Identification of CD73 as the Antigen of an Antigen-Unknown Monoclonal Antibody Established by Exosome Immunization, and Its Antibody–Drug Conjugate Exerts an Antitumor Effect on Glioblastoma Cell Lines
Abstract
:1. Introduction
2. Results and Discussions
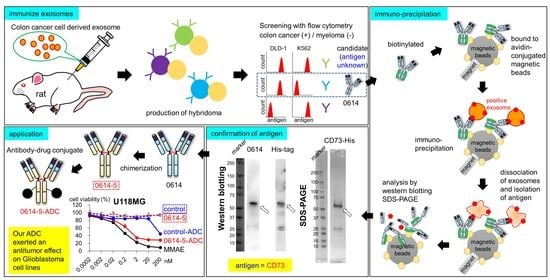
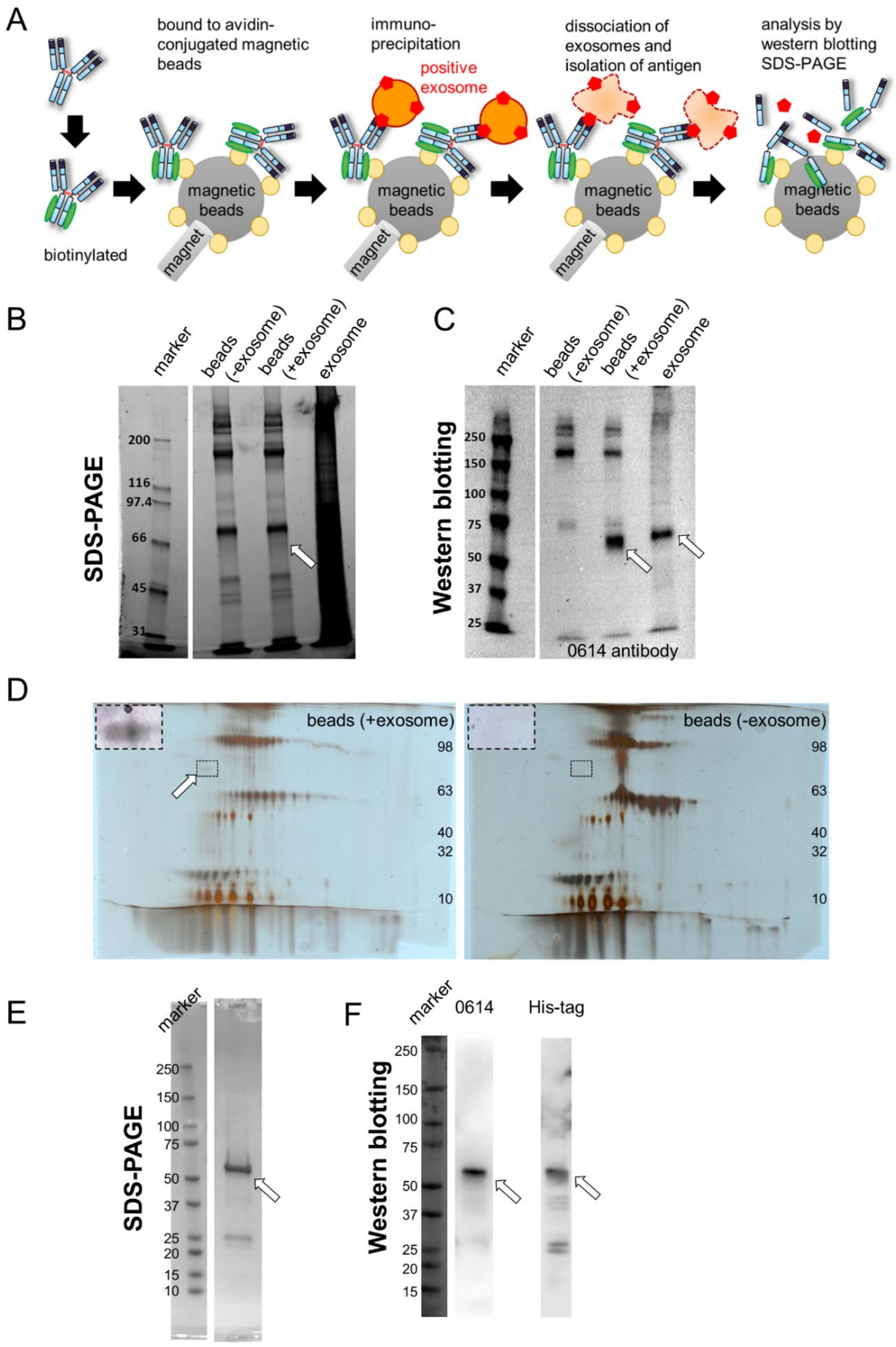
2.1. Identification of CD73 as the Target Antigen of Antibody Clone 0614
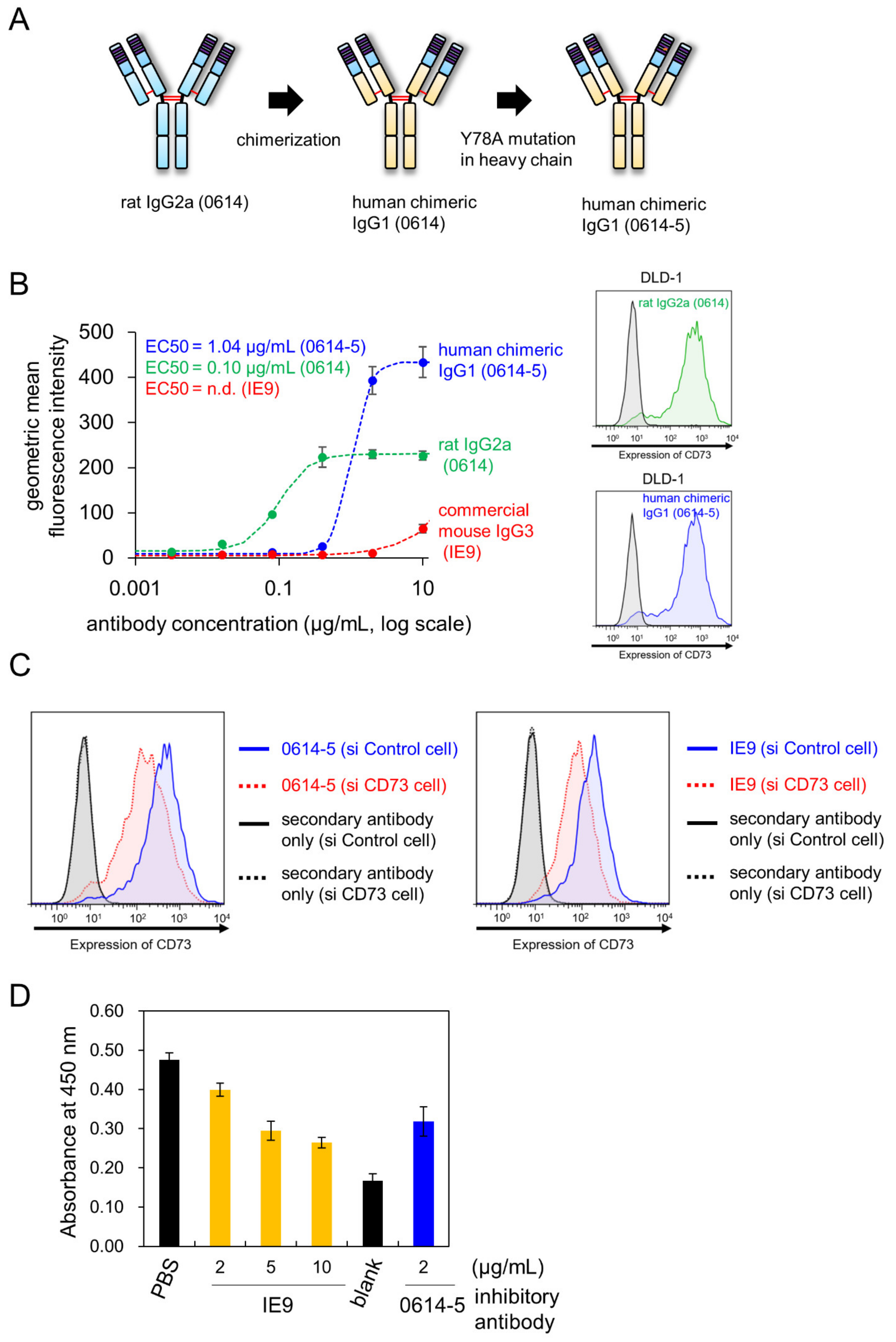
2.2. Characterization of Chimeric Antibody Clone 0614-5
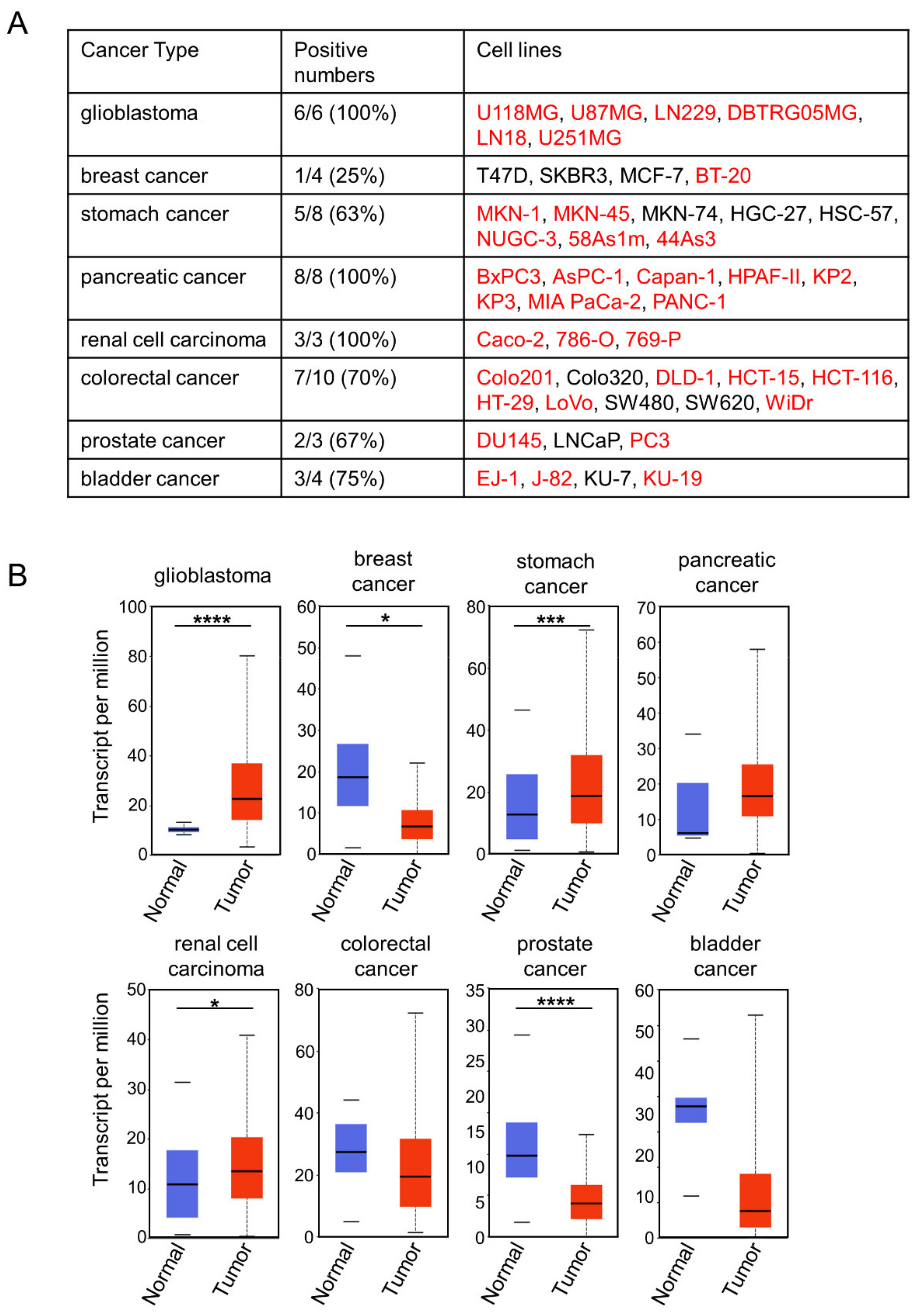
2.3. Choosing Cancer Types for Targeting by 0614-5 mAb
2.4. Application of Clone 0614-5 Antibody–Drug Conjugate in Glioblastoma Cell Lines
3. Materials and Methods
3.1. Cells and Cell Culture
3.2. Plasmid Construction
3.3. Antibodies and Recombinant Proteins
3.4. Isolation of Antigen of 0614 Antibody from Exosome Sample
3.5. Identification of Antigen of 0614 Antibody
3.6. Confirmation of Antigen of 0614 Antibody as CD73
3.7. Sandwich ELISA
3.8. Flow Cytometry
3.9. Database Analysis
3.10. In Vitro Cytotoxicity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADC | antibody-drug conjugate |
| AMP | adenosine monophosphate |
| ATP | adenosine triphosphate |
| CCLE | Cancer Cell Line Encyclopedia |
| CRC | colorectal cancer |
| EC50 | Effective Concentration 50 |
| ELISA | Enzyme-linked immunosorbent assays |
| EPR | Enhanced Permeability and Retention |
| GB | glioblastoma |
| IC50 | Inhibitory Concentration 50 |
| MMAE | monomethyl auristatin E |
| NT5E | 5'-nucleotidase ecto |
| PI | propidium iodide |
| TCGA | The Cancer Genome Atlas |
References
