Combined Ramipril and Black Seed Oil Dosage Forms Using Bioactive Self-Nanoemulsifying Drug Delivery Systems (BIO-SNEDDSs)
Abstract
:1. Introduction
2. Results
2.1. Ultra-Performance Liquid Chromatography (UPLC) for Quantification of RMP and THQ
2.2. Self-Emulsification Assessment
2.3. Experimentally Designed Phase Diagrams
2.3.1. Model Analysis
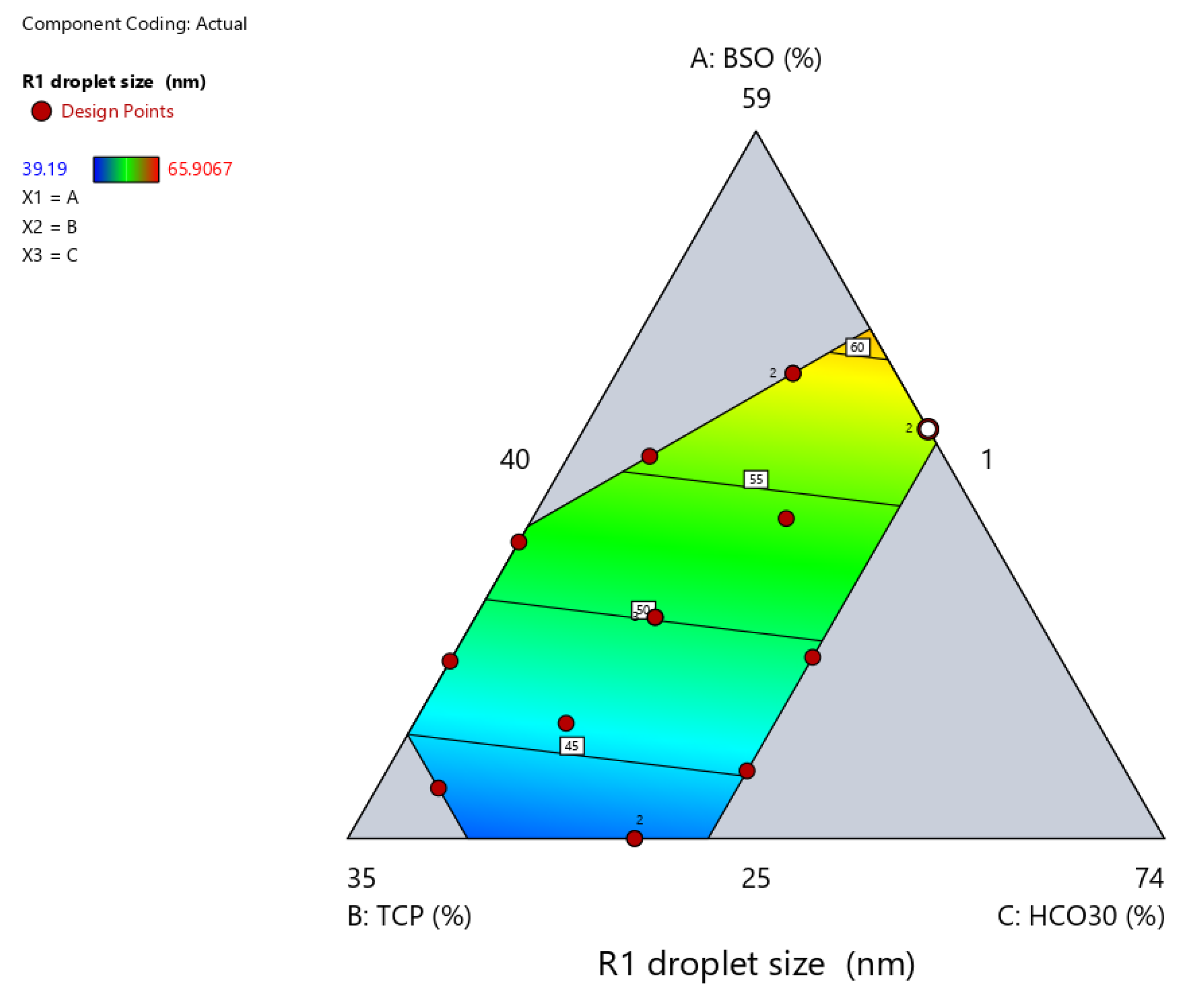
2.3.2. Droplet Size
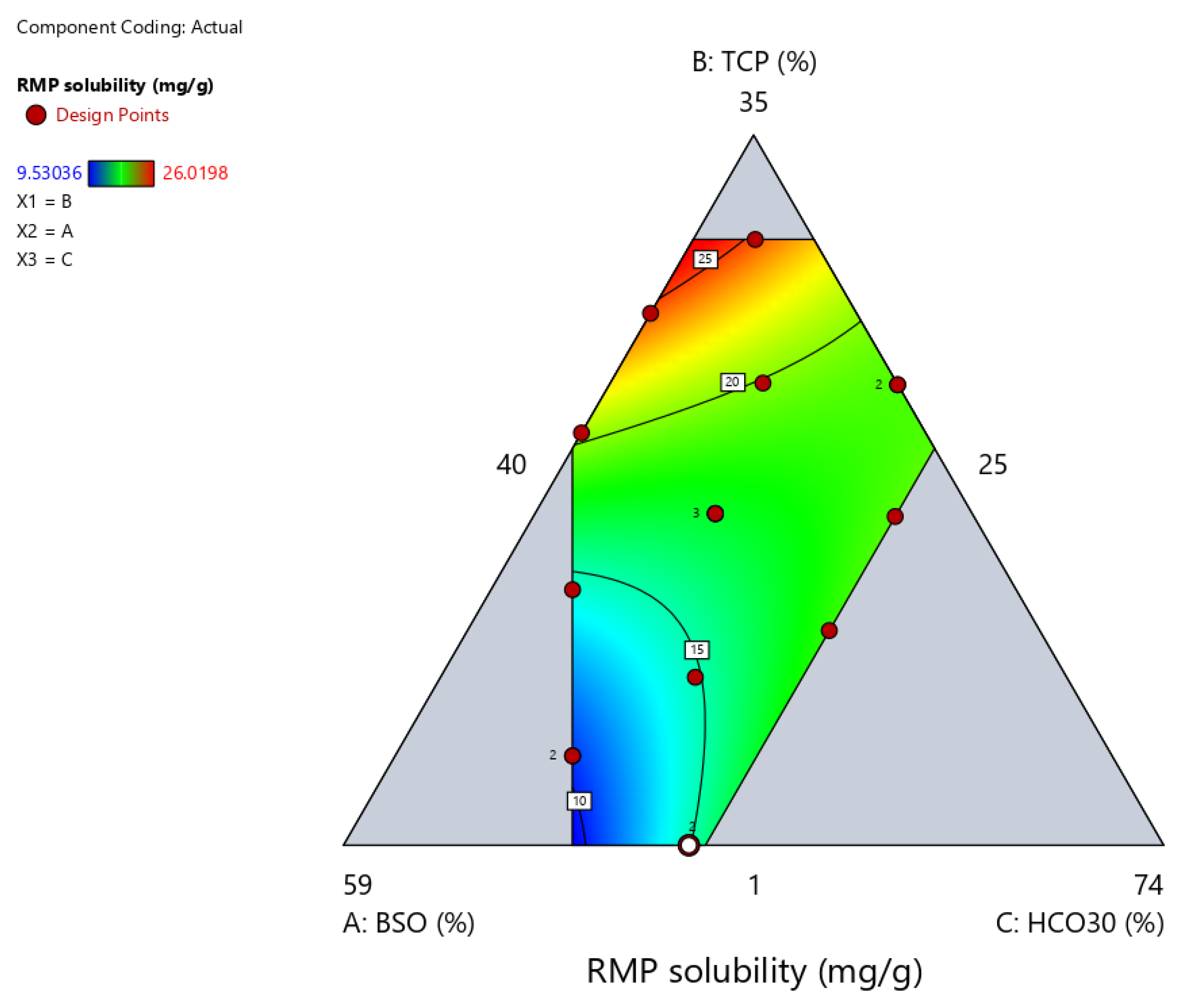
2.3.3. Apparent Solubility of RMP in Formulation
2.3.4. Zeta Potential (ZP) and the Release of RMP and THQ at 15 Min
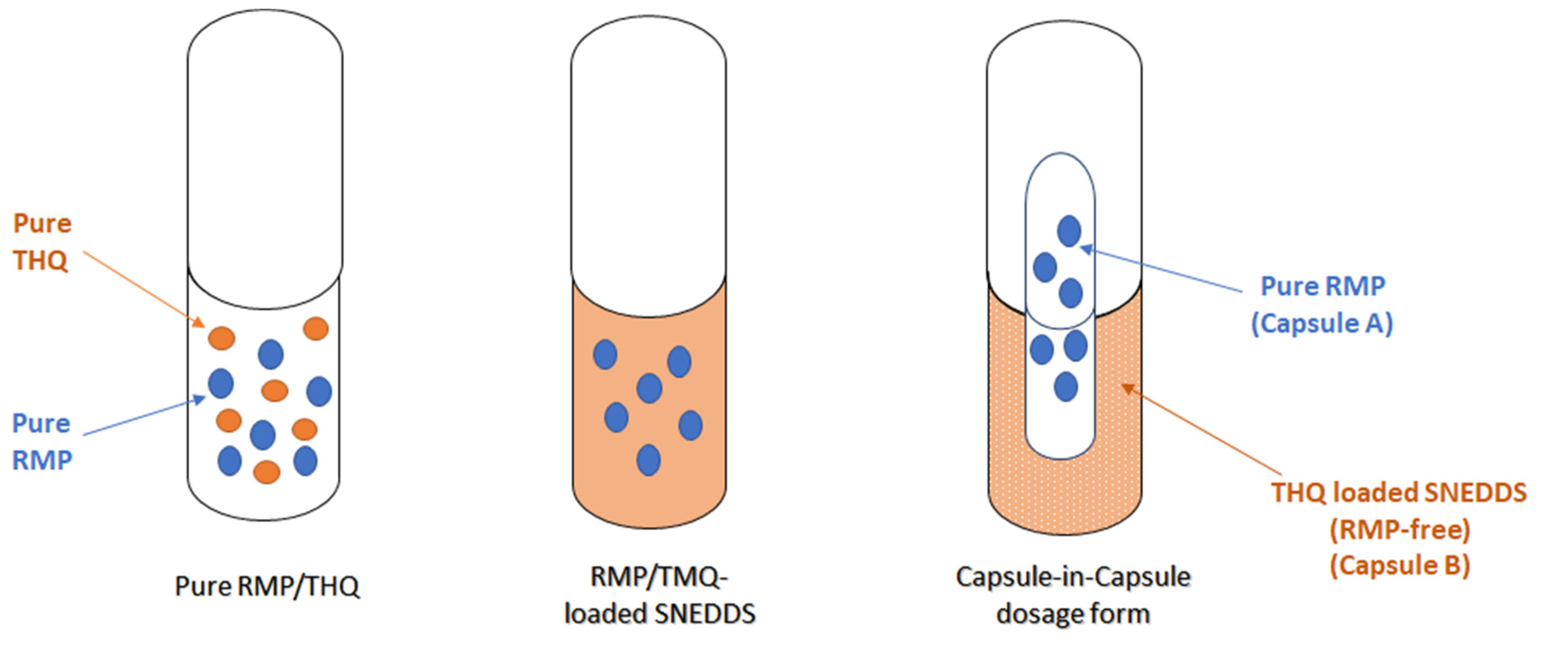
2.3.5. Optimization of the SNEDDS
2.3.6. Validation of the Experimental Model
2.4. In Vitro Dissolution
2.5. Accelerated Stability Study
3. Discussion
4. Materials and Methods
4.1. Plant Material and Extraction of Bioactive Oils
