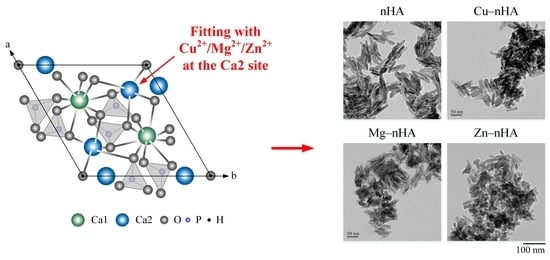
Morphological Changes, Antibacterial Activity, and Cytotoxicity Characterization of Hydrothermally Synthesized Metal Ions-Incorporated Nanoapatites for Biomedical Application
Abstract
:1. Introduction
2. Results and Discussion
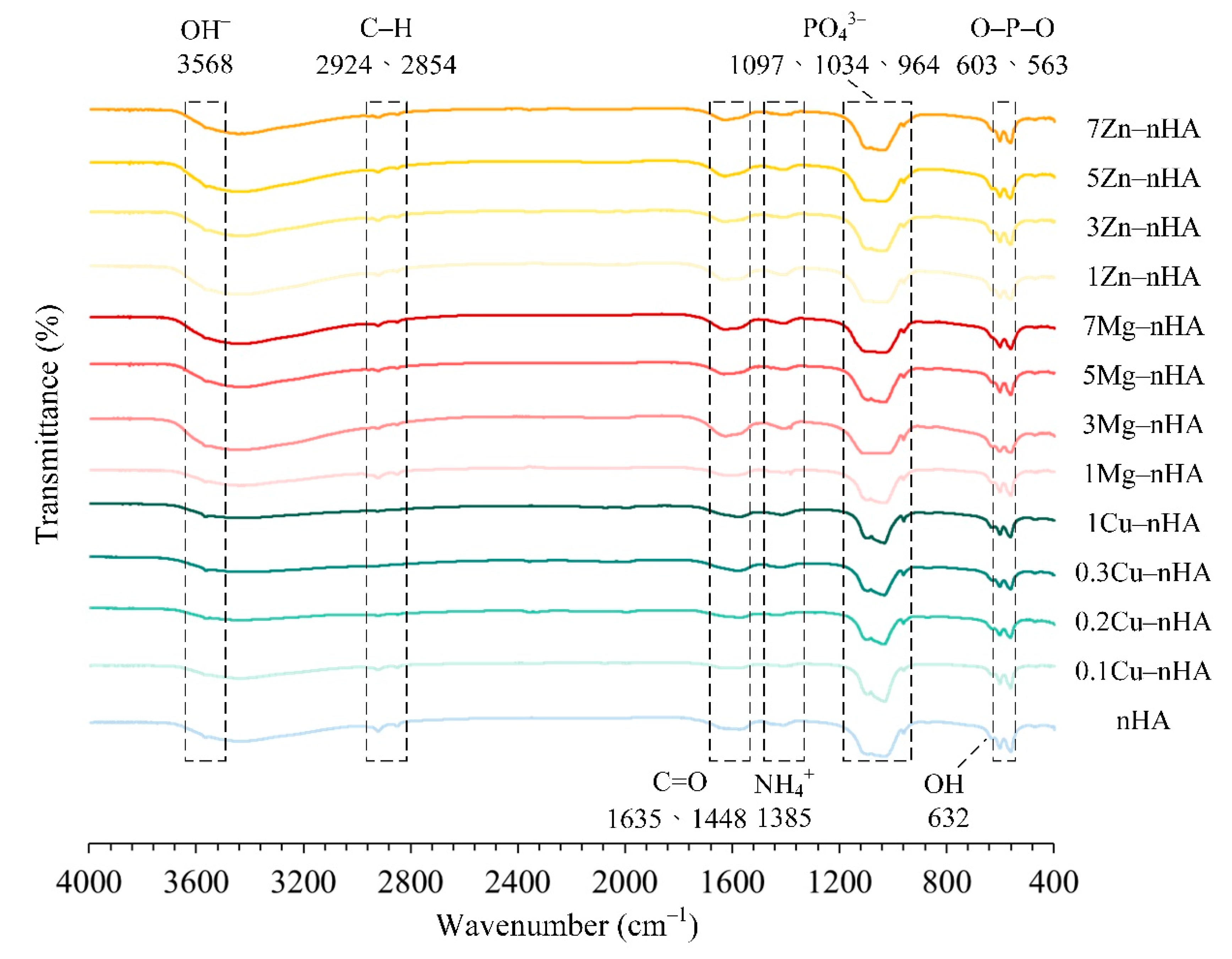
2.1. FT-IR Spectroscopic Analysis
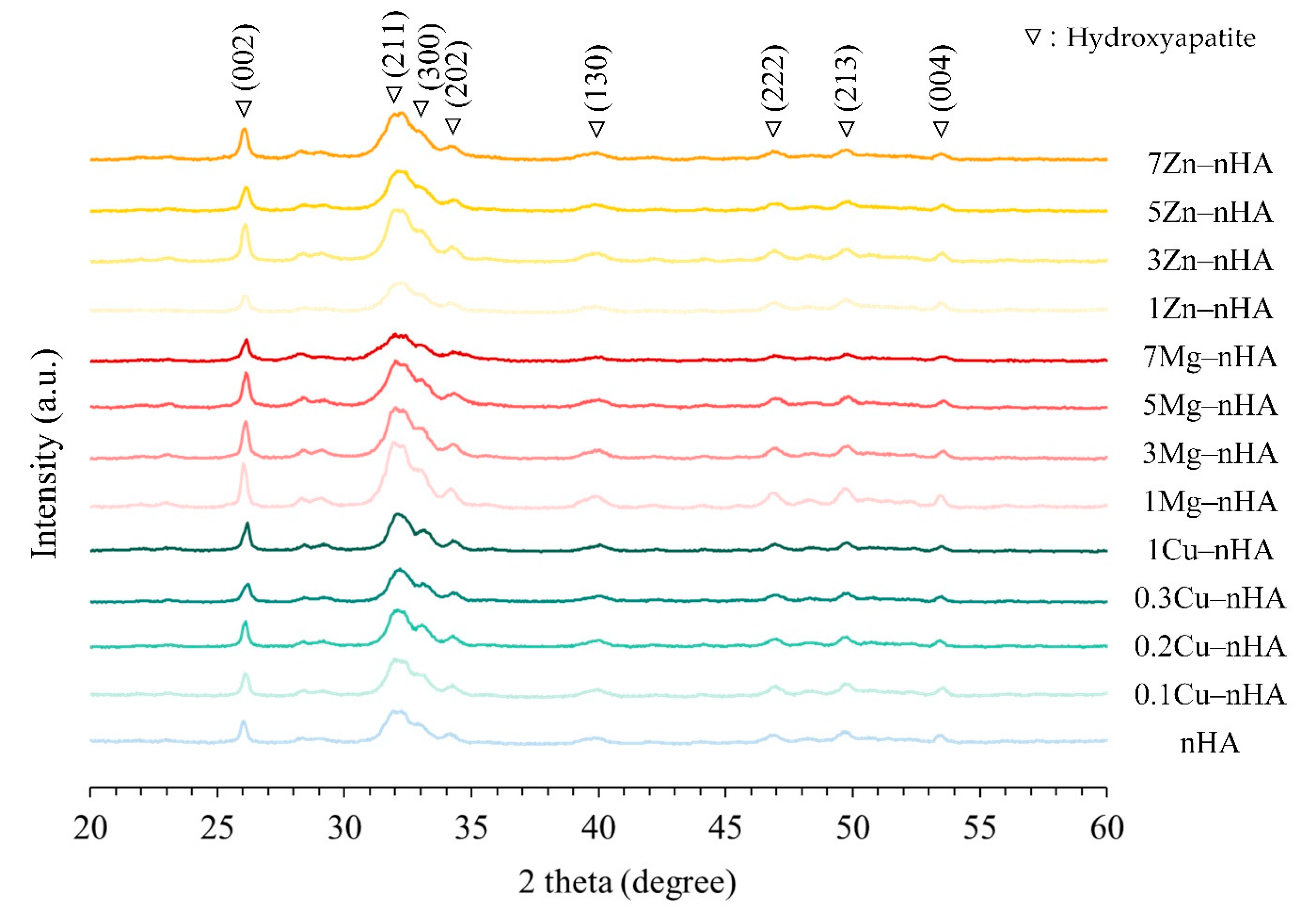
2.2. XRD Analysis
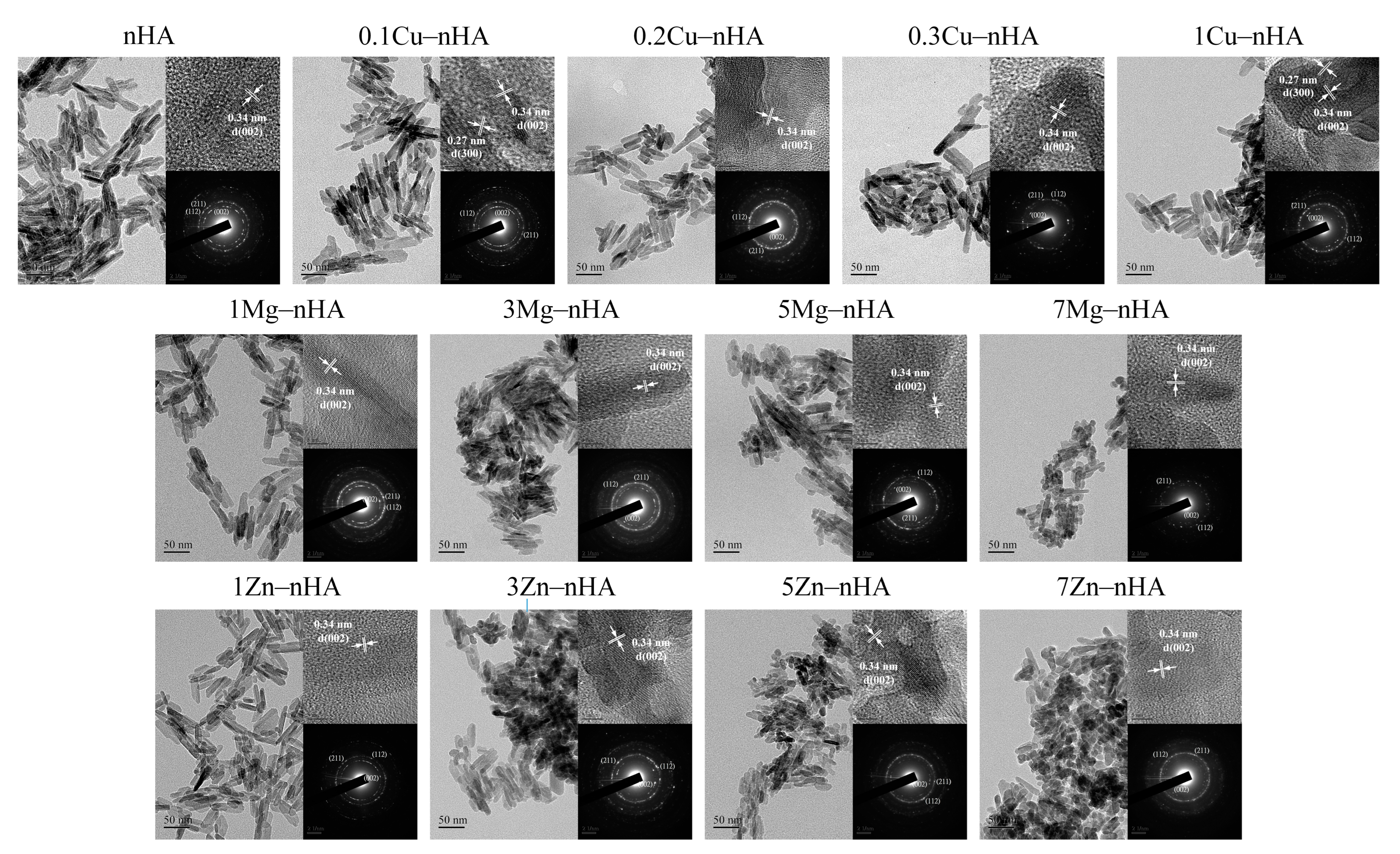
2.3. TEM Analysis
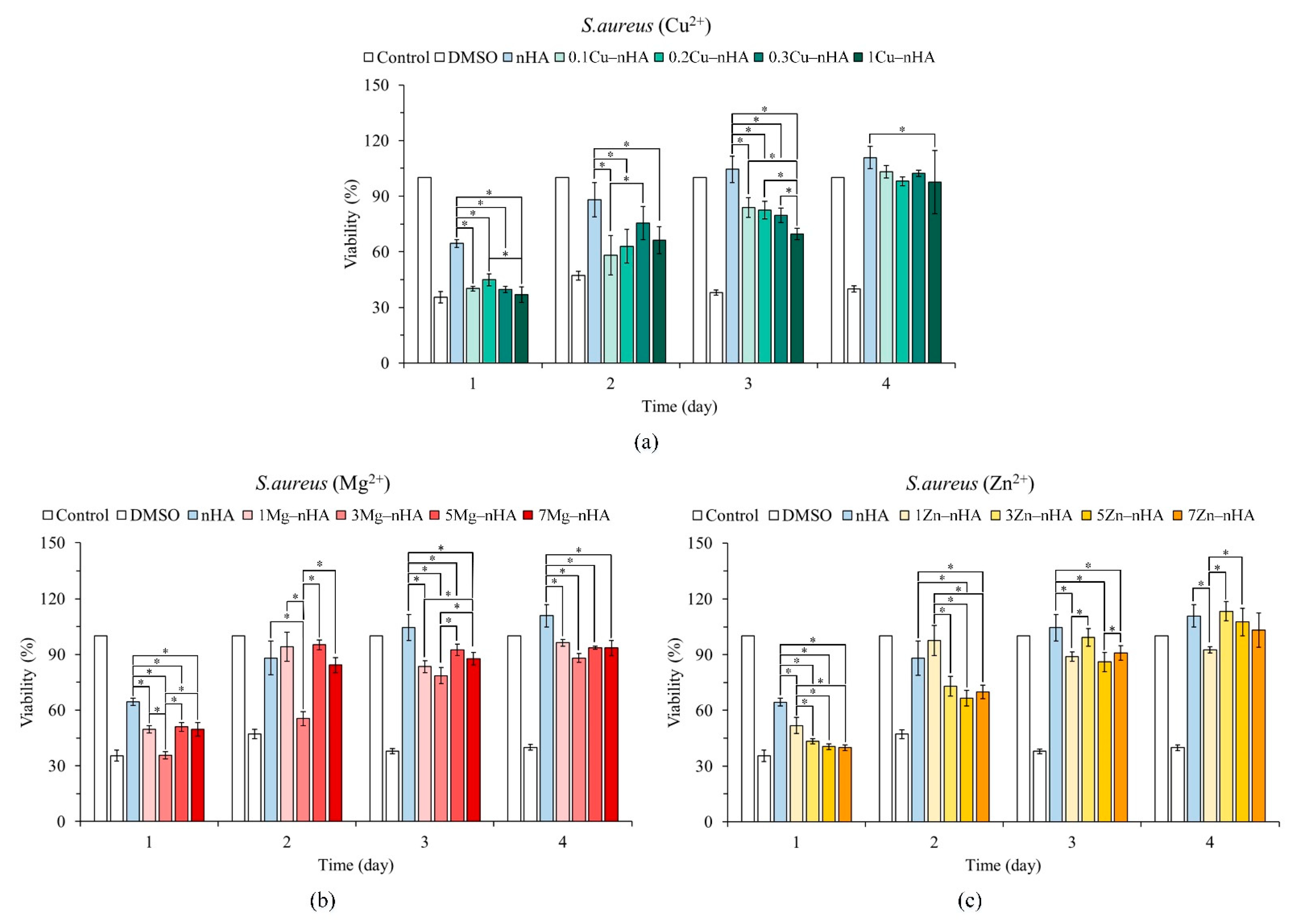
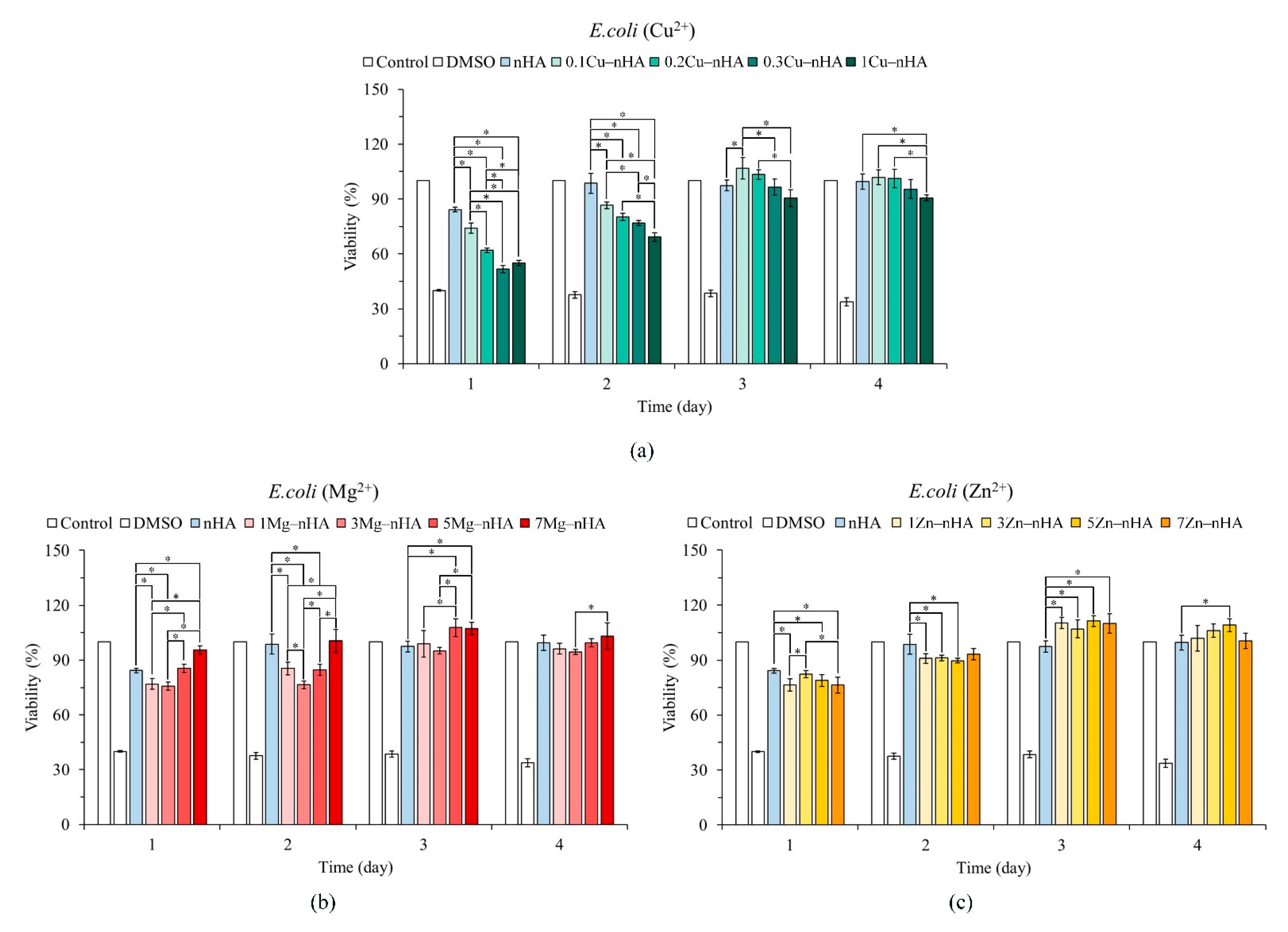
2.4. Antibacterial Activity
2.5. Cytotoxicity
3. Materials and Methods
3.1. Raw Materials
3.2. Preparation of Nano-Hydroxyapatite with Metal Ions (Cu2+, Mg2+, and Zn2+)
3.3. Characterization of Nanoapatites through Fourier Transform Infrared (FTIR) Spectroscopy, X-ray Diffraction (XRD) Analysis, and Transmission Electron Microscopy (TEM) Morphological Measurements
3.4. Antibacterial Abilities
3.5. In Vitro Cytotoxicity Tests
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, D.N.; Hauryliuk, V.; Atkinson, G.C.; O’Neill, A.J. Target protection as a key antibiotic resistance mechanism. Nat. Rev. Microbiol. 2020, 18, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Baldelli, A.; Etayash, H.; Oguzlu, H.; Mandal, R.; Jiang, F.; Hancock, R.E.W.; Pratap-Singh, A. Antimicrobial properties of spray-dried cellulose nanocrystals and metal oxide-based nanoparticles-in-microspheres. Chem. Eng. J. Adv. 2022, 10, 100273. [Google Scholar] [CrossRef]
- Langer, R. Drugs on target. Science 2001, 293, 58–59. [Google Scholar] [CrossRef]
- Daryan, S.H.; Javadpour, J.; Khavandi, A.; Erfan, M. Morphological evolution on the surface of hydrothermally synthesized hydroxyapatite microspheres in the presence of EDTMP. Ceram. Int. 2018, 44, 19743–19750. [Google Scholar] [CrossRef]
- Yu, H.-P.; Zhu, Y.-J.; Lu, B.-Q. Highly efficient and environmentally friendly microwave-assisted hydrothermal rapid synthesis of ultralong hydroxyapatite nanowires. Ceram. Int. 2018, 44, 12352–12356. [Google Scholar] [CrossRef]
- Zhong, Z.; Wu, X.; Wang, Y.; Li, M.; Li, Y.; Liu, X.; Zhang, X.; Lan, Z.; Wang, J.; Du, Y.; et al. Zn/Sr dual ions-collagen co-assembly hydroxyapatite enhances bone regeneration through procedural osteo-immunomodulation and osteogenesis. Bioact. Mater. 2021, 10, 195–206. [Google Scholar] [CrossRef]
- Palierse, E.; Hélary, C.; Krafft, J.-M.; Génois, I.; Masse, S.; Laurent, G.; Alvarez Echazu, M.I.; Selmane, M.; Casale, S.; Valentin, L.; et al. Baicalein-modified hydroxyapatite nanoparticles and coatings with antibacterial and antioxidant properties. Mater. Sci. Eng. C 2021, 118, 111537. [Google Scholar] [CrossRef]
- Vargas-Becerril, N.; Sánchez-Téllez, D.A.; Zarazúa-Villalobos, L.; González-García, D.M.; Álvarez-Pérez, M.A.; de León-Escobedo, C.; Téllez-Jurado, L. Structure of biomimetic apatite grown on hydroxyapatite (HA). Ceram. Int. 2020, 46, 28806–28813. [Google Scholar] [CrossRef]
