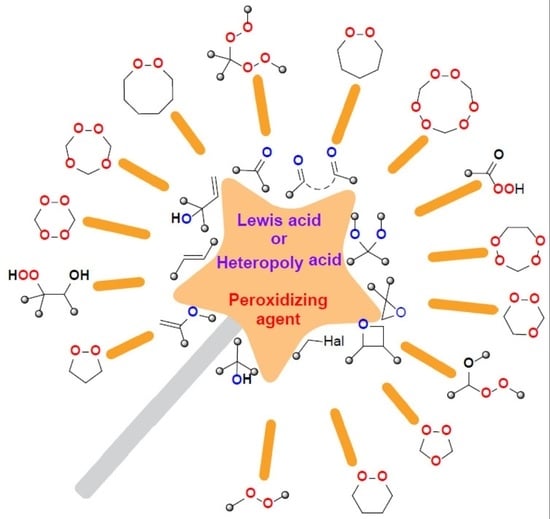
Lewis Acids and Heteropoly Acids in the Synthesis of Organic Peroxides
Abstract
:1. Introduction
2. Metal-Based Lewis Acids as Catalysts in the Synthesis of Organic Peroxides
2.1. Synthesis of Organic Peroxides Catalyzed by SnCl4, Me2SnCl2, SnCl2, and TiCl4
2.2. Peroxidation of Ketones and Aldehydes in the Presence of MeReO3
2.3. Sc(OTf)3, Yb(OTf)3, InCl3 and In(OTf)3 in the Synthesis of Organic Peroxides
2.4. Mercury Salts in the Synthesis of Peroxides
2.5. Other Metal-Based Lewis Acids
3. Non-Metal-Based Lewis Acids in the Synthesis of Organic Peroxides
3.1. Application of BF3·Et2O in the Synthesis of Organic Peroxides
3.2. Iodine in the Synthesis of Organic Peroxides
3.3. Synthesis of Peroxides Promoted by TMSOTf and TBDMSOTf
4. Heteropoly Acids in the Synthesis of Organic Peroxides
5. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matyjaszewski, K.; Davis, T.P. Handbook of Radical Polymerization; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Nesvadba, P. Radical polymerization in industry. In Encyclopedia of Radicals in Chemistry, Biology and Materials; John Wiley & Sons: Chichester, UK, 2012. [Google Scholar]
- Dluzneski, P.R. Peroxide vulcanization of elastomers. Rubber Chem. Technol. 2001, 74, 451–492. [Google Scholar] [CrossRef]
- Kruzelak, J.; Sykora, R.; Hudec, I. Vulcanization of rubber compounds with peroxide curing systems. Rubber Chem. Technol. 2017, 90, 60–88. [Google Scholar] [CrossRef]
- Global Organic Peroxide Market Growth 2019–2024. Available online: https://www.fiormarkets.com/report/global-organic-peroxide-market-growth-2019-2024-371362.html (accessed on 25 June 2021).
- Li, Y. Qinghaosu (artemisinin): Chemistry and pharmacology. Acta. Pharmacol. Sin. 2012, 33, 1141–1146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, W.E.; Peh, H.Y.; Chan, T.K.; Wong, W.S.F. Artemisinins: Pharmacological actions beyond anti-malarial. Pharmacol. Therapeut. 2014, 142, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.H.; Su, X.Z. Artemisinin: Discovery from the chinese herbal garden. Cell 2011, 146, 855–858. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.J.; Xia, Z.Q.; Wu, L.F. Study on the constituents of Artemisia-Annua L. 1. The isolation and identification of 11r-(-)-dihydroarteannuic acid. Acta. Chim. Sin. 1987, 45, 609–612. [Google Scholar]
- Vangapandu, S.; Jain, M.; Kaur, K.; Patil, P.; Patel, S.R.; Jain, R. Recent advances in antimalarial drug development. Med. Res. Rev. 2007, 27, 65–107. [Google Scholar] [CrossRef]
- White, N.J. Qinghaosu (Artemisinin): The price of success. Science 2008, 320, 330–334. [Google Scholar] [CrossRef]
- Nagelschmitz, J.; Voith, B.; Wensing, G.; Roemer, A.; Fugmann, B.; Haynes, R.K.; Kotecka, B.M.; Rieckmann, K.H.; Edstein, M.D. First assessment in humans of the safety, tolerability, pharmacokinetics, and ex vivo pharmacodynamic antimalarial activity of the new artemisinin derivative artemisone. Antimicrob. Agents Chemother. 2008, 52, 3085–3091. [Google Scholar] [CrossRef] [Green Version]
- Frohlich, T.; Karagoz, A.C.; Reiter, C.; Tsogoeva, S.B. Artemisinin-derived dimers: Potent antimalarial and anticancer agents. J. Med. Chem. 2016, 59, 7360–7388. [Google Scholar] [CrossRef]
- Capci, A.; Herrmann, L.; Kumar, H.M.S.; Frohlich, T.; Tsogoeva, S.B. Artemisinin-derived dimers from a chemical perspective. Med. Res. Rev. 2021, 41, 2927–2970. [Google Scholar] [CrossRef] [PubMed]
- Vennerstrom, J.L.; Fu, H.N.; Ellis, W.Y.; Ager, A.L.; Wood, J.K.; Andersen, S.L.; Gerena, L.; Milhous, W.K. Dispiro-1,2,4,5-tetraoxanes—A new class of antimalarial peroxides. J. Med. Chem. 1992, 35, 3023–3027. [Google Scholar] [CrossRef] [PubMed]
- Keiser, J.; Ingram, K.; Vargas, M.; Chollet, J.; Wang, X.F.; Dong, Y.X.; Vennerstrom, J.L. In Vivo Activity of Aryl Ozonides against Schistosoma Species. Antimicrob. Agents Chemother. 2012, 56, 1090–1092. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.X.; Chollet, J.; Vargas, M.; Mansour, N.R.; Bickle, Q.; Alnouti, Y.; Huang, J.G.; Keiser, J.; Vennerstrom, J.L. Praziquantel analogs with activity against juvenile Schistosoma mansoni. Bioorg. Med. Chem. Lett. 2010, 20, 2481–2484. [Google Scholar] [CrossRef] [PubMed]
- Cowan, N.; Yaremenko, I.A.; Krylov, I.B.; Terent’ev, A.O.; Keiser, J. Elucidation of the in vitro and in vivo activities of bridged 1,2,4-trioxolanes, bridged 1,2,4,5-tetraoxanes, tricyclic monoperoxides, silyl peroxides, and hydroxylamine derivatives against Schistosoma mansoni. Bioorg. Med. Chem. 2015, 23, 5175–5181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brecht, K.; Kirchhofer, C.; Bouitbir, J.; Trapani, F.; Keiser, J.; Krahenbuhl, S. Exogenous iron increases fasciocidal activity and hepatocellular toxicity of the synthetic endoperoxides OZ78 and MT04. Int. J. Mol. Sci. 2019, 20, 4880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.B.; Wang, X.F.; Chiu, F.C.K.; Haberli, C.; Shackleford, D.M.; Ryan, E.; Kamaraj, S.; Bulbule, V.J.; Wallick, A.I.; Dong, Y.X.; et al. Structure-activity relationship of antischistosomal ozonide carboxylic acids. J. Med. Chem. 2020, 63, 3723–3736. [Google Scholar] [CrossRef] [PubMed]
- Fisher, L.C.; Blackie, M.A.L. Tetraoxanes as antimalarials: Harnessing the endoperoxide. Mini Rev. Med. Chem. 2014, 14, 123–135. [Google Scholar] [CrossRef]
- Ghorai, P.; Dussault, P.H.; Hu, C.H. Synthesis of spiro-bisperoxyketals. Org. Lett. 2008, 10, 2401–2404. [Google Scholar] [CrossRef]
- Hao, H.D.; Wittlin, S.; Wu, Y.K. Potent antimalarial 1,2,4-trioxanes through perhydrolysis of epoxides. Chem. A Eur. J. 2013, 19, 7605–7619. [Google Scholar] [CrossRef]
- Jefford, C.W. Synthetic peroxides as potent antimalarials. News and views. Curr. Top. Med. Chem. 2012, 12, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Kuster, T.; Kriegel, N.; Stadelmann, B.; Wang, X.F.; Dong, Y.X.; Vennerstrom, J.L.; Keiser, J.; Hemphill, A. Amino ozonides exhibit in vitro activity against Echinococcus multilocularis metacestodes. Int. J. Antimicrob. Agents 2014, 43, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Opsenica, D.M.; Solaja, B.A. Antimalarial peroxides. J. Serb. Chem. Soc. 2009, 74, 1155–1193. [Google Scholar] [CrossRef]
- Solaja, B.A.; Terzic, N.; Pocsfalvi, G.; Gerena, L.; Tinant, B.; Opsenica, D.; Milhous, W.K. Mixed steroidal 1,2,4,5-tetraoxanes: Antimalarial and antimycobacterial activity. J. Med. Chem. 2002, 45, 3331–3336. [Google Scholar] [CrossRef]
- Vil’, V.A.; Yaremenko, I.A.; Ilovaisky, A.I.; Terent’ev, A.O. Peroxides with anthelmintic, antiprotozoal, fungicidal and antiviral bioactivity: Properties, synthesis and reactions. Molecules 2017, 22, 1881. [Google Scholar] [CrossRef] [Green Version]
- Dembitsky, V.M.; Gloriozova, T.A.; Poroikov, V.V. Natural peroxy anticancer agents. Mini Rev. Med. Chem. 2007, 7, 571–589. [Google Scholar] [CrossRef] [PubMed]
