Formulation and Characterization of O/W Nanoemulsions of Hemp Seed Oil for Protection from Steatohepatitis: Analysis of Hepatic Free Fatty Acids and Oxidation Markers
Abstract
:1. Introduction
2. Results and Discussion
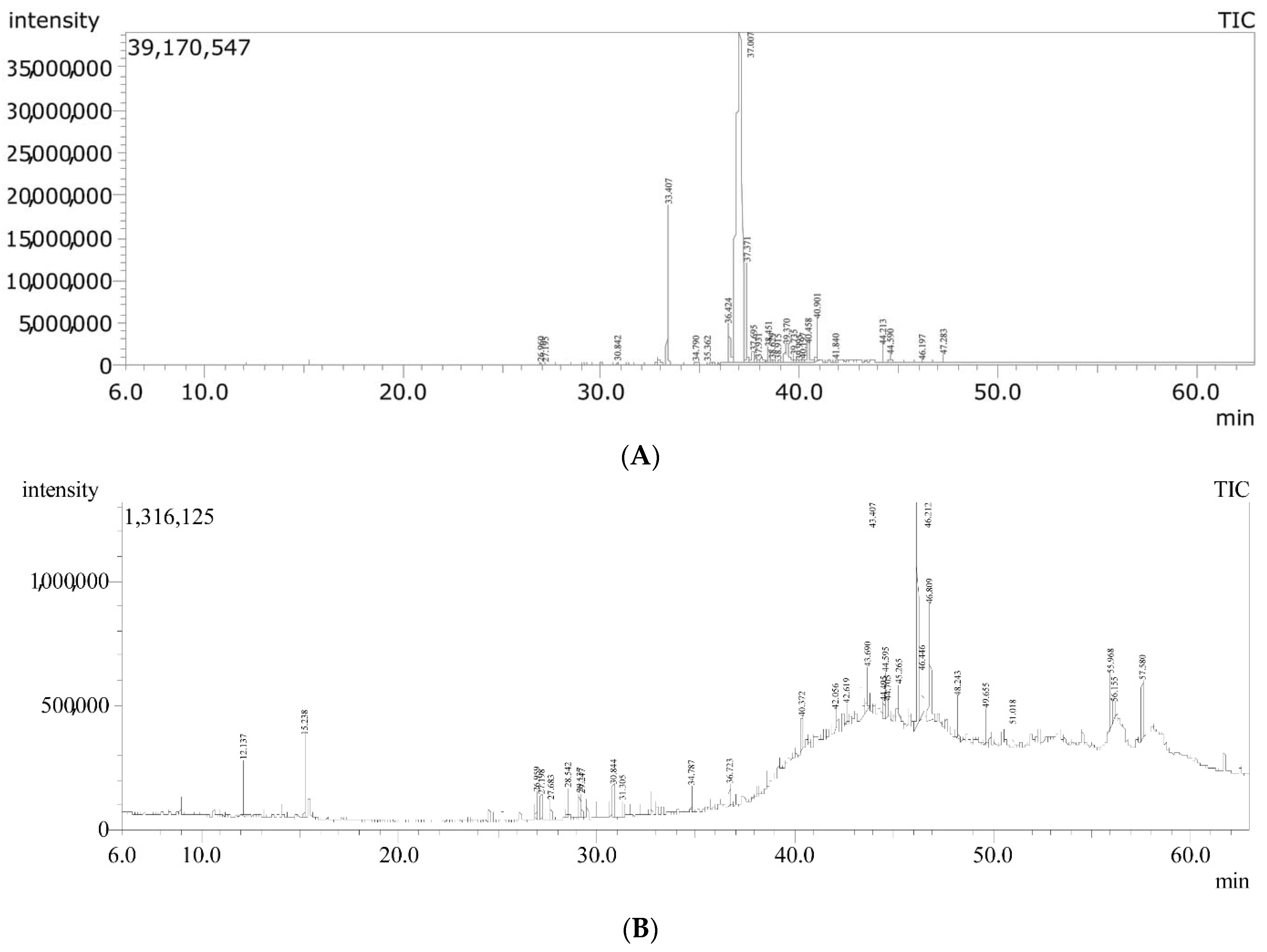
2.1. Hemp Seed Oil Characterization
2.2. Preparation of Hemp Seed Nanoemulsion
2.3. Effect of Surfactant Concentration on the Droplet Size, PDI, and ZP
2.4. Measurement of pH of Hemp Seed Oil Nanoemulsion Formulations
2.5. Viscosity Measurement of Hemp Seed Oil Nanoemulsion Formulations
2.6. Electrical Conductivity Measurement of Hemp Seed Oil Nanoemulsion Formulations
2.7. Selection of Best Hemp Seed Oil Nanoemulsion Formulation
The Surface Morphology of NEF#4
2.8. Protective Effect of NEF4 on Fatty Degeneration and Insulin Resistance in Rats
2.9. Protective Effect of NEF4 on Liver Histopathology in Rats
2.10. Protective Effect of NEMF4 on Hepatic Free Fatty Acids and Oxidation Parameters
3. Materials and Methods
3.1. Chemicals and Drugs
3.2. Characterization of the Content of Hemp Seed Oil
3.3. Preparation of Hemp Seed Oil Nanoemulsion
3.3.1. Measurement of the Droplet Size, Zeta Potential and Polydispersity Index of the O/W Nanoemulsion Preparations of Hemp Seed Oil
3.3.2. Evaluation of the pH of the Hemp Seed Oil Nanoemulsions
3.3.3. Viscosity Evaluation
3.3.4. Measuring Electrical Conductivity of the NEFs
3.3.5. The Selection of the Best Nanoemulsion Formulation
Transmission Electron Microscopy of the Best Formulation (TEM)
3.4. In Vivo Study of Hepatoprotective Activity
3.4.1. Experimental Animals
3.4.2. Experimental Design and Grouping
3.4.3. Tissue Collection and Sample Preparation
3.4.4. Measuring the Fasting Blood Glucose and Insulin and Calculation of HOMA-IR Index
3.4.5. Determination of Serum Liver Enzyme Activities
3.4.6. Determination of Sirt1 by Enzyme-Linked Immunoassay Kits
3.4.7. Assay of Free Fatty Acids, Malondialdehyde and GSH
3.4.8. Histopathological Examination of Liver Tissue
3.4.9. Immunohistochemistry and Image Analysis
3.5. Data Collection and Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ozogul, Y.; Boğa, E.K.; Akyol, I.; Durmus, M.; Ucar, Y.; Regenstein, J.M.; Köşker, A.R. Antimicrobial Activity of Thyme Essential Oil Nanoemulsions on Spoilage Bacteria of Fish and Food-Borne Pathogens. Food Biosci. 2020, 36, 100635. [Google Scholar] [CrossRef]
- Bendary, M.M.; Ibrahim, D.; Mosbah, R.A.; Mosallam, F.; Hegazy, W.A.; Awad, N.F.; Alshareef, W.A.; Alomar, S.Y.; Zaitone, S.A.; Abd El-Hamid, M.I. Thymol Nanoemulsion: A New Therapeutic Option for Extensively Drug Resistant Foodborne Pathogens. Antibiotics 2020, 10, 25. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hamid, M.I.; Ibrahim, S.M.; Eldemery, F.; El-Mandrawy, S.A.; Metwally, A.S.; Khalifa, E.; Elnahriry, S.S.; Ibrahim, D. Dietary Cinnamaldehyde Nanoemulsion Boosts Growth and Transcriptomes of Antioxidant and Immune Related Genes to Fight Streptococcus agalactiae Infection in Nile Tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2021, 113, 96–105. [Google Scholar] [CrossRef]
