Integrated Mechanisms of Polarity–Based Extracts of Cucumis melo L. Seed Kernels for Airway Smooth Muscle Relaxation via Key Signaling Pathways Based on WGCNA, In Vivo, and In Vitro Analyses
Abstract
1. Introduction
2. Results
2.1. Identification of Bioactive Compounds
2.2. Method Validation and Parameter Optimization for HPLC
2.3. Quantification of Phytochemical Compounds
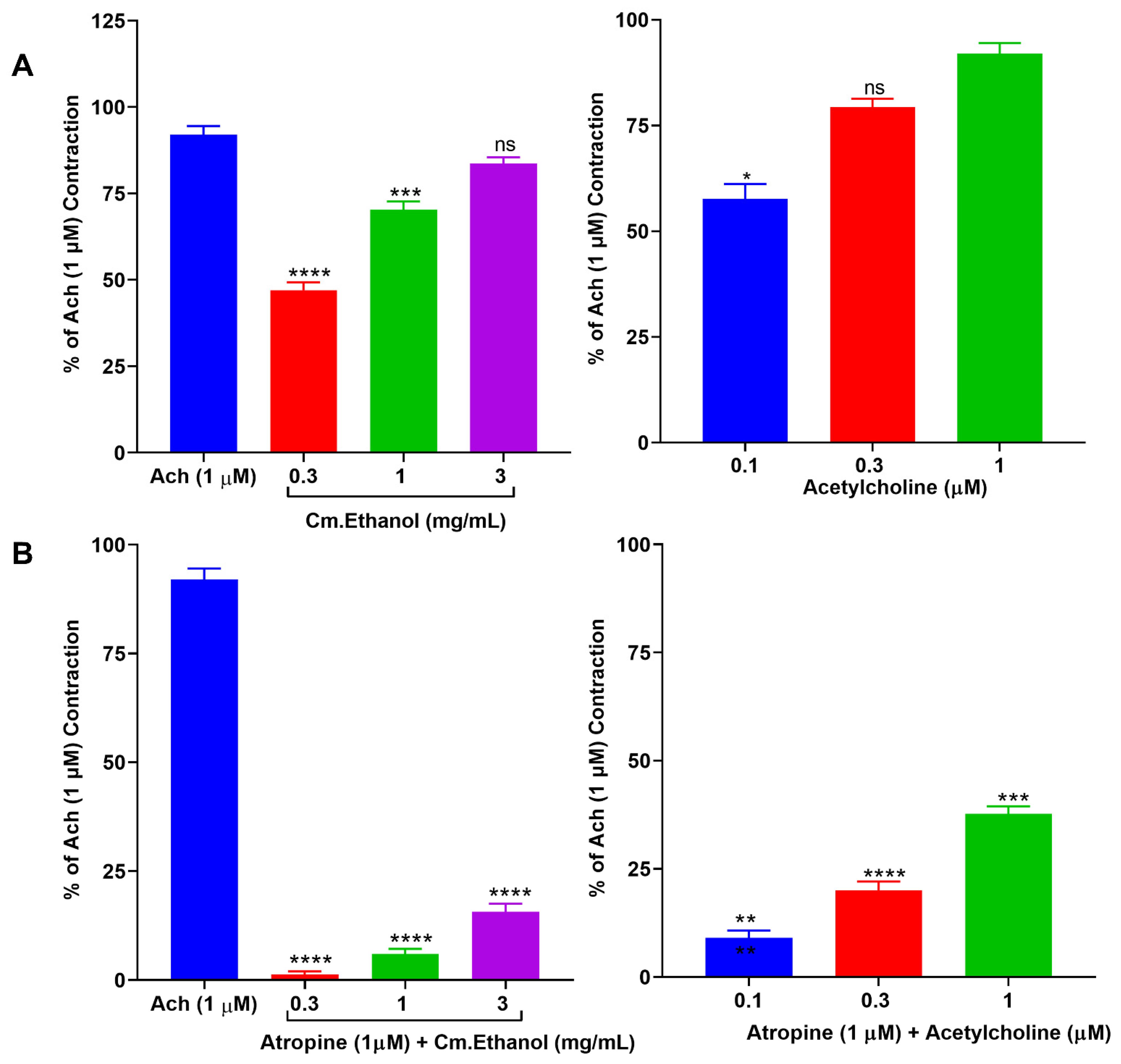
2.4. Isolated Tissue Experimentation
2.4.1. Effects on Isolated Rabbit Jejunum Preparation
2.4.2. Effect of Cm–Ethanol on Isolated Rat Ileum Preparations
2.4.3. Effect on Isolated Rabbit Tracheal Preparations
2.4.4. Effect on Isolated Rabbit Urinary Bladder Preparations
2.5. In Vivo Experiments
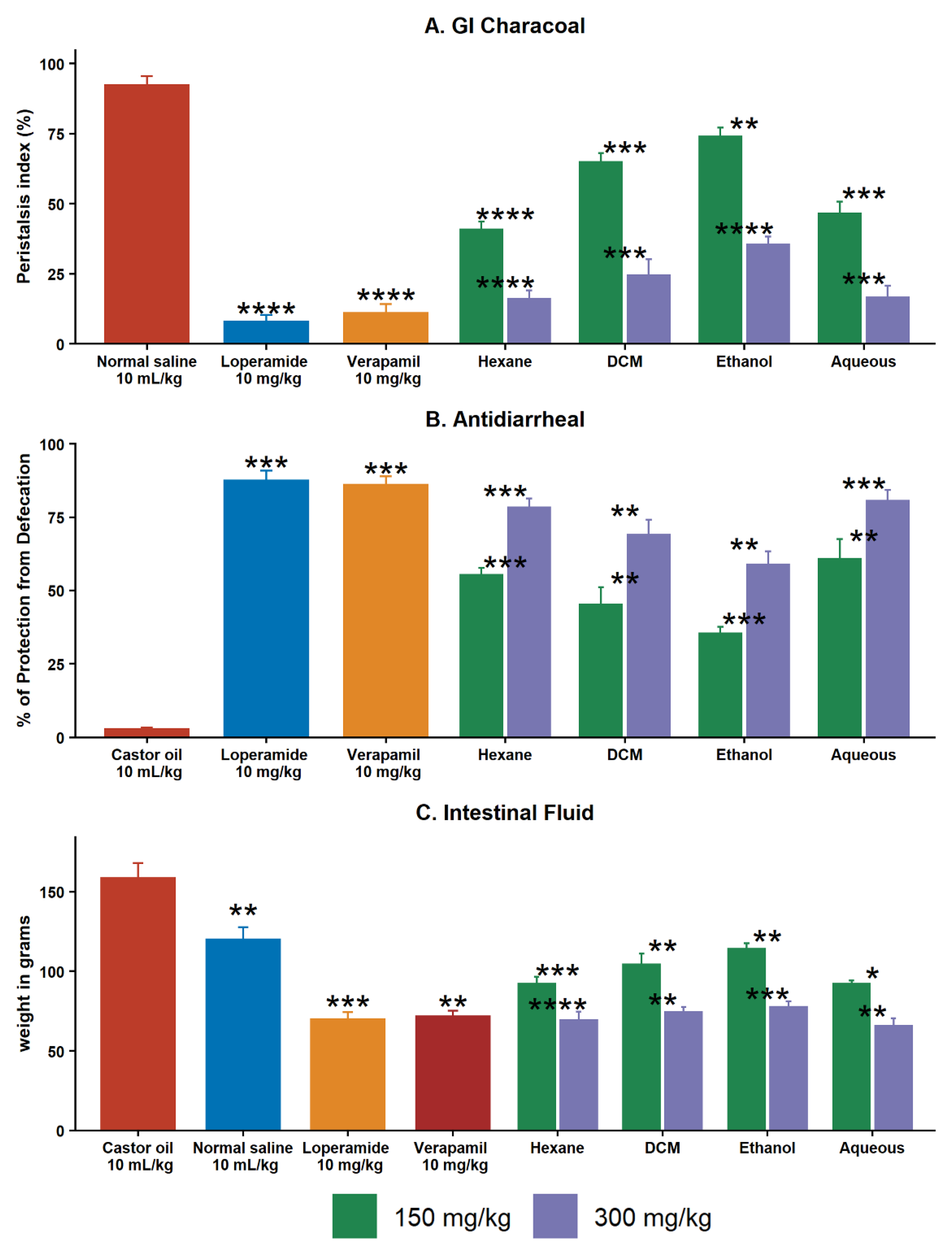
2.5.1. Effect on GI Charcoal Meal Intestinal Transit
2.5.2. Effect of Extracts on Castor Oil–Induced Diarrhea
2.5.3. Effect of Extracts on Intestinal Fluid Accumulation
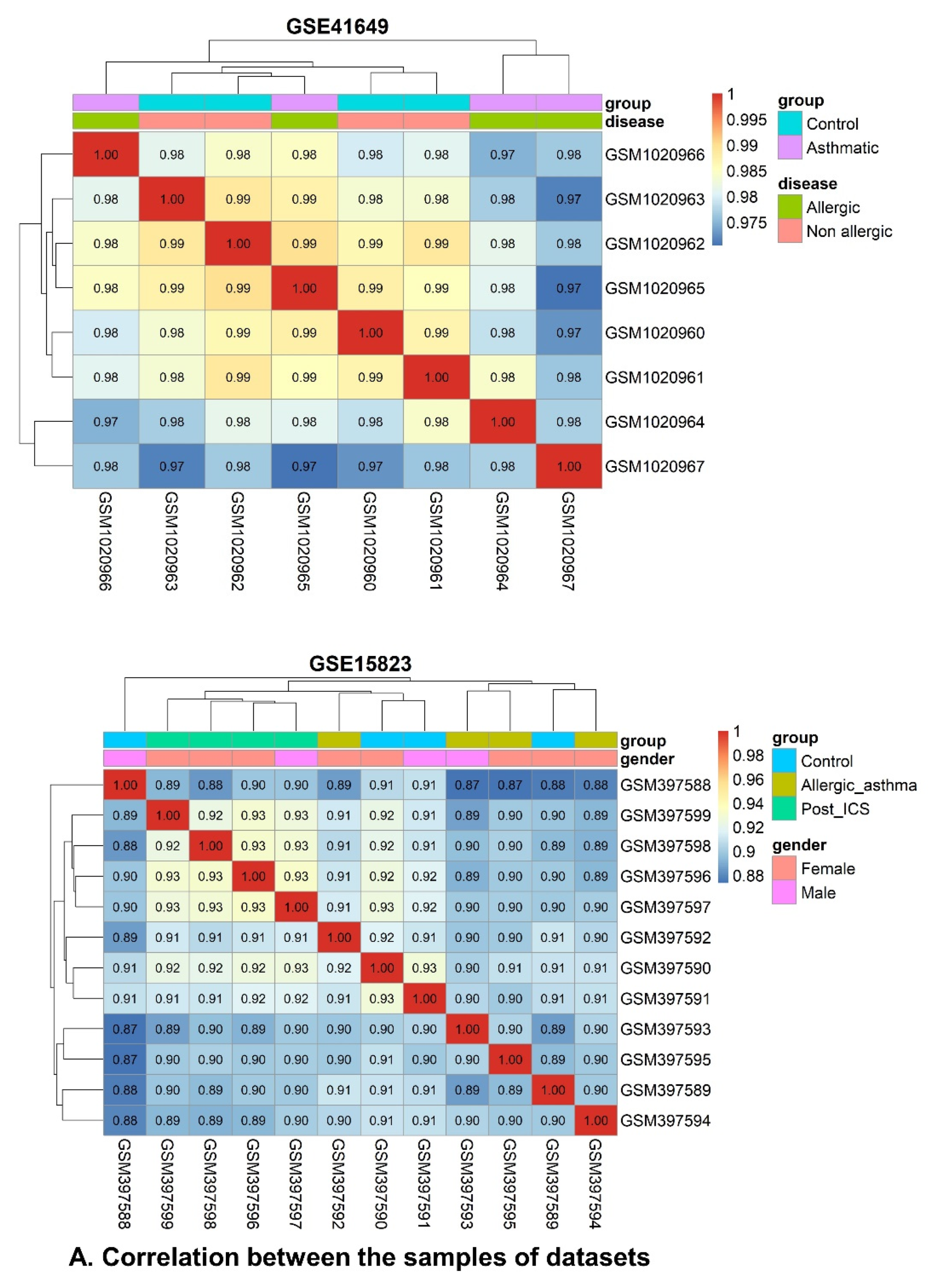
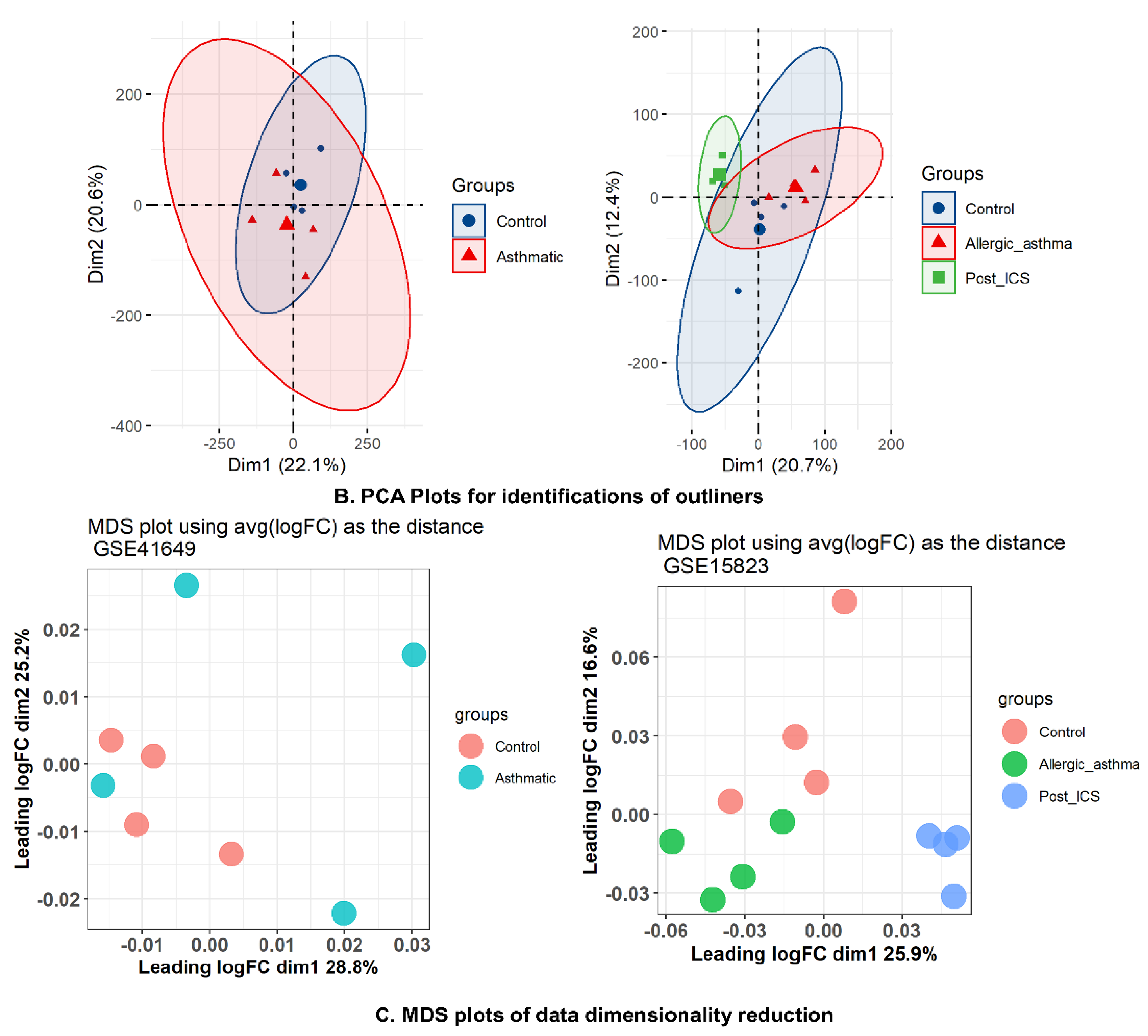
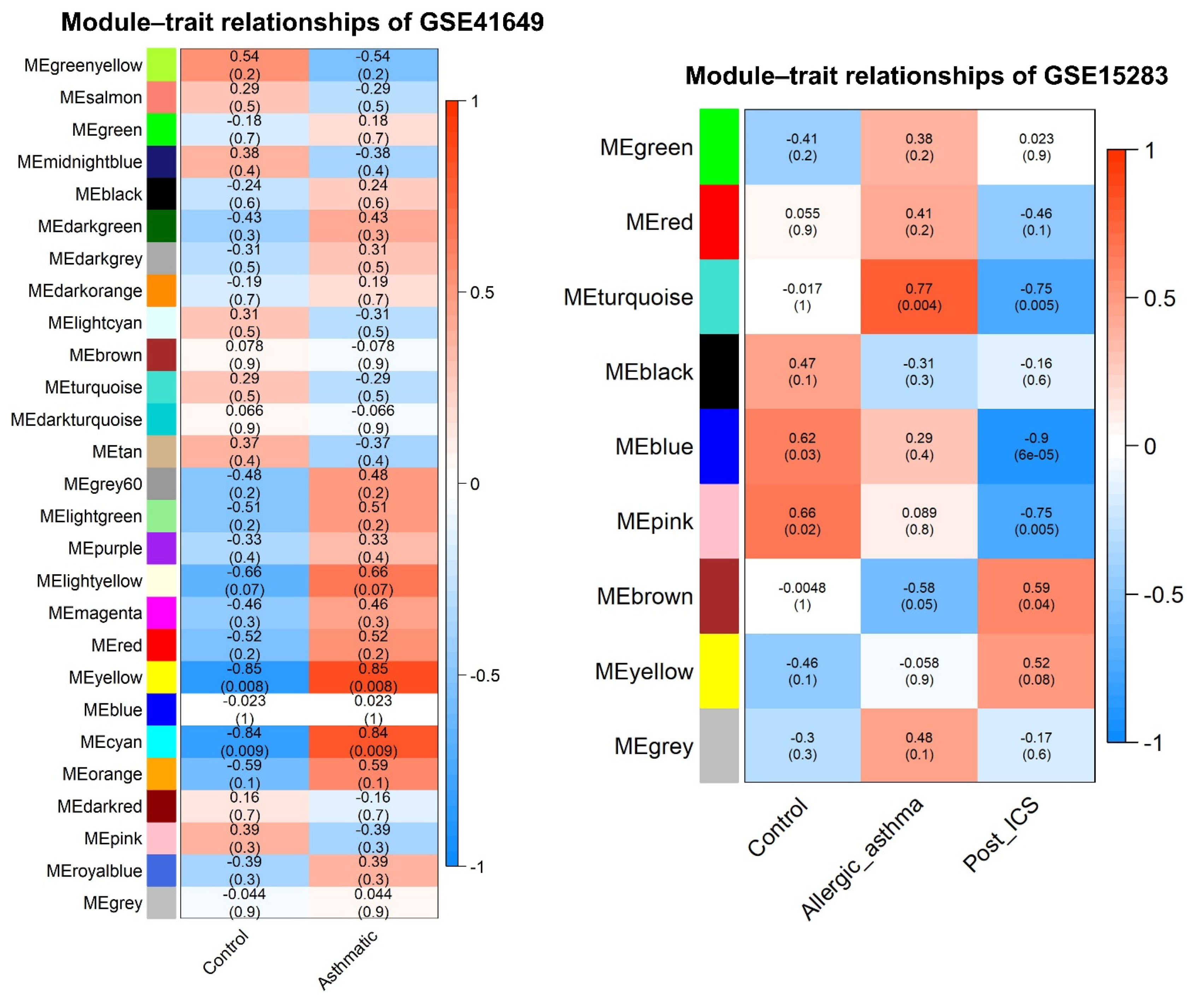
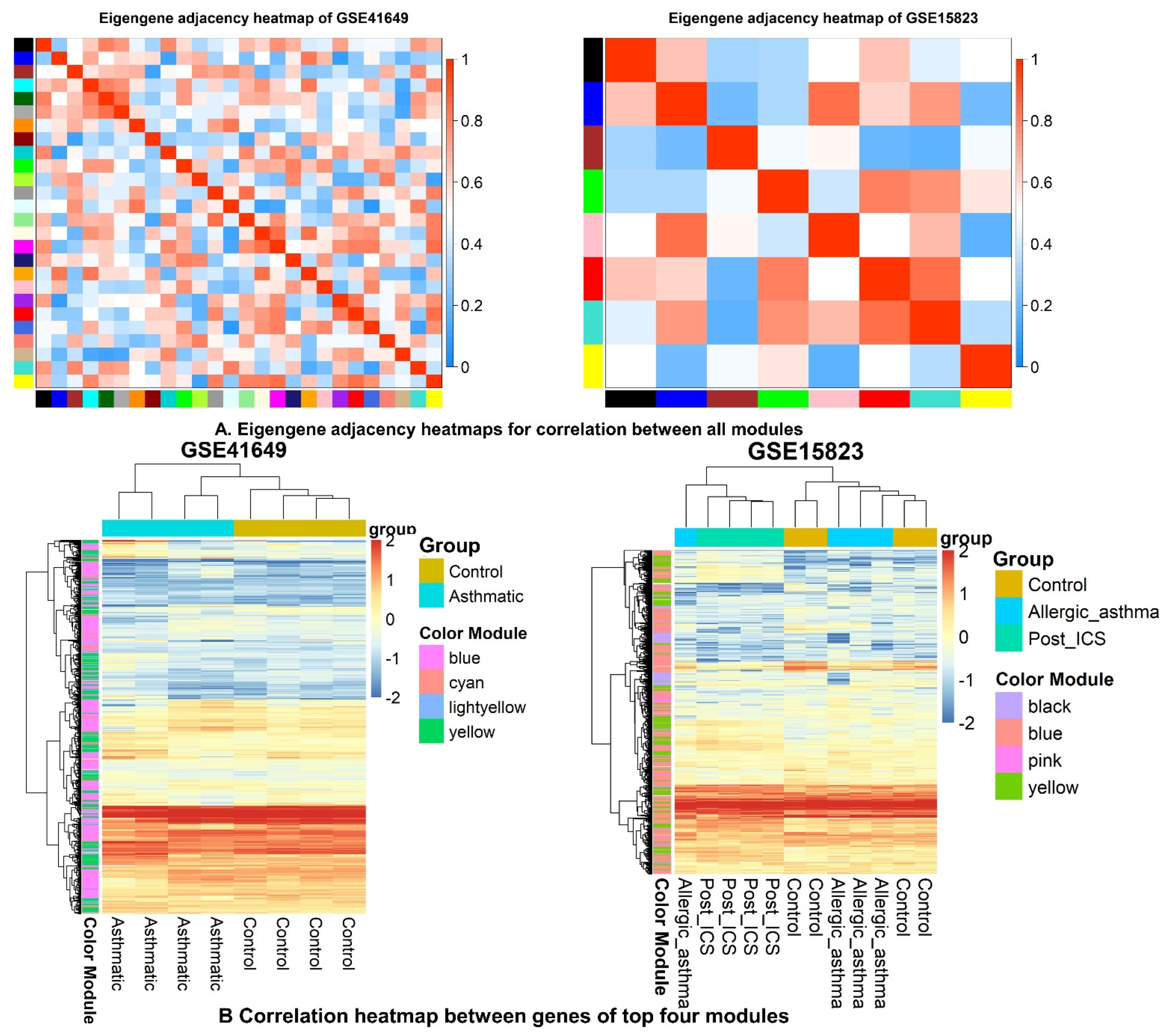
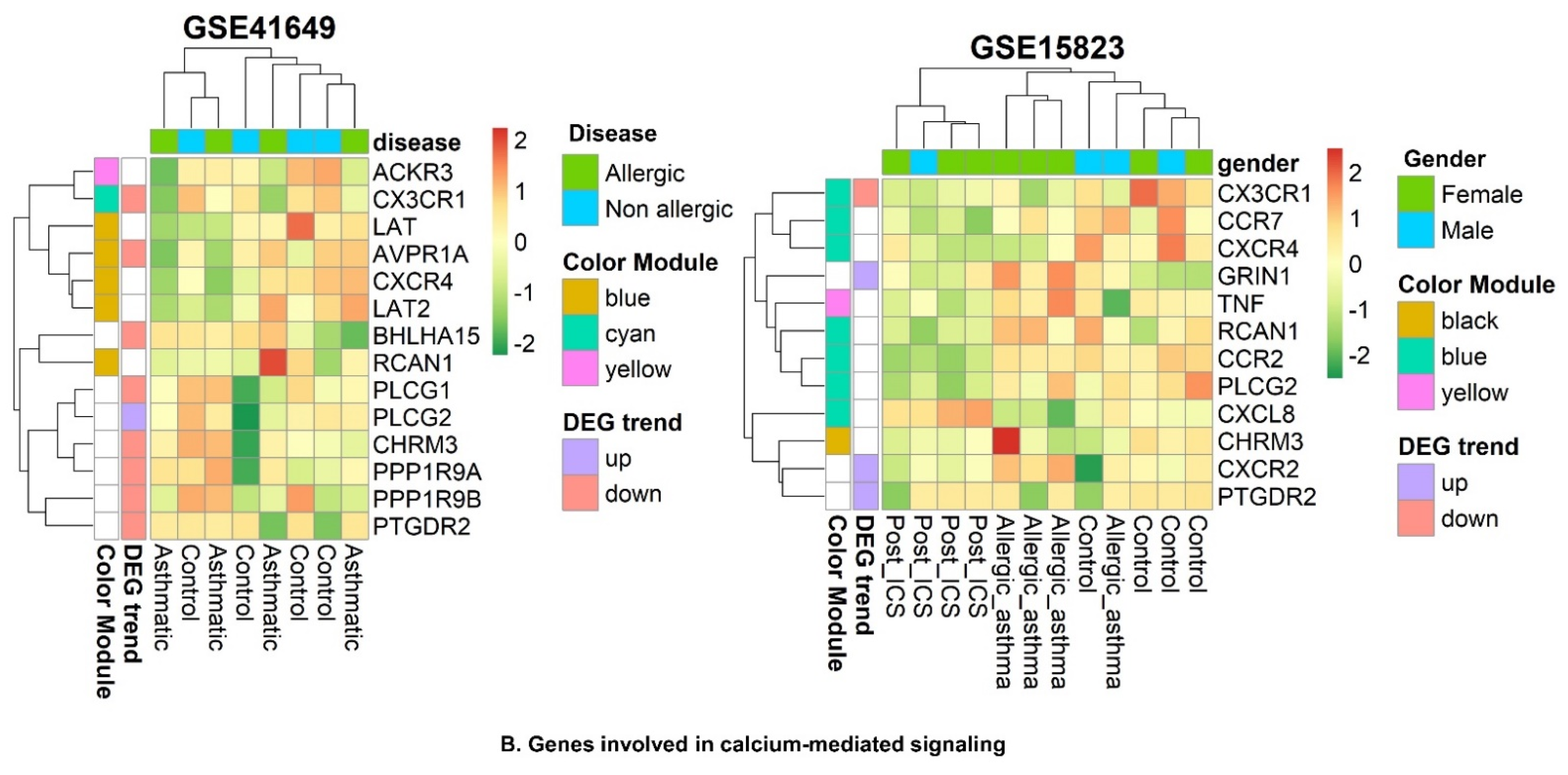
2.6. WGCNA and DEG Studies
2.6.1. Data Preprocessing and Standardization
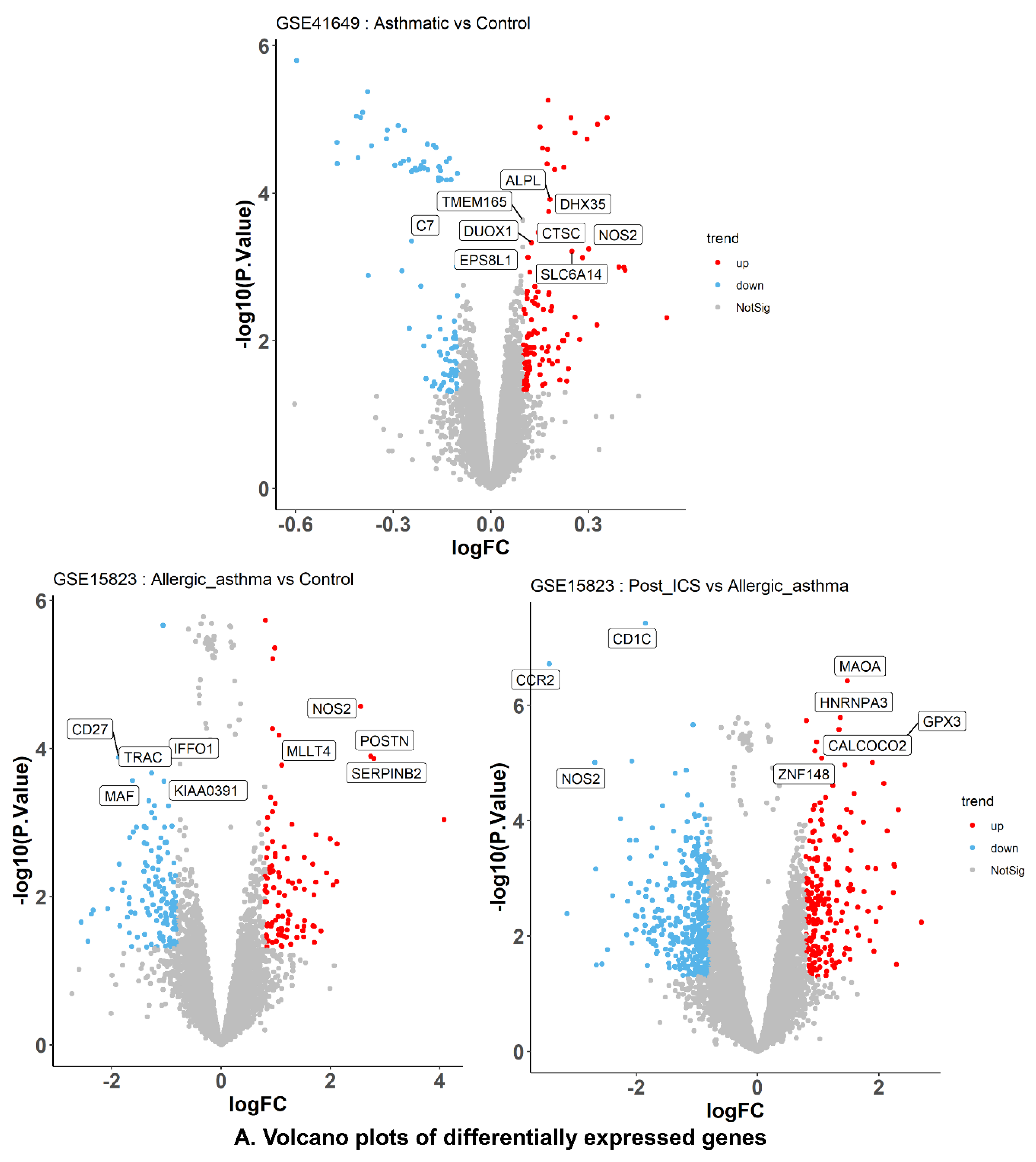
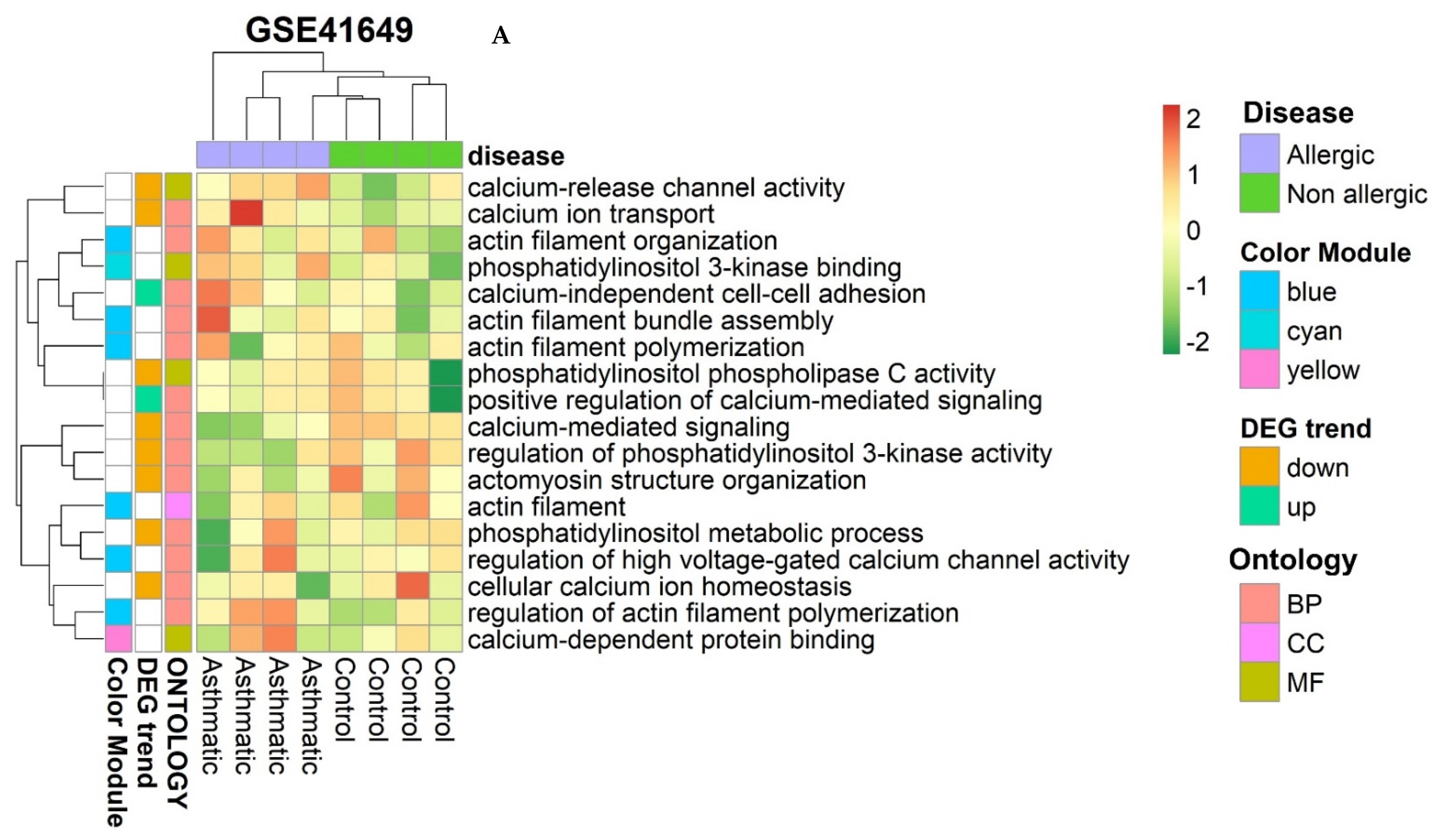
2.6.2. DEG Identification and Enrichment Analyses
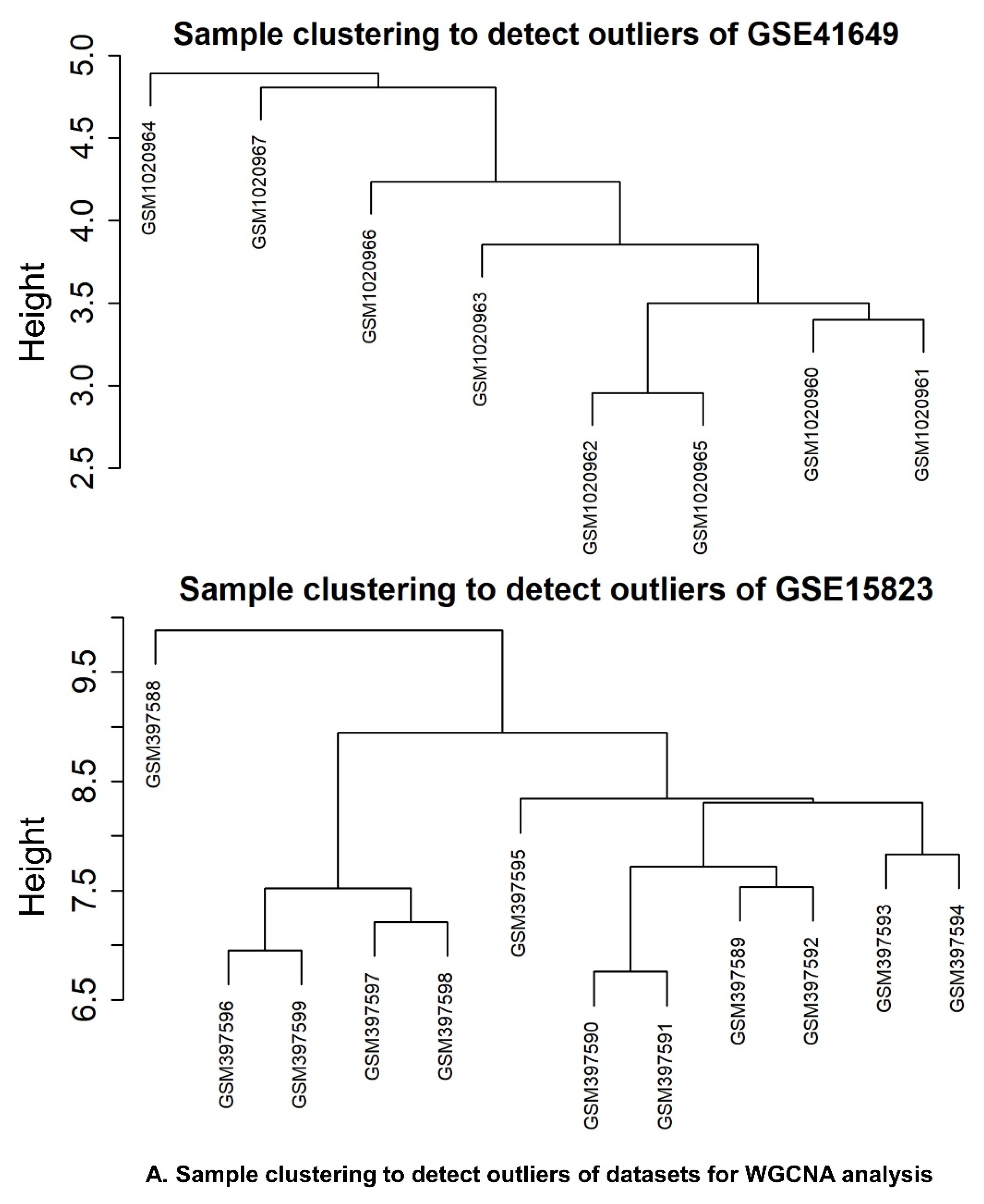
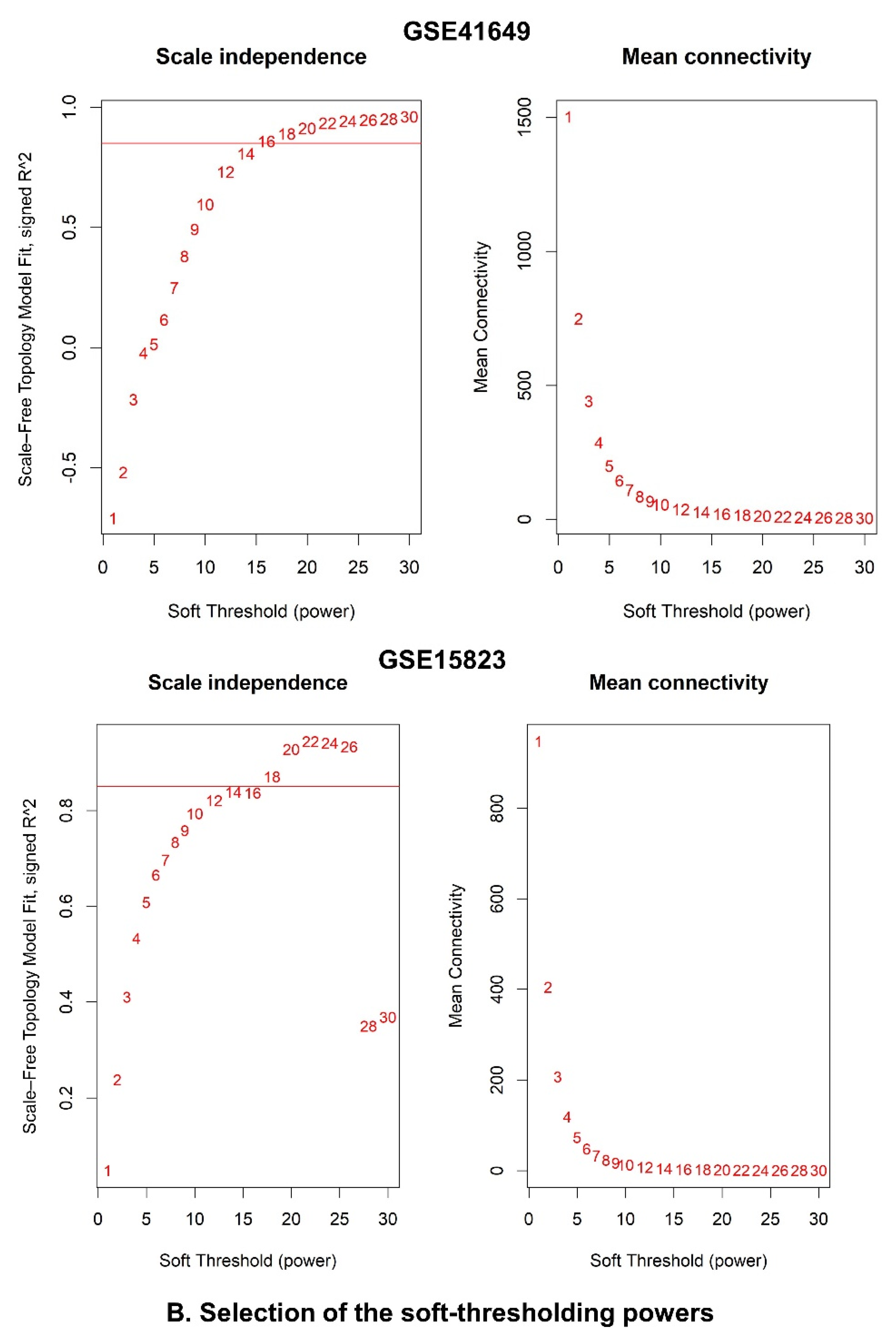
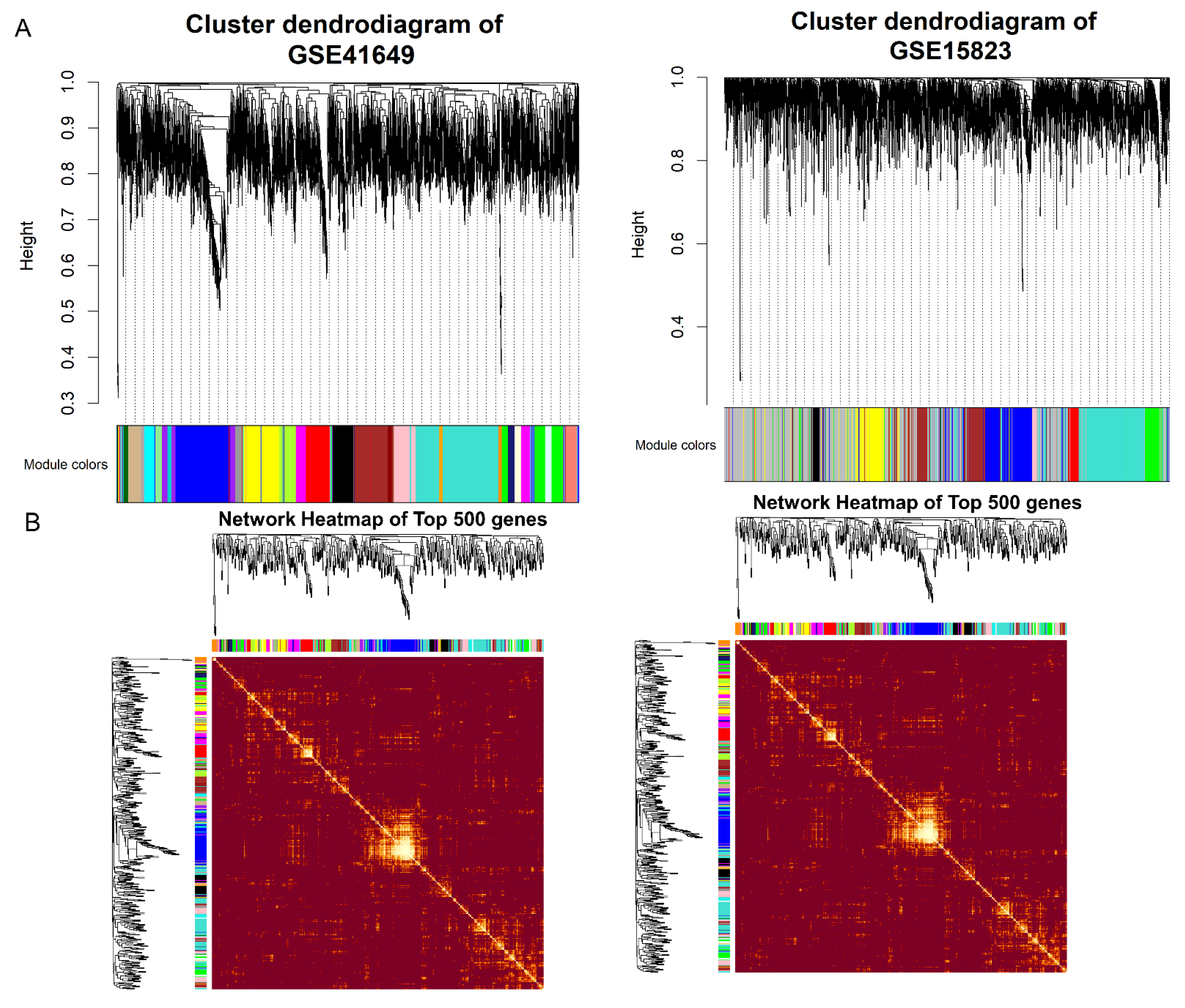
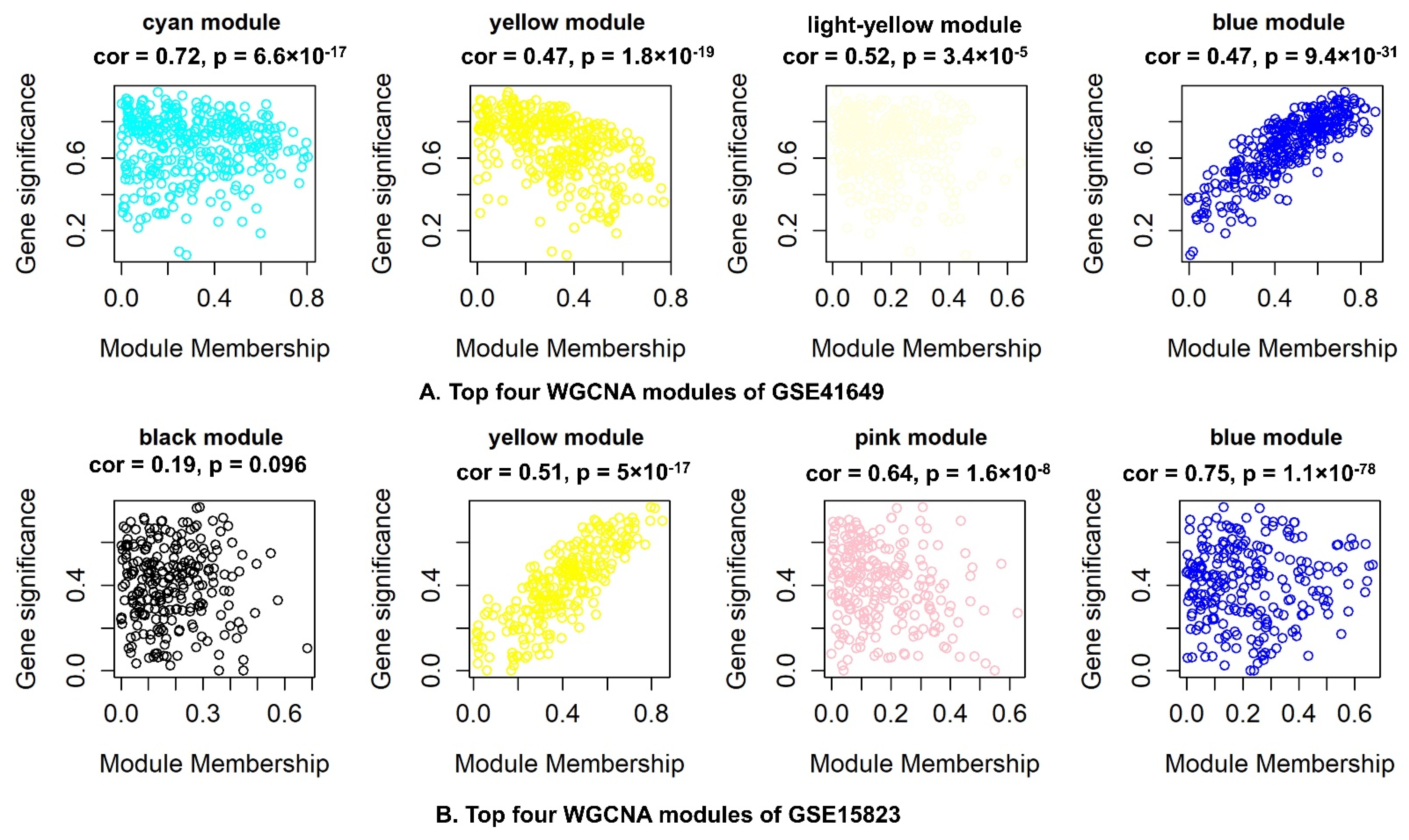
2.6.3. Identification of WGCNA Modules
2.6.4. Correlation between Modules and Clinical Traits
2.6.5. Hub–Gene Detection and Functional Pathway Enrichment
2.6.6. GSEA Analysis of Common Genes
2.6.7. Identification of Smooth Muscle Contraction Pathways
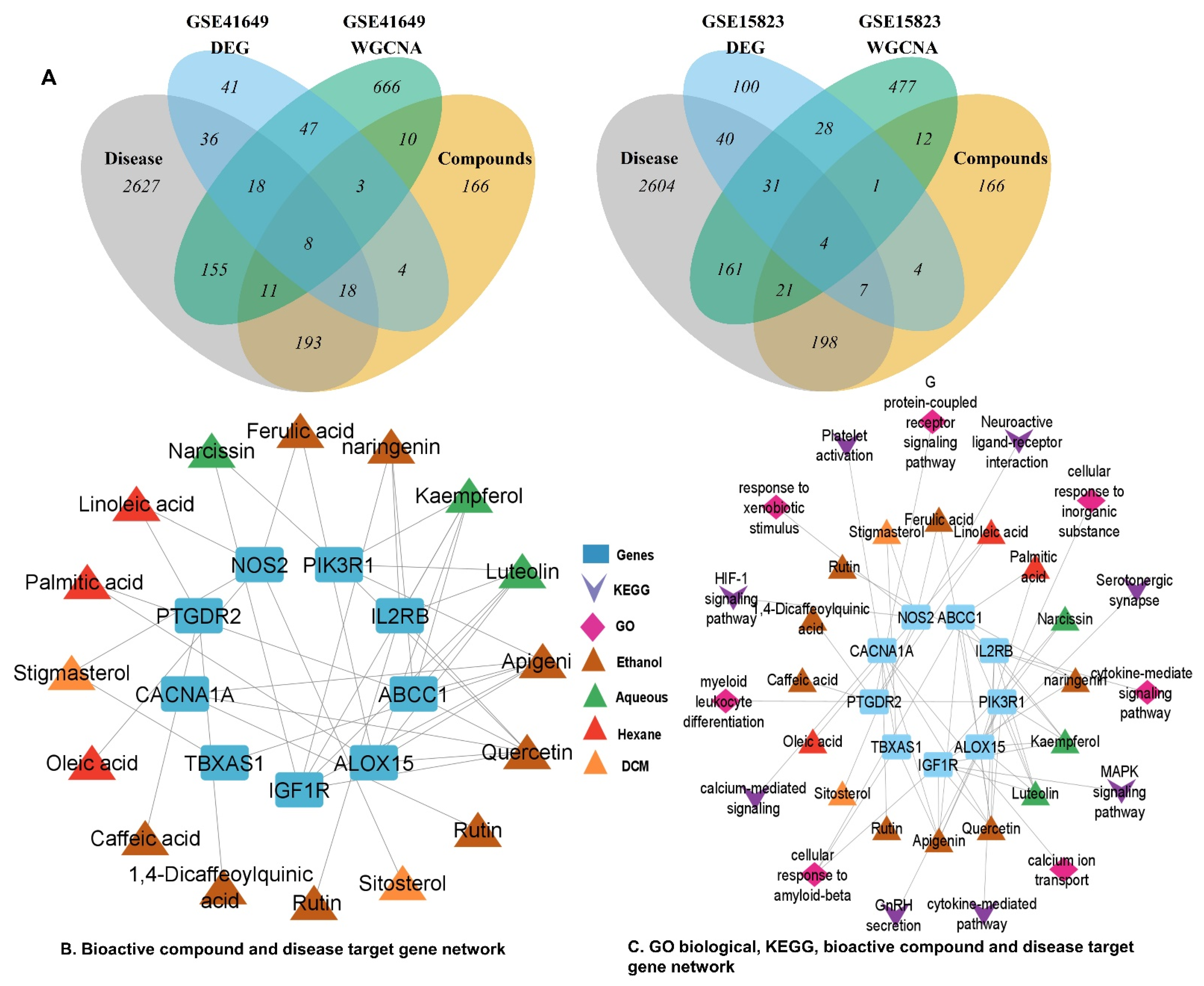
2.6.8. Identification of Potentially Active Genes for Bioactive Compounds and Disease
2.6.9. Identification of Key Genes
2.7. PPI and CTP Network Construction with Key Genes
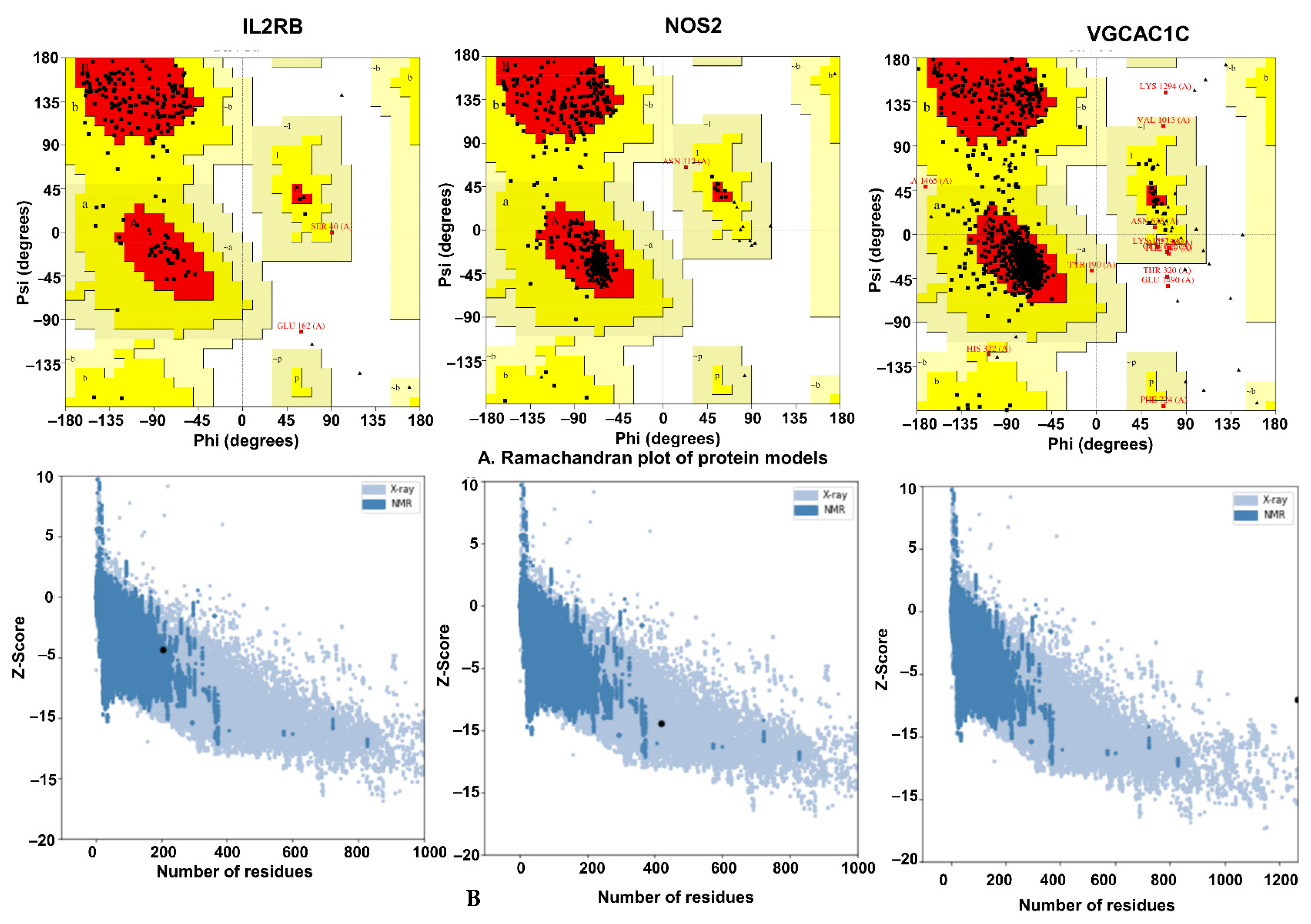
2.8. Protein Homology and Validation
2.8.1. Physicochemical Characteristics
2.8.2. Validation of Homology Modeling
2.9. Molecular Docking
3. Discussion
4. Materials and Methods
4.1. Preparation of Extract
4.2. Chemicals
4.3. Phytochemical Analysis of C. melo Seed Kernel Sequential Extracts
4.3.1. Preparation of Samples
4.3.2. LC/ESI–MS/MS Analysis
4.3.3. RP–HPLC Quantification and Method Validation
4.4. Ethical Committee Provision
4.5. Isolated Tissue Experimentation for Smooth Muscle Contraction
4.5.1. Isolated Rabbit Jejunum Preparations
4.5.2. Isolated Rabbit Tracheal Preparations
4.5.3. Isolated Urinary Bladder Preparations
4.6. In Vivo Experimentation
4.6.1. Evaluation of Maximum Tolerated Dose
4.6.2. Protocol and Design
- Group I was orally fed with normal saline (10 mL/kg).
- Group II was orally fed with castor oil or charcoal meal.
- Group III and IV were orally fed with 10 mg/kg of verapamil and loperamide.
- Groups V and VI were orally fed with 150 and 300 mg/kg of Cm–hexane.
- Groups VII and VIII were orally fed with 150 and 300 mg/kg of Cm–DCM.
- Groups IX and X were orally fed with 150 and 300 mg/kg of Cm–ethanol.
- Groups XI and XII were orally fed with 150 and 300 mg/kg of Cm–aqueous.
4.6.3. Charcoal Meal GI Transit Test
4.6.4. Castor Oil–Induced Diarrhea
4.6.5. Castor Oil–Induced Intestinal Fluid Accumulation
4.7. WGCNA and DEG Studies
4.7.1. Data Download and Preprocessing
4.7.2. Differentially Expressed Genes
4.7.3. Weighted Correlation Network Analysis
4.7.4. Functional Annotation and Pathway Enrichment
4.7.5. Gene Set Enrichment Analysis (GSEA)
4.7.6. Screening of Disease and Bioactive Compounds Associated with Target Genes
4.7.7. Detection of Key Genes
4.8. Construction of PPI, Bioactive Compounds, and Functional Enrichment Networks
4.9. Protein Homology Modeling
4.10. Molecular Docking
4.11. Software