- Dembitsky, V.M. Bioactive peroxides as potential therapeutic agents. Eur. J. Med. Chem. 2008, 43, 223–251. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, S.; Sharma, V.; Jaiswal, P.K.; Gaikwad, A.N.; Sinha, S.K.; Puri, S.K.; Sharon, A.; Maulik, P.R.; Chaturvedi, V. Stable Tricyclic Antitubercular Ozonides Derived from Artemisinin. Org. Lett. 2015, 17, 4948–4951. [Google Scholar] [CrossRef]
- Miller, M.J.; Walz, A.J.; Zhu, H.; Wu, C.R.; Moraski, G.; Mollmann, U.; Tristani, E.M.; Crumbliss, A.L.; Ferdig, M.T.; Checkley, L.; et al. Design, synthesis, and study of a mycobactin-artemisinin conjugate that has selective and potent activity against tuberculosis and malaria. J. Am. Chem. Soc. 2011, 133, 2076–2079. [Google Scholar] [CrossRef] [Green Version]
- Zhou, F.W.; Lei, H.S.; Fan, L.; Jiang, L.; Liu, J.; Peng, X.M.; Xu, X.R.; Chen, L.; Zhou, C.H.; Zou, Y.Y.; et al. Design, synthesis, and biological evaluation of dihydroartemisinin-fluoroquinolone conjugates as a novel type of potential antitubercular agents. Bioorg. Med. Chem. Lett. 2014, 24, 1912–1917. [Google Scholar] [CrossRef]
- Cusati, R.C.; Barbosa, L.C.A.; Maltha, C.R.A.; Demuner, A.J.; Oliveros-Bastidas, A.; Silva, A.A. Tetraoxanes as a new class of efficient herbicides comparable with commercial products. Pest Manag. Sci. 2015, 71, 1037–1048. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, L.C.A.; Maltha, C.R.A.; Cusati, R.C.; Teixeira, R.R.; Rodrigues, F.F.; Silva, A.A.; Drew, M.G.B.; Ismail, F.M.D. Synthesis and biological evaluation of new ozonides with improved plant growth regulatory activity. J. Agric. Food Chem. 2009, 57, 10107–10115. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, L.C.A.; Pereira, U.A.; Telxeira, R.R.; Maltha, C.R.A.; Fernandes, S.A.; Forlani, G. Synthesis and phytotoxic activity of ozonides. J. Agric. Food Chem. 2008, 56, 9434–9440. [Google Scholar] [CrossRef] [PubMed]
- Vil’, V.A.; Yaremenko, I.A.; Fomenkov, D.I.; Levitsky, D.O.; Fleury, F.; Terent’ev, A.O. Ion exchange resin-catalyzed synthesis of bridged tetraoxanes possessing in vitro cytotoxicity against HeLa cancer cells. Chem. Het. Comp. 2020, 56, 722–726. [Google Scholar] [CrossRef]
- Yaremenko, I.A.; Syromyatnikov, M.Y.; Radulov, P.S.; Belyakova, Y.Y.; Fomenkov, D.I.; Popov, V.N.; Terent’ev, A.O. Cyclic synthetic peroxides inhibit growth of entomopathogenic fungus Ascosphaera apis without toxic effect on bumblebees. Molecules 2020, 25, 1954. [Google Scholar] [CrossRef]
- Phillipson, D.W.; Rinehart, K.L. Antifungal peroxide-containing acids from two Caribbean sponges. J. Am. Chem. Soc. 1983, 105, 7735–7736. [Google Scholar] [CrossRef]
- Chen, Y.; McCarthy, P.J.; Harmody, D.K.; Schimoler-O’Rourke, R.; Chilson, K.; Selitrennikoff, C.; Pomponi, S.A.; Wright, A.E. New bioactive peroxides from marine sponges of the family Plakiniidae. J. Nat. Prod. 2002, 65, 1509–1512. [Google Scholar] [CrossRef]
- Biamonte, M.A.; Wanner, J.; Le Roch, K.G. Recent advances in malaria drug discovery. Bioorg. Med. Chem. Lett. 2013, 23, 2829–2843. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.F.; Dong, Y.X.; Wittlin, S.; Charman, S.A.; Chiu, F.C.K.; Chollet, J.; Katneni, K.; Mannila, J.; Morizzi, J.; Ryan, E.; et al. Comparative Antimalarial Activities and ADME Profiles of Ozonides (1,2,4-trioxolanes) OZ277, OZ439, and Their 1,2-Dioxolane, 1,2,4-Trioxane, and 1,2,4,5-Tetraoxane Isosteres. J. Med. Chem. 2013, 56, 2547–2555. [Google Scholar] [CrossRef]
- Fontaine, S.D.; Spangler, B.; Gut, J.; Lauterwasser, E.M.W.; Rosenthal, P.J.; Renslo, A.R. Drug delivery to the malaria parasite using an Arterolane-like scaffold. ChemMedChem 2015, 10, 47–51. [Google Scholar] [CrossRef] [Green Version]
- Siddiqui, G.; Giannangelo, C.; De Paoli, A.; Schuh, A.K.; Heimsch, K.C.; Anderson, D.; Brown, T.G.; MacRaild, C.A.; Wu, J.B.; Wang, X.F.; et al. Peroxide antimalarial drugs target redox homeostasis in Plasmodium falciparum infected red blood cells. ACS Infect. Dis. 2022, 8, 210–226. [Google Scholar] [CrossRef] [PubMed]
- Hamaluba, M.; van der Pluijm, R.W.; Weya, J.; Njuguna, P.; Ngama, M.; Kalume, P.; Mwambingu, G.; Ngetsa, C.; Wambua, J.; Boga, M.; et al. Arterolane-piperaquine-mefloquine versus arterolane-piperaquine and artemether-lumefantrine in the treatment of uncomplicated Plasmodium falciparum malaria in Kenyan children: A single-centre, open-label, randomised, non-inferiority trial. Lancet Infect. Dis. 2021, 21, 1395–1406. [Google Scholar] [CrossRef]
- Phyo, A.P.; Jittamala, P.; Nosten, F.H.; Pukrittayakamee, S.; Imwong, M.; White, N.J.; Duparc, S.; Macintyre, F.; Baker, M.; Mohrle, J.J. Antimalarial activity of artefenomel (OZ439), a novel synthetic antimalarial endoperoxide, in patients with Plasmodium falciparum and Plasmodium vivax malaria: An open-label phase 2 trial. Lancet Infect. Dis. 2016, 16, 61–69. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, A.K.; Miller, H.; Knox, K.; Kundu, M.; Henrickson, K.J.; Arav-Boger, R. Inhibition of human coronaviruses by antimalarial peroxides. ACS Infect. Dis. 2021, 7, 1985–1995. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Islam, M.S.; Wang, J.; Li, Y.; Chen, X. Traditional Chinese Medicine in the Treatment of Patients Infected with 2019-New Coronavirus (SARS-CoV-2): A Review and Perspective. Int. J. Biol. Sci. 2020, 16, 1708–1717. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Gilmore, K.; Ramirez, S.; Settels, E.; Gammeltoft, K.A.; Pham, L.V.; Fahnøe, U.; Feng, S.; Offersgaard, A.; Trimpert, J.; et al. In vitro efficacy of artemisinin-based treatments against SARS-CoV-2. Sci. Rep. 2021, 11, 14571. [Google Scholar] [CrossRef]
- Clennan, E.L.; Sram, J.P. Photochemical reactions in the interior of a zeolite. Part 5: The origin of the zeolite induced regioselectivity in the singlet oxygen ene reaction. Tetrahedron 2000, 56, 6945–6950. [Google Scholar] [CrossRef]
- Stratakis, M.; Orfanopoulos, M. Regioselectivity in the ene reaction of singlet oxygen with alkenes. Tetrahedron 2000, 56, 1595–1615. [Google Scholar] [CrossRef]
- Griesbeck, A.G.; El-Idreesy, T.T.; Fiege, M.; Brun, R. Synthesis of antimalarial 1,2,4-trioxanes via photooxygenation of a chiral allylic alcohol. Org. Lett. 2002, 4, 4193–4195. [Google Scholar] [CrossRef]
- Matsumoto, M.; Nasu, S.; Takeda, M.; Murakami, H.; Watanabe, N. Singlet oxygenation of 4-(4-tert-butyl-3,3-dimethyl-2,3-dihydrofuran-5-yl)-2-pyridone: Non-stereospecific 1,4-addition of singlet oxygen to a 1,3-diene system and thermal rearrangement of the resulting 1,4-endoperoxides to stable 1,2-dioxetanes. Chem. Commun. 2000, 821–822. [Google Scholar] [CrossRef]
- Lopez, D.; Quinoa, E.; Riguera, R. The [4 + 2] addition of singlet oxygen to thebaine: New access to highly functionalized morphine derivatives via opioid endoperoxides. J. Org. Chem. 2000, 65, 4671–4678. [Google Scholar] [CrossRef] [PubMed]
- Isayama, S.; Mukaiyama, T. Novel method for the preparation of triethylsilyl peroxides from olefins by the reaction with molecular-oxygen and triethylsilane catalyzed by bis(1,3-diketonato)Cobalt(II). Chem. Lett. 1989, 18, 573–576. [Google Scholar] [CrossRef]
