Therapeutic Efficacy of Arnica in Hamsters with Cutaneous Leishmaniasis Caused by Leishmania braziliensis and L. tropica
Abstract
:1. Introduction
2. Results and Discussion
2.1. In Vitro Cytotoxicity of AT and STLs against Human Macrophages, Skin Fibroblasts, and Liver Cells
2.2. In Vitro Antileishmanial Activity
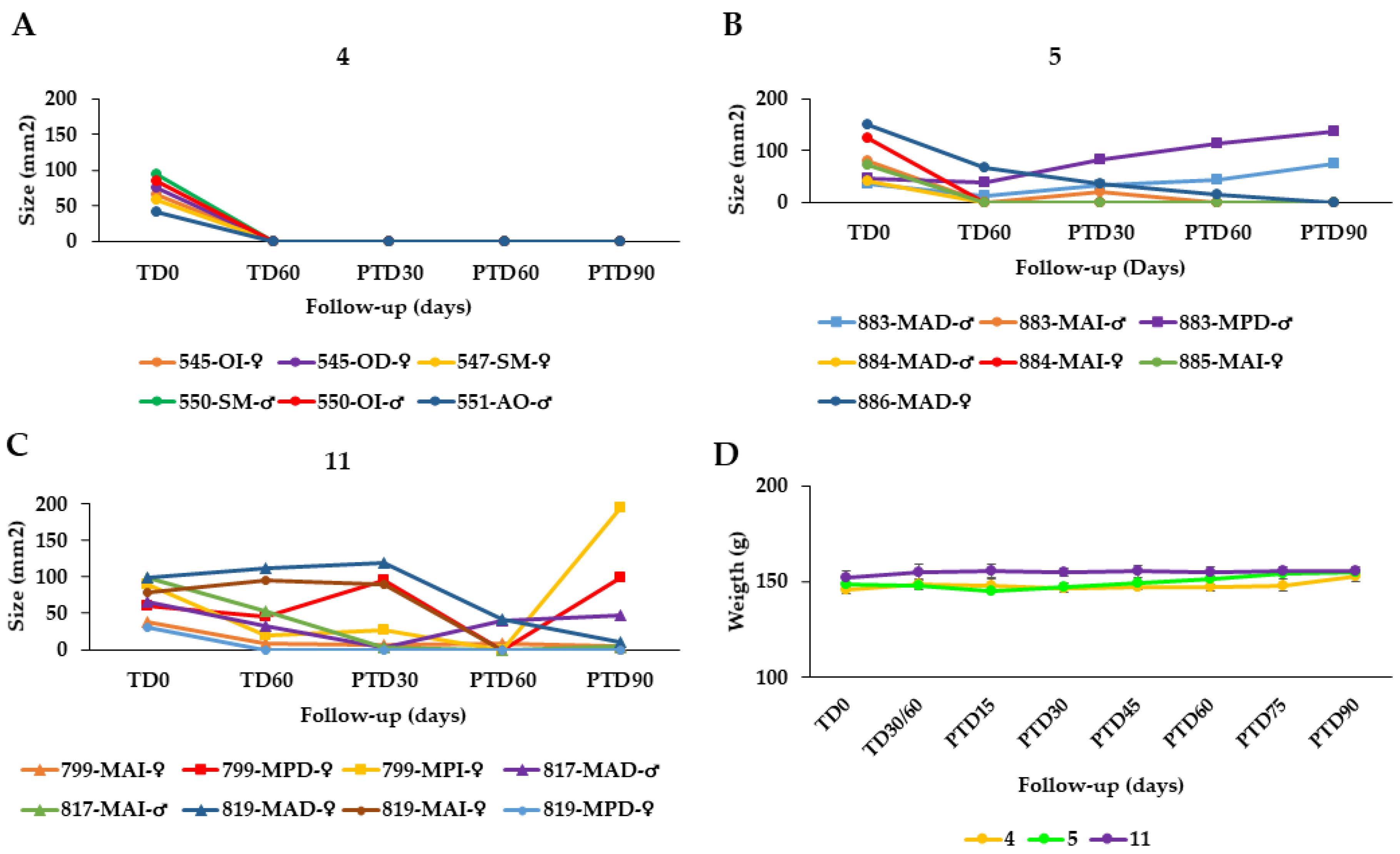
2.3. Response of Hamsters with CL Caused by L. braziliensis and L. tropica to Treatment with Arnica Tincture and other Formulations
2.4. Skin Penetration of STLs from AT and Semisolid Preparations
2.5. Residual Parasite Load in Cured Animals’ Skin
2.6. Expression of Growth Factors in the Skin of Cured Animals
2.7. Possible Influence of AT on Wound Healing
3. Materials and Methods
3.1. Arnica Tincture and STLs
3.2. Arnica Formulations
3.3. Human Cells and Culture
3.4. Parasites and Culture
3.5. Animals and Housing
3.6. In Vitro Cytotoxicity Assay
3.7. Infection of Human Macrophages (U937) with L. braziliensis and L. tropica and Exposure to Arnica Tincture and STLs
3.8. Intradermal Infection of Golden Hamsters with L. braziliensis and L. tropica and Therapeutic Schemes
3.9. Estimation of Parasite Load in Healed Skin Specimens by RT-qPCR
3.10. Expression of Growth Factors Associated with Wound Healing
3.11. In Vitro Wound Healing Assay (Scratch Test)
(confluent area (%) − untreated control (%))] × 100
3.12. Skin Penetration Experiments with Golden Hamster Skin in Franz Diffusion Cell
3.13. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Leishmaniasis. Available online: http://www.who.int/leishmaniasis/en/ (accessed on 10 April 2022).