- Dodd, R.B.; Wilkinson, T.; Schofield, D.J. Therapeutic Monoclonal Antibodies to Complex Membrane Protein Targets: Antigen Generation and Antibody Discovery Strategies. BioDrugs 2018, 32, 339–355. [Google Scholar] [CrossRef]
- To’a Salazar, G.; Huang, Z.; Zhang, N.; Zhang, X.G.; An, Z. Antibody Therapies Targeting Complex Membrane Proteins. Engineering 2021, 7, 1541–1551. [Google Scholar] [CrossRef]
- Yasunaga, M.; Saijou, S.; Hanaoka, S.; Anzai, T.; Tsumura, R.; Matsumura, Y. Significant antitumor effect of an antibody against TMEM180, a new colorectal cancer-specific molecule. Cancer Sci. 2019, 110, 761–770. [Google Scholar] [CrossRef]
- Tsuji, S.; Washimi, K.; Kageyama, T.; Yamashita, M.; Yoshihara, M.; Matsuura, R.; Yokose, T.; Kameda, Y.; Hayashi, H.; Morohoshi, T.; et al. HEG1 is a novel mucin-like membrane protein that serves as a diagnostic and therapeutic target for malignant mesothelioma. Sci. Rep. 2017, 7, 45768. [Google Scholar] [CrossRef] [PubMed]
- Hosen, N.; Matsunaga, Y.; Hasegawa, K.; Matsuno, H.; Nakamura, Y.; Makita, M.; Watanabe, K.; Yoshida, M.; Satoh, K.; Morimoto, S.; et al. The activated conformation of integrin β 7 is a novel multiple myeloma-specific target for CAR T cell therapy. Nat. Med. 2017, 23, 1436–1443. [Google Scholar] [CrossRef]
- Knapp, K.; Zebisch, M.; Pippel, J.; El-Tayeb, A.; Müller, C.E.; Sträter, N. Crystal structure of the human ecto-5′-nucleotidase (CD73): Insights into the regulation of purinergic signaling. Structure 2012, 20, 2161–2173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knapp, K.M.; Zebisch, M.; Sträter, N. Crystallization and preliminary X-ray analysis of the open form of human ecto-5′-nucleotidase (CD73). Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2012, 68, 1545–1549. [Google Scholar] [CrossRef] [PubMed]