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stern, J.; Pier, J.; Litonjua, A.A. Asthma Epidemiology and Risk Factors. Semin. Immunopathol. 2020, 42, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Michaeloudes, C.; Abubakar–Waziri, H.; Lakhdar, R.; Raby, K.; Dixey, P.; Adcock, I.M.; Mumby, S.; Bhavsar, P.K.; Chung, K.F. Molecular Mechanisms of Oxidative Stress in Asthma. Mol. Aspects Med. 2022, 85, 101026. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.F. New Treatments for Severe Treatment–Resistant Asthma: Targeting the Right Patient. Lancet Respir. Med. 2013, 1, 639–652. [Google Scholar] [CrossRef] [PubMed]
- Lambrecht, B.N.; Hammad, H.; Fahy, J.V. The Cytokines of Asthma. Immunity 2019, 50, 975–991. [Google Scholar] [CrossRef] [PubMed]
- Hellström, P.M. Pathophysiology of the Irritable Bowel Syndrome—Reflections of Today. Best Pract. Res. Clin. Gastroenterol. 2019, 40–41, 101620. [Google Scholar] [CrossRef]
- Camilleri, M.; Sellin, J.H.; Barrett, K.E. Pathophysiology, Evaluation, and Management of Chronic Watery Diarrhea. Gastroenterology 2017, 152, 515–532.e2. [Google Scholar] [CrossRef] [PubMed]
- Anbazhagan, A.N.; Priyamvada, S.; Alrefai, W.A.; Dudeja, P.K. Pathophysiology of IBD Associated Diarrhea. Tissue Barriers 2018, 6, e1463897. [Google Scholar] [CrossRef]
- Malysz, J.; Petkov, G.V. Urinary Bladder Smooth Muscle Ion Channels: Expression, Function, and Regulation in Health and Disease. Am. J. Physiol. Physiol. 2020, 319, F257–F283. [Google Scholar] [CrossRef]
- Duke, J.A. Melon (Cucumis melo L.). In Duke’s Handbook of Medicinal Plants of the Bible; CRC Press: New York, NY, USA, 2008; pp. 148–151. ISBN 9781420040463. [Google Scholar]
- Silva, M.A.; Albuquerque, T.G.; Alves, R.C.; Oliveira, M.B.P.P.; Costa, H.S. Melon (Cucumis melo L.) by–Products: Potential Food Ingredients for Novel Functional Foods? Trends Food Sci. Technol. 2020, 98, 181–189. [Google Scholar] [CrossRef]
- Mariod, A.A.; Saeed Mirghani, M.E.; Hussein, I. Cucumis Melo Var. Cantalupo Cantaloupe. In Unconv. Oilseeds Oil Sources; Academic Press: Cambridge, MA, USA, 2017; pp. 107–111, eISBN 9780128134337. [Google Scholar]
- Erhirhie, E.; Ekene, N. Medicinal Values on Citrullus Lanatus (Watermelon): Pharmacological Review. Int. J. Res. Pharm. Biomed. Sci. 2014, 4, 1305–1312. [Google Scholar]
- Siddiqui, W.A.; Shahzad, M.; Shabbir, A.; Ahmad, A. Evaluation of Anti–Urolithiatic and Diuretic Activities of Watermelon (Citrullus Lanatus) Using in Vivo and in Vitro Experiments. Biomed. Pharmacother. 2018, 97, 1212–1221. [Google Scholar] [CrossRef] [PubMed]
- Rahman, H.; Priyanka, P.; Lavanya, T.; Srilakshni, N.; Payili, R.K. A Review on Ethnobotany, Phytochemisrty and Pharmacology Of. Int. Res. J. Pharm. Appl. Sci. 2013, 3, 77–81. [Google Scholar]
- Rimando, A.M.; Perkins–Veazie, P.M. Determination of Citrulline in Watermelon Rind. J. Chromatogr. A 2005, 1078, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Reetu; Tomar, M. Watermelon: A Valuable Horticultural Crop with Nutritional Benefits. Pop. Kheti 2017, 5, 5–9. [Google Scholar]
- Gómez–García, R.; Campos, D.A.; Aguilar, C.N.; Madureira, A.R.; Pintado, M. Valorization of Melon Fruit (Cucumis melo L.) by–Products: Phytochemical and Biofunctional Properties with Emphasis on Recent Trends and Advances. Trends Food Sci. Technol. 2020, 99, 507–519. [Google Scholar] [CrossRef]
- Asif, H.M.; Akhtar, N.; Sultana, S.; Rehman, S.U.; Akram, M.; Rehman, U.J. Medicinal Properties of Cucumis Melo Linn. J. Pharm. Pharm. Sci. 2014, 2, 58–62. [Google Scholar]
- Patel, S.; Rauf, A. Edible Seeds from Cucurbitaceae Family as Potential Functional Foods: Immense Promises, Few Concerns. Biomed. Pharmacother. 2017, 91, 330–337. [Google Scholar] [CrossRef]
- Salehi, B.; Capanoglu, E.; Adrar, N.; Catalkaya, G.; Shaheen, S.; Jaffer, M.; Giri, L.; Suyal, R.; Jugran, A.K.; Calina, D.; et al. Cucurbits Plants: A Key Emphasis to Its Pharmacological Potential. Molecules 2019, 24, 1854. [Google Scholar] [CrossRef]
- Dixit, Y.; Kar, A. Protective Role of Three Vegetable Peels in Alloxan Induced Diabetes Mellitus in Male Mice. Plant Foods Hum. Nutr. 2010, 65, 284–289. [Google Scholar] [CrossRef]
- Wahid, M.; Ali, A.; Saqib, F.; Aleem, A.; Bibi, S.; Afzal, K.; Ali, A.; Baig, A.; Khan, S.A.; Bin Asad, M.H.H. Pharmacological Exploration of Traditional Plants for the Treatment of Neurodegenerative Disorders. Phyther. Res. 2020, 34, 3089–3112. [Google Scholar] [CrossRef]