- Isayama, S. An efficient method for the direct peroxygenation of various olefinic compounds with molecular-oxygen and triethylsilane catalyzed by a Cobalt(II) complex. Bull. Chem. Soc. Jpn. 1990, 63, 1305–1310. [Google Scholar] [CrossRef] [Green Version]
- Tokuyasu, T.; Kunikawa, S.; Masuyama, A.; Nojima, M. Co(III)-alkyl complex- and Co(III)-alkylperoxo complex-catalyzed triethylsilylperoxidation of alkenes with molecular oxygen and triethylsilane. Org. Lett. 2002, 4, 3595–3598. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, P.M.; Hindley, S.; Pugh, M.D.; Davies, J.; Bray, P.G.; Park, B.K.; Kapu, D.S.; Ward, S.A.; Stocks, P.A. Co(thd)(2): A superior catalyst for aerobic epoxidation and hydroperoxysilylation of unactivated alkenes: Application to the synthesis of spiro-1,2,4-trioxanes. Tetrahedron Lett. 2003, 44, 8135–8138. [Google Scholar] [CrossRef]
- Demarteau, J.; Debuigne, A.; Detrembleur, C. Organocobalt complexes as sources of carbon-centered radicals for organic and polymer chemistries. Chem. Rev. 2019, 119, 6906–6955. [Google Scholar] [CrossRef]
- Tokuyasu, T.; Kunikawa, S.; Abe, M.; Masuyama, A.; Nojima, M.; Kim, H.S.; Begum, K.; Wataya, Y. Synthesis of antimalarial yingzhaosu a analogues by the peroxidation of dienes with Co(II)/O2/Et3SiH. J. Org. Chem. 2003, 68, 7361–7367. [Google Scholar] [CrossRef]
- Sugamoto, K.; Matsushita, Y.; Matsui, T. Direct hydroperoxygenation of conjugated olefins catalyzed by cobalt(II) porphyrin. J. Chem. Soc. Perkin Trans. 1 1998, 3989–3998. [Google Scholar] [CrossRef]
- Gandhi, H.; O’Reilly, K.; Gupta, M.K.; Horgan, C.; O’Leary, E.M.; O’Sullivan, T.P. Advances in the synthesis of acyclic peroxides. RSC Adv. 2017, 7, 19506–19556. [Google Scholar] [CrossRef] [Green Version]
- Kawanishi, M.; Kotoku, N.; Itagaki, S.; Horii, T.; Kobayashi, M. Structure-activity relationship of anti-malarial spongean peroxides having a 3-methoxy-1,2-dioxane structure. Bioorg. Med. Chem. 2004, 12, 5297–5307. [Google Scholar] [CrossRef]
- Murakami, N.; Kawanishi, M.; Itagaki, S.; Horii, T.; Kobayashi, M. Facile construction of 6-carbomethoxymethyl-3-methoxy-1,2-dioxane, a core structure of spongean anti-malarial peroxides. Tetrahedron Lett. 2001, 42, 7281–7285. [Google Scholar] [CrossRef]
- Murakami, N.; Kawanishi, M.; Itagaki, S.; Horii, T.; Kobayashi, M. New readily accessible peroxides with high anti-malarial potency. Bioorg. Med. Chem. Lett. 2002, 12, 69–72. [Google Scholar] [CrossRef]
- Juaristi, E.; Gomes, G.D.; Terent’ev, A.O.; Notario, R.; Alabugin, I.V. Stereoelectronic Interactions as a Probe for the Existence of the Intramolecular alpha-Effect. J. Am. Chem. Soc. 2017, 139, 10799–10813. [Google Scholar] [CrossRef] [PubMed]
- Alabugin, I.V.; Kuhn, L.; Medvedev, M.G.; Krivoshchapov, N.V.; Vil’, V.A.; Yaremenko, I.A.; Mehaffy, P.; Yarie, M.; Terent’ev, A.O.; Zolfigol, M.A. Stereoelectronic power of oxygen in control of chemical reactivity: The anomeric effect is not alone. Chem. Soc. Rev. 2021, 50, 10253–10345. [Google Scholar] [CrossRef] [PubMed]
- Hassan, Z.; Stahlberger, M.; Rosenbaum, N.; Brase, S. Criegee intermediates beyond ozonolysis: Synthetic and mechanistic insights. Angew. Chem. Int. Ed. 2021, 60, 15138–15152. [Google Scholar] [CrossRef]
- Criegee, R. Mechanism of ozonolysis. Angew. Chem. Int. Ed. Engl. 1975, 14, 745–752. [Google Scholar] [CrossRef]
- Griesbaum, K.; Ovez, B.; Huh, T.S.; Dong, Y.X. Ozonolyses of O-methyloximes in the presence of acid-derivatives—A new access to substituted ozonides. Liebigs Ann. 1995, 1571–1574. [Google Scholar] [CrossRef]
- Griesbaum, K.; Liu, X.J.; Dong, Y.X. Diozonides from coozonolyses of suitable O-methyl oximes and ketones. Tetrahedron 1997, 53, 5463–5470. [Google Scholar] [CrossRef]
- Geletneky, C.; Berger, S. The mechanism of ozonolysis revisited by O-17-NMR spectroscopy. Eur. J. Org. Chem. 1998, 1998, 1625–1627. [Google Scholar] [CrossRef]
- Dong, Y.X.; Vennerstrom, J.L. Differentiation between 1,2,4,5-tetraoxanes and 1,2,4,5,7,8-hexaoxonanes using H-1 and C-13 NMR analyses. J. Heterocycl. Chem. 2001, 38, 463–466. [Google Scholar] [CrossRef]
- Dai, P.; Dussault, P.H. Intramolecular reactions of hydroperoxides and oxetanes: Stereoselective synthesis of 1,2-dioxolanes and 1,2-dioxanes. Org. Lett. 2005, 7, 4333–4335. [Google Scholar] [CrossRef] [PubMed]
- Yaremenko, I.A.; Radulov, P.S.; Medvedev, M.G.; Krivoshchapov, N.V.; Belyakova, Y.Y.; Korlyukov, A.A.; Ilovaisky, A.I.; Terent’ev, A.O.; Alabugin, I.V. How to Build Rigid Oxygen-Rich Tricyclic Heterocycles from Triketones and Hydrogen Peroxide: Control of Dynamic Covalent Chemistry with Inverse alpha-Effect. J. Am. Chem. Soc. 2020, 142, 14588–14607. [Google Scholar] [CrossRef] [PubMed]
- Terent’ev, A.O.; Borisov, D.A.; Chernyshev, V.V.; Nikishin, G.I. Facile and selective procedure for the synthesis of bridged 1,2,4,5-tetraoxanes; strong acids as cosolvents and catalysts for addition of hydrogen peroxide to beta-diketones. J. Org. Chem. 2009, 74, 3335–3340. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, P.M.; Amewu, R.K.; Nixon, G.L.; ElGarah, F.B.; Mungthin, M.; Chadwick, J.; Shone, A.E.; Vivas, L.; Lander, H.; Barton, V.; et al. Identification of a 1,2,4,5-tetraoxane antimalarial drug-development candidate (RKA 182) with superior properties to the semisynthetic artemisinins. Angew. Chem. Int. Ed. 2010, 49, 5693–5697. [Google Scholar] [CrossRef]
- Vennerstrom, J.L.; Dong, Y.X.; Andersen, S.L.; Ager, A.L.; Fu, H.N.; Miller, R.E.; Wesche, D.L.; Kyle, D.E.; Gerena, L.; Walters, S.M.; et al. Synthesis and antimalarial activity of sixteen dispiro-1,2,4,5-tetraoxanes: Alkyl-substituted 7,8,15,16-tetraoxadispiro [5.2.5.2]hexadecanes. J. Med. Chem. 2000, 43, 2753–2758. [Google Scholar] [CrossRef]
- Opsenica, D.; Pocsfalvi, G.; Milhous, W.K.; Solaja, B.A. Antimalarial peroxides: The first intramolecular 1,2,4,5-tetraoxane. J. Serb. Chem. Soc. 2002, 67, 465–471. [Google Scholar] [CrossRef]
- Belic, I.; Kastelicsuhadolc, T.; Kavcic, R.; Marsel, J.; Kramer, V.; Kralj, B. Peroxides of higher aliphatic ethers. Tetrahedron 1976, 32, 3045–3049. [Google Scholar] [CrossRef]
- Terent’ev, A.O.; Yaremenko, I.A.; Chernyshev, V.V.; Dembitsky, V.M.; Nikishin, G.I. Selective Synthesis of Cyclic Peroxides from Triketones and H2O2. J. Org. Chem. 2012, 77, 1833–1842. [Google Scholar] [CrossRef]
- Ramirez, A.; Woerpel, K.A. Synthesis of 1,2-dioxolanes by annulation reactions of peroxycarbenium ions with alkenes. Org. Lett. 2005, 7, 4617–4620. [Google Scholar] [CrossRef]
- Terent’ev, A.O.; Borisov, D.A.; Vil’, V.A.; Dembitsky, V.M. Synthesis of five- and six-membered cyclic organic peroxides: Key transformations into peroxide ring-retaining products. Beilstein J. Org. Chem. 2014, 10, 34–114. [Google Scholar] [CrossRef] [Green Version]
- Gomes, G.D.; Yaremenko, I.A.; Radulov, P.S.; Novikov, R.A.; Chernyshev, V.V.; Korlyukov, A.A.; Nikishin, G.I.; Alabugin, I.V.; Terent’ev, A.O. Stereoelectronic Control in the Ozone-Free Synthesis of Ozonides. Angew. Chem. Int. Ed. 2017, 56, 4955–4959. [Google Scholar] [CrossRef] [PubMed]