- Al-Kamel, M.A. Stigmata in cutaneous leishmaniasis: Historical and new evidence-based concepts. Our Derm. Online 2017, 8, 81–90. [Google Scholar] [CrossRef] [Green Version]
- Serbate, Á.; Margotto, C.; da Silva, M.F.; Silva, S.A.; Moreira, A.M.; Parreiras, M.A. Adverse reactions to meglumine antimoniate in Brazilian inpatients with visceral leishmaniases: A case series. J. Clin. Pharm. Ther. 2020, 45, 573–576. [Google Scholar] [CrossRef]
- Croft, S.L.; Yardley, V. Chemotherapy of leishmaniasis. Curr. Pharm. Des. 2002, 8, 319–342. [Google Scholar] [CrossRef]
- Chakravarty, J.; Sundar, S. Drug resistance in leishmaniasis. J. Global. Infect Dis. 2010, 2, 167–176. [Google Scholar] [CrossRef]
- European Medicines Agency-Community Herbal Monograph on Arnica montana L., Flos. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Herbal_-_Community_herbal_monograph/2014/07/WC500170007.pdf (accessed on 3 May 2022).
- Willuhn, G. Arnicae flos. In Herbal Drugs and Phytopharmaceuticals: A Handbook for Practice on a Scientific Basis, 3rd ed.; Wichtl, M., Ed.; Medpharm: Stuttgart, Germany; CRC Press: Boca Raton, FL, USA, 2004; pp. 54–59. [Google Scholar]
- Willuhn, G. Arnica flowers: Pharmacology, toxicology, and analysis of the sesquiterpene lactones—Their main active substances. In Phytomedicines of Europe—ACS Symposium Series 691; Lawson, L.D., Bauer, R., Eds.; American Chemical Society: Washington, DC, USA, 1998; Volume 691, pp. 118–132. [Google Scholar]
- European Directorate for the Quality of Medicines & HealthCare (EDQM). Arnicae tinctura. In European Pharmacopoeia, 10th ed.; Council of Europe: Strasbourg, France, 2020. [Google Scholar]
- Kriplani, P.; Guarve, K.; Baghael, U.S. Arnica montana L.—A plant of healing: Review. J. Pharm. Pharmacol. 2017, 69, 925–945. [Google Scholar] [CrossRef] [Green Version]
- Robledo, S.M.; Vélez, I.D.; Schmidt, T.J. Arnica Tincture Cures Cutaneous Leishmaniasis in Golden Hamsters. Molecules 2018, 23, 150. [Google Scholar] [CrossRef] [Green Version]
- Woerdenbag, H.J.; Merfort, I.; Schmidt, T.J.; Passreiter, C.M.; Willuhn, G.; Van Uden, W.; Pras, N.; Konings, A.W. Decreased helenalin-induced cytotoxicity by flavonoids from Arnica as studied in a human lung carcinoma cell line. Phytomedicine 1995, 2, 127–132. [Google Scholar] [CrossRef]
- Bamorovat, M.; Sharifi, I.; Tavakoli, R.; Jafarzadeh, A.; Khosravi, A. Determinants of Unresponsiveness to Treatment in Cutaneous Leishmaniasis: A Focus on Anthroponotic Form Due to Leishmania tropica. Front. Microbiol. 2021, 12, 638957. [Google Scholar] [CrossRef]
- Robledo, S.M.; Carrillo, L.M.; Daza, A.; Restrepo, A.M.; Muñoz, D.L.; Tobón, J.; Murillo, J.D.; López, A.; Ríos, C.; Mesa, C.V.; et al. Cutaneous leishmaniasis in the dorsal skin of hamsters: A useful model for the screening of antileishmanial drugs. J. Vis. Exp. 2012, 62, e3533. [Google Scholar] [CrossRef]