- Wahid, M.; Saqib, F.; Akhtar, S.; Ali, A.; Wilairatana, P.; Mubarak, M.S. Possible Mechanisms Underlying the Antispasmodic, Bronchodilator, and Antidiarrheal Activities of Polarity–Based Extracts of Cucumis Sativus L. Seeds in In Silico, In Vitro, and In Vivo Studies. Pharmaceuticals 2022, 15, 641. [Google Scholar] [CrossRef] [PubMed]
- Wahid, M.; Saqib, F. Scientific Basis for Medicinal Use of Citrullus Lanatus (Thunb.) in Diarrhea and Asthma: In Vitro, in Vivo and in Silico Studies. Phytomedicine 2022, 98, 153978. [Google Scholar] [CrossRef] [PubMed]
- Sirous, H.; Chemi, G.; Campiani, G.; Brogi, S. An Integrated in Silico Screening Strategy for Identifying Promising Disruptors of P53–MDM2 Interaction. Comput. Biol. Chem. 2019, 83, 107105. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, B.; Kollman, P.A. Binding of a Diverse Set of Ligands to Avidin and Streptavidin: An Accurate Quantitative Prediction of Their Relative Affinities by a Combination of Molecular Mechanics and Continuum Solvent Models. J. Med. Chem. 2000, 43, 3786–3791. [Google Scholar] [CrossRef]
- Du, X.; Li, Y.; Xia, Y.-L.; Ai, S.-M.; Liang, J.; Sang, P.; Ji, X.-L.; Liu, S.-Q. Insights into Protein–Ligand Interactions: Mechanisms, Models, and Methods. Int. J. Mol. Sci. 2016, 17, 144. [Google Scholar] [CrossRef]
- Yu, X. Prediction of Inhibitory Constants of Compounds against SARS–CoV 3CLpro Enzyme with 2D–QSAR Model. J. Saudi Chem. Soc. 2021, 25, 101262. [Google Scholar] [CrossRef]
- Soltani, R.; Hashemi, M.; Farazmand, A.; Asghari, G.; Heshmat–Ghahdarijani, K.; Kharazmkia, A.; Ghanadian, S.M. Evaluation of the Effects of Cucumis Sativus Seed Extract on Serum Lipids in Adult Hyperlipidemic Patients: A Randomized Double–Blind Placebo–Controlled Clinical Trial. J. Food Sci. 2017, 82, 214–218. [Google Scholar] [CrossRef]
- Rajasree, R.S.; Sibi, P.I.; Francis, F.; William, H. Phytochemicals of Cucurbitaceae Family—A Review. Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 113–123. [Google Scholar]
- Mallik, J.; Priyanka, D.; Sourav, D. Pharmacological Activity of Cucumis Sativus L.—A Complete Review. Asian J. Pharm. Res. Dev. 2013, 1, 1–6. [Google Scholar]
- Mukherjee, P.K.; Nema, N.K.; Maity, N.; Sarkar, B.K. Phytochemical and Therapeutic Potential of Cucumber. Fitoterapia 2013, 84, 227–236. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, S.S.; Li, X.L.; Wang, Z.F.; Zhao, L.Q. Inhibitory Effect of TongXie–YaoFang Formula on Colonic Contraction in Rats. World J. Gastroenterol. 2015, 21, 2912–2917. [Google Scholar] [CrossRef] [PubMed]
- Mahn, K.; Ojo, O.O.; Chadwick, G.; Aaronson, P.I.; Ward, J.P.T.; Lee, T.H. Ca2+ Homeostasis and Structural and Functional Remodelling of Airway Smooth Muscle in Asthma. Thorax 2010, 65, 547–552. [Google Scholar] [CrossRef]
- Athari, S.S. Targeting Cell Signaling in Allergic Asthma. Signal Transduct. Target. Ther. 2019, 4, 45. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.W.; Ko, W.C. Suppressive Effects of Rutin, Quercitrin, and Isoquercitrin on Atypical Allergic Asthma in an Animal Model. Med. Drug Discov. 2021, 12, 100106. [Google Scholar] [CrossRef]
- Liu, L.L.; Zhang, Y.; Zhang, X.F.; Li, F.H. Influence of Rutin on the Effects of Neonatal Cigarette Smoke Exposure–Induced Exacerbated MMP–9 Expression, Th17 Cytokines and NF–KB/INOS–Mediated Inflammatory Responses in Asthmatic Mice Model. Korean J. Physiol. Pharmacol. 2018, 22, 481–491. [Google Scholar] [CrossRef]
- Hall, J.E.; Hall, M.E. Excitation and Contraction of Smooth Muscle. In Guyton and Hall Textbook of Medical Physiology; Elsevier Health Sciences: Amsterdam, The Netherlands, 2020; pp. 101–109. ISBN 9780323597128. [Google Scholar]
- Sharkey, K.A.; MacNaughton, W.K. Gastrointestinal Motility and Water Flux, Emesis, and Biliary and Pancreatic Disease. In Goodman and Gilman’s The Pharmacological Basis of Therapeutics; Brunton, L.L., Hilal-Dandan, R., Knollmann, B.C., Eds.; McGraw-Hill: New York, NY, USA, 2018; pp. 921–944. ISBN 978-1-25-958474-9. [Google Scholar]
