A Shape-Adaptive Gallic Acid Driven Multifunctional Adhesive Hydrogel Loaded with Scolopin2 for Wound Repair
Abstract
:1. Introduction
2. Results
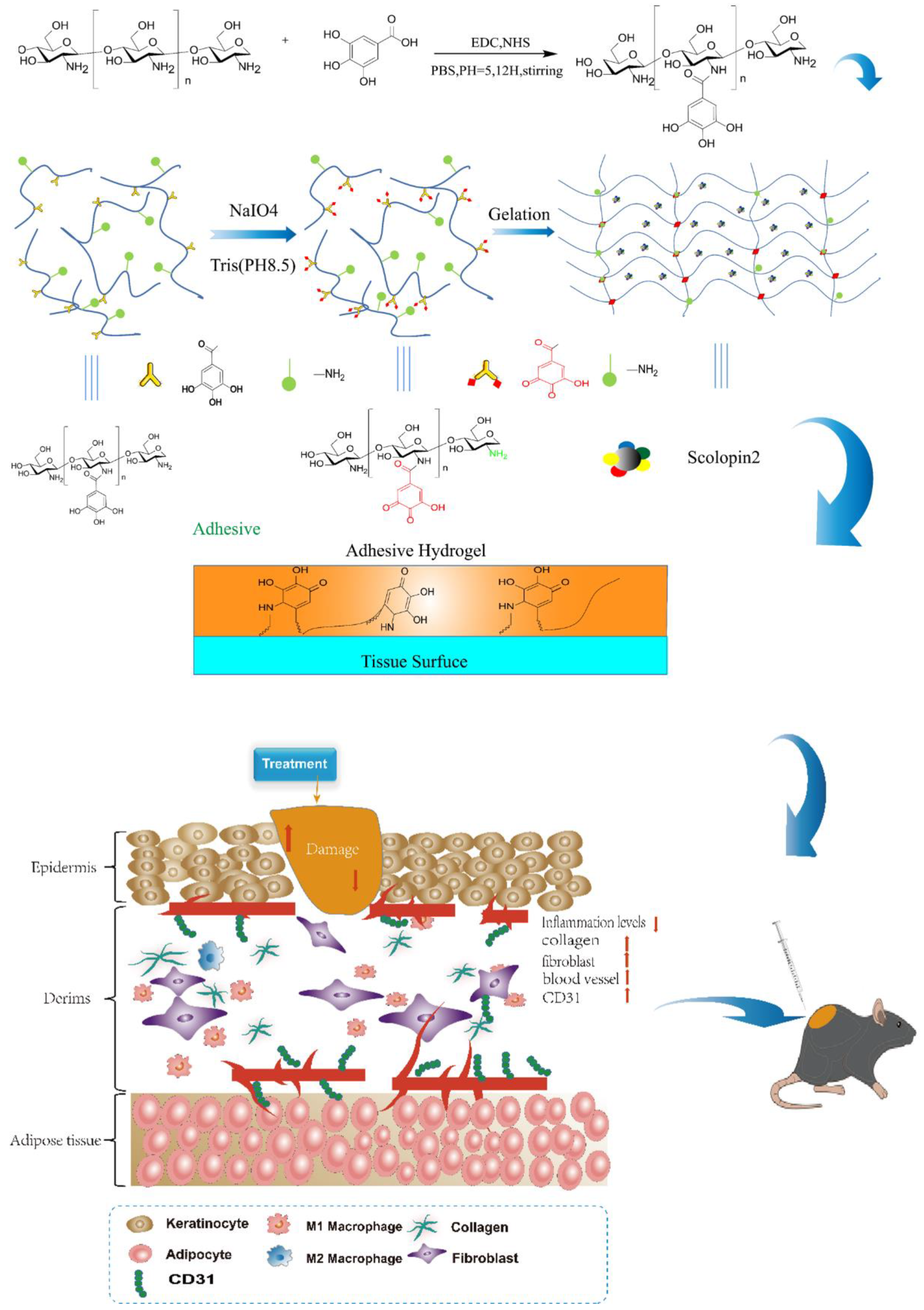
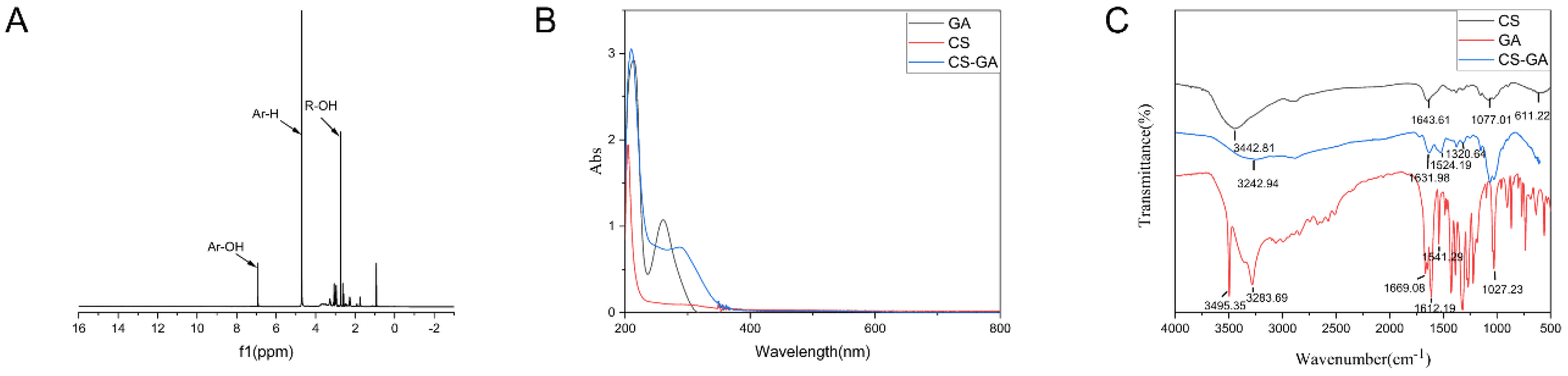
2.1. Characterization of Chitosan-Gallic Acid
2.1.1. Characterization
2.1.2. Antioxidant Performance of CS-GA
2.2. Characterization of Hydrogel
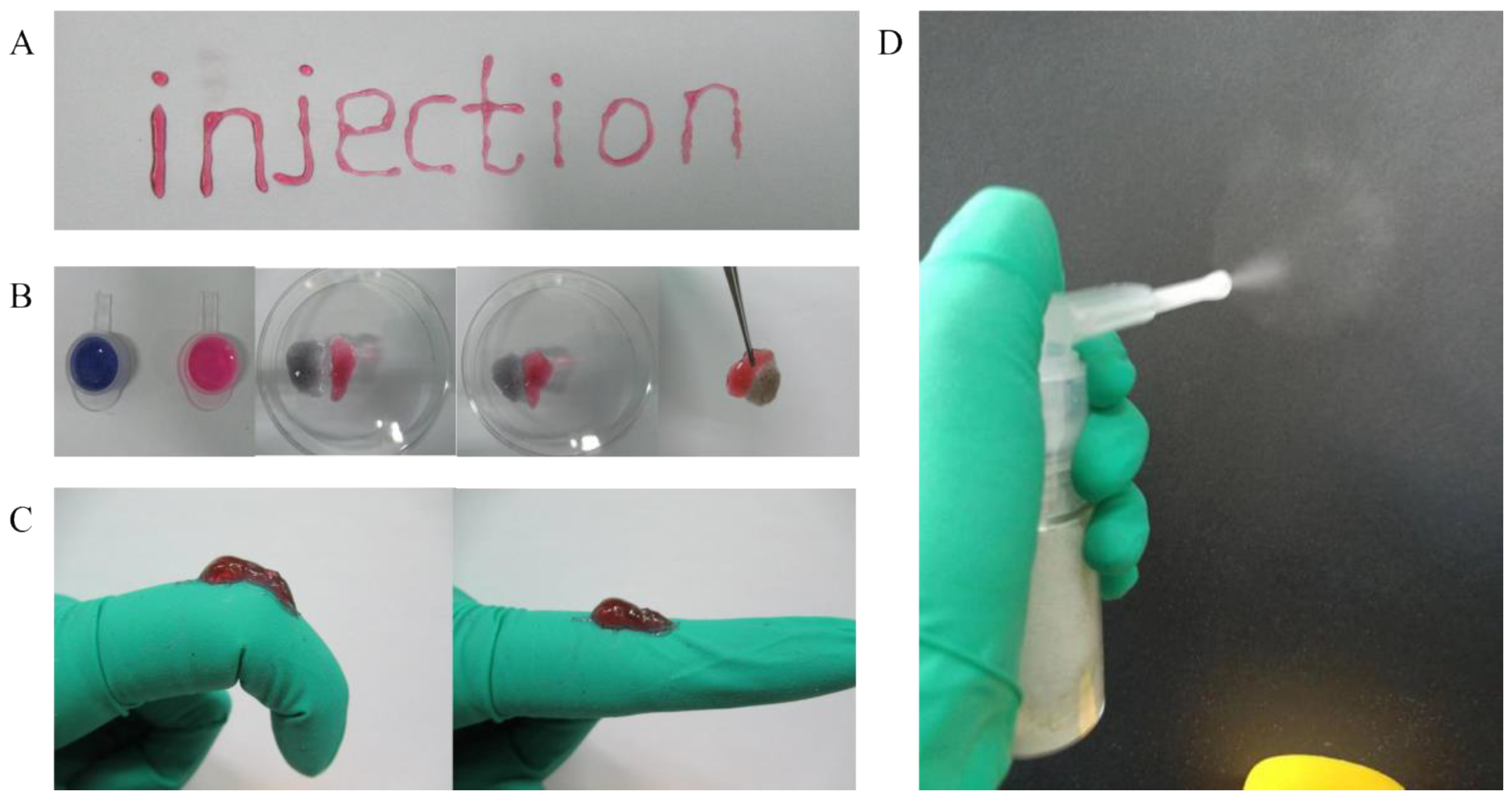
2.2.1. The Shape-Adaptive Performance of Hydrogel
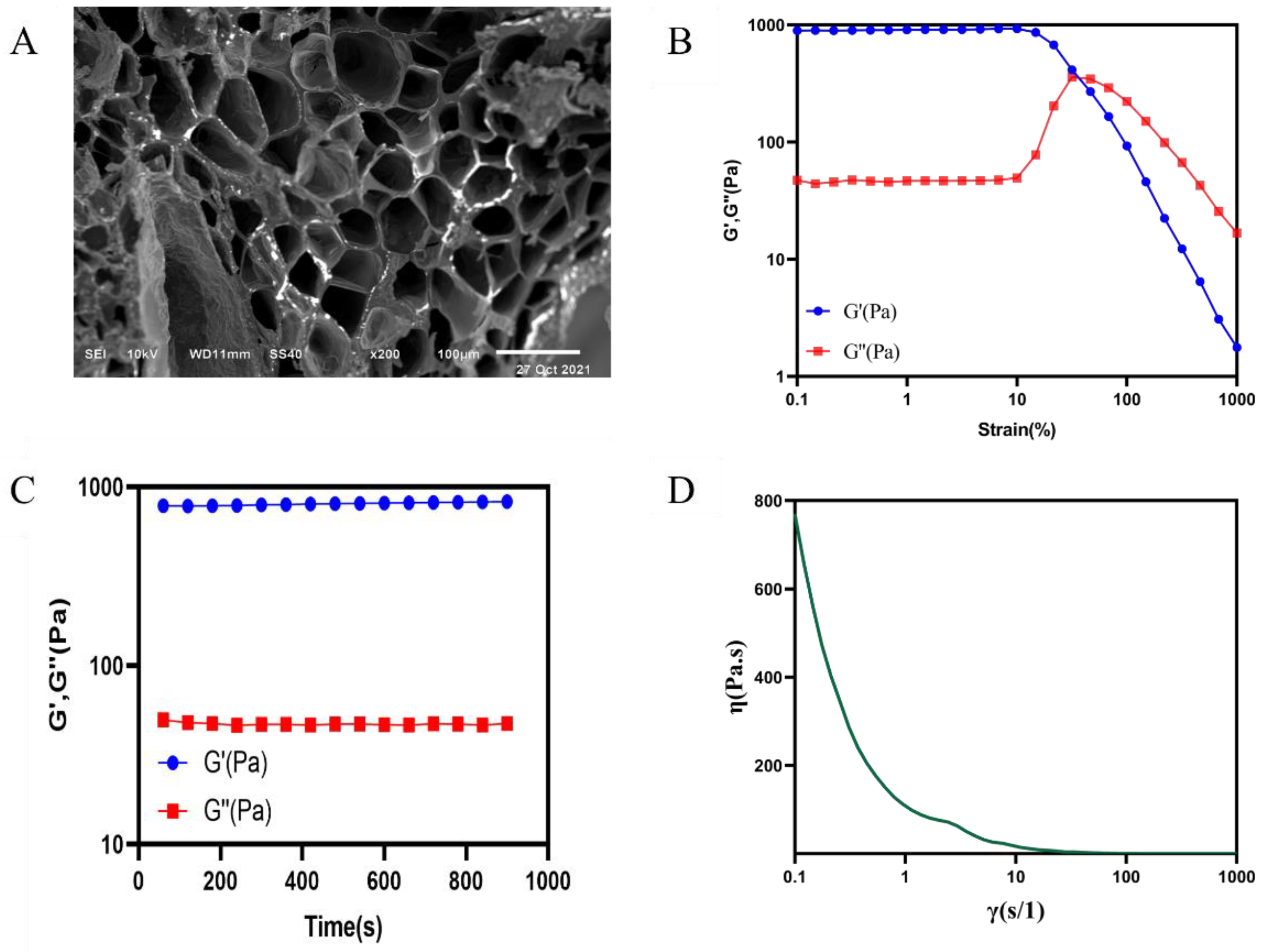
2.2.2. Scanning Electron Microscopy
2.2.3. Rheological Properties of the Hydrogel
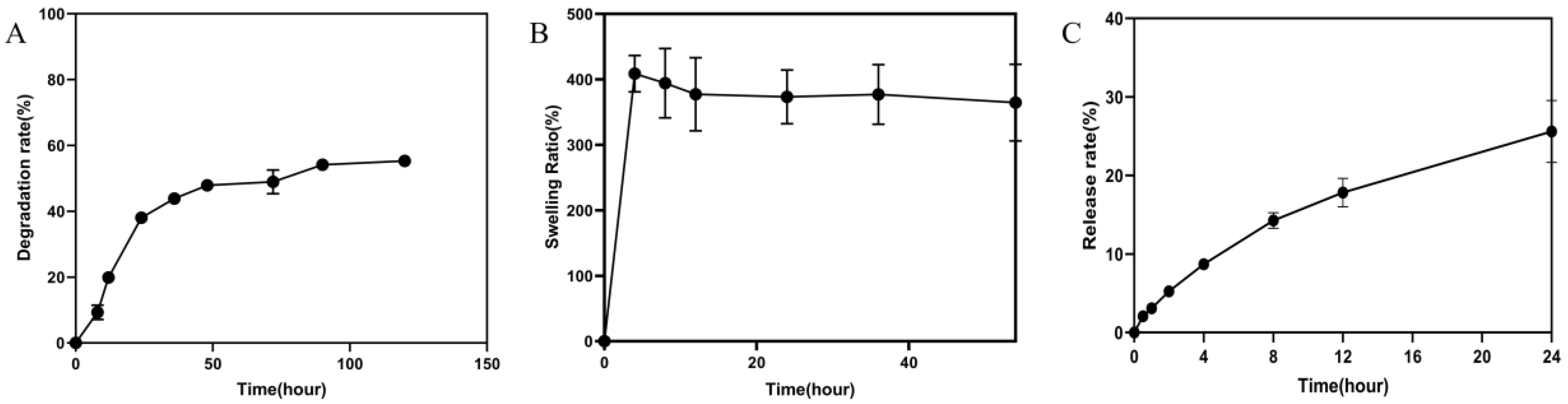
2.2.4. Degradation Rate and Swelling Ratio of Hydrogel
2.2.5. In Vitro Drug Release Study
2.2.6. Antimicrobial Assay
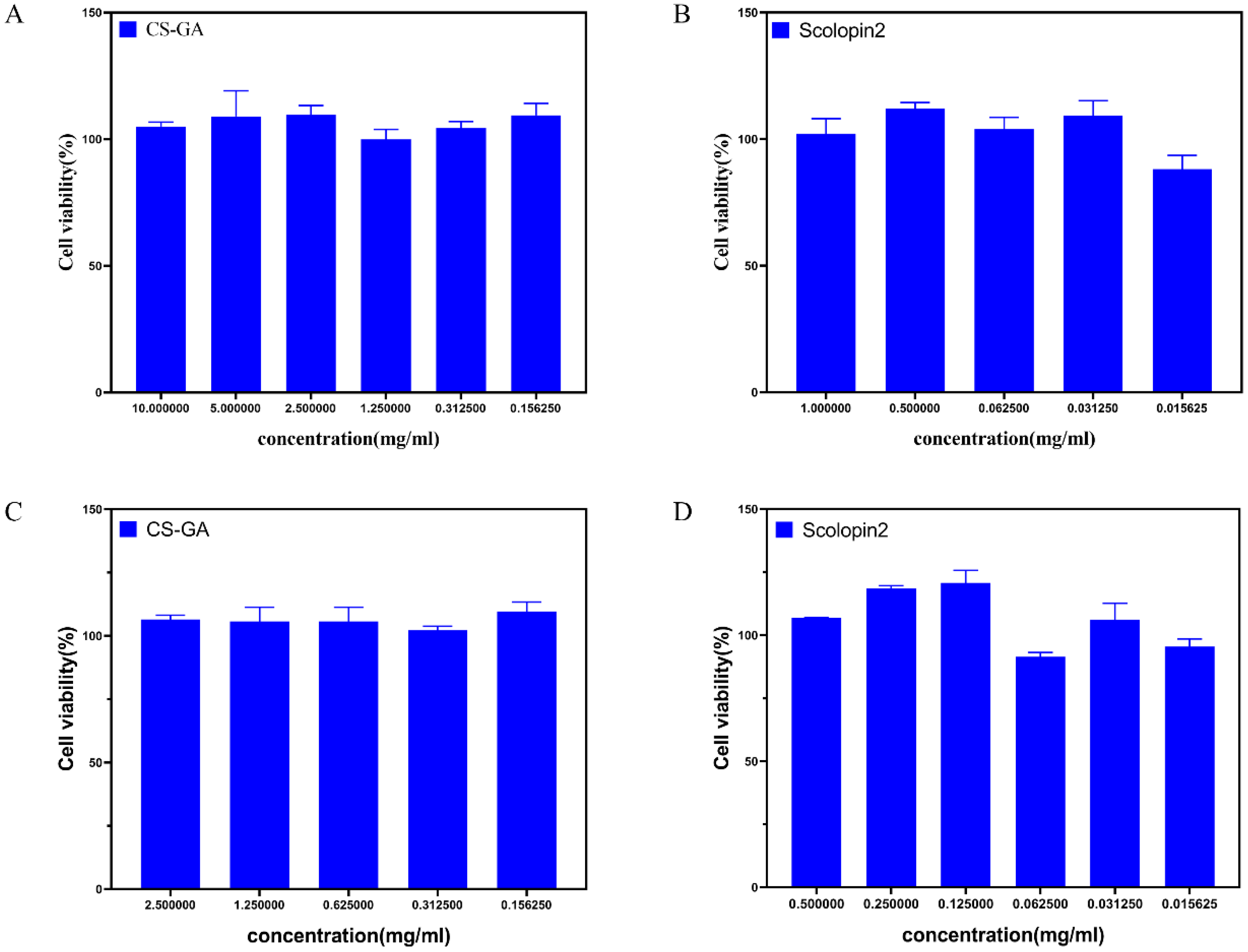
2.2.7. Cytocompatibility of Hydrogel
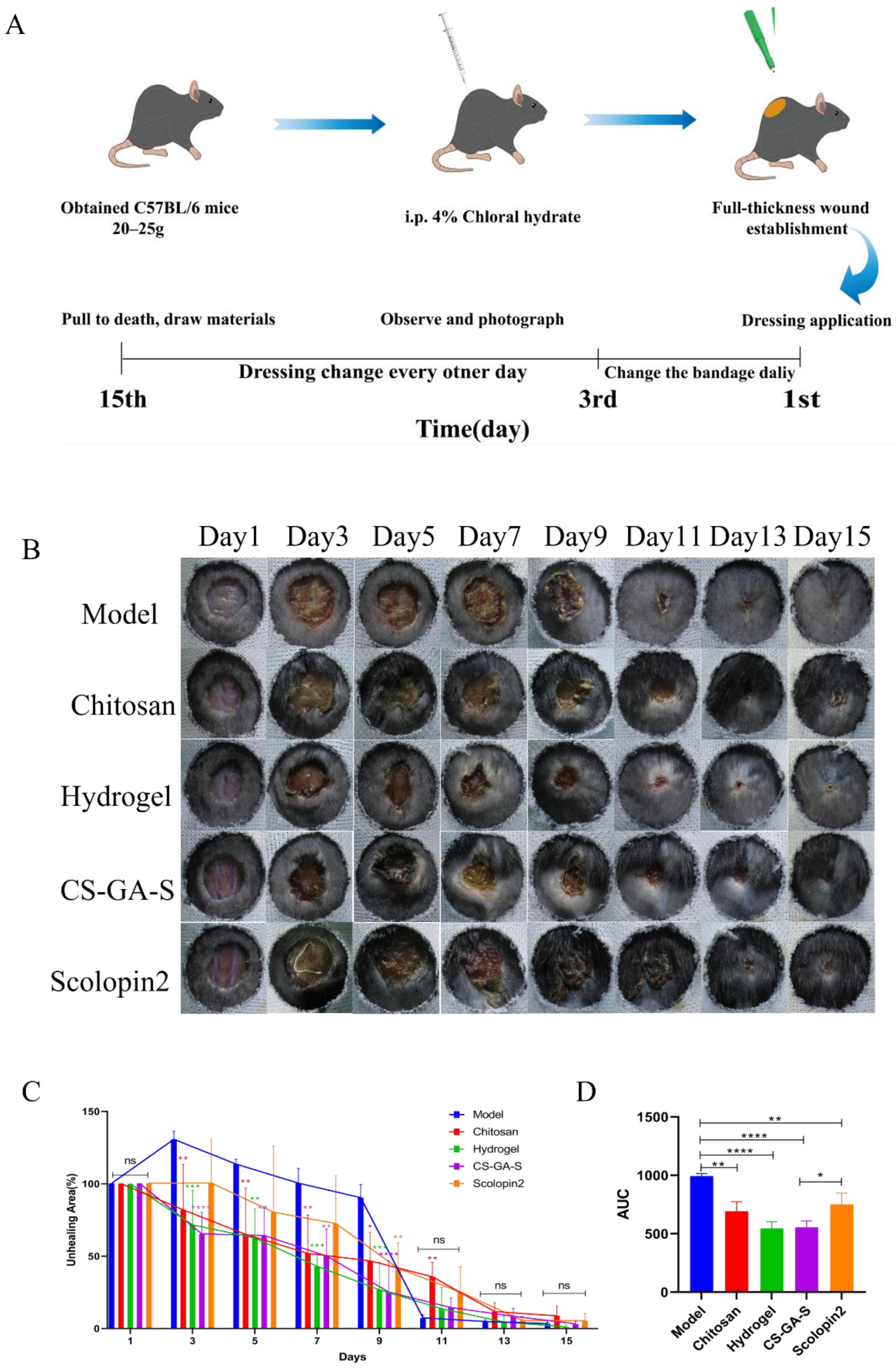
2.3. In Vivo Assessment of the Wound Healing Activity
2.3.1. Effect of Hydrogel on Wound Closure in Mice
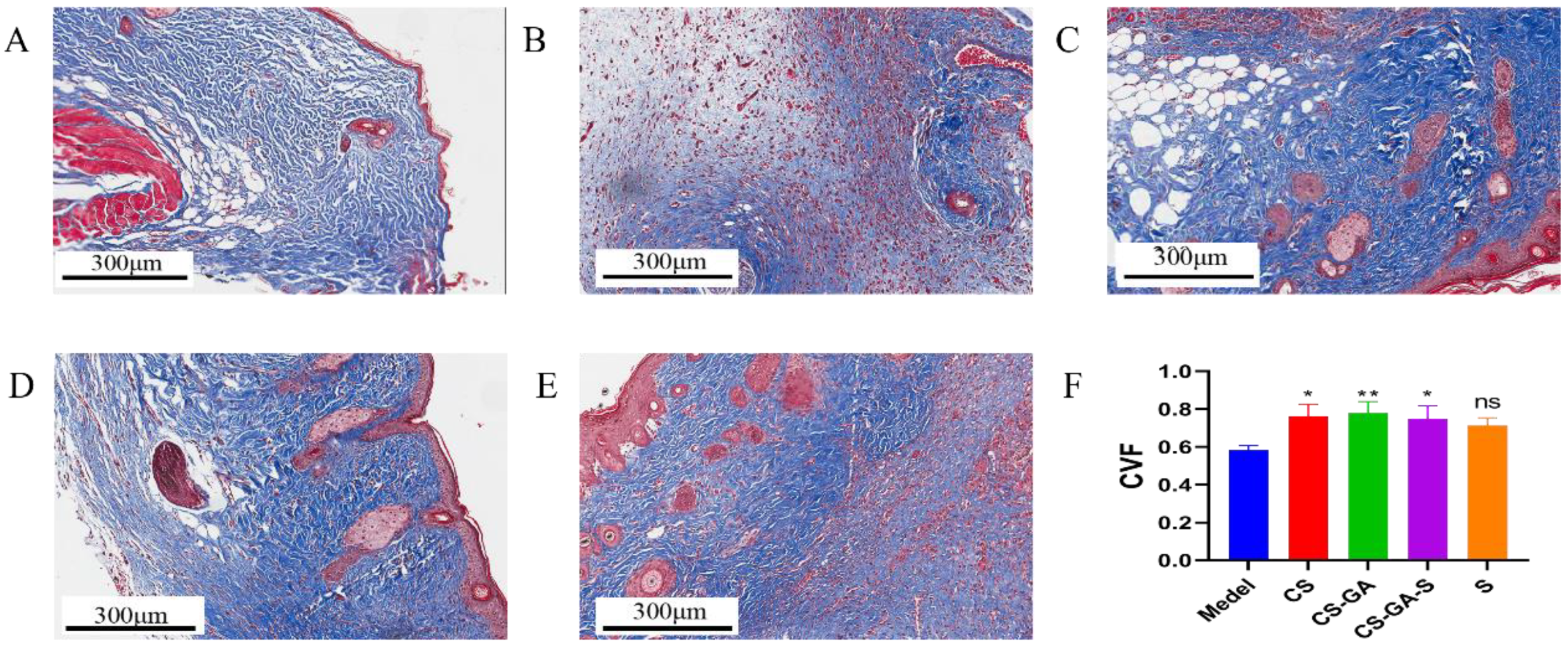
2.3.2. Histological Analysis
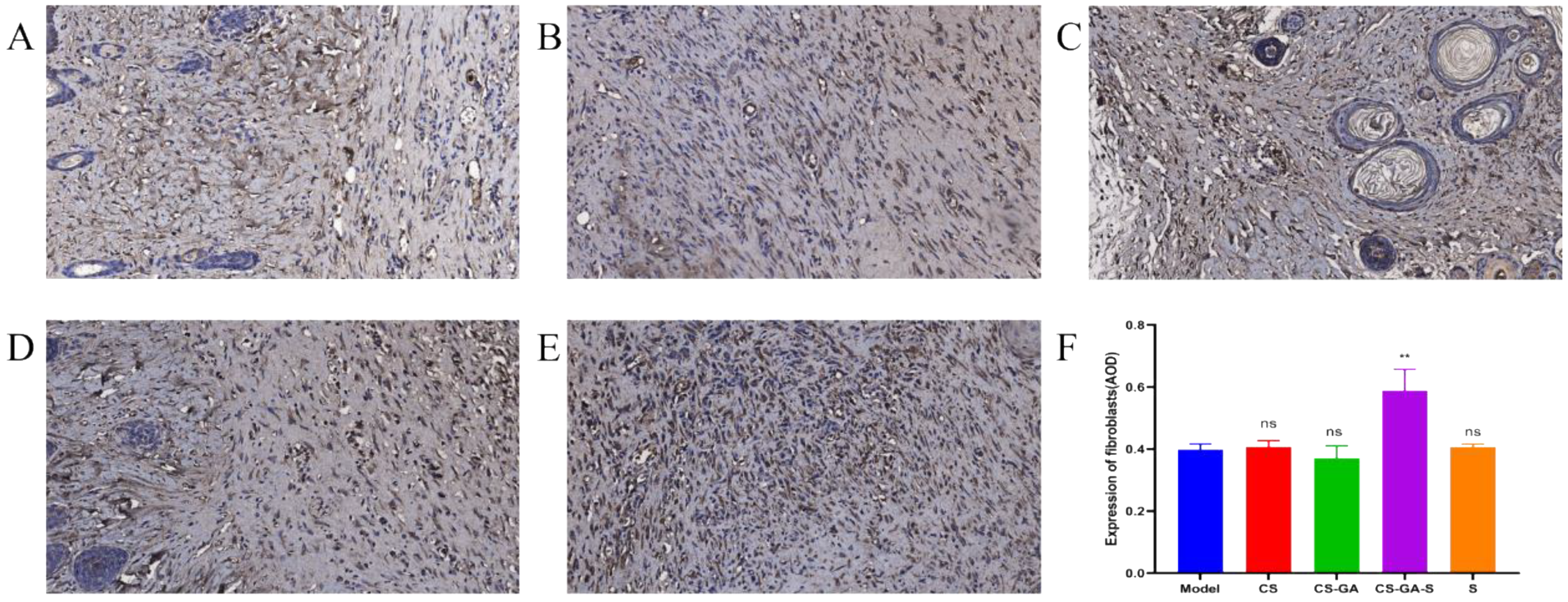
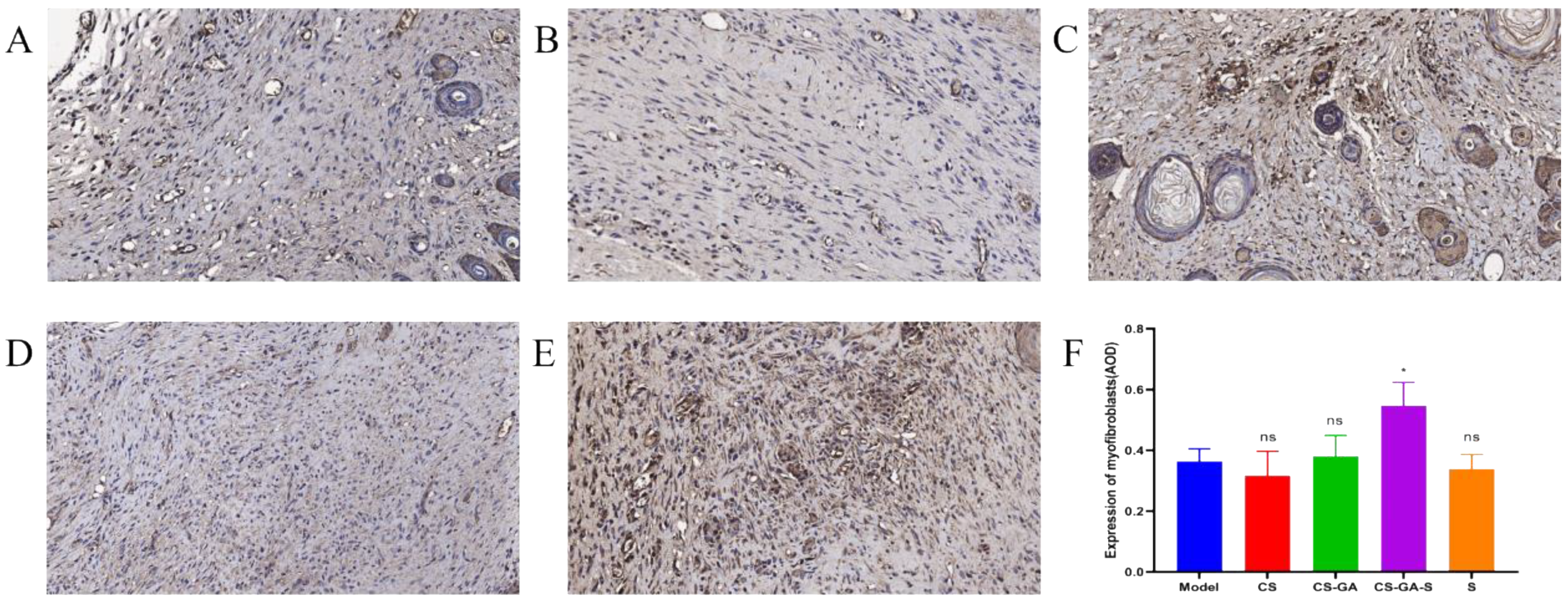
2.3.3. Immunohistochemistry
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis of GA
4.3. Characterization of GA
4.3.1. Characterization
4.3.2. Antioxidant Properties of the Chitosan-Gallic Acid
4.4. Preparation of Adhesive Hydrogel
4.5. Characterization of Adhesive Hydrogel
4.5.1. The Injectability of Hydrogel
4.5.2. The Self-Healing of Hydrogel
4.5.3. The Shape Adaptation of Hydrogel
4.5.4. The Sprayability of Hydrogel
4.5.5. Scanning Electron Microscopy (SEM)
4.5.6. Rheological Properties of Hydrogel
4.5.7. Degradation Rate of Hydrogel
