1. Introduction
Hearing loss and balance disorders affect 466 million individuals worldwide, with the figure estimated to rise to 900 million by 2050 [
1]. Various factors such as chronic infections, noise exposure, chronic diseases, the aging process, genetic mutations, and ototoxic drugs affect hearing loss and balance disorders. Hearing loss is predominantly an irreversible process, and there are currently no approved medications or treatments available that target inner ear sensory dysfunction or sensory recovery [
2,
3]. Cochlear implants and hearing aids are intended for the management of hearing loss but are not a complete cure. Therefore, the development of novel drug candidates and localized drug delivery methods for the treatment of hearing loss is an essential unmet need in the field. Nevertheless, localized drug delivery to the inner ear and cochlear structures affected by hearing loss is challenging, which severely hampers the advancement of effective drugs in clinical settings [
3,
4,
5].
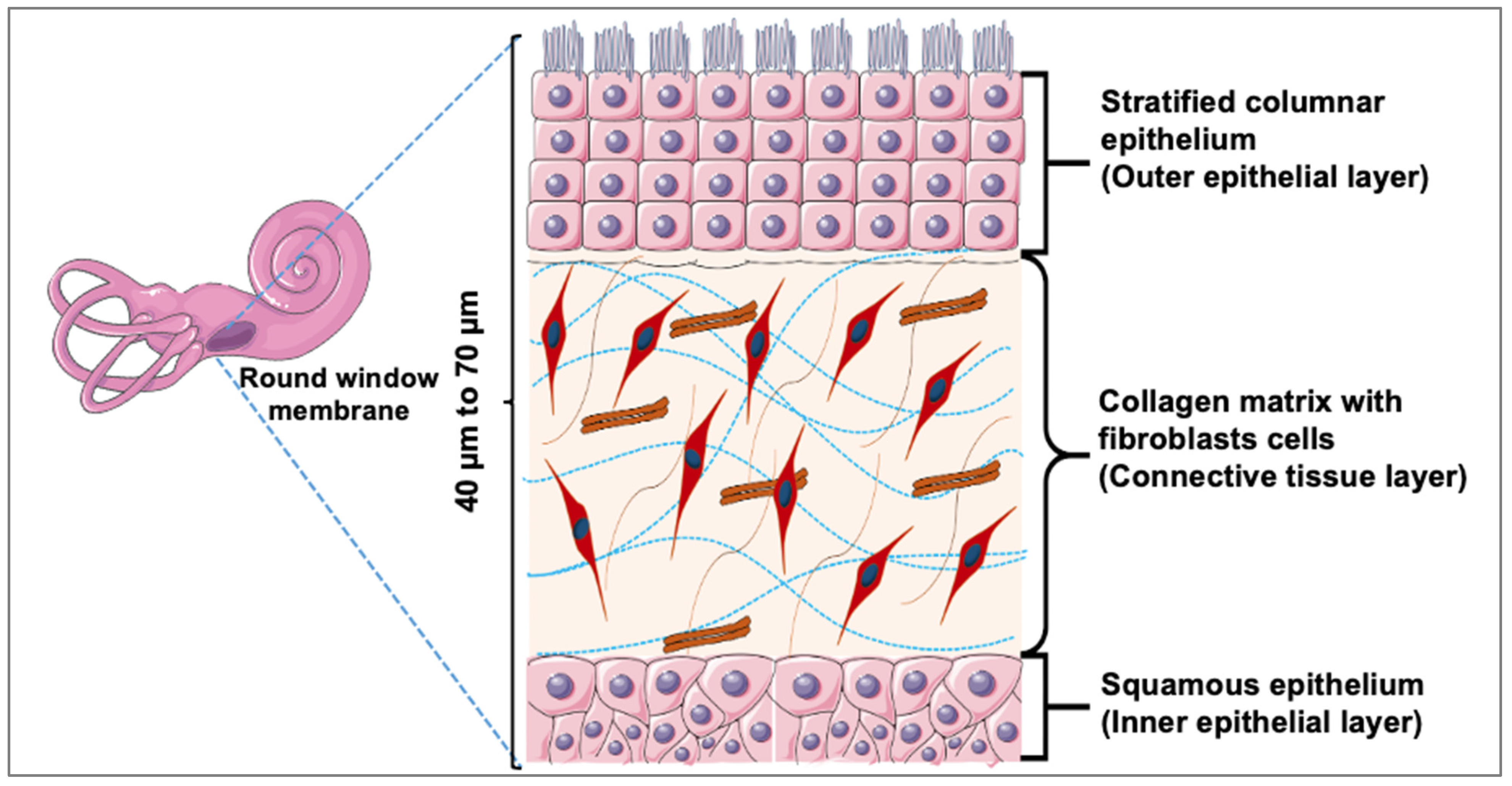
The round window membrane (RWM) is a non-osseous barrier that separates the middle and inner ear compartments, potentially making it an ideal portal for topically delivering medications to the inner ear [
6,
7]. As a result, understanding and anticipating RWM permeation efficiency could provide useful information for improving local drug delivery with clinical significance [
8,
9,
10]. Multiple studies have been conducted to assess the RWM permeability of various drugs in animal models, some supplemented with computational modeling. However, these preclinical models result in long drug development times, high costs, and in certain instances, have limited clinical translational relevance [
11,
12,
13,
14].
With the limitations of the existing preclinical models, numerous research areas have recently employed the use of three-dimensional (3D) in vitro models to accelerate and improve the drug discovery process [
15,
16,
17,
18,
19,
20,
21,
22]. This approach has been recognized by the Food and Drug Administration as a viable tool for drug discovery advancement [
16]. Similarly, the availability of physiologically relevant 3D models of the human inner ear structures could accelerate the discovery of therapeutic agents specifically targeting the structures affected by hearing loss. A viable model of the RWM could allow for rapid assessment of topically deliverable drugs or drug delivery systems. A simplified mouse tracheal cell layer model employed in one drug permeation study showed encouraging results and underlined the feasibility of this approach [
