Bruceine D Identified as a Drug Candidate against Breast Cancer by a Novel Drug Selection Pipeline and Cell Viability Assay
Abstract
1. Introduction
2. Results
2.1. Ferulic Acid, Resveratrol, Capecitabine, and Methotrexate Are Predicted to Reduce the Growth of MCF-7 Cells
2.2. Bruceine D, Narciclasine, Hydroxysafflor Yellow A, Ferulic Acid, and Salvianolic Acid B Are Predicted to Show the Strongest Perturbations of the Metabolism of MCF-7 Cells
2.3. 13 Natural Products Are Predicted to Affect the Metabolism of MCF-7 Cells Similarly to the Breast Cancer Drugs Capecitabine and Methotrexate
2.4. Narciclasine, Emodin, Scutellarein, Strychnine, Resveratrol, Chenodeoxycholic Acid, Chelerythrine, Tetrahydropalmatine, Osthole, and Glycyrrhizic Acid Are Predicted to Alter the Androgen and Estrogen Synthesis and Metabolism Pathway
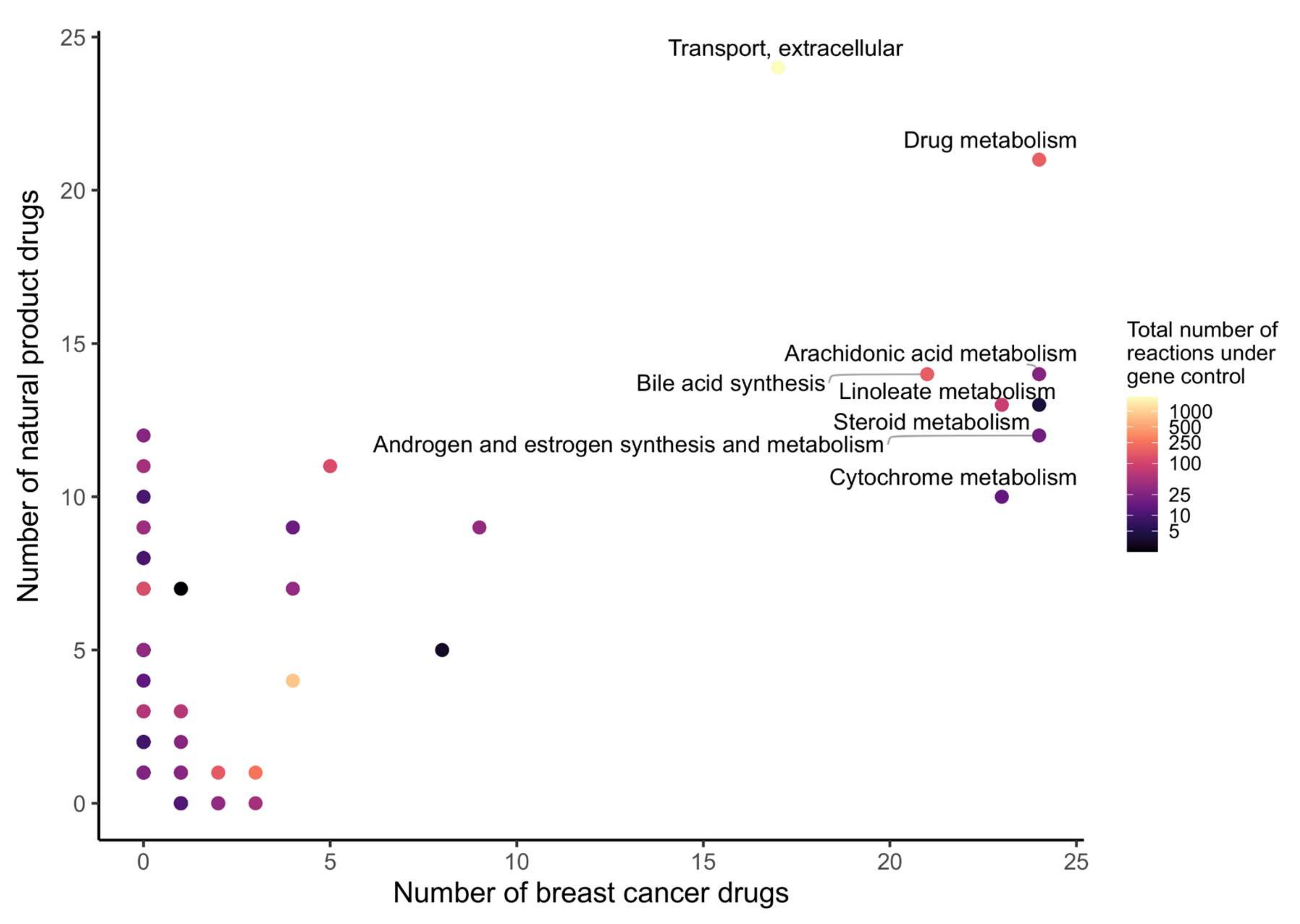
2.5. 23 Natural Products with Potential Anticancer Activity Emerged from the Different Stages of Analysis
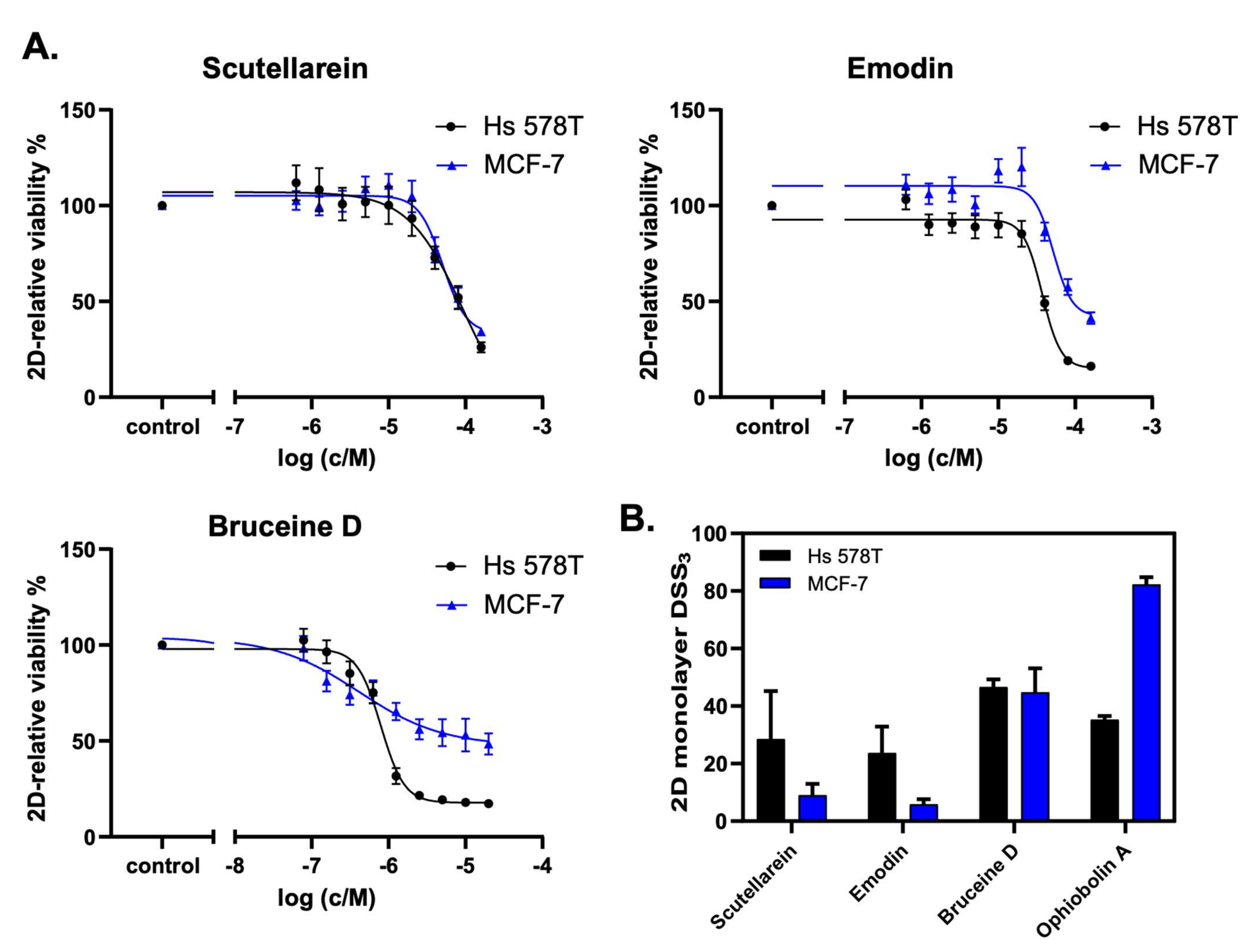
2.6. Scutellarein, Emodin, and Bruceine D Decrease the 2D Cell Viability of MCF-7 and Hs 578T Breast Cancer Cells
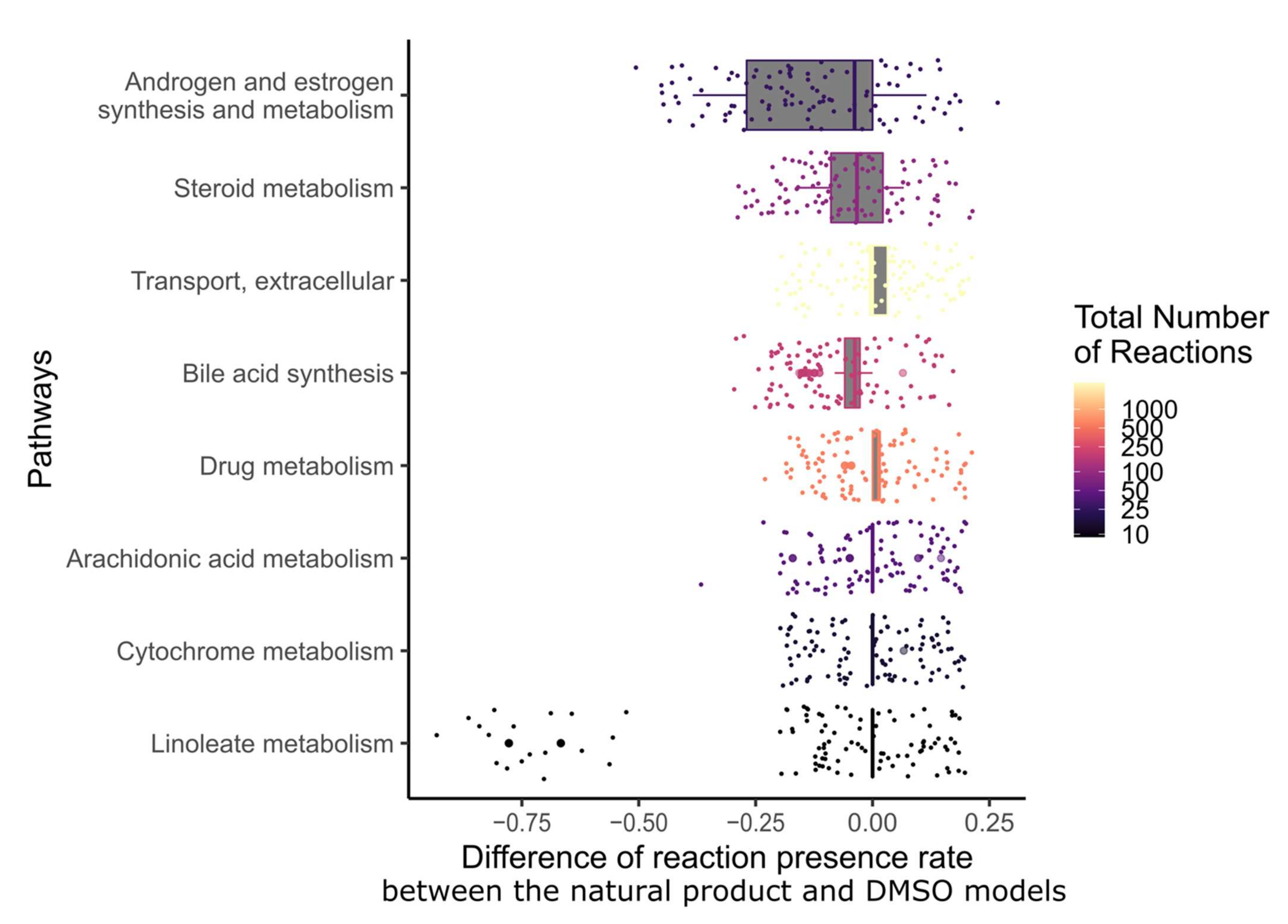
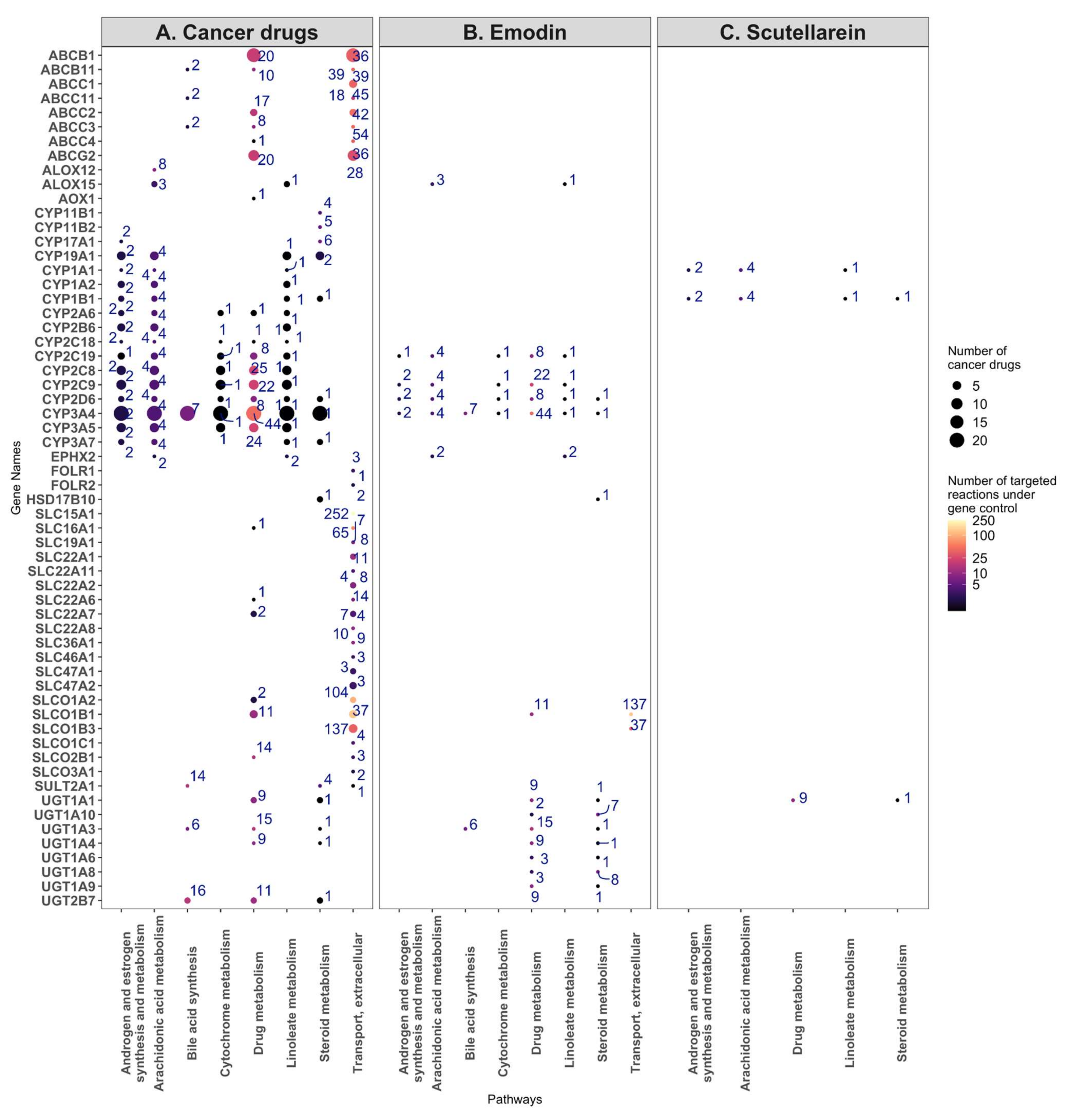
2.7. Targeting the Androgen and Estrogen Synthesis and Metabolism, as well as the Accumulation of ROS, Are the Two Main Modes of Action of Emodin, Bruceine D and Scutellarein
3. Discussion
4. Methods
4.1. Data Retrieval
4.2. Building of Context-Specific Metabolic Models via FASTCORMICS
4.3. Drug Deletion Prediction
4.3.1. Natural Products
4.3.2. Breast Cancer Drugs
4.3.3. Combination of the Natural Product and Breast Cancer Drugs
4.3.4. Single-Gene Deletion
4.4. Dissimilarity of the Natural Product Models to the DMSO Model
4.5. Similarity between the Natural Product and Breast Cancer Drug Model Fluxes
4.6. Pathway Analysis
4.7. Drug Selection
4.8. Experimental Validation
4.8.1. Natural Products and Inhibitors
4.8.2. Cell Culture
4.8.3. 2D Cell Viability Assay (2D Cell Proliferation)
4.8.4. Drug Sensitivity Score Determination and Data Analysis
4.9. Post-Experimental Analysis of the Pathways Affected by Bruceine D, Scutellarein, and Emodin
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Sinn, H.P.; Kreipe, H. A Brief Overview of the WHO Classification of Breast Tumors, 4th Edition, Focusing on Issues and Updates from the 3rd Edition. Breast Care 2013, 8, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Polyak, K. Breast cancer: Origins and evolution. J. Clin. Investig. 2007, 117, 3155–3163. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Hoffmann, A.D.; Liu, H.; Liu, X. Organotropism: New insights into molecular mechanisms of breast cancer metastasis. NPJ Precis. Oncol. 2018, 2, 4. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Zhang, M.; Wang, B.; Zhang, L.; Zhou, F.; Fang, M. Chemoresistance and Metastasis in Breast Cancer Molecular Mechanisms and Novel Clinical Strategies. Front. Oncol. 2021, 11, 658552. [Google Scholar] [CrossRef]
- Qi, F.; Zhao, L.; Zhou, A.; Zhang, B.; Li, A.; Wang, Z.; Han, J. The advantages of using traditional Chinese medicine as an adjunctive therapy in the whole course of cancer treatment instead of only terminal stage of cancer. Biosci. Trends 2015, 9, 16–34. [Google Scholar] [CrossRef]
- Wang, Z.-G.; Ren, J. Current status and future direction of Chinese herbal medicine. Trends Pharmacol. Sci. 2002, 23, 347–348. [Google Scholar] [CrossRef]