4.5.8. Swelling Ratio of Hydrogel
4.5.9. In Vitro Drug Release Study
4.5.10. Antibacterial Efficacy of Hydrogel
4.5.11. Cytocompatibility of Hydrogel
4.6. In Vivo Assessment of Wound Healing Activity
4.6.1. Animals
4.6.2. Excision Wound
4.6.3. Experimental Design
4.6.4. Histological Analysis
4.6.5. Immunohistochemistry
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schafer, M.; Werner, S. Oxidative stress in normal and impaired wound repair. Pharm. Res. 2008, 58, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Xu, M.; Meng, G.; Lu, Y. SIRT3 deficiency delays diabetic skin wound healing via oxidative stress and necroptosis enhancement. J. Cell Mol. Med. 2020, 24, 4415–4427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, H.; Li, F.; Shao, W.; Gao, J.; Ling, D. Promoting Angiogenesis in Oxidative Diabetic Wound Microenvironment Using a Nanozyme-Reinforced Self-Protecting Hydrogel. ACS Cent. Sci. 2019, 5, 477–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Yu, H.; Pan, H.; Zhou, X.; Yao, P. Nrf2 Suppression Delays Diabetic Wound Healing Through Sustained Oxidative Stress and Inflammation. Front. Pharmacol. 2019, 10, 1099. [Google Scholar] [CrossRef]
- Bl, A.; Minb, D.; Mm, E.; Sa, E.; Yh, B.; By, B.; Cl, C.; Nan, J. Efficiency of Traditional Chinese medicine targeting the Nrf2/HO-1 signaling pathway—ScienceDirect. Biomed. Pharmacother. 2020, 126, 110074. [Google Scholar]
- Chen, L.Y.; Cheng, H.L.; Kuan, Y.H.; Liang, T.J.; Chao, Y.Y.; Lin, H.C. Therapeutic Potential of Luteolin on Impaired Wound Healing in Streptozotocin-Induced Rats. Biomedicines 2021, 9, 761. [Google Scholar] [CrossRef]
