Analysis of Structure and Antioxidant Activity of Polysaccharides from Aralia continentalis
Abstract
:1. Introduction
2. Results
2.1. Extraction of the Polysaccharides from Three Parts of A. continentalis
2.2. Fractionation of Polysaccharides from A. continentalis
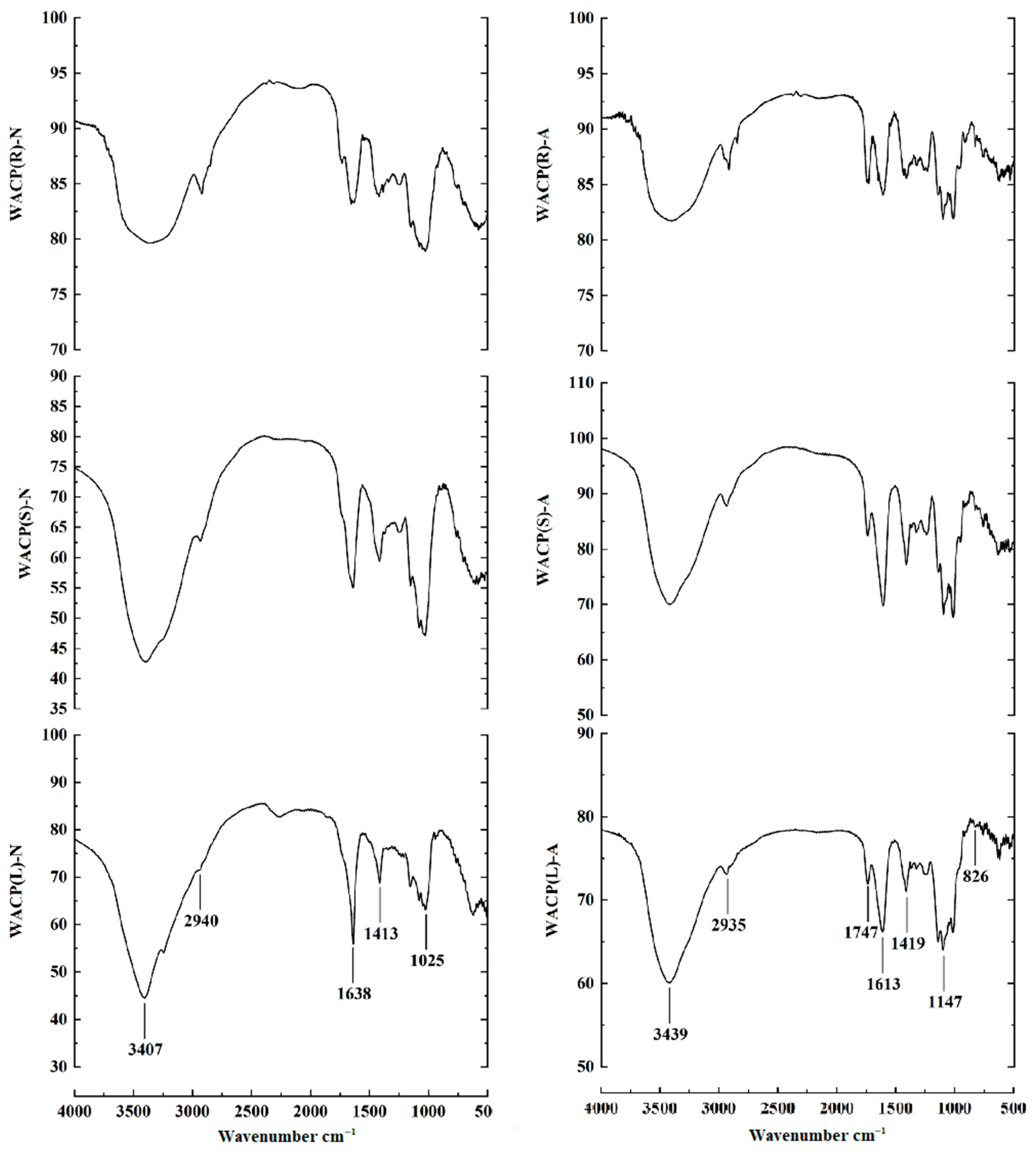
2.3. FTIR Spectra Analysis
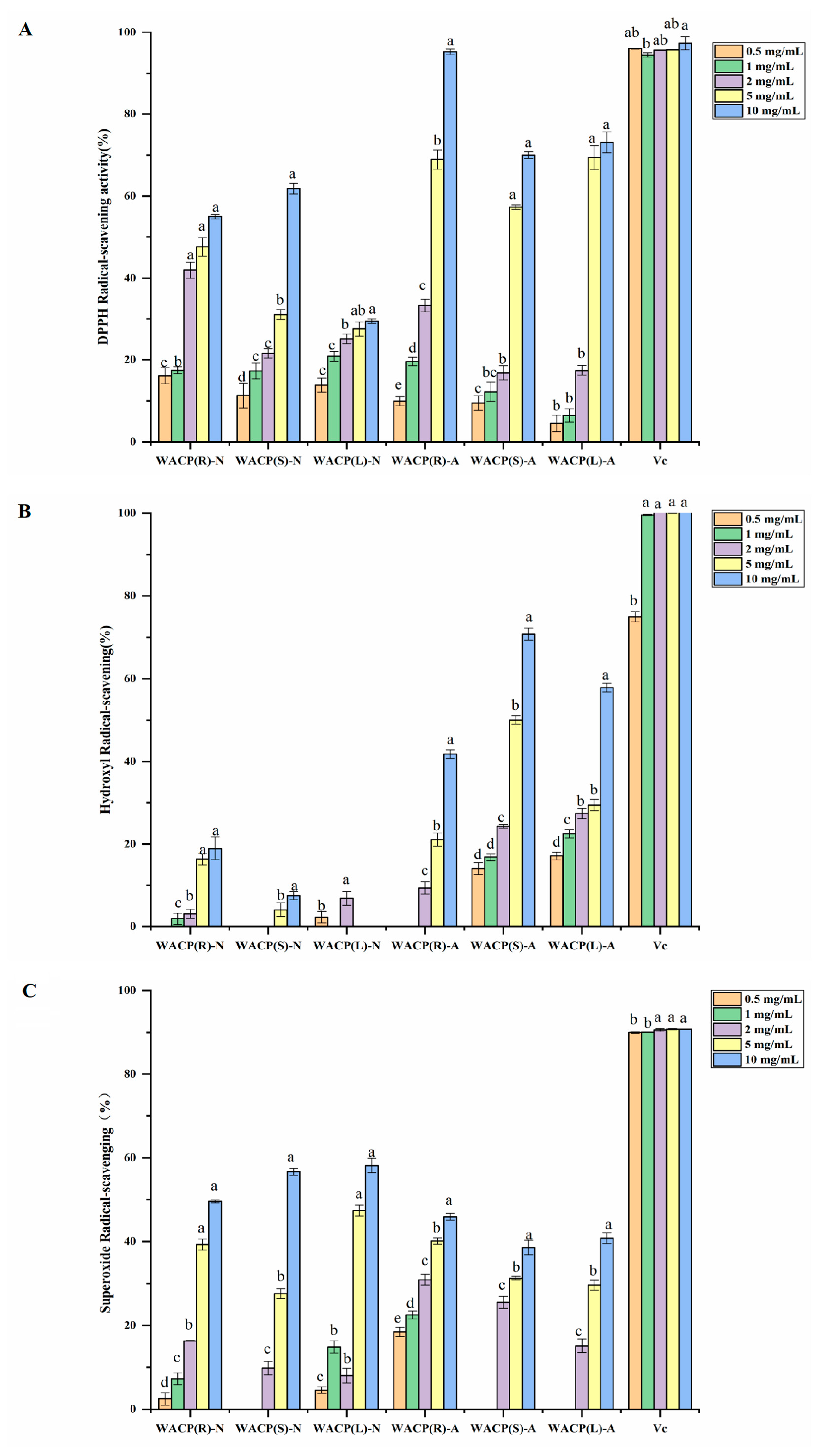
2.4. In Vitro Antioxidant Activity Assays of the Polysaccharides from A. continentalis
2.5. Structural Analysis of Acidic Polysaccharides from A. continentalis
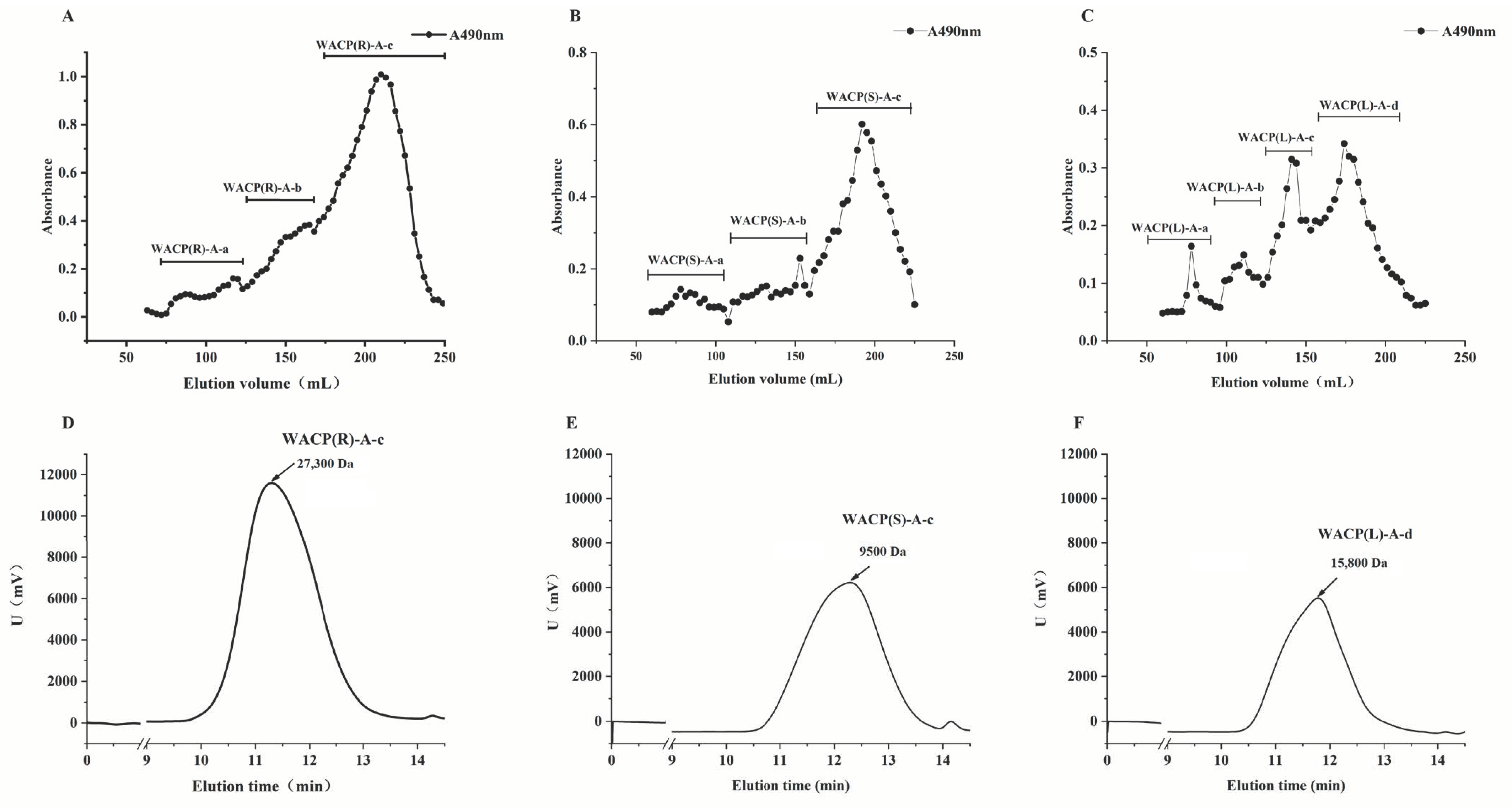
2.5.1. Purification of Acidic Polysaccharide Fractions
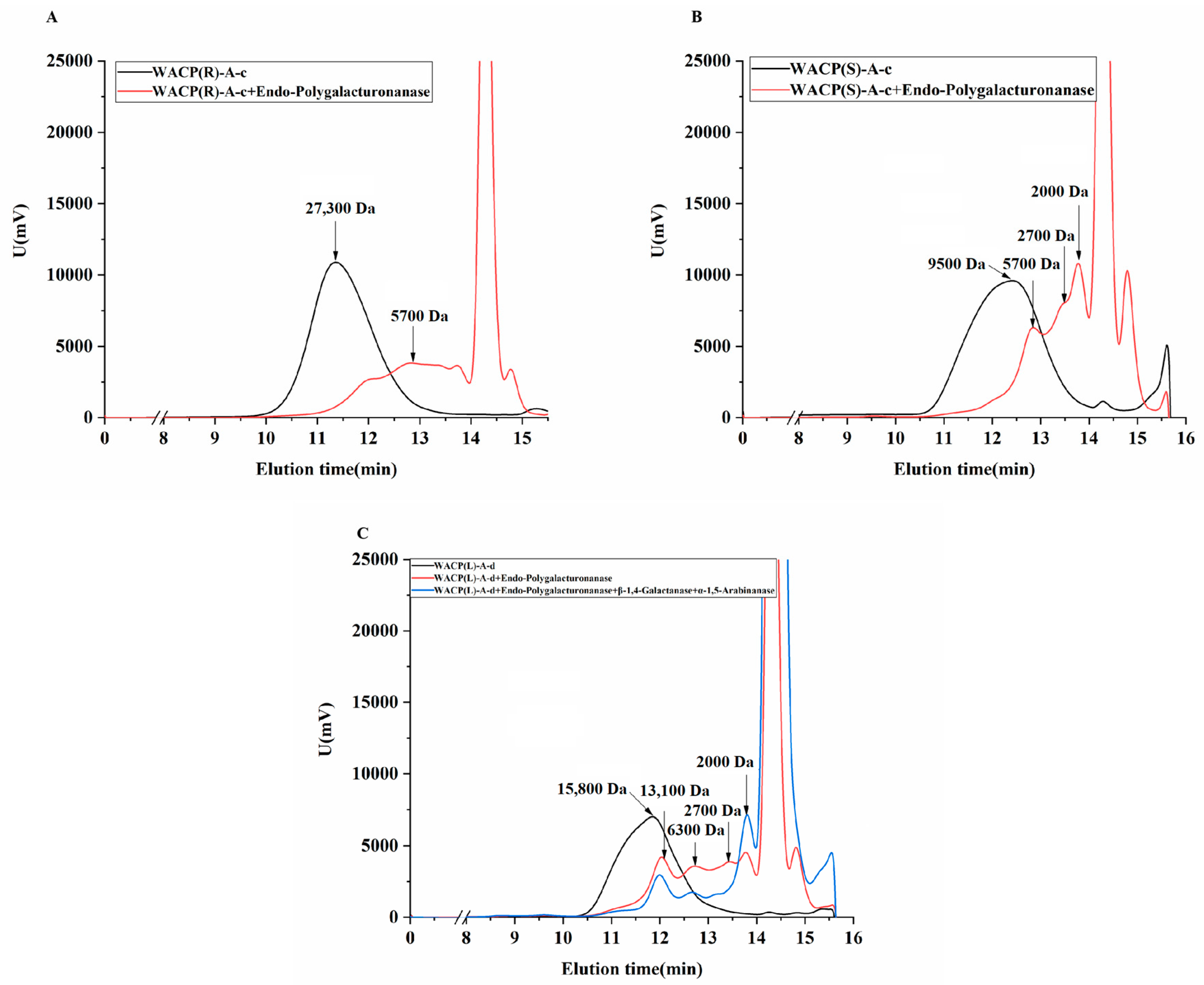
2.5.2. Enzymological Analysis
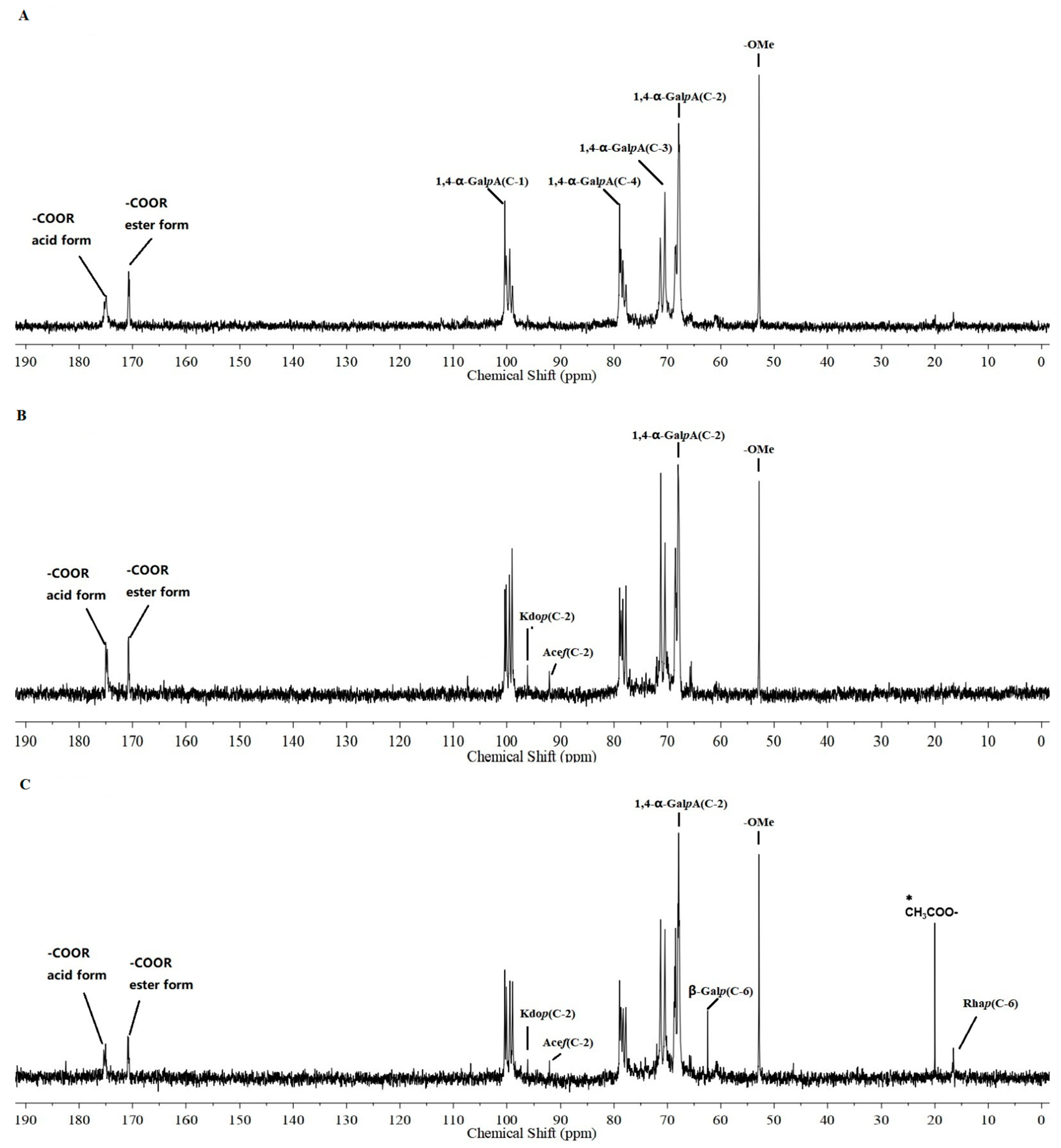
2.5.3. 13C NMR Spectra Analysis
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Polysaccharide Extraction and Purification
4.3. Chemical Composition Analysis
4.4. Monosaccharide Composition and Molecular Weights Analysis
4.5. Fourier Transform Infrared Spectroscopy
4.6. Enzymatic Hydrolysis Analysis
4.7. NMR Spectroscopy
4.8. Antioxidant Activity Analysis
4.8.1. DPPH Radical Scavenging Activity
4.8.2. Hydroxyl Radical Scavenging Activity
4.8.3. Superoxide Anion Radical Scavenging Activity
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Park, S.Y.; Kim, J.W. Cytotoxic polyacetylenes from Aralia cordata. Yakhak Hoeji 1995, 39, 681–688. [Google Scholar]
- Kim, J.S.; Kang, S.S. Saponins from the aerial parts of Aralia continentalis. Nat. Prod. Sci. 1998, 4, 45–50. [Google Scholar]
- Park, H.J.; Hong, M.S.; Lee, J.S.; Leem, K.H.; Kim, C.J.; Kim, J.W.; Lim, S. Effects of Aralia continentalis on Hyperalgesia with Peripheral Inflammation. Phytother. Res. 2005, 19, 511–513. [Google Scholar] [CrossRef] [PubMed]
