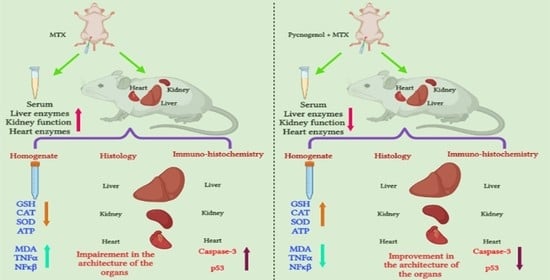
Protective Effect of Pycnogenol against Methotrexate-Induced Hepatic, Renal, and Cardiac Toxicity: An In Vivo Study
Abstract
1. Introduction
2. Results
2.1. Biochemical Analysis
2.2. Histological Analysis
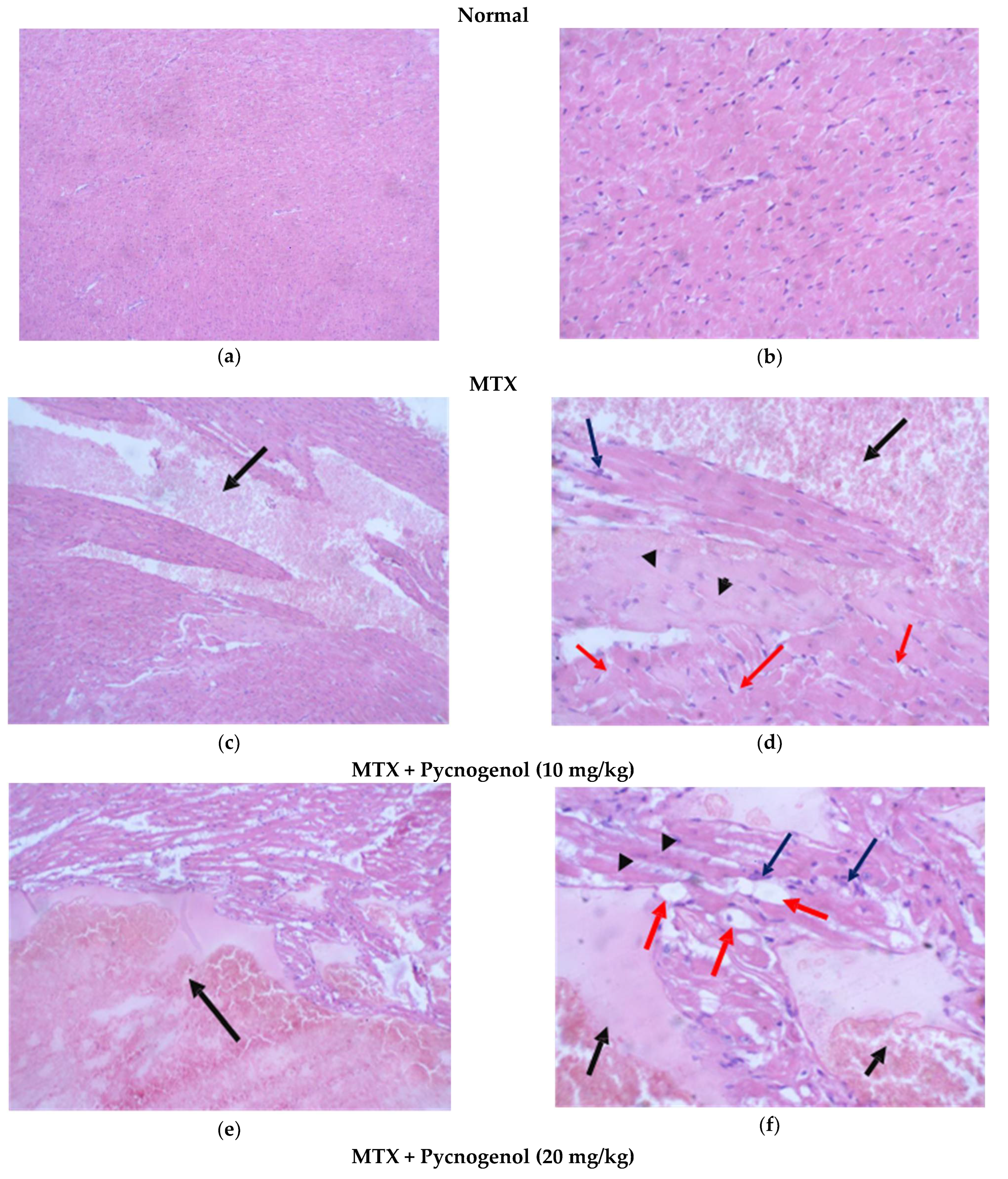
2.2.1. Histopathologic Analysis: Examination of H&E-Stained Sections for Liver, Kidney, and Heart to Examine the Architecture of the Cells
2.2.2. Immunohistochemical Analysis
3. Discussion
4. Materials and Methods
4.1. Animals and Grouping:
4.2. Chemicals and Reagents
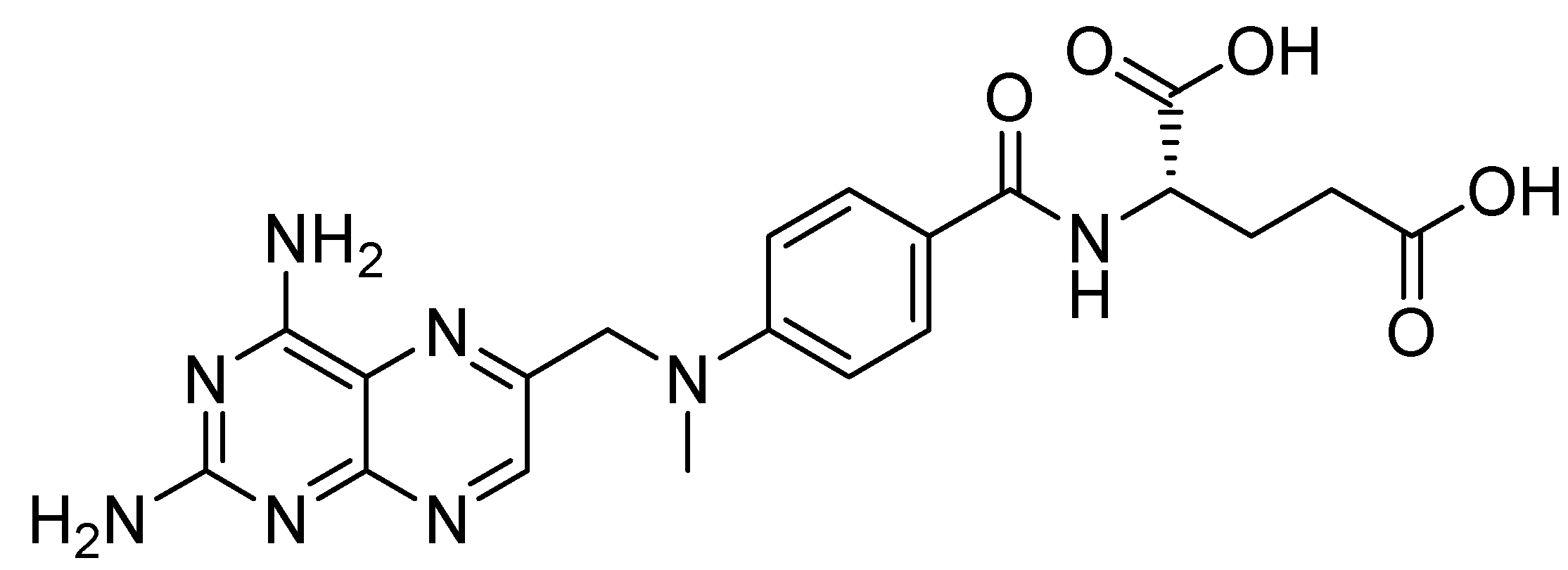
4.3. Induction of Toxicity by MTX
4.4. Collection of Blood and Tissue Samples
4.5. Biochemical Analysis
4.6. Histological Analysis:
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Parkin, D.M.; Bray, F.; Ferlay, J.; Pisani, P. Estimating the World Cancer Burden: Globocan 2000. Int. J. Cancer 2001, 94, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Farias, M.; Carrasco-Pozo, C. The Anti-Cancer Effect of Quercetin: Molecular Implications in Cancer Metabolism. Int. J. Mol. Sci. 2019, 20, 3177. [Google Scholar] [CrossRef] [PubMed]
- Cordero, M.D.; Sánchez-Alcázar, J.A.; Bautista-Ferrufino, M.R.; Carmona-López, M.I.; Illanes, M.; Ríos, M.J.; Garrido-Maraver, J.; Alcudia, A.; Navas, P.; de Miguel, M. Acute Oxidant Damage Promoted on Cancer Cells by Amitriptyline in Comparison with Some Common Chemotherapeutic Drugs. Anticancer. Drugs 2010, 21, 932–944. [Google Scholar] [CrossRef] [PubMed]
- El Azab, I.H.; Saied, E.M.; Osman, A.A.; Mehana, A.E.; Saad, H.A.; Elkanzi, N.A. Novel N-Bridged Pyrazole-1-Carbothioamides with Potential Antiproliferative Activity: Design, Synthesis, in Vitro and in Silico Studies. Future Med. Chem. 2021, 13, 1743–1766. [Google Scholar] [CrossRef]
- Koźmiński, P.; Halik, P.K.; Chesori, R.; Gniazdowska, E. Overview of Dual-Acting Drug Methotrexate in Different Neurological Diseases, Autoimmune Pathologies and Cancers. Int. J. Mol. Sci. 2020, 21, 3483. [Google Scholar] [CrossRef]
- Wei, C.-W.; Yu, Y.-L.; Chen, Y.-H.; Hung, Y.-T.; Yiang, G.-T. Anticancer Effects of Methotrexate in Combination with A-tocopherol and A-tocopherol Succinate on Triple-negative Breast Cancer. Oncol. Rep. 2019, 41, 2060–2066. [Google Scholar] [CrossRef]
- Saied, E.M.; El-Maradny, Y.A.; Osman, A.A.; Darwish, A.M.G.; Abo Nahas, H.H.; Niedbała, G.; Piekutowska, M.; Abdel-Rahman, M.A.; Balbool, B.A.; Abdel-Azeem, A.M. A Comprehensive Review about the Molecular Structure of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): Insights into Natural Products against COVID-19. Pharmaceutics 2021, 13, 1759. [Google Scholar] [CrossRef] [PubMed]
- Çakır, T.; Özkan, E.; Dulundu, E.; Topaloğlu, Ü.; Şehirli, A.Ö.; Ercan, F.; Şener, E.; Şener, G. Caffeic Acid Phenethyl Ester (CAPE) Prevents Methotrexate-Induced Hepatorenal Oxidative Injury in Rats. J. Pharm. Pharmacol. 2011, 63, 1566–1571. [Google Scholar] [CrossRef]
- Friedman, B.; Cronstein, B. Methotrexate Mechanism in Treatment of Rheumatoid Arthritis. Jt. Bone Spine 2019, 86, 301–307. [Google Scholar] [CrossRef]
- Saied, E.M.; Arenz, C. Inhibitors of Ceramidases. Chem. Phys. Lipids 2016, 197, 60–68. [Google Scholar] [CrossRef]
- Saied, E.M.; Arenz, C. Small Molecule Inhibitors of Ceramidases. Cell. Physiol. Biochem. 2014, 34, 197–212. [Google Scholar] [CrossRef] [PubMed]