23]. For the development of physiologically relevant in vitro RWM drug permeation models, a key step is understanding the membrane’s architecture and its impact on drug permeation. Histologically, the RWM consists of an outer epithelial layer, which interfaces with the air-filled middle ear; a central or middle layer, which is made up of elastic fibers and collagen-rich connective tissue; and an inner epithelial layer, which interacts with scala tympani—a fluid-filled cochlear compartment (
Figure 1) [
24,
25].
In the present study, we report the development and evaluation of such an engineered in vitro 3D RWM model for drug permeability evaluation studies. Individual RWM components were histologically characterized, their barrier properties were assessed, and their contributions to drug permeability were evaluated. A panel of four drugs with a range of lipophilic to hydrophilic partition coefficients (log P) was evaluated (Supplemental
Table S1). Our findings indicate that RWM drug diffusion rates are primarily dictated by the outer layer of stratified columnar epithelial cells, which we modeled with primary human small airway epithelial cells. These data seem in agreement with previous reports indicating that the outer epithelium is the primary contributor to the physiological RWM barrier properties [
26] and highlight the feasibility of using these engineered in vitro models as rapid, high-throughput screening tools for oto-therapeutic development.
3. Discussion
The present study sought to develop in vitro 3D RWM models to enable the rapid, high-throughput screening of novel drug candidates against inner ear pathologies. Our approach was to individually engineer and evaluate tissues corresponding to the constituent layers of the native RWM. For the outer layer, we employed human primary airway epithelial cells to form 3D constructs that would mirror the histology of the native tissue layer. For the middle connective tissue layer, we used primary human dermal fibroblasts and allowed them to mature into tissues via endogenous extracellular matrix secretion. For the inner layer, we attempted to model it using human primary renal proximal tubule epithelial cells, which have previously been studied for this purpose [
27], as potential representatives of the native tissue layer; however, no confluent monolayers were obtained, and the relevance of using these cells to model RWM components is unclear. Additionally, it has been suggested that this inner layer is not expected to play a significant role in RWM permeability relative to the outer epithelial layer [
28].