4.1.1. Collection and Extraction of Seeds
4.1.2. BSO Standardization
4.2. Chemical and Reagents
4.3. Ultra-Performance Liquid Chromatography (UPLC) for Quantification of Ramipril
4.4. Preparation of Drug-Free Liquid SNEDDSs
4.5. Self-Emulsification Assessment
- The blends of different excipients—such as mixtures of oils, surfactants, and/or cosolvents—were examined carefully to evaluate the mutual miscibility between the components.
- The spontaneity of the formulation was judged as “good” when the droplets easily spread in water, to form an emulsion, within 1 min. It was judged as “moderate” when the droplets took 1–5 min to completely spread in water. Finally, the formulation was judged as “poor” when the droplets tended to coalesce, needed high shear mixing, and/or took >5 min to completely spread in water.
- The homogeneity of the formulation was judged as “good” when the formulation was able to be dispersed in water without causing any phase separation. It was judged as “poor” when the formulation resulted in phase separation and/or oil floating upon aqueous dilution.
- For further characterization, the appearance of the formulations was also judged as turbid, bluish (semi-clear), or clear according to the degree of clarity of the formulation after aqueous dilution.
- Descriptive green, yellow, and red colors were utilized to easily distinguish between good, moderate, and poor performance of the formulations. According to the assessment criteria, the formulation was accepted as an SEDDS/SNEDDS only if it showed complete excipient miscibility, as well as at least moderate spontaneity and homogeneity.