- Yiang, G.-T.; Chou, P.-L.; Hung, Y.-T.; Chen, J.-N.; Chang, W.-J.; Yu, Y.-L.; Wei, C.-W. Vitamin C Enhances Anticancer Activity in Methotrexate-treated Hep3B Hepatocellular Carcinoma Cells. Oncol. Rep. 2014, 32, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.-M.; Foster, B.K.; Hui, S.K.; Xian, C.J. Prevention of Bone Growth Defects, Increased Bone Resorption and Marrow Adiposity with Folinic Acid in Rats Receiving Long-Term Methotrexate. PLoS ONE 2012, 7, e46915. [Google Scholar] [CrossRef]
- Yang, T.-M.; Qi, S.-N.; Zhao, N.; Yang, Y.-J.; Yuan, H.-Q.; Zhang, B.; Jin, S. Induction of Apoptosis through Caspase-Independent or Caspase-9-Dependent Pathway in Mouse and Human Osteosarcoma Cells by a New Nitroxyl Spin-Labeled Derivative of Podophyllotoxin. Apoptosis Int. J. Program. Cell Death 2013, 18, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Daim, M.M.; Khalifa, H.A.; Abushouk, A.I.; Dkhil, M.A.; Al-Quraishy, S.A. Diosmin Attenuates Methotrexate-Induced Hepatic, Renal, and Cardiac Injury: A Biochemical and Histopathological Study in Mice. Oxid. Med. Cell. Longev. 2017, 2017, 3281670. [Google Scholar] [CrossRef]
- Solomon, D.H.; Glynn, R.J.; Karlson, E.W.; Lu, F.; Corrigan, C.; Colls, J.; Xu, C.; MacFadyen, J.; Barbhaiya, M.; Berliner, N.; et al. Adverse Effects of Low-Dose Methotrexate: A Randomized Trial. Ann. Intern. Med. 2020, 172, 369–380. [Google Scholar] [CrossRef]
- Koch-Edelmann, S.; Banhart, S.; Saied, E.M.; Rose, L.; Aeberhard, L.; Laue, M.; Doellinger, J.; Arenz, C.; Heuer, D. The Cellular Ceramide Transport Protein CERT Promotes Chlamydia Psittaci Infection and Controls Bacterial Sphingolipid Uptake. Cell. Microbiol. 2017, 19, e12752. [Google Scholar] [CrossRef]
- Erdogan, E.; Ilgaz, Y.; Gurgor, P.N.; Oztas, Y.; Topal, T.; Oztas, E. Rutin Ameliorates Methotrexate Induced Hepatic Injury in Rats. Acta Cir. Bras. 2015, 30, 778–784. [Google Scholar] [CrossRef]
- Demiryilmaz, I.; Sener, E.; Cetin, N.; Altuner, D.; Suleyman, B.; Albayrak, F.; Akcay, F.; Suleyman, H. Biochemically and Histopathologically Comparative Review of Thiamine’s and Thiamine Pyrophosphate’s Oxidative Stress Effects Generated with Methotrexate in Rat Liver. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2012, 18, BR475–BR481. [Google Scholar] [CrossRef]
- Jahovic, N.; Sener, G.; Cevik, H.; Ersoy, Y.; Arbak, S.; Yeğen, B.C. Amelioration of Methotrexate-Induced Enteritis by Melatonin in Rats. Cell Biochem. Funct. 2004, 22, 169–178. [Google Scholar] [CrossRef]
- Banhart, S.; Saied, E.M.; Martini, A.; Koch, S.; Aeberhard, L.; Madela, K.; Arenz, C.; Heuer, D. Improved Plaque Assay Identifies a Novel Anti-Chlamydia Ceramide Derivative with Altered Intracellular Localization. Antimicrob. Agents Chemother. 2014, 58, 5537–5546. [Google Scholar] [CrossRef] [PubMed]
- Burmester, G.R.; Kaeley, G.S.; Kavanaugh, A.F.; Gabay, C.; MacCarter, D.K.; Nash, P.; Takeuchi, T.; Goss, S.L.; Rodila, R.; Chen, K.; et al. Treatment Efficacy and Methotrexate-Related Toxicity in Patients with Rheumatoid Arthritis Receiving Methotrexate in Combination with Adalimumab. RMD Open 2017, 3, e000465. [Google Scholar] [CrossRef] [PubMed]
- Kamen, B.A.; Nylen, P.A.; Camitta, B.M.; Bertino, J.R. Methotrexate Accumulation and Folate Depletion in Cells as a Possible Mechanism of Chronic Toxicity to the Drug. Br. J. Haematol. 1981, 49, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Rizk, F.H.; Saadany, A.A.E.; Dawood, L.; Elkaliny, H.H.; Sarhan, N.I.; Badawi, R.; Abd-Elsalam, S. Metformin Ameliorated Methotrexate-Induced Hepatorenal Toxicity in Rats in Addition to Its Antitumor Activity: Two Birds with One Stone. J. Inflamm. Res. 2018, 11, 421–429. [Google Scholar] [CrossRef]