- Dixon, D.; Edmonds, M. Managing Diabetic Foot Ulcers: Pharmacotherapy for Wound Healing. Drugs 2021, 81, 29–56. [Google Scholar] [CrossRef]
- Falanga, V. Wound healing and its impairment in the diabetic foot. Lancet 2005, 366, 1736–1743. [Google Scholar] [CrossRef]
- Bellot, G.L.; Dong, X.; Lahiri, A.; Sebastin, S.J.; Batinic-Haberle, I.; Pervaiz, S.; Puhaindran, M.E. MnSOD is implicated in accelerated wound healing upon Negative Pressure Wound Therapy (NPWT): A case in point for MnSOD mimetics as adjuvants for wound management. Redox Biol. 2019, 20, 307. [Google Scholar] [CrossRef]
- Fan, J.; Liu, H.; Wang, J.; Zeng, J.; Tan, Y.; Wang, Y.; Yu, X.; Li, W.; Wang, P.; Yang, Z.; et al. Procyanidin B2 improves endothelial progenitor cell function and promotes wound healing in diabetic mice via activating Nrf2. J. Cell Mol. Med. 2021, 25, 652–665. [Google Scholar] [CrossRef]
- Lee, H.; Hwang, J.S.; Lee, J.; Kim, J.I.; Lee, D.G. Scolopendin 2, a cationic antimicrobial peptide from centipede, and its membrane-active mechanism. Biochim. Biophys. Acta 2015, 1848, 634–642. [Google Scholar] [CrossRef] [Green Version]
- Peng, K.; Yi, K.; Lei, Z.; Wu, X.; Yu, H. Two novel antimicrobial peptides from centipede venoms. Toxicon 2009, 55, 274–279. [Google Scholar] [CrossRef]
- Heejeong, L.; Hwang, J.-S.; Lee, D.G. Scolopendin 2 leads to cellular stress response in Candida albicans. Apoptosis 2016, 21, 856–865. [Google Scholar]
- Lu, J.; Deng, Q.P.; Ren, W.H. Study on the antibacterial mechanism of antimicrobial peptide Scolopin2-NH2 from centipede. Biotechnol. Bull. 2018, 34, 12. [Google Scholar]