- Han, B.H.; Woo, E.R.; Park, M.H.; Han, Y.N. Studies on the anti-inflammatory activity of Aralia continentalis (III). Arch. Pharmacal Res. 1985, 8, 59–65. [Google Scholar] [CrossRef]
- Hong, R.; Sur, B.J.; Yeom, M.; Lee, B.; Kim, K.S.; Rodriguez, J.P.; Hahm, D.H. Anti-inflammatory and anti-arthritic effects of the ethanolic extract of Aralia continentalis Kitag. in IL-1β-stimulated human fibroblast-like synoviocytes and rodent models of polyarthritis and nociception. Phytomedicine 2018, 38, 45–56. [Google Scholar] [CrossRef]
- Lee, D.H.; Seo, B.R.; Kim, H.Y.; Gum, G.C.; Yu, H.H.; You, H.K. Inhibitory effect of Aralia continentalis on the cariogenic properties of Streptococcus mutans. J. Ethnopharmacol. 2011, 137, 979–984. [Google Scholar] [CrossRef]
- Park, D.S.; Huh, J.E.; Baek, Y.H. Therapeutic effect of Aralia cordata extracts on cartilage protection in collagenase-induced inflammatory arthritis rabbit model. J. Ethnopharmacol. 2009, 125, 207–217. [Google Scholar] [CrossRef]
- Kim, T.D.; Lee, J.Y.; Cho, B.J.; Park, T.W.; Kim, C.J. The analgesic and antiinflammatory effects of 7-oxosandaracopimaric acid isolated from the roots of Aralia cordata. Arch. Pharmacal Res. 2010, 33, 967–968. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.; Hong, R.W.; Lim, P.R.; Cho, D.C.; Yeom, M.J.; Lee, S.H.; Sung, K.; Lee, S.C.; Shim, I.S.; Lee, H.J.; et al. The ethanolic extract of Aralia continentalis ameliorates cognitive deficits via modifications of BDNF expression and anti-inflammatory effects in a rat model of post-traumatic stress disorder. BMC Complement. Altern. Med. Vol. 2019, 19, 11. [Google Scholar] [CrossRef]