- Jang, J.-H.; Oh, B.; Lee, E.-J. Crystalline hydroxyapatite/graphene oxide complex by low-temperature sol-gel synthesis and its characterization. Ceram. Int. 2021, 47, 27677–27684. [Google Scholar] [CrossRef]
- Liu, W.-C.; Wang, H.-Y.; Chen, L.-C.; Huang, S.-W.; Wu, C.; Chung, R.-J. Hydroxyapatite/tricalcium silicate composites cement derived from novel two-step sol-gel process with good biocompatibility and applications as bone cement and potential coating materials. Ceram. Int. 2019, 45, 5668–5679. [Google Scholar] [CrossRef]
- Sadetskaya, A.V.; Bobrysheva, N.P.; Osmolowsky, M.G.; Osmolovskaya, O.M.; Voznesenskiy, M.A. Correlative experimental and theoretical characterization of transition metal doped hydroxyapatite nanoparticles fabricated by hydrothermal method. Mater. Charact. 2021, 173, 110911. [Google Scholar] [CrossRef]
- Nosrati, H.; Mamoory, R.S.; Le, D.Q.S.; Bünger, C.E.; Emameh, R.Z.; Dabir, F. Gas injection approach for synthesis of hydroxyapatite nanorods via hydrothermal method. Mater. Charact. 2020, 159, 110071. [Google Scholar] [CrossRef]
- Zhang, C.; Uchikoshi, T.; Liu, L.; Kikuchi, M.; Ichinose, I. Nest-like microstructured biocompatible membrane fabricated by hydrothermally-synthesized hydroxyapatite (HAp) whiskers. J. Eur. Ceram. Soc. 2020, 40, 513–520. [Google Scholar] [CrossRef]
- Panda, S.; Biswas, C.K.; Paul, S. A comprehensive review on the preparation and application of calcium hydroxyapatite: A special focus on atomic doping methods for bone tissue engineering. Ceram. Int. 2021, 47, 28122–28144. [Google Scholar] [CrossRef]
- Jiang, J.; Long, Y.; Hu, X.; Hu, J.; Zhu, M.; Zhou, S. A facile microwave-assisted synthesis of mesoporous hydroxyapatite as an efficient adsorbent for Pb2+ adsorption. J. Solid State Chem. 2020, 289, 121491. [Google Scholar] [CrossRef]
- Gomez-Vazquez, O.M.; Correa-Piña, B.A.; Zubieta-Otero, L.F.; Castillo-Paz, A.M.; Londoño-Restrepo, S.M.; Rodriguez-García, M.E. Synthesis and characterization of bioinspired nano-hydroxyapatite by wet chemical precipitation. Ceram. Int. 2021, 47, 32775–32785. [Google Scholar] [CrossRef]
- Modolon, H.B.; Inocente, J.; Bernardin, A.M.; Klegues Montedo, O.R.; Arcaro, S. Nanostructured biological hydroxyapatite from Tilapia bone: A pathway to control crystallite size and crystallinity. Ceram. Int. 2021, 47, 27685–27693. [Google Scholar] [CrossRef]
- Sharifianjazi, F.; Esmaeilkhanian, A.; Moradi, M.; Pakseresht, A.; Asl, M.S.; Karimi-Maleh, H.; Jang, H.W.; Shokouhimehr, M.; Varma, R.S. Biocompatibility and mechanical properties of pigeon bone waste extracted natural nano-hydroxyapatite for bone tissue engineering. Mater. Sci. Eng. B 2021, 264, 114950. [Google Scholar] [CrossRef]
- Szcześ, A.; Hołysz, L.; Chibowski, E. Synthesis of hydroxyapatite for biomedical applications. Adv. Colloid Interface Sci. 2017, 249, 321–330. [Google Scholar] [CrossRef]