- Wu, C.; Lee, S.-L.; Taylor, C.; Li, J.; Chan, Y.-M.; Agarwal, R.; Temple, R.; Throckmorton, D.; Tyner, K. Scientific and Regulatory Approach to Botanical Drug Development: A U.S. FDA Perspective. J. Nat. Prod. 2020, 83, 552–562. [Google Scholar] [CrossRef]
- Balapure, A.K.; Kaushik, S.; Shyam, H.; Sharma, R. Genistein synergizes centchroman action in human breast cancer cells. Indian J. Pharmacol. 2016, 48, 637–642. [Google Scholar] [CrossRef]
- Charalambous, C.; Pitta, A.C.; Constantinou, A.I. Equol enhances tamoxifen’s anti-tumor activity by induction of caspase-mediated apoptosis in MCF-7 breast cancer cells. BMC Cancer 2013, 13, 238. [Google Scholar] [CrossRef]
- Shameer, S.; Vallarino, J.G.; Fernie, A.R.; Ratcliffe, R.G.; Sweetlove, L.J. Flux balance analysis of metabolism during growth by osmotic cell expansion and its application to tomato fruits. Plant J. 2020, 103, 68–82. [Google Scholar] [CrossRef]
- Cheung, C.Y.M.; Williams, T.C.R.; Poolman, M.G.; Fell, D.; Ratcliffe, R.G.; Sweetlove, L.J. A method for accounting for maintenance costs in flux balance analysis improves the prediction of plant cell metabolic phenotypes under stress conditions. Plant J. 2013, 75, 1050–1061. [Google Scholar] [CrossRef] [PubMed]
- Jerby, L.; Wolf, L.; Denkert, C.; Stein, G.Y.; Hilvo, M.; Oresic, M.; Geiger, T.; Ruppin, E. Metabolic Associations of Reduced Proliferation and Oxidative Stress in Advanced Breast Cancer. Cancer Res. 2012, 72, 5712–5720. [Google Scholar] [CrossRef]
- Gatto, F.; Miess, H.; Schulze, A.; Nielsen, J. Flux balance analysis predicts essential genes in clear cell renal cell carcinoma metabolism. Sci. Rep. 2015, 5, srep10738. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, M.P.; Bintener, T.; Ternes, D.; Kulms, D.; Haan, S.; Letellier, E.; Sauter, T. Identifying and targeting cancer-specific metabolism with network-based drug target prediction. EBioMedicine 2019, 43, 98–106. [Google Scholar] [CrossRef]
- Mardinoglu, A.; Agren, R.; Kampf, C.; Asplund, A.; Uhlen, M.; Nielsen, J. Genome-scale metabolic modelling of hepatocytes reveals serine deficiency in patients with non-alcoholic fatty liver disease. Nat. Commun. 2014, 5, 3083. [Google Scholar] [CrossRef]
- Pacheco, M.P.; John, E.; Kaoma, T.; Heinäniemi, M.; Nicot, N.; Vallar, L.; Bueb, J.-L.; Sinkkonen, L.; Sauter, T. Integrated metabolic modelling reveals cell-type specific epigenetic control points of the macrophage metabolic network. BMC Genom. 2015, 16, 809. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Wu, X.; Wang, X.; Su, J.; Zeng, H.; Zhao, J.; Lin, S.; Liu, R.; Li, H.; Li, X.; et al. The gene expression profiles in response to 102 traditional Chinese medicine (TCM) components: A general template for research on TCMs. Sci. Rep. 2017, 7, 352. [Google Scholar] [CrossRef]
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; Sayeeda, Z.; et al. DrugBank 5.0: A Major Update to the DrugBank Database for 2018. Nucleic Acids Res. 2018, 46, D1074–D1082. [Google Scholar] [CrossRef]
- Gallo, K.; Goede, A.; Eckert, A.; Moahamed, B.; Preissner, R.; Gohlke, B.-O. PROMISCUOUS 2.0: A resource for drug-repositioning. Nucleic Acids Res. 2020, 49, D1373–D1380. [Google Scholar] [CrossRef]