- Mahmood, Z.; Jahangir, M.; Liaquat, M.; Ahmad Shah, S.W.; Mumtaz Khan, M.; Stanley, R.; D’Arcy, B. Potential of Nano-Emulsions as Phytochemical Delivery System for Food Preservation. Pak. J. Pharm. Sci. 2017, 30, 2259–2263. [Google Scholar] [PubMed]
- Callaway, J.; Schwab, U.; Harvima, I.; Halonen, P.; Mykkänen, O.; Hyvönen, P.; Järvinen, T. Efficacy of Dietary Hempseed Oil in Patients with Atopic Dermatitis. J. Dermatol. Treat. 2005, 16, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Leizer, C.; Ribnicky, D.; Poulev, A.; Dushenkov, S.; Raskin, I. The Composition of Hemp Seed Oil and Its Potential as an Important Source of Nutrition. J. Nutraceuticals Funct. Med. Foods 2000, 2, 35–53. [Google Scholar] [CrossRef] [Green Version]
- Jarzębski, M.; Smulek, W.; Siejak, P.; Rezler, R.; Pawlicz, J.; Trzeciak, T.; Jarzębska, M.; Majchrzak, O.; Kaczorek, E.; Kazemian, P. Aesculus hippocastanum L. as a Stabilizer in Hemp Seed Oil Nanoemulsions for Potential Biomedical and Food Applications. Int. J. Mol. Sci. 2021, 22, 887. [Google Scholar] [CrossRef]
- Patel, R.P.; Joshi, J.R. An Overview on Nanoemulsion: A Novel Approach. Int. J. Pharm. Sci. Res. 2012, 3, 4640. [Google Scholar]
- Hosseini, S.F.; Ramezanzade, L.; McClements, D.J. Recent Advances in Nanoencapsulation of Hydrophobic Marine Bioactives: Bioavailability, Safety, and Sensory Attributes of Nano-Fortified Functional Foods. Trends Food Sci. Technol. 2021, 109, 322–339. [Google Scholar] [CrossRef]
- Kaushal, N.; Gupta, M.; Kulshreshtha, E. Hempseed (Cannabis sativa) Lipid Fractions Alleviate High-Fat Diet-Induced Fatty Liver Disease through Regulation of Inflammation and Oxidative Stress. Heliyon 2020, 6, e04422. [Google Scholar] [CrossRef]
- Chen, X.-Y.; Cai, C.-Z.; Yu, M.-L.; Feng, Z.-M.; Zhang, Y.-W.; Liu, P.-H.; Zeng, H.; Yu, C.-H. LB100 Ameliorates Nonalcoholic Fatty Liver Disease via the AMPK/Sirt1 Pathway. World J. Gastroenterol. 2019, 25, 6607. [Google Scholar] [CrossRef] [PubMed]
- Hazlehurst, J.M.; Woods, C.; Marjot, T.; Cobbold, J.F.; Tomlinson, J.W. Non-Alcoholic Fatty Liver Disease and Diabetes. Metabolism 2016, 65, 1096–1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boeckmans, J.; Natale, A.; Rombaut, M.; Buyl, K.; Rogiers, V.; De Kock, J.; Vanhaecke, T.; Rodrigues, R.M. Anti-NASH Drug Development Hitches a Lift on PPAR Agonism. Cells 2019, 9, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neuschwander-Tetri, B.A.; Caldwell, S.H. Nonalcoholic Steatohepatitis: Summary of an AASLD Single Topic Conference. Hepatology 2003, 37, 1202–1219. [Google Scholar] [CrossRef] [PubMed]
- Fisher, E.A.; Ginsberg, H.N. Complexity in the Secretory Pathway: The Assembly and Secretion of Apolipoprotein B-Containing Lipoproteins. J. Biol. Chem. 2002, 277, 17377–17380. [Google Scholar] [CrossRef] [Green Version]
