Astrocytes Reduce Store-Operated Ca2+ Entry in Microglia under the Conditions of an Inflammatory Stimulus and Muscarinic Receptor Blockade
Abstract
:1. Introduction
2. Results
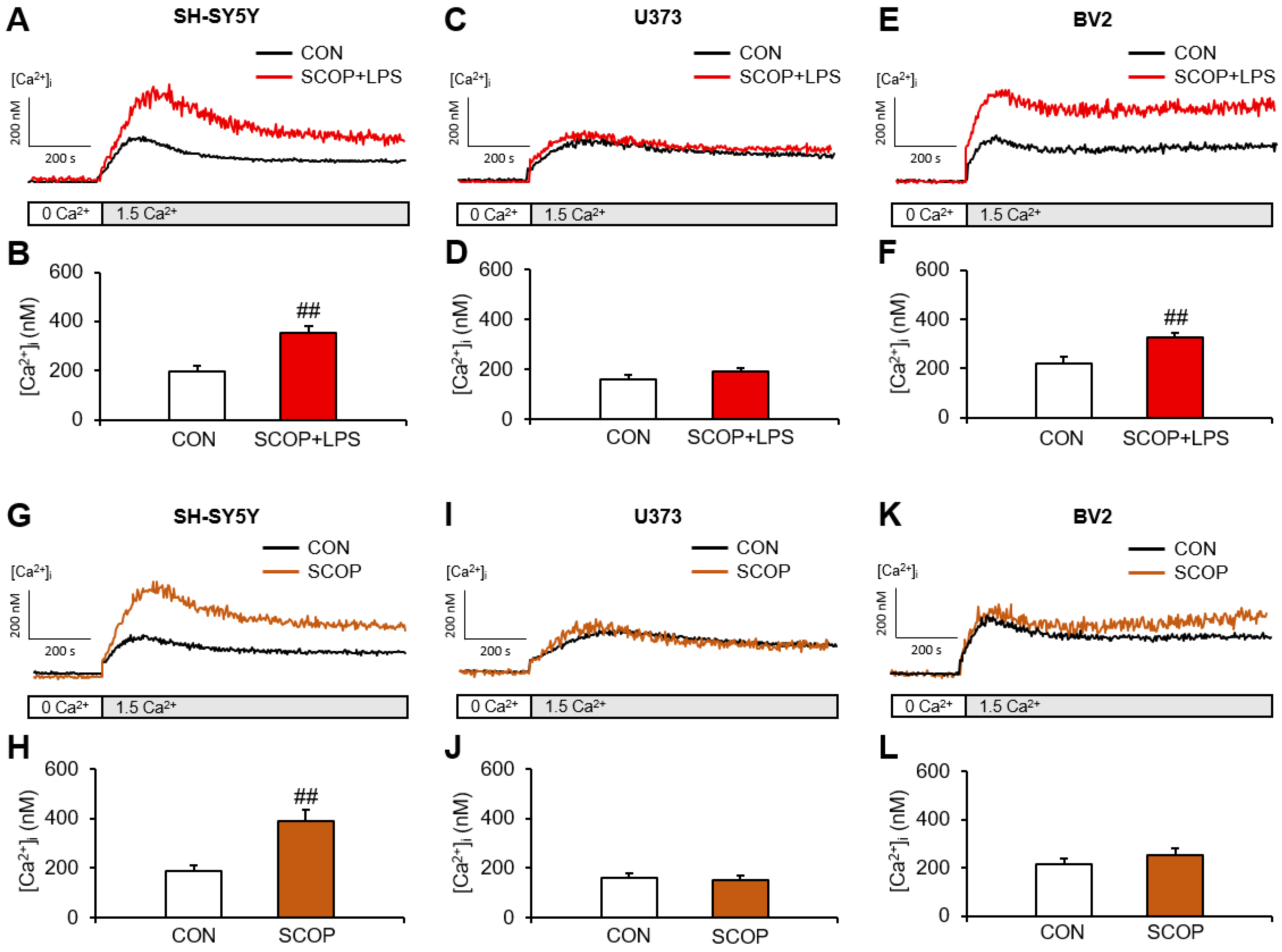
2.1. The Changes in SOCE Induced by LPS under Scopolamine-Pretreated Conditions or by Scopolamine Alone Are Different in SH-SY5Y, U373, and BV2 Cells
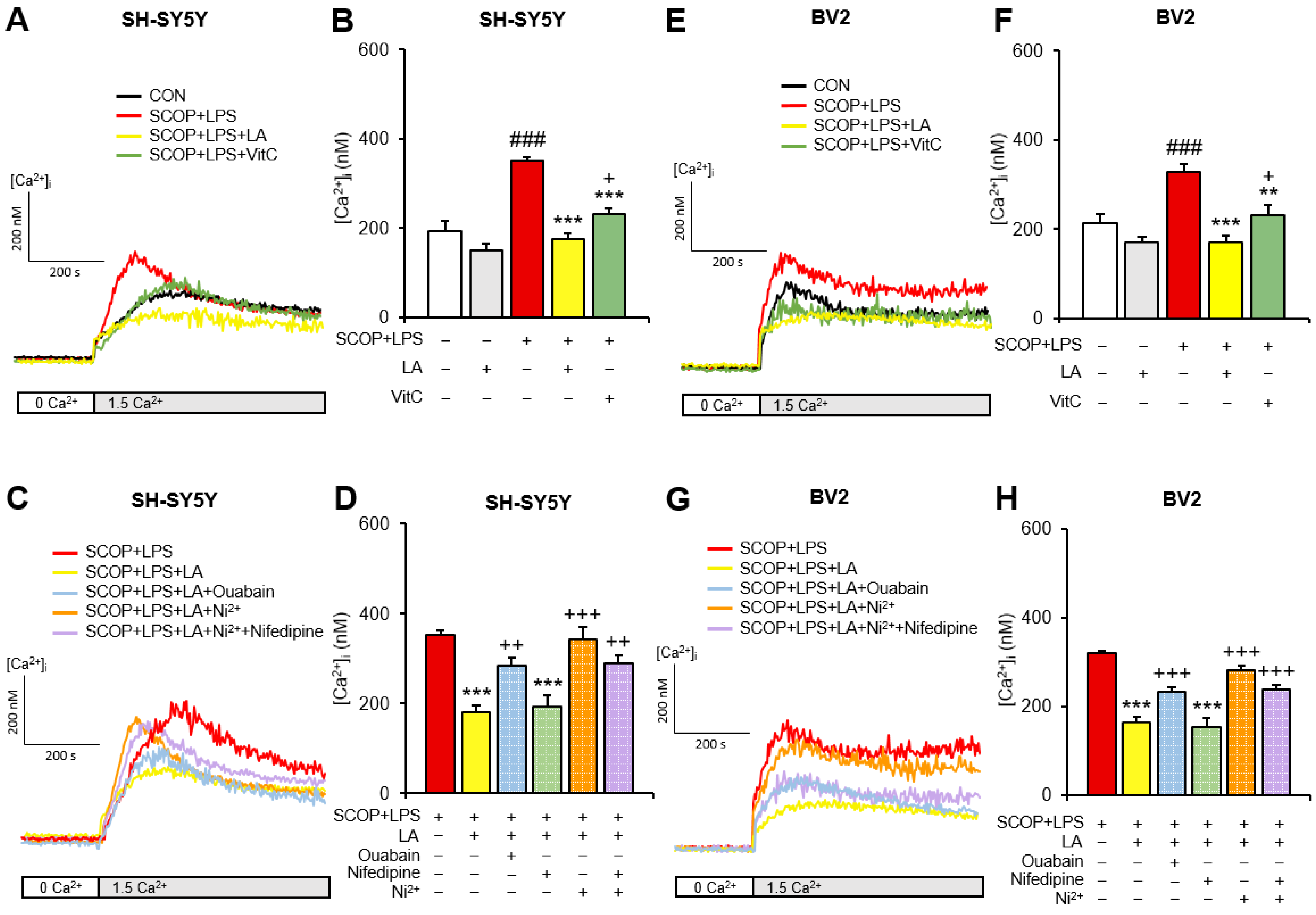
2.2. LA Decreases LPS Exposure-Induced Increases in SOCE in Scopolamine-Pretreated SH-SY5Y and BV2 Cells
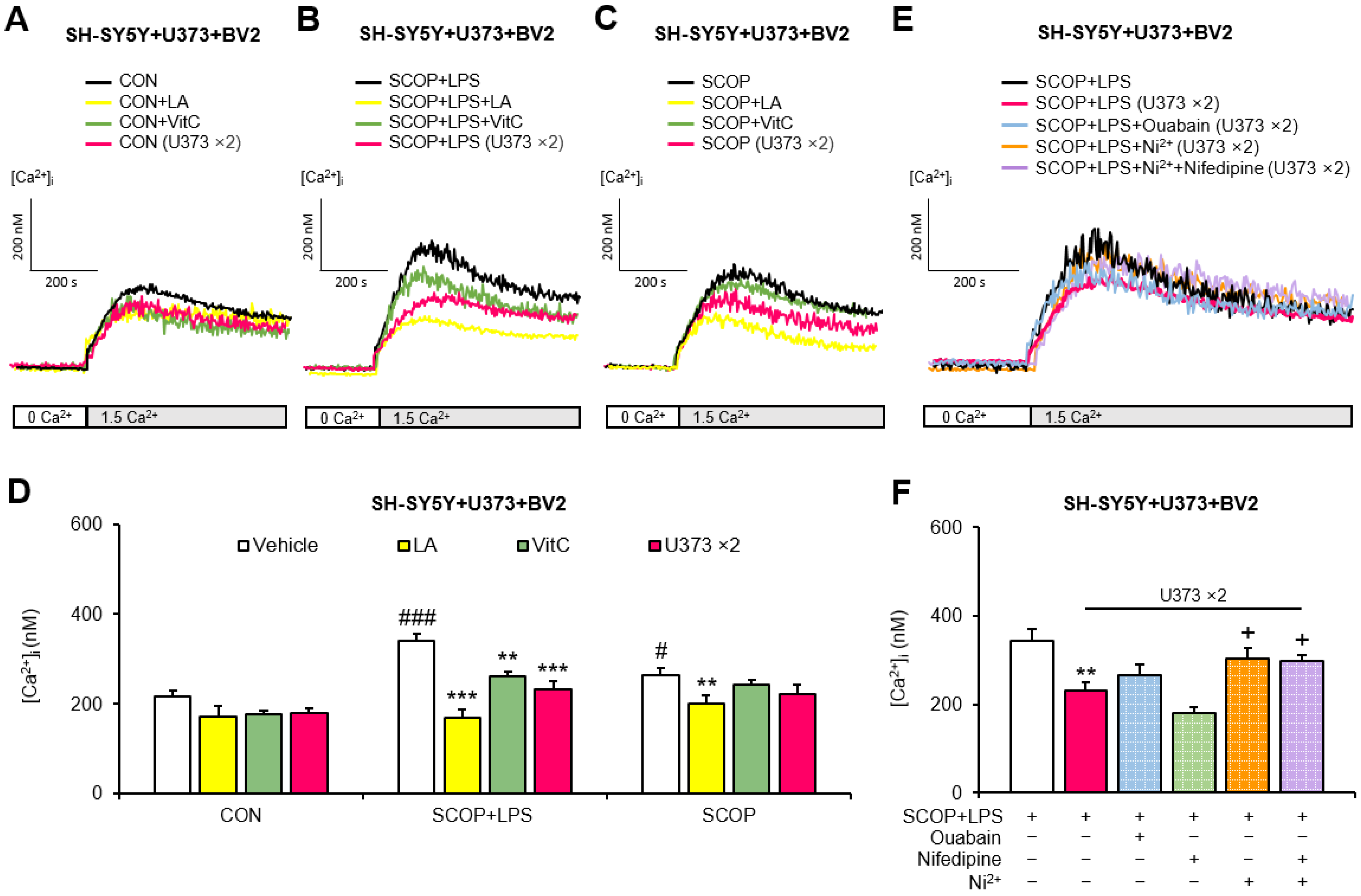
2.3. Doubling the Number of U373 Cells Inhibits the LPS Exposure-Induced Increases in SOCE in Scopolamine-Pretreated SH-SY5Y + U373 + BV2 Mixed Cells
2.4. Doubling the Number of U373 Cells Inhibits the LPS Exposure-Induced Increase in SOCE in the Scopolamine-Pretreated U373 + BV2 Mixed Cells
3. Discussion
4. Materials and Methods
4.1. Experimental Cell Preparations
4.2. Measurement of [Ca2+]i
4.3. Solutions and Chemicals
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sama, D.M.; Norris, C.M. Calcium dysregulation and neuroinflammation: Discrete and integrated mechanisms for age-related synaptic dysfunction. Ageing Res. Rev. 2013, 12, 982–995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AlFadly, E.D.; Elzahhar, P.A.; Tramarin, A.; Elkazaz, S.; Shaltout, H.; Abu-Serie, M.M.; Janockova, J.; Soukup, O.; Ghareeb, D.A.; El-Yazbi, A.F. Tackling neuroinflammation and cholinergic deficit in Alzheimer’s disease: Multi-target inhibitors of cholinesterases, cyclooxygenase-2 and 15-lipoxygenase. Eur. J. Med. Chem. 2019, 167, 161–186. [Google Scholar] [CrossRef]
- Gamage, R.; Wagnon, I.; Rossetti, I.; Childs, R.; Niedermayer, G.; Chesworth, R.; Gyengesi, E. Cholinergic modulation of glial function during aging and chronic neuroinflammation. Front. Cell. Neurosci. 2020, 14, 577912. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Morgan, T.E.; Rozovsky, I.; Finch, C.E. Aging and glial responses to lipopolysaccharide in vitro: Greater induction of IL-1 and IL-6, but smaller induction of neurotoxicity. Exp. Neurol. 2003, 182, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-Y.; Watson, M.L.; Gallagher, M.; Nicolle, M.M. Muscarinic receptor-mediated GTP–Eu binding in the hippocampus and prefrontal cortex is correlated with spatial memory impairment in aged rats. Neurobiol. Aging 2007, 28, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Galvin, V.C.; Yang, S.T.; Paspalas, C.D.; Yang, Y.; Jin, L.E.; Datta, D.; Morozov, Y.M.; Lightbourne, T.C.; Lowet, A.S.; Rakic, P. Muscarinic M1 receptors modulate working memory performance and activity via KCNQ potassium channels in the primate prefrontal cortex. Neuron 2020, 106, 649–661. [Google Scholar] [CrossRef] [PubMed]
