Assessment of Avermectins-Induced Toxicity in Animals
Abstract
:1. Introduction
2. Avermectins
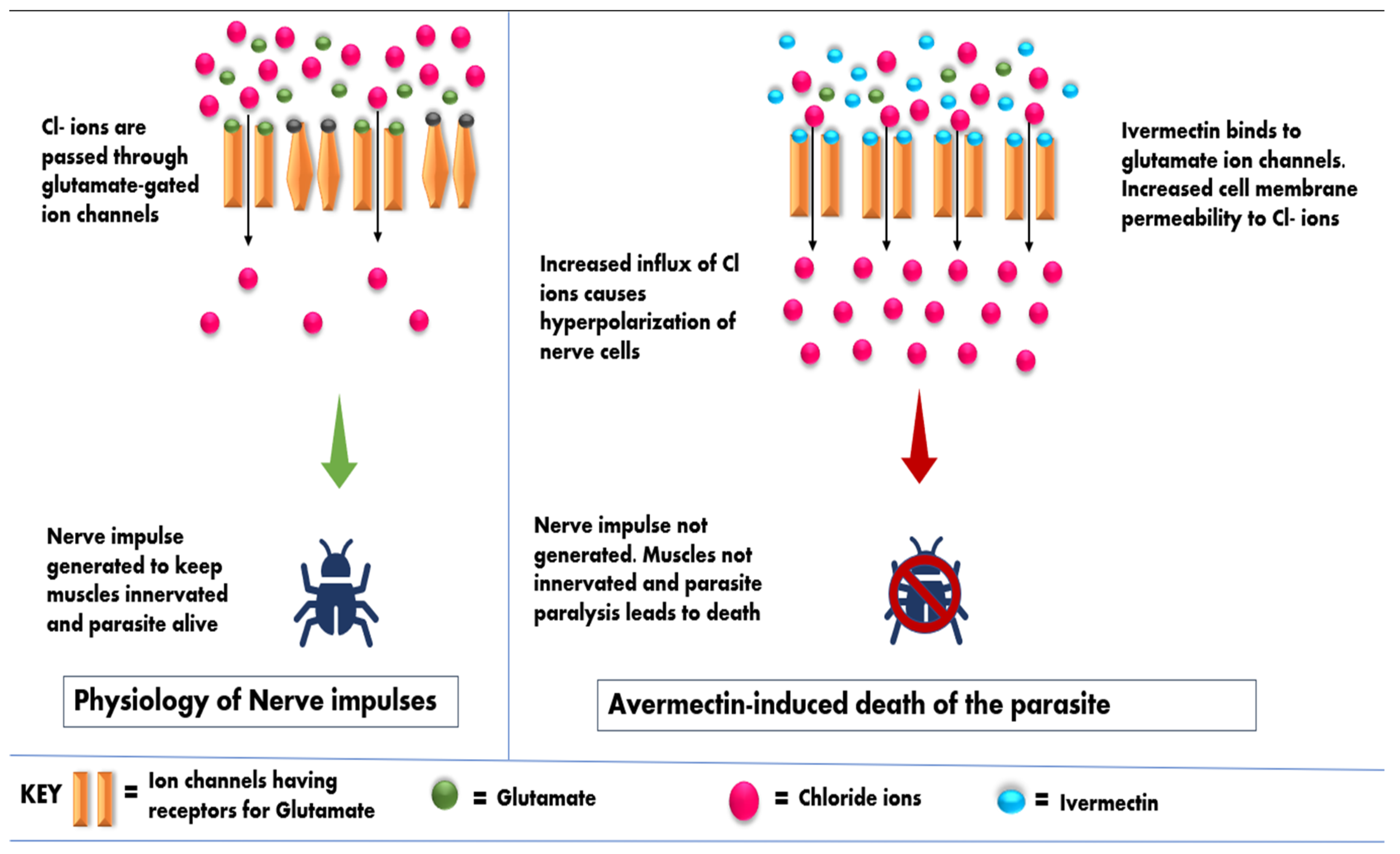
2.1. Mode of Avermectins Action against Parasites
2.2. Toxicity Studies
2.3. Nephrotoxicity
2.4. Hepatotoxicity
2.5. Neurotoxicity
2.6. Reproductive Toxicity
2.7. Endocrine Disruption
3. Missing Gaps and the Future Perspective
3.1. Milk Residues
3.2. Resistance
3.3. Ecotoxicity
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salman, M.; Abbas, R.Z.; Israr, M.; Abbas, A.; Mehmood, M.K.; Khan, M.K.; Shah, S. Repellent and acaricidal activity of essential oils and their components against Rhipicephalus ticks in cattle. Vet. Parasitol. 2020, 283, 109178. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Ijaz, M.; Ghaffar, A.; Oneeb, M.; Masud, A.; Durrani, A.Z.; Rashid, M.I. Species distribution and seasonal dynamics of equine tick infestation in two subtropical climate niches in Punjab, Pakistan. Pak. Vet. J. 2020, 40, 25–30. [Google Scholar]
- Jayawardene, K.L.T.; Palombo, E.A.; Boag, P.R. Natural Products Are a Promising Source for Anthelmintic Drug Discovery. Biomolecules 2021, 11, 1457. [Google Scholar] [CrossRef] [PubMed]
- Wolstenholme, A.J.; Neveu, C. The avermectin/milbemycin receptors of parasitic nematodes. Pestic. Biochem. Physiol. 2021, 181, 105010. [Google Scholar] [CrossRef] [PubMed]
- Alkenani, N.A.; Ahmed, M.M.M.; Al-Solami, H.M.; Anwar, Y.; Alghamdi, K.M.; Ahmad, M.S. Molecular Identification and bio-control of Mosquitoes using Black seeds extract in Jeddah. Pak. Vet. J. 2021, 41, 359–364. [Google Scholar] [CrossRef]
- Baz, M.M.; Hegazy, M.M.; Khater, H.F.; El-Sayed, Y.A. Comparative Evaluation of Five Oil-Resin Plant Extracts against The Mosquito Larvae, Culex pipiens Say (Diptera: Culicidae). Pak. Vet. J. 2021, 41, 191–196. [Google Scholar] [CrossRef]
- Abbas, R.Z.; Zaman, M.A.; Sindhu, D.; Sharif, M.; Rafique, A.; Saeed, Z.; Ahmad, M. Anthelmintic Effects and Toxicity Analysis of Herbal Dewormer against the Infection of Haemonchus contortus and Fasciola hepatica in Goat. Pak. Vet. J. 2020, 40, 455–460. [Google Scholar] [CrossRef]
- Štrbac, F.; Bosco, A.; Amadesi, A.; Rinaldi, L.; Stojanović, D.; Simin, N.; Ratajac, R. Ovicidal Potential of Five Different Essential Oils to Control Gastrointestinal Nematodes of Sheep. Pak. Vet. J. 2021, 41, 353–358. [Google Scholar] [CrossRef]
- Lasota, J.A.; Dybas, R.A. Avermectins, a novel class of compounds: Implications for use in arthropod pest control. Annu. Rev. Entomol. 1991, 36, 91–117. [Google Scholar] [CrossRef]
- Crump, A.; Omura, S. Ivermectin, “wonder drug” from Japan: The human use perspective. Proc. Jpn. Acad. Ser. B 2011, 87, 13–28. [Google Scholar] [CrossRef] [Green Version]
- Shoop, W.L.; Mrozik, H.; Fisher, M.H. Structure and activity of avermectins and milbemycins in animal health. Vet. Parasitol. 1995, 59, 139–156. [Google Scholar] [CrossRef]
- Pitterna, T.; Cassayre, J.; Huter, O.F.; Jung, P.M.; Maienfisch, P.; Kessabi, F.M.; Quaranta, L.; Tobler, H. New ventures in the chemistry of avermectins. Bioorg. Med. Chem. 2009, 17, 4085–4095. [Google Scholar] [CrossRef] [PubMed]
- Lumaret, J.P.; Errouissi, F.; Floate, K.; Rombke, J.; Wardhaugh, K. A review on the toxicity and non-target effects of macrocyclic lactones in terrestrial and aquatic environments. Curr. Pharm. Biotechnol. 2012, 13, 1004–1060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thuan, N.H.; Pandey, R.P.; Sohng, J.K. Recent advances in biochemistry and biotechnological synthesis of avermectins and their derivatives. Appl. Microbiol. Biotechnol. 2014, 98, 7747–7759. [Google Scholar] [CrossRef] [PubMed]
- Bishop, B.F.; Bruce, C.I.; Evans, N.A.; Goudie, A.C.; Gration, K.A.F.; Gibson, S.P.; Pacey, M.S.; Perry, D.A.; Walshe, N.D.A.; Witty, M.J. Selamectin: A novel broad-spectrum endectocide for dogs and cats. Vet. Parasitol. 2000, 91, 163–176. [Google Scholar] [CrossRef]
- Kose, L.P.; Gulçin, I.; Ozdemir, H.; Atasever, A.; Alwasel, S.H.; Supuran, C.T. The effects of some avermectins on bovine carbonic anhydrase enzyme. J. Enzyme Inhib. Med. Chem. 2016, 31, 773–778. [Google Scholar] [CrossRef] [Green Version]