4.6. Experimentally Designed Phase Diagrams
4.7. Apparent Solubility of RMP in SNEDDSs
4.8. Droplet Size and Zeta Potential
4.9. Preparation of the Optimized SNEDDS
4.10. In Vitro Dissolution Studies
4.11. Accelerated Stability Study
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naik, R.R.; Shakya, A.K.; Khalaf, N.A.; Abuhamdah, S.; Oriquat, G.A.; Maraqa, A. GC-MS Analysis and Biological Evaluation of Essential Oil of Zanthoxylum Rhesta (Roxb.) DC Pericarp. Jordan J. Pharm. Sci. 2015, 8, 181–193. [Google Scholar] [CrossRef]
- De Holanda Cavalcanti, S.C.; de Oliveira, R.d.R.B.; de Sousa, D.P. Antitumor Essential Oils: Progress in Medicinal Chemistry. In Bioactive Essential Oils and Cancer; de Sousa, D.P., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 111–124. [Google Scholar]
- Kazi, M.; Shahba, A.A.; Alrashoud, S.; Alwadei, M.; Sherif, A.Y.; Alanazi, F.K. Bioactive Self-Nanoemulsifying Drug Delivery Systems (Bio-SNEDDS) for Combined Oral Delivery of Curcumin and Piperine. Molecules 2020, 25, 1703. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-García, C.; Rubio-Guerra, A.F. Combination therapy in the treatment of hypertension. Drugs Context 2018, 7, 212531. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, D.; Zhang, W.; Xing, Y.; Guo, Y.; Wang, F.; Jia, J.; Yan, T.; Liu, Y.; Lin, S. ACE inhibitor benefit to kidney and cardiovascular outcomes for patients with non-dialysis chronic kidney disease stages 3–5: A network meta-analysis of randomised clinical trials. Drugs 2020, 80, 797–811. [Google Scholar] [CrossRef]
- Shafiq, S.; Shakeel, F. Enhanced stability of ramipril in nanoemulsion containing cremophor-EL: A technical note. Aaps Pharmscitech 2008, 9, 1097–1101. [Google Scholar] [CrossRef] [PubMed]
- Ekambaram, P.; Sathali, A.A.H. Formulation and evaluation of solid lipid nanoparticles of ramipril. J. Young Pharm. 2011, 3, 216–220. [Google Scholar] [CrossRef]
- Hussain, D.A.S.; Hussain, M.M. Nigella sativa (black seed) is an effective herbal remedy for every disease except death–a Prophetic statement which modern scientists confirm unanimously: A review. Adv. Med. Plant Res. 2016, 4, 27–57. [Google Scholar]
- Ahmad, A.; Husain, A.; Mujeeb, M.; Khan, S.A.; Najmi, A.K.; Siddique, N.A.; Damanhouri, Z.A.; Anwar, F. A review on therapeutic potential of Nigella sativa: A miracle herb. Asian Pac. J. Trop. Biomed. 2013, 3, 337–352. [Google Scholar] [CrossRef]
- Ali, S.; Hashim, A.; Shiekh, A.; Majid, S.; Rehman, M.U. Chapter 10-The cardioprotective effect of thymoquinone from Nigella sativa. In Black Seeds (Nigella Sativa); Khan, A., Rehman, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 239–252. [Google Scholar] [CrossRef]
- Nirmala, P. Formulation And Evaluation of Fast Dissolving Oral Films Incorporated With Ramipril and β-Cyclodextrin Complex. Int. J. Pharm. Sci. Drug Res. 2020, 12, 390–395. [Google Scholar] [CrossRef]
- Kalam, M.A.; Raish, M.; Ahmed, A.; Alkharfy, K.M.; Mohsin, K.; Alshamsan, A.; Al-Jenoobi, F.I.; Al-Mohizea, A.M.; Shakeel, F. Oral bioavailability enhancement and hepatoprotective effects of thymoquinone by self-nanoemulsifying drug delivery system. Mater. Sci. Eng. C 2017, 76, 319–329. [Google Scholar] [CrossRef]
- Alshadidi, A.; Shahba, A.A.; Sales, I.; Rashid, M.A.; Kazi, M. Combined Curcumin and Lansoprazole-Loaded Bioactive Solid Self-Nanoemulsifying Drug Delivery Systems (Bio-SSNEDDS). Pharmaceutics 2021, 14, 2. [Google Scholar] [CrossRef]
- Kazi, M.; Alhajri, A.; Alshehri, S.; Elzayat, E.; Meanazel, O.T.A.; Shakeel, F.; Noman, O.M.; Altamimi, M.A.; Alanazi, F. Enhancing Oral Bioavailability of Apigenin Using a Bioactive Self-Nanoemulsifying Drug Delivery System (Bio-SNEDDS): In Vitro, In Vivo and Stability Evaluations. Pharmaceutics 2020, 12, 749. [Google Scholar] [CrossRef] [PubMed]