With the 3D outer and middle layers modeled as AET and PFC, we next assessed the drug permeation properties of four different drugs with a wide range of permeability coefficients. Three of the drugs (DSP, ciprofloxacin HCl, and gentamicin sulfate) are currently used or have been historically used for the treatment of otic pathologies, while fluorescein was chosen because of its pronounced lipophilicity and ease of detection. The permeability data indicate that the RWM models allow selective drug permeation, which we postulate is largely dictated by log P and molecular weight [
29]. Highly lipophilic molecules such as fluorescein molecular weight (MW = 332.3 g/mol, log P = 3.88) [
30] or hydrophilic drugs such as gentamycin sulfate (MW = 1488.8 g/mol, log P = −3.1) [
31] showed decreased transmembrane permeation rates compared to the more lipophilic/hydrophilic balanced drugs such as DSP (MW = 516.4 g/mol, log P = 1.56) [
32] or ciprofloxacin HCl (MW = 367.8 g/mol, log P = −0.86) [
33], that more readily permeated the tissues.
Histologically, the outer layer of the human RWM consists of a permeable [
8], single cell layer outer epithelium, a middle connective tissue layer, and an inner layer of squamous cells towards the inner ear [
34]. Previous drug permeability reports suggest that in RWM, the outer epithelial layer is the key determinant of the membrane’s permeation properties [
26]. This finding seems to be reflected by our in vitro 3D models where the permeation through the AETs, representative of the native RWM outer layer, is the rate-limiting step, compared to the permeation through the PFCs, which represent the connective tissue, middle layer of the native RWM. Although we did not directly test a model of the inner layer, we are confident that the impact of such a construct on various drug permeation rates would be insignificant, as the human inner layer of the RWM was reported to be discontinuous and with loose junctions [
26]. Therefore, our data indicate that an outer layer-mimicking 3D model would be sufficient in vitro representation of the RWM in terms of drug permeability.