- Zhao, X.-Y.; Zhu, Y.-J.; Zhao, J.; Lu, B.-Q.; Chen, F.; Qi, C.; Wu, J. Hydroxyapatite nanosheet-assembled microspheres: Hemoglobin-templated synthesis and adsorption for heavy metal ions. J. Colloid Interface Sci. 2014, 416, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Arun Prakash, V.C.; Venda, I.; Thamizharasi, V.; Sathya, E. A new attempt on synthesis of spherical nano hydroxyapatite powders prepared by dimethyl sulfoxide—poly vinyl alcohol assisted microemulsion method. Mater. Chem. Phys. 2021, 259, 124097. [Google Scholar] [CrossRef]
- Alinda Shaly, A.; Hannah Priya, G.; Mary Linet, J. An outlook on the mechanical attributes and load curve analysis of hydrothermally acquired hydroxyapatite bioceramic nanoparticles. Phys. B Condens. Matter. 2020, 590, 412223. [Google Scholar] [CrossRef]
- Lo, Y.-S.; Chang, C.-C.; Lin, P.-C.; Lin, S.-P.; Wang, C.-L. Direct growth of structurally controllable hydroxyapatite coating on Ti-6Al-4V through a rapid hydrothermal synthesis. Appl. Surf. Sci. 2021, 556, 149672. [Google Scholar] [CrossRef]
- Chesley, M.; Kennard, R.; Roozbahani, S.; Kim, S.M.; Kukk, K.; Mason, M. One-step hydrothermal synthesis with in situ milling of biologically relevant hydroxyapatite. Mater. Sci. Eng. C 2020, 113, 110962. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Gracida, N.O.; Esparza-González, S.C.; Castillo-Martínez, N.A.; Serrano-Medina, A.; Olivas-Armendariz, I.; Campos-Múzquiz, L.G.; Múzquiz-Ramos, E.M. Synergism in novel silver-copper/hydroxyapatite composites for increased antibacterial activity and biocompatibility. Ceram. Int. 2020, 46, 20215–20225. [Google Scholar] [CrossRef]
- Tao, B.; Lin, C.; Guo, A.; Yu, Y.; Qin, X.; Li, K.; Tian, H.; Yi, W.; Lei, D.; Chen, L. Fabrication of copper ions-substituted hydroxyapatite/polydopamine nanocomposites with high antibacterial and angiogenesis effects for promoting infected wound healing. J. Ind. Eng. Chem. 2021, 104, 345–355. [Google Scholar] [CrossRef]
- Ressler, A.; Žužić, A.; Ivanišević, I.; Kamboj, N.; Ivanković, H. Ionic substituted hydroxyapatite for bone regeneration applications: A review. Open Ceram. 2021, 6, 100122. [Google Scholar] [CrossRef]
- Liu, T.; Jin, M.; Zhang, Y.; Weng, W.; Wang, T.; Yang, H.; Zhou, L. K+/Sr2+/Na+ triple-doped hydroxyapatites/GelMA composite hydrogel scaffold for the repair of bone defects. Ceram. Int. 2021, 47, 30929–30937. [Google Scholar] [CrossRef]
- Carrera, K.; Huerta, V.; Orozco, V.; Matutes, J.; Fernández, P.; Graeve, O.A.; Herrera, M. Formation of vacancy point-defects in hydroxyapatite nanobelts by selective incorporation of Fe3+ ions in Ca(II) sites. A CL and XPS study. Mater. Sci. Eng. B 2021, 271, 115308. [Google Scholar] [CrossRef]
- Paluch, E.; Sobierajska, P.; Okińczyc, P.; Widelski, J.; Duda-Madej, A.; Krzyżanowska, B.; Krzyżek, P.; Ogórek, R.; Szperlik, J.; Chmielowiec, J.; et al. Nanoapatites doped and co-doped with noble metal ions as modern antibiofilm materials for biomedical applications against drug-resistant clinical strains of Enterococcus faecalis VRE and Staphylococcus aureus MRSA. Int. J. Mol. Sci. 2022, 23, 1533. [Google Scholar] [CrossRef] [PubMed]
