Lyotropic Liquid Crystalline Nanostructures as Drug Delivery Systems and Vaccine Platforms
Abstract
:1. Introduction
2. Self-Assembly and Formation of Liquid Crystalline Nanostructures
3. Engineering of Non-Lamellar Lyotropic Liquid Crystalline Nanoparticles (Cubosomes and Hexosomes)
3.1. Materials and Preparation Methods
3.2. Characterization Methods
3.3. The Influence of the Environmental and Formulation Parameters in the Liquid Crystalline Nanostructure
4. Non-Lamellar Lyotropic Liquid Crystals as Drug Delivery Nanosystems
4.1. Controlling Drug Release Kinetic
4.2. Non-Lamellar Lyotropic Liquid Crystalline Nanosystems (LLCN) for Anticancer Therapy
4.3. Non-Lamellar LLCN Improving Oral Bioavailability
4.4. Non-Lamellar LLCN for Skin Administration
4.5. Non-Lamellar LLCN for Ocular, Brain, and Pulmonary Delivery
4.6. Injectable Non-Lamellar Liquid Crystalline Depot Systems for Sustained Delivery
4.7. Non-Lamellar LLCN as Vaccines
5. Development of Stimuli-Responsive Non-Lamellar LLCN
5.1. pH-Responsive Non-Lamellar LLCN
5.1.1. pH-Responsive Non-Lamellar LLCN Employing pH-Responsive Polymers
5.1.2. pH-Responsive Non-Lamellar LLCN Employing pH-Responsive Molecules (Lipid or Surfactant)
5.2. Thermoresponsive Non-Lamellar LLCN
5.3. Dual Stimuli-Responsive (pH and Temperature) Non-Lamellar LLCN
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Azmi, I.D.M.; Moghimi, S.M.; Yaghmur, A. Cubosomes and hexosomes as versatile platforms for drug delivery. Ther. Deliv. 2015, 6, 1347–1364. [Google Scholar] [CrossRef] [PubMed]
- Mulet, X.; Boyd, B.J.; Drummond, C.J. Advances in drug delivery and medical imaging using colloidal lyotropic liquid crystalline dispersions. J. Colloid Interface Sci. 2013, 393, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, C.V. Lipid Self-Assemblies and Nanostructured Emulsions for Cosmetic Formulations. Cosmetics 2016, 3, 37. [Google Scholar] [CrossRef] [Green Version]
- Israelachvili, J.N. Intermolecular and Surfaces Forces, 2nd ed.; Academic Press: Cambridge, MA, USA, 1991. [Google Scholar]
- Jonsson, B.; Lindman, B.; Holmberg, K.; Kronberg, B. Surfactants and Polymers in Aqueous Solutions; Wiley: Chichester, UK, 2001. [Google Scholar]
- Karami, Z.; Hamidi, M. Cubosomes: Remarkable drug delivery potential. Drug Discov. Today 2016, 21, 789–801. [Google Scholar] [CrossRef]
- Lancelot, A.; Sierra, T.; Serrano, J.L. Nanostructured liquid-crystalline particles for drug delivery. Expert Opin. Drug Deliv. 2014, 11, 547–564. [Google Scholar] [CrossRef]
- van ‘t Hag, L.; Gras, S.L.; Conn, C.E.; Drummond, C.J. Lyotropic liquid crystal engineering moving beyond binary compositional space—Ordered nanostructured amphiphile self-assembly materials by design. Chem. Soc. Rev. 2017, 46, 2705–2731. [Google Scholar] [CrossRef]
- Chountoulesi, M.; Pippa, N.; Pispas, S.; Chrysina, E.D.; Forys, A.; Trzebicka, B.; Demetzos, C. Cubic lyotropic liquid crystals as drug delivery carriers: Physicochemical and morphological studies. Int. J. Pharm. 2018, 550, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Seddon, J.M.; Templer, R.H. Cubic phases of self-assembled amphiphilic aggregates. Philos. Trans. R. Soc. A 1993, 344, 377–401. [Google Scholar]
- Briggs, J.; Chung, H.; Caffrey, M. The temperature-composition phase diagram and mesophase structure characterization of the monoolein/water system. J. Phys. II 1996, 6, 723–751. [Google Scholar] [CrossRef] [Green Version]
- Barauskas, J.; Misiunas, A.; Gunnarsson, T.; Tiberg, F.; Johnsson, M. “Sponge” Nanoparticle Dispersions in Aqueous Mixtures of Diglycerol Monooleate, Glycerol Dioleate, and Polysorbate 80. Langmuir 2006, 22, 6328–6334. [Google Scholar] [CrossRef]
- Rakotoarisoa, M.; Angelov, B.; Espinoza, S.; Khakurel, K.; Bizien, T.; Angelova, A. Cubic Liquid Crystalline Nanostructures Involving Catalase and Curcumin: BioSAXS Study and Catalase Peroxidatic Function after Cubosomal Nanoparticle Treatment of Differentiated SH-SY5Y Cells. Molecules 2019, 24, 3058. [Google Scholar] [CrossRef] [Green Version]
- Kulkarni, C.V.; Wachter, W.; Iglesias-Salto, G.; Engelskirchen, S.; Ahualli, S. Monoolein: A magic lipid? Phys. Chem. Chem. Phys. 2011, 13, 3004–3021. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, P.; Gui, S. Cubic and Hexagonal Liquid Crystals as Drug Delivery Systems. Biomed. Res. Int. 2014, 2014, 815981. [Google Scholar] [CrossRef] [PubMed]
- Hinton, T.M.; Grusche, F.; Achary, D.; Shukla, R.; Bansal, V.; Waddington, L.J.; Monaghan, P.; Muir, B.W. Bicontinuous cubic phase nanoparticle lipid chemistry affects toxicity in cultured cells. Toxicol. Res. 2014, 3, 11–22. [Google Scholar] [CrossRef]
- Chong, J.Y.T.; Mulet, X.; Boyd, B.J.; Drummond, C.J. Steric Stabilizers for Cubic Phase Lyotropic Liquid Crystal Nanodispersions (Cubosomes). In Advances in Planar Lipid Bilayers Liposomes, 1st ed.; Iglič, A., Kulkarni, C.V., Rappolt, M., Eds.; Elsevier: Waltham, MA, USA, 2015; pp. 131–187. [Google Scholar]
- Chong, J.Y.T.; Mulet, X.; Waddington, L.J.; Boyd, B.J.; Drummond, C.J. Steric stabilisation of self-assembled cubic lyotropic liquid crystalline nanoparticles: High throughput evaluation of triblock polyethylene oxide-polypropylene oxide-polyethylene oxide copolymers. Soft Matter 2011, 7, 4768–4777. [Google Scholar] [CrossRef]
- Chong, J.Y.T.; Mulet, X.; Waddington, L.J.; Boyd, B.J.; Drummond, C.J. High-Throughput Discovery of Novel Steric Stabilizers for Cubic Lyotropic Liquid Crystal Nanoparticle Dispersions. Langmuir 2012, 28, 9223–9232. [Google Scholar] [CrossRef]
- Zhai, J.; Hinton, T.M.; Waddington, L.J.; Fong, C.; Tran, N.; Mulet, X.; Drummond, C.J.; Muir, B.W. Lipid-PEG Conjugates Sterically Stabilize and Reduce the Toxicity of Phytantriol-Based Lyotropic Liquid Crystalline Nanoparticles. Langmuir 2015, 31, 10871–10880. [Google Scholar] [CrossRef]
- Chountoulesi, M.; Perinelli, D.R.; Forys, A.; Bonacucina, G.; Trzebicka, B.; Pispas, S.; Demetzos, C. Liquid crystalline nanoparticles for drug delivery: The role of gradient and block copolymers on the morphology, internal organisation and release profile. Eur. J. Pharm. Biopharm. 2021, 158, 21–34. [Google Scholar] [CrossRef]
- Zabara, M.; Senturk, B.; Gontsarik, M.; Ren, Q.; Rottmar, M.; Maniura-Weber, K.; Mezzenga, R.; Bolisetty, S.; Salentinig, S. Multifunctional nano-biointerfaces: Cytocompatible antimicrobial nanocarriers from stabilizer-free cubosomes. Adv. Funct. Mater. 2019, 29, 1904007. [Google Scholar] [CrossRef]
- Spicer, P.T.; Hayden, K.L. Novel Process for Producing Cubic Liquid Crystalline Nanoparticles (Cubosomes). Langmuir 2001, 17, 5748–5756. [Google Scholar] [CrossRef]
- Akhlaghi, S.P.; Ribeiro, I.R.; Boyd, B.J.; Loh, W. Impact of Preparation Method and Variables on the Internal Structure, Morphology, and Presence of Liposomes in Phytantriol-Pluronic® F127 Cubosomes. Colloids Surf. B Biointerfaces 2016, 145, 845–853. [Google Scholar] [CrossRef]