For AETs, two different culture times were evaluated. The barrier properties (TEER values) of the tissues at the two culture points were statistically significant. Day 14 tissues appeared to have a higher selectivity towards drugs based on their log P and MW; however, compared to other tissue constructs are known for their barrier properties (i.e., skin or the blood-brain-barrier) [
35,
36] the observed TEER differences between the two culture times do not seem pharmacokinetically relevant. The day 21 AETs seemed less selective for drugs, although they still showed the lowest permeation rate for the drug with the highest lipophilicity (fluorescein). The final selection of the most representative in vitro model will have to rely on future studies of human RWM drug permeation.
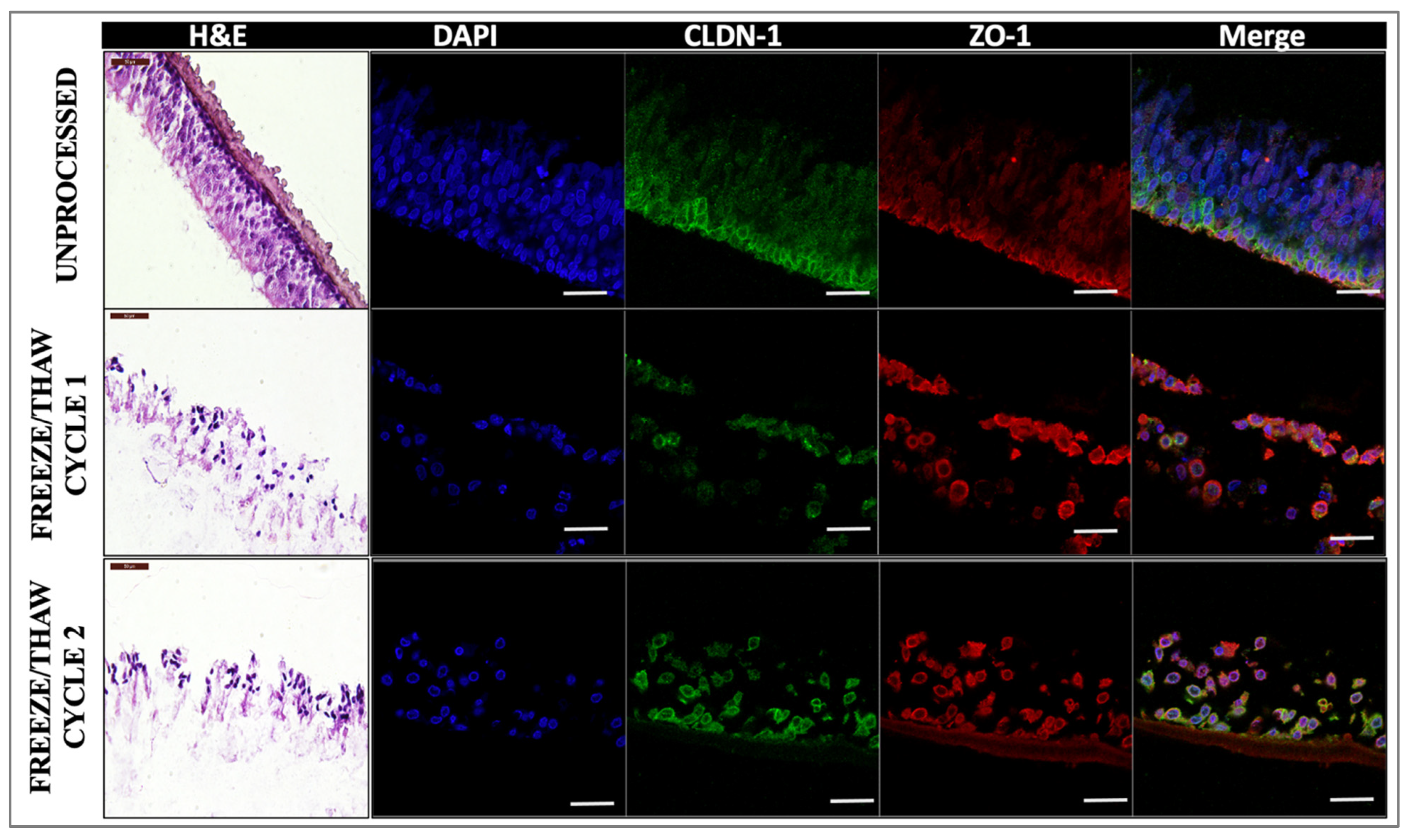
We also assessed the impact of freeze/thaw cycles on the tissue integrity and drug permeation properties of our in vitro 3D models as in situ tissue drug permeation studies showed that in the tympanic membrane (TM), the barrier properties of tissues are affected by such processing parameters. Our results indicated that freeze/thawing does indeed affect the quality of the tissue [
37]; however, the drug diffusion properties are not significantly altered, most likely due to the permeable nature of the outer layer of RWM compared to the multicellular outer layer structures of the TM [
8,
29].
Overall, we showed that engineering an outer layer 3D model is sufficient for the assessment of the permeability properties of different drugs, as observed in native tissues [
26,
38]. These models histologically mimic the outer layer of the RWM. Moreover, the models, especially the day 14 constructs, seem to discern between different drugs based on their partition coefficient and molecular weight, in agreement with previously published reports [
29]. Considering the robustness of the 3D AET constructs, we believe that these tissues could represent a reliable tool for the rapid, high-throughput screening of novel topically deliverable drug candidates targeting various inner ear pathologies. If validated with human RWM studies, such in vitro models have the capability of accelerating the discovery of novel, effective, topically deliverable therapeutics and could minimize the need for animal models in the early stages of drug development and discovery.