- Zeng, X.; Zhang, P.; He, W.; Qin, C.; Chen, S.; Tao, L.; Wang, Y.; Tan, Y.; Gao, D.; Wang, B.; et al. NPASS: Natural product activity and species source database for natural product research, discovery and tool development. Nucleic Acids Res. 2018, 46, D1217–D1222. [Google Scholar] [CrossRef]
- Aprile, G.; Mazzer, M.; Moroso, S.; Puglisi, F. Pharmacology and therapeutic efficacy of capecitabine: Focus on breast and colorectal cancer. Anti-Cancer Drugs 2009, 20, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Hannoodee, M.; Mittal, M. Methotrexate; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Brunk, E.; Sahoo, S.; Zielinski, D.C.; Altunkaya, A.; Dräger, A.; Mih, N.; Gatto, F.; Nilsson, A.; Gonzalez, G.A.P.; Aurich, M.K.; et al. Recon3D enables a three-dimensional view of gene variation in human metabolism. Nat. Biotechnol. 2018, 36, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Nelson, E.R.; Wardell, S.E.; Jasper, J.S.; Park, S.; Suchindran, S.; Howe, M.K.; Carver, N.J.; Pillai, R.V.; Sullivan, P.M.; Sondhi, V.; et al. 27-Hydroxycholesterol Links Hypercholesterolemia and Breast Cancer Pathophysiology. Science 2013, 342, 1094–1098. [Google Scholar] [CrossRef]
- Choi, E.J.; Jung, J.Y.; Kim, G.-H. Genistein inhibits the proliferation and differentiation of MCF-7 and 3T3-L1 cells via the regulation of ERα expression and induction of apoptosis. Exp. Ther. Med. 2014, 8, 454–458. [Google Scholar] [CrossRef]
- McNamara, K.; Moore, N.L.; Hickey, T.; Sasano, H.; Tilley, W.D. Complexities of androgen receptor signalling in breast cancer. Endocr. -Relat. Cancer 2014, 21, T161–T181. [Google Scholar] [CrossRef]
- Otter, J.; D’Orazio, J.L. Strychnine Toxicity; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Cao, C.; Huang, W.; Zhang, N.; Wu, F.; Xu, T.; Pan, X.; Peng, C.; Han, B. Narciclasine induces autophagy-dependent apoptosis in triple-negative breast cancer cells by regulating the AMPK-ULK1 axis. Cell Prolif. 2018, 51, e12518. [Google Scholar] [CrossRef] [PubMed]
- Duke, J.A. Dr. Duke’s Phytochemical and Ethnobotanical Databases; Routledge: London, UK, 1992. [Google Scholar]
- Corsello, S.; Bittker, J.A.; Liu, Z.; Gould, J.; McCarren, P.; Hirschman, J.E.; Johnston, S.E.; Vrcic, A.; Wong, B.; Khan, M.; et al. The Drug Repurposing Hub: A next-generation drug library and information resource. Nat. Med. 2017, 23, 405–408. [Google Scholar] [CrossRef]
- Sin, Z.W.; Bhardwaj, V.; Pandey, A.; Garg, M. A brief overview of antitumoral actions of bruceine D. Explor. Target. Anti-Tumor Ther. 2020, 1, 200–217. [Google Scholar] [CrossRef]
- Najumudeen, A.K.; Jaiswal, A.; Lectez, B.; Oetken-Lindholm, C.; Guzmán, C.; Siljamäki, E.; Posada, I.M.D.; Lacey, E.; Aittokallio, T.; Abankwa, D. Cancer stem cell drugs target K-ras signaling in a stemness context. Oncogene 2016, 35, 5248–5262. [Google Scholar] [CrossRef]
- Okutachi, S.; Manoharan, G.B.; Kiriazis, A.; Laurini, C.; Catillon, M.; McCormick, F.; Yli-Kauhaluoma, J.; Abankwa, D. A Covalent Calmodulin Inhibitor as a Tool to Study Cellular Mechanisms of K-Ras-Driven Stemness. Front. Cell Dev. Biol. 2021, 9, 665673. [Google Scholar] [CrossRef] [PubMed]
- Yadav, B.; Pemovska, T.; Szwajda, A.; Kulesskiy, E.; Kontro, M.; Karjalainen, R.; Majumder, M.M.; Malani, D.; Murumägi, A.; Knowles, J.; et al. Quantitative scoring of differential drug sensitivity for individually optimized anticancer therapies. Sci. Rep. 2014, 4, 5193. [Google Scholar] [CrossRef] [PubMed]
