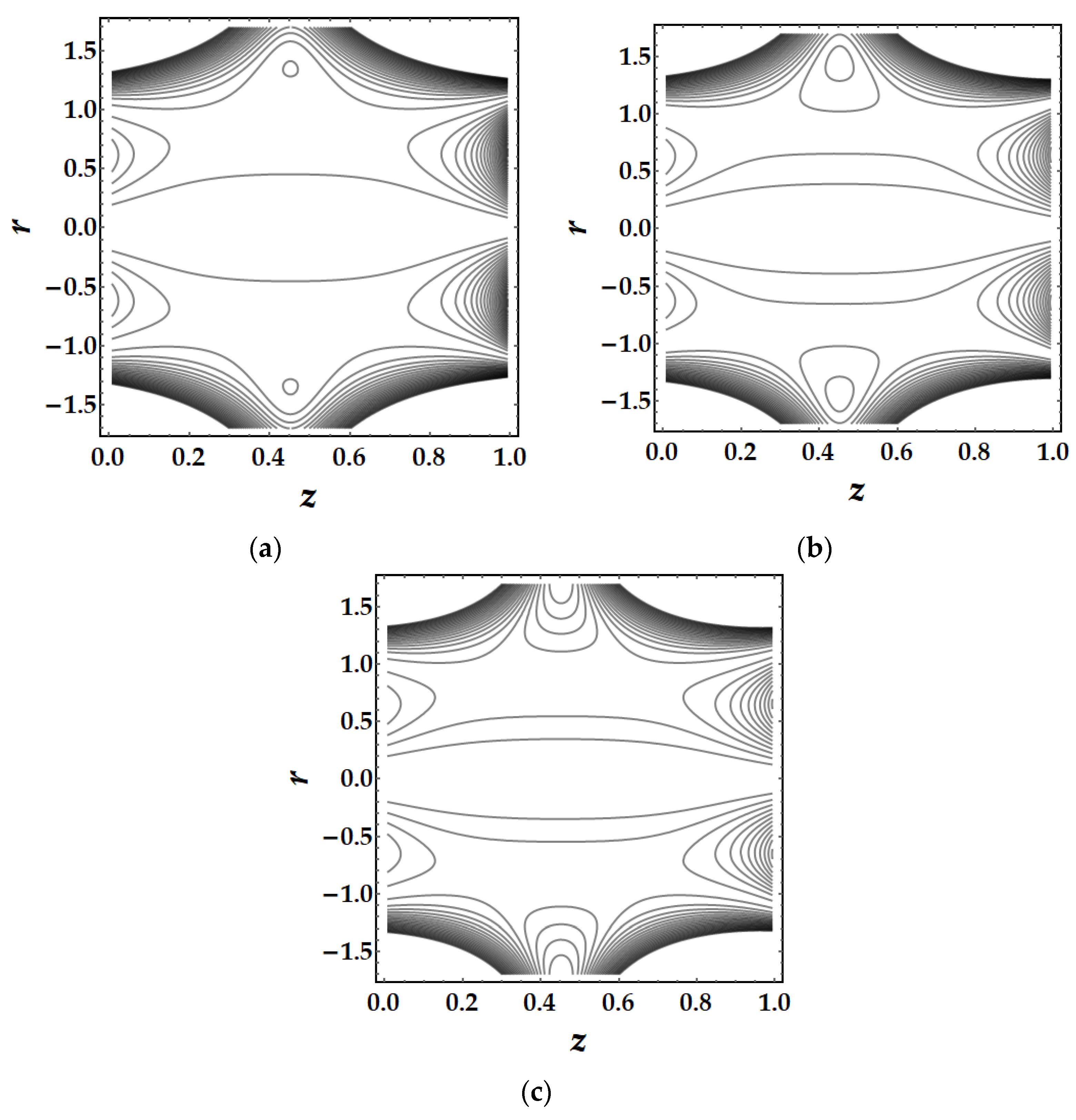
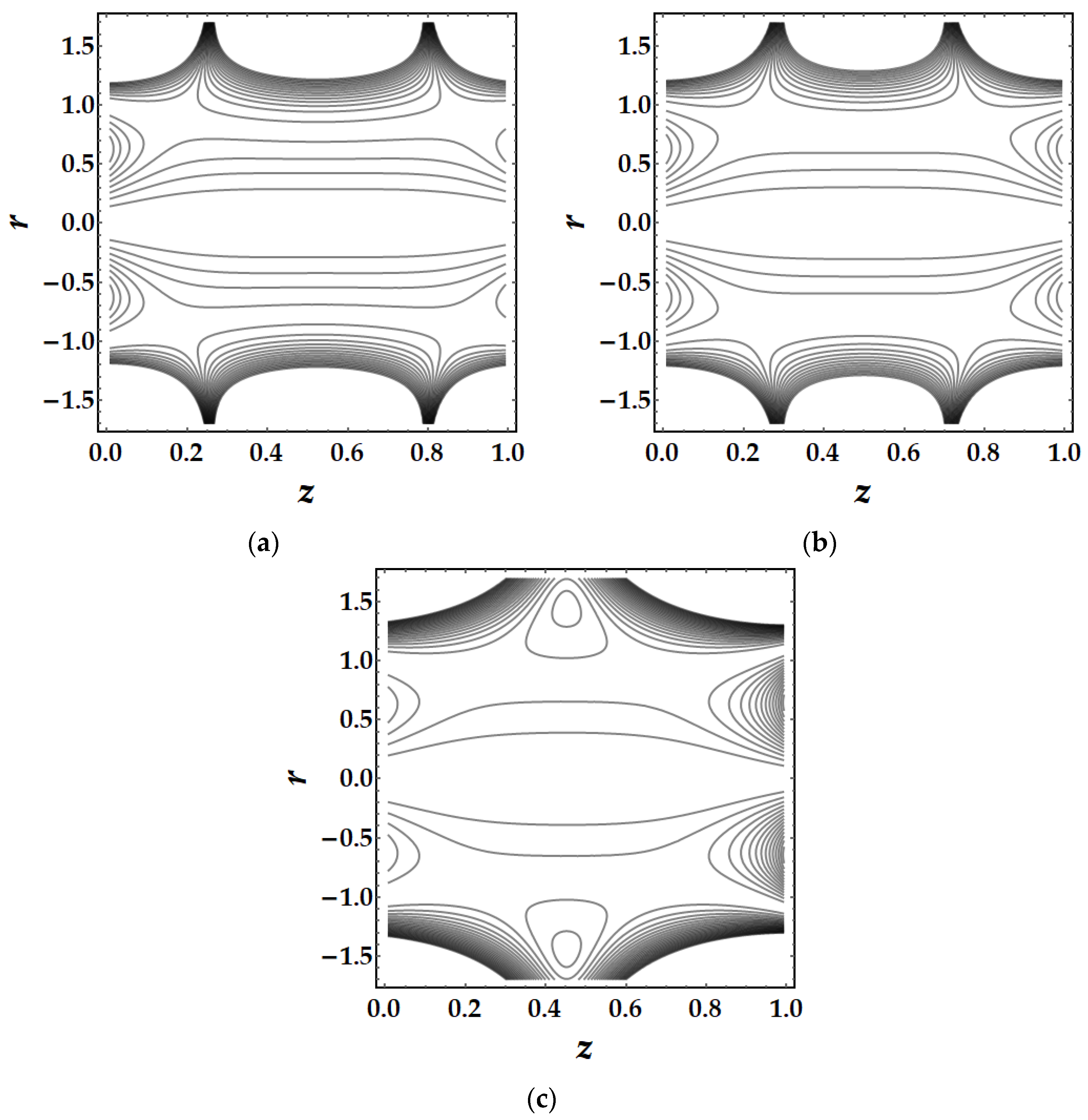
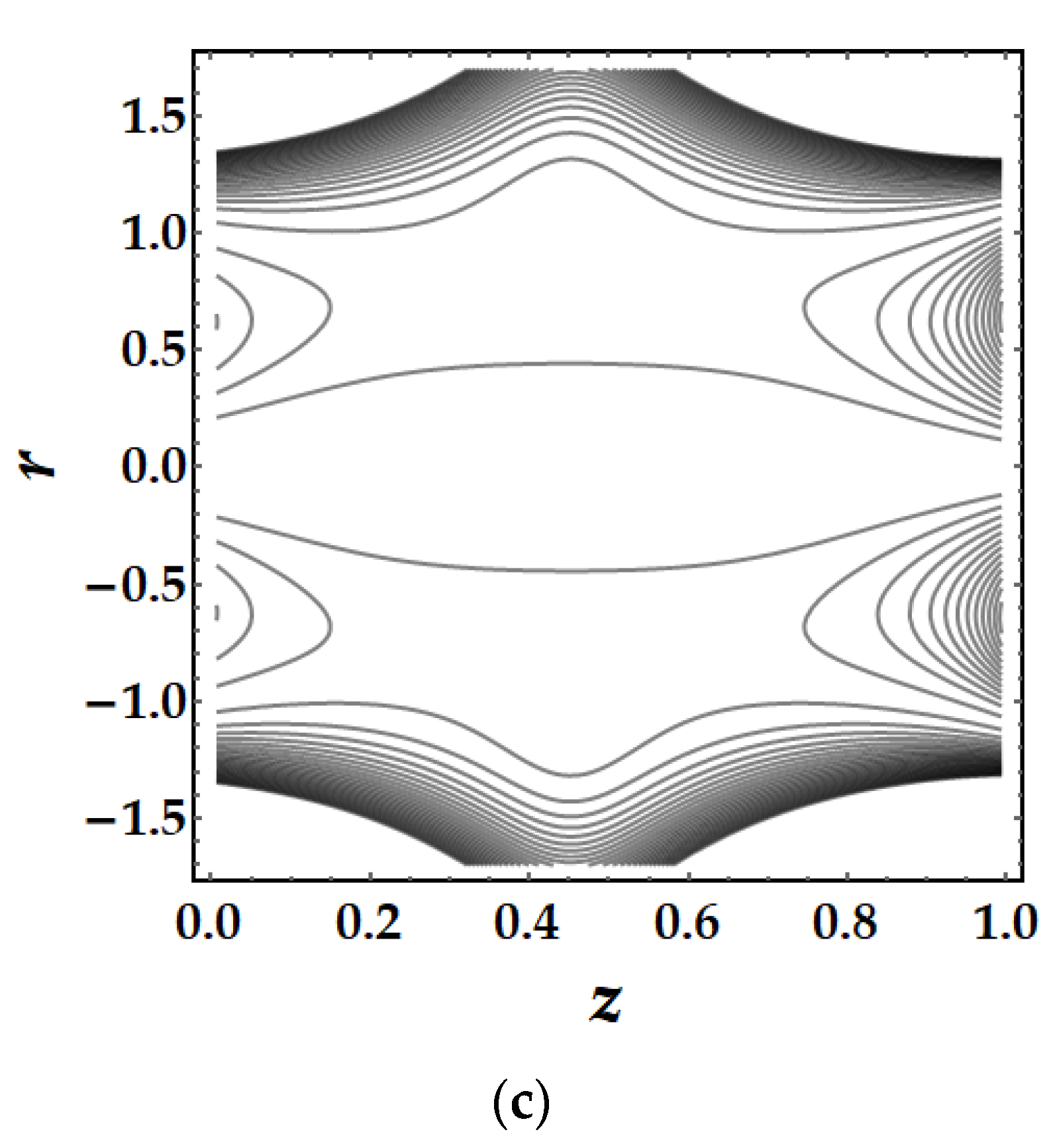
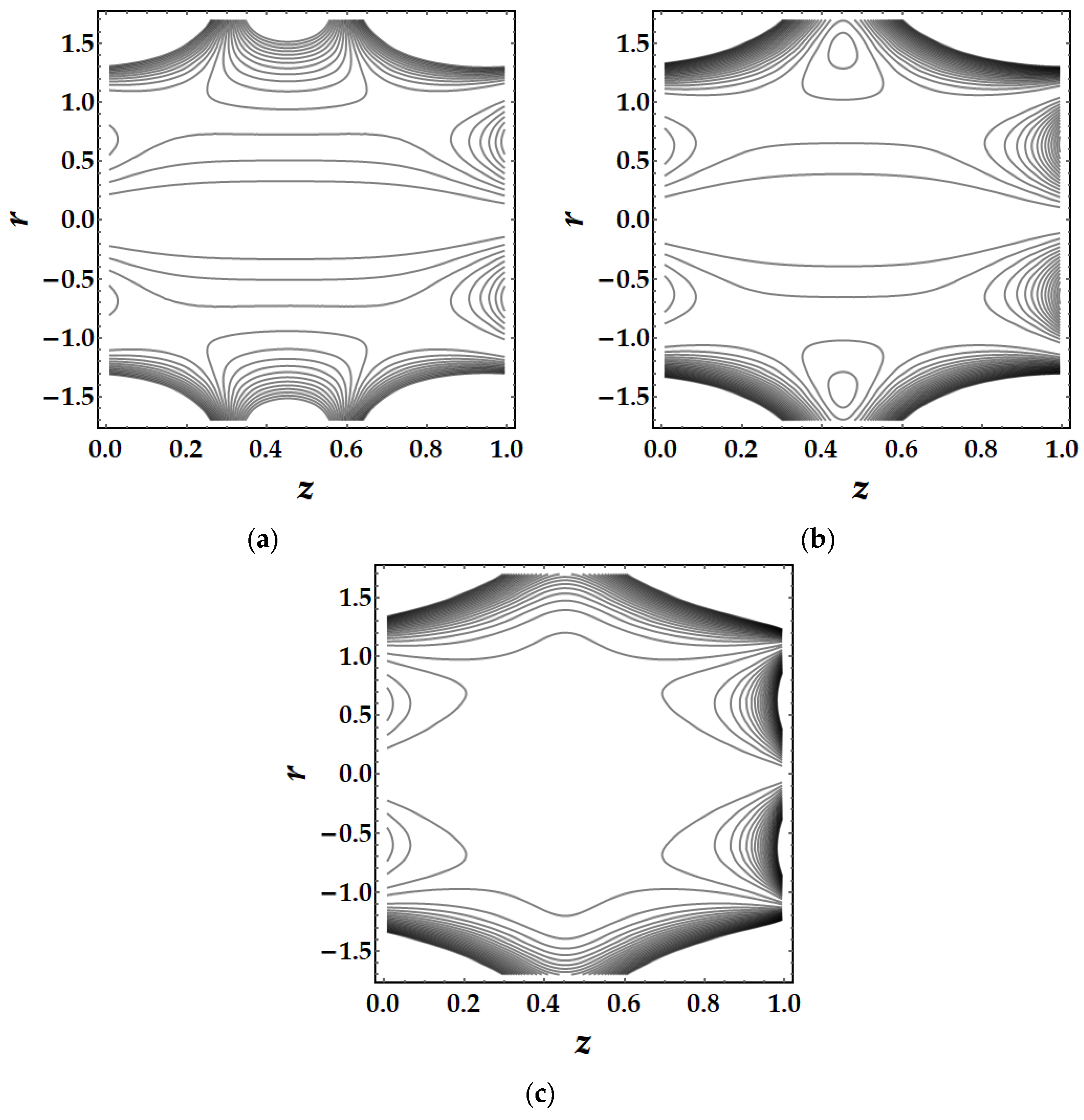
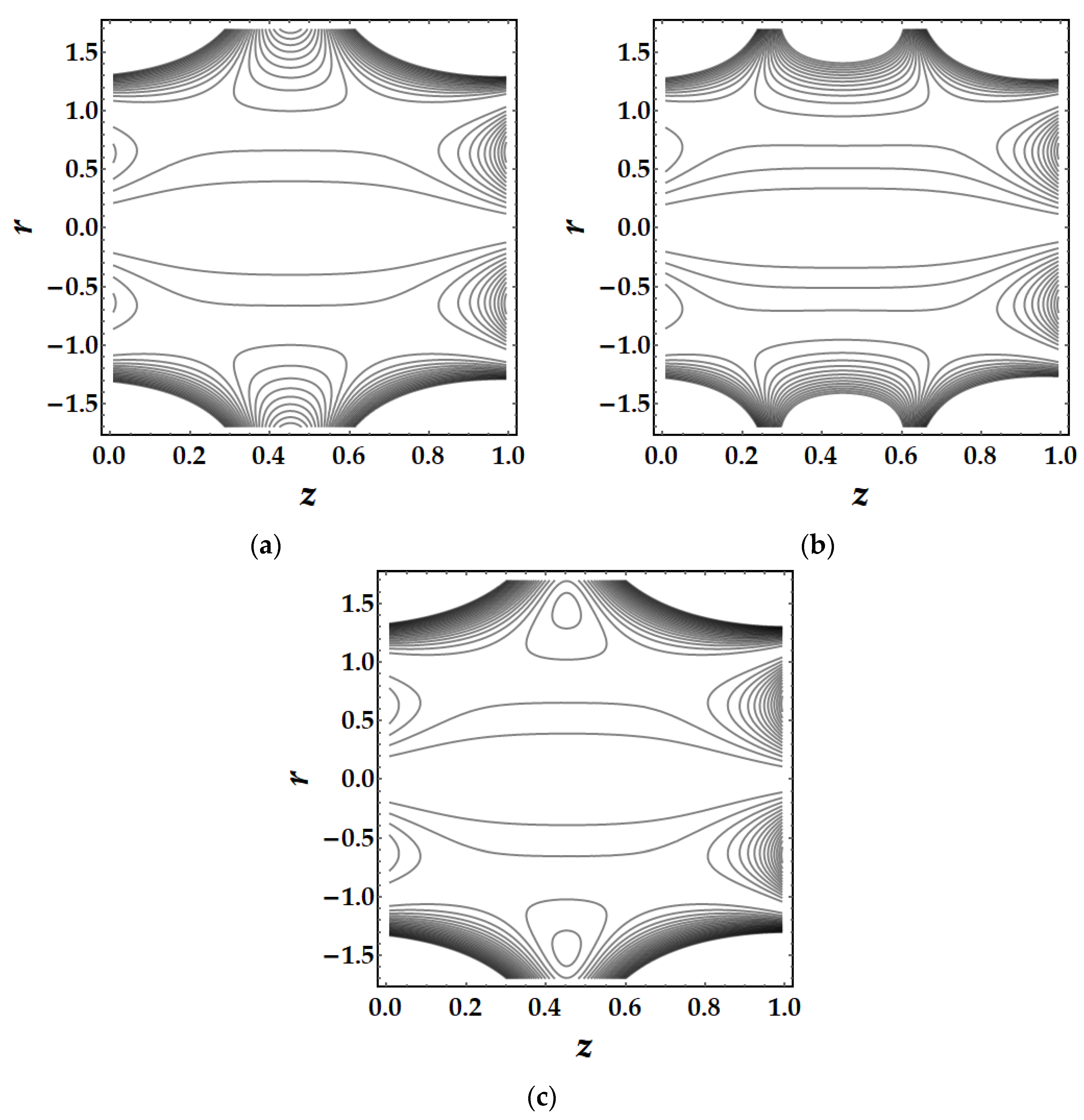
4.5. Trapping Mechanism
5. Conclusions
- (i)
- The Sutterby fluid parameter opposes the flow negligibly, whereas the Hartmann number and thermal Grashof number strengthen the flow field. In addition, the thermal Grashof number and the Hartmann number exhibit a decreasing tendency closer to the walls.
- (ii)
- It is also observed that the addition of gold nanoparticles (mono nanofluids) results in a greater magnitude of velocity than copper nanofluids.
- (iii)
- The thermal profile exhibits a diminishing trend owing to greater values of the Sutterby fluid parameter, whereas a rising trend is detected due to the significant impact of the magnetic field, Brinkman number, and thermal Grashof number.
- (iv)
- Copper nanoparticles (in the absence of gold nanoparticles) are observed to deplete the thermal profile substantially more than gold nanoparticles. Nevertheless, the thermal profile is enhanced by the presence of both nanoparticles (hybrid nanofluids).
- (v)
- It is observed that the impedance profile has a dual pattern for various values of the Sutterby fluid parameter, but the thermal Grashof number and magnetic field exhibit a uniformly declining tendency.
- (vi)
- When the impact of both nanoparticles rises, the impedance profile grows while the amplitude of the impedance profile for mono nanofluids decreases.
- (vii)
- For greater values of the Sutterby fluid parameter, the wall shear stress has been observed to rise considerably, whereas the inverse is true for the Hartmann number and the thermal Grashof number.