- Angelova, A.; Garamus, V.M.; Angelov, B.; Tian, Z.; Li, Y.; Zou, A. Advances in structural design of lipid-based nanoparticle carriers for delivery of macromolecular drugs, phytochemicals and antitumor agents. Adv. Colloid Interface Sci. 2017, 249, 331–345. [Google Scholar] [CrossRef]
- Rajabalaya, R.; Musa, M.N.; Kifli, N.; David, S.R. Oral and transdermal drug delivery systems: Role of lipid-based lyotropic liquid crystals. Drug Des. Dev. Ther. 2011, 11, 393–406. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.H.; Lim, S.; Shim, J.; Song, E.J.; Chang, J.S.; Jin, K.S.; Cho, E.C. A Simple Evaporation Method for the Large Scale Production of Liquid Crystalline Lipid Nanoparticles with Various Internal Structures. ACS Appl. Mater. Interfaces 2015, 7, 20438–20446. [Google Scholar] [CrossRef]
- Kuntsche, J.; Horst, J.C.; Bunjes, H. Cryogenic transmission electron microscopy (cryo-TEM) for studying the morphology of colloidal drug delivery systems. Int. J. Pharm. 2011, 417, 120–137. [Google Scholar] [CrossRef]
- Boyd, B.J.; Rizwan, S.B.; Dong, Y.D.; Hook, S.; Rades, T. Self-assembled geometric liquid-crystalline nanoparticles imaged in three dimensions: Hexosomes are not necessarily flat hexagonal prisms. Langmuir 2007, 23, 12461–12464. [Google Scholar] [CrossRef] [PubMed]
- Chountoulesi, M.; Perinelli, D.R.; Pippa, N.; Chrysostomou, V.; Forys, A.; Otulakowski, L.; Bonacucina, G.; Trzebicka, B.; Pispas, S.; Demetzos, C. Physicochemical, morphological and thermal evaluation of lyotropic lipidic liquid crystalline nanoparticles: The effect of stimuli-responsive polymeric stabilizer. Colloids Surf. A Physicochem. Eng. Asp. 2020, 595, 124678. [Google Scholar] [CrossRef]
- Pippa, N.; Dokoumetzidis, A.; Demetzos, C.; Macheras, P. On the ubiquitous presence of fractals and fractal concepts in pharmaceutical sciences: A review. Int. J. Pharm. 2013, 456, 340–352. [Google Scholar] [CrossRef]
- Pippa, N.; Merkouraki, M.; Pispas, S.; Demetzos, C. DPPC:MPOx chimeric advanced Drug Delivery nanosystems (chi-aDDnSs): Physicochemical and structural characterization, stability and drug release studies. Int. J. Pharm. 2013, 450, 1–10. [Google Scholar] [CrossRef]
- Pippa, N.; Kaditi, E.; Pispas, S.; Demetzos, C. DPPC/poly(2-methyl-2- oxazoline)-grad-poly(2-phenyl-2-oxazoline) chimeric nanostructures as potential drug nanocarriers. J. Nanopart. Res. 2013, 15, 1685. [Google Scholar] [CrossRef]
- Pippa, N.; Pispas, S.; Demetzos, C. The fractal hologram and elucidation of the structure of liposomal carriers in aqueous and biological media. Int. J. Pharm. 2012, 430, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Pippa, N.; Pispas, S.; Demetzos, C. The delineation of the morphology of charged liposomal vectors via a fractal analysis in aqueous and biological media: Physicochemical and self-assembly studies. Int. J. Pharm. 2012, 437, 264–274. [Google Scholar] [CrossRef]
- Pippa, N.; Psarommati, F.; Pispas, S.; Demetzos, C. The shape/morphology balance: A study of stealth liposomes via fractal analysis and drug encapsulation. Pharm. Res. 2013, 30, 2385–2395. [Google Scholar] [CrossRef]
- Fong, C.; Zhai, J.; Drummond, C.J.; Tran, N. Micellar Fd3m cubosomes from monoolein—long chain unsaturated fatty acid mixtures: Stability on temperature and pH response. J. Colloid Interface Sci. 2020, 566, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Chountoulesi, M.; Pippa, N.; Chrysostomou, V.; Pispas, S.; Chrysina, E.D.; Forys, A.; Otulakowski, L.; Trzebicka, B.; Demetzos, C. Stimuli-Responsive Lyotropic Liquid Crystalline Nanosystems with Incorporated Poly(2-Dimethylamino Ethyl Methacrylate)-b-Poly(Lauryl Methacrylate) Amphiphilic Block Copolymer. Polymers 2019, 11, 1400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.; Dong, Y.D.; Hanley, T.L.; Boyd, B.J. Sensitivity of Nanostructure in Charged Cubosomes to Phase Changes Triggered by Ionic Species in Solution. Langmuir 2013, 29, 14265–14273. [Google Scholar] [CrossRef]
- Awad, T.S.; Okamoto, Y.; Masum, S.M.; Yamazaki, M. Formation of cubic phases from large unilamellar vesicles of dioleoylphosphatidylglycerol/monoolein membranes induced by low concentrations of Ca2+. Langmuir 2005, 21, 11556–11561. [Google Scholar] [CrossRef]
- Negrini, R.; Mezzenga, R. pH-Responsive Lyotropic Liquid Crystals for Controlled Drug Delivery. Langmuir 2011, 27, 5296–5303. [Google Scholar] [CrossRef]
- Kluzek, M.; Tyler, A.I.I.; Wang, S.; Chen, R.; Marques, C.; Thalmann, F.; Seddon, J.; Schmutz, M. Influence of a pH-sensitive polymer on the structure of monoolein cubosomes. Soft Matter 2017, 13, 7571–7577. [Google Scholar] [CrossRef] [PubMed]
- Azmi, I.D.M.; Wu, L.; Wibroe, P.P.; Nilsson, C.; Østergaard, J.; Stürup, S.; Gammelgaard, B.; Urtti, A.; Moghimi, S.M.; Yaghmur, A. Modulatory effect of human plasma on the internal nanostructure and size characteristics of liquid-crystalline nanocarriers. Langmuir 2015, 31, 5042–5049. [Google Scholar] [CrossRef] [PubMed]
- Azmi, I.D.M.; Wibroe, P.P.; Wu, L.P.; Kazem, A.I.; Amenitsch, H.; Moghimi, S.M.; Yaghmur, A. A structurally diverse library of safe-by-design citrem-phospholipid lamellar and non-lamellar liquid crystalline nano-assemblies. J. Control. Release 2016, 239, 1–9. [Google Scholar] [CrossRef]
- Tan, A.; Lam, Y.Y.; Sun, X.; Boyd, B. Monocytic Cell-Induced Phase Transformation of Circulating Lipid-Based Liquid Crystalline Nanosystems. Materials 2020, 13, 1013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, Y.D.; Larson, I.; Barnes, T.J.; Prestidge, C.A.; Allen, S.; Chen, X.; Roberts, C.J.; Boyd, B.J. Understanding the Interfacial Properties of Nanostructured Liquid Crystalline Materials for Surface-Specific Delivery Applications. Langmuir 2012, 28, 13485–13495. [Google Scholar] [CrossRef]
- Tan, A.; Hong, L.; Du, J.D.; Boyd, B.J. Self-Assembled Nanostructured Lipid Systems: Is There a Link between Structure and Cytotoxicity? Adv. Sci. 2019, 6, 1801223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, Y.D.; Larson, I.; Hanley, T.; Boyd, B.J. Bulk and Dispersed Aqueous Phase Behavior of Phytantriol: Effect of Vitamin E Acetate and F127 Polymer on Liquid Crystal Nanostructure. Langmuir 2006, 22, 9512–9518. [Google Scholar] [CrossRef] [PubMed]
- Yaghmur, A.; Tran, B.V.; Moghimi, S.M. Non-Lamellar Liquid Crystalline Nanocarriers for Thymoquinone Encapsulation. Molecules 2020, 25, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Liang, X.; Ma, P.; Tao, Y.; Wu, X.; Wu, X.; Chu, X.; Gui, S. Phytantriol-based in situ liquid crystals with long-term release for intra-articular administration. AAPS PharmSciTech 2015, 16, 846–854. [Google Scholar] [CrossRef] [Green Version]
- Mionić Ebersold, M.; Petrović, M.; Fong, W.K.; Bonvin, D.; Hofmann, H.; Milošević, I. Hexosomes with Undecylenic Acid Efficient against Candida albicans. Nanomaterials 2018, 8, 91. [Google Scholar] [CrossRef] [Green Version]