- Blum, J.; Lockwood, D.N.J.; Visser, L.; Harms, G.; Bailey, M.S.; Caumes, E.; Clerinx, J.; van Thiel, P.P.A.M.; Morizot, G.; Hatz, C.; et al. Local or systemic treatment for New World cutaneous leishmaniasis? Re-evaluating the evidence for the risk of mucosal leishmaniasis. Int. Health 2012, 4, 153–163. [Google Scholar] [CrossRef]
- Mendonça, M.G.; de Brito, M.E.F.; Rodrigues, E.H.G.; Bandeira, V.; Jardim, M.L.; Abath, F.G.C. Persistence of Leishmania Parasites in Scars after Clinical Cure of American Cutaneous Leishmaniasis: Is There a Sterile Cure? J. Infect. Dis. 2004, 189, 1018–1023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robson, M.C. Growth factors as wound healing agents. Curr. Opin. Biotechnol. 1991, 2, 863–867. [Google Scholar] [CrossRef]
- Steed, D.L. The role of growth factors in wound healing. Surg. Clin. N. Am. 1997, 77, 575–586. [Google Scholar] [CrossRef]
- Montoya, A.; Yepes, L.; Bedoya, A.; Henao, R.; Delgado, G.; Vélez, I.D.; Robledo, S.M. Transforming Growth Factor Beta (TGFβ1) and Epidermal Growth Factor (EGF) as Biomarkers of Leishmania (V) braziliensis Infection and Early Therapeutic Response in Cutaneous Leishmaniasis: Studies in Hamsters. Front. Cell Infect. Microbiol. 2018, 8, 350. [Google Scholar] [CrossRef] [PubMed]
- Raja, S.K.; Garcia, M.S.; Isseroff, R.R. Wound reepithelialization: Modulating keratinocyte migration in wound healing. Front Biosci. 2007, 12, 2849–2868. [Google Scholar] [CrossRef] [Green Version]
- Powers, C.J.; McLeskey, S.W.; Wellstein, A. Fibroblast growth factors, their receptors and signaling. Endocr. Relat. Cancer 2000, 7, 165–197. [Google Scholar] [CrossRef] [Green Version]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Perspective article: Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef]
- Jürgens, F.M.; Herrmann, F.C.; Robledo, S.M.; Schmidt, T.J. Dermal Absorption of Sesquiterpene Lactones from Arnica Tincture. Pharmaceutics 2022, 14, 742. [Google Scholar] [CrossRef]
- Jürgens, F.M.; Behrens, M.; Humpf, H.-U.; Robledo, S.M.; Schmidt, T.J. In Vitro Metabolism of Helenalin Acetate and 11α,13-Dihydrohelenalin Acetate: Natural Sesquiterpene Lactones from Arnica. Metabolites 2022, 12, 88. [Google Scholar] [CrossRef]
- Wagner, S.; Merfort, I. Skin penetration behaviour of sesquiterpene lactones from different Arnica preparations using a validated GC-MSD method. J. Pharm. Biomed. Anal. 2007, 43, 32–38. [Google Scholar] [CrossRef]