- Vil, V.A.; Gomes, G.D.; Bityukov, O.V.; Lyssenko, K.A.; Nikishin, G.I.; Alabugin, I.V.; Terent’ev, A.O. Interrupted Baeyer-Villiger rearrangement: Building a stereoelectronic trap for the Criegee intermediate. Angew. Chem. Int. Ed. 2018, 57, 3372–3376. [Google Scholar] [CrossRef] [PubMed]
- Terent’ev, A.O.; Yaremenko, I.A.; Vil’, V.A.; Dembitsky, V.M.; Nikishin, G.I. Boron Trifluoride as an Efficient Catalyst for the Selective Synthesis of Tricyclic Monoperoxides from beta,delta-Triketones and H2O2. Synthesis 2013, 45, 246–250. [Google Scholar] [CrossRef] [Green Version]
- Dussault, P.H.; Lee, H.J.; Niu, Q.J. Peroxycarbenium-mediated C-C bond formation: Synthesis of cyclic peroxides from monoperoxyketals. J. Org. Chem. 1995, 60, 784–785. [Google Scholar] [CrossRef]
- Radulov, P.S.; Belyakova, Y.Y.; Demina, A.A.; Nikishin, G.I.; Yaremenko, I.A.; Terent’ev, A.O. Selective synthesis of cyclic triperoxides from 1,1-dihydroperoxydi(cycloalkyl)peroxides and acetals using SnCl4. Russ. Chem. Bull. 2019, 68, 1289–1292. [Google Scholar] [CrossRef]
- Terent’ev, A.O.; Kutkin, A.V.; Platonov, M.M.; Starikova, Z.A.; Ogibin, Y.N.; Nikishin, G.I. Synthesis of 1,1’-bishydroperoxydi(cycloalkyl) peroxides by homocoupling of 11-15-membered gem-bis (hydroperoxy)cycloalkanes in the presence of boron trifluoride. Russ. Chem. Bull. 2005, 54, 1214–1218. [Google Scholar] [CrossRef]
- Zmitek, K.; Zupan, M.; Stavber, S.; Iskra, J. The effect of iodine on the peroxidation of carbonyl compounds. J. Org. Chem. 2007, 72, 6534–6540. [Google Scholar] [CrossRef]
- Azarifar, D.; Khosravi, K.; Soleimanei, F. Stannous chloride dihydrate: A novel and efficient catalyst for the synthesis of gem-dihydroperoxides from ketones and aldehydes. Synthesis 2009, 15, 2553–2556. [Google Scholar] [CrossRef]
- Singh, K.; Bera, T.; Jaiswal, V.; Biswas, S.; Mondal, B.; Das, D.; Saha, J. Lewis acid catalyzed nucleophilic ring opening and 1,3-bisfunctionalization of donor-acceptor cyclopropanes with hydroperoxides: Access to highly functionalized peroxy/(alpha-heteroatom substituted)peroxy compounds. J. Org. Chem. 2019, 84, 710–725. [Google Scholar] [CrossRef]
- Yaremenko, I.A.; Vil, V.A.; Demchuk, D.V.; Terent’ev, A.O. Rearrangements of organic peroxides and related processes. Beilstein J. Org. Chem. 2016, 12, 1647–1748. [Google Scholar] [CrossRef] [Green Version]
- Terent’ev, A.O.; Pastukhova, Z.Y.; Yaremenko, I.A.; Novikov, R.A.; Demchuk, D.V.; Bruk, L.G.; Levitsky, D.O.; Fleury, F.; Nikishin, G.I. Selective transformation of tricyclic peroxides with pronounced antischistosomal activity into 2-hydroxy-1,5-diketones using iron (II) salts. Tetrahedron 2016, 72, 3421–3426. [Google Scholar] [CrossRef]
- Bartlett, P.D.; Cotman, J.D. Migration aptitude as a criterion of ionic mechanism in the rearrangement of mono-p-nitrotriphenylmethyl hydroperoxide. J. Am. Chem. Soc. 1950, 72, 3095–3099. [Google Scholar] [CrossRef]
- Hüttel, R.; Schmid, H.; Ross, H. Alkylhydroperoxyde aus alkylhalogeniden, II. Chem. Ber. 1959, 92, 699–704. [Google Scholar] [CrossRef]
- Jiang, H.; Chu, G.; Gong, H.; Qiao, Q.D. Tin chloride catalysed oxidation of acetone with hydrogen peroxide to tetrameric acetone peroxide. J. Chem. Res. 1999, 288–289. [Google Scholar] [CrossRef]
- Pettinari, C.; Marchetti, F.; Cingolani, A.; Drozdov, A.; Troyanov, S. Unexpected synthesis of (bis(diphenylphosphinoyl)ethane)center dot 2(2,2-dihydroperoxypropane) 1:2 adduct: A new route to stable organic dihydroperoxides. Chem. Commun. 2000, 1901–1902. [Google Scholar] [CrossRef]
- Yan, X.; Qiao, C.H.; Guo, Z.W. Tin(IV) chloride promoted reaction of oxiranes with hydrogen peroxide. Synlett 2013, 24, 502–506. [Google Scholar] [CrossRef]
- Dussault, P.H.; Lee, I.Q. Peroxycarbenium-mediated C-C bond formation: Synthesis of peroxides from monoperoxy ketals. J. Am. Chem. Soc. 1993, 115, 6458–6459. [Google Scholar] [CrossRef]
- Dussault, P.H.; Zope, U. Hydroperoxide-mediated C-C bond formation: Synthesis of 1,2-dioxolanes from alkoxyhydroperoxides in the presence of Lewis-acids. Tetrahedron Lett. 1995, 36, 3655–3658. [Google Scholar] [CrossRef]
- Dussault, P.H.; Lee, I.Q.; Lee, H.J.; Lee, R.J.; Niu, Q.J.; Schultz, J.A.; Zope, U.R. Peroxycarbenium-mediated C-C bond formation: Aplications to the synthesis of hydroperoxides and peroxides. J. Org. Chem. 2000, 65, 8407–8414. [Google Scholar] [CrossRef]
- Dussault, P.H.; Liu, X.J. SnCl4-mediated reaction of ozonides with allyltrimethylsilane: Formation of 1,2-dioxolanes. Tetrahedron Lett. 1999, 40, 6553–6556. [Google Scholar] [CrossRef]
- Dussault, P.H.; Lee, H.J.; Liu, X.J. Selectivity in Lewis acid-mediated fragmentations of peroxides and ozonides: Application to the synthesis of alkenes, homoallyl ethers, and 1,2-dioxolanes. J. Chem. Soc. Perkin Trans. 1 2000, 3006–3013. [Google Scholar] [CrossRef]
- Pinet, A.; Nguyen, T.L.; Bernadat, G.; Figadere, B.; Ferrie, L. Synthesis of 3,5-disubstituted 1,2-dioxolanes through the use of acetoxy peroxyacetals. Org. Lett. 2019, 21, 4729–4733. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.F.; Dong, Y.X.; Wittlin, S.; Creek, D.; Chollet, J.; Charman, S.A.; Santo Tomas, J.; Scheurer, C.; Snyder, C.; Vennerstrom, J.L. Spiro- and dispiro-1,2-dioxolanes: Contribution of iron(II)-mediated one-electron vs. two-electron reduction to the activity of antimalarial peroxides. J. Med. Chem. 2007, 50, 5840–5847. [Google Scholar] [CrossRef] [PubMed]
- Hurlocker, B.; Miner, M.R.; Woerpel, K.A. Synthesis of Silyl Monoperoxyketals by Regioselective Cobalt-Catalyzed Peroxidation of Silyl Enol Ethers: Application to the Synthesis of 1,2-Dioxolanes. Org. Lett. 2014, 16, 4280–4283. [Google Scholar] [CrossRef] [PubMed]
- Martyn, D.C.; Ramirez, A.P.; Berattie, M.J.; Cortese, J.F.; Patel, V.; Rush, M.A.; Woerpel, K.A.; Clardy, J. Synthesis of spiro-1,2-dioxolanes and their activity against Plasmodium falciparum. Bioorg. Med. Chem. Lett. 2008, 18, 6521–6524. [Google Scholar] [CrossRef]
- Zhao, Q.J.; Vargas, M.; Dong, Y.X.; Zhou, L.; Wang, X.F.; Sriraghavan, K.; Keiser, J.; Vennerstrom, J.L. Structure-activity relationship of an ozonide carboxylic acid (OZ78) against Fasciola hepatica. J. Med. Chem. 2010, 53, 4223–4233. [Google Scholar] [CrossRef] [Green Version]
- Dussault, P.H.; Lee, R.J.; Schultz, J.A.; Suh, Y.S. Reaction of peroxyacetals with silyl ketene acetals: Synthesis of 3-peroxyalkanoates and 3-peroxyalkanals. Tetrahedron Lett. 2000, 41, 5457–5460. [Google Scholar] [CrossRef]
- Dai, P.; Trullinger, T.K.; Liu, X.J.; Dussault, P.H. Asymmetric synthesis of 1,2-dioxolane-3-acetic acids: Synthesis and configurational assignment of plakinic acid A. J. Org. Chem. 2006, 71, 2283–2292. [Google Scholar] [CrossRef]
- Marson, C.M.; Khan, A.; Porter, R.A. Stereocontrolled formation of epoxy peroxide functionality appended to a lactam ring. J. Org. Chem. 2001, 66, 4771–4775. [Google Scholar] [CrossRef]