- Gronier, B.; Perry, K.W.; Rasmussen, K. Activation of the mesocorticolimbic dopaminergic system by stimulation of muscarinic cholinergic receptors in the ventral tegmental area. Psychopharmacology 2000, 147, 347–355. [Google Scholar] [CrossRef]
- Kékesi, O.; Liang, H.; Münch, G.; Morley, J.W.; Gyengesi, E.; Buskila, Y. The differential impact of acute microglia activation on the excitability of cholinergic neurons in the mouse medial septum. Brain Struct. Funct. 2019, 224, 2297–2309. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Rodríguez, M.; de la Fuente, C.; García-Durillo, M.; García-Rodríguez, C.; Villalobos, C.; Núñez, L. Aging and amyloid β oligomers enhance TLR4 expression, LPS-induced Ca2+ responses, and neuron cell death in cultured rat hippocampal neurons. J. Neuroinflamm. 2017, 14, 24. [Google Scholar] [CrossRef] [Green Version]
- Pchitskaya, E.; Popugaeva, E.; Bezprozvanny, I. Calcium signaling and molecular mechanisms underlying neurodegenerative diseases. Cell Calcium 2018, 70, 87–94. [Google Scholar] [CrossRef]
- Majewski, L.; Kuznicki, J. SOCE in neurons: Signaling or just refilling? Biochim. Biophys. Acta Mol. Cell Res. 2015, 1853, 1940–1952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Wu, S. Impairment of store-operated calcium entry: Implications in Alzheimer’s neurodegeneration. Curr. Alzheimer Res. 2020, 17, 1088–1094. [Google Scholar] [CrossRef]
- Mitra, R.; Hasan, G. Store-operated Ca2+ entry regulates neuronal gene expression and function. Curr. Opin. Neurobiol. 2022, 73, 102520. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ryskamp, D.; Birnbaumer, L.; Bezprozvanny, I. Inhibition of TRPC1-dependent store-operated calcium entry improves synaptic stability and motor performance in a mouse model of Huntington’s disease. J. Huntingt. Dis. 2018, 7, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, W.; Zhang, L.; Liu, W.-B.; Fei, Z. Inhibition of store-operated calcium entry attenuates MPP+-induced oxidative stress via preservation of mitochondrial function in PC12 cells: Involvement of Homer1a. PLoS ONE 2013, 8, e83638. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Guo, S.; Luo, L.; Zheng, Y.; Gan, S.; Kang, X.; Wu, X.; Zhu, S. Emerging roles of microglia in neuro-vascular unit: Implications of microglia-neurons interactions. Front. Cell. Neurosci. 2021, 15, 706025. [Google Scholar] [CrossRef]