- Asci, H.; Ozmen, O.; Ellidag, H.Y.; Aydin, B.; Bas, E.; Yilmaz, N. The Impact of Gallic Acid on the Methotrexate-Induced Kidney Damage in Rats. J. Food Drug Anal. 2017, 25, 890–897. [Google Scholar] [CrossRef]
- Collenburg, L.; Beyersdorf, N.; Wiese, T.; Arenz, C.; Saied, E.M.; Becker-Flegler, K.A.; Schneider-Schaulies, S.; Avota, E. The Activity of the Neutral Sphingomyelinase Is Important in T Cell Recruitment and Directional Migration. Front. Immunol. 2017, 8, 1007. [Google Scholar] [CrossRef]
- Bedoui, Y.; Guillot, X.; Sélambarom, J.; Guiraud, P.; Giry, C.; Jaffar-Bandjee, M.C.; Ralandison, S.; Gasque, P. Methotrexate an Old Drug with New Tricks. Int. J. Mol. Sci. 2019, 20, 5023. [Google Scholar] [CrossRef]
- Taylor, P.C.; Balsa Criado, A.; Mongey, A.-B.; Avouac, J.; Marotte, H.; Mueller, R.B. How to Get the Most from Methotrexate (MTX) Treatment for Your Rheumatoid Arthritis Patient?—MTX in the Treat-to-Target Strategy. J. Clin. Med. 2019, 8, 515. [Google Scholar] [CrossRef]
- Mashour, N.H.; Lin, G.I.; Frishman, W.H. Herbal Medicine for the Treatment of Cardiovascular Disease: Clinical Considerations. Arch. Intern. Med. 1998, 158, 2225–2234. [Google Scholar] [CrossRef]
- Tokac, M.; Bacanli, M.; Dumlu, E.G.; Aydin, S.; Engin, M.; Bozkurt, B.; Yalcin, A.; Erel, Ö.; Kilic, M.; Basaran, N. The Ameliorative Effects of Pycnogenol® on Liver Ischemia-Reperfusion Injury in Rats. Turk. J. Pharm. Sci. 2017, 14, 257–263. [Google Scholar] [CrossRef]
- D’Andrea, G. Pycnogenol: A Blend of Procyanidins with Multifaceted Therapeutic Applications? Fitoterapia 2010, 81, 724–736. [Google Scholar] [CrossRef] [PubMed]
- Rezzani, R.; Porteri, E.; De Ciuceis, C.; Bonomini, F.; Rodella, L.F.; Paiardi, S.; Boari, G.E.M.; Platto, C.; Pilu, A.; Avanzi, D.; et al. Effects of Melatonin and Pycnogenol on Small Artery Structure and Function in Spontaneously Hypertensive Rats. Hypertension 2010, 55, 1373–1380. [Google Scholar] [CrossRef] [PubMed]
- Park, C.-M.; Kim, H.-Y.; Jeon, D.; Shin, Y.-J.; Kim, I.-H.; Choi, S.-J.; Kim, K.C.; Lee, K.; Kim, S.-H.; Kim, M.-S. Anti-Fibrotic Effect of Pycnogenol® in a Polyhexamethylene Guanidine-Treated Mouse Model. Respir. Physiol. Neurobiol. 2022, 296, 103802. [Google Scholar] [CrossRef] [PubMed]
- Simpson, T.; Kure, C.; Stough, C. Assessing the Efficacy and Mechanisms of Pycnogenol® on Cognitive Aging From In Vitro Animal and Human Studies. Front. Pharmacol. 2019, 10, 694. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Tong, X.; Wei, Y.-L.; Zhao, L.; Xu, J.-Y.; Qin, L.-Q. Effect of Pycnogenol Supplementation on Blood Pressure: A Systematic Review and Meta-Analysis. Iran. J. Public Health 2018, 47, 779–787. [Google Scholar] [PubMed]
- Oliff, H. Scientific and Clinical Monograph for Pycnogenol®, 2019 Update. Available online: https://abc.herbalgram.org/site/SPageServer/;jsessionid=00000000.app20113b?NONCE_TOKEN=51E51ADE1CE774A9077BC429D2BDFE66&pagename=Pycnogenol (accessed on 31 May 2021).
- Grimm, T.; Skrabala, R.; Chovanová, Z.; Muchová, J.; Sumegová, K.; Liptáková, A.; Ďuračková, Z.; Högger, P. Single and Multiple Dose Pharmacokinetics of Maritime Pine Bark Extract (Pycnogenol) after Oral Administration to Healthy Volunteers. BMC Clin. Pharmacol. 2006, 6, 4. [Google Scholar] [CrossRef]
- Uhlenhut, K.; Högger, P. Facilitated Cellular Uptake and Suppression of Inducible Nitric Oxide Synthase by a Metabolite of Maritime Pine Bark Extract (Pycnogenol). Free Radic. Biol. Med. 2012, 53, 305–313. [Google Scholar] [CrossRef]
- Uddin, N.; Ahmed, S.; Khan, A.M.; Mazharol Hoque, M.; Halim, M.A. Halogenated Derivatives of Methotrexate as Human Dihydrofolate Reductase Inhibitors in Cancer Chemotherapy. J. Biomol. Struct. Dyn. 2020, 38, 901–917. [Google Scholar] [CrossRef]