- Baek, Y.H.; Huh, J.E.; Lee, J.D.; Choi, D.Y.; Park, D.S. Effect of Aralia cordata extracts on cartilage protection and apoptosis inhibition. Biol. Pharm. Bull. 2006, 29, 1423–1430. [Google Scholar] [CrossRef] [Green Version]
- Zou, Y.F.; Zhang, Y.Y.; Paulsen, B.S.; Rise, F.; Yin, Z.Q. Structural features of pectic polysaccharides from stems of two species of Radix codonopsis and their antioxidant activities. Int. J. Biol. Macromol. 2020, 159, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.D.; Chen, H.; Liu, H.H.; Yang, X.H.; Guo, P.H.; Cao, X.; Cai, Y.; Xu, H.W.; Yang, J.T. Structure characterization and antioxidant activity analysis of polysaccharides from Lanzhou lily. Front. Nutr. 2022, 9, 976607. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Qiu, Z.; Li, L.; Vidyarthi, S.K.; Zhang, R. Structural characterization and antioxidant activities of one neutral polysaccharide and three acid polysaccharides from Ziziphus jujuba cv. Hamidazao: A comparison. Carbohydr. Polym. 2021, 261, 117879. [Google Scholar] [CrossRef] [PubMed]
- Paiheerding, M.; Khayrulla, B.; Aytursun, A.; Haibaier, H.; Rehebati, N.; Haji, A.A.; Abulimiti, Y. Structural characterization and antioxidant activities of a water soluble polysaccharide isolated from Glycyrrhiza glabra. Int. J. Biol. Macromol. 2020, 144, 751–759. [Google Scholar]
- Che, Q.F. Study on the Anti-oxidative Effect of Polysaccharide in the Roots of Aralia continentalis Kitag (AKSP). J. Anhui Agric. Sci. 2010, 38, 4047–4048. [Google Scholar]