- Hirschey, M.D. Old Enzymes, New Tricks: Sirtuins Are NAD+-Dependent De-Acylases. Cell Metab. 2011, 14, 718–719. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Liu, M.; Yu, H.; Wang, W.; Han, L.; Chen, Q.; Ruan, J.; Wen, S.; Zhang, Y.; Wang, T. Mangiferin Improves Hepatic Lipid Metabolism Mainly through Its Metabolite-Norathyriol by Modulating SIRT-1/AMPK/SREBP-1c Signaling. Front. Pharmacol. 2018, 9, 201. [Google Scholar] [CrossRef]
- Mahmoud, A.R.; Ali, F.E.; Abd-Elhamid, T.H.; Hassanein, E.H. Coenzyme Q10 Protects Hepatocytes from Ischemia Reperfusion-Induced Apoptosis and Oxidative Stress via Regulation of Bax/Bcl-2/PUMA and Nrf-2/FOXO-3/Sirt-1 Signaling Pathways. Tissue Cell 2019, 60, 1–13. [Google Scholar] [CrossRef]
- Schultze, S.M.; Hemmings, B.A.; Niessen, M.; Tschopp, O. PI3K/AKT, MAPK and AMPK Signalling: Protein Kinases in Glucose Homeostasis. Expert Rev. Mol. Med. 2012, 14, e1. [Google Scholar] [CrossRef] [Green Version]
- Liou, C.-J.; Lee, Y.-K.; Ting, N.-C.; Chen, Y.-L.; Shen, S.-C.; Wu, S.-J.; Huang, W.-C. Protective Effects of Licochalcone A Ameliorates Obesity and Non-Alcoholic Fatty Liver Disease via Promotion of the Sirt-1/AMPK Pathway in Mice Fed a High-Fat Diet. Cells 2019, 8, 447. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Chen, D.; Gong, Q.; Xu, Q.; Pan, D.; Lu, F.; Tang, Q. Elucidation of SIRT-1/PGC-1α-Associated Mitochondrial Dysfunction and Autophagy in Nonalcoholic Fatty Liver Disease. Lipids Health Dis. 2021, 20, 40. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Li, Y.; Wang, J.; Wu, B.; Li, J. Effect of Omega-3 Polyunsaturated Fatty Acids to Reverse Biopsy-Proven Parenteral Nutrition-Associated Liver Disease in Adults. Clin. Nutr. 2012, 31, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Callaway, J.C. Hempseed as a Nutritional Resource: An Overview. Euphytica 2004, 140, 65–72. [Google Scholar] [CrossRef]
- Kumar, N.; Mandal, A. Surfactant Stabilized Oil-in-Water Nanoemulsion: Stability, Interfacial Tension, and Rheology Study for Enhanced Oil Recovery Application. Energy Fuels 2018, 32, 6452–6466. [Google Scholar] [CrossRef]
- Pengon, S.; Chinatangkul, N.; Limmatvapirat, C.; Limmatvapirat, S. The Effect of Surfactant on the Physical Properties of Coconut Oil Nanoemulsions. Asian J. Pharm. Sci. 2018, 13, 409–414. [Google Scholar] [CrossRef]
- Liu, T.; Gao, Z.; Zhong, W.; Fu, F.; Li, G.; Guo, J.; Shan, Y. Preparation, Characterization, and Antioxidant Activity of Nanoemulsions Incorporating Lemon Essential Oil. Antioxidants 2022, 11, 650. [Google Scholar] [CrossRef]
- Jarzębski, M.; Fathordoobady, F.; Guo, Y.; Xu, M.; Singh, A.; Kitts, D.D.; Kowalczewski, P.L.; Jeżowski, P.; Pratap Singh, A. Pea Protein for Hempseed Oil Nanoemulsion Stabilization. Molecules 2019, 24, 4288. [Google Scholar] [CrossRef] [Green Version]
- Smulek, W.; Siejak, P.; Fathordoobady, F.; Masewicz, L.; Guo, Y.; Jarzębska, M.; Kitts, D.D.; Kowalczewski, P.L.; Baranowska, H.M.; Stangierski, J. Whey Proteins as a Potential Co-Surfactant with Aesculus hippocastanum L. as a Stabilizer in Nanoemulsions Derived from Hempseed Oil. Molecules 2021, 26, 5856. [Google Scholar] [CrossRef]