- Gilani, A.H.; Ghayur, M.N.; Saify, Z.S.; Ahmed, S.P.; Choudhary, M.I.; Khalid, A. Presence of Cholinomimetic and Acetylcholinesterase Inhibitory Constituents in Betel Nut. Life Sci. 2004, 75, 2377–2389. [Google Scholar] [CrossRef]
- Saqib, F.; Janbaz, K.H. Rationalizing Ethnopharmacological Uses of Alternanthera Sessilis: A Folk Medicinal Plant of Pakistan to Manage Diarrhea, Asthma and Hypertension. J. Ethnopharmacol. 2016, 182, 110–121. [Google Scholar] [CrossRef]
- Wallace, J.L.; Sharkey, K.A. Pharmacotherapy of Gastric Acidity, Peptic Ulcers, and Gastroesophageal Reflux Disease. In Goodman and Gilman’s The Pharmacological Basis of Therapeutics; Brunton, L.L., Hilal-Dandan, R., Knollmann, B.C., Eds.; McGraw-Hill: New York, NY, USA, 2018; pp. 1309–1322. ISBN 978-0-07-176939-6. [Google Scholar]
- Saqib, F.; Janbaz, K.H. Ethnopharmacological Basis for Folkloric Claims of Anagallis Arvensis Linn. (Scarlet Pimpernel) as Prokinetic, Spasmolytic and Hypotensive in Province of Punjab, Pakistan. J. Ethnopharmacol. 2021, 267, 113634. [Google Scholar] [CrossRef]
- Gilani, A.H.; Jabeen, Q.; Ghayur, M.N.; Janbaz, K.H.; Akhtar, M.S. Studies on the Antihypertensive, Antispasmodic, Bronchodilator and Hepatoprotective Activities of the Carum Copticum Seed Extract. J. Ethnopharmacol. 2005, 98, 127–135. [Google Scholar] [CrossRef]
- Janbaz, K.H.; Nisa, M.; Saqib, F.; Imran, I.; Zia-Ul-Haq, M.; De Feo, V. Bronchodilator, Vasodilator and Spasmolytic Activities of Methanolic Extract of Myrtus Communis L. J. Physiol. Pharmacol. 2013, 64, 479–484. [Google Scholar]
- Miyazaki, E.; Yabu, H.; Sunano, S. Excitation and Contraction of the Smooth Muscle. Jpn. J. Smooth Muscle Res. 1971, 7, 83–97. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Janbaz, K.H.; Zaeem Ahsan, M.; Saqib, F.; Imran, I.; Zia-Ul-Haq, M.; Abid Rashid, M.; Jaafar, H.Z.E.; Moga, M. Scientific Basis for Use of Pyrus Pashia Buch.-Ham. Ex D. Don. Fruit in Gastrointestinal, Respiratory and Cardiovascular Ailments. PLoS ONE 2015, 10, e0118605. [Google Scholar] [CrossRef]
- Chan, H.J.; Ji, Y.L.; Chul, H.C.; Chang, J.K. Anti-Asthmatic Action of Quercetin and Rutin in Conscious Guinea-Pigs Challenged with Aerosolized Ovalbumin. Arch. Pharm. Res. 2007, 30, 1599–1607. [Google Scholar] [CrossRef]
- Agbor, G.A.; Longo, F.; Makong, E.A.; Tarkang, P.A. Evaluation of the Antidiarrheal and Antioxidant Properties of Justicia Hypocrateriformis. Pharm. Biol. 2014, 52, 1128–1133. [Google Scholar] [CrossRef] [PubMed]
- Iwao, I.; Terada, Y. On the Mechanism of Diarrhea Due to Castor Oil. Jpn. J. Pharmacol. 1962, 12, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Atta, A.H.; Mouneir, S.M. Antidiarrhoeal Activity of Some Egyptian Medicinal Plant Extracts. J. Ethnopharmacol. 2004, 92, 303–309. [Google Scholar] [CrossRef]
- Gaginella, T.S.; Phillips, S.F. Ricinoleic Acid: Current View of an Ancient Oil. Am. J. Dig. Dis. 1975, 20, 1171–1177. [Google Scholar] [CrossRef] [PubMed]
- Yakubu, M.T.; Salimon, S.S. Antidiarrhoeal Activity of Aqueous Extract of Mangifera Indica L. Leaves in Female Albino Rats. J. Ethnopharmacol. 2015, 163, 135–141. [Google Scholar] [CrossRef]
- Reynolds, I.J.; Gould, R.J.; Snyder, S.H. Loperamide: Blockade of Calcium Channels as a Mechanism for Antidiarrheal Effects. J. Pharmacol. Exp. Ther. 1984, 231, 628–632. [Google Scholar]
- Crowe, A.; Wong, P. Potential Roles of P–Gp and Calcium Channels in Loperamide and Diphenoxylate Transport. Toxicol. Appl. Pharmacol. 2003, 193, 127–137. [Google Scholar] [CrossRef]
- Wahid, M.; Saqib, F.; Qamar, M.; Ziora, Z.M. Antispasmodic Activity of the Ethanol Extract of Citrullus Lanatus Seeds: Justifying Ethnomedicinal Use in Pakistan to Treat Asthma and Diarrhea. J. Ethnopharmacol. 2022, 295, 115314. [Google Scholar] [CrossRef] [PubMed]
- Wahid, M.; Saqib, F.; Ahmedah, H.T.; Gavris, C.M.; De Feo, V.; Hogea, M.; Moga, M.; Chicea, R. Cucumis Sativus l. Seeds Ameliorate Muscular Spasm-Induced Gastrointestinal and Respiratory Disorders by Simultaneously Inhibiting Calcium Mediated Signaling Pathway. Pharmaceuticals 2021, 14, 1197. [Google Scholar] [CrossRef]