- Nayak, D.; Roth, T.L.; McGavern, D.B. Microglia development and function. Annu. Rev. Immunol. 2014, 32, 367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McBean, G.J. Cysteine, glutathione, and thiol redox balance in astrocytes. Antioxidants 2017, 6, 62. [Google Scholar] [CrossRef] [Green Version]
- Durkee, C.; Kofuji, P.; Navarrete, M.; Araque, A. Astrocyte and neuron cooperation in long-term depression. Trends Neurosci. 2021, 44, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Seol, G.H.; Lee, Y.H.; Kang, P.; You, J.H.; Park, M.; Min, S.S. Randomized controlled trial for Salvia sclarea or Lavandula angustifolia: Differential effects on blood pressure in female patients with urinary incontinence undergoing urodynamic examination. J. Altern. Complement. Med. 2013, 19, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Letizia, C.; Cocchiara, J.; Lalko, J.; Api, A. Fragrance material review on linalyl acetate. Food Chem. Toxicol. 2003, 41, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.K.; Hsieh, Y.S.; Han, A.Y.; Kwon, S.; Kang, P.; Seol, G.H. Sex-specific susceptibility to type 2 diabetes mellitus and preventive effect of linalyl acetate. Life Sci. 2020, 260, 118432. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.K.; Kwon, S.; Hsieh, Y.S.; Han, A.Y.; Seol, G.H. Linalyl acetate restores colon contractility and blood pressure in repeatedly stressed-ulcerative colitis rats. Environ. Health Prev. Med. 2022, 27, 27. [Google Scholar] [CrossRef] [PubMed]
- Seo, E.; Shin, Y.K.; Hsieh, Y.S.; Lee, J.-M.; Seol, G.H. Linalyl acetate as a potential preventive agent against muscle wasting in rheumatoid arthritis rats chronically exposed to nicotine. J. Pharmacol. Sci. 2021, 147, 27–32. [Google Scholar] [CrossRef] [PubMed]
- You, J.H.; Kang, P.; Min, S.S.; Seol, G.H. Bergamot essential oil differentially modulates intracellular Ca2+ levels in vascular endothelial and smooth muscle cells: A new finding seen with fura-2. J. Cardiovasc. Pharmacol. 2013, 61, 324–328. [Google Scholar] [CrossRef]
- Hsieh, Y.S.; Shin, Y.K.; Seol, G.H. Protection of the neurovascular unit from calcium-related ischemic injury by linalyl acetate. Chin. J. Physiol. 2021, 64, 88. [Google Scholar]