- Sungpud, C.; Panpipat, W.; Chaijan, M.; Sae Yoon, A. Techno-Biofunctionality of Mangostin Extract-Loaded Virgin Coconut Oil Nanoemulsion and Nanoemulgel. PLoS ONE 2020, 15, e0227979. [Google Scholar] [CrossRef] [Green Version]
- Mahdi, W.A.; Alam, P.; Alshetaili, A.; Alshehri, S.; Ghoneim, M.M.; Shakeel, F. Product Development Studies of Cranberry Seed Oil Nanoemulsion. Processes 2022, 10, 393. [Google Scholar] [CrossRef]
- Lewińska, A. Optimizing the Process Design of Oil-in-Water Nanoemulsion for Delivering Poorly Soluble Cannabidiol Oil. Processes 2021, 9, 1180. [Google Scholar] [CrossRef]
- Garcia, F.L.; Ma, S.; Dave, A.; Acevedo-Fani, A. Structural and Physicochemical Characteristics of Oil Bodies from Hemp Seeds (Cannabis sativa L.). Foods 2021, 10, 2930. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Sun, D.; Li, C.; Liu, Q.; Xu, J. Formation and Stability of Paraffin Oil-in-Water Nano-Emulsions Prepared by the Emulsion Inversion Point Method. J. Colloid Interface Sci. 2006, 303, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.; Nair, A.B.; Jacob, S.; Patel, R.K.; Shah, H.; Shehata, T.M.; Morsy, M.A. Nanoemulsion Based Vehicle for Effective Ocular Delivery of Moxifloxacin Using Experimental Design and Pharmacokinetic Study in Rabbits. Pharmaceutics 2019, 11, 230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dapčević Hadnadjev, T.; Dokić, P.; Krstonošić, V.; Hadnadjev, M. Influence of Oil Phase Concentration on Droplet Size Distribution and Stability of Oil-in-Water Emulsions. Eur. J. Lipid Sci. Technol. 2013, 115, 313–321. [Google Scholar] [CrossRef]
- Demisli, S.; Mitsou, E.; Pletsa, V.; Xenakis, A.; Papadimitriou, V. Development and Study of Nanoemulsions and Nanoemulsion-Based Hydrogels for the Encapsulation of Lipophilic Compounds. Nanomaterials 2020, 10, 2464. [Google Scholar] [CrossRef] [PubMed]
- Yousef, S.A.; Mohammed, Y.H.; Namjoshi, S.; Grice, J.E.; Benson, H.A.; Sakran, W.; Roberts, M.S. Mechanistic Evaluation of Enhanced Curcumin Delivery through Human Skin in Vitro from Optimised Nanoemulsion Formulations Fabricated with Different Penetration Enhancers. Pharmaceutics 2019, 11, 639. [Google Scholar] [CrossRef] [Green Version]
- Maccelli, A.; Vitanza, L.; Imbriano, A.; Fraschetti, C.; Filippi, A.; Goldoni, P.; Maurizi, L.; Ammendolia, M.G.; Crestoni, M.E.; Fornarini, S. Satureja Montana L. Essential Oils: Chemical Profiles/Phytochemical Screening, Antimicrobial Activity and o/w Nanoemulsion Formulations. Pharmaceutics 2019, 12, 7. [Google Scholar] [CrossRef] [Green Version]
- Zaitone, S.; Hassan, N.; El-Orabi, N.; El-Awady, E.-S. Pentoxifylline and Melatonin in Combination with Pioglitazone Ameliorate Experimental Non-Alcoholic Fatty Liver Disease. Eur. J. Pharmacol. 2011, 662, 70–77. [Google Scholar] [CrossRef]