- Parisi, D.P.; Santos, S.A.; Cabral, D.; Queiroz-Hazarbassanov, N.; Florio, J.C.; Bernardi, M.M.; Kirsten, T.B. Therapeutical doses of ivermectin and its association with stress disrupt motor and social behaviors of juvenile rats and serotonergic and dopaminergic systems. Res. Vet. Sci. 2019, 124, 149–157. [Google Scholar] [CrossRef]
- Bordes, L.; Dumont, N.; Lespine, A.; Souil, E.; Sutra, J.F.; Preévot, F.; Grisez, C.; Romanos, L.; Dailledouze, A.; Jacquiet, P. First report of multiple resistance to eprinomectin and benzimidazole in Haemonchus contortus on a dairy goat farm in France. Parasitol. Int. 2020, 76, 102063. [Google Scholar] [CrossRef]
- Ahmed, A.E.; Al-Kahtani, M.A.; Khalil, A.M.; Alshehri, A.S.; Elghoneimy, A.A.; Elbehairi, S.E.I.; Alfaifi, M.Y.; Shati, A.A.; Morsy, K.S.; Alshehri, M.A.; et al. Co-administration of vitamin E and selenium in vivo and in vitro ameliorates the toxic effects caused by ivermection and doramectin. Vet. Med. 2020, 65, 71–83. [Google Scholar] [CrossRef]
- Hurtt, M.E.; Cappon, G.D.; Browning, A. Proposal for a tiered approach to developmental toxicity testing for veterinary pharmaceutical products for food-producing animals. Food Chem. Toxicol. 2003, 41, 611–619. [Google Scholar] [CrossRef]
- Chung, K.; Yang, C.C.; Wu, M.L.; Deng, J.F.; Tsai, W.J. Agricultural avermectins: An uncommon but potentially fatal cause of pesticide poisoning. Ann. Emerg. Med. 1999, 34, 51–57. [Google Scholar] [CrossRef]
- Gonzalez, P.; González, F.; Ueno, K. Ivermectin in human medicine, an overview of the current status of its clinical applications. Curr. Pharm. Biotechnol. 2012, 13, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- El-Saber Batiha, G.; Alqahtani, A.; Ilesanmi, O.B.; Saati, A.A.; El-Mleeh, A.; Hetta, H.F.; Magdy Beshbishy, A. Avermectin derivatives, pharmacokinetics, therapeutic and toxic dosages, mechanism of action, and their biological effects. Pharmaceuticals 2020, 13, 196. [Google Scholar] [CrossRef]
- Rendic, S.P. Metabolism and interactions of Ivermectin with human cytochrome P450 enzymes and drug transporters, possible adverse and toxic effects. Arch. Toxicol. 2021, 95, 1535–1546. [Google Scholar] [CrossRef]
- Zhang, P.; Li, Y.; Xu, W.; Cheng, J.; Zhang, C.; Gao, J.; Zhang, Y. Immunotoxicity induced by Ivermectin is associated with NF-κB signaling pathway on macrophages. Chemosphere 2022, 289, 133087. [Google Scholar] [CrossRef] [PubMed]
- Yas-Natan, E.; Shamir, M.; Kleinbart, S.; Aroch, I. Doramectin toxicity in a collie. Vet. Rec. 2003, 153, 718. [Google Scholar] [PubMed]
- Lobetti, R.G.; Caldwell, P. Doramectin toxicity in a group of lions (Panthera leo). J. S. Afr. Vet. Assoc. 2012, 83, 89–92. [Google Scholar] [CrossRef] [Green Version]
- El Maghraby, M.M.; El Maghraby, N.M.; Salama, A.A.; Abdlmonem, A.S.; Authman, E.A.; Abdelmohsen, E.A.; ElTras, M.A.-E.; Barseem, O.N.; Awad, S.R.; Matter, A.A.; et al. Protective effects of vitamin E and grape seed oil against acute hepatorenal ivermectin toxicity in mice: Biochemical and histopathological studies. GSC Biol. Pharm. Sci. 2019, 7, 87–94. [Google Scholar] [CrossRef]
- Guizelini, C.C.; Pupin, R.C.; Möck, T.B.M.; Morais, D.R.; Arredondo, J.A.C.; Robalinho, L.L.; Gimelli, A.; de Lemos, R.A.A. Approaches for a field diagnosis of abamectin poisoning in calves. Pesqui. Vet. Bras. 2020, 40, 155–157. [Google Scholar] [CrossRef]
- Hopper, K.; Aldrich, J.; Haskins, S.C. Ivermectin toxicity in 17 collies. J. Vet. Intern. Med. 2002, 16, 89–94. [Google Scholar] [CrossRef]
- Lacau-Mengido, I.M.; Mejía, M.E.; Díaz-Torga, G.S.; Iglesias, A.G.; Formía, N.; Libertun, C.; Becú-Villalobos, D. Endocrine studies in ivermectin-treated heifers from birth to puberty. J. Anim. Sci. 2000, 78, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Sadek, K.M.; Shaheen, H.M. The biochemical effects of ivermectin on reproductive hormones and mineral homeostasis in Baladi cows post parturition. Vet. Arh. 2015, 85, 95–103. [Google Scholar]