- Azarifar, D.; Khosravi, K. Trans-3,5-dihydroperoxy-3,5-dimethyl-1,2-dioxolane as a novel and efficient reagent for selective sulfoxidation of sulfides under catalyst-free condition. Eur. J. Chem. 2010, 1, 15–19. [Google Scholar] [CrossRef]
- Azarifar, D.; Khosravi, K. AlCl3.6H2O as a catalyst for simple and efficient synthesis of gem-dihydroperoxides from ketones and aldehydes using aqueous H2O2. J. Iran. Chem. Soc. 2011, 8, 1006–1013. [Google Scholar] [CrossRef]
- Gamage, N.D.H.; Stiasny, B.; Kratz, E.G.; Stierstorfer, J.; Martin, P.D.; Cisneros, G.A.; Klapotke, T.M.; Winter, C.H. Energetic materials trends in 5-and 6-membered cyclic peroxides containing hydroperoxy and hydroxy substituents. Eur. J. Inorg. Chem. 2016, 2016, 5036–5043. [Google Scholar] [CrossRef]
- Jost, C.; Wahl, G.; Kleinhenz, D.; Sundermeyer, J. Peroxo complexes of molybdenum, tungsten and rhenium with phase transfer active ligands: Catalysts for the oxidation of olefins and aromatics by hydrogen peroxide and bistrimethylsilyl peroxide. In Peroxide Chemistry; Wiley-VCH: Weinheim, Germany, 2000; pp. 341–364. [Google Scholar] [CrossRef]
- Nabavizadeh, S.M. Thermodynamic studies of the binding of bidentate nitrogen donors with methyltrioxorhenium (MTO) in CHCl3 solution. Dalton Trans. 2005, 1644–1648. [Google Scholar] [CrossRef] [PubMed]
- Hwang, T.; Goldsmith, B.R.; Peters, B.; Scott, S.L. Water-catalyzed activation of H2O2 by methyltrioxorhenium: A combined computational-experimental study. Inorg. Chem. 2013, 52, 13904–13917. [Google Scholar] [CrossRef]
- Zmitek, K.; Stavber, S.; Zupan, M.; Bonnet-Delpon, D.; Charneau, S.; Grellier, P.; Iskra, J. Synthesis and antimalarial activities of novel 3,3,6,6-tetraalkyl-1,2,4,5-tetraoxanes. Bioorg. Med. Chem. 2006, 14, 7790–7795. [Google Scholar] [CrossRef]
- Atheaya, H.; Khan, S.I.; Mamgain, R.; Rawat, D.S. Synthesis, thermal stability, antimalarial activity of symmetrically and asymmetrically substituted tetraoxanes. Bioorg. Med. Chem. Lett. 2008, 18, 1446–1449. [Google Scholar] [CrossRef]
- Kumar, N.; Khan, S.I.; Atheaya, H.; Mamgain, R.; Rawat, D.S. Synthesis and in vitro antimalarial activity of tetraoxane-amine/amide conjugates. Eur. J. Med. Chem. 2011, 46, 2816–2827. [Google Scholar] [CrossRef]
- Iskra, J.; Bonnet-Delpon, D.; Begue, J.P. One-pot synthesis of non-symmetric tetraoxanes with the H2O2/MTO/fluorous alcohol system. Tetrahedron Lett. 2003, 44, 6309–6312. [Google Scholar] [CrossRef]
- Ellis, G.L.; Amewu, R.; Sabbani, S.; Stocks, P.A.; Shone, A.; Stanford, D.; Gibbons, P.; Davies, J.; Vivas, L.; Charnaud, S.; et al. Two-step synthesis of achiral dispiro-1,2,4,5-tetraoxanes with outstanding antimalarial activity, low toxicity, and high-stability profiles. J. Med. Chem. 2008, 51, 2170–2177. [Google Scholar] [CrossRef]
- Liu, H.H.; Jin, H.X.; Zhang, Q.; Wu, Y.K.; Kim, H.S.; Wataya, Y. Synthesis and in vitro antimalarial activity of several simple analogues of peroxyplakoric acid. Chin. J. Chem. 2005, 23, 1469–1473. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, Y. Simplified analogues of qinghaosu (artemisinin). Tetrahedron 2007, 63, 10407–10414. [Google Scholar] [CrossRef]
- Singh, K.; Kumar, P.; Jagadeesh, C.; Patel, M.; Das, D.; Saha, J. An approach to alpha- and beta-amino peroxides via lewis acid catalyzed ring opening-peroxidation of donor-acceptor aziridines and n-activated aziridines. Adv. Synth. Catal. 2020, 362, 4130–4137. [Google Scholar] [CrossRef]
- Dussault, P.H.; Trullinger, T.K.; Noor-e-Ain, F. Opening of substituted oxetanes with H2O2 and alkyl hydroperoxides: Stereoselective approach to 3-peroxyalcohols and 1,2,4-trioxepanes. Org. Lett. 2002, 4, 4591–4593. [Google Scholar] [CrossRef] [PubMed]
- Pinet, A.; Cojean, S.; Nguyen, L.T.; Vasquez-Ocmin, P.; Maciuk, A.; Loiseau, P.M.; Le Pape, P.; Figadere, B.; Ferrie, L. Anti-protozoal and anti-fungal evaluation of 3,5-disubstituted 1,2-dioxolanes. Bioorg. Med. Chem. Lett. 2021, 47, 128196. [Google Scholar] [CrossRef]
- Pinet, A.; Figadere, B.; Ferrie, L. Access to functionalized 3,5-disubstituted 1,2-dioxolanes under mild conditions through indium(III) chloride/trimethylsilyl chloride or scandium(III) triflate catalysis. Adv. Synth. Catal. 2020, 362, 1190–1194. [Google Scholar] [CrossRef]
- Eske, A.; Ecker, S.; Fendinger, C.; Goldfuss, B.; Jonen, M.; Lefarth, J.; Neudorfl, J.M.; Spilles, M.; Griesbeck, A.G. Spirofused and annulated 1,2,4-trioxepane-, 1,2,4-trioxocane-, and 1,2,4-trioxonane-cyclohexadienones: Cyclic peroxides with unusual ring conformation dynamics. Angew. Chem. Int. Ed. 2018, 57, 13770–13774. [Google Scholar] [CrossRef] [PubMed]
- Bloodworth, A.J.; Griffin, I.M. Oxymetallation. Part VI. Halogenodemercuration of peroxymercurials derived from αβ-unsaturated esters and ketones. J. Chem. Soc. Perkin Trans. 1 1974, 688–695. [Google Scholar] [CrossRef]
- Bloodworth, A.J.; Courtneidge, J.L. Oxymetalation. Part 17. t-Butyl peroxymercuriation and subsequent demercuriation of phenylcyclopropane. J. Chem. Soc. Perkin Trans. 1 1982, 1807–1809. [Google Scholar] [CrossRef]
- Bloodworth, A.J.; Loveitt, M.E. Peroxymercuration of dienes-simple route to new cyclic peroxides. J. Chem. Soc. Chem. Commun. 1976, 94–95. [Google Scholar] [CrossRef]
- Adam, W.; Bloodworth, A.J.; Eggelte, H.J.; Loveitt, M.E. Regioselective synthesis of isomeric bicyclic peroxides. Angew. Chem. Int. Ed. 1978, 17, 209–210. [Google Scholar] [CrossRef]
- Bloodworth, A.J.; Loveitt, M.E. Oxymetalation. Part 11. Synthesis of cyclic secondary alkyl peroxides via peroxymercuration of alpha,omega-dienes. J. Chem. Soc. Perkin Trans. 1 1978, 522–530. [Google Scholar] [CrossRef]
- Nixon, J.R.; Cudd, M.A.; Porter, N.A. Cyclic peroxides by intra-molecular peroxymercuration of unsaturated hydroperoxides. J. Org. Chem. 1978, 43, 4048–4052. [Google Scholar] [CrossRef]
- Bloodworth, A.J.; Spencer, M.D. Oxymetalation. XXII. Hydroperoxymercuriation using 30% hydrogen peroxide. J. Organomet. Chem. 1990, 386, 299–304. [Google Scholar] [CrossRef]
- Bloodworth, A.J.; Cooksey, C.J.; Korkodilos, D. Synthesis of alkyl hydroperoxides by hydroperoxymercuriation and reduction. J. Chem. Soc. Chem. Commun. 1992, 926–927. [Google Scholar] [CrossRef]
- Bloodworth, A.J.; Korkodilos, D. Mercury(II)-mediated cyclization of hydroperoxyalkylcyclopropanes—A new route to cyclic peroxides. Tetrahedron Lett. 1991, 32, 6953–6956. [Google Scholar] [CrossRef]
- Bloodworth, A.J.; Bothwell, B.D.; Collins, A.N.; Maidwell, N.L. A short synthesis of naturally occurring and other analogues of plakinic acids that contain the 1,2-dioxolane group. Tetrahedron Lett. 1996, 37, 1885–1888. [Google Scholar] [CrossRef]
- Liu, Y.H.; Zhang, Z.H.; Li, T.S. Efficient conversion of epoxides into beta-hydroperoxy alcohols catalyzed by antimony trichloride/SiO2. Synthesis 2008, 20, 3314–3318. [Google Scholar] [CrossRef]
- Azarifar, D.; Khosravi, K.; Soleimanei, F. Mild and efficient strontium chloride hexahydrate-catalyzed conversion of ketones and aldehydes into corresponding gem-dihydroperoxides by aqueous H2O2. Molecules 2010, 15, 1433–1441. [Google Scholar] [CrossRef] [Green Version]