- Gentile, C.L.; Pagliassotti, M.J. The Role of Fatty Acids in the Development and Progression of Nonalcoholic Fatty Liver Disease. J. Nutr. Biochem. 2008, 19, 567–576. [Google Scholar] [CrossRef] [Green Version]
- Tavares De Almeida, I.; Cortez-Pinto, H.; Fidalgo, G.; Rodrigues, D.; Camilo, M.E. Plasma Total and Free Fatty Acids Composition in Human Non-Alcoholic Steatohepatitis. Clin. Nutr. 2002, 21, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Neuschwander-Tetri, B.A.; Ford, D.A.; Acharya, S.; Gilkey, G.; Basaranoglu, M.; Tetri, L.H.; Brunt, E.M. Dietary Trans-Fatty Acid Induced NASH Is Normalized Following Loss of Trans-Fatty Acids from Hepatic Lipid Pools. Lipids 2012, 47, 941–950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Han, L.; Zhu, L.; Yu, Y. Free Fatty Acids, Not Triglycerides, Are Associated with Non-Alcoholic Liver Injury Progression in High Fat Diet Induced Obese Rats. Lipids Health Dis. 2016, 15, 27. [Google Scholar] [CrossRef] [Green Version]
- Sumida, Y.; Niki, E.; Naito, Y.; Yoshikawa, T. Involvement of Free Radicals and Oxidative Stress in NAFLD/NASH. Free Radic. Res. 2013, 47, 869–880. [Google Scholar] [CrossRef] [PubMed]
- El Sayed, A.M.; Basam, S.M.; El-Naggar, E.-M.b.A.; Marzouk, H.S.; El-Hawary, S. LC–MS/MS and GC–MS Profiling as Well as the Antimicrobial Effect of Leaves of Selected Yucca Species Introduced to Egypt. Sci. Rep. 2020, 10, 17778. [Google Scholar] [CrossRef]
- Luo, Q.; Yan, X.; Bobrovskaya, L.; Ji, M.; Yuan, H.; Lou, H.; Fan, P. Anti-Neuroinflammatory Effects of Grossamide from Hemp Seed via Suppression of TLR-4-Mediated NF-ΚB Signaling Pathways in Lipopolysaccharide-Stimulated BV2 Microglia Cells. Mol. Cell. Biochem. 2017, 428, 129–137. [Google Scholar] [CrossRef]
- Youssef, A.A.A.; Cai, C.; Dudhipala, N.; Majumdar, S. Design of Topical Ocular Ciprofloxacin Nanoemulsion for the Management of Bacterial Keratitis. Pharmaceuticals 2021, 14, 210. [Google Scholar] [CrossRef]
- Mostafa, D.M.; Abd El-Alim, S.H.; Asfour, M.H.; Al-Okbi, S.Y.; Mohamed, D.A.; Awad, G. Transdermal Nanoemulsions of Foeniculum vulgare Mill. Essential Oil: Preparation, Characterization and Evaluation of Antidiabetic Potential. J. Drug Deliv. Sci. Technol. 2015, 29, 99–106. [Google Scholar] [CrossRef]
- Falleh, H.; Jemaa, M.B.; Neves, M.A.; Isoda, H.; Nakajima, M.; Ksouri, R. Formulation, Physicochemical Characterization, and Anti-E. coli Activity of Food-Grade Nanoemulsions Incorporating Clove, Cinnamon, and Lavender Essential Oils. Food Chem. 2021, 359, 129963. [Google Scholar] [CrossRef]
- Srivastava, S.; Singh, S.; Saraf, S.A.; Chourasia, M.K.; Mathew, J.; Pandey, A.C. Encapsulation of Baicalein in Cinnamon Essential Oil Nanoemulsion for Enhanced Anticancer Efficacy Against MDA-MB-231 Cells. BioNanoScience 2021, 11, 1049–1060. [Google Scholar] [CrossRef]