- Kazi, M.; Nasr, F.A.; Noman, O.; Alharbi, A.; Alqahtani, M.S.; Alanazi, F.K. Development, Characterization Optimization, and Assessment of Curcumin-Loaded Bioactive Self-Nanoemulsifying Formulations and Their Inhibitory Effects on Human Breast Cancer MCF-7 Cells. Pharmaceutics 2020, 12, 1107. [Google Scholar] [CrossRef] [PubMed]
- Bergström, C.A.S.; Larsson, P. Computational prediction of drug solubility in water-based systems: Qualitative and quantitative approaches used in the current drug discovery and development setting. Int. J. Pharm. 2018, 540, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Chevillard, F.; Lagorce, D.; Reynès, C.; Villoutreix, B.O.; Vayer, P.; Miteva, M.A. In Silico Prediction of Aqueous Solubility: A Multimodel Protocol Based on Chemical Similarity. Mol. Pharm. 2012, 9, 3127–3135. [Google Scholar] [CrossRef] [PubMed]
- Shahba, A.A.; Mohsin, K.; Alanazi, F.K. The Studies of Phase Equilibria and Efficiency Assessment for Self-Emulsifying Lipid-Based Formulations. AAPS PharmSciTech 2012, 13, 522–533. [Google Scholar] [CrossRef]
- Mohsin, K.; Long, M.A.; Pouton, C.W. Design of lipid-based formulations for oral administration of poorly water-soluble drugs: Precipitation of drug after dispersion of formulations in aqueous solution. J. Pharm. Sci. 2009, 98, 3582–3595. [Google Scholar] [CrossRef]
- Dahan, A.; Hoffman, A. Rationalizing the selection of oral lipid based drug delivery systems by an in vitro dynamic lipolysis model for improved oral bioavailability of poorly water soluble drugs. J. Control. Release 2008, 129, 1–10. [Google Scholar] [CrossRef]
- Bahloul, B.; Lassoued, M.A.; Seguin, J.; Lai-Kuen, R.; Dhotel, H.; Sfar, S.; Mignet, N. Self-emulsifying drug delivery system developed by the HLB-RSM approach: Characterization by transmission electron microscopy and pharmacokinetic study. Int. J. Pharm. 2015, 487, 56–63. [Google Scholar] [CrossRef]
- Shahba, A.A.-W.; Mohsin, K.; Alanazi, F.K.; Abdel-Rahman, S.I. Optimization of self-nanoemulsifying formulations for weakly basic lipophilic drugs: Role of acidification and experimental design. Braz. J. Pharm. Sci. 2016, 52, 653–667. [Google Scholar] [CrossRef] [Green Version]
- Bahloul, B.; Lassoued, M.A.; Sfar, S. A novel approach for the development and optimization of self emulsifying drug delivery system using HLB and response surface methodology: Application to fenofibrate encapsulation. Int. J. Pharm. 2014, 466, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Buya, A.B.; Terrasi, R.; Mbinze, J.K.; Muccioli, G.G.; Beloqui, A.; Memvanga, P.B.; Préat, V. Quality-by-Design-Based Development of a Voxelotor Self-Nanoemulsifying Drug-Delivery System with Improved Biopharmaceutical Attributes. Pharmaceutics 2021, 13, 1388. [Google Scholar] [CrossRef]
- Mahmoud, E.A.; Bendas, E.R.; Mohamed, M.I. Preparation and evaluation of self-nanoemulsifying tablets of carvedilol. AAPS PharmSciTech 2009, 10, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Zainol, S.; Basri, M.; Basri, H.B.; Shamsuddin, A.F.; Abdul-Gani, S.S.; Karjiban, R.A.; Abdul-Malek, E. Formulation Optimization of a Palm-Based Nanoemulsion System Containing Levodopa. Int. J. Mol. Sci. 2012, 13, 13049–13064. [Google Scholar] [CrossRef] [PubMed]
- Valicherla, G.R.; Dave, K.M.; Syed, A.A.; Riyazuddin, M.; Gupta, A.P.; Singh, A.; Wahajuddin; Mitra, K.; Datta, D.; Gayen, J.R. Formulation optimization of Docetaxel loaded self-emulsifying drug delivery system to enhance bioavailability and anti-tumor activity. Sci. Rep. 2016, 6, 26895. [Google Scholar] [CrossRef]