- Bai, R.; Yong, H.; Zhang, X.; Liu, J.; Liu, J. Structural characterization and protective effect of gallic acid grafted O-carboxymethyl chitosan against hydrogen peroxide-induced oxidative damage. Int. J. Biol. Macromol. 2020, 143, 49–59. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Z.; Mo, X. Rapid in situ cross-linking of hydrogel adhesives based on thiol-grafted bio-inspired catechol-conjugated chitosan. J. Biomater. Appl. 2017, 32, 612–621. [Google Scholar] [CrossRef]
- Liu, B.; Zhou, C.; Zhang, Z.; Roland, J.D.; Lee, B.P. Antimicrobial Property of Halogenated Catechols. Chem. Eng. J. 2020, 403, 126340. [Google Scholar] [CrossRef]
- Wang, B.; Jeon, Y.S.; Park, H.S.; Kim, J.H. Self-healable mussel-mimetic nanocomposite hydrogel based on catechol-containing polyaspartamide and graphene oxide. Mater. Sci. Eng. Mater. Biol. Appl. 2016, 69, 160–170. [Google Scholar] [CrossRef]
- Bwa, C.; Jh, C.; Ry, B.; Ky, B.; Lma, C. Electrochemically deposition of catechol-chitosan hydrogel coating on coronary stent with robust copper ions immobilization capability and improved interfacial biological activity. Int. J. Biol. Macromol. 2021, 181, 435–443. [Google Scholar]
- Yang, B.; Song, J.; Jiang, Y.; Li, M.; Gu, Z. Injectable Adhesive Self-Healing Multicross-Linked Double-Network Hydrogel Facilitates Full-Thickness Skin Wound Healing. ACS Appl. Mater. Interfaces 2020, 12, 57782–57797. [Google Scholar] [CrossRef]
- Song, F.; Zhang, J.; Lu, J.; Cheng, Y.; Tao, Y.; Shao, C.; Wang, H. A mussel-inspired flexible chitosan-based bio-hydrogel as a tailored medical adhesive. Int. J. Biol. Macromol. 2021, 189, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Man, Z.; Sidi, L.; Xubo, Y.; Jin, Z.; Xin, H. An in situ catechol functionalized ε-polylysine/polyacrylamide hydrogel formed by hydrogen bonding recombination with high mechanical property for hemostasis. Int. J. Biol. Macromol. 2021, 191, 714–726. [Google Scholar] [CrossRef] [PubMed]