- Abdelaziz, R.M.; Abdelazem, A.Z.; Hashem, K.S.; Attia, Y.A. Protective Effects of Hesperidin against MTX-Induced Hepatotoxicity in Male Albino Rats. Naunyn. Schmiedebergs Arch. Pharmacol. 2020, 393, 1405–1417. [Google Scholar] [CrossRef]
- Cağlar, Y.; Özgür, H.; Matur, I.; Dündar Yenilmez, E.; Tuli, A.; Gönlüşen, G.; Polat, S. Ultrastructural Evaluation of the Effect of N-Acetylcysteine on Methotrexate Nephrotoxicity in Rats. Histol. Histopathol. 2013, 28. [Google Scholar] [CrossRef]
- Al-Taher, A.Y.; Morsy, M.A.; Rifaai, R.A.; Zenhom, N.M.; Abdel-Gaber, S.A. Paeonol Attenuates Methotrexate-Induced Cardiac Toxicity in Rats by Inhibiting Oxidative Stress and Suppressing TLR4-Induced NF-ΚB Inflammatory Pathway. Mediators Inflamm. 2020, 2020, e8641026. [Google Scholar] [CrossRef] [PubMed]
- Atta, M.S.; Farrag, F.A.; Almadaly, E.A.; Ghoneim, H.A.; Hafez, A.S.; Al Jaouni, S.K.; Mousa, S.A.; El-Far, A.H. Transcriptomic and Biochemical Effects of Pycnogenol in Ameliorating Heat Stress-Related Oxidative Alterations in Rats. J. Therm. Biol. 2020, 93, 102683. [Google Scholar] [CrossRef] [PubMed]
- Dar, A.; Fehaid, A.; Alkhatani, S.; Alarifi, S.; Alqahtani, W.; Albasher, G.; Almeer, R.; Alfarraj, S.; Moneim, A.A. The Protective Role of Luteolin against the Methotrexate-Induced Hepato-Renal Toxicity via Its Antioxidative, Anti-Inflammatory, and Anti-Apoptotic Effects in Rats. Hum. Exp. Toxicol. 2021, 40, 1194–1207. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Kim, W.R.; Poterucha, J.J. Evaluation of Elevated Liver Enzymes. Clin. Liver Dis. 2012, 16, 183–198. [Google Scholar] [CrossRef]
- Dalaklioglu, S.; Genc, G.E.; Aksoy, N.H.; Akcit, F.; Gumuslu, S. Resveratrol Ameliorates Methotrexate-Induced Hepatotoxicity in Rats via Inhibition of Lipid Peroxidation. Hum. Exp. Toxicol. 2013, 32, 662–671. [Google Scholar] [CrossRef]
- Howard, S.C.; McCormick, J.; Pui, C.-H.; Buddington, R.K.; Harvey, R.D. Preventing and Managing Toxicities of High-Dose Methotrexate. The Oncologist 2016, 21, 1471–1482. [Google Scholar] [CrossRef]
- Hu, S.; Belcaro, G.; Cesarone, M.R.; Feragalli, B.; Cotellese, R.; Dugall, M.; Scipione, C.; Scipione, V.; Maione, C. Central Cardiovascular Calcifications: Supplementation with Pycnogenol® and Centellicum®: Variations over 12 Months. Minerva Cardioangiol. 2020, 68, 22–26. [Google Scholar] [CrossRef]
- Kolli, V.K.; Abraham, P.; Isaac, B.; Selvakumar, D. Neutrophil Infiltration and Oxidative Stress May Play a Critical Role in Methotrexate-Induced Renal Damage. Chemotherapy 2009, 55, 83–90. [Google Scholar] [CrossRef]
- Chauhan, P.; Sharma, H.; Kumar, U.; Mayachari, A.; Sangli, G.; Singh, S. Protective Effects of Glycyrrhiza Glabra Supplementation against Methotrexate-Induced Hepato-Renal Damage in Rats: An Experimental Approach. J. Ethnopharmacol. 2020, 263, 113209. [Google Scholar] [CrossRef]
- Gosselt, H.R.; Muller, I.B.; Jansen, G.; van Weeghel, M.; Vaz, F.M.; Hazes, J.M.W.; Heil, S.G.; de Jonge, R. Identification of Metabolic Biomarkers in Relation to Methotrexate Response in Early Rheumatoid Arthritis. J. Pers. Med. 2020, 10, 271. [Google Scholar] [CrossRef]
- Hassanein, E.H.M.; Mohamed, W.R.; Khalaf, M.M.; Shalkami, A.S.; Sayed, A.M.; Hemeida, R.A.M. Diallyl Disulfide Ameliorates Methotrexate-induced Nephropathy in Rats: Molecular Studies and Network Pharmacology Analysis. J. Food Biochem. 2021, 45. [Google Scholar] [CrossRef] [PubMed]