- Piazzini, V.; Monteforte, E.; Luceri, C.; Bigagli, E.; Bilia, A.R.; Bergonzi, M.C. Nanoemulsion for Improving Solubility and Permeability of Vitex Agnus-Castus Extract: Formulation and In Vitro Evaluation Using PAMPA and Caco-2 Approaches. Drug Deliv. 2017, 24, 380–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esteban, P.P.; Jenkins, A.T.A.; Arnot, T.C. Elucidation of the Mechanisms of Action of Bacteriophage K/Nano-Emulsion Formulations against S. aureus via Measurement of Particle Size and Zeta Potential. Colloids Surf. B Biointerfaces 2016, 139, 87–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocha-Filho, P.A.; Ferrari, M.; Maruno, M.; Souza, O.; Gumiero, V. In Vitro and in Vivo Evaluation of Nanoemulsion Containing Vegetable Extracts. Cosmetics 2017, 4, 32. [Google Scholar] [CrossRef] [Green Version]
- Mostafa, N.M. Antibacterial Activity of Ginger (Zingiber officinale) Leaves Essential Oil Nanoemulsion against the Cariogenic Streptococcus mutans. J. Appl. Pharm. Sci. 2018, 8, 34–41. [Google Scholar]
- Fuentes, K.; Matamala, C.; Martínez, N.; Zúñiga, R.N.; Troncoso, E. Comparative Study of Physicochemical Properties of Nanoemulsions Fabricated with Natural and Synthetic Surfactants. Processes 2021, 9, 2002. [Google Scholar] [CrossRef]
- Kamairudin, N.; Hoong, S.S.; Abdullah, L.C.; Ariffin, H.; Biak, D.R.A. Optimisation of Epoxide Ring-Opening Reaction for the Synthesis of Bio-Polyol from Palm Oil Derivative Using Response Surface Methodology. Molecules 2021, 26, 648. [Google Scholar] [CrossRef]
- Hasssanzadeh, H.; Alizadeh, M.; Bari, M.R. Formulation of Garlic Oil-in-Water Nanoemulsion: Antimicrobial and Physicochemical Aspects. IET Nanobiotechnol. 2018, 12, 647–652. [Google Scholar] [CrossRef]
- Seibert, J.B.; Rodrigues, I.V.; Carneiro, S.P.; Amparo, T.R.; Lanza, J.S.; Frézard, F.J.G.; de Souza, G.H.B.; dos Santos, O.D.H. Seasonality Study of Essential Oil from Leaves of Cymbopogon densiflorus and Nanoemulsion Development with Antioxidant Activity. Flavour Fragr. J. 2019, 34, 5–14. [Google Scholar] [CrossRef] [Green Version]
- Pan, M.; Song, Y.-L.; Xu, J.-M.; Gan, H.-Z. Melatonin Ameliorates Nonalcoholic Fatty Liver Induced by High-Fat Diet in Rats. J. Pineal Res. 2006, 41, 79–84. [Google Scholar] [CrossRef]
- Lima, E.A.; Silveira, L.S.; Masi, L.N.; Crisma, A.R.; Davanso, M.R.; Souza, G.I.; Santamarina, A.B.; Moreira, R.G.; Roque Martins, A.; de Sousa, L.G.O. Macadamia Oil Supplementation Attenuates Inflammation and Adipocyte Hypertrophy in Obese Mice. Mediat. Inflamm. 2014, 2014, 870634. [Google Scholar] [CrossRef] [Green Version]
- Atteia, H.H.; Alzahrani, S.; El-Sherbeeny, N.A.; Youssef, A.M.; Farag, N.E.; Mehanna, E.T.; Elhawary, R.; Ibrahim, G.A.; Elmistekawy, A.; Zaitone, S.A. Evening Primrose Oil Ameliorates Hyperleptinemia and Reproductive Hormone Disturbances in Obese Female Rats: Impact on Estrus Cyclicity. Front. Endocrinol. 2020, 10, 942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis Model Assessment: Insulin Resistance and β-Cell Function from Fasting Plasma Glucose and Insulin Concentrations in Man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, A.H.; Jacobson, K.A.; Rose, J.; Zeller, R. Hematoxylin and Eosin Staining of Tissue and Cell Sections. Cold Spring Harb. Protoc. 