- Tao, W.; Wang, G.; Pei, X.; Sun, W.; Wang, M. Chitosan Oligosaccharide Attenuates Lipopolysaccharide-Induced Intestinal Barrier Dysfunction through Suppressing the Inflammatory Response and Oxidative Stress in Mice. Antioxidants 2022, 11, 1384. [Google Scholar] [CrossRef]
- Muhammad, T.; Ali, T.; Ikram, M.; Khan, A.; Alam, S.I.; Kim, M.O. Melatonin rescue oxidative stress-mediated neuroinflammation/neurodegeneration and memory impairment in scopolamine-induced amnesia mice model. J. Neuroimmune Pharmacol. 2019, 14, 278–294. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, B.J.; Irrinki, K.M.; Mallilankaraman, K.; Lien, Y.-C.; Wang, Y.; Bhanumathy, C.D.; Subbiah, R.; Ritchie, M.F.; Soboloff, J.; Baba, Y. S-glutathionylation activates STIM1 and alters mitochondrial homeostasis. J. Cell Biol. 2010, 190, 391–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grupe, M.; Myers, G.; Penner, R.; Fleig, A. Activation of store-operated ICRAC by hydrogen peroxide. Cell Calcium 2010, 48, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsieh, Y.S.; Kwon, S.; Lee, H.S.; Seol, G.H. Linalyl acetate prevents hypertension-related ischemic injury. PLoS ONE 2018, 13, e0198082. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.S.; Shin, Y.K.; Han, A.Y.; Kwon, S.; Seol, G.H. Linalyl acetate prevents three related factors of vascular damage in COPD-like and hypertensive rats. Life Sci. 2019, 232, 116608. [Google Scholar] [CrossRef] [PubMed]
- Noh, D.; Choi, J.G.; Huh, E.; Oh, M.S. Tectorigenin, a flavonoid-based compound of leopard lily rhizome, attenuates UV-B-induced apoptosis and collagen degradation by inhibiting oxidative stress in human keratinocytes. Nutrients 2018, 10, 1998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enciu, A.-M.; Popescu, B.O. Is there a causal link between inflammation and dementia? BioMed Res. Int. 2013, 2013, 316495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pahwa, R.; Goyal, A.; Bansal, P.; Jialal, I. Chronic Inflammation; StatPearls Publishing: Treasure Island, FL, USA, 2018. [Google Scholar]
- Sermersheim, M.; Kenney, A.D.; Lin, P.-H.; McMichael, T.M.; Cai, C.; Gumpper, K.; Adesanya, T.A.; Li, H.; Zhou, X.; Park, K.-H. MG53 suppresses interferon-β and inflammation via regulation of ryanodine receptor-mediated intracellular calcium signaling. Nat. Commun. 2020, 11, 3624. [Google Scholar] [CrossRef] [PubMed]