- Alghananim, A.; Özalp, Y.; Mesut, B.; Serakinci, N.; Özsoy, Y.; Güngör, S. A Solid Ultra Fine Self-Nanoemulsifying Drug Delivery System (S-SNEDDS) of Deferasirox for Improved Solubility: Optimization, Characterization, and In Vitro Cytotoxicity Studies. Pharmaceuticals 2020, 13, 162. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.H.; Shanmugam, S.; Thapa, P.; Lee, E.-S.; Balakrishnan, P.; Baskaran, R.; Yoon, S.-K.; Choi, H.-G.; Yong, C.S.; Yoo, B.K.; et al. Novel self-nanoemulsifying drug delivery system for enhanced solubility and dissolution of lutein. Arch. Pharmacal Res. 2010, 33, 417–426. [Google Scholar] [CrossRef]
- Alhasani, K.F.; Kazi, M.; Ibrahim, M.A.; Shahba, A.A.; Alanazi, F.K. Self-nanoemulsifying ramipril tablets: A novel delivery system for the enhancement of drug dissolution and stability. Int. J. Nanomed. 2019, 14, 5435–5448. [Google Scholar] [CrossRef] [PubMed]
- Odovic, J.V.; Markovic, B.D.; Trbojevic-Stankovic, J.; Vladimirov, S.M.; Karljikovic-Rajic, K.D. Evaluation of ACE inhibitors lipophilicity using in silico and chromatographically obtained hydrophobicity parameters/Procena lipofilnosti ace inhibitora primenom in silico i hromatografski dobijenih parametara hidrofobnosti. Hem. Ind. 2013, 67, 209–217. [Google Scholar] [CrossRef]
- Porter, C.; Pouton, C.; Cuine, J.; Charman, W. Enhancing intestinal drug solubilisation using lipid-based delivery systems☆. Adv. Drug Deliv. Rev. 2008, 60, 673–691. [Google Scholar] [CrossRef]
- Pouton, C.; Porter, C. Formulation of lipid-based delivery systems for oral administration: Materials, methods and strategies☆. Adv. Drug Deliv. Rev. 2008, 60, 625–637. [Google Scholar] [CrossRef]
- Saifee, M.; Zarekar, S.; Rao, V.U.; Zaheer, Z.; Soni, R.; Burande, S. Formulation and in vitro evaluation of solid-self-emulsifying drug delivery system (SEDDS) of glibenclamide. Am. J. Adv. Drug Deliv. 2013, 1, 323–340. [Google Scholar]
- Tian, Y.; Chen, L.; Zhang, W. Influence of ionic surfactants on the properties of nanoemulsions emulsified by nonionic surfactants span 80/tween 80. J. Dispers. Sci. Technol. 2016, 37, 1511–1517. [Google Scholar] [CrossRef]
- Silva, H.D.; Cerqueira, M.A.; Vicente, A.A. Influence of surfactant and processing conditions in the stability of oil-in-water nanoemulsions. J. Food Eng. 2015, 167, 89–98. [Google Scholar] [CrossRef]
- Singh, M.K.; Pooja, D.; Kulhari, H.; Jain, S.K.; Sistla, R.; Chauhan, A.S. Poly (amidoamine) dendrimer-mediated hybrid formulation for combination therapy of ramipril and hydrochlorothiazide. Eur. J. Pharm. Sci. 2017, 96, 84–92. [Google Scholar] [CrossRef]
- Zaid, A.N.; Ghanem, M.; Maqboul, L.; Zaid, H.; Mahasne, A. Biowaiver Eligibility of a Lower Strength Ramipril/Hydrochlorothiazide Immediate Release Tablets Using a New Validated HPLC Analytical Method. Drug Res. 2016, 66, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, E.; Laosa, O.; Guerra, P.; Duque, B.; Mosquera, B.; Borobia, A.M.; Lei, S.H.; Carcas, A.J.; Frias, J. Acceptability and characteristics of 124 human bioequivalence studies with active substances classified according to the Biopharmaceutic Classification System. Br. J. Clin. Pharm. 2010, 70, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, T.; Plakogiannis, F.M. Development and oral bioavailability assessment of a supersaturated self-microemulsifying drug delivery system (SMEDDS) of albendazole. J. Pharm. Pharmacol. 2010, 62, 1112–1120. [Google Scholar] [CrossRef] [PubMed]
- Shahba, A.A.; Tashish, A.Y.; Alanazi, F.K.; Kazi, M. Combined self-nanoemulsifying and solid dispersion systems showed enhanced cinnarizine release in hypochlorhydria/achlorhydria dissolution model. Pharmaceutics 2021, 13, 627. [Google Scholar] [CrossRef]
- Shahba, A.A.; Mohsin, K.; Alanazi, F.K. Novel self-nanoemulsifying drug delivery systems (SNEDDS) for oral delivery of cinnarizine: Design, optimization, and in-vitro assessment. AAPS PharmSciTech 2012, 13, 967–977. [Google Scholar] [CrossRef]