- Nicolas, P.; Maia, M.F.; Bassat, Q.; Kobylinski, K.C.; Monteiro, W.; Rabinovich, N.R.; Menéndez, C.; Bardají, A.; Chaccour, C. Safety of oral ivermectin during pregnancy: A systematic review and meta-analysis. Lancet Glob. Health 2020, 8, e92–e100. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, A.E.; Alshehri, A.; Al-Kahtani, M.A.; Elbehairi, S.E.I.; Alshehri, M.A.; Shati, A.A.; Alfaifi, M.Y.; Al-Doaiss, A.A.; Taha, R.; Morsy, K.; et al. Vitamin E and selenium administration synergistically mitigates ivermectin and doramectin-induced testicular dysfunction in male Wistar albino rats. Biomed. Pharmacother. 2020, 124, 109841. [Google Scholar] [CrossRef]
- Ikeda, H.; Omura, S. Avermectin biosynthesis. Chem. Rev. 1997, 97, 2591–2610. [Google Scholar] [CrossRef]
- Bai, S.H.; Ogbourne, S. Eco-toxicological effects of the avermectin family with a focus on abamectin and ivermectin. Chemosphere 2016, 154, 204–214. [Google Scholar] [CrossRef]
- Laing, R.; Gillan, V.; Devaney, E. Ivermectin–old drug, new tricks? Trends Parasitol. 2017, 33, 463–472. [Google Scholar] [CrossRef] [Green Version]
- Khalil, M.S.; Darwesh, D.M. Avermectins: The promising solution to control plant parasitic nematodes. J. Plant Sci. Phytopathol. 2019, 3, 81–85. [Google Scholar] [CrossRef] [Green Version]
- Lynagh, T.; Lynch, J.W. Ivermectin binding sites in human and invertebrate Cys-loop receptors. Trends Pharmacol. Sci. 2012, 33, 432–441. [Google Scholar] [CrossRef] [Green Version]
- Caglayan, C.; Gulcin, I. The toxicological effects of some avermectins on goat liver carbonic anhydrase enzyme. J. Biochem. Mol. Toxicol. 2018, 32, 22010. [Google Scholar] [CrossRef]
- Abongwa, M.; Martin, R.J.; Robertson, A.P. A brief review on the mode of action of antinematodal drugs. Acta Vet. 2017, 67, 137–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barragry, T.B. A review of the pharmacology and clinical uses of ivermectin. Can. Vet. J. 1987, 28, 512. [Google Scholar] [PubMed]
- Martin, R.J. Modes of action of anthelmintic drugs. Vet. J. 1997, 154, 11–34. [Google Scholar] [CrossRef]
- Ashour, D.S. Ivermectin: From theory to clinical application. Int. J. Antimicrob. Agents 2019, 54, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Omura, S. A splendid gift from the earth: The origins and impact of the avermectins (Nobel Lecture). Angew. Chem. Int. 2016, 55, 10190–10209. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elhady, H.K.; Abou-Elghar, G.E. Abamectin induced biochemical and histopathological changes in the albino rat, Rattus norvegicus. J. Plant Prot. Res. 2013, 53, 263–270. [Google Scholar] [CrossRef]
- Rodrigues, D.d.C.; Buzullini, C.; Pereira, T.A.; Curz, B.C.; Gomes, L.V.C.; Soares, V.E.; Bastos, T.S.A.; Couto, L.F.M.; Lopes, W.D.Z.; de Oliveira, G.P.; et al. Avermectin toxicity in bovines less than thirty days old. Res. Vet. Sci. 2018, 118, 403–412. [Google Scholar] [CrossRef] [Green Version]
- Perez-Cogollo, L.C.; Rodriguez-Vivas, R.I.; Basto-Estrella, G.S.; Reyes-Novelo, E.; Martinez-Morales, I.; Ojeda-Chi, M.M.; Favila, M.E. Toxicidad y efectos adversos de las lactonas macrocíclicas sobre los escarabajos estercoleros: Una revisión. Rev. Mex. Biodiv. 2018, 89, 1293–1314. [Google Scholar] [CrossRef] [Green Version]
- Trailović, S.M.; Nedeljković, J.T. Central and peripheral neurotoxic effects of ivermectin in rats. J. Vet. Med. Sci. 2011, 73, 591. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; You, T.Z.; Zhu, W.J.; Qu, J.P.; Liu, C.; Zhao, B.; Li, S. Antioxidant response and histopathological changes in brain tissue of pigeon exposed to avermectin. Ecotoxicology 2013, 22, 1241–1254. [Google Scholar] [CrossRef]
- Nasr, H.M.; El-Demerdash, F.M.; El-Nagar, W.A. Neuro and renal toxicity induced by chlorpyrifos and abamectin in rats. Environ. Sci. Pollut. Res. 2016, 23, 1852–1859. [Google Scholar] [CrossRef] [PubMed]
- Lankas, G.R.; Wise, L.D.; Cartwright, M.E.; Pippert, T.; Umbenhauer, D.R. Placental P-glycoprotein deficiency enhances susceptibility to chemically induced birth defects in mice. Reprod. Toxicol. 1998, 12, 457–463. [Google Scholar] [CrossRef]