- Zweifel, M.; Thürlimann, B.; Riniker, S.; Weder, P.; Von Moos, R.; Pagani, O.; Bigler, M.; Rothgiesser, K.M.; Pilop, C.; Hawle, H.; et al. Phase I trial of the androgen receptor modulator CR1447 in breast cancer patients. Endocr. Connect. 2017, 6, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Tian, B.; Zhang, M.; Gao, X.; Jie, L.; Liu, P.; Li, J. Diagnostic and prognostic value of thymidylate synthase expression in breast cancer. Clin. Exp. Pharmacol. Physiol. 2021, 48, 279–287. [Google Scholar] [CrossRef]
- Ozer, U.; Barbour, K.W.; Clinton, S.A.; Berger, F.G. Oxidative Stress and Response to Thymidylate Synthase-Targeted Antimetabolites. Mol. Pharmacol. 2015, 88, 970–981. [Google Scholar] [CrossRef]
- Li, M.; Jin, C.; Xu, M.; Zhou, L.; Li, D.; Yin, Y. Bifunctional enzyme ATIC promotes propagation of hepatocellular carcinoma by regulating AMPK-mTOR-S6 K1 signaling. Cell Commun. Signal. 2017, 15, 52. [Google Scholar] [CrossRef] [PubMed]
- Robey, R.W.; Pluchino, K.M.; Hall, M.D.; Fojo, A.T.; Bates, S.E.; Gottesman, M.M. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat. Rev. Cancer 2018, 18, 452–464. [Google Scholar] [CrossRef]
- Mohan, C.D.; Liew, Y.Y.; Jung, Y.Y.; Rangappa, S.; Preetham, H.D.; Chinnathambi, A.; Alahmadi, T.A.; Alharbi, S.A.; Lin, Z.-X.; Rangappa, K.S.; et al. Brucein D modulates MAPK signaling cascade to exert multi-faceted anti-neoplastic actions against breast cancer cells. Biochimie 2021, 182, 140–151. [Google Scholar] [CrossRef]
- Androutsopoulos, V.P.; Ruparelia, K.; Arroo, R.R.J.; Tsatsakis, A.M.; Spandidos, D. CYP1-mediated antiproliferative activity of dietary flavonoids in MDA-MB-468 breast cancer cells. Toxicology 2009, 264, 162–170. [Google Scholar] [CrossRef]
- Luo, C.; Wang, Y.; Wei, C.; Chen, Y.; Ji, Z. The anti-migration and anti-invasion effects of Bruceine D in human triple-negative breast cancer MDA-MB-231 cells. Exp. Ther. Med. 2020, 19, 273–279. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, X.; Zhou, Q.; Lu, Y.; Zhang, H.; Chen, Q.; Zhao, M.; Su, S. Inhibitory effect of emodin on migration, invasion and metastasis of human breast cancer MDA-MB-231 cells in vitro and in vivo. Oncol. Rep. 2015, 33, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Ni, G.; Tang, Y.; Li, M.; He, Y.; Rao, G. Synthesis of Scutellarein Derivatives with a Long Aliphatic Chain and Their Biological Evaluation against Human Cancer Cells. Molecules 2018, 23, 310. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, J.; Liu, X.; Xing, K.; Wang, Y.; Li, F.; Yao, L. Resveratrol-Induced Cell Inhibition of Growth and Apoptosis in MCF7 Human Breast Cancer Cells Are Associated with Modulation of Phosphorylated Akt and Caspase-9. Appl. Biochem. Biotechnol. 2006, 135, 181–192. [Google Scholar] [CrossRef]
- Liang, Z.-J.; Wan, Y.; Zhu, D.-D.; Wang, M.-X.; Jiang, H.-M.; Huang, D.-L.; Luo, L.-F.; Chen, M.-J.; Yang, W.-P.; Li, H.-M.; et al. Resveratrol Mediates the Apoptosis of Triple Negative Breast Cancer Cells by Reducing POLD1 Expression. Front. Oncol. 2021, 11, 569295. [Google Scholar] [CrossRef] [PubMed]
- Poschner, S.; Maier-Salamon, A.; Zehl, M.; Wackerlig, J.; Dobusch, D.; Meshcheryakova, A.; Mechtcheriakova, D.; Thalhammer, T.; Pachmann, B.; Jäger, W. Resveratrol Inhibits Key Steps of Steroid Metabolism in a Human Estrogen-Receptor Positive Breast Cancer Model: Impact on Cellular Proliferation. Front. Pharmacol. 2018, 9, 742. [Google Scholar] [CrossRef]