- Miyake, T.; Shirakawa, H.; Kusano, A.; Sakimoto, S.; Konno, M.; Nakagawa, T.; Mori, Y.; Kaneko, S. TRPM2 contributes to LPS/IFNγ-induced production of nitric oxide via the p38/JNK pathway in microglia. Biochem. Biophys. Res. Commun. 2014, 444, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Hopp, S.C.; D’Angelo, H.M.; Royer, S.E.; Kaercher, R.M.; Crockett, A.M.; Adzovic, L.; Wenk, G.L. Calcium dysregulation via L-type voltage-dependent calcium channels and ryanodine receptors underlies memory deficits and synaptic dysfunction during chronic neuroinflammation. J. Neuroinflamm. 2015, 12, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Sevilla, D.F.; Núñez, A.; Buño, W. Muscarinic receptors, from synaptic plasticity to its role in network activity. Neuroscience 2021, 456, 60–70. [Google Scholar] [CrossRef]
- Dennis, S.H.; Pasqui, F.; Colvin, E.M.; Sanger, H.; Mogg, A.J.; Felder, C.C.; Broad, L.M.; Fitzjohn, S.M.; Isaac, J.T.; Mellor, J.R. Activation of muscarinic M1 acetylcholine receptors induces long-term potentiation in the hippocampus. Cereb. Cortex 2016, 26, 414–426. [Google Scholar] [CrossRef] [Green Version]
- Hornick, A.; Schwaiger, S.; Rollinger, J.M.; Vo, N.P.; Prast, H.; Stuppner, H. Extracts and constituents of Leontopodium alpinum enhance cholinergic transmission: Brain ACh increasing and memory improving properties. Biochem. Pharmacol. 2008, 76, 236–248. [Google Scholar] [CrossRef] [Green Version]
- Sun, D.; Unnithan, R.R.; French, C. Scopolamine Impairs Spatial Information Recorded With “Miniscope” Calcium Imaging in Hippocampal Place Cells. Front. Neurosci. 2021, 15, 299. [Google Scholar] [CrossRef]
- Wong-Guerra, M.; Jiménez-Martin, J.; Pardo-Andreu, G.L.; Fonseca-Fonseca, L.A.; Souza, D.O.; de Assis, A.M.; Ramirez-Sanchez, J.; Del Valle, R.M.-S.; Nuñez-Figueredo, Y. Mitochondrial involvement in memory impairment induced by scopolamine in rats. Neurol. Res. 2017, 39, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Baghel, M.S.; Thakur, M.K. Vdac1 downregulation causes mitochondrial disintegration leading to hippocampal neurodegeneration in scopolamine-induced amnesic mice. Mol. Neurobiol. 2019, 56, 1707–1718. [Google Scholar] [CrossRef] [PubMed]
- Puangmalai, N.; Thangnipon, W.; Soi-Ampornkul, R.; Suwanna, N.; Tuchinda, P.; Nobsathian, S. Neuroprotection of N-benzylcinnamide on scopolamine-induced cholinergic dysfunction in human SH-SY5Y neuroblastoma cells. Neural Regen. Res. 2017, 12, 1492. [Google Scholar] [PubMed]