- Das, B.; Krishnaiah, M.; Veeranjaneyulu, B.; Ravikanth, B. A simple and efficient synthesis of gem-dihydroperoxides from ketones using aqueous hydrogen peroxide and catalytic ceric ammonium nitrate. Tetrahedron Lett. 2007, 48, 6286–6289. [Google Scholar] [CrossRef]
- Sashidhara, K.V.; Avula, S.R.; Singh, L.R.; Palnati, G.R. A facile and efficient Bi(III) catalyzed synthesis of 1,1-dihydroperoxides and 1,2,4,5-tetraoxanes. Tetrahedron Lett. 2012, 53, 4880–4884. [Google Scholar] [CrossRef]
- Wang, X.F.; Zhao, Q.J.; Vargas, M.; Dong, Y.X.; Sriraghavan, K.; Keiser, J.; Vennerstrom, J.L. The activity of dispiro peroxides against Fasciola hepatica. Bioorg. Med. Chem. Lett. 2011, 21, 5320–5323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miura, M.; Nojima, M. Formation of 3,6-dialkyl-1,2,4,5-tetraoxans and related cyclic bis-(peroxides) by the action of antimony pentachloride or chlorosulfonic acid on ozonides. J. Chem. Soc. Chem. Commun. 1979, 467–468. [Google Scholar] [CrossRef]
- Harris, J.R.; Waetzig, S.R.; Woerpel, K.A. Palladium(II)-catalyzed cyclization of unsaturated hydroperoxides for the synthesis of 1,2-dioxanes. Org. Lett. 2009, 11, 3290–3293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell, E.R.; Vaughan, W.E. Preparation of Organic Hydroperoxides. US2630456A, 3 March 1953. [Google Scholar]
- Davies, A.G.; Foster, R.V.; White, A.M. 314. Organic peroxides. Part I. The preparation of alkyl hydroperoxides from hydrogen peroxide. J. Chem. Soc. 1953, 1541–1547. [Google Scholar] [CrossRef]
- Barthen, P.; Frank, W. The ‘super acid’ BF3.H2O stabilized by 1,4-dioxane: New preparative aspects and the crystal structure of BH3H2O.C4H8O2. Acta Crystallogr. Sect. E Crystallogr. Commun. 2019, 75, 1787–1791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.T.; Zhang, X.H.; Ling, X.G.; He, C.; Huang, R.F.; Pan, J.; Li, J.Q.; Xiong, Y. Superacid BF3-H2O promoted benzylation of arenes with benzyl alcohols and acetates initiated by trace water. RSC Adv. 2014, 4, 30768–30774. [Google Scholar] [CrossRef]
- Prakash, G.K.S.; Paknia, F.; Mathew, T.; Mloston, G.; Joschek, J.P.; Olah, G.A. Fluoroanalogs of DDT: Superacidic BF3-H2O catalyzed facile synthesis of 1,1,1-trifluoro-2,2-diarylethanes and 1,1-difluoro-2,2-diarylethanes. Org. Lett. 2011, 13, 4128–4131. [Google Scholar] [CrossRef]
- Prakash, G.K.S.; Mathew, T.; Hoole, D.; Esteves, P.M.; Wang, Q.; Rasul, G.; Olah, G.A. N-Halosuccinimide/BF3-H2O, efficient electrophilic halogenating systems for aromatics. J. Am. Chem. Soc. 2004, 126, 15770–15776. [Google Scholar] [CrossRef]
- Prakash, G.K.S.; Panja, C.; Shakhmin, A.; Shah, E.; Mathew, T.; Olah, G.A. BF3-H2O catalyzed hydroxyalkylation of aromatics with aromatic aldehydes and dicarboxaldehydes: Efficient synthesis of triarylmethanes, diarylmethylbenzaldehydes, and anthracene derivatives. J. Org. Chem. 2009, 74, 8659–8668. [Google Scholar] [CrossRef]
- Tkach, V.S.; Suslov, D.S.; Myagmarsuren, G.; Gubaydulina, O.V.; Bykov, M.V.; Umanets, V.A. Highly effective catalysts for the addition polymerization of norbornene: Zerovalent-nickel complex/H2O/BF3.OEt2. Catal. Commun. 2009, 10, 1813–1815. [Google Scholar] [CrossRef]
- Gross, A. Production of Per-Fatty Acids. US2806045A, 10 September 1957. [Google Scholar]
- Ropp, W.S. Monoperoxyacetals. US2776319A, 1 January 1957. [Google Scholar]
- Bourgeois, M.J.; Montaudon, E.; Maillard, B. Novel synthesis of asymmetric alkyl peroxides using tertiary alcohols. Tetrahedron 1993, 49, 2477–2484. [Google Scholar] [CrossRef]
- Terent’ev, A.O.; Kutkin, A.V.; Platonov, M.M.; Ogibin, Y.N.; Nikishin, G.I. A new method for the synthesis of bishydroperoxides based on a reaction of ketals with hydrogen peroxide catalyzed by boron trifluoride complexes. Tetrahedron Lett. 2003, 44, 7359–7363. [Google Scholar] [CrossRef]
- Terent’ev, A.O.; Kutkin, A.V.; Platonov, M.M.; Vorontsov, I.I.; Antipin, M.Y.; Ogibin, Y.N.; Nikishin, G.I. Synthesis of peroxide compounds by the BF3-catalyzed reaction of acetals and enol ethers with H2O2. Russ. Chem. Bull. 2004, 53, 681–687. [Google Scholar] [CrossRef]
- Terent’ev, A.O.; Kutkin, A.V.; Troizky, N.A.; Ogibin, Y.N.; Nikishin, G.I. Synthesis of geminal bisperoxides by acid-catalyzed reaction of acetals and enol ethers with tert-butyl hydroperoxide. Synthesis 2005, 13, 2215–2219. [Google Scholar] [CrossRef]
- Zhang, Q.; Jin, H.X.; Liu, H.H.; Wu, Y.K. Synthesis of a nitro analogue of plakoric acid. Chin. J. Chem. 2006, 24, 1190–1195. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Y.; Wu, Y.K. Synthesis of a 1,2,7,8-tetraoxa-spiro [5.5]undecane. Chin. J. Chem. 2007, 25, 1304–1308. [Google Scholar] [CrossRef]
- Bartoschek, A.; El-Idreesy, T.; Griesbeck, A.G.; Hoinck, L.O.; Lex, J.; Miara, C.; Neudorfl, J.M. A family of new 1,2,4-trioxanes by photooxygenation of allylic alcohols in sensitizer-doped polymers and secondary reactions. Synthesis 2005, 14, 2433–2444. [Google Scholar] [CrossRef]
- Griesbeck, A.G.; El-Idreesy, T.T.; Hoinck, L.O.; Lex, J.; Brun, R. Novel spiroanellated 1,2,4-trioxanes with high in vitro antimalarial activities. Bioorg. Med. Chem. Lett. 2005, 15, 595–597. [Google Scholar] [CrossRef]
- Griesbeck, A.G.; Hoinck, L.O.; Lex, J.; Neudorfl, J.; Blunk, D.; El-Idreesy, T.T. 1,2,5,10,11,14-hexaoxadispiro [5.2.5.2]hexadecanes: Novel spirofused bis-trioxane peroxides. Molecules 2008, 13, 1743–1758. [Google Scholar] [CrossRef]
- Griesbeck, A.G.; El-Idreesy, T.T.; Lex, J. Singlet oxygen addition to chiral allylic alcohols and subsequent peroxyacetalization with beta-naphthaldehyde: Synthesis of diastereomerically pure 3-beta-naphthyl-substituted 1,2,4-trioxanes. Tetrahedron 2006, 62, 10615–10622. [Google Scholar] [CrossRef]
- Yoshida, M.; Miura, M.; Nojima, M.; Kusabayashi, S. Synthesis and decomposition of E- and Z-3,3,5-trisubstituted 1,2-dioxolanes. J. Am. Chem. Soc. 1983, 105, 6279–6285. [Google Scholar] [CrossRef]
- Terent’ev, A.O.; Kutkin, A.V.; Starikova, Z.A.; Antipin, M.Y.; Ogibin, Y.N.; Nikishina, G.I. New preparation of 1,2,4,5-tetraoxanes. Synthesis 2004, 2004, 2356–2366. [Google Scholar] [CrossRef]
- Hamann, H.J.; Hecht, M.; Bunge, A.; Gogol, M.; Liebscher, J. Synthesis and antimalarial activity of new 1,2,4,5-tetroxanes and novel alkoxy-substituted 1,2,4,5-tetroxanes derived from primary gem-dihydroperoxides. Tetrahedron Lett. 2011, 52, 107–111. [Google Scholar] [CrossRef]
- Niesen, A.; Barthel, A.; Kluge, R.; Kowitzsch, A.; Strohl, D.; Schwarz, S.; Csuk, R. Antitumoractive endoperoxides from triterpenes. Pharm. Med. Chem. 2009, 342, 569–576. [Google Scholar] [CrossRef]
- Terent’ev, A.O.; Platonov, M.M.; Sonneveld, E.J.; Peschar, R.; Chernyshev, V.V.; Starikova, Z.A.; Nikishin, G.I. New preparation of 1,2,4,5,7,8-hexaoxonanes. J. Org. Chem. 2007, 72, 7237–7243. [Google Scholar] [CrossRef]