- Rowan, A.N. Guide for the Care and Use of Laboratory Animals; National Academies Press: Washington, DC, USA, 1979; Volume 8, ISBN 0309186633. [Google Scholar]
- Elasoru, S.E.; Rhana, P.; de Oliveira Barreto, T.; Naves de Souza, D.L.; Menezes-Filho, J.E.R.; Souza, D.S.; Loes Moreira, M.V.; Gomes Campos, M.T.; Adedosu, O.T.; Roman-Campos, D.; et al. Andrographolide Protects against Isoproterenol-Induced Myocardial Infarction in Rats through Inhibition of L-Type Ca2+ and Increase of Cardiac Transient Outward K+ Currents. Eur. J. Pharmacol. 2021, 906, 174194. [Google Scholar] [CrossRef]
- Chauhan, V.; Singh, M.P. Immuno-Informatics Approach to Design a Multi-Epitope Vaccine to Combat Cytomegalovirus Infection. Eur. J. Pharm. Sci. 2020, 147, 105279. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Kang, L.; Zhao, W.; Wu, S.; Ding, L.; Zheng, F.; Liu, J.; Li, J. Identification of Novel Umami Peptides from Myosin via Homology Modeling and Molecular Docking. Food Chem. 2021, 344, 128728. [Google Scholar] [CrossRef] [PubMed]
- Subhani, S.; Jayaraman, A.; Jamil, K. Homology Modelling and Molecular Docking of MDR1 with Chemotherapeutic Agents in Non-Small Cell Lung Cancer. Biomed. Pharmacother. 2015, 71, 37–45. [Google Scholar] [CrossRef] [PubMed]

| Fraction | Analytes | λ (nm) | Rt (min) | Linear Regression Data | LOD µg/mL | LOQ µg/mL | Concentration µg/g | Precision * (RSD %) | Recovery * | Analytes + Extracts (µg/g) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Range µg/mL | Equation | r2 | Inter–Day | Intra–Day | Mean | RSD % | 50 µg | 100 µg | |||||||
| DCM | Umbelliferone | 270 | 5.3 | 7.81–500 | y =147.14x + 22.15 | 0.9996 | 0.69 | 2.08 | 319.97 | 0.94 | 1.14 | 98.65 | 1.30 | 369.45 | 419.09 |
| Stigmasterol | 18.2 | 7.81–500 | y =163.15x + 28.64 | 0.9998 | 0.61 | 1.84 | 324.69 | 1.88 | 1.03 | 98.26 | 0.86 | 374.17 | 423.80 | ||
| β–sitosterol | 19.5 | 7.81–500 | y = 105.11x + 9.81 | 0.9993 | 0.59 | 1.79 | 216.78 | 0.63 | 0.71 | 99.34 | 1.71 | 266.41 | 316.11 | ||
| Ethanol | Caffeic acid | 280 | 8.3 | 7.81–500 | y =209.19x + 21.41 | 0.9999 | 0.44 | 1.32 | 341.27 | 1.07 | 0.30 | 99.02 | 0.76 | 390.72 | 440.34 |
| Rutin | 9.7 | 7.81–500 | y =263.12x + 20.66 | 0.9999 | 0.47 | 1.44 | 752.61 | 1.25 | 1.49 | 99.31 | 0.67 | 801.49 | 850.82 | ||
| Quercetin | 13.8 | 7.81–500 | y =181.13x + 32.08 | 0.9994 | 0.66 | 2.02 | 768.89 | 1.69 | 1.28 | 99.60 | 0.99 | 817.74 | 867.07 | ||
| Apigenin | 17.7 | 7.81–500 | y =228.17x + 18.10 | 0.9999 | 0.27 | 0.87 | 572.91 | 1.29 | 1.62 | 98.85 | 0.94 | 622.04 | 671.50 | ||
| Ferulic acid | 22.9 | 7.81–500 | y =220.98x + 28.21 | 0.9999 | 0.35 | 1.05 | 417.12 | 1.63 | 1.33 | 99.38 | 1.14 | 466.47 | 516.03 | ||
| 1,4–Dicaffeoylquinic acid | 320 | 7.2 | 7.81–500 | y =198.31x + 28.16 | 0.9997 | 0.39 | 1.18 | 610.34 | 0.90 | 1.75 | 99.25 | 0.71 | 659.42 | 708.85 | |
| Aqueous | Kaempferol | 280 | 19.4 | 7.81–500 | y =158.19x + 21.65 | 0.9999 | 0.34 | 1.03 | 791.37 | 0.92 | 1.83 | 98.72 | 1.27 | 840.19 | 889.50 |
| Luteolin | 12.4 | 7.81–500 | y =174.19x + 21.61 | 0.9999 | 0.41 | 1.26 | 624.71 | 1.92 | 1.56 | 99.16 | 0.78 | 673.77 | 723.19 | ||
| Hesperidin | 320 | 17.2 | 7.81–500 | y =234.21x + 15.73 | 0.9997 | 0.22 | 0.66 | 645.14 | 0.93 | 1.17 | 99.15 | 0.95 | 694.17 | 743.58 | |
| Narcissin | 12.5 | 7.81–500 | y =220.16x + 28.19 | 0.9999 | 0.38 | 1.16 | 596.78 | 1.12 | 2.25 | 98.97 | 0.99 | 645.87 | 695.32 | ||
| Cm–Hexane (mg/mL) | Cm–DCM (mg/mL) | Cm–Ethanol (mg/mL) | Cm–Aqueous (mg/mL) | Verapamil (µM) | |
|---|---|---|---|---|---|
| Jejunum | |||||
| Spontaneous | 0.1767 (0.1244–0.2636; 95% CI) | 0.2490 (0.1873–0.3344; 95% CI) | Spasmogenic | 4.662 (2.125–16.28; 95% CI) | 0.04725 (0.03764–0.05968; 95% CI) |
| K+ (80 mM) | 0.2437 (0.1636–0.3969; 95% CI) | 0.3128 (0.2484–0.3982; 95% CI) | 2.089 (1.218–4.141; 95% CI) | 0.5439 (0.4209–0.7075; 95% CI) | 0.1194 (0.09889–0.1445; 95% CI) |
| K+ (25 mM) | 0.1112 (0.08209–0.1532; 95% CI) | 0.1926 (0.1281–0.2919; 95% CI) | 1.076 (0.8378–1.402; 95% CI) | 0.2654 (0.1822–0.3938; 95% CI) | 0.01595 (0.01303–0.01956; 95% CI). |
| Trachea | |||||