- Lin, S.-C.; Chu, P.-Y.; Liao, W.-T.; Wu, M.-Y.; Tsui, K.-H.; Lin, L.-T.; Huang, C.-H.; Chen, L.-L.; Li, C.-J. Glycyrrhizic acid induces human MDA-MB-231 breast cancer cell death and autophagy via the ROS-mitochondrial pathway. Oncol. Rep. 2018, 39, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Inoue, A.; Zhu, Y.; Tanji, M.; Kiyama, R. Activation of rapid signaling pathways and the subsequent transcriptional regulation for the proliferation of breast cancer MCF-7 cells by the treatment with an extract of Glycyrrhiza glabra root. Food Chem. Toxicol. 2007, 45, 2470–2478. [Google Scholar] [CrossRef]
- Zhang, Z.; Feng, Y.; Li, Z.-Y.; Cao, X.-Z. Antiproliferative and apoptotic activity of glycyrrhizinic acid in MCF-7 human breast cancer cells and evaluation of its effect on cell cycle, cell migration and m-TOR/PI3K/Akt signalling pathway. Arch. Med Sci. 2019, 15, 174–182. [Google Scholar] [CrossRef]
- ElKhazendar, M.; Chalak, J.; El-Huneidi, W.; Vinod, A.; Abdel-Rahman, W.M.; Abu-Gharbieh, E. Antiproliferative and proapoptotic activities of ferulic acid in breast and liver cancer cell lines. Trop. J. Pharm. Res. 2019, 18, 2571–2576. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, D.; Jiang, R.; Li, H.; Wan, J.; Li, H. Ferulic acid exerts antitumor activity and inhibits metastasis in breast cancer cells by regulating epithelial to mesenchymal transition. Oncol. Rep. 2016, 36, 271–278. [Google Scholar] [CrossRef]
- Chang, C.J.; Chiu, J.H.; Tseng, L.M.; Chang, C.H.; Chien, T.M.; Wu, C.W.; Lui, W.Y.; Wu, C.W. Modulation of HER2 expression by ferulic acid on human breast cancer MCF7 cells. Eur. J. Clin. Investig. 2006, 36, 588–596. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Y.; Guan, Y.; Wang, Z.; Zhang, L. Hydroxysafflor yellow A induces apoptosis in MCF-7 cells by blocking NFκB/p65 pathway and disrupting mitochondrial transmembrane potential. RSC Adv. 2014, 4, 47576–47586. [Google Scholar] [CrossRef]
- Sha, W.; Zhou, Y.; Ling, Z.-Q.; Xie, G.; Pang, X.; Wang, P.; Gu, X. Antitumor properties of Salvianolic acid B against triple-negative and hormone receptor-positive breast cancer cells via ceramide-mediated apoptosis. Oncotarget 2018, 9, 36331–36343. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Katary, M.A.; Abdelsayed, R.; Alhashim, A.; Abdelhasib, M.; Elmarakby, A.A. Salvianolic Acid B Slows the Progression of Breast Cancer Cell Growth via Enhancement of Apoptosis and Reduction of Oxidative Stress, Inflammation, and Angiogenesis. Int. J. Mol. Sci. 2019, 20, 5653. [Google Scholar] [CrossRef] [PubMed]
- Sougiannis, A.T.; Enos, R.T.; VanderVeen, B.N.; Velazquez, K.T.; Kelly, B.; McDonald, S.; Cotham, W.; Chatzistamou, I.; Nagarkatti, M.; Fan, D.; et al. Safety of natural anthraquinone emodin: An assessment in mice. BMC Pharmacol. Toxicol. 2021, 22, 9. [Google Scholar] [CrossRef] [PubMed]
- Jayasuriya, H.; Koonchanok, N.M.; Geahlen, R.; McLaughlin, J.L.; Chang, C.-J. Emodin, a Protein Tyrosine Kinase Inhibitor from Polygonum cuspidatum. J. Nat. Prod. 1992, 55, 696–698. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Zhou, N.; Ying, P.; Zhang, T.; Liang, R.; Jiang, X. Emodin promotes apoptosis of human endometrial cancer through regulating the MAPK and PI3K/AKT pathways. Open Life Sci. 2019, 13, 489–496. [Google Scholar] [CrossRef]