- Shahba, A.A.; Ahmed, A.R.; Mohsin, K.; Abdel-Rahman, S.I.; Alanazi, F.K. Solidification of cinnarizine self-nanoemulsifying drug delivery systems by fluid bed coating: Optimization of the process and formulation variables. Pharmazie 2017, 72, 143–151. [Google Scholar]
- Arora, S.; Ali, J.; Ahuja, A.; Khar, R.K.; Baboota, S. Floating drug delivery systems: A review. AAPS PharmSciTech 2005, 6, E372–E390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aulton, M. Aulton’s Pharmaceutics: The Design and Manufacture of Medicines, 3rd ed.; Aulton, M., Ed.; Churchill Livingstone: Edinburgh, Scotland, 2007. [Google Scholar]
- El Maghraby, G.M.; Elzayat, E.M.; Alanazi, F.K. Development of modified in situ gelling oral liquid sustained release formulation of dextromethorphan. Drug Dev. Ind. Pharm. 2012, 38, 971–978. [Google Scholar] [CrossRef]
- Shi, S.; Chen, H.; Cui, Y.; Tang, X. Formulation, stability and degradation kinetics of intravenous cinnarizine lipid emulsion. Int. J. Pharm. 2009, 373, 147–155. [Google Scholar] [CrossRef]








| Formulation Code | Oil | Cosurfactant | Surfactant | Self-Emulsification Assessment | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BSO | I308 | I988 | TCP | SR-P80 | Kr-EL | Kr-EL (HP) | T80 | T20 | Kr-H40 | T85 | HCO30 | Miscibility | Time (min) | Appearance | Homogeneity | Overall Assessment | |
| F1 | 35 | 15 | 50 | IM | - | X | |||||||||||
| F2 | 35 | 15 | 50 | IM | NA | X | |||||||||||
| F3 | 35 | 15 | 50 | IM | NA | X | |||||||||||
| F4 | 35 | 15 | 50 | IM | NA | X | |||||||||||
| F5 | 35 | 15 | 50 | IM | NA | X | |||||||||||
| F6 | 35 | 15 | 50 | IM | NA | X | |||||||||||
| F7 | 35 | 15 | 50 | IM | NA | X | |||||||||||
| F8 | 35 | 15 | 50 | M | <1 | T | H | √ | |||||||||
| F9 | 45 | 10 | 45 | M | <1 | T | H | √ | |||||||||
| F10 | 50 | 50 | M | <1 | T | OF | X | ||||||||||
| F11 | 35 | 15 | 50 | M | 1–2 | H | √ | ||||||||||
| F12 | 35 | 15 | 50 | M | <1 | C | H | √ | |||||||||
| F13 | 45 | 10 | 45 | M | <1 | SC | H | √ | |||||||||
| F14 | 50 | 50 | M | <1 | SC | H | √ | ||||||||||
| F15 | 35 | 15 | 50 | M | 1–2 | C | H | √ | |||||||||
| F16 | 45 | 10 | 45 | M | <1 | SC | H | √ | |||||||||
| Run | Independent Variables | Dependent Variables (Responses) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| A: BSO (%) | B: TCP (%) | C: HCO-30 (%) | R1: Droplet Size (nm) | R2: Polydispersity Index (PDI) | R3: Zeta Potential (mV) | R4: Apparent RMP Solubility (Day 0) (mg/g) | R5: RMP Release (%) at 15 min | R6: THQ Release (%) at 15 min | R7: Apparent RMP Solubility (Day 10) (mg/g) | R7 Formulation Gelling at 30 min | |
| 1 | 47.4 | 5.3 | 47.4 | 65.9 | 0.213 | −25.5 | 9.5 | 72.8 | 65.7 | 4.0 | No |
| 2 | 33.7 | 11.3 | 55.0 | 46.3 | 0.229 | −17.4 | 20.0 | 58.1 | 42.6 | 6.7 | No |
| 3 | 25.0 | 23.0 | 52.0 | 41.4 | 0.237 | −32.7 | 17.4 | 89.0 | 77.6 | 12.8 | Yes |
| 4 | 35.6 | 16.9 | 47.5 | 51.5 | 0.189 | −19.7 | 14.4 | 71.0 | 73.4 | 7.6 | Yes |
| 5 | 25.0 | 23.0 | 52.0 | 48.9 | 0.220 | −15.3 | 18.8 | 75.8 | 65.5 | 10.9 | No |
| 6 | 30.5 | 23.1 | 46.3 | 52.4 | 0.200 | −25.0 | 22.9 | 70.0 | 70.4 | 8.0 | No |
| 7 | 33.5 | 26.5 | 40.0 | 46.8 | 0.202 | −21.6 | 26.0 | 67.7 | 63.0 | 11.9 | Yes |
| 8 | 28.3 | 16.7 | 55.0 | 43.0 | 0.258 | −31.3 | 19.8 | 82.9 | 88.3 | 7.2 | No |
| 9 | 40.4 | 9.0 | 50.6 | 50.7 | 0.184 | −22.5 | 13.6 | 74.7 | 62.9 | 6.2 | Yes |
| 10 | 35.6 | 16.9 | 47.5 | 50.7 | 0.213 | −21.0 | 18.3 | 77.0 | 82.9 | 6.8 | Yes |
| 11 | 39.3 | 20.7 | 40.0 | 47.6 | 0.150 | −21.5 | 18.5 | 80.6 | 86.3 | 8.9 | Yes |
| 12 | 43.4 | 13.2 | 43.4 | 56.1 | 0.148 | −23.4 | 14.9 | 82.4 | 77.1 | 4.6 | No |
| 13 | 47.4 | 5.3 | 47.4 | 60.6 | 0.139 | −27.2 | 13.0 | 65.4 | 78.9 | 0.9 | Yes |
| 14 | 27.4 | 30.0 | 42.6 | 39.2 | 0.230 | −24.8 | 23.9 | 95.8 | 90.1 | 14.2 | No |
| 15 | 35.6 | 16.9 | 47.5 | 49.6 | 0.208 | −15.4 | 16.6 | 85.5 | 95.7 | 9.9 | No |