- Griesbeck, A.G.; Blunk, D.; El-Idreesy, T.T.; Raabe, A. Bicyclic peroxides and perorthoesters with 1,2,4-trioxane structures. Angew. Chem. Int. Ed. 2007, 46, 8883–8886. [Google Scholar] [CrossRef]
- Yaremenko, I.A.; Gomes, G.D.; Radulov, P.S.; Belyakova, Y.Y.; Vilikotskiy, A.E.; Vil’, V.A.; Korlyukov, A.A.; Nikishin, G.I.; Alabugin, I.V.; Terent’ev, A.O. Ozone-Free Synthesis of Ozonides: Assembling Bicyclic Structures from 1,5-Diketones and Hydrogen Peroxide. J. Org. Chem. 2018, 83, 4402–4426. [Google Scholar] [CrossRef]
- Coghi, P.; Yaremenko, I.A.; Prommana, P.; Radulov, P.S.; Syroeshkin, M.A.; Wu, Y.J.; Gao, J.Y.; Gordillo, F.M.; Mok, S.; Wong, V.K.W.; et al. Novel Peroxides as Promising Anticancer Agents with Unexpected Depressed Antimalarial Activity. ChemMedChem 2018, 13, 902–908. [Google Scholar] [CrossRef]
- Yaremenko, I.A.; Coghi, P.; Prommana, P.; Qiu, C.L.; Radulov, P.S.; Qu, Y.Q.; Belyakova, Y.Y.; Zanforlin, E.; Kokorekin, V.A.; Wu, Y.Y.J.; et al. Synthetic peroxides promote apoptosis of cancer cells by inhibiting p-glycoprotein ABCB5. ChemMedChem 2020, 15, 1118–1127. [Google Scholar] [CrossRef]
- Vil, V.A.; Gomes, G.D.; Ekimova, M.V.; Lyssenko, K.A.; Syroeshkin, M.A.; Nikishin, G.I.; Alabugin, I.V.; Terent’ev, A.O. Five roads that converge at the cyclic peroxy-criegee intermediates: BF3-catalyzed synthesis of beta-hydroperoxy-beta-peroxylactones. J. Org. Chem. 2018, 83, 13427–13445. [Google Scholar] [CrossRef]
- Vil, V.A.; Barsegyan, Y.A.; Barsukov, D.V.; Korlyukov, A.A.; Alabugin, I.V.; Terent’ev, A.O. Peroxycarbenium Ions as the “Gatekeepers” in Reaction Design: Assistance from Inverse Alpha-Effect in Three-Component beta-Alkoxy-beta-peroxylactones Synthesis. Chem. A Eur. J. 2019, 25, 14460–14468. [Google Scholar] [CrossRef] [PubMed]
- Vil, V.A.; Barsegyan, Y.A.; Kuhn, L.; Ekimova, M.V.; Semenov, E.A.; Korlyukov, A.A.; Terent’ev, A.O.; Alabugin, I.V. Synthesis of unstrained Criegee intermediates: Inverse alpha-effect and other protective stereoelectronic forces can stop Baeyer-Villiger rearrangement of gamma-hydroperoxy-gamma-peroxylactones. Chem. Sci. 2020, 11, 5313–5322. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Holte, D.; Zoller, J.; Umemiya, S.; Simke, L.R.; Baran, P.S. Total Synthesis of Verruculogen and Fumitremorgin A Enabled by Ligand-Controlled C-H Borylation. J. Am. Chem. Soc. 2015, 137, 10160–10163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bulfield, D.; Huber, S.M. Halogen bonding in organic synthesis and organocatalysis. Chem. A Eur. J. 2016, 22, 14434–14450. [Google Scholar] [CrossRef]
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. The halogen bond. Chem. Rev. 2016, 116, 2478–2601. [Google Scholar] [CrossRef] [Green Version]
- Mikherdov, A.S.; Novikov, A.S.; Boyarskiy, V.P.; Kukushkin, V.Y. The halogen bond with isocyano carbon reduces isocyanide odor. Nat. Commun. 2020, 11, 2921. [Google Scholar] [CrossRef]
- Guha, S.; Kazi, I.; Mukherjee, P.; Sekar, G. Halogen-bonded iodonium ion catalysis: A route to alpha-hydroxy ketones via domino oxidations of secondary alcohols and aliphatic C-H bonds with high selectivity and control. Chem. Commun. 2017, 53, 10942–10945. [Google Scholar] [CrossRef]
- Jungbauer, S.H.; Walter, S.M.; Schindler, S.; Rout, L.; Kniep, F.; Huber, S.M. Activation of a carbonyl compound by halogen bonding. Chem. Commun. 2014, 50, 6281–6284. [Google Scholar] [CrossRef]
- Liu, X.L.; Ma, S.; Toy, P.H. Halogen bond-catalyzed friedel-crafts reactions of aldehydes and ketones using a bidentate halogen bond donor catalyst: Synthesis of symmetrical bis(indolyl)methanes. Org. Lett. 2019, 21, 9212–9216. [Google Scholar] [CrossRef]
- Breugst, M.; von der Heiden, D. Mechanisms in iodine catalysis. Chem. A Eur. J. 2018, 24, 9187–9199. [Google Scholar] [CrossRef] [PubMed]
- Terent’ev, A.O.; Krylov, I.B.; Borisov, D.A.; Nikishin, G.I. A new approach to the synthesis of vicinal iodoperoxyalkanes by the reaction of alkenes with iodine and hydroperoxides. Synthesis 2007, 2007, 2979–2986. [Google Scholar] [CrossRef]
- Dussault, P.H.; Davies, D.R. Synthesis of 1,2-dioxanes, 1,2,4-trioxanes, and 1,2,4-trioxepanes via cyclizations of unsaturated hydroperoxyacetals. Tetrahedron Lett. 1996, 37, 463–466. [Google Scholar] [CrossRef]
- Tropina, V.I.; Krivykh, O.V.; Sadchikova, N.P.; Terent’ev, A.O.; Krylov, I.B. Synthesis and antimicrobial activity of geminal bis-hydroperoxides. Pharm. Chem. J. 2010, 44, 248–250. [Google Scholar] [CrossRef]
- Terent’ev, A.O.; Borisov, A.M.; Platonov, M.M.; Starikova, Z.A.; Chernyshev, V.V.; Nikishin, G.I. Reaction of enol ethers with the I2-H2O2 system: Synthesis of 2-iodo-1-methoxy hydroperoxides and their deperoxidation and demethoxylation to 2-iodo ketones. Synthesis 2009, 24, 4159–4166. [Google Scholar] [CrossRef]
- Terent’ev, A.O.; Zdvizhkov, A.T.; Kulakova, A.N.; Novikov, R.A.; Arzumanyan, A.V.; Nikishin, G.I. Reactions of mono- and bicyclic enol ethers with the I2-hydroperoxide system. RSC Adv. 2014, 4, 7579–7587. [Google Scholar] [CrossRef]
- Terent’ev, A.O.; Platonov, M.M.; Krylov, I.B.; Chernyshev, V.V.; Nikishin, G.I. Synthesis of 1-hydroperoxy-1’-alkoxyperoxides by the iodine-catalyzed reactions of geminal bishydroperoxides with acetals or enol ethers. Org. Biomol. Chem. 2008, 6, 4435–4441. [Google Scholar] [CrossRef]
- Zdvizhkov, A.T.; Terent’ev, A.O.; Radulov, P.S.; Novikov, R.A.; Tafeenko, V.A.; Chernyshev, V.V.; Ilovaisky, A.I.; Levitsky, D.O.; Fleury, F.; Nikishin, G.I. Transformation of 2-allyl-1,3-diketones to bicyclic compounds containing 1,2-dioxolane and tetrahydrofuran rings using the I-2/H2O2 system. Tetrahedron Lett. 2016, 57, 949–952. [Google Scholar] [CrossRef]
- Terent’ev, A.O.; Platonov, M.M.; Krylov, I.B.; Nikishin, G.I. Synthesis of 1,2,4,5,7,8-hexaoxonanes by iodine-catalyzed reactions of bis(1-hydroperoxycycloalkyl) peroxides with ketals. Russ. Chem. Bull. 2009, 58, 335–338. [Google Scholar] [CrossRef]
- Jefford, C.W.; Jaber, A.; Boukouvalas, J. Efficient preparation of 1,2,4,5-tetroxanes from bis(trimethylsilyl) peroxide and carbonyl-compounds. Synthesis 1988, 5, 391–393. [Google Scholar] [CrossRef]
- McCullough, K.J.; Nonami, Y.; Masuyama, A.; Nojima, M.; Kim, H.S.; Wataya, Y. Synthesis, crystal structure and antimalarial activity of novel 1,2,5,6-tetraoxacycloalkanes from 2,3-dihydroperoxy-2-phenylnorbornane. Tetrahedron Lett. 1999, 40, 9151–9155. [Google Scholar] [CrossRef]
- Opsenica, D.; Pocsfalvi, G.; Juranic, Z.; Tinant, B.; Declercq, J.P.; Kyle, D.E.; Milhous, W.K.; Solaja, B.A. Cholic acid derivatives as 1,2,4,5-tetraoxane carriers: Structure and antimalarial and antiproliferative activity. J. Med. Chem. 2000, 43, 3274–3282. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Begum, E.; Ogura, N.; Wataya, Y.; Nonami, Y.; Ito, T.; Masuyama, A.; Nojima, M.; McCullough, K.J. Antimalarial activity of novel 1,2,5,6-tetraoxacycloalkanes and 1,2,5-trioxacycloalkanes. J. Med. Chem. 2003, 46, 1957–1961. [Google Scholar] [CrossRef] [PubMed]