| K+ (80 mM) | 0.9567 (0.6366–1.482; 95% CI) | 2.596 (1.855–3.796; 95% CI) | 4.055 (2.838–5.896; 95% CI) | 1.448 (1.186–1.783; 95% CI) | 0.2564 (0.2111–0.3118; 95% CI) |
| CCh (1 µM) | 0.5497 (0.4305–0.7191; 95% CI) | 0.8250 (0.5890–1.176; 95% CI) | 3.393 (2.357–5.184; 95% CI) | 0.1972 (0.1556–0.2510; 95% CI) | 0.06765 (0.05113–0.09052; 95% CI) |
| K+ (25 mM) | 0.05417 (0.03680–0.07970; 95% CI) | 0.7764 (0.5116–1.312; 95% CI) | 1.726 (1.166–2.714; 95% CI) | 0.2748 (0.2116–0.3606; 95% CI) | 0.01278 (0.01017–0.01608; 95% CI). |
| Urinary Bladder | |||||
| K+ (80 mM) | 0.5107 (0.3928–0.6686; 95% CI) | 1.124 (0.8837–1.451; 95% CI) | 1.607 (1.293–2.022; 95% CI) | 0.2199 (0.1667–0.2924; 95% CI) | 0.1036 (0.08172–0.1330; 95% CI) |
| CCh (1 µM) | 0.2540 (0.1859–0.3521; 95% CI) | 0.4637 (0.3606–0.6092; 95% CI) | 0.6436 (0.5133–0.8126; 95% CI) | 0.7115 (0.04493–0.1140; 95% CI) | 0.1371 (0.1092–0.1723; 95% CI) |
| K+ (25 mM) | 0.4152 (0.3313–0.5281) | 0.7650 (0.5168–1.161; 95% CI) | 1.461 (1.185–1.821; 95% CI) | 0.1602 (0.1195–0.2154; 95% CI) | 0.00944 (0.007502–0.01187; 95% CI) |
| Parameters | IL2RB | NOS2 | VGCAC1C |
|---|---|---|---|
| Molecular weight | 61,117.20 | 131,117.23 | 248,976.62 |
| Number of amino acid residues | 551 | 1153 | 2221 |
| Maximum amino acid residues | 12.5% Leu, 10.5% Pro, 8.9% Ser | 10.1% Leu, 6.8% Ser, 6.6% Glu | 10.2% Leu, 7.7% Ala, 7.0% Ile |
| Negatively charged residues (Asp + Glu) | 62 | 128 | 240 |
| Positively charged residues (Arg + Lys) | 40 | 134 | 255 |
| Theoretical isoelectric point (pI) | 4.93, indicating its acidic nature | 8.20, indicating its basic nature | 6.13, indicating its acidic nature |
| Instability index * | 58.62 | 48.97 | 48.87 |
| Aliphatic index | 81.78 | 79.56 | 92.53 |
| GRAVY score | −0.273 | −0.385 | −0.052 |
| Extinction coefficient (M−1cm−1) ** | 109,150 | 172,255 | 244,660 |
| Compounds | Docking Score (kcal/mol) | Glide Energy kcal/mol) | ∆G Bind (kcal/mol) | pKi (µM) | ∆G Hbond (kcal/mol) | ∆G vdW (kcal/mol) | Residue–Ligand Interactions with Distance (Å) | |
|---|---|---|---|---|---|---|---|---|
| Hydrogen Bonds | Electrostatic/Hydrophobic Bonds | |||||||
| Interleukin–2 Receptor Subunit β (IL2RB) | ||||||||
| Rutin | −6.82 | −41.93 | −36.20 | −12.49 | −2.39 | −25.23 | Conventional hydrogen bond: Asn87 (1.78 Å), Asn43 (1.85 Å), Asn43 (1.90 Å), Arg131 (1.60 Å), Carbon hydrogen bond: Ser45 (2.49 Å), Asn87 (2.56 Å), Asn43 (3.03 Å) | Pi–Cation; Pi–Donor hydrogen bond: Arg131 (4.03 Å), Pi–Sulfur: Met133 (4.25 Å), Pi–Alkyl: Pro222 (4.91 Å), Pro222 (4.88 Å), Arg131 (5.47 Å) |
| Luteolin | −6.22 | −35.82 | −38.97 | −13.70 | −2.36 | −24.03 | Conventional hydrogen bond: Arg131 (1.62 Å), Phe37 (2.33 Å), Carbon hydrogen bond: Met133 (2.88 Å), Ala134 (1.84 Å), Arg131 (2.32 Å) | Pi–Sigma: Pro222 (2.61 Å), Pi–Cation: Arg131 (4.30 Å), Pi–Sulfur: Met133 (4.22 Å), Pi–Alkyl: Met133 (5.06 Å), Pro222 (4.45 Å) |
| Quercetin | −3.85 | −42.28 | −28.68 | −9.23 | −2.25 | −29.05 | Conventional hydrogen bond: Ser45 (2.91 Å), Asn87 (1.91 Å), Arg131 (2.18 Å), Arg131 (2.54 Å), Phe37 (3.05 Å), Arg131 (3.10 Å), Asn43 (2.41 Å), Carbon hydrogen bond: Ser45 (2.67 Å) | Pi–Sigma: Pro222 (2.72 Å), Pi–Sulfur: Met133 (5.76 Å), Alkyl: Val47 (4.63 Å), Pi–Alkyl: Ala134 (4.67 Å), Pro222 (4.34 Å), Met133 (5.12 Å) |
| Umbelliferone | −3.52 | −26.52 | −35.59 | −12.23 | −1.10 | −20.30 | Conventional hydrogen bond: Ala134 (2.17 Å), Arg131 (1.64 Å), Carbon hydrogen bond: Pro222 (3.07 Å) | Pi–Cation: Arg131 (4.57 Å), Pi–Sulfur: Met133 (3.73 Å), Met133 (3.77 Å), Pi–Alkyl: Pro222 (4.87 Å) |
| Kaempferol | −3.40 | −31.55 | −30.08 | −9.83 | −1.81 | −24.49 | Conventional hydrogen bond: Arg131 (2.05 Å), Arg131 (1.71 Å), Trp223 (2.37 Å), Carbon hydrogen bond: Ser45 (2.60 Å) | Pi–Cation: Arg131 (4.46 Å), Pi–Sulfur: Met133 (4.24 Å), Met133 (3.98 Å), Pi–Alkyl: Pro222 (5.35 Å), Ala134 (3.71 Å), Pro222 (4.56 Å) |
| Ferulic acid | −3.27 | −22.50 | −20.23 | −5.56 | −1.12 | −21.03 | Conventional hydrogen bond: Arg131 (2.25 Å), Arg131 (1.91 Å), Carbon hydrogen bond: Phe37 (2.48 Å), Asn43 (2.74 Å), Asn43 (2.58 Å), Phe37 (2.73 Å) | Pi–Cation: Arg131pi–Sulfur: Met133 (3.88 Å) |