2008, 2008, pdb-prot4986. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.; Onozato, M.; Shores, N.; Yagi, Y. Automated Quantification of Liver Steatosis by WSI Based Image Analysis. In Analytical Cellular Pathology, Proceedings of the 1st Congress of the International Academy of Digital Pathology, Quebec City, QC, Canada, 3–5 August 2011; IOS Press: Amsterdam, The Netherlands, 2011; Volume 34, p. 188. [Google Scholar]
- Williams, A.S.; Kang, L.; Zheng, J.; Grueter, C.; Bracy, D.P.; James, F.D.; Pozzi, A.; Wasserman, D.H. Integrin A1-Null Mice Exhibit Improved Fatty Liver When Fed a High Fat Diet despite Severe Hepatic Insulin Resistance. J. Biol. Chem. 2015, 290, 6546–6557. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| Fatty Acid Methyl Esters | % w/w * | Base Ion (m/z) |
|---|---|---|
| (9Z,11E,13E)-octadeca-9,11,13-trienoic acid methyl ester | 0.05 | 79.05 |
| (Z)-Methyl heptadec-9-enoate | 0.07 | 55.05 |
| 10-Heptadecen-8-ynoic acid, methyl ester, (E) | 0.15 | 79 |
| 13-Docosenoic acid, methyl ester, (Z) | 0.06 | 55.05 |
| 7,10,13-Eicosatrienoic acid, methyl ester | 0.17 | 67.05 |
| 9,11-Octadecadienoic acid, methyl ester, (E,E) | 0.75 | 95.05 |
| 9,12,15-Octadecatrienoic acid, methyl ester, (Z,Z,Z) | 73.7 | 79.05 |
| 9-Hexadecenoic acid, methyl ester, (Z) | 0.23 | 55.05 |
| cis-11-Eicosenoic acid, methyl ester | 0.98 | 55.05 |
| Docosanoic acid, methyl ester | 0.61 | 74 |
| Heptadecanoic acid, methyl ester | 0.08 | 74 |
| Hexadecanoic acid, methyl ester | 7.66 | 74.05 |
| Methyl γ-linolenate | 3.98 | 79.05 |
| Methyl 18-methylnonadecanoate | 1.45 | 74.05 |
| Methyl stearate | 3.68 | 74 |
| Tetracosanoic acid, methyl ester | 0.3 | 74 |
| Tricosanoic acid, methyl ester | 0.11 | 74 |
| Compound | % w/w * | Base Ion (m/z) |
|---|---|---|
| Benzene, (1,3,3-trimethylnonyl)– | 1.84 | 105.1 |
| Benzene, (1-butylheptyl)– | 1.18 | 91.05 |
| Benzene, (1-butyloctyl)– | 1.15 | 91.1 |
| Benzene, (1-ethylnonyl)– | 1.01 | 91.05 |
| Benzene, (1-methyldecyl)– | 1.56 | 105.1 |
| Benzene, (1-pentylheptyl)– | 1.22 | 91.1 |
| Benzene, (1-pentyloctyl)– | 1.04 | 91.1 |
| Benzene, (1-propyloctyl)– | 1.23 | 91.05 |
| Decyl oleate | 1.16 | 57.05 |
| Diisooctyl phthalate | 3.98 | 149.1 |
| Dodecane | 3.83 | 57.05 |
| Eicosane | 3.49 | 57.05 |
| Glycidyl (Z)-9-Heptadecenoate | 13.72 | 55.05 |
| Glycidyl palmitate | 2.02 | 57.05 |
| Heneicosane | 1.07 | 57.1 |
| Heneicosane | 1.16 | 57.05 |
| Heneicosane | 1.43 | 57.1 |
| Heneicosane, 10-methyl– | 0.93 | 57.1 |
| Heptadecane | 1.08 | 57.1 |
| Hexatriacontane | 2.43 | 57.05 |
| Hexatriacontane | 1.32 | 57.05 |
| Hexatriacontane | 1.73 | 57.1 |
| Oleoyl chloride | 14.28 | 55.05 |
| Oxalic acid, cyclohexylmethyl octadecyl ester | 1.13 | 97.1 |
| Silane, diethylheptyloxyoctadecyloxy– | 4.37 | 441.4 |