- Heuts, D.P.H.M.; Weissenborn, M.J.; Olkhov, R.V.; Shaw, A.M.; Gummadova, J.; Levy, C.; Scrutton, N.S. Crystal Structure of a Soluble Form of Human CD73 with Ecto-5′-Nucleotidase Activity. ChemBioChem 2012, 13, 2384–2391. [Google Scholar] [CrossRef] [PubMed]
- Kobie, J.J.; Shah, P.R.; Yang, L.; Rebhahn, J.A.; Fowell, D.J.; Mosmann, T.R. T Regulatory and Primed Uncommitted CD4 T Cells Express CD73, Which Suppresses Effector CD4 T Cells by Converting 5′-Adenosine Monophosphate to Adenosine. J. Immunol. 2006, 177, 6780–6786. [Google Scholar] [CrossRef] [PubMed]
- Antonioli, L.; Pacher, P.; Vizi, E.S.; Haskó, G. CD39 and CD73 in immunity and inflammation. Trends. Mol. Med. 2013, 19, 355–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perrot, I.; Michaud, H.A.; Giraudon-Paoli, M.; Augier, S.; Docquier, A.; Gros, L.; Courtois, R.; Déjou, C.; Jecko, D.; Becquart, O.; et al. Blocking Antibodies Targeting the CD39/CD73 Immunosuppressive Pathway Unleash Immune Responses in Combination Cancer Therapies. Cell Rep. 2019, 27, 2411–2425.e9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clayton, A.; Al-Taei, S.; Webber, J.; Mason, M.D.; Tabi, Z. Cancer Exosomes Express CD39 and CD73, Which Suppress T Cells through Adenosine Production. J. Immunol. 2011, 187, 676–683. [Google Scholar] [CrossRef]
- Schuler, P.; Saze, Z.; Hong, C.; Muller, L.; Gillespie, D.; Cheng, D.; Harasymczuk, M.; Mandapathil, M.; Lang, S.; Jackson, E.; et al. Human CD4+ CD39+ regulatory T cells produce adenosine upon co-expression of surface CD73 or contact with CD73+ exosomes or CD73+ cells. Clin. Exp. Immunol. 2014, 177, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Turiello, R.; Pinto, A.; Morello, S. CD73: A Promising Biomarker in Cancer Patients. Front. Pharmacol. 2020, 11, 609931. [Google Scholar] [CrossRef] [PubMed]