- DeMarco, J.H.; Heard, D.J.; Fleming, G.J.; Lock, B.A.; Scase, T.J. Ivermectin toxicosis after topical administration in dog-faced fruit bats (Cynopterus brachyotis). J. Zoo Wildl. Med. 2002, 33, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Al-Jassim, K.B.; Jawad, A.A.D.H.; Al-Masoudi, E.A.; Majeed, S.K. Histopathological and biochemical effects of ivermectin on kidney functions, lung and the ameliorative effects of vitamin c in rabbits (Lupus cuniculus). Basrah J. Vet. Res. 2016, 14, 110–124. [Google Scholar]
- Moqbel, F.S.; Al-Eryani, M.A.; Al Galil, F.M.A. Histopathological and biochemical effects of abamectin on kidney in male albino rats. J. Entomol. Zool. Stud. 2017, 5, 245–249. [Google Scholar]
- Abdel-Daim, M.M.; Abdellatief, S.A. Attenuating effects of caffeic acid phenethyl ester and betaine on abamectin-induced hepatotoxicity and nephrotoxicity. Environ. Sci. Pollut. Res. 2018, 25, 15909–15917. [Google Scholar] [CrossRef]
- Eissa, F.I.; Zidan, N.A. Haematological, biochemical and histopathological alterations induced by abamectin and Bacillus thuringiensis in male albino rats. Acta Biol. 2010, 61, 33–44. [Google Scholar] [CrossRef] [Green Version]
- Magdy, B.W.; Mohamed, F.E.; Amin, A.S.; Rana, S.S. Ameliorative effect of antioxidants (vitamins C and E) against abamectin toxicity in liver, kidney and testis of male albino rats. J. Basic Appl. Zool. 2016, 77, 69–82. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, M.L.R.; Mattei, R. Toxicity assessment of the antiparasitic ivermectin. Toxicity Assess. 1988, 3, 379–384. [Google Scholar] [CrossRef]
- Dey, S.; Kurade, N.P.; Khurana, K.L.; Dan, A. Clinicobiochemical changes in ivermectin toxicity in Doberman pinscher pups. J. Parasit. Dis. 2017, 41, 580–583. [Google Scholar] [CrossRef]
- Abdou, K.A.; Sharkawy, A.A. Some toxicological studies on ivermectin in goats. In Proceeding of the 20 Annual Meeting of the Egyptian Society of Toxicology, Alexandria, Egypt, 18–19 February 2004; pp. 18–19. [Google Scholar]
- GabAllh, M.S.; El-mashad, A.B.E.; Amin, A.A.; Darweish, M.M. Pathological studies on effects of ivermectin on male and female rabbits. Benha Vet. Med. J. 2017, 32, 104–112. [Google Scholar] [CrossRef]
- El-Shafey, A.A.M.; Seliem, M.M.E.; El-Mahrouky, F.; Gabr, W.M.; Kandil, R.A. Some physiological and biochemical effects of oshar extract and abamectin biocide on male albino rats. J. Am. Sci. 2011, 7, 254–261. [Google Scholar]
- Fahim, H.E.; Ahmed, O.M.; Boules, M.W.; Ahmed, H.Y. Nephrotoxic effects of abamectin and Calotropis procera latex and leaf extract in male albino rats. Am. J. Med. Med. Sci. 2016, 6, 73–86. [Google Scholar] [CrossRef]
- Zhu, W.J.; Zhang, Z.W.; Wang, X.S.; Xu, S.W.; Li, M.; Li, S. Effects of avermectin on microsomal cytochrome P450 enzymes in the liver and kidneys of pigeons. Environ. Toxicol. Pharmacol. 2014, 38, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Zanoli, J.C.C.; Maioli, M.A.; Medeiros, H.C.; Mingatto, F.E. Abamectin affects the bioenergetics of liver mitochondria: A potential mechanism of hepatotoxicity. Toxicol. Vitro 2012, 26, 51–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bischoff, K.; Mukai, M.; Ramaiah, S.K. Liver toxicity. In Veterinary Toxicology: Basic and Clinical Principles, 3rd ed.; Gupta, R.C., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 239–257. [Google Scholar]
- Fisher, M.H.; Mrozik, H. The chemistry and pharmacology of avermectins. Annu. Rev. Pharmacol. Toxicol. 1992, 32, 537–553. [Google Scholar] [CrossRef]
- Hou, X.; Jiang, H.; Ding, S.; Zhang, S.; Li, X.; Shen, J. Multiresidue analysis of avermectins in cattle liver by liquid chromatography/tandem mass spectrometry. J. AOAC Int. 2006, 89, 1110–1115. [Google Scholar] [CrossRef] [Green Version]
- Celis-Giraldo, C.T.; Ordonez, D.; Roa, L.; Cuervo-Escobar, S.A.; Garzon-Rodriguez, D.; Alarcon-Caballero, M.; Merchan, L.F. Preliminary study of ivermectin residues in bovine livers in the Bogota Savanna. Rev. Mex. Cienc. Pecu. 2020, 11, 311–325. [Google Scholar] [CrossRef]