| γ-Sitosterol | 4.95 | 43.05 |
| Formulation No | Hemp Seed Oil % (v/v) | Surfactant (Tween 80% v/v) | Propylene Glycol % (v/v) | Water % (v/v) |
|---|---|---|---|---|
| NEF#1 | 40 | 2.5 | 5 | 52.5 |
| NEF#2 | 40 | 5 | 5 | 50 |
| NEF#3 | 40 | 7.5 | 5 | 47.5 |
| NEF#4 | 40 | 10 | 5 | 45 |
| Formulations No | Droplet Size (nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|
| NEF#1 | 140.9 ± 5.62 | 0.493 ± 0.02 | −10.32 ± 1.35 |
| NEF#2 | 121.3 ± 3.41 | 0.438 ± 0.01 | −12.67 ± 2.68 |
| NEF#3 | 98.6 ± 9.54 | 0.312 ± 0.03 | −19.26 ± 2.92 |
| NEF#4 | 80.7 ± 3.96 | 0.249 ± 0.06 | −21.94 ± 1.13 |
| Δ BWt. % | Glucose (mg/dL) | Insulin (µIU/L) | HOMA-IR Index | |
|---|---|---|---|---|
| Normal | 18.59 ± 6.76 | 76.4 ± 2.30 | 12 ± 2.92 | 1.99 ± 0.38 |
| NASH control | 51.43 ± 8.17 * | 96.2 ± 2.77 * | 47.6 ± 6.80 * | 11.28 ± 1.42 * |
| NASH + Hemp seed oil | 44.48 ± 3.78 # | 84 ± 5.24 # | 30 ± 6.04 # | 6.35 ± 1.39 # |
| NASH+ Hemp seed oil NEF#4 | 32.34 ± 6.74 #$ | 78 ± 2.23 # | 22.2 ± 5.2 #$ | 4.29 ± 1.11 #$ |
| Alanine Aminotransferase (IU/L) | Aspartate Aminotransferase (IU/L) | |
|---|---|---|
| Normal | 40.8 ± 3.35 | 61.6 ± 2.97 |
| NASH control | 85.4 ± 7.89 * | 97 ± 6.78 * |
| NASH + Hemp seed oil | 62.6 ± 9.76 # | 83.6 ± 6.73 # |
| NASH+ Hemp seed oil NEF4 | 56 ± 5.48 #$ | 68.4 ± 5.13 #$ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qushawy, M.; Mortagi, Y.; Alshaman, R.; Mokhtar, H.I.; Hisham, F.A.; Alattar, A.; Liang, D.; Enan, E.T.; Eltrawy, A.H.; Alamrani, Z.H.; et al. Formulation and Characterization of O/W Nanoemulsions of Hemp Seed Oil for Protection from Steatohepatitis: Analysis of Hepatic Free Fatty Acids and Oxidation Markers. Pharmaceuticals 2022, 15, 864. https://doi.org/10.3390/ph15070864
Qushawy M, Mortagi Y, Alshaman R, Mokhtar HI, Hisham FA, Alattar A, Liang D, Enan ET, Eltrawy AH, Alamrani ZH, et al. Formulation and Characterization of O/W Nanoemulsions of Hemp Seed Oil for Protection from Steatohepatitis: Analysis of Hepatic Free Fatty Acids and Oxidation Markers. Pharmaceuticals. 2022; 15(7):864. https://doi.org/10.3390/ph15070864
Chicago/Turabian StyleQushawy, Mona, Yasmin Mortagi, Reem Alshaman, Hatem I. Mokhtar, Fatma Azzahraa Hisham, Abdullah Alattar, Dong Liang, Eman T. Enan, Amira H. Eltrawy, Zainab H. Alamrani, and et al. 2022. "Formulation and Characterization of O/W Nanoemulsions of Hemp Seed Oil for Protection from Steatohepatitis: Analysis of Hepatic Free Fatty Acids and Oxidation Markers" Pharmaceuticals 15, no. 7: 864. https://doi.org/10.3390/ph15070864
APA StyleQushawy, M., Mortagi, Y., Alshaman, R., Mokhtar, H. I., Hisham, F. A., Alattar, A., Liang, D., Enan, E. T., Eltrawy, A. H., Alamrani, Z. H., Alshmrani, S. A., & Zaitone, S. A. (2022). Formulation and Characterization of O/W Nanoemulsions of Hemp Seed Oil for Protection from Steatohepatitis: Analysis of Hepatic Free Fatty Acids and Oxidation Markers. Pharmaceuticals, 15(7), 864. https://doi.org/10.3390/ph15070864