- Akbar, S.; Anwar, A.; Ayish, A.; Elliott, J.M.; Squires, A.M. Phytantriol based smart nano-carriers for drug delivery applications. Eur. J. Pharm. Sci. 2017, 101, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.; Milleret, G.; Nagaraj, M. Liquid crystal nanoparticles for commercial drug delivery. Liq. Cryst. Rev. 2017, 5, 69–85. [Google Scholar] [CrossRef]
- Nazaruk, E.; Majkowska-Pilip, A.; Bilewicz, R. Lipidic Cubic-Phase Nanoparticles—Cubosomes for Efficient Drug Delivery to Cancer Cells. Chem. Plus Chem. 2017, 82, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.Y.; Nguyen, T.H.; Hanley, T.; Boyd, B.J. Nanostructure of liquid crystalline matrix determines in vitro sustained release and in vivo oral absorption kinetics for hydrophilic model drugs. Int. J. Pharm. 2009, 365, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Boyd, B.J.; Whittaker, D.V.; Khoo, S.M.; Davey, G. Lyotropic Liquid Crystalline Phases Formed from Glycerate Surfactants as Sustained Release Drug Delivery Systems. Int. J. Pharm. 2006, 309, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Esposito, E.; Cortesi, R.; Drechsler, M.; Paccamiccio, L.; Mariani, P.; Contado, C.; Stellin, E.; Menegatti, E.; Bonina, F.; Puglia, C. Cubosome dispersions as delivery systems for percutaneous administration of indomethacin. Pharm. Res. 2005, 22, 2163–2173. [Google Scholar] [CrossRef]
- Clogston, J.; Craciun, G.; Hart, D.J.; Caffrey, M. Controlling Release from the Lipidic Cubic Phase by Selective Alkylation. J. Control. Release 2005, 102, 441–461. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, C.V.; Vishwapathi, V.K.; Quarshie, A.; Moinuddin, Z.; Page, J.; Kendrekar, P.; Mashele, S.S. Self-Assembled Lipid Cubic Phase and Cubosomes for the Delivery of a Model Drug (Aspirin). Langmuir 2017, 33, 9907–9915. [Google Scholar] [CrossRef] [PubMed]
- Mansour, M.; Kamel, A.O.; Mansour, S.; Mortada, N.D. Novel polyglycerol-dioleate based cubosomal dispersion with tailored physical characteristics for controlled delivery of ondansetron. Colloids Surf. B Biointerfaces 2017, 156, 44–54. [Google Scholar] [CrossRef]
- Sagalowicz, L.; Mezzenga, R.; Leser, M.E. Investigating reversed liquid crystalline mesophases. Curr. Opin. Colloid Interface Sci. 2006, 11, 224–229. [Google Scholar] [CrossRef] [Green Version]
- Cribier, S.; Gulik, A.; Fellmann, P.; Vargas, R.; Devaux, P.F.; Luzzati, V. Cubic phases of lipid containing systems. A translational diffusion study by fluorescence recovery after photobleaching. J. Mol. Biol. 1993, 229, 517–525. [Google Scholar] [CrossRef]
- Fong, W.K.; Hanley, T.; Boyd, B.J. Stimuli responsive liquid crystals provide ’on demand’ drug delivery in vitro and in vivo. J. Control. Release 2009, 135, 218–226. [Google Scholar] [CrossRef]
- Phan, S.; Fong, W.K.; Kirby, N.; Hanley, T.; Boyd, B.J. Evaluating the link between self-assembled mesophase structure and drug release. Int. J. Pharm. 2011, 421, 176–182. [Google Scholar] [CrossRef]
- Boyd, B.J. Characterisation of Drug Release from Cubosomes Using the Pressure Ultrafiltration Method. Int. J. Pharm. 2003, 260, 239–247. [Google Scholar] [CrossRef]
- Yaghmur, A.; Laggner, P.; Sartori, B.; Rappolt, M. Calcium triggered Lα-H2 phase transition monitored by combined rapid mixing and time-resolved synchrotron SAXS. PLoS ONE 2008, 3, e2072. [Google Scholar] [CrossRef] [PubMed]
- Muir, B.W.; Zhen, G.; Gunatillake, P.; Hartley, P.G. Salt induced lamellar to bicontinuous cubic phase transitions in cationic nanoparticles. J. Phys. Chem. B 2012, 116, 3551–3556. [Google Scholar] [CrossRef] [PubMed]
- Leal, C.; Bouxsein, N.F.; Ewert, K.K.; Safinya, C.R. Highly Efficient Gene Silencing Activity of siRNA Embedded in a Nanostructured Gyroid Cubic Lipid Matrix. J. Am. Chem. Soc. 2010, 132, 16841–16847. [Google Scholar] [CrossRef] [Green Version]
- Astolfi, P.; Giorgini, E.; Adamo, F.C.; Vita, F.; Logrippo, S.; Francescangeli, O.; Pisani, M. Effects of a cationic surfactant incorporation in phytantriol bulk cubic phases and dispersions loaded with the anticancer drug 5-fluorouracil. J. Mol. Liq. 2019, 286, 110954. [Google Scholar] [CrossRef]
- Zabara, A.; Negrini, N.; Baumann, P.; Onaca-Fischer, O.; Mezzenga, R. Reconstitution of OmpF membrane protein on bended lipid bilayers: Perforated hexagonal mesophases. Chem. Commun. 2014, 50, 2642–2645. [Google Scholar] [CrossRef]
- Engblom, J.; Miezis, Y.; Nylander, T.; Razumas, V.; Larsson, K. On the swelling of monoolein liquid-crystalline aqueous phases in the presence of distearoylphosphatidylglycerol. Prog. Colloid Polym. Sci. 2000, 116, 9–15. [Google Scholar]
- Angelov, B.; Angelova, A.; Ollivon, M.; Bourgaux, C.; Campitelli, A. Diamond-type lipid cubic phase with large water channels. J. Am. Chem. Soc. 2003, 125, 7188–7189. [Google Scholar] [CrossRef] [PubMed]
- Negrini, R.; Mezzenga, R. Diffusion, molecular separation, and drug delivery from lipid mesophases with tunable water channels. Langmuir 2012, 28, 16455–16462. [Google Scholar] [CrossRef]
- Barriga, H.M.G.; Ces, O.; Law, R.V.; Seddon, J.M.; Brooks, N.J. Engineering swollen cubosomes using cholesterol and anionic lipids. Langmuir 2019, 35, 16521–16527. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Tran, N.; Rashid, M.H.; Le, T.C.; Yarovsky, I.; Conn, C.E.; Drummond, C.J. Toward cell membrane biomimetic lipidic cubic phases: A high-throughput exploration of lipid compositional space. ACS Appl. Bio Mater. 2019, 2, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Zabara, A.; Chong, J.T.Y.; Martiel, I.; Stark, L.; Cromer, B.A.; Speziale, C.; Drummond, C.J.; Mezzenga, R. Design of ultra-swollen lipidic mesophases for the crystallization of membrane proteins with large extracellular domains. Nat. Commun. 2018, 9, 544. [Google Scholar] [CrossRef] [Green Version]
- Cervin, C.; Tinzl, M.; Johnsson, M.; Abrahamsson, P.A.; Tiberg, F.; Dizeyi, N. Properties and Effects of a Novel Liquid Crystal Nanoparticle Formulation of Docetaxel in a Prostate Cancer Mouse Model. Eur. J. Pharm. Sci. 2010, 41, 369–375. [Google Scholar] [CrossRef]
- Jain, V.; Swarnakar, N.K.; Mishra, P.R.; Verma, A.; Kaul, A.; Mishra, A.K.; Jain, N.K. Paclitaxel Loaded PEGylated Gleceryl Monooleate Based Nanoparticulate Carriers in Chemotherapy. Biomaterials 2012, 33, 7206–7220. [Google Scholar] [CrossRef]
- Zhai, J.; Luwor, R.B.; Ahmed, N.; Escalona, R.; Tan, F.H.; Fong, C.; Ratcliffe, J.; Scoble, J.A.; Drummond, C.J.; Tran, N. Paclitaxel-Loaded Self-Assembled Lipid Nanoparticles as Targeted Drug Delivery Systems for the Treatment of Aggressive Ovarian Cancer. ACS Appl. Mater. Interfaces 2018, 10, 25174–25185. [Google Scholar] [CrossRef] [PubMed]