- Hsu, D.Z.; Hsu, C.H.; Huang, B.M.; Liu, M.Y. Abamectin effects on aspartate aminotransferase and nitric oxide in rats. Toxicology 2001, 165, 189–193. [Google Scholar] [CrossRef]
- El-Hamid, S.R.A.; Refaie, A.A. Ameliorative effect of Silybum marianum extract against avermectin induced toxicity in adult male rats. JASMR 2009, 4, 25–31. [Google Scholar]
- Zhu, W.J.; Li, M.; Liu, C.; Qu, J.P.; Min, Y.H.; Xu, S.W.; Li, S. Avermectin induced liver injury in pigeon: Mechanisms of apoptosis and oxidative stress. Ecotoxicol. Environ. Saf. 2013, 98, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.S.; Liu, C.; Khoso, P.A.; Zheng, W.; Li, M.; Li, S. Autophagy response in the liver of pigeon exposed to avermectin. Environ. Sci. Pollut. Res. 2017, 24, 12767–12777. [Google Scholar] [CrossRef] [PubMed]
- Khaldoun-Oularbi, H.; Richeval, C.; Djenas, N.; Lhermitte, M.; Humbert, L.; Baz, A. Effect of sub-acute exposure to abamectin “insecticide” on liver rats (Rattus norvegicus). Ann. Toxicol. Anal. 2013, 25, 63–70. [Google Scholar] [CrossRef] [Green Version]
- Dadarkar, S.S.; Deore, M.D.; Gatne, M.M. Comparative evaluation of acute toxicity of ivermectin by two methods after single subcutaneous administration in rats. Regul. Toxicol. Pharmacol. 2007, 47, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Lankas, G.R.; Cartwright, M.E.; Umbenhauer, D. P-glycoprotein deficiency in a subpopulation of CF-1 mice enhances avermectin-induced neurotoxicity. Toxicol. Appl. Pharmacol. 1997, 143, 357–365. [Google Scholar] [CrossRef]
- Krolewiecki, A.J.; Alvarez, L.I. Ivermectin for the treatment of soil-transmitted helmithiases. Curr. Treat. Options Infect. Dis. 2019, 11, 252–266. [Google Scholar] [CrossRef]
- Bates, N. Poisons affecting the neurological system. Vet. Nurs. 2020, 11, 116–125. [Google Scholar] [CrossRef]
- Didier, A.; Loor, F. The abamectin derivative ivermectin is a potent P-glycoprotein inhibitor. Anti-Cancer Drugs 1996, 7, 745–751. [Google Scholar] [CrossRef]
- Edwards, G. Ivermectin: Does P-glycoprotein play a role in neurotoxicity? Filaria J. 2003, 2, S8. [Google Scholar] [CrossRef] [Green Version]
- Olsen, R.W.; Snowman, A.M. Avermectin B1a modulation of γ-aminobutyric acid/benzodiazepine receptor binding in mammalian brain. J. Neurochem. 1985, 44, 1074–1082. [Google Scholar] [CrossRef]
- Payne, G.T.; Soderlund, D.M. Activation of γ-aminobutyric acid insensitive chloride channels in mouse brain synaptic vesicles by avermectin B1a. J. Biochem. Toxicol. 1991, 6, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Merola, V.M.; Eubig, P.A. Toxicology of avermectins and milbemycins (macrocylic lactones) and the role of P-glycoprotein in dogs and cats. Vet. Clin. N. Am. Small Anim. Pract. 2012, 42, 313–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.J.; Sun, B.H.; Ping Qu, J.; Xu, S.; Li, S. Avermectin induced inflammation damage in king pigeon brain. Chemosphere 2013, 93, 2528–2534. [Google Scholar] [CrossRef] [PubMed]
- Seaman, J.T.; Eagleson, J.S.; Carrigan, M.J.; Webb, R.F. Avermectin B1 toxicity in a herd of Murray Grey cattle. Aust. Vet. J. 1987, 64, 284–285. [Google Scholar] [CrossRef] [PubMed]
- Kanungo, D.; Pfeil, R. Triforine. In Pesticide Residues in Food 2014—EVALUATIONS 2014, Proceedings of the Joint FAO/WHO Meeting on Pesticide Residues, Rome, Italy, 16–25 September 2014; FAO: Rome, Italy, 2014; pp. 451–514. [Google Scholar]
- Tranquilli, W.J.; Paul, A.J.; Todd, K.S. Assessment of toxicosis induced by high-dose administration of milbemycin oxime in collies. Am. J. Vet. Res. 1991, 52, 1170–1172. [Google Scholar]
- Laube, C.; van den Bos, W.; Fandakova, Y. The relationship between pubertal hormones and brain plasticity: Implications for cognitive training in adolescence. Dev. Cogn. Neurosci. 2020, 42, 100753. [Google Scholar] [CrossRef]