- Bulut, Ş.; Uslu, H.; Özdemir, B.H.; Bulut, Ö.E. Expression of Caspase-3, P53 and Bcl-2 in Generalized Aggressive Periodontitis. Head Face Med. 2006, 2, 17. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Koyun, E.; Karadag, R.; Ozkanli, S.; Oguztuzun, S.; Kocdogan, A.K.; Ozsoy, I. Caspase-3, P53 and Bcl-2 Expression in Basal Cell Carcinoma of the Eyelid. Adv. Dermatol. Allergol. Dermatol. Alergol. 2020, 37, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-Y.; Yang, P.-M.; Chang, Y.-F.; Marquez, V.E.; Chen, C.-C. Methotrexate Induces Apoptosis through P53/P21-Dependent Pathway and Increases E-Cadherin Expression through Downregulation of HDAC/EZH2. Biochem. Pharmacol. 2011, 81, 510–517. [Google Scholar] [CrossRef]
- Yang, I.-H.; Shin, J.-A.; Kim, L.-H.; Kwon, K.H.; Cho, S.-D. The Caspase 3-Dependent Apoptotic Effect of Pycnogenol in Human Oral Squamous Cell Carcinoma HSC-3 Cells. J. Clin. Biochem. Nutr. 2016, 58, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Verlaet, A.; van der Bolt, N.; Meijer, B.; Breynaert, A.; Naessens, T.; Konstanti, P.; Smidt, H.; Hermans, N.; Savelkoul, H.; Teodorowicz, M. Toll-Like Receptor-Dependent Immunomodulatory Activity of Pycnogenol®. Nutrients 2019, 11, 214. [Google Scholar] [CrossRef]
- BeciT, M.; Aydin, S. An In Vitro Study on the Interactions of Pycnogenol® with Cisplatin in Human Cervical Cancer Cells. Turk. J. Pharm. Sci. 2020, 17, 1–6. [Google Scholar] [CrossRef]
- Harati, K.; Slodnik, P.; Chromik, A.M.; Behr, B.; Goertz, O.; Hirsch, T.; Kapalschinski, N.; Klein-Hitpass, L.; Kolbenschlag, J.; Uhl, W.; et al. Pro-Apoptotic Effects of Pycnogenol on HT1080 Human Fibrosarcoma Cells. Int. J. Oncol. 2015, 46, 1629–1636. [Google Scholar] [CrossRef][Green Version]
- Bayomy, N.A.; Abdelaziz, E.Z.; Said, M.A.; Badawi, M.S.; El-Bakary, R.H. Effect of Pycnogenol and Spirulina on Vancomycin-Induced Renal Cortical Oxidative Stress, Apoptosis, and Autophagy in Adult Male Albino Rat. Can. J. Physiol. Pharmacol. 2016, 94, 838–848. [Google Scholar] [CrossRef]
- Kralova, E.; Jankyova, S.; Mucaji, P.; Gresakova, E.; Stankovicova, T. Pycnogenol® and Its Fractions Influence the Function of Isolated Heart in Rats with Experimental Diabetes Mellitus. Pathol. Res. Pract. 2015, 211, 156–161. [Google Scholar] [CrossRef]
- Singh, K.; Malviya, A.; Bhori, M.; Marar, T. An in Vitro Study of the Ameliorative Role of α-Tocopherol on Methotrexate-Induced Oxidative Stress in Rat Heart Mitochondria. J. Basic Clin. Physiol. Pharmacol. 2012, 23. [Google Scholar] [CrossRef] [PubMed]
- Eryılmaz, U.; Aksun, S.; Demirci, B. Protective Effect of Pycnogenol® on Cisplatin Induced-Cardiotoxicity in Rats. Meandros Med. Dent. J. 2018, 19, 192–197. [Google Scholar] [CrossRef]
- Rašković, A.; Bukumirović, N.; Paut Kusturica, M.; Milić, N.; Čabarkapa, V.; Borišev, I.; Čapo, I.; Miljković, D.; Stilinović, N.; Mikov, M. Hepatoprotective and Antioxidant Potential of Pycnogenol® in Acetaminophen-induced Hepatotoxicity in Rats. Phytother. Res. 2018, ptr.6251. [Google Scholar] [CrossRef] [PubMed]
- Xiao, P.; Zhang, K.; Tao, Z.; Liu, N.; Ge, B. Cardioprotective Effect of Pycnogenol in Ischemic-Reperfusion Injury (IRI) in Rats. Cell. Mol. Biol. 2017, 63, 49–53. [Google Scholar] [CrossRef]
- Ozoner, B.; Yuceli, S.; Aydin, S.; Yazici, G.N.; Sunar, M.; Arslan, Y.K.; Coban, T.A.; Suleyman, H. Effects of Pycnogenol on Ischemia/Reperfusion-Induced Inflammatory and Oxidative Brain Injury in Rats. Neurosci. Lett. 2019, 704, 169–175. [Google Scholar] [CrossRef]