- (viii)
- The trapping mechanism demonstrates that the fluid parameters influence the size and frequency of the bolus. However, for other parameters, the trapped bolus manifested for certain values, although for converging and non-tapered arteries, it did not.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alanazi, F.K.; Radwan, A.A.; Alsarra, I. Biopharmaceutical applications of nanogold. Saudi Pharm. J. 2010, 18, 179–193. [Google Scholar] [CrossRef] [Green Version]
- Di Guglielmo, C.; López, D.R.; De Lapuente, J.; Mallafre, J.M.L.; Suàrez, M.B. Embryotoxicity of Cobalt Ferrite and Gold Nanoparticles: A First in Vitro Approach. Reprod. Toxicol. 2010, 30, 271–276. [Google Scholar] [CrossRef]
- Tartaj, P.; Morales, M.a.d.P.; Veintemillas-Verdaguer, S.; Gonz lez-Carre, O.T.; Serna, C.J. The Preparation of Magnetic Nanoparticles for Applications in Biomedicine. J. Phys. D Appl. Phys. 2003, 36, R182–R197. [Google Scholar] [CrossRef]
- Faraji, M.; Yamini, Y.; Rezaee, M. Magnetic Nanoparticles: Synthesis, Stabilization, Functionalization, Characterization, and Applications. J. Iran. Chem. Soc. 2010, 7, 1–37. [Google Scholar] [CrossRef]
- Siddiqui, A.A.; Turkyilmazoglu, M. Natural convection in the ferrofluid enclosed in a porous and permeable cavity. Int. Commun. Heat Mass Transf. 2020, 113, 104499. [Google Scholar] [CrossRef]
- Piao, Y.; Kim, J.; Na, H.B.; Kim, D.; Baek, J.S.; Ko, M.K.; Lee, J.H.; Shokouhimehr, M.; Hyeon, T. Wrap-Bake-Peel Process for Nanostructural Transformation from Beta-FeOOH Nanorods to Biocompatible Iron Oxide Nanocapsules. Nat. Mater. 2008, 7, 242–247. [Google Scholar] [CrossRef]
- Liu, C.; Wu, X.; Klemmer, T.; Shukla, N.; Weller, D.; Roy, A.G.; Tanase, M.; Laughlin, D. Reduction of Sintering during Annealing of FePt Nanoparticles Coated with Iron Oxide. Chem. Mater. 2005, 17, 620–625. [Google Scholar] [CrossRef]
- Park, J.-I.; Cheon, J. Synthesis of “Solid Solution” and “Core-Shell” Type Cobalt−Platinum Magnetic Nanoparticles via Transmetalation Reactions. J. Am. Chem. Soc. 2001, 123, 5743–5746. [Google Scholar] [CrossRef]
- Grancharov, S.G.; Zeng, H.; Sun, S.; Wang, S.X.; Obrien, S.; Murray, C.B.; Kirtley, A.J.R.; Held, G.A. Bio-functionalization of Monodisperse Magnetic Nanoparticles and Their Use as Biomolecular Labels in a Magnetic Tunnel Junction Based Sensor. J. Phys. Chem. B 2005, 109, 13030–13035. [Google Scholar] [CrossRef] [Green Version]
- Hartono, D.; Hody, S.; Yang, K.-L.; Yung, L.-Y.L. The Effect of Cholesterol on Protein-Coated Gold Nanoparticle Binding to Liquid Crystal-Supported Models of Cell Membranes. Biomaterials 2010, 31, 3008–3015. [Google Scholar] [CrossRef]
- Sidik, N.A.C.; Adamu, I.M.; Jamil, M.M.; Kefayati, G.H.; Mamat, R.; Najafi, G. Recent progress on hybrid nanofluids in heat transfer applications: A comprehensive review. Int. Commun. Heat Mass Transf. 2016, 78, 68–79. [Google Scholar] [CrossRef]
- Gerosa, C.; Crisponi, G.; Nurchi, V.M.; Saba, L.; Cappai, R.; Cau, F.; Faa, G.; Van Eyken, P.; Scartozzi, M.; Floris, G.; et al. Gold Nanoparticles: A New Golden Era in Oncology? Pharmaceuticals (Basel) 2020, 13, 192. [Google Scholar] [CrossRef]
- Ganeshkumar, M.; Sastry, T.P.; Kumar, M.S.; Dinesh, M.G.; Kannappan, S.; Suguna, L. Sun light mediated synthesis of gold nanoparticles as carrier for 6-mercaptopurine: Preparation, characterization and toxicity studies in zebrafish embryo model. Mater. Res. Bull. 2012, 47, 2113–2119. [Google Scholar] [CrossRef]