- Celik-Ozenci, C.; Tasatargil, A.; Tekcan, M.; Sati, L.; Gungor, E.; Isbir, M.; Demir, R. Effects of abamectin exposure on male fertility in rats: Potential role of oxidative stress-mediated poly (ADP-ribose) polymerase (PARP) activation. Regul. Toxicol. Pharmacol. 2011, 61, 310–317. [Google Scholar] [CrossRef]
- Dixon, R.L.; Lee, I.P. Possible role of the blood-testicular barrier in dominant lethal testing. Environ. Health Perspect. 1973, 6, 59–63. [Google Scholar] [CrossRef]
- Elbetieha, A.; Da’as, S.I. Assessment of antifertility activities of abamectin pesticide in male rats. Ecotoxicol. Environ. Saf. 2003, 55, 307–313. [Google Scholar] [CrossRef]
- Ferri, R.; Silva, A.T.; Cabral, D.; Moreira, N.; Spinosa, H.S.; Bernardi, M.M. Doramectin reduces sexual behavior and penile erection in male rats. Neurotoxicol. Teratol. 2013, 39, 63–68. [Google Scholar] [CrossRef]
- Lankas, G.R.; Gordon, L.R. Ivermectin and abamectin. Toxicology 1989, 13, 10–142. [Google Scholar]
- Chamberlain, P.L.; Fowler, B.A.; Sexton, M.J.; Peggins, J.O.; von Bredow, J. Preliminary studies of offspring exposure to phenylbutazone and ivermectin during the perinatal period in a Holstein cow–calf model. Toxicol. Appl. Pharmacol. 2003, 187, 198–208. [Google Scholar] [CrossRef]
- Sánchez-Bayo, F. Insecticides mode of action in relation to their toxicity to non-target organisms. J. Environ. Anal. Toxicol. 2012, S4, 2. [Google Scholar] [CrossRef] [Green Version]
- Nassar, A.M.K. Comparative endocrine disrupting effects of abamectin and indoxacarb insecticides. Int. J. Pharmacol. Toxicol. 2016, 4, 89–92. [Google Scholar] [CrossRef] [Green Version]
- European Food Safety Authority (EFSA). Peer review of the pesticide risk assessment of the active substance abamectin. EFSA J. 2016, 14, e04491. [Google Scholar] [CrossRef]
- Moreira, N.; Bernardi, M.M.; Spinosa, H.D.S. Ivermectin reduces sexual behavior in female rats. Neurotoxicol. Teratol. 2014, 43, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.F.; Meligi, N.M. Effects of sublethal abamectin exposure on some hormonal profiles and testicular histopathology in male albino rats and the possible ameliorative role of Eruca sativa. Environ. Sci. Pollut. Res. 2017, 24, 24690–24697. [Google Scholar] [CrossRef] [PubMed]
- Mansour, S.A.; Abbassy, M.A.; Shaldam, H.A. Zinc ameliorate oxidative stress and hormonal disturbance induced by methomyl, abamectin, and their mixture in male rats. Toxics 2017, 5, 37. [Google Scholar] [CrossRef] [Green Version]
- Ewence, A.; Brescia, S.; Johnson, I.; Rumsby, P.C. An approach to the identification and regulation of endocrine disrupting pesticides. Food Chem. Toxicol. 2015, 78, 214–220. [Google Scholar] [CrossRef]
- Prichard, R.K.; Geary, T.G. Perspectives on the utility of moxidectin for the control of parasitic nematodes in the face of developing anthelmintic resistance. Int. J. Parasitol. Drugs Drug Resist. 2019, 10, 69–83. [Google Scholar] [CrossRef]
- Shoop, W.L.; Demontigny, P.; Fink, D.W.; Williams, J.B.; Egerton, J.R.; Mrozik, H.; Fisher, M.H.; Skelly, B.J.; Turner, M.J. Efficacy in sheep and pharmacokinetics in cattle that led to the selection of eprinomectin as a topical endectocide for cattle. Int. J. Parasitol. 1996, 26, 1227–1235. [Google Scholar] [CrossRef]
- Prichard, R.; Menez, C.; Lespine, A. Moxidectin and the avermectins: Consanguinity but not identity. Int. J. Parasitol. Drugs Drug Resist. 2012, 2, 134–153. [Google Scholar] [CrossRef] [PubMed]
- Sturgess-Osborne, C.; Burgess, S.; Mitchell, S.; Wall, R. Multiple resistance to macrocyclic lactones in the sheep scab mite Psoroptes ovis. Vet. Parasitol. 2019, 272, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Redman, E.; Sargison, N.; Whitelaw, F.; Jackson, F.; Morrison, A.; Bartley, D.J.; Gilleard, J.S. Introgression of ivermectin resistance genes into a susceptible Haemonchus contortus strain by multiple backcrossing. PLoS Pathog. 2012, 8, 1002534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howe, K.L.; Bolt, B.J.; Cain, S.; Chan, J.; Chen, W.J.; Davis, P.; Done, J.; Down, T.; Gao, S.; Grove, C.; et al. WormBase 2016: Expanding to enable helminth genomic research. Nucleic Acids Res. 2016, 44, 774–780. [Google Scholar] [CrossRef] [Green Version]