- Zeng, N.; Gao, X.; Hu, Q.; Song, Q.; Xia, H.; Liu, Z.; Gu, G.; Jiang, M.; Pang, Z.; Chen, H.; et al. Lipid-based liquid crystalline nanoparticles as oral drug delivery vehicles for poorly water-soluble drugs: Cellular interaction and in vivo absorption. Int. J. Nanomed. 2012, 7, 3703–3718. [Google Scholar]
- Nasr, M.; Ghorab, M.K.; Abdelazem, A. In Vitro and In Vivo Evaluation of Cubosomes Containing 5-Fluorouracil for Liver Targeting. Acta Pharm. Sin. B 2015, 5, 79–88. [Google Scholar] [CrossRef] [Green Version]
- Caltagirone, C.; Falchi, A.M.; Lampis, S.; Lippolis, V.; Meli, V.; Monduzzi, M.; Prodi, L.; Schmidt, J.; Sgarzi, M.; Talmon, Y.; et al. Cancer-Cell-Targeted Theranostic Cubosomes. Langmuir 2014, 30, 6228–6236. [Google Scholar] [CrossRef]
- Zhang, L.; Li, J.; Tian, D.; Sun, L.; Wang, X.; Tian, M. Theranostic combinatorial drug-loaded coated cubosomes for enhanced targeting and efficacy against cancer cells. Cell Death Dis. 2020, 11, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Astolfi, P.; Giorgini, E.; Gambini, V.; Rossi, B.; Vaccari, L.; Vita, F.; Francescangeli, O.; Marchini, C.; Pisani, M. Lyotropic Liquid-Crystalline Nanosystems as Drug Delivery Agents for 5-Fluorouracil: Structure and Cytotoxicity. Langmuir 2017, 33, 12369–12378. [Google Scholar] [CrossRef]
- Gong, X.; Moghaddam, M.J.; Sagnella, S.M.; Conn, C.E.; Mulet, X.; Danon, S.J.; Waddington, L.J.; Drummond, C.J. Nanostructured self-assembly materials from neat and aqueous solutions of C18 lipid pro-drug analogues of capecitabine—A chemotherapy agent. Focus on nanoparticulate cubosomesTM of the oleyl analogue. Soft Matter 2011, 7, 5764–5776. [Google Scholar] [CrossRef]
- Sagnella, S.M.; Gong, X.; Moghaddam, M.J.; Conn, C.E.; Kimpton, K.; Waddington, L.J.; Krodkiewska, I.; Drummond, C.J. Nanostructured nanoparticles of self-assembled lipid pro-drugs as a route to improved chemotherapeutic agents. Nanoscale 2011, 3, 919–924. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Tan, Y.; Chen, M.; Dian, L.; Shan, Z.; Peng, X.; Wu, C. Development of amphotericin B-loaded cubosomes through the SolEmuls technology for enhancing the oral bioavailability. AAPS PharmSciTech 2012, 13, 1483–1491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, T.H.; Hanley, T.; Porter, C.J.H.; Boyd, B.J. Nanostructured liquid crystalline particles provide long duration sustained-release effect for a poorly water soluble drug after oral administration. J. Control. Release 2011, 153, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Kim, J.; Um, Y.J.; Kwon, C.I.; Jeong, Y.S. Self-Assembled “Nanocubicle” as a Carrier for Peroral Insulin Delivery. Diabetologia 2002, 45, 448–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, J.; Wu, W.; Lu, Y.; Yin, Z.; Hu, F. Pharmacokinetics and Enhanced Oral Bioavailability in Beagle Dogs of Cyclosporine a Encapsulated in Glyceryl Monooleate/Poloxamer 407 Cubic Nanoparticles. Int. J. Nanomed. 2010, 5, 13–23. [Google Scholar]
- Nasr, M.; Dawoud, M. Sorbitol Based Powder Precursor of Cubosomes as an Oral Delivery System for Improved Bioavailability of Poorly Water Soluble Drugs. J. Drug Deliv. Sci. Technol. 2016, 35, 106–113. [Google Scholar] [CrossRef]
- Ali, M.A.; Kataoka, N.; Ranneh, A.H.; Iwao, Y.; Noguchi, S.; Oka, T.; Itai, S. Enhancing the Solubility and Oral Bioavailability of Poorly Water-Soluble Drugs Using Monoolein Cubosomes. Chem. Pharm. Bull. 2017, 65, 42–48. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.W.; Chen, M.W.; Yang, M.H.; Chen, J.; Fang, W.J.; Xu, P. Evaluating the Potential of Cubosomal Nanoparticles for Oral Delivery of Amphotericin B in Treating Fungal Infection. Int. J. Nanomed. 2014, 9, 327–336. [Google Scholar]
- von Halling Laier, C.; Gibson, B.; Moreno, J.A.S.; Rades, T.; Hook, S.; Nielsen, L.H.; Boisen, A. Microcontainers for protection of oral vaccines, in vitro and in vivo evaluation. J. Control. Release 2019, 294, 91–101. [Google Scholar] [CrossRef] [Green Version]
- Swarnakar, N.K.; Thanki, K.; Jain, S. Enhanced antitumor efficacy and counterfeited cardiotoxicity of combinatorial oral therapy using doxorubicin- and coenzyme Q10-liquid crystalline nanoparticles in comparison with intravenous adriamycin. Nanomedicine 2014, 10, 1231–1241. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Chen, H.; Huang, Z.; Zhu, J.; Wan, F.; Peng, T.; Pan, X.; Huang, Y.; Wu, C. Taste-masking and colloidal-stable cubosomes loaded with Cefpodoxime proxetil for pediatric oral delivery. Int. J. Pharm. 2020, 575, 118875. [Google Scholar] [CrossRef] [PubMed]
- Rarokar, N.R.; Saoji, S.D.; Raut, N.A.; Taksande, J.B.; Khedekar, P.B.; Dave, V.S. Nanostructured cubosomes in a thermoresponsive depot system: An alternative approach for the controlled delivery of docetaxel. AAPS PharmSciTech 2015, 17, 436–445. [Google Scholar] [CrossRef] [Green Version]
- Lapteva, M.; Kalia, Y.N. Microstructured Bicontinuous Phase Formulations: Their Characterization and Application in Dermal and Transdermal Drug Delivery. Expert Opin. Drug Delivery 2013, 10, 1043–1059. [Google Scholar] [CrossRef] [PubMed]
- Morsi, N.M.; Abdelbary, G.A.; Ahmed, M.A. Silver Sulfadiazine Based Cubosome Hydrogels for Topical Treatment of Burns: Development and In Vitro/In Vivo Characterization. Eur. J. Pharm. Biopharm. 2014, 86, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, V.; Korat, V.; Baldaniya, L.; Gohel, M.; Gandhi, T.; Patel, N. Development and Characterization of Novel Hydrogel Containing Antimicrobial Drug for Treatment of Burns. Int. J. Pharm. Investig. 2016, 6, 158–168. [Google Scholar] [PubMed] [Green Version]
- Boge, L.; Hallstensson, K.; Ringstad, L.; Johansson, J.; Andersson, T.; Davoudi, M.; Larsson, P.T.; Mahlapuu, M.; Håkansson, J.; Andersson, M. Cubosomes for topical delivery of the antimicrobial peptide LL-37. Eur. J. Pharm. Biopharm. 2019, 134, 60–67. [Google Scholar] [CrossRef]
- Bender, J.; Ericson, M.B.; Merclin, N.; Iani, V.; Rosen, A.; Engstrom, S.; Moan, J. Lipid Cubic Phases for Improved Topical Drug Delivery in Photodynamic Therapy. J. Control. Release 2005, 106, 350–360. [Google Scholar] [CrossRef]
- Yu, X.; Du, L.N.; Zhu, L.F.; Liu, X.Y.; Zhang, B.L.; Fu, G.Y.; Jin, Y.G. Melanoma Therapy with Transdermal Mitoxantrone Cubic Phases. Drug Deliv. 2016, 23, 1565–1570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bazylińska, U.; Kulbacka, J.; Schmidt, J.; Talmon, Y.; Murgia, S. Polymer-free cubosomes for simultaneous bioimaging and photodynamic action of photosensitizers in melanoma skin cancer cells. J. Colloid. Interface Sci. 2018, 522, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Salah, S.; Mahmoud, A.A.; Kamel, A.O. Etodolac Transdermal Cubosomes for the Treatment of Rheumatoid Arthritis: Ex Vivo Permeation and in Vivo Pharmacokinetic Studies. Drug Deliv. 2017, 24, 846–856. [Google Scholar] [CrossRef] [Green Version]