- Jefford, C.W.; Rossier, J.C.; Richardson, G.D. The reaction of trimethylsilyl alpha-trimethylsilylperoxy esters with ketones and aldehydes—A simple, efficient synthesis of 1,2,4-trioxan-5-one. J. Chem. Soc. Chem. Commun. 1983, 1064–1065. [Google Scholar] [CrossRef]
- Jefford, C.W.; Currie, J.; Richardson, G.D.; Rossier, J.C. The synthesis of 1,2,4-trioxan-5-ones. Helv. Chim. Acta 1991, 74, 1239–1246. [Google Scholar] [CrossRef]
- Jefford, C.W.; Boukouvalas, J.; Kohmoto, S. Reactions of cyclic peroxides with aldehydes and ketones catalyzed by trimethylsilyl trifluoromethanesulfonate—An efficient synthesis of 1,2,4-trioxanes. J. Chem. Soc. Chem. Commun. 1984, 523–524. [Google Scholar] [CrossRef]
- Jefford, C.W.; Kohmoto, S.; Jaggi, D.; Timari, G.; Rossier, J.C.; Rudaz, M.; Barbuzzi, O.; Gerard, D.; Burger, U.; Kamalaprija, P.; et al. Synthesis, structure, and antimalarial activity of some enantiomerically pure, cis-fused cyclopenteno-1,2,4-trioxanes. Helv. Chim. Acta 1995, 78, 647–662. [Google Scholar] [CrossRef]
- Dechy-Cabaret, O.; Robert, A.; Meunier, B. Synthesis and stereochemical study of a trioxaquine prepared from cis-bicyclo [3.3.0]octane-3,7-dione. Comptes Rendus Chim. 2002, 5, 297–302. [Google Scholar] [CrossRef]
- Dechy-Cabaret, O.; Benoit-Vical, F.; Loup, C.; Robert, A.; Gornitzka, H.; Bonhoure, A.; Vial, H.; Magnaval, J.F.; Seguela, J.P.; Meunier, B. Synthesis and antimalarial activity of trioxaquine derivatives. Chem. A Eur. J. 2004, 10, 1625–1636. [Google Scholar] [CrossRef]
- Ushigoe, Y.; Masuyama, A.; Nojima, M.; McCullough, K.J. New methods for the synthesis of oxy-functionalized 1,2,4-trioxanes and 1,2,4-trioxepanes from unsaturated hydroperoxy acetals. Tetrahedron Lett. 1997, 38, 8753–8756. [Google Scholar] [CrossRef]
- Jefford, C.W.; Jin, S.J.; Kamalaprija, P.; Burger, U.; Bernardinelli, G. Synthesis of 1,2-dioxanes from an endoperoxide. Tetrahedron Lett. 1992, 33, 7129–7132. [Google Scholar] [CrossRef]
- O’Neill, P.M.; Rawe, S.L.; Storr, R.C.; Ward, S.A.; Posner, G.H. Lewis acid catalysed rearrangements of unsaturated bicyclic [2.2.n] endoperoxides in the presence of vinyl silanes; access to novel Fenozan BO-7 analogues. Tetrahedron Lett. 2005, 46, 3029–3032. [Google Scholar] [CrossRef]
- Tokuyasu, T.; Ito, T.; Masuyama, A.; Nojima, M. Synthesis of 3-hydroperoxy (or hydroxy)substituted 1,2-dioxanes and 1,2-dioxepanes by the ozonolysis of unsaturated hydroperoxy acetals. Heterocycles 2000, 53, 1293–1304. [Google Scholar] [CrossRef]
- Posner, G.H.; Oh, C.H.; Milhous, W.K. Olefin oxidative cleavage and dioxetane formation using triethylsilyl hydrotrioxide—Applications to preparation of potent antimalarial 1,2,4-trioxanes. Tetrahedron Lett. 1991, 32, 4235–4238. [Google Scholar] [CrossRef]
- Posner, G.H.; Oh, C.H.; Gerena, L.; Milhous, W.K. Extraordinarily potent antimalarial compounds—New, structurally simple, easily synthesized, tricyclic 1,2,4-trioxanes. J. Med. Chem. 1992, 35, 2459–2467. [Google Scholar] [CrossRef] [PubMed]
- Posner, G.H.; Oh, C.H.; Gerena, L.; Milhous, W.K. Synthesis and antimalarial activities of structurally simplified 1,2,4-trioxanes related to Artemisinin. Heteroat. Chem. 1995, 6, 105–116. [Google Scholar] [CrossRef]
- Cointeaux, L.; Berrien, J.F.; Mahuteau, J.; Huu-Dau, M.E.T.; Ciceron, L.; Danis, M.; Mayrargue, J. A short synthesis of antimalarial peroxides. Bioorg. Med. Chem. 2003, 11, 3791–3794. [Google Scholar] [CrossRef]
- Terent’ev, A.O.; Yaremenko, I.A.; Vil, V.A.; Moiseev, I.K.; Kon’kov, S.A.; Dembitsky, V.M.; Levitsky, D.O.; Nikishin, G.I. Phosphomolybdic and phosphotungstic acids as efficient catalysts for the synthesis of bridged 1,2,4,5-tetraoxanes from β-diketones and hydrogen peroxide. Org. Biomol. Chem. 2013, 11, 2613–2623. [Google Scholar] [CrossRef]
- Yaremenko, I.A.; Terent’ev, A.O.; Vil, V.A.; Novikov, R.A.; Chernyshev, V.V.; Tafeenko, V.A.; Levitsky, D.O.; Fleury, F.; Nikishin, G.I. Approach for the preparation of various classes of peroxides based on the reaction of triketones with H2O2: First examples of ozonide rearrangements. Chem. A Eur. J. 2014, 20, 10160–10169. [Google Scholar] [CrossRef]
- Khosravi, K.; Zendehdel, M.; Naserifar, S.; Tavakoli, F.; Khalaji, K.; Asgari, A. Heteropoly acid/NaY zeolite as a reusable solid catalyst for highly efficient synthesis of gem-dihydroperoxides and 1,2,4,5-tetraoxanes. J. Chem. Res. 2016, 40, 744–749. [Google Scholar] [CrossRef]
- Tarlani, A.; Riahi, A.; Abedini, M.; Amini, M.M.; Muzart, J. Catalytic condensation process for the preparation of organic peroxides from tert-butyl hydroperoxide and benzylic alcohols. Appl. Catal. A Gen. 2006, 315, 150–152. [Google Scholar] [CrossRef]
- Li, Y.; Hao, H.-D.; Zhang, Q.; Wu, Y. A broadly applicable mild method for the synthesis of gem-diperoxides from corresponding ketones or 1,3-dioxolanes. Org. Lett. 2009, 11, 1615–1618. [Google Scholar] [CrossRef] [PubMed]
- Han, W.B.; Wu, Y.K. Facile perhydrolysis of oxetanes catalyzed by molybdenum species. Org. Lett. 2014, 16, 5706–5709. [Google Scholar] [CrossRef] [PubMed]
- Zdvizhkov, A.T.; Radulov, P.S.; Novikov, R.A.; Tafeenko, V.A.; Chernyshev, V.V.; Ilovaisky, A.I.; Terent’ev, A.O.; Nikishin, G.I. Convenient synthesis of furo [2,3-c][1,2]dioxoles from 1-aryl-2-allylalkane-1,3-diones. Mendeleev Commun. 2020, 30, 607–609. [Google Scholar] [CrossRef]
- Terent’ev, A.O.; Yaremenko, I.A.; Glinushkin, A.P.; Nikishin, G.I. Synthesis of peroxides from beta,delta-triketones under heterogeneous conditions. Russ. J. Org. Chem. 2015, 51, 1681–1687. [Google Scholar] [CrossRef]
- Yaremenko, I.A.; Syroeshkin, M.A.; Levitsky, D.O.; Fleury, F.; Terent’ev, A.O. Cyclic peroxides as promising anticancer agents: In vitro cytotoxicity study of synthetic ozonides and tetraoxanes on human prostate cancer cell lines. Med. Chem. Res. 2017, 26, 170–179. [Google Scholar] [CrossRef]
































































































































Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaremenko, I.A.; Radulov, P.S.; Belyakova, Y.Y.; Fomenkov, D.I.; Tsogoeva, S.B.; Terent’ev, A.O. Lewis Acids and Heteropoly Acids in the Synthesis of Organic Peroxides. Pharmaceuticals 2022, 15, 472. https://doi.org/10.3390/ph15040472
Yaremenko IA, Radulov PS, Belyakova YY, Fomenkov DI, Tsogoeva SB, Terent’ev AO. Lewis Acids and Heteropoly Acids in the Synthesis of Organic Peroxides. Pharmaceuticals. 2022; 15(4):472. https://doi.org/10.3390/ph15040472
Chicago/Turabian StyleYaremenko, Ivan A., Peter S. Radulov, Yulia Yu. Belyakova, Dmitriy I. Fomenkov, Svetlana B. Tsogoeva, and Alexander O. Terent’ev. 2022. "Lewis Acids and Heteropoly Acids in the Synthesis of Organic Peroxides" Pharmaceuticals 15, no. 4: 472. https://doi.org/10.3390/ph15040472
APA StyleYaremenko, I. A., Radulov, P. S., Belyakova, Y. Y., Fomenkov, D. I., Tsogoeva, S. B., & Terent’ev, A. O. (2022). Lewis Acids and Heteropoly Acids in the Synthesis of Organic Peroxides. Pharmaceuticals, 15(4), 472. https://doi.org/10.3390/ph15040472