- Laing, R.; Martinelli, A.; Tracey, A.; Holroyd, N.; Gilleard, J.S.; Cotton, J.A. Haemonchus contortus: Genome structure, organization and comparative genomics. Adv. Parasitol. 2016, 93, 569–598. [Google Scholar] [CrossRef]
- Jensen, J.; Scott-Fordsmand, J.J. Ecotoxicity of the veterinary pharmaceutical ivermectin tested in a soil multi-species (SMS) system. Environ. Pollut. 2012, 171, 133–139. [Google Scholar] [CrossRef]
- McKellar, Q.A. Ecotoxicology and residues of anthelmintic compounds. Vet. Parasitol. 1997, 72, 413–435. [Google Scholar] [CrossRef]
- Litskas, V.D.; Karamanlis, X.N.; Batzias, G.C.; Tsiouris, S.E. Are the parasiticidal avermectins resistant to dissipation in the environment? The case of eprinomectin. Environ. Int. 2013, 60, 48–55. [Google Scholar] [CrossRef]
- King, K.L. The potential for avermectins to affect the nutrient economy of grazed pastures. Vet. Parasitol. 1993, 48, 261–271. [Google Scholar] [CrossRef]

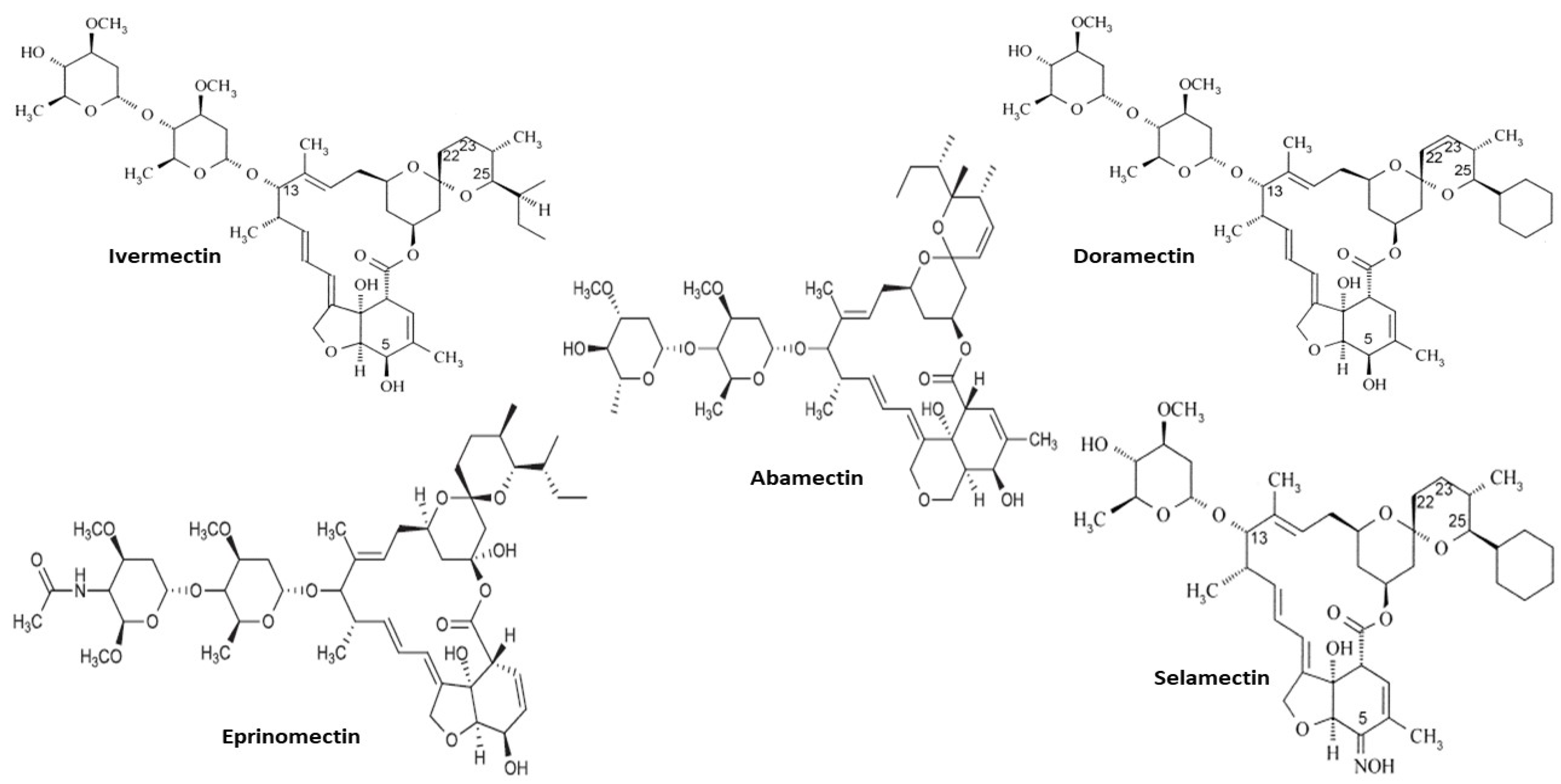

| Drug | Dose | Animal | Outcome | Reference |
|---|---|---|---|---|
| Ivermectin | One drop 1% topically | Bats | Proliferative glomerulonephritis, tubular necrosis | [53] |
| 2 mg/Kg BW (2 injections with 15 days interval) subcutaneously | Goats | Glomerular necrosis, degeneration of tubular epithelium, necrosis of capillary tuft, elevated blood levels of uric acid, urea, creatinine, and glucose | [61] | |
| Weekly 0.5 mg/Kg BW for 8 weeks subcutaneously | Rabbit | Subcapsular tubules vacuolation, glomerular atrophy, elevated serum creatinine | [54] | |
| Weekly 0.4 mg/Kg BW for 4 weeks subcutaneously | Rabbit | Congested blood vessels, tubular degeneration, desquamation and necrosis of tubular epithelium, hyaline casts, leucocytic infiltration, and cystic dilatation of tubules | [62] | |
| 6.5 mg/Kg BW (1/5th of LD50) single dose orally | Mice | Elevated levels of creatinine and urea, renal edema, necrosis and karyorrhexis of tubular epithelium, Bowman’s space narrowing | [28] | |
| Abamectin | 0.181 mg/Kg BW (1/100 of LD50) for 30 days orally | Rats | Elevated levels of creatinine and uric acid, nephritis | [57] |
| 2.18 mg/Kg BW (1/4 of LD50) single dose orally | Rats | Elevated levels of creatinine, uric acid, and urea | [63] | |
| 0.44 mg/Kg BW (1/20 of LD50) for 4 weeks orally | Rats | Elevated levels of creatinine, uric acid and urea, induced oxidative stress, necrosis, congestion, edema and nephritis | [64] | |
| 10 mg/Kg BW for 6 weeks orally | Rats | Elevated levels of creatinine, uric acid and urea, glomerular and tubular necrosis, hemorrhages in cortex | [58] | |
| 30 mg/Kg BW for 30 days orally | Rats | Elevated levels of creatinine and urea, oxidative stress | [51] | |
| 0.1 mg/Kg BW for 15 days Intraperitoneal | Rats | Elevated levels of creatinine and urea, renal degeneration, congested blood vessels and renal casts | [55] | |
| 2 mg/Kg BW (1/100 of LD50) for 5 days, Oral | Rats | Elevated levels of creatinine and urea, edema, hemorrhages, mononuclear cell penetration, glomerular atrophy and tubular necrosis | [56] | |
| Avermectin 1a | 20 mg/Kg feed for 60 days, Oral | Pigeons | Reduced cytochrome P450 concentration, Tubular swelling, vascular degeneration | [65] |
| Drug | Dose | Animal | Outcome | Reference |
|---|---|---|---|---|
| Abamectin | 5 mg/Kg BW | Rats | ↑Serum AST, ↑serum nitric oxide (NO) | [71] |
| 10µM | Rat | ↓Liver mitochondrial respiration, inhibition of ATP synthesis | [66] | |
| 2.13 mg/day per animal orally for 28 days | Rats | ↑Glucose, ↑ASAT, ↑ALAT, Histopathological changes of liver | [75] | |
| 10 mg/Kg BW orally | Rats | ↑ALT, ↑AST, ↑ acid phosphatase (AP), ↑total protein, ↑albumin | [58] | |
| 0.283 nM Inhibition constant | In-vitro goat liver | Carbonic anhydrase inhibition | [40] | |
| 0.4 mg/kg SC | Calves | Liver swollen | [29] | |
| Avermectin 1a | 20 mg/Kg feed | Pigeons | Inhibition of cytochrome P450 enzyme | [65] |
| Avermectin B1a | 20 mg/kg diet | Pigeons | Chromatin aggregation, mitochondrial damage | [73] |