- Wessler, I.; Reinheimer, T.; Klapproth, H.; Schneider, F.-J.; Racké, K.; Hammer, R. Mammalian glial cells in culture synthesize acetylcholine. Naunyn-Schmiedebergs Arch. Pharmacol. 1997, 356, 694–697. [Google Scholar] [CrossRef] [PubMed]
- Atwood, B.K.; Lopez, J.; Wager-Miller, J.; Mackie, K.; Straiker, A. Expression of G protein-coupled receptors and related proteins in HEK293, AtT20, BV2, and N18 cell lines as revealed by microarray analysis. BMC Genom. 2011, 12, 14. [Google Scholar] [CrossRef] [Green Version]
- Moon, P.-D.; Han, N.-R.; Lee, J.S.; Kim, H.-M.; Jeong, H.-J. Effects of linalyl acetate on thymic stromal lymphopoietin production in mast cells. Molecules 2018, 23, 1711. [Google Scholar] [CrossRef] [Green Version]
- Hritcu, L.; Cioanca, O.; Hancianu, M. Effects of lavender oil inhalation on improving scopolamine-induced spatial memory impairment in laboratory rats. Phytomedicine 2012, 19, 529–534. [Google Scholar] [CrossRef]
- Schuwald, A.M.; Nöldner, M.; Wilmes, T.; Klugbauer, N.; Leuner, K.; Müller, W.E. Lavender oil-potent anxiolytic properties via modulating voltage dependent calcium channels. PLoS ONE 2013, 8, e59998. [Google Scholar] [CrossRef] [Green Version]
- Fairless, R.; Williams, S.K.; Diem, R. Dysfunction of neuronal calcium signalling in neuroinflammation and neurodegeneration. Cell Tissue Res. 2014, 357, 455–462. [Google Scholar] [CrossRef]
- Molinaro, P.; Viggiano, D.; Nisticò, R.; Sirabella, R.; Secondo, A.; Boscia, F.; Pannaccione, A.; Scorziello, A.; Mehdawy, B.; Sokolow, S. Na+–Ca2+ exchanger (NCX3) knock-out mice display an impairment in hippocampal long-term potentiation and spatial learning and memory. J. Neurosci. 2011, 31, 7312–7321. [Google Scholar] [CrossRef]
- Sibarov, D.A.; Bolshakov, A.E.; Abushik, P.A.; Krivoi, I.I.; Antonov, S.M. Na+, K+-ATPase functionally interacts with the plasma membrane Na+, Ca2+ exchanger to prevent Ca2+ overload and neuronal apoptosis in excitotoxic stress. J. Pharmacol. Exp. Ther. 2012, 343, 596–607. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Cao, L.; Cao, X.; Zhu, M.; Zhang, X.; Wu, Z.; Xiong, S.; Xie, Z.; Yang, Y.; Chen, J. DR-region of Na+/K+ ATPase is a target to treat excitotoxicity and stroke. Cell Death Dis. 2018, 10, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, Y.; Hu, J.; Li, J.; Yang, Z.; Xin, X.; Wang, J.; Ding, J.; Geng, M. Effect of acidic oligosaccharide sugar chain on scopolamine-induced memory impairment in rats and its related mechanisms. Neurosci. Lett. 2005, 374, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Kip, S.N.; Strehler, E.E. Rapid downregulation of NCX and PMCA in hippocampal neurons following H2O2 oxidative stress. Ann. N. Y. Acad. Sci. 2007, 1099, 436–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waller, R.; Murphy, M.; Garwood, C.J.; Jennings, L.; Heath, P.R.; Chambers, A.; Matthews, F.E.; Brayne, C.; Ince, P.G.; Wharton, S.B. Metallothionein-I/II expression associates with the astrocyte DNA damage response and not Alzheimer-type pathology in the aging brain. Glia 2018, 66, 2316–2323. [Google Scholar] [CrossRef] [Green Version]
- Turati, J.; Ramírez, D.; Carniglia, L.; Saba, J.; Caruso, C.; Quarleri, J.; Durand, D.; Lasaga, M. Antioxidant and neuroprotective effects of mGlu3 receptor activation on astrocytes aged in vitro. Neurochem. Int. 2020, 140, 104837. [Google Scholar] [CrossRef]
- Ju, Y.H.; Bhalla, M.; Hyeon, S.J.; Oh, J.E.; Yoo, S.; Chae, U.; Kwon, J.; Koh, W.; Lim, J.; Park, Y.M. Astrocytic urea cycle detoxifies Aβ-derived ammonia while impairing memory in Alzheimer’s disease. Cell Metab. 2022, 34, 1104–1120. [Google Scholar] [CrossRef]
- Sun, L.; Li, Y.; Jia, X.; Wang, Q.; Li, Y.; Hu, M.; Tian, L.; Yang, J.; Xing, W.; Zhang, W. Neuroprotection by IFN-γ via astrocyte-secreted IL-6 in acute neuroinflammation. Oncotarget 2017, 8, 40065. [Google Scholar] [CrossRef] [Green Version]
- Lull, M.E.; Block, M.L. Microglial activation and chronic neurodegeneration. Neurotherapeutics 2010, 7, 354–365. [Google Scholar] [CrossRef] [Green Version]
- Brawek, B.; Schwendele, B.; Riester, K.; Kohsaka, S.; Lerdkrai, C.; Liang, Y.; Garaschuk, O. Impairment of in vivo calcium signaling in amyloid plaque-associated microglia. Acta Neuropathol. 2014, 127, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Sanz, J.M.; Chiozzi, P.; Ferrari, D.; Colaianna, M.; Idzko, M.; Falzoni, S.; Fellin, R.; Trabace, L.; Di Virgilio, F. Activation of microglia by amyloid β requires P2X7 receptor expression. J. Immunol. 2009, 182, 4378–4385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henn, A.; Lund, S.; Hedtjärn, M.; Schrattenholz, A.; Pörzgen, P.; Leist, M. The suitability of BV2 cells as alternative model system for primary microglia cultures or for animal experiments examining brain inflammation. ALTEX Altern. Anim. Exp. 2009, 26, 83–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stansley, B.; Post, J.; Hensley, K. A comparative review of cell culture systems for the study of microglial biology in Alzheimer’s disease. J. Neuroinflamm. 