- Wang, K.-J.; Meng, X.-Y.; Chen, J.-F.; Wang, K.-Y.; Zhou, C.; Yu, R.; Ma, Q. Emodin Induced Necroptosis and Inhibited Glycolysis in the Renal Cancer Cells by Enhancing ROS. Oxidative Med. Cell. Longev. 2021, 2021, 8840590. [Google Scholar] [CrossRef]
- Dong, X.; Ni, B.; Fu, J.; Yin, X.; You, L.; Leng, X.; Liang, X.; Ni, J. Emodin induces apoptosis in human hepatocellular carcinoma HepaRG cells via the mitochondrial caspase-dependent pathway. Oncol. Rep. 2018, 40, 1985–1993. [Google Scholar] [CrossRef]
- Shi, L.; Wu, Y.; Lv, D.L.; Feng, L. Scutellarein selectively targets multiple myeloma cells by increasing mitochondrial superoxide production and activating intrinsic apoptosis pathway. Biomed. Pharmacother. 2019, 109, 2109–2118. [Google Scholar] [CrossRef]
- Fan, J.; Ren, D.; Wang, J.; Liu, X.; Zhang, H.; Wu, M.; Yang, G. Bruceine D induces lung cancer cell apoptosis and autophagy via the ROS/MAPK signaling pathway in vitro and in vivo. Cell Death Dis. 2020, 11, 126. [Google Scholar] [CrossRef] [PubMed]
- Lai, Z.-Q.; Ip, S.-P.; Liao, H.-J.; Lu, Z.; Xie, J.-H.; Su, Z.-R.; Chen, Y.-L.; Xian, Y.-F.; Leung, P.-S.; Lin, Z.-X. Brucein D, a Naturally Occurring Tetracyclic Triterpene Quassinoid, Induces Apoptosis in Pancreatic Cancer through ROS-Associated PI3K/Akt Signaling Pathway. Front. Pharmacol. 2017, 8, 936. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Jeong, J.; Seong, S.; Kim, W. In Silico Evaluation of Natural Compounds for an Acidic Extracellular Environment in Human Breast Cancer. Cells 2021, 10, 2673. [Google Scholar] [CrossRef] [PubMed]
- Stompor-Gorący, M. The Health Benefits of Emodin, a Natural Anthraquinone Derived from Rhubarb—A Summary Update. Int. J. Mol. Sci. 2021, 22, 9522. [Google Scholar] [CrossRef]
- Ha, S.E.; Kim, S.M.; Vetrivel, P.; Kim, H.H.; Bhosale, P.B.; Heo, J.D.; Lee, H.J.; Kim, G.S. Inhibition of Cell Proliferation and Metastasis by Scutellarein Regulating PI3K/Akt/NF-κB Signaling through PTEN Activation in Hepatocellular Carcinoma. Int. J. Mol. Sci. 2021, 22, 8841. [Google Scholar] [CrossRef] [PubMed]
- Hoxhaj, G.; Manning, B.D. The PI3K–AKT network at the interface of oncogenic signalling and cancer metabolism. Nat. Rev. Cancer 2020, 20, 74–88. [Google Scholar] [CrossRef]
- Xia, Y.; Shen, S.; Verma, I.M. NF-κB, an Active Player in Human Cancers. Cancer Immunol. Res. 2014, 2, 823–830. [Google Scholar] [CrossRef]
- Huang, R.; Chen, H.; Liang, J.; Li, Y.; Yang, J.; Luo, C.; Tang, Y.; Ding, Y.; Liu, X.; Yuan, Q.; et al. Dual Role of Reactive Oxygen Species and their Application in Cancer Therapy. J. Cancer 2021, 12, 5543–5561. [Google Scholar] [CrossRef]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
- Kamei, H.; Koide, T.; Kojima, T.; Hashimoto, Y.; Hasegawa, M. Inhibition of Cell Growth in Culture by Quinones. Cancer Biother. Radiopharm. 1998, 13, 185–188. [Google Scholar] [CrossRef]
- Guo, F.; Yang, F.; Zhu, Y.-H. Scutellarein from Scutellaria barbata induces apoptosis of human colon cancer HCT116 cells through the ROS-mediated mitochondria-dependent pathway. Nat. Prod. Res. 2019, 33, 2372–2375. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhao, G.; Zhang, C.; Yang, C.; Zeng, X.; Li, J.; Zhu, K.; Zhao, S.; Lu, H.; Yin, D.; et al. Comparison of the roles of estrogens and androgens in breast cancer and prostate cancer. J. Cell. Biochem. 2020, 121, 2756–2769. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Karthik, G.-M.; Lövrot, J.; Haglund, F.; Rosin, G.; Katchy, A.; Zhang, X.; Viberg, L.; Frisell, J.; Williams, C.; et al. Estrogen Receptor β as a Therapeutic Target in Breast Cancer Stem Cells. JNCI J. Natl. Cancer Inst. 2017, 109, djw236. [Google Scholar] [CrossRef]