4. Materials and Methods
4.1. Tissue Cultures
Primary human small airway epithelial cells (HSAEC; normal (ATCC PCS301010, Manassas, VA, USA) were cultured in airway epithelial cell basal media supplemented with bronchial epithelial cell growth kit (ATCC PCS300040, Manassas, VA, USA) components. PneumaCult™-ALI complete base medium (PALI, Manassas, VA, USA) (PneumaCult™-ALI 10X Supplement + PneumaCult™-ALI Basal Medium supplemented with PneumaCult™-ALI Maintenance Supplement Heparin Solution and Hydrocortisone Stock Solution) was used for air–liquid interface (ALI). Cells were grown in T-75 flasks up to 80% confluence in an incubator at 37 °C, with 5% CO2. The culture medium was changed every two days. Once cells were 70–80% confluent, they were released, transferred to a conical tube, and pelleted via centrifugation at room temperature (RT) for 5 min at 150 RCF. The supernatant was aspirated, and cells were resuspended in fresh PALI. PALI was added to the 12-well plates (basal chamber), and cell suspension (105 cells/insert) was placed on the cell culture insert (apical chamber). Media change was performed after 24 h in both basal and apical chambers, and 72 h after plating, media change was conducted, and media was added only to the basal chamber. Full media change was performed in the basal chamber using PALI every 2 days, leaving the apical chamber empty. The tissues were allowed to grow for different time points (7, 14, and 21 days after establishing ALI), and at the end of each time point, experiments were performed. Excess mucus was removed from the apical surface by washing the cells with DPBS at RT.
Primary human dermal fibroblasts (HDF, human, primary, ATCC PCS-201-012, Manassas, VA, USA) were grown for 28 days to form a uniform layer of HDF cells. Morphology of the HDF cells was analyzed by staining the cells for actin filaments [
39,
40].
4.2. Histology, Immuno-Labeling, and Imaging of Tissues
Fixing solution (4% formaldehyde + 1% acetic acid in PBS) was used to fix the tissues overnight and then processed and paraffin-embedded using a Leica ASP300S tissue processor. Sections of 6 µm were taken on glass slides and were either stained with hematoxylin and eosin (H&E) or deparaffinized and rehydrated for immuno-labeling in a Leica Autostainer XL.
For immunostaining, deparaffinized tissues were washed with PBS and permeabilized with 0.5% Triton X-100 in PBS for 20 min, washed 3 × in PBS, and blocked with 3% BSA in PBS for 30 min at RT. After blocking cells were washed with PBS thrice and were stained with primary antibodies overnight at 4C, washed 3 × in PBS, and then incubated with secondary antibodies prepared in 3% BSA in PBS for 1 h at RT. After secondary antibody staining, cells were washed with PBS twice and counterstained with DAPI for 15 min and washed thrice with DPBS. After washing cells were mounted using Mowiol + DABCO mounting media (10% w/v Mowiol 4-88, 25% w/v glycerol, 0.1 M Tris (pH 8.5), and 2.5% w/v 1,4-diazobicyclo-[2.2.2]-octane) on coverslips. Tissue was observed under the confocal microscope Leica Stellaris 5 Confocal Microscope (Leica, Germany).
Primary antibodies goat anti-human ZO-1 (Invitrogen, PA5-19090, Waltham, MA, USA), rabbit anti-human CLDN-1 (Invitrogen, 51-9000, Waltham, MA, USA), and rabbit anti-human Occludin (Invitrogen, 40-4700, Waltham, MA, USA) were diluted 1:200. Secondary antibody donkey anti-goat 647 (Invitrogen, A32849, Waltham, MA, USA) was diluted 1:500 and donkey anti-rabbit 555 (Invitrogen, A32794, Waltham, MA, USA) was diluted 1:1000. DAPI (Thermo Scientific, 62248, Waltham, MA, USA) was diluted 1:1000.
To evaluate the morphology of human dermal fibroblast tissue, it was stained with actin dye (ActinRed 555, Invitrogen, R371121 drop/mL, Waltham, MA, USA) for actin filaments and counter stained with DAPI for nuclei. Extracellular matrix deposition was assessed for collagen I (Anti-Collagen Type I antibody, Rockland, 600-401-103S Pottstown, PA, USA) and fibronectin (Fibronectin Polyclonal Antibody, Invitrogen, PA5-29578, Waltham, MA, USA).