- Im, T.J.; Kang, M.J.; Seo, D.W.; Lee, J. Effect of Cubic Liquid Crystalline Systems on Skin Localization of Oregonin and Hirsutanonol. Biomol. Ther. 2008, 16, 226–230. [Google Scholar] [CrossRef] [Green Version]
- Seo, S.R.; Kang, G.; Ha, J.W.; Kim, J.C. In Vivo Hair Growth- Promoting Efficacies of Herbal Extracts and Their Cubosomal Suspensions. J. Ind. Eng. Chem. 2013, 19, 1331–1339. [Google Scholar] [CrossRef]
- Lopes, L.B.; Ferreira, D.A.; de Paula, D.; Garcia, M.T.J.; Thomazini, J.A.; Fantini, M.C.A.; Bentley, M.V.L. Reverse Hexagonal Phase Nanodispersion of Monoolein and Oleic Acid for Topical Delivery of Peptides: In Vitro and In Vivo Skin Penetration of Cyclosporin, A. Pharm. Res. 2006, 23, 1332–1342. [Google Scholar] [CrossRef]
- de Carvalho Vicentini, F.T.M.; Depieri, L.V.; Polizello, A.C.M.; Del Ciampo, J.O.; Spadaro, A.C.C.; Fantini, M.C.A.; Vitória Lopes Badra Bentley, M. Liquid crystalline phase nanodispersions enable skin delivery of siRNA. Eur. J. Pharm. Biopharm. 2013, 83, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Estracanholli, E.A.; Praça, F.S.G.; Cintra, A.B.; Pierre, M.B.R.; Lara, M.G. Liquid crystalline systems for transdermal delivery of celecoxib: In Vitro drug release and skin permeation studies. AAPS PharmSciTech 2014, 15, 1468–1475. [Google Scholar] [CrossRef] [Green Version]
- Muller-Goymann, C.C. Physicochemical characterization of colloidal drug delivery systems such as reverse micelles, vesicles, liquid crystals and nanoparticles for topical administration. Eur. J. Pharm. Biopharm. 2004, 58, 343–356. [Google Scholar] [CrossRef]
- Gan, L.; Han, S.; Shen, J.; Zhu, J.; Zhu, C.; Zhang, X.; Gan, Y. Self-Assembled Liquid Crystalline Nanoparticles as a Novel Ophthalmic Delivery System for Dexamethasone: Improving Preocular Retention and Ocular Bioavailability. Int. J. Pharm. 2010, 396, 179–187. [Google Scholar] [CrossRef]
- Han, S.; Shen, J.Q.; Gan, Y.; Geng, H.M.; Zhang, X.X.; Zhu, C.L.; Gan, L. Novel Vehicle Based on Cubosomes for Ophthalmic Delivery of Flurbiprofen with Low Irritancy and High Bioavailability. Acta Pharmacol. Sin. 2010, 31, 990–998. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, L.; Wu, W.; Wang, B.; Wang, Z.; Xin, H.; Xu, Q. A Potential Carrier Based on Liquid Crystal Nanoparticles for Ophthalmic Delivery of Pilocarpine Nitrate. Int. J. Pharm. 2013, 455, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Li, J.; Wu, L.; Wang, B.; Wang, Z.; Xu, Q.; Xin, H. Ophthalmic Delivery of Brinzolamide by Liquid Crystalline Nanoparticles: In Vitro and In Vivo Evaluation. AAPS PharmSciTech 2013, 14, 1063–1071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Lu, Y.; Zhong, Y.; Wang, Q.; Wu, W.; Gao, S. Ocular delivery of cyclosporine A based on glyceryl monooleate/poloxamer 407 liquid crystalline nanoparticles: Preparation, characterization, in vitro corneal penetration and ocular irritation. J. Drug Target. 2012, 20, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wang, S.; Fang, S.; Wang, J.; Chen, J.; Huang, X.; He, X.; Liu, C. Liquid Crystalline Nanoparticles as an Ophthalmic Delivery System for Tetrandrine: Development, Characterization, and In Vitro and In Vivo Evaluation. Nanoscale Res. Lett. 2016, 11, 254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.; Peng, T.; Li, Y.; Zhan, Z.; Zeng, Y.; Huang, Y.; Pan, X.; Wu, C.Y.; Wu, C. Ocular Cubosome Drug Delivery System for Timolol Maleate: Preparation, Characterization, Cytotoxicity, Ex Vivo, and In Vivo Evaluation. AAPS PharmSciTech 2017, 18, 2919–2926. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, W.S.; Hosny, K.M. Development and optimization of ocular in situ gels loaded with ciprofloxacin cubic liquid crystalline nanoparticles. J. Drug Deliv. Sci. Technol. 2020, 57, 101710. [Google Scholar] [CrossRef]
- Azhari, H.; Strauss, M.; Hook, S.; Boyd, B.J.; Rizwan, S.B. Stabilising Cubosomes with Tween 80 as a Step Towards Targeting Lipid Nanocarriers to the Blood–Brain Barrier. Eur. J. Pharm. Biopharm. 2016, 104, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, F.E.; Elsayed, I.; Gad, M.K.; Badr, A.; Mohamed, M.I. Investigating the Cubosomal Ability for Transnasal Brain Targeting: In Vitro Optimization, Ex Vivo Permeation and In Vivo Biodistribution. Int. J. Pharm. 2015, 490, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Elnaggar, Y.S.; Etman, S.M.; Abdelmonsif, D.A.; Abdallah, O.Y. Novel Piperine-Loaded Tween-Integrated Monoolein Cubosomes as Brain-Targeted Oral Nanomedicine in Alzheimer’s Disease: Pharmaceutical, Biological, and Toxicological Studies. Int. J. Nanomed. 2015, 10, 5459–5473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, H.; Li, J.; Zhang, Q.; Yan, X.; Guo, L.; Gao, X.; Qiu, M.; Jiang, X.; Lai, R.; Chen, H. A novel small odorranalectin-bearing cubosomes: Preparation, brain delivery and pharmacodynamic study on amyloid-beta25--35-treated rats following intranasal administration. Eur. J. Pharm. Biopharm. 2012, 80, 368–378. [Google Scholar] [CrossRef]
- Patil, S.S.; Mahadik, K.R.; Paradkar, A.R. Liquid crystalline phase as a probe for crystal engineering of lactose: Carrier for pulmonary drug delivery. Eur. J. Pharm. Sci. 2015, 68, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.L.; Ki, M.H.; Joo, M.K.; An, S.W.; Hwang, K.M.; Park, E.S. An injectable liquid crystal system for sustained delivery of entecavir. Int. J. Pharm. 2015, 490, 265–272. [Google Scholar]
- Ki, M.H.; Lim, J.L.; Ko, J.Y.; Park, S.H.; Kim, J.E.; Cho, H.J.; Park, E.S.; Kim, D.D. A new injectable liquid crystal system for one month delivery of leuprolide. J. Control. Release 2014, 185, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Báez-Santos, Y.M.; Otte, A.; Mun, E.A.; Soh, B.K.; Song, C.G.; Lee, Y.; Park, K. Formulation and characterization of a liquid crystalline hexagonal mesophase region of phosphatidylcholine, sorbitan monooleate, and tocopherol acetate for sustained delivery of leuprolide acetate. Int. J. Pharm. 2016, 514, 314–321. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Liang, X.; Jiang, X.; Guo, J.; Tao, Y.; Wang, S.; Cao, Y.; Gui, S. Development and Evaluation of Minocycline Hydrochloride-Loaded In Situ Cubic Liquid Crystal for Intra-Periodontal Pocket Administration. Molecules 2018, 23, 2275. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, L.; Raftopoulos, K.N.; Tandrup Schmidt, S.; Schneider, F.; Dietz, H.; Rades, T.; Franzyk, H.; Pedersen, A.E.; Papadakis, C.M.; Christensen, D.; et al. Immune responses induced by nano-self-assembled lipid adjuvants based on a monomycoloyl glycerol analogue after vaccination with the Chlamydia trachomatis major outer membrane protein. J. Control Release 2018, 285, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, L.; Kyriakos, K.; Schneider, F.; Dietz, H.; Winter, G.; Papadakis, C.M.; Hubert, M. Characterization of Lipid-Based Hexosomes as Versatile Vaccine Carriers. Mol. Pharm. 2016, 13, 3945–3954. [Google Scholar] [CrossRef] [PubMed]