- Liu, Y.J.; Tian, X.Y.; Li, F.; Su, Z. Putification, Identification and Anti-oxidation Activity of Polysaccharide AKSP2 of Roof of Aralia continentalis Kitag. J. Food Sci. Biotechnol. 2008, 27, 35–39. [Google Scholar]
- Zheng, C.; Ye, W.; Sanchez, N.P.; Li, C.; Lei, D.; Wang, Y. Development and field deployment of a mid-infrared methane sensor without pressure control using interband cascade laser absorption spectroscopy. Sens. Actuators B Chem. 2017, 244, 365–372. [Google Scholar] [CrossRef] [Green Version]
- Chan, M.K.; Yu, Y.; Wulamu, S.; Wang, Y.; Wang, Q.D.; Zhou, Y.F. Structural analysis of water-soluble polysaccharides isolated from Panax notoginseng. Int. J. Biol. Macromol. 2020, 155, 155,376–385. [Google Scholar] [CrossRef]
- Chen, X.; Qi, Y.; Zhu, C.; Wang, Q. Effect of ultrasound on the properties and antioxidant activity of hawthorn pectin. Int. J. Biol. Macromol. 2019, 131, 273–281. [Google Scholar] [CrossRef]
- Ebringerova, A.; Hromadkova, Z.; Alfoldi, J.; Berth, G. Structural and solution properties of corn cob heteroxylans. Carbohydr. Polym. 1992, 19, 99–105. [Google Scholar] [CrossRef]
- Ren, J.L.; Sun, R.C.; Liu, C.F.; Cao, Z.N.; Luo, W. Acetylation of wheat straw hemicelluloses in ionic liquid using iodine as a catalyst. Carbohydr. Polym. 2007, 70, 406–414. [Google Scholar] [CrossRef]
- Shakhmatov, E.G.; Atukmaev, K.V.; Makarova, E.N. Structural characteristics of pectic polysaccharides and arabinogalactan proteins from Heracleum sosnowskyi Manden. Carbohydr. Polym. 2016, 136, 1358. [Google Scholar] [CrossRef]
- Vriesmann, L.C.; Petkowicz, C. Polysaccharides from the pulp of cupuassu (Theobroma grandiflorum): Structural characterization of a pectic fraction. Carbohydr. Polym. 2009, 77, 72–79. [Google Scholar] [CrossRef]