- Aydın, S.; Bacanlı, M.; Anlar, H.G.; Çal, T.; Arı, N.; Ündeğer Bucurgat, Ü.; Başaran, A.A.; Başaran, N. Preventive Role of Pycnogenol® against the Hyperglycemia-Induced Oxidative Stress and DNA Damage in Diabetic Rats. Food Chem. Toxicol. 2019, 124, 54–63. [Google Scholar] [CrossRef]
- Rohdewald, P. Update on the Clinical Pharmacology of Pycnogenol®. Med. Res. Arch. 2015, 11, 1–11. [Google Scholar] [CrossRef]
- Khan, M.M.; Kempuraj, D.; Thangavel, R.; Zaheer, A. Protection of MPTP-Induced Neuroinflammation and Neurodegeneration by Pycnogenol. Neurochem. Int. 2013, 62, 379–388. [Google Scholar] [CrossRef]
- Gao, B.; Chang, C.; Zhou, J.; Zhao, T.; Wang, C.; Li, C.; Gao, G. Pycnogenol Protects Against Rotenone-Induced Neurotoxicity in PC12 Cells Through Regulating NF-ΚB-INOS Signaling Pathway. DNA Cell Biol. 2015, 34, 643–649. [Google Scholar] [CrossRef]
- Shin, N.-R.; Ryu, H.-W.; Ko, J.-W.; Park, J.-W.; Kwon, O.-K.; Oh, S.-R.; Kim, J.-C.; Shin, I.-S.; Ahn, K.-S. A Standardized Bark Extract of Pinus Pinaster Aiton (Pycnogenol®) Attenuated Chronic Obstructive Pulmonary Disease via Erk-Sp1 Signaling Pathway. J. Ethnopharmacol. 2016, 194, 412–420. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, Y.A.; Yokozawa, T. Pycnogenol Modulates Apoptosis by Suppressing Oxidative Stress and Inflammation in High Glucose-Treated Renal Tubular Cells. Food Chem. Toxicol. 2011, 49, 2196–2201. [Google Scholar] [CrossRef] [PubMed]
- Jafari, F.; Goudarzvand, M.; Hajikhani, R.; Qorbani, M.; Solati, J. Pycnogenol Ameliorates Motor Function and Gene Expressions of NF-ƘB and Nrf2 in a 6-Hydroxydopamine-Induced Experimental Model of Parkinson’s Disease in Male NMRI Mice. Naunyn. Schmiedebergs Arch. Pharmacol. 2022, 395, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Sakalli Çetin, E.; Tetiker, H.; İlhan Çelik, Ö.; Yılmaz, N.; Ciğerci, İ.H. Methotrexate-Induced Nephrotoxicity in Rats: Protective Effect of Mistletoe (Viscum album L.) Extract. Complement. Med. Res. 2017, 24, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Kei, S. Serum Lipid Peroxide in Cerebrovascular Disorders Determined by a New Colorimetric Method. Clin. Chim. Acta 1978, 90, 37–43. [Google Scholar] [CrossRef]
- Samaha, D.; Hamdo, H.H.; Cong, X.; Schumacher, F.; Banhart, S.; Aglar, Ö.; Möller, H.M.; Heuer, D.; Kleuser, B.; Saied, E.M.; et al. Liposomal FRET Assay Identifies Potent Drug-Like Inhibitors of the Ceramide Transport Protein (CERT). Chem. Eur. J. 2020, 26, 16616–16621. [Google Scholar] [CrossRef]
- Beutler, E.; Duron, O.; Kelly, B.M. Improved Method for the Determination of Blood Glutathione. J. Lab. Clin. Med. 1963, 61, 882–888. [Google Scholar]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques; Churchill Livingstone: London, UK; New York, NJ, USA, 2002; ISBN 978-0-443-06435-7. [Google Scholar]
- Ishak, K.; Baptista, A.; Bianchi, L.; Callea, F.; De Groote, J.; Gudat, F.; Denk, H.; Desmet, V.; Korb, G.; MacSween, R.N. Histological Grading and Staging of Chronic Hepatitis. J. Hepatol. 1995, 22, 696–699. [Google Scholar] [CrossRef]
- Cao, Z.; Cooper, M.E.; Wu, L.L.; Cox, A.J.; Jandeleit-Dahm, K.; Kelly, D.J.; Gilbert, R.E. Blockade of the Renin-Angiotensin and Endothelin Systems on Progressive Renal Injury. Hypertens. Dallas Tex 1979 2000, 36, 561–568. [Google Scholar] [CrossRef]
- Pieters, T.T.; Falke, L.L.; Nguyen, T.Q.; Verhaar, M.C.; Florquin, S.; Bemelman, F.J.; Kers, J.; Vanhove, T.; Kuypers, D.; Goldschmeding, R.; et al. Histological Characteristics of Acute Tubular Injury during Delayed Graft Function Predict Renal Function after Renal Transplantation. Physiol. Rep. 2019, 7, e14000. [Google Scholar] [CrossRef]