- Silvane, L.; Celias, D.P.; Romagnoli, P.A.; Maletto, B.A.; Sanchez Vallecillo, M.F.; Chiapello, L.S.; Palma, S.D.; Allemandi, D.A.; Sanabria, R.E.F.; Pruzzo, C.I.; et al. A Vaccine Based on Kunitz-Type Molecule Confers Protection Against Fasciola hepatica Challenge by Inducing IFN-γ and Antibody Immune Responses Through IL-17A Production. Front Immunol. 2020, 11, 2087. [Google Scholar] [CrossRef]
- Qiu, T.; Gu, P.; Wusiman, A.; Ni, H.; Xu, S.; Zhang, Y.; Zhu, T.; He, J.; Liu, Z.; Hu, Y.; et al. Immunoenhancement effects of chitosan-modified ginseng stem-leaf saponins-encapsulated cubosomes as an ajuvant. Colloids Surf. B Biointerfaces 2021, 204, 111799. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Luo, L.; Zheng, S.; Niu, Y.; Bo, R.; Huang, Y.; Xing, J.; Li, Z.; Wang, D. Cubosome nanoparticles potentiate immune properties of immunostimulants. Int. J. Nanomed. 2016, 11, 3571–3583. [Google Scholar]
- Liu, Z.; Yu, L.; Gu, P.; Bo, R.; Xu, S.; Wusiman, A.; Liu, J.; Hu, Y.; Wang, D. Surface-Engineered Cubosomes Serve as a Novel Vaccine Adjuvant to Modulate Innate Immunity and Improve Adaptive Immunity in vivo. Int. J. Nanomed. 2020, 15, 8595–8608. [Google Scholar] [CrossRef]
- Liu, Z.; Ni, H.; Yu, L.; Xu, S.; Bo, R.; Qiu, T.; Gu, P.; Zhu, T.; He, J.; Wusiman, A.; et al. Adjuvant activities of CTAB-modified Polygonatum sibiricum polysaccharide cubosomes on immune responses to ovalbumin in mice. Int. J. Biol. Macromol. 2020, 148, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.H.; Beiss, V.; Fiering, S.N.; Steinmetz, N.F. COVID-19 Vaccine Frontrunners and Their Nanotechnology Design. ACS Nano 2020, 14, 12522–12537. [Google Scholar] [CrossRef] [PubMed]
- Formica, N.; Mallory, R.; Albert, G.; Robinson, M.; Plested, J.S.; Cho, I.; Robertson, A.; Dubovsky, F.; Glenn, G.M. Different dose regimens of a SARS-CoV-2 recombinant spike protein vaccine (NVX-CoV2373) in younger and older adults: A phase 2 randomized placebocontrolled trial. PLoS Med. 2021, 18, e1003769. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson, K.L.; Song, H.; Stertman, L.; Liu, Y.; Flyer, D.C.; Massare, M.J.; Xu, R.H.; Zhou, B.; Lu, H.; Kwilas, S.A.; et al. Matrix-M adjuvant enhances antibody, cellular and protective immune responses of a Zaire Ebola/Makona virus glycoprotein (GP) nanoparticle vaccine in mice. Vaccine 2016, 34, 1927–1935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keech, C.; Albert, G.; Cho, I.; Robertson, A.; Reed, P.; Neal, S.; Plested, J.S.; Zhu, M.; Cloney-Clark, S.; Zhou, H.; et al. Phase 1–2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N. Engl. J. Med. 2020, 383, 2320–2332. [Google Scholar] [CrossRef]
- Reimer, J.M.; Karlsson, K.H.; Lövgren-Bengtsson, K.; Magnusson, S.E.; Fuentes, A.; Stertman, L. Matrix-M™ adjuvant induces local recruitment, activation and maturation of central immune cells in absence of antigen. PLoS ONE 2012, 7, e41451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fong, W.K.; Negrini, R.; Vallooran, J.J.; Mezzenga, R.; Boyd, B.J. Responsive self-assembled nanostructured lipid systems for drug delivery and diagnostics. J. Colloid Interface Sci. 2016, 484, 320–339. [Google Scholar] [CrossRef]
- Liu, J.; Huang, Y.; Kumar, A.; Tan, A.; Jin, S.; Mozhi, A.; Liang, X.J. pH-Sensitive nano-systems for drug delivery in cancer therapy. Biotechnol. Adv. 2014, 32, 693–710. [Google Scholar] [CrossRef] [PubMed]
- Kanamala, M.; Wilson, W.R.; Yang, M.; Palmer, B.D.; Wu, Z. Mechanisms and biomaterials in pH-responsive tumour targeted drug delivery: A review. Biomaterials 2016, 85, 152–167. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.K.; Kim, J.C. Complex Coacervation-Controlled Release from Monoolein Cubic Phase Containing Silk Fibroin and Alginate. Biomacromolecules 2011, 12, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Crisci, A.; Hay, D.N.T.; Seifert, S.; Firestone, M.A. pH- and Ionic-Strength-Induced Structural Changes in Poly(acrylic acid)-Lipid-Based Self-Assembled Materials. Macromol. Symp. 2009, 281, 126–134. [Google Scholar] [CrossRef]
- Negrini, R.; Fong, W.K.; Boyd, B.J.; Mezzenga, R. pH-responsive lyotropic liquid crystals and their potential therapeutic role in cancer treatment. Chem. Commun. 2015, 51, 6671–6674. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, R.; Gontsarik, M.; Yaghmur, A.; Salentinig, S. pH-Responsive Nano-Self-Assemblies of the Anticancer Drug 2-Hydroxyoleic Acid. Langmuir 2019, 35, 7954–7961. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Angelova, A.; Hu, F.; Garamus, V.M.; Peng, C.; Li, N.; Liu, J.; Liu, D.; Zou, A. pH Responsiveness of Hexosomes and Cubosomes for Combined Delivery of Brucea javanica Oil and Doxorubicin. Langmuir 2019, 35, 14532–14542. [Google Scholar] [CrossRef]
- Ribeiro, I.R.; Immich, M.F.; Lundberg, D.; Poletto, F.; Loh, W. Physiological neutral pH drives a gradual lamellar-to-reverse cubic-to-reverse hexagonal phase transition in phytantriol-based nanoparticles. Colloids Surf. B Biointerfaces 2019, 177, 204–210. [Google Scholar] [CrossRef]
- Qiu, H.; Caffrey, M. The Phase Diagram of the Monoolein/Water System: Metastability and Equilibrium Aspects. Biomaterials 2000, 21, 223–234. [Google Scholar] [CrossRef]
- de Campo, L.; Yaghmur, A.; Sagalowicz, L.; Leser, M.E.; Watzke, H.; Glatter, O. Reversible Phase Transitions in Emulsified Nanostructured Lipid Systems. Langmuir 2004, 20, 5254–5261. [Google Scholar] [CrossRef] [PubMed]
- Muller, F.; Salonen, A.; Glatter, O. Phase behavior of Phytantriol/water bicontinuous cubic Pn3m cubosomes stabilized by Laponite disc-like particles. J. Colloid Interface Sci. 2010, 342, 392–398. [Google Scholar] [CrossRef]
- Mariani, P.; Luzzati, V.; Delacroix, H. Cubic phases of lipid-containing systems. Structure analysis and biological implications. J. Mol. Biol. 1988, 204, 165–189. [Google Scholar] [CrossRef]
- Luzzati, V.; Vargas, R.; Mariani, P.; Gulik, A.; Delacroix, H. Cubic Phases of Lipid-containing Systems: Elements of a Theory and Biological Connotations. J. Mol. Biol. 1993, 229, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Barriga, H.M.; Tyler, A.I.; McCarthy, N.L.; Parsons, E.S.; Ces, O.; Law, R.V.; Seddon, J.M.; Brooks, N.J. Temperature and pressure tuneable swollen bicontinuous cubic phases approaching nature’s length scales. Soft Matter 2015, 11, 600–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dabkowska, A.P.; Hirst, C.; Valldeperas, M.; Clifton, L.A.; Montis, C.; Nöjd, S.; Gentile, L.; Wang, M.; Pálsson, G.K.; Lages, S.; et al. Temperature responsive lipid liquid crystal layers with embedded nanogels. Chem. Commun. 2017, 53, 1417–1420. [Google Scholar] [CrossRef]
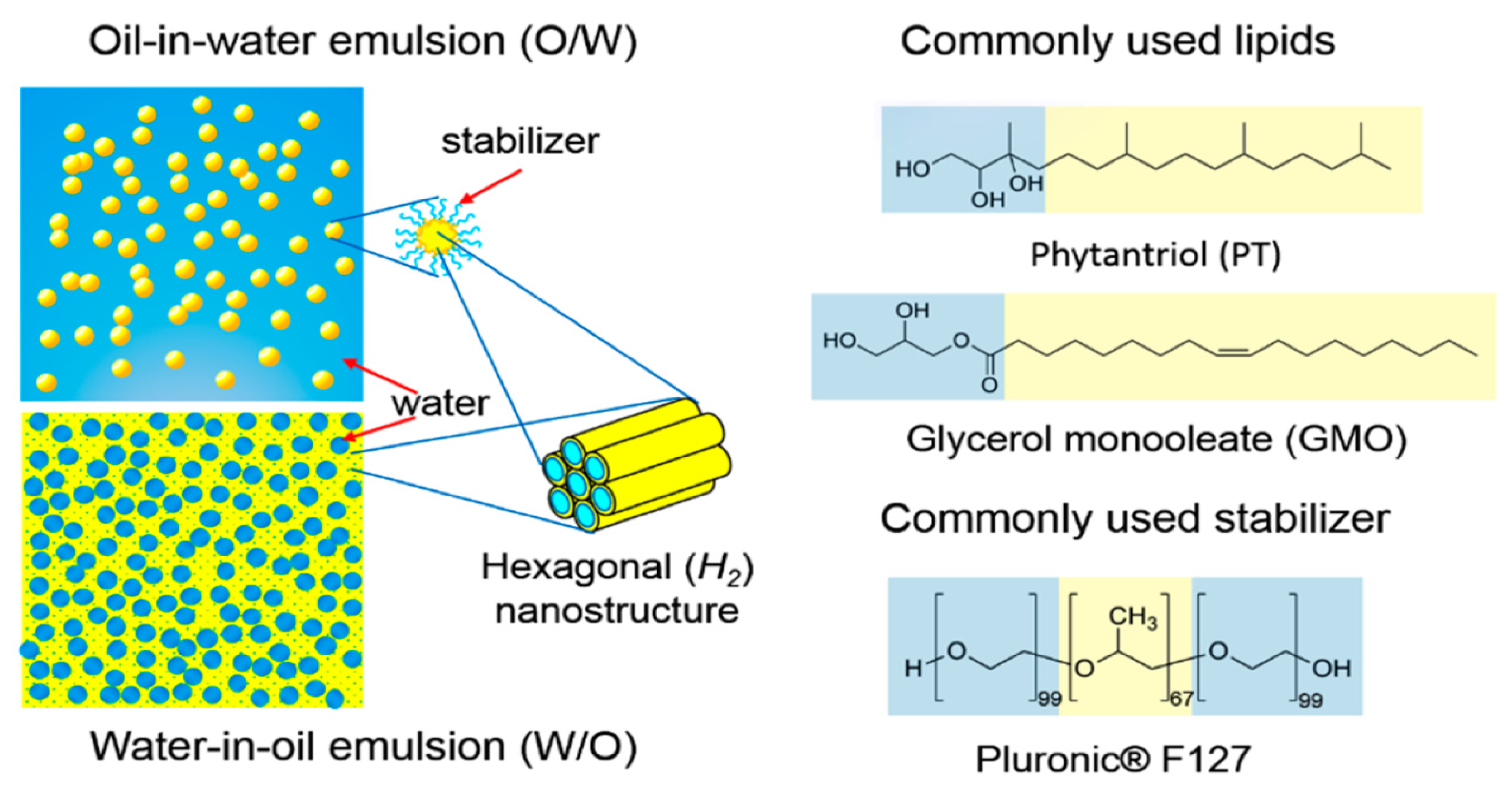
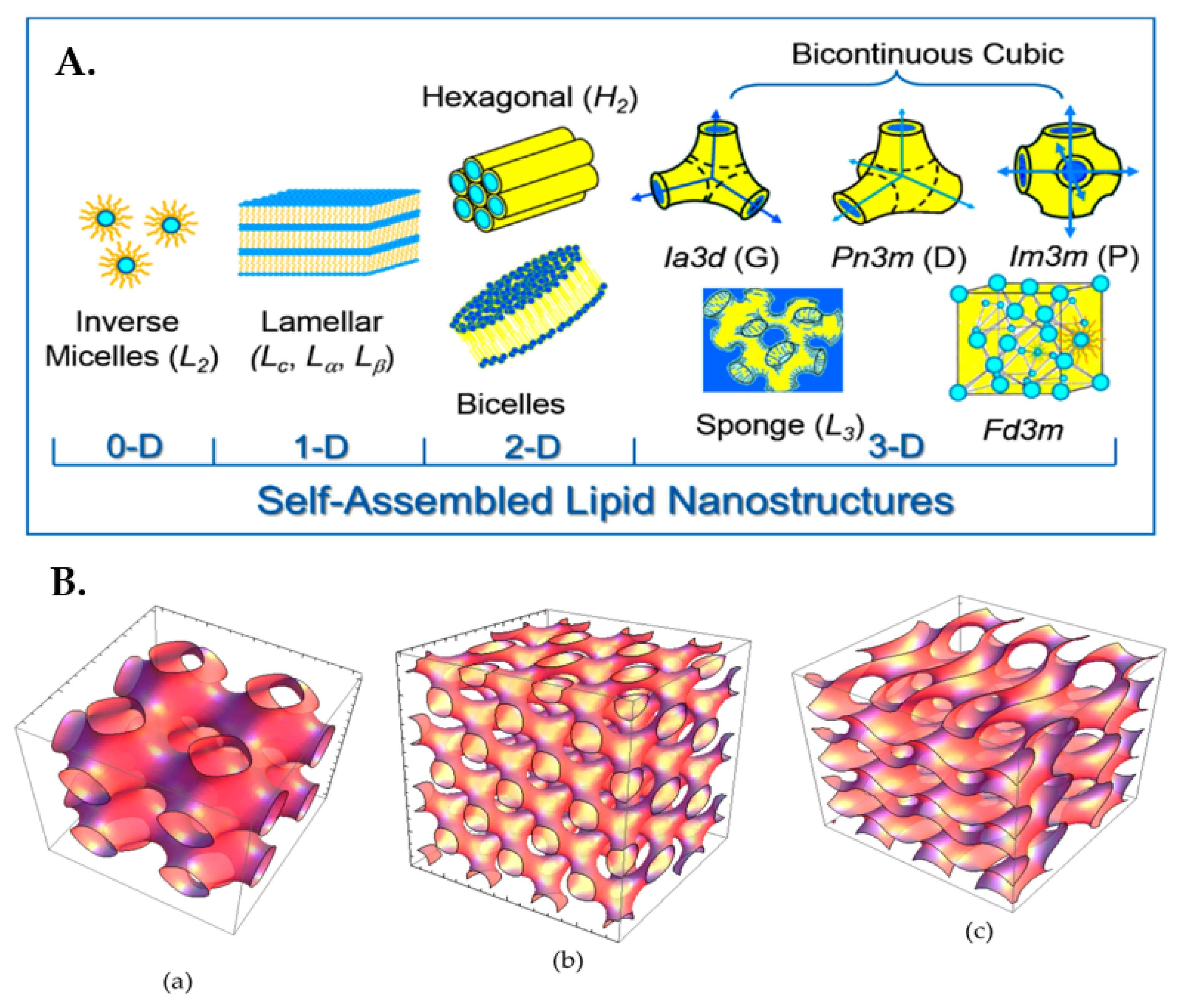



| Formulation | Therapeutic Agent | Therapy | Route of Administration | Notes | Reference |