- Karas, D.; Ulrichová, J.; Valentová, K. Galloylation of polyphenols alters their biological activity. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. 2017, 105, 223–240. [Google Scholar] [CrossRef]
- Liang, Y.; Li, Z.; Huang, Y.; Yu, R.; Guo, B. Dual-Dynamic-Bond Cross-Linked Antibacterial Adhesive Hydrogel Sealants with On-Demand Removability for Post-Wound-Closure and Infected Wound Healing. ACS Nano 2021, 15, 7078–7093. [Google Scholar] [CrossRef]
- Du, D.; Chen, X.; Shi, C.; Zhang, Z.; Shi, D.; Kaneko, D.; Kaneko, T.; Hua, Z. Mussel-Inspired Epoxy Bioadhesive with Enhanced Interfacial Interactions for Wound Repair. Acta Biomater. 2021, 136, 223–232. [Google Scholar] [CrossRef]
- Bz, A.; Jh, B.; Ms, B.; Yl, B.; Bgab, C. Injectable self-healing supramolecular hydrogels with conductivity and photo-thermal antibacterial activity to enhance complete skin regeneration. Chem. Eng. J. 2020, 400, 125994. [Google Scholar]
- Zhao, X.; Liang, Y.; Huang, Y.; He, J.; Han, Y.; Guo, B. Physical Double-Network Hydrogel Adhesives with Rapid Shape Adaptability, Fast Self-Healing, Antioxidant and NIR/pH Stimulus-Responsiveness for Multidrug-Resistant Bacterial Infection and Removable Wound Dressing. Adv. Funct. Mater. 2020, 30, 1910748. [Google Scholar] [CrossRef]
- Dong, J.; Chen, L.; Zhang, Y.; Jayaswal, N.; Mezghani, I.; Zhang, W.; Veves, A. Mast Cells in Diabetes and Diabetic Wound Healing. Adv. Ther. 2020, 37, 4519–4537. [Google Scholar] [CrossRef]