- Jokinen, M.P.; Lieuallen, W.G.; Boyle, M.C.; Johnson, C.L.; Malarkey, D.E.; Nyska, A. Morphologic Aspects of Rodent Cardiotoxicity in a Retrospective Evaluation of National Toxicology Program Studies. Toxicol. Pathol. 2011, 39, 850–860. [Google Scholar] [CrossRef]
- Mohamed, D.I.; Alaa El-Din Aly El-Waseef, D.; Nabih, E.S.; El-Kharashi, O.A.; Abd El-Kareem, H.F.; Abo Nahas, H.H.; Abdel-Wahab, B.A.; Helmy, Y.A.; Alshawwa, S.Z.; Saied, E.M. Acetylsalicylic Acid Suppresses Alcoholism-Induced Cognitive Impairment Associated with Atorvastatin Intake by Targeting Cerebral MiRNA155 and NLRP3: In Vivo, and In Silico Study. Pharmaceutics 2022, 14, 529. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, D.I.; Abou-Bakr, D.A.; Ezzat, S.F.; El-Kareem, H.F.A.; Nahas, H.H.A.; Saad, H.A.; Mehana, A.E.; Saied, E.M. Vitamin D3 Prevents the Deleterious Effects of Testicular Torsion on Testis by Targeting MiRNA-145 and ADAM17: In Silico and In Vivo Study. Pharmaceuticals 2021, 14, 1222. [Google Scholar] [CrossRef] [PubMed]













| Parameter | Normal Group | MTX Group | MTX + Pycnogenol (10 mg/kg) Group | MTX + Pycnogenol (20 mg/kg) Group | MTX + Pycnogenol (30 mg/kg) Group |
|---|---|---|---|---|---|
| ALT (U/L) | 35.33 ± 2.5 E | 104.00 ± 4.0 A | 84.00 ± 2.0 B | 61.00 ± 1.0 C | 46.00 ± 1.0 D |
| AST (U/L) | 41.03 ± 0.2 E | 136.93 ± 3.6 A | 101.00 ± 3.0 B | 78.00 ± 2.0 C | 55.67 ± 2.5 D |
| Urea (mg/dL) | 36.00 ± 2.0 E | 64.00 ± 1.0 A | 51.00 ± 1.0 B | 43.00 ± 1.0 C | 39.00 ± 1.0 D |
| Creatinine (mg/dL) | 0.50 ± 0.1 B | 1.87 ± 0.4 A | 0.90 ± 0.1 B | 0.70 ± 0.1 B | 0.60 ± 0.1 B |
| Troponin (ng/mL) | 0.94 ± 0.1 D | 4.57 ± 0.4 A | 2.08 ± 0.1 B | 1.72 ± 0.1 C | 1.07 ± 0.1 D |
| CK (U/L) | 12.27 ± 0.3 E | 56.10 ± 0.1 A | 35.00 ± 0.1 B | 23.07 ± 0.1 C | 16.47 ± 0.5 D |
| Ck-MB (U/L) | 10.50 ± 0.1 E | 36.50 ± 0.1 A | 23.70 ± 0.6 B | 16.23 ± 1.1 C | 12.07 ± 0.2 D |
| Organs and Scoring Index | Normal | MTX | MTX + Pycnogenol (10 mg/kg) | MTX + Pycnogenol (20 mg/kg) | MTX + Pycnogenol (30 mg/kg) | |
|---|---|---|---|---|---|---|
| Liver | Hepatic activity index Grading: | 0 | 6 | 6 | 0 | 0 |
| -Confluent necrosis | Absent (0) | Necrosis and multiple portal-central bridging (5) | Necrosis and multiple portal-central bridging (5) | Absent (0) | Absent (0) | |
| -Lytic necrosis, apoptosis, and focal inflammation | Absent (0) | One focus or less per 10X objective (1) | One focus or less per 10X objective (1) | Absent (0) | Absent (0) | |
| -Piecemeal necrosis | Absent (0) | Absent (0) | Absent (0) | Absent (0) | Absent (0) | |
| -Portal inflammation | Absent (0) | Absent (0) | Absent (0) | Absent (0) | Absent (0) | |
| Other changes | No changes | Fatty change and hydropic degeneration | Fatty change and hydropic degeneration | Mild fatty change and hydropic degeneration | No evidence of hepatocyte injury | |
| Kidney | Tubular injury | Absent (0) | Acute tubular injury in more than 50% of tubules (3) | Acute tubular injury in 10–50% of tubules (2) | Absent (0) | Absent (0) |
| Glomerular injury | Absent (0) | 25–50% mesangial expansion and sclerotizing glomerulus (2) | 25–50% mesangial expansion and sclerotizing glomerulus (2) | 25–50% mesangial expansion and sclerotizing glomerulus (2) | 25–50% mesangial expansion and sclerotizing glomerulus (2) | |
| Total score | 0 | 5 | 4 | 2 | 2 | |
| Heart | Myocyte vacuolization | Absent (0) | Mild (2) | Moderate (3) | Absent (0) | Absent (0) |
| Myocyte necrosis | Absent (0) | Mild (2) | Mild (2) | Minimal (1) | Absent (0) | |