| Doramectin | 0.153 nM Inhibition constant | In-vitro goat liver | Carbonic anhydrase inhibition | [40] |
| Eprinomectin | 0.232 nM Inhibition constant | In-vitro goat liver | Carbonic anhydrase inhibition | [40] |
| Ivermectin | 50 mg/Kg Single dose LD50 | Rats | Congested and haemorrhagic liver with centrilobar necrosis | [76] |
| Drug | Dose | Animal | Outcome | Reference |
|---|---|---|---|---|
| Avermectin | 20 mg/Kg diet | Pigeon | Increased expression of inflammatory factors, histological changes in cerebellum, cerebrum, and optic lobe | [85] |
| Avermectin1a | 20 mg/Kg diet | Pigeon | Oxidative damage shown in brain and serum | [50] |
| Avermectin B1 | 120–200 µg/Kg | Murray Grey cattle | Incoordination, swaying gait, salivation, lingual paralysis and blindness | [86] |
| Abamectin | 6 mg/Kg orally | Rats | Lowered weight of brain, decreased splay reflex, reduced motor activity | [87] |
| 30 mg/Kg orally | Rats | Changes in antioxidant defense markers of brain | [51] | |
| Ivermectin | 120 µg/Kg | Dog | Ataxia, mydriasis, hypersalivation | [88] |
| 0.8 mg/Kg subcutaneously for 8 weeks | Rabbits | Meningitis and brain degeneration | [62] | |
| 1 mg/Kg subcutaneously | Rats | Increased serotonergic and dopaminergic system activity in association with stress | [17] | |
| Doramectin | 200 µg/Kg Subcutaneous | Border collie Dog | Ataxia, fever, tachypnoea, head pressing, hypersalivation, lack of menace response, and blindness | [26] |
| 0.2–0.5 mg/Kg plus horse carcass treated with doramectin | Lion | Ataxia, mydriasis, hallucinations, and death | [27] |
| Drug | Dose | Animal | Outcome | Reference |
|---|---|---|---|---|
| Abamectin | 2.175 mg/Kg orally | Male Rats | ↑WBCs count, ↓RBCs count, ↓haemoglobin, altered serum enzymes levels, reduced sperm count and motility | [63] |
| 10 mg/Kg orally once a week for 210 days | Male Rats | Decreased fertility, reduced number of offspring, histopathological changes in testes, degeneration of spermatogonia cells | [46] | |
| 10 mg/Kg of BW orally | Male Rats | Intratubular edema in testes, degenerated and reduced number of spermatozoa | [58] | |
| Doramectin | 0.3 mg/Kg | Male Rats | Impaired sexual behaviour | [79] |
| 0.2 mg/Kg subcutaneously | Male Rats | Apoptosis of cells, focal degeneration areas in testes, necrotic spermatocytes, and decreased Sertoli cells count | [34] | |
| Ivermectin | 200 µg/Kg subcutaneously | Pregnant Cows | Transfer of drug in milk and colostrum, Accumulation of drug in calf plasma | [95] |
| 0.4 mg/Kg subcutaneously | Rabbits | Thickened testicular capsule, testicular edema, degenerated spermatogenic cells, atritic follicles and degenerated ova, desquamation of uterus glands | [62] | |
| 0.2 mg/Kg subcutaneously | Male Rats | Apoptosis of cells, focal degeneration areas in testes, necrotic spermatocytes, and decreased Sertoli cells count | [34] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salman, M.; Abbas, R.Z.; Mehmood, K.; Hussain, R.; Shah, S.; Faheem, M.; Zaheer, T.; Abbas, A.; Morales, B.; Aneva, I.; et al. Assessment of Avermectins-Induced Toxicity in Animals. Pharmaceuticals 2022, 15, 332. https://doi.org/10.3390/ph15030332
Salman M, Abbas RZ, Mehmood K, Hussain R, Shah S, Faheem M, Zaheer T, Abbas A, Morales B, Aneva I, et al. Assessment of Avermectins-Induced Toxicity in Animals. Pharmaceuticals. 2022; 15(3):332. https://doi.org/10.3390/ph15030332
Chicago/Turabian StyleSalman, Muhammad, Rao Zahid Abbas, Khalid Mehmood, Riaz Hussain, Sehar Shah, Mehwish Faheem, Tean Zaheer, Asghar Abbas, Bernardo Morales, Ina Aneva, and et al. 2022. "Assessment of Avermectins-Induced Toxicity in Animals" Pharmaceuticals 15, no. 3: 332. https://doi.org/10.3390/ph15030332
APA StyleSalman, M., Abbas, R. Z., Mehmood, K., Hussain, R., Shah, S., Faheem, M., Zaheer, T., Abbas, A., Morales, B., Aneva, I., & Martínez, J. L. (2022). Assessment of Avermectins-Induced Toxicity in Animals. Pharmaceuticals, 15(3), 332. https://doi.org/10.3390/ph15030332