- Wu, D.; Cui, L.; Yang, G.; Sun, L.; Zhou, Y.F. Preparing rhamnogalacturonan II domains from seven plant pectins using Penicillium oxalicum degradation and their structural comparison. Carbohydr. Polym. 2018, 180, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Correa-Ferreira, M.L.; Ferreira, D.M.; Dallazen, J.L.; Silva, A.M.S.; Werner, M.F.D.P.; Petkowicz, C.L.O. Gastroprotective effects and structural characterization of a pectic fraction isolated from Artemisia campestris subsp maritima. Int. J. Biol. Macromol. 2017, 107, 2395–2403. [Google Scholar] [CrossRef] [PubMed]
- Atmodjo, M.A.; Hao, Z.; Mohnen, D. Evolving views of pectin biosynthesis. Annu. Rev. Plant Biol. 2013, 64, 747–779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bulu, M.; Dhrubo, J.S.; Beduin, M.; Amit, K.N. Antioxidant potential of herbal polysaccharides: An overview on recent researches. Sens. Int. 2022, 3, 100158. [Google Scholar]
- Zhong, Q.W.; Wei, B.; Wang, S.J.; Ke, S.Z.; Chen, J.W.; Zhang, H.W.; Wang, H. The Antioxidant Activity of Polysaccharides Derived from Marine Organisms: An Overview. Mar. Drugs 2019, 17, 674. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Shuai, M.; Ma, P.; Huang, J.; Yan, S. Purification, chemical analysis and antioxidative activity of polysaccharides from ph-modified citrus pectin after dialyzation. LWT-Food Sci. Technol. 2020, 128, e109513. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, P.H.; Bai, X.Y.; Liu, P.; Yang, Y.; Huang, J. Fractionation and antioxidant activities of the water-soluble polysaccharides from Lonicera japonica Thunb. Int. J. Biol. Macromol. 2020, 151, 1058–1066. [Google Scholar] [CrossRef]