|---|---|---|---|---|---|
| GMO:F127 | Doxorubicin | Anticancer (glioblastoma) | In vitro | pH-dependent drug release | Nazaruk et al. [54] |
| Phosphatidyl choline:glycerol dioleate:Tween 80 | Docetaxel | Anticancer (prostate cancer) | Intravenous | Better tumor regression compared to commercial | Cervin et al. [77] |
| GMO:F127:mPEG2kDSPE | Paclitaxel | Anticancer | Intravenous | PEGylation enhances the safety and efficacy of GMO systems | Jain et al. [78] |
| GMO:F127 | 5-Fluorouracil | Anticancer | Subcutaneous | Enhanced biodistribution | Nasr et al. [81] |
| MO:F127:DSPE-PEG-maleimide:EGFR antibodies | Paclitaxel | Anticancer (ovarian cancer) | Intraperitoneal | Enhanced cancer cytotoxicity | Zhai et al. [79] |
| GMO:F108 | Camptothecin | Anticancer | In vitro | Increased targeting | Caltagirone et al. [82] |
| MO:F127 | Cisplatin, Paclitacel, Dual | Anticancer | In vitro | Sustained drug release | Zhang et al. [83] |
| PHYT:DOTAP:F127 | 5-Fluorouracil | Anticancer | In vitro | Enhanced cytotoxicity in breast cancer cells | Astolfi et al. [84] |
| 5-FCOle:F127:ethanol | 5-Fluorouracil | Anticancer | Via orogastric gavage | Self-assembled amphiphile prodrugs | Gong et al. [85] |
| 5-FCPal/5-FCOle/5-FCPhy:F127 | 5-fluorouracil | Anticancer | Via orogastric gavage | Self-assembled amphiphile prodrugs | Sangella et al. [86] |
| Soy phosphatidylcholine: glycerol dioleate:Tween 80 | Paclitaxel | Anticancer | Oral | Enhanced oral bioavailability than commercial | Zeng et al. [80] |
| GMO:F127 into gelling system (F127, F68, HPMC K4M) | Docetaxel | Anticancer | - | Thermoresponsive depot system | Rarokar et al. [97] |
| Odorranalectin-decorated-GMO-F127 | Gly14-humanin (S14G-HN) peptide | Alzheimer’s therapy | Intranasal (to brain) | Enhanced therapeutic effects | Wu et al. [123] |
| GMO:F127:Tween 80 in gellan gum or polyox gel | Risperidone | Schizophrenia | Intranasal (to brain) | Enhanced bioavailability and permeation | Abdelrahman et al. [121] |
| GMO:F127:Tween 80 or Cremophor RH 40 | Piperine | Alzheimer’s therapy | Intranasal (to brain) | Sustained drug release | Elanggar et al. [122] |
| PHYT:F127 | Amphotericin B | Antifungal | Oral | Enhanced oral bioavailability | Yang et al. [87] |
| PHYT:F68 | Doxorubicin-CoQ10 | Anticancer | Oral | Preventing cardiotoxicity | Swarnakar et al. [95] |
| GMO:F127:ethanol:Propylene glycol | Insulin | Diabetes | Oral | Taken up by Caco-2 cells | Chung et al. [89] |
| GMO:F127 | Cyclosporine A | Antibiotics | Oral | Enhanced oral bioavailability compared to commercial | Lai et al. [90] |
| GMO:F127 | Amphotericin B | Antifungal | Oral | Enhanced permeation in Caco-2 cells, enhanced oral bioavailability | Yang et al. [93] |
| GMO:F127:sorbitol | Tamoxifen | Anticancer | Oral | Enhanced oral bioavailability | Nasr and Dawoud [91] |
| PHYT:F127 | Cinarizine | Model drug | Oral | Sustained drug release | Nguyen et al. [88] |
| GMO:F127 | Spironolactone, nifedipine | Antihypertensive | Oral | Enhanced oral bioavailability | Ali et al. [92] |
| PHYT:F127:propylene glycol | Cefpodoxime proxetil | Antibiotic | Oral | Taste-making for pediatric patients | Fan et al. [96] |
| GMO:dextran:Eudragit® L100–55 microcontainers | OVA and Quil-A | Vaccine | Oral | Improve the humoral response to oral boosters | von Halling Laier et al. [94] |
| GMO:F127:glycerol | Dexamethasone | Anti-inflammatory | Ocular | Enhanced bioavailability and unaffected corneal structure | Gan et al. [112] |
| GMO:F127:glycerol | Flurbiprofen | Anti-inflammatory | Ocular | Enhanced bioavailability compared with eye drops | Han et al. [113] |
| GMO:F127 | Pilocarpin nitrate | Glaucoma | Ocular | Prolonged effect compared with commercial and controlled delivery | Li et al. [114] |
| GMO:F127 | Brinzolamide | Glaucoma | Ocular | Better ocular bioavailability, and patient compliance compared to commercial | Wu et al. [115] |
| GMO:F127:glycerine | Timolol | Glaucoma | Ocular | Enhanced corneal permeability and bioavailability compared to commercial | Huang et al. [118] |
| GMO:F127 | Cyclosporine A | Antibiotics | Ocular | Excellent ocular tolerance | Chen et al. [116] |
| GMO:F127 | Tetrandrine | Glaucoma | Ocular | Enhanced ocular bioavailability | Liu et al. [117] |
| PHYT:F127 in thermo-gelling chitosan solution | Ciprofloxacin | Antimicrobial | Ocular | Improved eye permeation, prolonged ocular retention time, and enhanced antimicrobial activity compared to commercial | Alharbi et al. [119] |
| GMO:oleic acid: polyethylenimine (PEI)/oleylamine (OAM) | siRNA | Various | Topical skin | Without skin irritation | de Carvalho Vicentini et al. [109] |
| GMO:Tween 20 | Celecoxib | Anti-inflammatory | In vitro skin | Enhanced skin permeation | Estracanholli et al. [110] |
| MO:F127 | Cyclosporin A | Antibiotic | Topical skin | Without skin irritation | Lopes et al. [108] |
| GMO:F127:polyvinyl alcohol in chitosan/carbopol 940 hydrogels (cubogels) | Silver sulfadiazine | Burn therapy | Topical skin | Least side effects and better compliance than commercial | Morsi et al. [99] |
| GMO:F127 in carbopol 940/aloe vera hydrogels (cubogels) | Silver sulfadiazine and aloe vera | Burn therapy | Topical skin | Better bio adhesion and superior burn healing than commercial | Thakkar et al. [100] |
| GMO/PHYT:propylene glycol | δ-Aminolevulinic acid | Photodynamic therapy | Topical skin | Enhanced drug penetration | Bender et al. [102] |
| GMO:ethanol | Mitoxantrone | Melanoma | Topical skin | Non-invasion and no severe side effects | Yu et al. [103] |
| GMO:phospholipids:propylene glycol | Chlorin e6 or meso-Tetraphenylporphine-Mn(III) chloride | Photodynamic therapy (PDT) of melanoma | Topical skin | Biocompatible polymer-free cubosomes for potential application in both PDT and bioimaging | Bazylińska et al. [104] |
| GMO:F127:ethanol | Oregonin and Hirsutanonol | Atopic dermatitis | Topical skin | Enhanced skin permeation | Im et al. [106] |
| GMO:F127 | Herbal extracts | Hair loss | Topical skin | Enhanced skin permeation | Seo et al. [107] |
| GMO:F127 | Etodolac | Rheumatoid arthritis | Topical skin | Enhanced bioavailability | Salah et al. [105] |
| GMO:F127 | antimicrobial peptide LL-37 | Bacterial infections | Topical skin | Enhanced bactericidal effect without irritation | Boge et al. [101] |
| GMO:lactose | Salbutamol sulfate | Dry powder inhaler (DPI) formulation | Pulmonary | aerosolisation parameters as in commercial | Patil et al. [124] |
| Sorbitan monooleate:Tween 20:tocopherol acetate: phosphatidylcholine | Leuprolide acetate | In situ gelling system | Injectable | Sustained drug release (prostate cancer, endometriosis, and central precocious puberty) | Báez-Santos et al. [127] Ki et al. [126] |
| Sorbitan monooleate:Tween 20:tocopherol acetate: phosphatidylcholine | Entecavir | In situ gelling system | Injectable | Sustained drug release (hepatitis B) | Kim et al. [125] |