- Pulido, S.A.; Muñoz, D.L.; Restrepo, A.M.; Mesa, C.V.; Alzate, J.F.; Vélez, I.D.; Robledo, S.M. Improvement of the green fluorescent protein reporter system in Leishmania spp. for the in vitro and in vivo screening of antileishmanial drugs. Acta Trop. 2012, 122, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Ladopoulos, T.; Ntais, P.; Tsirigotakis, N.; Dokianakis, E.; Antoniou, M. The proliferation potential of promastigotes of the main Leishmania species of the old world in NNN culture medium prepared using blood of four different mammals. Exp. Parasitol. 2015, 157, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Ribeiro-Romão, R.P.; Saavedra, A.F.; Da-Cruz, A.M.; Pinto, E.F.; Moreira, O.C. Development of real-time PCR assays for evaluation of immune response and parasite load in golden hamster (Mesocricetus auratus) infected by Leishmania (Viannia) braziliensis. Parasit. Vectors 2016, 9, 361. [Google Scholar] [CrossRef] [Green Version]
- Fox, L.T.; Mazumder, A.; Dwivedi, A.; Gerber, M.; du Plessis, J.; Hamman, J.H. In vitro wound healing and cytotoxic activity of the gel and whole-leaf materials from selected aloe species. J. Ethnopharmacol. 2017, 200, 1–7. [Google Scholar] [CrossRef]




| Product | U937 LC50 (µg/mL) | Detroit 551 LC50 (µg/mL) | Hep G2 LC50 (µg/mL) |
|---|---|---|---|
| Tincture (total STLs) | >96 | 300 ± 4 | 380 ± 1 |
| helenalin | 5.75 ± 0.20 | 218 ± 44 | 19.1 ± 2.9 |
| helenalin acetate | 5.66 ± 0.08 | 59.8 ± 3.6 | 0.300 ± 0.080 |
| helenalin isobutyrate | 5.65 ± 0.01 | 69.5 ± 6.3 | 0.200 ± 0.010 |
| helenalin methacrylate | 5.68 ± 0.66 | 57.8 ± 9.2 | 1.20 ± 0.20 |
| helenalin tiglate | 5.80 ± 0.10 | 234 ± 44 | 52.2 ± 0.1 |
| 11α, 13-dihydrohelenalin acetate | 8.23 ± 0.40 | 206 ± 35 | 41.0 ± 7.1 |
| 11α, 13-dihydrohelenalin isovalerate | 10.1 ± 1.3 | 0.800 ± 0.020 | 0.600 ± 0.090 |
| meglumine antimoniate | 416 ± 23 | 492 ± 32 | 226 ± 29 |
| doxorubicin | 0.800 ± 0.100 | 2.00 ± 0.00 | 5.42 ± 0.10 |
| Product | L. braziliensis | L. tropica | ||
|---|---|---|---|---|
| EC50 (µg/mL) | SI | EC50 (µg/mL) | SI | |
| Tincture (total STLs) | 4.85 ± 0.19 | >19.8 | 5.94 ± 1.17 | >16.2 |
| helenalin | 2.02 ± 0.07 | 2.8 | 3.76 ± 0.78 | 1.5 |
| helenalin acetate | 1.86 ± 0.01 | 3.0 | 5.33 ± 0.17 | 1.1 |
| helenalin isobutyrate | 1.85 ± 0.03 | 3.1 | 2.20 ± 0.29 | 2.6 |
| helenalin methacrylate | 2.16 ± 0.13 | 2.6 | 2.40 ± 0.07 | 2.4 |
| helenalin tiglate | 2.14 ± 0.06 | 2.7 | 2.32 ± 0.14 | 2.5 |
| 11α, 13-dihydrohelenalin acetate | 2.44 ± 0.10 | 3.4 | 3.50 ± 0.09 | 2.4 |
| 11α, 13-dihydrohelenalin isovalerate | 3.04 ± 0.16 | 3.3 | 3.86 ± 0.29 | 2.6 |
| meglumine antimoniate | 13.7 ± 0.9 | 30.4 | 50.2 ± 1.3 | 8.3 |
| Formulation | Leishmania Species | Group | Dose | Cure n/m (%) | Improvement n/m (%) | Failure n/m (%) |
|---|---|---|---|---|---|---|
| Tincture | L. braziliensis | 1 | 40 µL AT = 19.2 μg STL/1× d/60 d | 8/11 (72.2) | 2/11 (18.2) | 1/11 (9.1) |
| 2 | 40 µL AT = 19.2 μg STL/2× d/60 d | 7/8 (87.5) | 1/8 (12.5) | 0 (0) | ||
| 3 | 80 µL AT = 38.4 μg STL/2× d/60 d | 6/9 (66.7) | 2/9 (22.2) | 1/9 (11.1) | ||
| L. tropica | 4 | 40 µL AT = 19.2 μg STL/2× d/60 d | 6/6 (100) | 0 (0) | 0 (0) | |