- Mohd Yusoff, M.F.; Abu Kasim, N.H.; Himratul-Aznita, W.H.; Saidin, S.; Genasan, K.; Kamarul, T.; Radzi, Z. Physicochemical, antibacterial and biocompatibility assessments of silver incorporated nano-hydroxyapatite synthesized using a novel microwave-assisted wet precipitation technique. Mater. Charact. 2021, 178, 111169. [Google Scholar] [CrossRef]
- Li, G.; Huang, J.; Wei, J.; Liu, C.; Zuo, Y.; Li, J.; Li, Y. Fabrication of strontium and simvastatin loaded hydroxyapatite microspheres by one-step approach. Mater. Lett. 2021, 300, 130234. [Google Scholar] [CrossRef]
- Khvostov, M.V.; Borisova, M.S.; Bulina, N.V.; Makarova, S.V.; Dumchenko, N.B.; Tolstikova, T.G.; Lyakhov, N.Z. The influence of zinc and silicate ions on biological properties of hydroxyapatite synthesized by a mechanochemical method. Ceram. Int. 2021, 47, 9495–9503. [Google Scholar] [CrossRef]
- Bensalem, A.; Kucukosman, O.K.; Raszkiewicz, J.; Topkaya, F. Synthesis, characterization, bactericidal activity, and mechanical properties of hydroxyapatite nano powders impregnated with silver and zinc oxide nanoparticles (Ag-ZnO-Hap). Ceram. Int. 2021, 47, 21319–21324. [Google Scholar] [CrossRef]
- Czechowska, J. Self-assembling, hybrid hydroxyapatite-methylcellulose granules, modified with nano-silver. Mater. Lett. 2021, 300, 130156. [Google Scholar] [CrossRef]
- Szurkowska, K.; Kolmas, J. Hydroxyapatites enriched in silicon—Bioceramic materials for biomedical and pharmaceutical applications. Prog. Nat. Sci. 2017, 27, 401–409. [Google Scholar] [CrossRef]
- Ai, F.; Chen, L.; Yan, J.; Yang, K.; Li, S.; Duan, H.; Cao, C.; Li, W.; Zhou, K. Hydroxyapatite scaffolds containing copper for bone tissue engineering. J. Sol-Gel Sci. Technol. 2020, 95, 168–179. [Google Scholar] [CrossRef]
- Shanmugam, S.; Gopal, B. Copper substituted hydroxyapatite and fluorapatite: Synthesis, characterization and antimicrobial properties. Ceram. Int. 2014, 40, 15655–15662. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, Y.; Jiang, D.; Jiao, Y.; Wu, Y.; Peng, Z.; Zhou, J.; Wu, J.; Dong, Z. Synthesis and characterization of a bi-functional hydroxyapatite/Cu-doped TiO2 composite coating. Ceram. Int. 2019, 45, 6693–6701. [Google Scholar] [CrossRef]
- Correa-Piña, B.A.; Gomez-Vazquez, O.M.; Londoño-Restrepo, S.M.; Zubieta-Otero, L.F.; Millan-Malo, B.M.; Rodriguez-García, M.E. Synthesis and characterization of nano-hydroxyapatite added with magnesium obtained by wet chemical precipitation. Prog. Nat. Sci. 2021, 31, 575–582. [Google Scholar] [CrossRef]
- Kulanthaivel, S.; Mishra, U.; Agarwal, T.; Giri, S.; Pal, K.; Pramanik, K.; Banerjee, I. Improving the osteogenic and angiogenic properties of synthetic hydroxyapatite by dual doping of bivalent cobalt and magnesium ion. Ceram. Int. 2015, 41, 11323–11333. [Google Scholar] [CrossRef]
- Dittler, M.L.; Unalan, I.; Grünewald, A.; Beltrán, A.M.; Grillo, C.A.; Destch, R.; Gonzalez, M.C.; Boccaccini, A.R. Bioactive glass (45S5)-based 3D scaffolds coated with magnesium and zinc-loaded hydroxyapatite nanoparticles for tissue engineering applications. Colloids Surf. B Biointerfaces 2019, 182, 110346. [Google Scholar] [CrossRef]