- Bacci, S. Fine Regulation during Wound Healing by Mast Cells, a Physiological Role Not Yet Clarified. Int. J. Mol. Sci. 2022, 23, 1820. [Google Scholar] [CrossRef]
- Woodley, D.T. Distinct Fibroblasts in the Papillary and Reticular Dermis: Implications for Wound Healing. Derm. Clin. 2017, 35, 95–100. [Google Scholar] [CrossRef]
- Abbasi, S.; Sinha, S.; Labit, E.; Rosin, N.L.; Yoon, G.; Rahmani, W.; Jaffer, A.; Sharma, N.; Hagner, A.; Shah, P. Distinct Regulatory Programs Control the Latent Regenerative Potential of Dermal Fibroblasts during Wound Healing. Cell Stem Cell 2020, 27, 396–412. [Google Scholar] [CrossRef]
- Kuroda, K.; Tajima, S. Proliferation of HSP47-positive skin fibroblasts in dermatofibroma. J. Cutan. Pathol. 2007, 35, 21–26. [Google Scholar] [CrossRef]
- Qiang, L.; Yang, S.; Cui, Y.-H.; He, Y.-Y. Keratinocyte autophagy enables the activation of keratinocytes and fibroblastsand facilitates wound healing. Autophagy 2020, 17, 2128–2143. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Chen, L.; Bond, J.E.; Medina, M.A.; Ren, L.; Kokosis, G.; Selim, A.M.; Levinson, H. Myofibroblasts contribute to but are not necessary for wound contraction. Lab. Investig. 2015, 95, 1429–1438. [Google Scholar] [CrossRef] [Green Version]
- Bergmeier, V.; Etich, J.; Pitzler, L.; Frie, C.; Koch, M.; Fischer, M.; Rappl, G.; Abken, H.; Tomasek, J.J.; Brachvogel, B. Identification of a myofibroblast-specific expression signature in skin wounds. Matrix Biol. 2018, 65, 59–74. [Google Scholar] [CrossRef]
- Wang, Y.; Mack, J.A.; Maytin, E.V. CD44 inhibits alpha-SMA gene expression via a novel G-actin/MRTF-mediated pathway that intersects with TGFbetaR/p38MAPK signaling in murine skin fibroblasts. J. Biol. Chem. 2019, 294, 12779–12794. [Google Scholar] [CrossRef]