| PHYT:ethanol:vit E | Sinomenine hydrochloride | In situ gelling system | Intra-articular | Sustained drug release (rheumatoid arthritis) | Chen et al. [50] |
| PHYT:propylene glycol | Minocycline hydrochloride | In situ gelling system | Intra-periodontal pocket | Sustained drug release (periodontitis) | Yang et al. [128] |
| Stabilizer-free GMO | Antimicrobial peptide LL-37 | Bacterial infections | In vitro | Antimicrobial, cytocompatible | Zabara et al. [22] |
| Monoglycerides (dimodan):F127 | Undecylenic acid | Fungal infections (Candida albicans) | In vitro | Inhibition of fungal growth and filamentation, non-toxic in human cells | Mionić Ebersold et al. [51] |
| MO:F127 | Indomethacin | Anti-inflammatory | Percutaneous | Depot effect on the epidermis | Esposito et al. [57] |
| Lipidic Host Matrice | Additive | Phase Transition | Triggering Parameter | Reference |
|---|---|---|---|---|
| MO | linoleic acid | Im3m ↔ HII (*) | pH | Negrini and Mezzenga [41] |
| MO:F127 | poly(L-lysine-iso-phthalamide) grafted with L-phenylalanine | Disruption of Im3m | pH | Kluzek et al. [42] |
| Monolinolein (MLO) | pyridinylmethyl linoleate | Pn3m ↔ HII (*) | pH | Negrini et al. [146] |
| MO | hydrophobically modified alginate (HmAL) and hydrophobically modified silk fibroin (HmSF) | Cubic with coacervate in water channels (*) | pH | Kwon and Kim [144] |
| GMO:F127 | 2-hydroxyoleic acid (2OHOA) | Pn3m, HII ↔ Pn3m, Im3m ↔ Lamellar | pH | Prajapati et al. [147] |
| GMO:oleic acid:F127 | Brucea javanica oil, doxorubicin | HII → Pn3m, Im3m → Microemulsion (*) | pH | Li et al. [148] |
| PHYT:F127 | decyl betainate chloride cleavable surfactant | lamellar-to-Im3m-to-HII (*) | pH | Ribeiro et al. [149] |
| 1,2-Dimyristoyl-sn-glycero-3-phosphocholine (DMPC) | poly(acrylic acid)-dimyristoyl-sn-glycero-3-phosphoethanolamine (PAA-DMPE) | Swollen lamellar to cubic | pH, ionic strength | Crisci et al. [145] |
| GMO/PHYT:F127 | poly(2-(dimethylamino)ethyl methacrylate)-b-poly(lauryl methacrylate) (PDMAEMA-b-PLMA) | Structure dependant on formulation, pH-responsive ζ-potential, and fractal dimension | pH, temperature | Chountoulesi et al. [30,38] |
| MO | fatty acid (oleic acid/vaccenic acid/gondoic acid/erucic acid/nervonic acid) | Fd3m → HII | pH, ionic strength, Temperature | Fong et al. [37] |
| GMO:diglycerol monoleate (DGMO) | poly(N-isopropylacrylamide) (pNIPAM) nanoparticles | Pn3m | Temperature | Dabkowska et al. [156] |
| Monolinolein (MLO):F127 | - | Pn3m ↔ HII ↔ L2 | Temperature | de Campo et al. [151] |
| PHYT | laponite | Pn3m ↔ L2 | Temperature | Muller et al. [152] |
| GMO/PHYT | vitamin E acetate | Pn3m ↔ HII (*) | Temperature | Fong et al. [63] |
| MO:cholesterol | dioleoyl-phosphatidylserine (DOPS) or dioleoyl-phosphatidylglycerol (DOPG) | Highly swollen Im3m (varying lattice parameter) | Temperature and pressure | Barriga et al. [155] |
| MO | dioleoyl-phosphatidylglycerol (DOPG) | Lα → cubic | Ionic strength (Ca2+ cations) | Awad et al. [40] |
| PHYT:F127 | sodium bis(2-ethylhexyl)sulfosuccinate (AOT), didodecyldimethylammonium bromide (DDAB) | Lamellar ↔ Im3m ↔ Pn3m | Ionic surfactant content, ionic strength | Liu et al. [39] |
| PHYT:F127 | didodecyldimethylammonium bromide (DDAB) | Liposomes → cubosomes | Ionic strength | Muir et al. [67] |
| MO | dioleoyl-phosphatidylglycerol (DOPG) | Lα → HII | Ionic strength (Ca2+ cations) | Yaghmur et al. [66] |
| PHYT:F127:5-fluorouracil | didodecyldimethylammonium bromide (DDABr) | Pn3m ↔ Im3m ↔ Lamellar | Ionic content | Astolfi et al. [69] |
| Monolinolein (MLO) | outer membrane protein F (OmpF) | Topological interconnectivities between the aqueous nanochannels (*) | Protein incorporation | Zabara et al. [70] |
| MO | distearoylphosphatidylglycerol (DSPG) | Swollen cubic phases | Charged lipid | Engblom et al. [71] |
| MO | sucrose stearate | Pn3m → Im3m | Hydration-enhancing effect | Negrini and Mezzenga [73] |
| Monoacylglycerols and phospholipids | - | Ultra-swollen bicontinuous cubic phases of Ia3d, Pn3m and Im3m | Crystallize proteins with small extracellular domains (ECDs) | Zabara et al. [76] |
| MO:cholesterol | 1,2-dioleoyl-sn-glycero-3-phospho-l-serine (sodium salt) (DOPS), 1,2-dioleoyl-sn-glycero-3-phospho-(1′-rac-glycerol) (sodium salt) (DOPG), 1,2-dioleoyl-sn-glycero-3-phosphate (sodium salt) (DOPA) charged phospholipids | Swollen cubosomes | Electrostatic tuning | Barriga et al. [74] |
| MO:cholesterol | phospholipids with PC, PE, and PS headgroups and saturated chain lengths from C12 to C18 [lauryl (C12); myristyl (C14); palmityl (C16); stearyl (C18)] except for the singly unsaturated (C18:1) oleoyl chain | Swollen cubosomes | Increase in curvature of the MO bilayer | Sarkar et al. [75] |
| GMO:vit E | d-α-tocopheryl poly(ethylene glycol)2000 succinate (TPGS-PEG2000) and thymoquinone | Coexisting Fd3m and HII-to-Fd3m or inverse micellar (L2) | Lipids ratio, presence, and concentration of stabilizer and drug | Yaghmur et al. [49] |
| PHYT:F108 | 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-n-[amino(polyethylene glycol)-2000] (DSPE-PEG2000) and vitamin E acetate | Cubosomes → HII hexosomes → time-dependent growth of swollen hexagonal phase | Human monocytic cells (THP-1) | Tan et al. [45] |
| MO:TPEG1000 amphiphile:fish oil:curcumin | Catalase enzyme | Pn3m and Im3m→ Im3m | Enzyme presence | Rakotoarisoa et al. [13] |
| PHYT:F127 | - | Pn3m with HII → neat HII | Human plasma | Azmi et al. [43] |
| Soy phosphatidylcholine: citrem | - | Pn3m, HII → swollen ones | Human plasma | Azmi et al. [44] |
| GMO/PHYT:F127 | Fetal bovine serum | Acute size reduction | Serum proteins | Chountoulesi et al. [9] |
| PHYT | Vitamin E acetate | Suppresses the temperature of QII-to-HII-to-L2 transitions | Vitamin E acetate | Dong et al. [48] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chountoulesi, M.; Pispas, S.; Tseti, I.K.; Demetzos, C. Lyotropic Liquid Crystalline Nanostructures as Drug Delivery Systems and Vaccine Platforms. Pharmaceuticals 2022, 15, 429. https://doi.org/10.3390/ph15040429
Chountoulesi M, Pispas S, Tseti IK, Demetzos C. Lyotropic Liquid Crystalline Nanostructures as Drug Delivery Systems and Vaccine Platforms. Pharmaceuticals. 2022; 15(4):429. https://doi.org/10.3390/ph15040429
Chicago/Turabian StyleChountoulesi, Maria, Stergios Pispas, Ioulia K. Tseti, and Costas Demetzos. 2022. "Lyotropic Liquid Crystalline Nanostructures as Drug Delivery Systems and Vaccine Platforms" Pharmaceuticals 15, no. 4: 429. https://doi.org/10.3390/ph15040429
APA StyleChountoulesi, M., Pispas, S., Tseti, I. K., & Demetzos, C. (2022). Lyotropic Liquid Crystalline Nanostructures as Drug Delivery Systems and Vaccine Platforms. Pharmaceuticals, 15(4), 429. https://doi.org/10.3390/ph15040429