- Furko, M.; Horváth, Z.E.; Sulyok, A.; Kis, V.K.; Balázsi, K.; Mihály, J.; Balázsi, C. Preparation and morphological investigation on bioactive ion-modified carbonated hydroxyapatite-biopolymer composite ceramics as coatings for orthopaedic implants. Ceram. Int. 2021, 48, 760–768. [Google Scholar] [CrossRef]
- Mardziah, C.M.; Ramesh, S.; Tan, C.Y.; Chandran, H.; Sidhu, A.; Krishnasamy, S.; Purbolaksono, J. Zinc-substituted hydroxyapatite produced from calcium precursor derived from eggshells. Ceram. Int. 2021, 47, 33010–33019. [Google Scholar] [CrossRef]
- Maleki-Ghaleh, H.; Hossein Siadati, M.; Fallah, A.; Zarrabi, A.; Afghah, F.; Koc, B.; Dalir Abdolahinia, E.; Omidi, Y.; Barar, J.; Akbari-Fakhrabadi, A.; et al. Effect of zinc-doped hydroxyapatite/graphene nanocomposite on the physicochemical properties and osteogenesis differentiation of 3D-printed polycaprolactone scaffolds for bone tissue engineering. Chem. Eng. J. 2021, 426, 131321. [Google Scholar] [CrossRef]
- Lin, D.-J.; Lin, H.-L.; Haung, S.-M.; Liu, S.-M.; Chen, W.-C. Effect of pH on the In Vitro Biocompatibility of Surfactant-Assisted Synthesis and Hydrothermal Precipitation of Rod-Shaped Nano-Hydroxyapatite. Polymers 2021, 13, 2994. [Google Scholar] [CrossRef]
- Chen, W.-C.; Cheng, I.-T.; Chang, K.-C.; Haung, S.-M.; Chen, J.-C.; Shih, C.-J. Heparin as a biomimetic template on nanoapatite rods with tunable aspect ratio: Synthesis and biocompatibility. J. Aust. Ceram. Soc. 2021, 57, 825–834. [Google Scholar] [CrossRef]
- Chen, J.; Liu, J.; Deng, H.; Yao, S.; Wang, Y. Regulatory synthesis and characterization of hydroxyapatite nanocrystals by a microwave-assisted hydrothermal method. Ceram. Int. 2020, 46, 2185–2193. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Gupta, A.; Verma, M.; Murugan, P.A.; Sengupta, P.; Matheshwaran, S.; Manna, I.; Balani, K. Site-specific antibacterial efficacy and cyto/hemo-compatibility of zinc substituted hydroxyapatite. Ceram. Int. 2019, 45, 12225–12233. [Google Scholar] [CrossRef]
- Haung, S.-M.; Chen, J.-C.; Chang, K.-C.; Ko, C.-L.; Lin, D.-J.; Chen, W.-C. Synthesis of nanorod apatites with templates at critical micelle concentrations and in vitro evaluation of cytotoxicity and antimicrobial activity. J. Asian Ceram. Soc. 2021, 9, 995–1006. [Google Scholar] [CrossRef]
- Mardziah, C.M.; Ramesh, S.; Abdul Wahid, M.F.; Chandran, H.; Sidhu, A.; Krishnasamy, S.; Purbolaksono, J. Effect of zinc ions on the structural characteristics of hydroxyapatite bioceramics. Ceram. Int. 2020, 46, 13945–13952. [Google Scholar] [CrossRef]
- Bulina, N.V.; Vinokurova, O.B.; Eremina, N.V.; Prosanov, I.Y.; Khusnutdinov, V.R.; Chaikina, M.V. Features of solid-phase mechanochemical synthesis of hydroxyapatite doped by copper and zinc ions. J. Solid State Chem. 2021, 296, 121973. [Google Scholar] [CrossRef]