- Kallis, P.J.; Friedman, A.J. Collagen Powder in Wound Healing. J. Drugs Dermatol. JDD 2018, 17, 403–408. [Google Scholar]
- Zhao, X.; Pei, D.; Yang, Y.; Xu, K.; Yu, J.; Zhang, Y.; Zhang, Q.; He, G.; Zhang, Y.; Li, A.; et al. Green Tea Derivative Driven Smart Hydrogels with Desired Functions for Chronic Diabetic Wound Treatment. Adv. Funct. Mater. 2021, 31, 2009442. [Google Scholar] [CrossRef]
- Xia, Q.; Wang, L.; Xu, C.; Mei, J.; Li, Y. Effects of germination and high hydrostatic pressure processing on mineral elements, amino acids and antioxidants in vitro bioaccessibility, as well as starch digestibility in brown rice (Oryza sativa L.). Food Chem. 2017, 214, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Bui, H.L.; Cao, T.; Lee, W.Y.; Huang, S.C.; Huang, C.J. Dopamine-Initiated Photopolymerization for a Versatile Catechol-Functionalized Hydrogel. ACS Appl. Bio Mater. 2021, 4, 6268–6279. [Google Scholar] [CrossRef] [PubMed]
- Guyot, C.; Adoungotchodo, A.; Taillades, W.; Cerruti, M.; Lerouge, S. A catechol-chitosan-based adhesive and injectable hydrogel resistant to oxidation and compatible with cell therapy. J. Mater. Chem. 2021, 9, 8406–8416. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.Y.; Wang, Y.; Li, G.; Xiao, C.H.; Chen, X. Injectable self-repairing hydrogel based on imine borate and borate ester bond and its multiple response properties. J. High Mol. 2019, 50, 505–515. [Google Scholar]
- Puthia, M.; Butrym, M.; Petrlova, J.; Strömdahl, A.-C.; Andersson, M.; Kjellström, S.; Schmidtchen, A. A dual-action peptide-containing hydrogel targets wound infection and inflammation. Sci. Transl. Med. 2020, 12, eaax6601. [Google Scholar] [CrossRef]