| 16 | 44.7 | 1.0 | 54.3 | 54.2 | 0.196 | −19.1 | 12.5 | 62.8 | 67.0 | 2.8 | Yes |
| 17 | 44.7 | 1.0 | 54.3 | 57.6 | 0.192 | −36.7 | 16.3 | 82.9 | 82.3 | 3.2 | Yes |
| Responses | Selected Model | F-Value | p-Value | Lack of Fit p-Value | Adjusted R2 | Predicted R2 | Adequate Precision |
|---|---|---|---|---|---|---|---|
| R1 droplet size | Linear model | 17.86 | 0.0001 | 0.2740 | 0.6782 | 0.5676 | 10.284 |
| R2 polydispersity index (PDI) | Linear model | 13.40 | 0.0005 | 0.8693 | 0.6079 | 0.4675 | 8.969 |
| R3 ZP | Mean model | NA | NA | 0.9416 | 0.0000 | −0.1289 | NA * |
| R4 apparent solubility of RMP | Quadratic | 11.85 | 0.0004 | 0.5338 | 0.7722 | 0.6222 | 11.562 |
| R5 RMP release at 15 min | Linear model | 1.55 | 0.2460 | 0.4732 | 0.0646 | −0.2835 | 3.4337 |
| R6 THQ release at 15 min | Mean | NA | NA | 0.2543 | 0.0000 | −0.1289 | NA * |
| Component | Gradient in Reals | Component Effect | Gradient Standard Error | Approx. t for H₀ Gradient = 0 | Prob > |t| (p-Value) | Gradient in Pseudo |
|---|---|---|---|---|---|---|
| A-BSO | 73.24 | 14.74 | 14.38 | 5.09 | 0.0002 | 24.90 |
| B-TCP | −48.63 | −14.10 | 11.46 | −4.24 | 0.0008 | −16.53 |
| C-HCO30 | −23.79 | −3.56 | 19.99 | −1.19 | 0.2537 | −8.09 |
| Sum of Squares | df | Mean Square | F-Value | p-Value | ||
|---|---|---|---|---|---|---|
| Model | 253.72 | 5 | 50.74 | 11.85 | 0.0004 | Significant |
| Linear mixture ⁽*⁾ | 189.21 | 2 | 94.60 | 22.08 | 0.0001 | |
| AB | 3.48 | 1 | 3.48 | 0.81 | 0.3866 | |
| AC | 1.42 | 1 | 1.42 | 0.33 | 0.5766 | |
| BC | 31.23 | 1 | 31.23 | 7.29 | 0.0207 | |
| Residual | 47.12 | 11 | 4.28 | |||
| Lack of fit | 25.09 | 6 | 4.18 | 0.95 | 0.5338 | Not significant |
| Pure error | 22.03 | 5 | 4.41 | |||
| Total correlation | 300.84 | 16 |
| Response | Predicted Mean | n | 95% PI Low | Data Mean | 95% PI High | Data SD |
|---|---|---|---|---|---|---|
| R1 droplet size * | 46.73 | 3 | 40.30 | 47.47 | 53.14 | 0.99 |
| R2 polydispersity index (PDI) * | 0.189 | 3 | 0.156 | 0.203 | 0.222 | 0.010 |
| R3ZP | −23.54 | 4 | −30.502 | −30.13 | −16.57 | 2.28 |
| R4 apparent solubility of RMP * | 25.37 | 4 | 21.47 | 25.57 | 29.28 | 4.43 |
| R5 RMP release at 15 min | 81.93 | 3 | 66.14 | 78.91 | 97.73 | 6.33 |
| R6 THQ release at 15 min | 74.68 | 3 | 57.40 | 86.22 | 91.95 | 8.48 |
| File Version | 13.0.11.0 | ||
|---|---|---|---|
| Study type | Mixture | Subtype | Randomized |
| Design type | I-optimal (coordinate exchange) | Runs | 17.00 |
| Design model | Special cubic | Blocks | No Blocks |
| Variable | Name | Units | Minimum | Maximum | Mixture Component Coding | Coded Low | Coded High | Mean | Standard Deviation |
|---|---|---|---|---|---|---|---|---|---|
| A | BSO | % | 25 | 47.36 | L_Pseudo | +0 ↔ 25 | +0.72 ↔ 49.5 | 36.32 | 7.53 |
| B | TCP | % | 1 | 30 | L_Pseudo | +0 ↔ 1 | +0.85 ↔ 30 | 15.29 | 8.77 |
| C | HCO30 | % | 40 | 55 | L_Pseudo | +0 ↔ 40 | +0.44 ↔ 55 | 48.39 | 4.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahba, A.A.-W.; Sherif, A.Y.; Elzayat, E.M.; Kazi, M. Combined Ramipril and Black Seed Oil Dosage Forms Using Bioactive Self-Nanoemulsifying Drug Delivery Systems (BIO-SNEDDSs). Pharmaceuticals 2022, 15, 1120. https://doi.org/10.3390/ph15091120
Shahba AA-W, Sherif AY, Elzayat EM, Kazi M. Combined Ramipril and Black Seed Oil Dosage Forms Using Bioactive Self-Nanoemulsifying Drug Delivery Systems (BIO-SNEDDSs). Pharmaceuticals. 2022; 15(9):1120. https://doi.org/10.3390/ph15091120
Chicago/Turabian StyleShahba, Ahmad Abdul-Wahhab, Abdelrahman Y. Sherif, Ehab M. Elzayat, and Mohsin Kazi. 2022. "Combined Ramipril and Black Seed Oil Dosage Forms Using Bioactive Self-Nanoemulsifying Drug Delivery Systems (BIO-SNEDDSs)" Pharmaceuticals 15, no. 9: 1120. https://doi.org/10.3390/ph15091120