- Ning, X.; Liu, Y.; Jia, M.; Wang, Q.; Sun, L. Pectic polysaccharides from Radix Sophorae Tonkinensis exhibit significant antioxidant effects. Carbohydr. Polym. 2021, 262, 117925. [Google Scholar] [CrossRef] [PubMed]
- Han, B.H.; Yong, N.H.; Han, K.A.; Park, M.H.; Lee, E.O. Studies on the anti-inflammatory activity of Aralia continentalis (i). Arch. Pharmacal Res. 1983, 6, 17–23. [Google Scholar] [CrossRef]
- Lim, H.; Jung, H.A.; Choi, J.S.; Kim, Y.S.; Kang, S.S.; Kim, H.P. Anti-inflammatory activity of the constituents of the roots of Aralia continentalis. Arch. Pharmacal Res. 2009, 32, 1237–1243. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Oh, H.L.; Lee, C.H. Effects of Aralia continentalis root extract on cell proliferation and apoptosis in human promyelocytic leukemia HL-60 cells. J. Microbiol. Biotechnol. 2006, 16, 1399–1404. [Google Scholar]
- Yong, P.H.; Choi, J.H.; Jeong, H.G. Protective effect of the Aralia continentalis root extract against carbon tetrachloride-induced hepatotoxicity in mice. Food Chem. Toxicol. 2009, 47, 75–81. [Google Scholar]
- Dubois, M.K.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F.A. A colorimetric method for the determination of sugars. Nature 1951, 168, 167. [Google Scholar] [CrossRef]
- Blumenkranz, B. New method for quantitative determination of uronic acid. Anal. Biochem. 1993, 54, 484–489. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Bi, H.; Li, X.; Ni, W.; Han, H. Total fractionation and characterization of the water-soluble polysaccharides isolated from Panax ginseng C. A. Meyer. Carbohydr. Polym. 2009, 77, 544–552. [Google Scholar] [CrossRef]