| 5 | 80 µL AT = 38.4 μg STL/2× d/60 d | 5/7 (66.7) | 0 (0) | 2/7 (11.1) | ||
| DOC Arnica 21,5% Creme (DAC) | L. braziliensis | 6 | 40 mg = 4.0 μg STL/2× d/60 d | 4/11 (36.3) | 2/11 (18.2) | 5/11 (45.4) |
| Arnica Salbe S (Kneipp) (ASK) | 7 | 40 mg = 4.0 μg STL/2× d/60 d | 6/11 (54.5) | 2/11 (18.2) | 3/11 (27.3) | |
| STL enriched Arnica cream (EAC) | 8 | 40 mg = 16.0 μg STL/2× d/60 d | 3/5 (60) | 1 (20) | 1 (20) | |
| STL enriched Arnica Gel (EAG) | 9 | 40 mg = 19.2 μg STL/2× d/60 d | 4/6 (66.7) | 2/6 (33.3) | 0 (0) | |
| Positive controls: Meglumine antimoniate (MA; intralesional injection) | L. braziliensis | 10 | 200 μg/every 3 d/30 d | 5/8 (62.5) | 1/8 (12.5) | 2/8 (25) |
| L. tropica | 11 | 200 μg/every 3 d/30 d | 3/8 (37.5) | 2/8 (25) | 3/8 (37.5) |
| Group | ALT (U/L) (R.V. 22–128) | CREATININE (mg/dL) (R.V. 0.4–1.0) | BUN (mg/dL) (R.V. 12–26) | |||
|---|---|---|---|---|---|---|
| TD0 | TD8 | TD0 | TD8 | TD0 | TD8 | |
| 1 | 62.7 ± 4.6 | 78.3 ± 11.8 | 0.4 ± 0.02 | 0.5 ± 0.05 | 19.0 ± 2.7 | 18.7 ± 0.6 |
| 2 | 57.0 ± 8.7 | 80.3 ± 13.3 | 0.4 ± 0.01 | 0.5 ± 0.02 | 24.8 ± 3.6 | 22.7 ± 0.6 |
| 3 | 45.3 ± 5.0 | 61.2 ± 1.8 | 0.5 ± 0.02 | 0.5 ± 0.03 | 14.2 ± 2.4 | 23.6 ± 2.3 |
| 4 | 58.1 ± 2.6 | 75.4 ± 4.8 | 0.4 ± 0.04 | 0.4 ± 0.03 | 15.3 ± 1.3 | 21.3 ± 4.8 |
| 5 | 47.4 ± 1.2 | 68.7 ± 4.4 | 0.6 ± 0.01 | 0.3 ± 0.02 | 21.3 ± 3.3 | 17.5 ± 6.8 |
| 6 | 57.3 ± 4.7 | 61.3 ± 4.2 | 0.7 ± 0.02 | 0.4 ± 0.01 | 16.2 ± 4.7 | 22.1 ± 3.3 |
| 7 | 67.2 ± 3.4 | 73.4 ± 5.6 | 0.5 ± 0.03 | 0.5 ± 0.03 | 15.7 ± 2.1 | 19.5 ± 1.7 |
| 8 | 60.5 ± 2.3 | 75.3 ± 5.6 | 0.4 ± 0.02 | 0.3 ± 0.02 | 24.2 ± 1.5 | 21.0 ± 2.8 |
| 9 | 64.9 ± 3.7 | 58.2 ± 2.9 | 0.6 ± 0.03 | 0.4 ± 0.03 | 23.3 ± 1.1 | 20.3 ± 2.7 |
| 10 | 43.3 ± 11.3 | 49.5 ± 10.9 | 0.5 ± 1.0 | 0.5 ± 0.05 | 21.1 ± 2.5 | 23.3 ± 2.3 |
| 11 | 48.5 ± 7.3 | 62.0 ± 14.5 | 0.5 ± 0.08 | 0.05 ± 0.07 | 20.3 ± 3.8 | 19.8 ± 3.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robledo, S.M.; Murillo, J.; Arbeláez, N.; Montoya, A.; Ospina, V.; Jürgens, F.M.; Vélez, I.D.; Schmidt, T.J. Therapeutic Efficacy of Arnica in Hamsters with Cutaneous Leishmaniasis Caused by Leishmania braziliensis and L. tropica. Pharmaceuticals 2022, 15, 776. https://doi.org/10.3390/ph15070776
Robledo SM, Murillo J, Arbeláez N, Montoya A, Ospina V, Jürgens FM, Vélez ID, Schmidt TJ. Therapeutic Efficacy of Arnica in Hamsters with Cutaneous Leishmaniasis Caused by Leishmania braziliensis and L. tropica. Pharmaceuticals. 2022; 15(7):776. https://doi.org/10.3390/ph15070776
Chicago/Turabian StyleRobledo, Sara M., Javier Murillo, Natalia Arbeláez, Andrés Montoya, Victoria Ospina, Franziska M. Jürgens, Iván D. Vélez, and Thomas J. Schmidt. 2022. "Therapeutic Efficacy of Arnica in Hamsters with Cutaneous Leishmaniasis Caused by Leishmania braziliensis and L. tropica" Pharmaceuticals 15, no. 7: 776. https://doi.org/10.3390/ph15070776