- Alcedo, K.P.; Bowser, J.L.; Snider, N.T. The elegant complexity of mammalian ecto-5′-nucleotidase (CD73). Trends Cell Biol. 2021, 31, 829–842. [Google Scholar] [CrossRef] [PubMed]
- Terp, M.G.; Olesen, K.A.; Arnspang, E.C.; Lund, R.R.; Lagerholm, B.C.; Ditzel, H.J.; Leth-Larsen, R. Anti-human CD73 monoclonal antibody inhibits metastasis formation in human breast cancer by inducing clustering and internalization of CD73 expressed on the surface of cancer cells. J. Immunol. 2013, 191, 4165–4173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohta, M.; Toyama, K.; Gutterman, D.D.; Campbell, W.B.; Lemaître, V.; Teraoka, R.; Miura, H. Ecto-5′-nucleotidase, CD73, is an endothelium-derived hyperpolarizing factor synthase. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 629–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandrashekar, D.S.; Bashel, B.; Balasubramanya, S.A.H.; Creighton, C.J.; Ponce-Rodriguez, I.; Chakravarthi, B.V.S.K.; Varambally, S. UALCAN: A Portal for Facilitating Tumor Subgroup Gene Expression and Survival Analyses. Neoplasia 2017, 19, 649–658. [Google Scholar] [CrossRef]
- Bhaskaran, M.; Devegowda, V.G.; Gupta, V.K.; Shivachar, A.; Bhosale, R.R.; Arunachalam, M.; Vaishnavi, T. Current Perspectives on Therapies, Including Drug Delivery Systems, for Managing Glioblastoma Multiforme. ACS Chem. Neurosci. 2020, 11, 2962–2977. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Matosevic, S. NT5E/CD73 as Correlative Factor of Patient Survival and Natural Killer Cell Infiltration in Glioblastoma. J. Clin. Med. 2019, 8, 1526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghandi, M.; Huang, F.W.; Jané-Valbuena, J.; Kryukov, G.V.; Lo, C.C.; McDonald, E.R.; Barretina, J.; Gelfand, E.T.; Bielski, C.M.; Li, H.; et al. Next-generation characterization of the Cancer Cell Line Encyclopedia. Nature 2019, 569, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar]
- Duncan, R. The dawning era of polymer therapeutics. Nat. Rev. Drug Discov. 2003, 2, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Tarin, D. Cancer Drug Delivery Systems Based on the Tumor Microenvironment; Springer: Tokyo, Japan, 2019. [Google Scholar]
- Yasunaga, M.; Manabe, S.; Tsuji, A.; Furuta, M.; Ogata, K.; Koga, Y.; Saga, T.; Matsumura, Y. Development of Antibody-Drug Conjugates Using DDS and Molecular Imaging. Bioengineering 2017, 4, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, R.; Liu, L.; Xing, Y.; Meng, T.; Ma, L.; Pei, J.; Cong, Y.; Zhang, X.; Ren, Z.; Wang, X.; et al. Dual mechanisms of novel CD73-targeted antibody and antibody–drug conjugate in inhibiting lung tumor growth and promoting antitumor immune-effector function. Mol. Cancer Ther. 2020, 19, 2340–2352. [Google Scholar] [CrossRef] [PubMed]
- Shevchenko, A.; Wilm, M.; Vorm, O.; Mann, M. Mass spectrometric sequencing of proteins from silver-stained polyacrylamide gels. Anal. Chem. 1996, 68, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Koga, Y.; Manabe, S.; Aihara, Y.; Sato, R.; Tsumura, R.; Iwafuji, H.; Furuya, F.; Fuchigami, H.; Fujiwara, Y.; Hisada, Y.; et al. Antitumor effect of