- Yan, J.; Zhu, L.; Qu, Y.; Qu, X.; Mu, M.; Zhang, M. Analyses of active antioxidant polysaccharides from four edible mushrooms. Int. J. Biol. Macromol. 2018, 123, 945–956. [Google Scholar] [CrossRef]
- Yang, X.; Wang, R.; Zhang, S.; Zhu, W.; Tang, J.; Liu, J. Polysaccharides from Panax japonicus C.A. Meyer and their antioxidant activities. Carbohydr. Polym. 2014, 101, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dai, L.; Kong, X.; Chen, L. Characterization and in vitro antioxidant activities of polysaccharides from Pleurotus ostreatus. Int. J. Biol. Macromol. 2012, 51, 259–265. [Google Scholar] [CrossRef] [PubMed]
| WACP (R) | WACP (S) | WACP (L) | |
|---|---|---|---|
| Yield (%) | 3.6 | 12.9 | 8.2 |
| Total sugar (%) | 68.5 | 43.9 | 45.9 |
| Protein (%) | 1.5 | 3.2 | 1.1 |
| Uronic acid (%) | 30.2 | 50.0 | 24.5 |
| Ash (%) | 10.2 | 14.0 | 12.8 |
| Sugar composition (%) | |||
| Glucose (Glc) | 34.5 | 28.5 | 44.7 |
| Galactose (Gal) | 21.4 | 16.9 | 11.7 |
| Mannose (Man) | - | - | - |
| Rhamnose (Rha) | 4.0 | - | - |
| Glucuronic acid (GlcA) | - | - | - |
| Galacturonic acid (GalA) | 23.5 | 25.71 | 14.7 |
| Arabinose (Ara) | 15.2 | 10.55 | 3.7 |
| Fucose (Fuc) | - | - | - |
| Fractions | Yield (%) | Monosaccharide Composition (Molar%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Glc | Gal | Man | Rha | GlcA | GalA | Ara | ||
| WACP(R)-N | 40.8 | 44.0 | 21.9 | - | - | - | 1.5 | 18.1 |
| WACP(S)-N | 41.2 | 38.5 | 32.6 | 2.3 | - | - | 4.0 | 16.3 |
| WACP(L)-N | 32.2 | 67.4 | 13.1 | 1.8 | - | 1.1 | - | 7.2 |
| WACP(R)-A | 59.2 | - | 10.0 | - | - | - | 71.2 | 10.1 |