4.3. Permeation Devices
An in-house permeation device was employed for drug permeation studies, and transepithelial electrical resistance (TEER) measurements were used to identify potential leakage between the interface of the cell culture insert and the tissue layers. The permeation devices were designed in Fusion360 software (Autodesk, San Rafael, CA, USA), exported to PreForm software, and then printed using Clear V4 resin on a Form 3 photopolymer 3D printer (Formlabs, Somerville, MA, USA). The permeation device was made to fit within a standard 6-well cell culture plate serving as the receiver chamber and holding a standard 24-well plate cell culture insert. The donor chamber (6 mm inner diameter) is placed over the tissue-containing insert, which is placed into the base unit of the permeation device. The donor and receiver chambers are sealed off by the top unit, which has a 6 mm inner diameter “donor” chamber, which is put over the tissue and fastened in place with three stainless steel M2 bolts screwed into base unit nuts that had been press-fit into the base unit. While the second electrode is being placed into the donor chamber, a secondary channel going to the receiver chamber allows for the placement of a TEER electrode.
4.4. TEER Measurements
TEER is widely accepted as an index of the integrity of tight junctions. TEER was measured for the airway tissue prior to permeation testing with a Millicell ERS-2-volt ohmmeter (Millipore, MERS00002, Burlington, MA, USA). The tissue culture insert was placed gently with the help of tweezers into the well. To measure the TEER in the insert, 1500 µL of DPBS was placed into the well of a 12-well plate. Then, 500 µL of DPBS was added into the insert (over top of tissue), and the electrode was placed (MERSSTX01). The longer electrode was placed outside of the insert; the shorter electrode was placed into the insert over the tissue, and the electrode was not touching the base of the well. The values were normalized with the surface area and expressed as Ω.cm2.
4.5. Drug Preparation
Fluorescein (Fluka Analytical, 32615-25G-R, St. Louis, MO, USA) was prepared at 10 mM concentration (equivalent to 3.323 mg/mL) and diluted to 100 µM. pH was adjusted to 8.5 ± 0.5 with 1 M hydrochloric acid. Dexamethasone 21-phosphate disodium (Alfa Aesar, J64083, Tewksbury, MA, USA) was prepared at 2.63 mg/mL (equivalent to 0.2% w/v dexamethasone) in phosphate-buffered saline (PBS). Ciprofloxacin hydrochloride monohydrate (Alfa Aesar, J61970, Tewksbury, MA, USA) was prepared at 2.33 mg/mL (equivalent to 0.2% w/v ciprofloxacin) in PBS, and the pH was adjusted to 3.5 ± 0.5 with 1 M hydrochloric acid. Gentamicin Sulfate was prepared at 3.62 mg/mL equivalent to 3 mg/mL) in PBS. The given concentration for all four drugs was chosen on the basis of clinically available formulations except fluorescein.
4.6. Drug Permeation Testing
After TEER measurement, airway tissue was placed into a 6-well plate containing 2500 µL PBS (receiver solution), and 200 µL of drug solution was placed into the donor chamber, being sure that no air bubbles became trapped above or below the tissue.
At each time point (1, 2, 4, 6, 8, and 24 h), the device was removed from the well, allowed to drip, then transferred to a new well containing fresh donor solution. To ensure a consistent gradient, 100 µL of drug solution was removed from the donor channel and replaced with the fresh drug at each time point. All receiver solutions were collected immediately in microfuge tubes and stored at −20 °C until analysis. Any noticeable decrease in donor solution level in tissue indicated a leak or compromised tissue, and the sample was excluded from the study.
Fluorescein was used as a model drug for drug permeability testing, and it can be traced easily due to its fluorescent property. The permeability of Fluorescein was quantified using Citation. Concentrations of Fluorescein were determined by measuring the fluorescence intensity of the apical samples using a Cytation 5 (Biotek, Winooski, VT, USA) microplate reader (ex. 490 nm; em. 515 nm) against a standard curve. Other drugs were quantified using the HPLC method.
4.7. High-Performance Liquid Chromatography
The flux measurement and drug permeability in the time-dependent permeation were determined using HPLC. All samples were filtered with 0.22 µm PVDF syringe filters prior to HPLC analysis. All reagents used were HPLC-grade; the buffers were freshly made and filtered at 0.1 µm prior to use. HPLC was performed using an Agilent 1260 Infinity II system (Agilent Technologies Inc., Santa Clara, CA, USA).