| Apigenin | −2.97 | −30.28 | −28.44 | −9.12 | −1.81 | −25.27 | Conventional hydrogen bond Arg131 (2.06 Å), Arg131 (1.72 Å), Trp223 (2.39 Å), Carbon hydrogen bond: Ser45 (2.62 Å) | Pi–Cation: Arg131 (4.46 Å), Pi–Sulfur: Met133 (4.24 Å), Met133 (3.99 Å), Pi–Alkyl: Pro222 (5.32 Å), Ala134 (3.71 Å), Pro222 (4.56 Å) |
| Verapamil | −1.60 | −37.75 | −46.89 | −17.13 | −1.10 | −36.91 | Conventional hydrogen bond: Ser45 (2.76 Å), Arg131 (2.03 Å), Carbon hydrogen bond: Ser45 (2.62 Å), Ser45 (2.69 Å), Asn43 (2.50 Å), Phe37 (2.55 Å), Asn43 (2.68 Å), Phe37 (2.72 Å), Arg131 (2.61 Å) | Pi–Cation; Pi–Donor hydrogen bond: Arg131 (4.13 Å), Pi–Sigma: Val79 (2.94 Å), Pi–Sulfur: Met133 (4.45 Å), Alkyl: Val47 (4.59 Å), Val79 (4.57 Å), Val79 (4.78 Å), Pi–Alkyl: Ala85 (3.89 Å) |
| Nitric Oxide Synthase 2 (NOS2) | ||||||||
| Rutin | −14.69 | −75.74 | −18.93 | −4.99 | −4.77 | −56.65 | Conventional hydrogen bond: Ile201 (1.92 Å), Glu377 (2.07 Å), Pro350 (2.61 Å), Cys200 (3.03 Å), Arg199 (1.63 Å), Asn370 (1.84 Å), Arg199 (2.99 Å), Carbon hydrogen bond: Gly371 (2.95 Å), Glu377 (2.55 Å), Arg199 (2.51 Å), Arg199 (2.98 Å), Trp463 (2.56 Å) | Pi–Anion: Cys200 (3.87 Å), Pi–Pi Stacked: Trp194 (4.22 Å), Trp194 (3.65 Å), Trp194 (5.32 Å), Trp194 (4.13 Å), Phe369 (4.34 Å), Phe369 (4.14 Å), Pi–Alkyl: Cys200 (4.21 Å), Ala197 (4.32 Å), Cys200 (4.66 Å) |
| Quercetin | −10.50 | −58.68 | −42.09 | −15.05 | −2.24 | −44.52 | Conventional hydrogen bond: Tyr491 (2.45 Å), Glu377 (2.32 Å), Glu377 (2.16 Å), Tyr489 (1.79 Å), Cys200 (2.14 Å), Carbon hydrogen bond: Arg199 (2.65 Å), Glu377 (2.85 Å) | Pi–Sigma: Met355 (2.45 Å), Pi–Sulfur: Met355 (4.27 Å), Met355 (4.41 Å), Pi–Pi Stacked: Trp194 (4.29 Å), Phe369 (3.93 Å), Pi–Pi T–Shaped: Tyr491 (5.55 Å), Pi–Alkyl: Ala197 (4.42 Å), Arg199 (5.23 Å), Cys200 (5.44 Å), Ala197 (4.78 Å), Arg199 (4.40 Å), Ala197 (4.79 Å), Cys200 (4.47 Å) |
| Luteolin | −9.09 | −40.07 | −47.68 | −17.48 | −1.01 | −32.84 | Conventional hydrogen bond: Tyr491 (2.44 Å), Tyr489 (1.81 Å), Cys200 (2.01 Å), Carbon hydrogen bond: Arg199 (2.63 Å) | Pi–Sulfur: Met355 (4.27 Å), Met355 (4.41 Å), Pi–Pi Stacked: Trp194 (4.31 Å), Phe369 (3.98 Å), Pi–Pi T–Shaped: Tyr491 (5.47 Å), Pi–Alkyl: Ala197 (4.38 Å), Arg199 (5.19 Å), Ala197 (4.86 Å), Arg199 (4.39 Å), Ala197 (4.73 Å), Cys200 (4.41 Å) |
| Kaempferol | −8.14 | −39.60 | −25.84 | −7.99 | −0.75 | −36.79 | Conventional hydrogen bond: Ser242 (1.95 Å), Asn370 (2.30 Å), Carbon hydrogen bond: Gly371 (2.78 Å) | Pi–Donor hydrogen bond: Tyr489 (3.07 Å), Trp372 (2.38 Å), Pi–Pi Stacked: Trp194 (3.53 Å), Trp194 (3.92 Å), Trp194 (3.94 Å), Trp194 (5.27 Å), Phe369 (3.94 Å), Pi–Pi T–Shaped: Trp372 (5.07 Å), Trp372 (5.53 Å), Pi–Alkyl: Cys200 (4.91 Å), Ala197 (5.20 Å), Cys200 (4.60 Å) |
| Apigenin | −7.98 | −36.73 | −38.28 | −13.40 | −0.85 | −34.03 | Conventional hydrogen bond: Tyr491 (2.61 Å), Tyr489 (1.79 Å), Carbon hydrogen bond: Arg199 (2.67 Å) | Pi–Donor hydrogen bond: Trp194 (2.89 Å), Pi–Sigma: Met355 (2.46 Å), Pi–Sulfur: Met355 (4.32 Å), Met355 (4.38 Å), Pi–Pi Stacked: Trp194 (4.21 Å), Trp194 (5.69 Å), Phe369 (3.89 Å), Pi–Pi T–Shaped: Tyr491 (5.62 Å), Pi–Alkyl: Ala197 (4.31 Å), Arg199 (5.32 Å), Cys200 (5.34 Å), Ala197 (4.73 Å), Arg199 (4.41 Å), Ala197 (4.83 Å), Cys200 (4.57 Å) |
| Umbelliferone | −6.83 | −25.99 | −28.30 | −9.06 | −0.96 | −23.40 | Conventional hydrogen bond: Asn370 (1.73 Å) | Pi–Pi Stacked: Trp194 (4.04 Å), Trp194 (3.58 Å), Trp194 (5.43 Å), Trp194 (3.92 Å), Phe369 (3.80 Å), Phe369 (4.46 Å), Pi–Alkyl: Ala197 (5.08 Å), Cys200 (4.77 Å) |
| Verapamil | −6.08 | −55.87 | −60.12 | −22.88 | −1.57 | −58.63 | Salt bridge; Attractive charge: Glu377 (2.50 Å), Hydrogen bond: Trp372 (2.79 Å), Carbon hydrogen bond: Arg199 (3.07 Å), Glu377 (2.62 Å), Tyr373 (3.02 Å), Trp372 (2.80 Å) | Pi–Sigma: Gly202 (2.59 Å), Pi–Sulfur: Cys200 (3.99 Å), Pi–Pi T–Shaped: Phe369 (4.51 Å), Trp372 (5.53 Å), Alkyl: Met374 (4.92 Å), Met434 (3.95 Å), Pi–Alkyl: Trp194 (4.06 Å), Trp194 (3.66 Å), Trp194 (5.26 Å), Trp194 (3.58 Å), Trp194 (5.02 Å), Phe369 (3.51 Å), Trp372 (4.19 Å), Trp372 (4.16 Å), Trp372 (3.62 Å), Tyr489 (4.38 Å), Cys200 (5.19 Å) |