2012, 9, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawrimore, C.J.; Coleman, L.G.; Zou, J.; Crews, F.T. Ethanol induction of innate immune signals across BV2 microglia and SH-SY5Y neuroblastoma involves induction of IL-4 and IL-13. Brain Sci. 2019, 9, 228. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Fu, Y.; Zhang, Y.; Liu, F.; Rose, G.M.; He, X.; Yi, X.; Ren, R.; Li, Y.; Zhang, Y. Butein attenuates the cytotoxic effects of LPS-stimulated microglia on the SH-SY5Y neuronal cell line. Eur. J. Pharmacol. 2020, 868, 172858. [Google Scholar] [CrossRef]
- Pandur, E.; Tamási, K.; Pap, R.; Varga, E.; Miseta, A.; Sipos, K. Fractalkine induces hepcidin expression of BV-2 microglia and causes iron accumulation in SH-SY5Y cells. Cell. Mol. Neurobiol. 2019, 39, 985–1001. [Google Scholar] [CrossRef] [Green Version]
- Han, A.Y.; Ha, S.M.; Shin, Y.K.; Seol, G.H. Ginsenoside Rg-1 prevents elevated cytosolic Ca2+ via store-operated Ca2+ entry in high-glucose–stimulated vascular endothelial and smooth muscle cells. BMC Complement. Med. Ther. 2022, 22, 166. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.J.; Shin, Y.K.; Seo, E.; Seol, G.H. Astrocytes Reduce Store-Operated Ca2+ Entry in Microglia under the Conditions of an Inflammatory Stimulus and Muscarinic Receptor Blockade. Pharmaceuticals 2022, 15, 1521. https://doi.org/10.3390/ph15121521
Kim YJ, Shin YK, Seo E, Seol GH. Astrocytes Reduce Store-Operated Ca2+ Entry in Microglia under the Conditions of an Inflammatory Stimulus and Muscarinic Receptor Blockade. Pharmaceuticals. 2022; 15(12):1521. https://doi.org/10.3390/ph15121521
Chicago/Turabian StyleKim, Yoo Jin, You Kyoung Shin, Eunhye Seo, and Geun Hee Seol. 2022. "Astrocytes Reduce Store-Operated Ca2+ Entry in Microglia under the Conditions of an Inflammatory Stimulus and Muscarinic Receptor Blockade" Pharmaceuticals 15, no. 12: 1521. https://doi.org/10.3390/ph15121521
APA StyleKim, Y. J., Shin, Y. K., Seo, E., & Seol, G. H. (2022). Astrocytes Reduce Store-Operated Ca2+ Entry in Microglia under the Conditions of an Inflammatory Stimulus and Muscarinic Receptor Blockade. Pharmaceuticals, 15(12), 1521. https://doi.org/10.3390/ph15121521