- Stobiecka, M.; Hepel, M. Double-shell gold nanoparticle-based DNA-carriers with poly-l-lysine binding surface. Biomaterials 2011, 32, 3312–3321. [Google Scholar] [CrossRef]
- Guo, Q.; Guo, Q.; Yuan, J.; Zeng, J. Biosynthesis of Gold Nanoparticles Using a Kind of Flavonol: Dihydromyricetin. Colloids Surf. A Physicochem. Eng. Asp. 2014, 441, 127–132. [Google Scholar] [CrossRef]
- Glavee, G.N.; Klabunde, K.J.; Sorensen, C.M.; Hadjipanayis, G.C. Borohydride reduction of cobalt ions in water. Chemistry leading to nanoscale metal, boride, or borate particles. Langmuir 1993, 9, 162–169. [Google Scholar] [CrossRef]
- Liz-Marzán, L.M.; Touriño, I.L. Reduction and Stabilization of Silver Nanoparticles in Ethanol by Nonionic Surfactants. Langmuir 1996, 12, 3585–3589. [Google Scholar] [CrossRef]
- Jana, N.R.; Gearheart, L.; Murphy, C.J. Evidence for Seed-Mediated Nucleation in the Chemical Reduction of Gold Salts to Gold Nanoparticles. Chem. Mater. 2001, 13, 2313–2322. [Google Scholar] [CrossRef]
- Patel, K.; Kapoor, S.; Dave, D.P.; Mukherjee, T. Synthesis of nanosized silver colloids by microwave dielectric heating. J. Chem. Sci. 2005, 117, 53–60. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Chang, S.-S.; Lee, C.-L.; Wang, C.R.C. Gold Nanorods: Electrochemical Synthesis and Optical Properties. J. Phys. Chem. B 1997, 101, 6661–6664. [Google Scholar] [CrossRef]
- Mallik, K.; Mandal, M.; Pradhan, A.N.; Pal, T. Seed Mediated Formation of Bimetallic Nanoparticles by UV Irradiation: A Photochemical Approach for the Preparation of “Core−Shell” Type Structures. Nano Lett. 2001, 1, 319–322. [Google Scholar] [CrossRef]
- Ardahaie, S.S.; Amiri, A.J.; Amouei, A.; Hosseinzadeh, K.; Ganji, D. Investigating the effect of adding nanoparticles to the blood flow in presence of magnetic field in a porous blood arterial. Informatics Med. Unlocked 2018, 10, 71–81. [Google Scholar] [CrossRef]
- Ellahi, R.; Zeeshan, A.; Hussain, F.; Asadollahi, A. Peristaltic Blood Flow of Couple Stress Fluid Suspended with Nanoparticles under the Influence of Chemical Reaction and Activation Energy. Symmetry 2019, 11, 276. [Google Scholar] [CrossRef] [Green Version]
- Elmaboud, Y.A.; Mekheimer, K.S.; Emam, T.G. Numerical Examination of Gold Nanoparticles as a Drug Carrier on Peristaltic Blood Flow Through Physiological Vessels: Cancer Therapy Treatment. BioNanoScience 2019, 9, 952–965. [Google Scholar] [CrossRef]
- Seikh, A.H.; Akinshilo, A.; Taheri, M.H.; Rahimi-Gorji, M.; Alharthi, N.H.; Khan, I.; Khan, A.R. Influence of the nanoparticles and uniform magnetic field on the slip blood flows in arterial vessels. Phys. Scr. 2019, 94, 125218. [Google Scholar] [CrossRef]
- Mekheimer, K.S.; Abo-Elkhair, R.E.; Moawad, A.M.A. Electrothermal Transport via Gold Nanoparticles as Antimicrobials of Blood? Ow through an Electro-Osmosis Artery with Overlapping Stenosis. Int. J. Fluid Mech. Res. 2020, 47, 135–152. [Google Scholar] [CrossRef]
- Zhang, L.; Bhatti, M.M.; Marin, M.; Mekheimer, K.S. Entropy Analysis on the Blood Flow through Anisotropically Tapered Arteries Filled with Magnetic Zinc-Oxide (ZnO) Nanoparticles. Entropy 2020, 22, 1070. [Google Scholar] [CrossRef]
- Umadevi, C.; Dhange, M.; Haritha, B.; Sudha, T. Flow of blood mixed with copper nanoparticles in an inclined overlapping stenosed artery with magnetic field. Case Stud. Therm. Eng. 2021, 25, 100947. [Google Scholar] [CrossRef]
- Turrina, C.; Berensmeier, S.; Schwaminger, S. Bare Iron Oxide Nanoparticles as Drug Delivery Carrier for the Short Cationic Peptide Lasioglossin. Pharmaceuticals 2021, 14, 405. [Google Scholar] [CrossRef]