- Stanczyk, F.Z. The 2-/16α-Hydroxylated Estrogen Ratio-Breast Cancer Risk Hypothesis: Insufficient Evidence for its Support. J. Steroid Biochem. Mol. Biol. 2020, 201, 105685. [Google Scholar] [CrossRef] [PubMed]
- Haas, S.; Pierl, C.; Harth, V.; Pesch, B.; Rabstein, S.; Brüning, T.; Ko, Y.; Hamann, U.; Justenhoven, C.; Brauch, H.; et al. Expression of xenobiotic and steroid hormone metabolizing enzymes in human breast carcinomas. Int. J. Cancer 2006, 119, 1785–1791. [Google Scholar] [CrossRef]
- Mitra, R.; Guo, Z.; Milani, M.; Mesaros, C.; Rodriguez, M.; Nguyen, J.; Luo, X.; Clarke, D.; Lamba, J.; Schuetz, E.; et al. CYP3A4 Mediates Growth of Estrogen Receptor-positive Breast Cancer Cells in Part by Inducing Nuclear Translocation of Phospho-Stat3 through Biosynthesis of (±)-14,15-Epoxyeicosatrienoic Acid (EET). J. Biol. Chem. 2011, 286, 17543–17559. [Google Scholar] [CrossRef]
- Barrett, T.; Wilhite, S.E.; Ledoux, P.; Evangelista, C.; Kim, I.F.; Tomashevsky, M.; Marshall, K.A.; Phillippy, K.H.; Sherman, P.M.; Holko, M.; et al. NCBI GEO: Archive for functional genomics data sets—Update. Nucleic Acids Res. 2013, 41, D991–D995. [Google Scholar] [CrossRef]
- McCall, M.N.; Bolstad, B.M.; Irizarry, R.A. Frozen robust multiarray analysis (fRMA). Biostatistics 2010, 11, 242–253. [Google Scholar] [CrossRef]
- Greenhalgh, K.; Ramiro-Garcia, J.; Heinken, A.; Ullmann, P.; Bintener, T.; Pacheco, M.P.; Baginska, J.; Shah, P.; Frachet, A.; Halder, R.; et al. Integrated In Vitro and In Silico Modeling Delineates the Molecular Effects of a Synbiotic Regimen on Colorectal-Cancer-Derived Cells. Cell Rep. 2019, 27, 1621–1632.e9. [Google Scholar] [CrossRef]
- Vlassis, N.; Pacheco, M.P.; Sauter, T. Fast Reconstruction of Compact Context-Specific Metabolic Network Models. PLOS Comput. Biol. 2014, 10, e1003424. [Google Scholar] [CrossRef]
- Robinson, J.L.; Kocabaş, P.; Wang, H.; Cholley, P.-E.; Cook, D.; Nilsson, A.; Anton, M.; Ferreira, R.; Domenzain, I.; Billa, V.; et al. An atlas of human metabolism. Sci. Signal. 2020, 13, 624. [Google Scholar] [CrossRef] [PubMed]
- Mudunuri, U.; Che, A.; Yi, M.; Stephens, R.M. bioDBnet: The biological database network. Bioinformatics 2009, 25, 555–556. [Google Scholar] [CrossRef] [PubMed]
- Heirendt, L.; Arreckx, S.; Pfau, T.; Mendoza, S.N.; Richelle, A.; Heinken, A.; Haraldsdóttir, H.S.; Wachowiak, J.; Keating, S.M.; Vlasov, V.; et al. Creation and analysis of biochemical constraint-based models using the COBRA Toolbox v.3.0. Nat. Protoc. 2019, 14, 639–702. [Google Scholar] [CrossRef] [PubMed]
- Gudmundsson, S.; Thiele, I. Computationally efficient flux variability analysis. BMC Bioinform. 2010, 11, 489. [Google Scholar] [CrossRef]
- Gillen, J.E.; Tse, T.; Ide, N.C.; McCray, A.T. Design, implementation and management of a web-based data entry system for Clin-icalTrials.gov. Stud. Health Technol. Inform. 2004, 107, 1466–1470. [Google Scholar]
- Potdar, S.; Ianevski, A.; Mpindi, J.-P.; Bychkov, D.; Fiere, C.; Ianevski, P.; Yadav, B.; Wennerberg, K.; Aittokallio, T.; Kallioniemi, O.; et al. Breeze: An integrated quality control and data analysis application for high-throughput drug screening. Bioinformatics 2020, 36, 3602–3604. [Google Scholar] [CrossRef] [PubMed]