Dexamethasone sodium Phosphate: 50 µL of the sample was injected into an isocratic mobile phase of 75% 0.01 M potassium phosphate buffer (pH 7.58) and 25% acetonitrile flowing at 1.5 mL/min through a Gemini 3 µm 100 × 4.6 mm reverse-phase C18 column (Phenomenex, 00D-4439-E0) protected by a Security Guard C18 4 × 3.0 mm cartridge (Phenomenex, AJ0-7597) at 25 °C. The retention time of DSP at 1.9 min was used to quantify the area under the curve (AUC) of absorbance at 240 nm.
Ciprofloxacin: 50 µL of the sample was injected into an isocratic mobile phase of 80% 0.02 M potassium phosphate buffer (pH to 2.7 with orthophosphoric acid) and 20% acetonitrile flowing at 1.0 nmL/min through a Gemini 5 µm 150 × 3 mm reverse-phase C18 column (Phenomenex, 00F-4435-Y0, Torrance, CA, USA) protected by a Security Guard C18 4 × 2.0 mm cartridge (Phenomenex, AJ0-7596, Torrance, CA, USA) at 25 °C. The retention time of ciprofloxacin at 1.75 min was used to quantify the area under the curve (AUC) of absorbance at 277 nm.
Gentamicin Sulfate: Gentamicin was pre-column derivatized using the o-phthalaldehyde reaction. The filtered sample of 50 μL is combined with 55 μL of isopropanol and 20 μL of Reagent B (1 mL HPLC-grade methanol, 19 mL Reagent A, 0.4 mL thioglycolic acid, 200 mg O-phthalaldehyde, pH adjusted to 10.4 using 8 N potassium hydroxide), vortexed for 10 s and then incubated at 60 °C for 15 min. The reagent A is 0.4 M boric acid adjusted to a pH of 10.4 with 8N potassium hydroxide. Following the derivatization procedure, the samples were subjected to HPLC analysis. Then, 50 µL of the sample was injected into an isocratic mobile phase of 100% (5 g/L sodium 1-heptane sulfonate monohydrate in 70:25:5 methanol–water–acetic acid) flowing at 0.8 mL/min through a Gemini 5 µm 150 × 3 mm reverse-phase C18 column (Phenomenex, 00F-4435-Y0, Torrance, CA, USA) protected by a Security Guard C18 4 × 2. 0 mm cartridge (Phenomenex, AJ0-7596) at 25 °C. The retention time of C-components of gentamicin at C1a − 7.9 min; C2a − 10.3 min; C2 − 11.9 min were used to quantify the area under the curve (AUC) of absorbance at 330 nm.
4.8. Analysis of Flux and Apparent Permeability Coefficient
Following the amount of drug permeated through tissues was determined, the flux was calculated using where D is the drug permeated since the previous time point (nmoles), A is the permeable area (cm2), and ΔT is the elapsed time (hours) since the previous time point.
Using Fick’s law, the apparent permeability coefficient (P
app, cmhr-1) was calculated:
where C
r is the receiver solution concentration (considered constant at 0 since receiver solution was replaced at each time point and results confirm receiver solution remained negligible), and Flux
ss is the average of the flux values once a steady state has been reached. C
d is the donor solution concentration (nmol.cm
−3), which is assumed to be constant due to excess and regular replenishment of donor solution.
4.9. Tissue Freeze/Thawing Process
For freeze/thawing processing, samples (n = 4) were frozen for 24 h at −20 °C (cycle 1) and for cycle 2, frozen for 24 h, after 24 h allowed to thaw for 30 min, then refrozen for an additional 24 h at −20 °C. Post-freezing, samples were taken out and allowed to thaw completely. Subsequently, tissue integrity and drug permeability analyses were performed as described above. Unprocessed samples (not frozen/thawed) were used as controls.
4.10. Statistical Analyses
All experiments were run in triplicates or greater, and all data were presented as mean ± standard deviation of the mean. Student t-tests were performed for 2-group comparisons, while one-way ANOVA with multiple comparisons was performed for more than 2-group comparisons with confidence limits of 95% considered significant.