- Stipniece, L.; Wilson, S.; Curran, J.M.; Chen, R.; Salma-Ancane, K.; Sharma, P.K.; Meenan, B.J.; Boyd, A.R. Strontium substituted hydroxyapatite promotes direct primary human osteoblast maturation. Ceram. Int. 2021, 47, 3368–3379. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Sergi, R.; Bellucci, D.; Candidato, R.T.; Lusvarghi, L.; Bolelli, G.; Pawlowski, L.; Candiani, G.; Altomare, L.; De Nardo, L.; Cannillo, V. Bioactive Zn-doped hydroxyapatite coatings and their antibacterial efficacy against Escherichia coli and Staphylococcus aureus. Surf. Coat. Technol. 2018, 352, 84–91. [Google Scholar] [CrossRef]
- George, A.; Raj, D.M.A.; Raj, A.D.; Irudayaraj, A.A.; Arumugam, J.; Senthil kumar, M.; Prabu, H.J.; Sundaram, S.J.; Al-Dhabi, N.A.; Arasu, M.V.; et al. Temperature effect on CuO nanoparticles: Antimicrobial activity towards bacterial strains. Surf. Interfaces 2020, 21, 100761. [Google Scholar] [CrossRef]
- Jose, S.; Senthilkumar, M.; Elayaraja, K.; Haris, M.; George, A.; Raj, A.D.; Sundaram, S.J.; Bashir, A.K.H.; Maaza, M.; Kaviyarasu, K. Preparation and characterization of Fe doped n-hydroxyapatite for biomedical application. Surf. Interfaces 2021, 25, 101185. [Google Scholar] [CrossRef]
- El-Sherif, A.A.; Eldebss, T.M.A. Synthesis, spectral characterization, solution equilibria, in vitro antibacterial and cytotoxic activities of Cu(II), Ni(II), Mn(II), Co(II) and Zn(II) complexes with Schiff base derived from 5-bromosalicylaldehyde and 2-aminomethylthiophene. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 79, 1803–1814. [Google Scholar] [CrossRef]
- Lv, Y.; Chen, Y.; Zheng, Y.; Li, Q.; Lei, T.; Yin, P. Evaluation of the antibacterial properties and in-vitro cell compatibilities of doped copper oxide/hydroxyapatite composites. Colloids Surf. B Biointerfaces 2022, 209, 112194. [Google Scholar] [CrossRef]
- Iso, I. 10993–5: 2009; Biological evaluation of medical devices—part 5: Tests for in vitro cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- Sivaraj, D.; Vijayalakshmi, K.; Ganeshkumar, A.; Rajaram, R. Tailoring Cu substituted hydroxyapatite/functionalized multiwalled carbon nanotube composite coating on 316L SS implant for enhanced corrosion resistance, antibacterial and bioactive properties. Int. J. Pharm. 2020, 590, 119946. [Google Scholar] [CrossRef] [PubMed]
- Arul, K.T.; Kolanthai, E.; Manikandan, E.; Bhalerao, G.M.; Chandra, V.S.; Ramya, J.R.; Mudali, U.K.; Nair, K.G.M.; Kalkura, S.N. Green synthesis of magnesium ion incorporated nanocrystalline hydroxyapatite and their mechanical, dielectric and photoluminescence properties. Mater. Res. Bull. 2015, 67, 55–62. [Google Scholar] [CrossRef]
- Tang, Y.; Chappell, H.F.; Dove, M.T.; Reeder, R.J.; Lee, Y.J. Zinc incorporation into hydroxylapatite. Biomaterials 2009, 30, 2864–2872. [Google Scholar] [CrossRef]