- Jangde, R.; Elhassan, G.O.; Khute, S.; Singh, D.; Singh, M.; Sahu, R.K.; Khan, J. Hesperidin-Loaded Lipid Polymer Hybrid Nanoparticles for Topical Delivery of Bioactive Drugs. Pharmaceuticals 2022, 15, 211. [Google Scholar] [CrossRef]
- Waters, M.; Hopf, J.; Tam, E.; Wallace, S.; Chang, J.; Bennett, Z.; Aquino, H.; Roeder, P.; Stack, M.S.; Nallathamby, P.D. Biocompatible, Multi-Mode, Fluorescent, T2 MRI Contrast Magnetoelectric-Silica Nanoparticles (MagSiNs), for On-Demand Doxorubicin Delivery to Metastatic Cancer Cells. Pharmaceuticals 2022, 15, 1216. [Google Scholar] [CrossRef]
- Zeeshan, A.; Shehzad, N.; Atif, M.; Ellahi, R.; Sait, S.M. Electromagnetic Flow of SWCNT/MWCNT Suspensions in Two Immiscible Water- and Engine-Oil-Based Newtonian Fluids through Porous Media. Symmetry 2022, 14, 406. [Google Scholar] [CrossRef]
- Alshomrani, A.S.; Ullah, M.Z.; Capizzano, S.S.; Khan, W.A.; Khan, M. Interpretation of Chemical Reactions and Activation Energy for Unsteady 3D Flow of Eyring–Powell Magneto-Nanofluid. Arab. J. Sci. Eng. 2019, 44, 579–589. [Google Scholar] [CrossRef]
- Shehzad, N.; Zeeshan, A.; Shakeel, M.; Ellahi, R.; Sait, S.M. Effects of Magnetohydrodynamics Flow on Multilayer Coatings of Newtonian and Non-Newtonian Fluids through Porous Inclined Rotating Channel. Coatings 2022, 12, 430. [Google Scholar] [CrossRef]
- Abuzaid, D.; Ullah, M.Z. Numerical simulation for bio-convective flow of Sutterby nanofluid by a rotating disk. Phys. Scr. 2021, 96, 084007. [Google Scholar] [CrossRef]
- Izadi, M.; Sheremet, M.; Alshehri, H.M.; Ambreen, T.; Doranehgard, M.H. Numerical study on charging process inside a grid-structure thermal storage. J. Energy Storage 2021, 45, 103522. [Google Scholar] [CrossRef]
- Taheri, M.H.; Pourmehran, O.; Sarafraz, M.M.; Ahookhosh, K.; Farnoud, A.; Cui, X. Effect of swirling flow and particle-release pattern on drug delivery to human tracheobronchial airways. Biomech. Model. Mechanobiol. 2021, 20, 2451–2469. [Google Scholar] [CrossRef]
- Pourmehran, O.; Gorji, T.B.; Gorji-Bandpy, M. Magnetic drug targeting through a realistic model of human tracheobronchial airways using computational fluid and particle dynamics. Biomech. Model. Mechanobiol. 2016, 15, 1355–1374. [Google Scholar] [CrossRef]
- Usman, M.; Gul, T.; Khan, A.; Alsubie, A.; Ullah, M.Z. Electromagnetic couple stress film flow of hybrid nanofluid over an unsteady rotating disc. Int. Commun. Heat Mass Transf. 2021, 127, 105562. [Google Scholar] [CrossRef]
- Turkyilmazoglu, M. Equivalence of Ratio and Residual Approaches in the Homotopy Analysis Method and Some Applications in Nonlinear Science and Engineering. Comput. Model. Eng. Sci. 2019, 120, 63–81. [Google Scholar] [CrossRef]
- Gibanov, N.S.; Sheremet, M.A.; Oztop, H.F.; Al-Salem, K. MHD natural convection and entropy generation in an open cavity having different horizontal porous blocks saturated with a ferrofluid. J. Magn. Magn. Mater. 2018, 452, 193–204. [Google Scholar] [CrossRef]
- Elelamy, A.F.; Elgazery, N.S.; Ellahi, R. Blood Flow of MHD Non-Newtonian Nanofluid with Heat Transfer And Slip Effects: Application of Bacterial Growth in Heart Valve. Int. J. Numer. Methods Heat Fluid Flow 2020, 30, 4883–4908. [Google Scholar] [CrossRef]
- Ishtiaq, F.; Ellahi, R.; Bhatti, M.M.; Alamri, S.Z. Insight in Thermally Radiative Cilia-Driven Flow of Electrically Conducting Non-Newtonian Jeffrey Fluid under the Influence of Induced Magnetic Field. Mathematics 2022, 10, 2007. [Google Scholar] [CrossRef]