| WACP(S)-A | 58.8 | 2.4 | 11.0 | - | 6.6 | 2.7 | 65.3 | 8.3 |
| WACP(L)-A | 67.8 | 3.2 | 26.7 | 5.0 | 7.8 | 5.4 | 36.1 | 15.7 |
| Fractions | Yield (%) | Monosaccharide Composition (Molar%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Glc | Gal | Man | Rha | GlcA | GalA | Ara | ||
| WACP(R)-A-c | 75.3 | 1.0 | 5.3 | - | 3.0 | 1.0 | 84.5 | 4.5 |
| WACP(S)-A-c | 76.6 | 3.0 | 5.5 | - | 3.1 | 1.1 | 83.0 | 4.4 |
| WACP(L)-A-d | 46.6 | 10.1 | 19.3 | - | 11.5 | 2.1 | 44.8 | 12.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.-b.; Hong, H.-l.; Liu, L.-y.; Zhou, J.-n.; Wang, Y.; Li, Y.-m.; Zhai, L.-y.; Shi, Z.-h.; Zhao, J.; Liu, D. Analysis of Structure and Antioxidant Activity of Polysaccharides from Aralia continentalis. Pharmaceuticals 2022, 15, 1545. https://doi.org/10.3390/ph15121545
Hu Y-b, Hong H-l, Liu L-y, Zhou J-n, Wang Y, Li Y-m, Zhai L-y, Shi Z-h, Zhao J, Liu D. Analysis of Structure and Antioxidant Activity of Polysaccharides from Aralia continentalis. Pharmaceuticals. 2022; 15(12):1545. https://doi.org/10.3390/ph15121545
Chicago/Turabian StyleHu, Yan-bo, Hui-li Hong, Li-yang Liu, Jia-ning Zhou, Yue Wang, Yi-ming Li, Li-yuan Zhai, Zeng-hui Shi, Jun Zhao, and Duo Liu. 2022. "Analysis of Structure and Antioxidant Activity of Polysaccharides from Aralia continentalis" Pharmaceuticals 15, no. 12: 1545. https://doi.org/10.3390/ph15121545
APA StyleHu, Y.-b., Hong, H.-l., Liu, L.-y., Zhou, J.-n., Wang, Y., Li, Y.-m., Zhai, L.-y., Shi, Z.-h., Zhao, J., & Liu, D. (2022). Analysis of Structure and Antioxidant Activity of Polysaccharides from Aralia continentalis. Pharmaceuticals, 15(12), 1545. https://doi.org/10.3390/ph15121545