antitissue factor antibody-MMAE conjugate in human pancreatic tumor xenografts. Int. J. Cancer 2015, 137, 1457–1466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anzai, T.; Saijou, S.; Takashima, H.; Hara, M.; Hanaoka, S.; Matsumura, Y.; Yasunaga, M. Identification of CD73 as the Antigen of an Antigen-Unknown Monoclonal Antibody Established by Exosome Immunization, and Its Antibody–Drug Conjugate Exerts an Antitumor Effect on Glioblastoma Cell Lines. Pharmaceuticals 2022, 15, 837. https://doi.org/10.3390/ph15070837
Anzai T, Saijou S, Takashima H, Hara M, Hanaoka S, Matsumura Y, Yasunaga M. Identification of CD73 as the Antigen of an Antigen-Unknown Monoclonal Antibody Established by Exosome Immunization, and Its Antibody–Drug Conjugate Exerts an Antitumor Effect on Glioblastoma Cell Lines. Pharmaceuticals. 2022; 15(7):837. https://doi.org/10.3390/ph15070837
Chicago/Turabian StyleAnzai, Takahiro, Shinji Saijou, Hiroki Takashima, Misato Hara, Shingo Hanaoka, Yasuhiro Matsumura, and Masahiro Yasunaga. 2022. "Identification of CD73 as the Antigen of an Antigen-Unknown Monoclonal Antibody Established by Exosome Immunization, and Its Antibody–Drug Conjugate Exerts an Antitumor Effect on Glioblastoma Cell Lines" Pharmaceuticals 15, no. 7: 837. https://doi.org/10.3390/ph15070837
APA StyleAnzai, T., Saijou, S., Takashima, H., Hara, M., Hanaoka, S., Matsumura, Y., & Yasunaga, M. (2022). Identification of CD73 as the Antigen of an Antigen-Unknown Monoclonal Antibody Established by Exosome Immunization, and Its Antibody–Drug Conjugate Exerts an Antitumor Effect on Glioblastoma Cell Lines. Pharmaceuticals, 15(7), 837. https://doi.org/10.3390/ph15070837