| Ferulic acid | −5.95 | −26.82 | −8.19 | −0.33 | −0.19 | −33.16 | Conventional hydrogen bond: Ser242 (2.73 Å) | Pi–Pi Stacked: Trp194 (3.49 Å), Trp194 (4.08 Å), Phe369 (4.55 Å), Alkyl: Leu209 (4.53 Å), Ile244 (4.44 Å), Pi–Alkyl: Trp194 (4.94 Å), Phe369 (4.29 Å), Tyr489 (4.49 Å), Cys200 (5.15 Å) |
| Voltage–dependent L–type calcium channel subunit alpha–1C (VGCAC1C) | ||||||||
| Rutin | −9.07 | −57.33 | −20.14 | −5.52 | −2.79 | −45.06 | Conventional hydrogen bond: Asn741 (1.69 Å), Ser1132 (1.73 Å), Glu1135 (2.21 Å), Gly1463 (3.00 Å), Glu363 (1.96 Å), Carbon hydrogen bond: Gly705 (2.98 Å), Asn741 (2.99 Å), Asn741 (2.79 Å) | Alkyl: Leu744 (4.82 Å), Leu745 (4.44 Å), Met1178 (5.29 Å), Pi–Alkyl: Phe748 (5.44 Å) |
| Quercetin | −7.58 | −48.23 | −40.37 | −14.30 | −1.05 | −47.92 | Conventional hydrogen bond Ile738 (2.57 Å), Asn741 (1.92 Å), Thr361 (1.90 Å), Carbon hydrogen bond: Ile738 (2.86 Å) | Pi–Pi T–Shaped: Phe737 (5.24 Å), Phe737 (5.29 Å), Alkyl: Ile738 (4.12 Å), Pi–Alkyl: Leu397 (4.87 Å), Ile360 (4.71 Å), Val396 (5.33 Å) |
| Apigenin | −6.12 | −32.47 | −24.50 | −7.41 | −0.78 | −29.70 | Conventional hydrogen bond: Ile360 (2.04 Å), Asn741 (2.71 Å) | Pi–Donor Hydrogen Bond: Tyr742 (3.29 Å), Pi–Alkyl: Val275 (5.46 Å), Val400 (5.07 Å), Ala272 (5.42 Å), Val400 (4.33 Å) |
| Luteolin | −5.78 | −42.83 | −16.03 | −3.73 | −2.57 | −28.94 | Conventional hydrogen bond: Asn741 (2.11 Å), Ile360 (1.84 Å), Glu1135 (1.90 Å), Glu1135 (2.55 Å), Thr1462 (3.04 Å), Gly1463 (1.98 Å) | Pi–Alkyl: Leu397 (4.90 Å), Met362 (5.05 Å) |
| Verapamil | −5.77 | −56.89 | −25.64 | −7.91 | −0.81 | −48.66 | Salt bridge; Attractive charge: Glu363 (3.12 Å), Glu1135 (2.41 Å), Attractive Charge: Glu706 (5.02 Å), Carbon hydrogen bond: Glu363 (2.60 Å), Glu1135 (2.71 Å), Thr704 (2.49 Å), Glu363 (2.65 Å), Glu1135 (2.73 Å), Asn741 (2.59 Å), Ile360 (2.69 Å), Ser1132 (2.80 Å), Ser1132 (2.86 Å) | Pi–Pi T–Shaped: Phe737 (5.70 Å), Tyr1508 (5.67 Å), Alkyl: Ala1512 (3.85 Å), Met362 (5.13 Å), Pi–Alkyl: Phe737 (4.51 Å), Phe1134 (4.76 Å), Tyr1508 (5.15 Å), Leu397 (4.71 Å) |
| Kaempferol | −5.75 | −35.56 | −30.99 | −10.23 | −1.59 | −21.96 | Conventional hydrogen bond: Asn741 (1.63 Å), Ile360 (1.80 Å), Thr704 (1.74 Å) | Pi–Pi T–Shaped: Phe737 (5.49 Å), Phe737 (5.20 Å), Pi–Alkyl: Leu397 (4.55 Å), Leu397 (4.59 Å) |
| Umbelliferone | −4.57 | −23.01 | −25.39 | −7.80 | −0.24 | −23.76 | Conventional hydrogen bond: Asn741 (2.86 Å), Carbon hydrogen bond: Thr704 (2.79 Å) | Pi–Alkyl: Leu744 (4.38 Å), Ala1174 (5.01 Å), Leu744 (4.24 Å), Ala1174 (5.25 Å) |
| Ferulic acid | −3.85 | −17.57 | −10.73 | −1.43 | 0.00 | −22.75 | Carbon hydrogen bond: Ser393 (2.43 Å) | Pi–Alkyl: Phe737 (4.68 Å), Leu397 (4.70 Å) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wahid, M.; Saqib, F.; Ali, A.; Alshammari, A.; Alharbi, M.; Rauf, A.; Mubarak, M.S. Integrated Mechanisms of Polarity–Based Extracts of Cucumis melo L. Seed Kernels for Airway Smooth Muscle Relaxation via Key Signaling Pathways Based on WGCNA, In Vivo, and In Vitro Analyses. Pharmaceuticals 2022, 15, 1522. https://doi.org/10.3390/ph15121522
Wahid M, Saqib F, Ali A, Alshammari A, Alharbi M, Rauf A, Mubarak MS. Integrated Mechanisms of Polarity–Based Extracts of Cucumis melo L. Seed Kernels for Airway Smooth Muscle Relaxation via Key Signaling Pathways Based on WGCNA, In Vivo, and In Vitro Analyses. Pharmaceuticals. 2022; 15(12):1522. https://doi.org/10.3390/ph15121522
Chicago/Turabian StyleWahid, Muqeet, Fatima Saqib, Anam Ali, Abdulrahman Alshammari, Metab Alharbi, Abdur Rauf, and Mohammad S. Mubarak. 2022. "Integrated Mechanisms of Polarity–Based Extracts of Cucumis melo L. Seed Kernels for Airway Smooth Muscle Relaxation via Key Signaling Pathways Based on WGCNA, In Vivo, and In Vitro Analyses" Pharmaceuticals 15, no. 12: 1522. https://doi.org/10.3390/ph15121522
APA StyleWahid, M., Saqib, F., Ali, A., Alshammari, A., Alharbi, M., Rauf, A., & Mubarak, M. S. (2022). Integrated Mechanisms of Polarity–Based Extracts of Cucumis melo L. Seed Kernels for Airway Smooth Muscle Relaxation via Key Signaling Pathways Based on WGCNA, In Vivo, and In Vitro Analyses. Pharmaceuticals, 15(12), 1522. https://doi.org/10.3390/ph15121522