| Mononuclear cells infiltration | Absent (0) | Mild (2) | Mild (2) | Absent (0) | Absent (0) | |
| Fibrosis | Absent (0) | Absent (0) | Absent (0) | Absent (0) | Absent (0) | |
| Lobular Architecture | Score |
|---|---|
| Normal (absence of fibrosis) | 0 |
| Fibrous expansion of some portal areas | 1 |
| Fibrous expansion of most portal areas, with portal–portal septa | 2 |
| Fibrous extension of portal spaces with portal–portal and portal–central septa, with possible nodule formation | 3 |
| Cirrhosis with predominant nodular areas in relation to the remaining lobules | 4 |
| A. Periportal or periseptal interface hepatitis (piecemeal necrosis) | Degree |
| Absent Mild (focal, few portal areas) Mild/moderate (focal, most portal areas) Moderate (continuous, around 60% of tracts or septa) Severe (continuous, >50% of tracts or septa) | 0 1 2 3 4 |
| B. Confluent necrosis | Degree |
| Absent 0 Focal confluent necrosis 1 Zone 3 necrosis in some areas Zone 3 necrosis in most areas Zone 3 necrosis + occasional portal–central (P-C) bridging Zone 3 necrosis + multiple P-C bridging Panacinar or multiacinar necrosis | 0 1 2 3 4 5 6 |
| C. Focal (spotty) lytic necrosis, apoptosis, and focal inflammation | Degree |
| Absent One focus or less per 10x objective Two to four foci per 10x objective Five to ten foci per 10x objective More than ten foci per 10x objective | 0 1 2 3 4 |
| D. Portal inflammation | Degree |
| Absence of portal lymphocytes Mild number of portal lymphocytes Moderate number of portal lymphocytes Marked number of portal lymphocytes Strongly marked number of portal lymphocytes | 0 1 2 3 4 |
| Lesion | Grade |
| No lesion | 0 |
| Acute tubular injury in 10% of tubules | 1 |
| Acute tubular injury in 10–50% of tubules | 2 |
| Acute tubular injury in more than 50% of tubules | 3 |
| Complete atrophy and thyroidization of the tubules | 4 |
| Lesion | Grade |
| No sclerosis | 0 |
| 1–25% mesangial expansion and sclerosing glomerulus | 1 |
| 25–50% mesangial expansion and sclerotizing glomerulus | 2 |
| 50–75% mesangial expansion and sclerosing glomerulus | 3 |
| 75–100% mesangial expansion and sclerotizing glomerulus | 4 |
| Lesion | Grade |
|---|---|
| No lesion | 0 |
| Lesions involved less than 10% of the heart section | 1 |
| Lesions involved 11–40% of the heart section | 2 |
| Lesions involved 41–80% of the heart section | 3 |
| Lesions involved more than 81% of the heart section | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Abkal, F.; Abdel-Wahab, B.A.; El-Kareem, H.F.A.; Moustafa, Y.M.; Khodeer, D.M. Protective Effect of Pycnogenol against Methotrexate-Induced Hepatic, Renal, and Cardiac Toxicity: An In Vivo Study. Pharmaceuticals 2022, 15, 674. https://doi.org/10.3390/ph15060674
Al-Abkal F, Abdel-Wahab BA, El-Kareem HFA, Moustafa YM, Khodeer DM. Protective Effect of Pycnogenol against Methotrexate-Induced Hepatic, Renal, and Cardiac Toxicity: An In Vivo Study. Pharmaceuticals. 2022; 15(6):674. https://doi.org/10.3390/ph15060674
Chicago/Turabian StyleAl-Abkal, Faten, Basel A. Abdel-Wahab, Hanaa F. Abd El-Kareem, Yasser M. Moustafa, and Dina M. Khodeer. 2022. "Protective Effect of Pycnogenol against Methotrexate-Induced Hepatic, Renal, and Cardiac Toxicity: An In Vivo Study" Pharmaceuticals 15, no. 6: 674. https://doi.org/10.3390/ph15060674
APA StyleAl-Abkal, F., Abdel-Wahab, B. A., El-Kareem, H. F. A., Moustafa, Y. M., & Khodeer, D. M. (2022). Protective Effect of Pycnogenol against Methotrexate-Induced Hepatic, Renal, and Cardiac Toxicity: An In Vivo Study. Pharmaceuticals, 15(6), 674. https://doi.org/10.3390/ph15060674