- Radovanović, Ž.; Jokić, B.; Veljović, D.; Dimitrijević, S.; Kojić, V.; Petrović, R.; Janaćković, D. Antimicrobial activity and biocompatibility of Ag+- and Cu2+-doped biphasic hydroxyapatite/α-tricalcium phosphate obtained from hydrothermally synthesized Ag+- and Cu2+-doped hydroxyapatite. Appl. Surf. Sci. 2014, 307, 513–519. [Google Scholar] [CrossRef]
- Yao, F.; Xie, W.; Yang, M.; Zhang, H.; Gu, H.; Du, A.; Naik, N.; Young, D.P.; Lin, J.; Guo, Z. Interfacial polymerized copolymers of aniline and phenylenediamine with tunable magnetoresistance and negative permittivity. Mater. Today Phys. 2021, 21, 100502. [Google Scholar] [CrossRef]
- Dar, U.A.; Salunke-Gawali, S.; Shinde, D.; Bh, S.; Satpute, S. Thermal and Spectral Studies of Transition Metal Complexes of 2-Bromo-3-Hydroxynaphthalene-1, 4-Dione: Evaluation of Antibacterial Activity Against Six Bacterial Strains. Eng. Sci. 2021, 15, 105–115. [Google Scholar] [CrossRef]
- Panthi, G.; Ranjit, R.; Khadka, S.; Gyawali, K.R.; Kim, H.-Y.; Park, M. Characterization and antibacterial activity of rice grain-shaped ZnS nanoparticles immobilized inside the polymer electrospun nanofibers. Adv. Compos. Hybrid Mater. 2020, 3, 8–15. [Google Scholar] [CrossRef]
- Singh, M.; Bajaj, N.K.; Bhardwaj, A.; Singh, P.; Kumar, P.; Sharma, J. Study of photocatalytic and antibacterial activities of graphene oxide nanosheets. Adv. Compos. Hybrid Mater. 2018, 1, 759–765. [Google Scholar] [CrossRef]
- Kumar, S.; Gautam, C.; Chauhan, B.S.; Srikrishna, S.; Yadav, R.S.; Rai, S.B. Enhanced mechanical properties and hydrophilic behavior of magnesium oxide added hydroxyapatite nanocomposite: A bone substitute material for load bearing applications. Ceram. Int. 2020, 46, 16235–16248. [Google Scholar] [CrossRef]

| Samples | (002) | (211) | (300) | (202) | (130) | (222) | (213) | (004) |
|---|---|---|---|---|---|---|---|---|
| nHA/control | 0.319 | 1.074 | 0.429 | 0.368 | 0.792 | 0.547 | 0.431 | 0.340 |
| 0.1Cu−nHA | 0.321 | 1.048 | 0.336 | 0.400 | 0.826 | 0.535 | 0.443 | 0.345 |
| 0.2Cu−nHA | 0.304 | 0.834 | 0.522 | 0.384 | 0.724 | 0.533 | 0.424 | 0.333 |
| 0.3Cu−nHA | 0.378 | 0.861 | 0.464 | 0.347 | 0.696 | 0.577 | 0.425 | 0.378 |
| 1Cu−nHA | 0.324 | 0.818 | 0.538 | 0.367 | 0.705 | 0.527 | 0.390 | 0.351 |
| 1Mg−nHA | 0.318 | 1.042 | 0.362 | 0.297 | 0.812 | 0.542 | 0.435 | 0.332 |
| 3Mg−nHA | 0.322 | 0.924 | 0.485 | 0.387 | 0.828 | 0.529 | 0.430 | 0.342 |
| 5Mg−nHA | 0.345 | 0.956 | 0.442 | 0.442 | 0.827 | 0.518 | 0.410 | 0.384 |
| 7Mg−nHA | 0.356 | 0.855 | 0.579 | 0.597 | 0.777 | 0.597 | 0.434 | 0.477 |
| 1Zn−nHA | 0.349 | 0.934 | 0.337 | 0.337 | 0.851 | 0.534 | 0.517 | 0.393 |
| 3Zn−nHA | 0.338 | 1.053 | 0.350 | 0.269 | 0.836 | 0.549 | 0.464 | 0.332 |
| 5Zn−nHA | 0.374 | 1.082 | 0.334 | 0.361 | 0.866 | 0.621 | 0.510 | 0.361 |
| 7Zn−nHA | 0.369 | 1.128 | 0.318 | 0.401 | 0.884 | 0.562 | 0.472 | 0.395 |
| Samples | Longitudinal Length (nm) | Transverse Width (nm) | Length-to-Width Aspect Ratio | Plane Ratio of (002) to (300) FWHM Values |
|---|---|---|---|---|
| nHA/control | 48.76 ± 10.50 | 10.44 ± 2.38 | 4.85 ± 1.39 | 0.74 |
| 0.1Cu−nHA | 47.07 ± 7.33 | 11.80 ± 2.67 | 4.15 ± 0.97 | 0.96 |