| Reactions | Metabolites | Genes | |
|---|---|---|---|
| Median | 1895 ± 87 | 1593 ± 66 | 1036 ± 49 |
| Min | 1593 | 1353 | 908 |
| Max | 2169 | 1790 | 1128 |
| Drug Deletion | Dissimilarity to DMSO Model | Similarity to Cancer Drug Models | Pathway Analysis | Anticancer Activity (Dr. Duke Database) | Mode of Action (Drug Repurposing Hub Database) | Clinical Phase | |
|---|---|---|---|---|---|---|---|
| Ferulic Acid | 1 | 1 | 1 | Antioxidant | Phase 2 | ||
| Glycyrrhizic acid | 1 | 1 | |||||
| Resveratrol | 1 | 1 | Antitumor, Antioxidant, Apoptotic, Antiangiogenic | Cytochrome P450 inhibitor, SIRT activator | Launched | ||
| Scutellarein | 1 | Antioxidant, Cancer-preventive | |||||
| Strychnine | 1 | Antioxidant | Acetylcholine receptor antagonist | Preclinical | |||
| Narciclasine | 1 | 1 | |||||
| Hydroxysafflor yellow A | 1 | 1 | Anti-tumor agent | Preclinical | |||
| Salvianolic Acid B | 1 | 1 | EGFR inhibitor, metalloproteinase inhibitor | Phase 2 | |||
| Daidzin | 1 | Antioxidant, Cancer-preventive | Antioxidant | Phase 1 | |||
| Macrozamin | 1 | ||||||
| Chelerythrine | 1 | Antimitotic | |||||
| Chenodeoxycholic acid | 1 | 11-ß hydroxysteroid dehydrogenase inhibitor, FXR agonist | Launched | ||||
| Emodin | 1 | Antitumor, Immunosuppressant | 11-ß hydroxysteroid dehydrogenase inhibitor | Preclinical | |||
| Tetrahydropalmatine | 1 | ||||||
| Bacopaside I | 1 | ||||||
| Ethyl caffeate | 1 | ||||||
| Ginsenoside Rb1 | 1 | ||||||
| Hypaconitine | 1 | ||||||
| Salidroside | 1 | Beta-amyloid protein neurotoxicity inhibitor | Preclinical | ||||
| Salvianic acid A sodium | 1 | Matrix metalloprotease inhibitor | |||||
| Schizandrin | 1 | Antioxidant | |||||
| Bruceine D | 1 | Glycine receptor antagonist | Preclinical | ||||
| Osthole | 1 | Calcium channel blocker | Preclinical |
| Perturbations | Number of Replicates |
|---|---|
| DMSO | 6 |
| A mixture of four natural products (tanshinone IIA, salvianic acid A sodium, protocatechuic aldehyde, salvianolic acid B) | 2 |
| 101 natural products | 2 (except for glycyrrhizic acid that has 4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cipriani, C.; Pacheco, M.P.; Kishk, A.; Wachich, M.; Abankwa, D.; Schaffner-Reckinger, E.; Sauter, T. Bruceine D Identified as a Drug Candidate against Breast Cancer by a Novel Drug Selection Pipeline and Cell Viability Assay. Pharmaceuticals 2022, 15, 179. https://doi.org/10.3390/ph15020179
Cipriani C, Pacheco MP, Kishk A, Wachich M, Abankwa D, Schaffner-Reckinger E, Sauter T. Bruceine D Identified as a Drug Candidate against Breast Cancer by a Novel Drug Selection Pipeline and Cell Viability Assay. Pharmaceuticals. 2022; 15(2):179. https://doi.org/10.3390/ph15020179
Chicago/Turabian StyleCipriani, Claudia, Maria Pires Pacheco, Ali Kishk, Maryem Wachich, Daniel Abankwa, Elisabeth Schaffner-Reckinger, and Thomas Sauter. 2022. "Bruceine D Identified as a Drug Candidate against Breast Cancer by a Novel Drug Selection Pipeline and Cell Viability Assay" Pharmaceuticals 15, no. 2: 179. https://doi.org/10.3390/ph15020179
APA StyleCipriani, C., Pacheco, M. P., Kishk, A., Wachich, M., Abankwa, D., Schaffner-Reckinger, E., & Sauter, T. (2022). Bruceine D Identified as a Drug Candidate against Breast Cancer by a Novel Drug Selection Pipeline and Cell Viability Assay. Pharmaceuticals, 15(2), 179. https://doi.org/10.3390/ph15020179