- Gan, D.; Xing, W.; Jiang, L.; Fang, J.; Zhao, C.; Ren, F.; Fang, L.; Wang, K.; Lu, X. Plant-inspired adhesive and tough hydrogel based on Ag-Lignin nanoparticles-triggered dynamic redox catechol chemistry. Nat. Commun. 2019, 10, 1487. [Google Scholar] [CrossRef] [Green Version]
- Duan, L.; Yuan, Q.; Xiang, H.; Yang, X.; Liu, L.; Li, J. Fabrication and characterization of a novel collagen-catechol hydrogel. J. Biomater. Appl. 2017, 32, 862–870. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Zheng, T.; Wu, C.; Wang, J.; Ye, F.; Cui, M.; Sun, S.; Zhang, Y.; Li, Y.; Dong, Z. A Shape-Adaptive Gallic Acid Driven Multifunctional Adhesive Hydrogel Loaded with Scolopin2 for Wound Repair. Pharmaceuticals 2022, 15, 1422. https://doi.org/10.3390/ph15111422
Chen H, Zheng T, Wu C, Wang J, Ye F, Cui M, Sun S, Zhang Y, Li Y, Dong Z. A Shape-Adaptive Gallic Acid Driven Multifunctional Adhesive Hydrogel Loaded with Scolopin2 for Wound Repair. Pharmaceuticals. 2022; 15(11):1422. https://doi.org/10.3390/ph15111422
Chicago/Turabian StyleChen, Huan, Tingting Zheng, Chenyang Wu, Jinrui Wang, Fan Ye, Mengyao Cui, Shuhui Sun, Yun Zhang, Ying Li, and Zhengqi Dong. 2022. "A Shape-Adaptive Gallic Acid Driven Multifunctional Adhesive Hydrogel Loaded with Scolopin2 for Wound Repair" Pharmaceuticals 15, no. 11: 1422. https://doi.org/10.3390/ph15111422
APA StyleChen, H., Zheng, T., Wu, C., Wang, J., Ye, F., Cui, M., Sun, S., Zhang, Y., Li, Y., & Dong, Z. (2022). A Shape-Adaptive Gallic Acid Driven Multifunctional Adhesive Hydrogel Loaded with Scolopin2 for Wound Repair. Pharmaceuticals, 15(11), 1422. https://doi.org/10.3390/ph15111422