| 0.2Cu−nHA | 42.65 ± 10.87 | 10.99 ± 2.17 | 3.92 ± 0.81 | 0.58 |
| 0.3Cu−nHA | 42.73 ± 10.74 | 10.38 ± 2.82 | 4.43 ± 1.72 | 0.81 |
| 1Cu−nHA | 41.66 ± 9.75 | 11.91 ± 2.77 | 3.58 ± 0.82 * | 0.60 |
| 1Mg−nHA | 46.16 ± 9.23 * | 10.32 ± 2.15 | 4.62 ± 1.19 | 0.88 |
| 3Mg−nHA | 37.03 ± 7.60 | 6.80 ± 1.74 * | 5.69 ± 1.52 * | 0.66 |
| 5Mg−nHA | 36.48 ± 8.49 | 9.62 ± 2.46 | 3.94 ± 1.06 | 0.78 |
| 7Mg−nHA | 34.99 ± 9.65 | 9.06 ± 1.95 | 3.96 ± 1.12 | 0.61 |
| 1Zn−nHA | 45.69 ± 8.54 * | 12.15 ± 2.62 | 3.91 ± 1.04 | 1.04 |
| 3Zn−nHA | 39.37 ± 8.33 * | 10.04 ± 2.34 * | 4.09 ± 1.18 | 0.97 |
| 5Zn−nHA | 31.92 ± 5.21 | 9.30 ± 2.14 * | 3.57 ± 0.86 | 1.12 |
| 7Zn−nHA | 30.53 ± 4.80 | 12.05 ± 2.62 | 2.62 ± 0.58 * | 1.16 |
| Abbreviated Names | Ca(NO3)2•4H2O (mmol) | Contents of Metal Ion in Morality | [M/(Ca + M)] × 100% a | (NH4)2HPO4 (mmol) | (Ca + M)/P (Atomic Ratio) |
|---|---|---|---|---|---|
| nHA/control | 9.000 | − | − | 5.400 | 1.670 |
| Cu(NO3)2•3H2O (mmol) | |||||
| 0.1Cu–nHA | 8.991 | 0.009 | 0.1 | 5.400 | 1.670 |
| 0.2Cu−nHA | 8.982 | 0.018 | 0.2 | 5.400 | 1.670 |
| 0.3Cu−nHA | 8.973 | 0.027 | 0.3 | 5.400 | 1.670 |
| 1Cu−nHA | 8.910 | 0.090 | 1.0 | 5.400 | 1.670 |
| Mg(NO3)2•6H2O (mmol) | |||||
| 1Mg−nHA | 8.910 | 0.090 | 1.0 | 5.400 | 1.670 |
| 3Mg−nHA | 8.730 | 0.270 | 3.0 | 5.400 | 1.670 |
| 5Mg−nHA | 8.550 | 0.450 | 5.0 | 5.400 | 1.670 |
| 7Mg−nHA | 8.370 | 0.630 | 7.0 | 5.400 | 1.670 |
| Zn(NO3)2 •6H2O (mmol) | |||||
| 1Zn−nHA | 8.910 | 0.090 | 1.0 | 5.400 | 1.670 |
| 3Zn−nHA | 8.730 | 0.270 | 3.0 | 5.400 | 1.670 |
| 5Zn−nHA | 8.550 | 0.450 | 5.0 | 5.400 | 1.670 |
| 7Zn−nHA | 8.370 | 0.630 | 7.0 | 5.400 | 1.670 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.-M.; Liu, S.-M.; Chen, W.-C.; Ko, C.-L.; Shih, C.-J.; Chen, J.-C. Morphological Changes, Antibacterial Activity, and Cytotoxicity Characterization of Hydrothermally Synthesized Metal Ions-Incorporated Nanoapatites for Biomedical Application. Pharmaceuticals 2022, 15, 885. https://doi.org/10.3390/ph15070885
Huang S-M, Liu S-M, Chen W-C, Ko C-L, Shih C-J, Chen J-C. Morphological Changes, Antibacterial Activity, and Cytotoxicity Characterization of Hydrothermally Synthesized Metal Ions-Incorporated Nanoapatites for Biomedical Application. Pharmaceuticals. 2022; 15(7):885. https://doi.org/10.3390/ph15070885
Chicago/Turabian StyleHuang, Ssu-Meng, Shih-Ming Liu, Wen-Cheng Chen, Chia-Ling Ko, Chi-Jen Shih, and Jian-Chih Chen. 2022. "Morphological Changes, Antibacterial Activity, and Cytotoxicity Characterization of Hydrothermally Synthesized Metal Ions-Incorporated Nanoapatites for Biomedical Application" Pharmaceuticals 15, no. 7: 885. https://doi.org/10.3390/ph15070885
APA StyleHuang, S.-M., Liu, S.-M., Chen, W.-C., Ko, C.-L., Shih, C.-J., & Chen, J.-C. (2022). Morphological Changes, Antibacterial Activity, and Cytotoxicity Characterization of Hydrothermally Synthesized Metal Ions-Incorporated Nanoapatites for Biomedical Application. Pharmaceuticals, 15(7), 885. https://doi.org/10.3390/ph15070885