- Ibrahim, M. Concentration-dependent viscosity effect on magnetonano peristaltic flow of Powell-Eyring fluid in a divergent-convergent channel. Int. Commun. Heat Mass Transf. 2022, 134, 105987. [Google Scholar] [CrossRef]
- Akram, J.; Akbar, N.S.; Tripathi, D. Blood-based graphene oxide nanofluid flow through capillary in the presence of electromagnetic fields: A Sutterby fluid model. Microvasc. Res. 2020, 132, 104062. [Google Scholar] [CrossRef]
- Bhatti, M.M.; Lu, D.Q. Head-on Collision Between Two Hydroelastic Solitary Waves in Shallow Water. Qual. Theory Dyn. Syst. 2018, 17, 103–122. [Google Scholar] [CrossRef]
- Turkyilmazoglu, M. Is homotopy perturbation method the traditional Taylor series expansion. Hacet. J. Math. Stat. 2015, 44, 651–657. [Google Scholar] [CrossRef]
- Ellahi, R. The effects of MHD and temperature dependent viscosity on the flow of non-Newtonian nanofluid in a pipe: Analytical solutions. Appl. Math. Model. 2013, 37, 1451–1467. [Google Scholar] [CrossRef]
- Bhatti, M.M.; Zeeshan, A.; Bashir, F.; Sait, S.M.; Ellahi, R. Sinusoidal motion of small particles through a Darcy-Brinkman-Forchheimer microchannel filled with non-Newtonian fluid under electro-osmotic forces. J. Taibah Univ. Sci. 2021, 15, 514–529. [Google Scholar] [CrossRef]
- Ebaid, A. Remarks on the homotopy perturbation method for the peristaltic flow of Jeffrey fluid with nano-particles in an asymmetric channel. Comput. Math. Appl. 2014, 68, 77–85. [Google Scholar] [CrossRef]
- Bhatti, M.M.; Bég, O.A.; Ellahi, R.; Abbas, T. Natural Convection Non-Newtonian EMHD Dissipative Flow Through a Microchannel Containing a Non-Darcy Porous Medium: Homotopy Perturbation Method Study. Qual. Theory Dyn. Syst. 2022, 21, 97. [Google Scholar] [CrossRef]
- SubramanyamReddy, A.; Srinivas, S.; Jagadeshkumar, K. Blood-Gold/Copper nanofluid Flow between Expanding or Contracting Permeable Walls with Slip Effects. Mater. Today 2019, 9, 351–360. [Google Scholar]
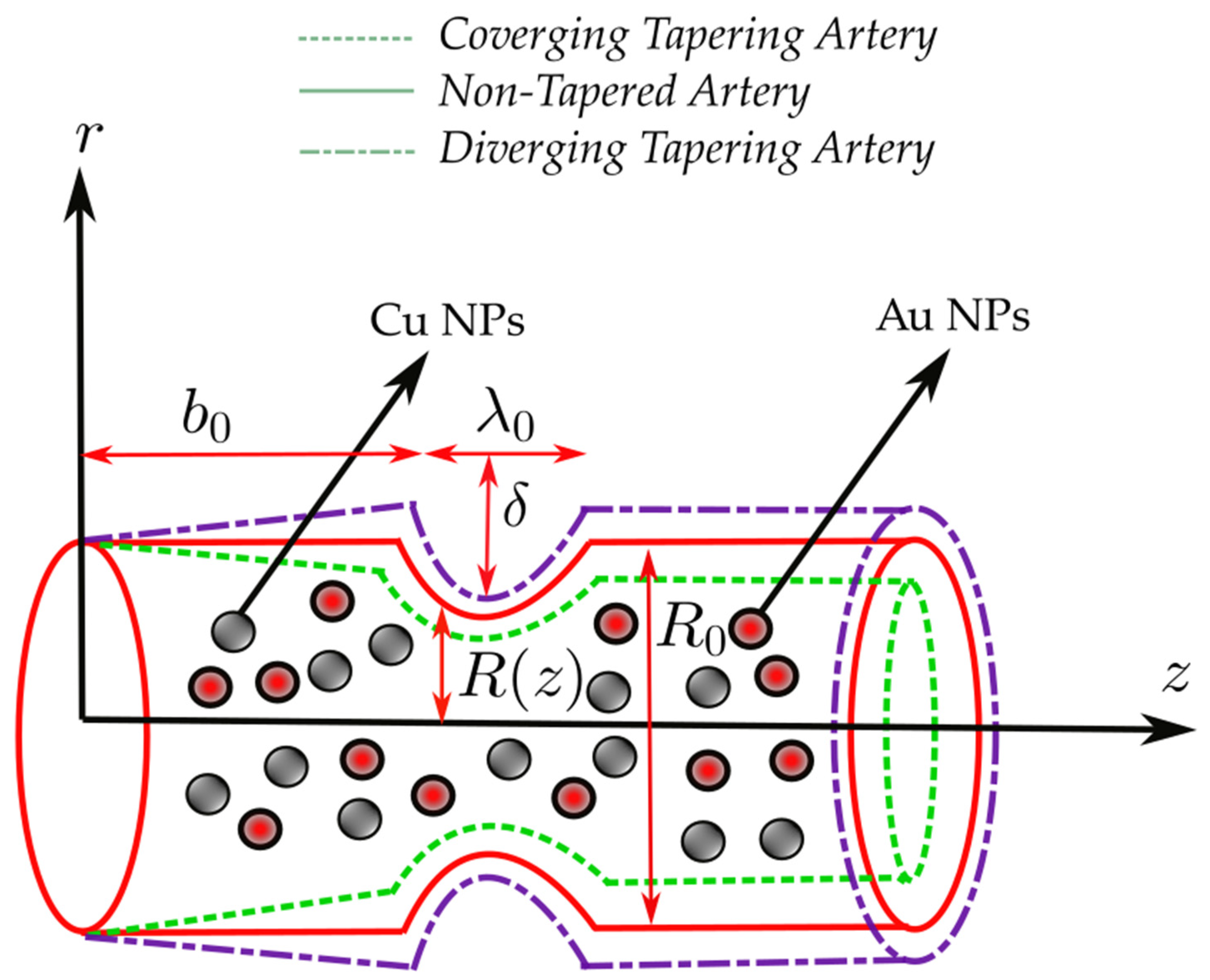








| Nanofluid | Hybrid Nanofluid | |
|---|---|---|
| Dynamic viscosity | ||
| Density | ||
| Electrical conductivity | ||
| Thermal conductivity | ||
| Heat capacity | ||
| Thermal expansion |
| Physical Characteristics | Base Fluid (Blood) | Copper Nanoparticles | Gold Nanoparticles |
|---|---|---|---|
| 3617 | 385 | 129.1 | |
| 1050 | 8933 | 19300 | |
| 1.33 | |||
| 0.18 | 16.65 10−6 | 0.0000142 | |
| 0.52 | 400 | 320 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhatti, M.M.; Sait, S.M.; Ellahi, R. Magnetic Nanoparticles for Drug Delivery through Tapered Stenosed Artery with Blood Based Non-Newtonian Fluid. Pharmaceuticals 2022, 15, 1352. https://doi.org/10.3390/ph15111352
Bhatti MM, Sait SM, Ellahi R. Magnetic Nanoparticles for Drug Delivery through Tapered Stenosed Artery with Blood Based Non-Newtonian Fluid. Pharmaceuticals. 2022; 15(11):1352. https://doi.org/10.3390/ph15111352
Chicago/Turabian StyleBhatti, Muhammad Mubashir, Sadiq M. Sait, and Rahmat Ellahi. 2022. "Magnetic Nanoparticles for Drug Delivery through Tapered Stenosed Artery with Blood Based Non-Newtonian Fluid" Pharmaceuticals 15, no. 11: 1352. https://doi.org/10.3390/ph15111352
APA StyleBhatti, M. M., Sait, S. M., & Ellahi, R. (2022). Magnetic Nanoparticles for Drug Delivery through Tapered Stenosed Artery with Blood Based Non-Newtonian Fluid. Pharmaceuticals, 15(11), 1352. https://doi.org/10.3390/ph15111352








