Recent Development of LDL-Based Nanoparticles for Cancer Therapy
Abstract
:1. Introduction
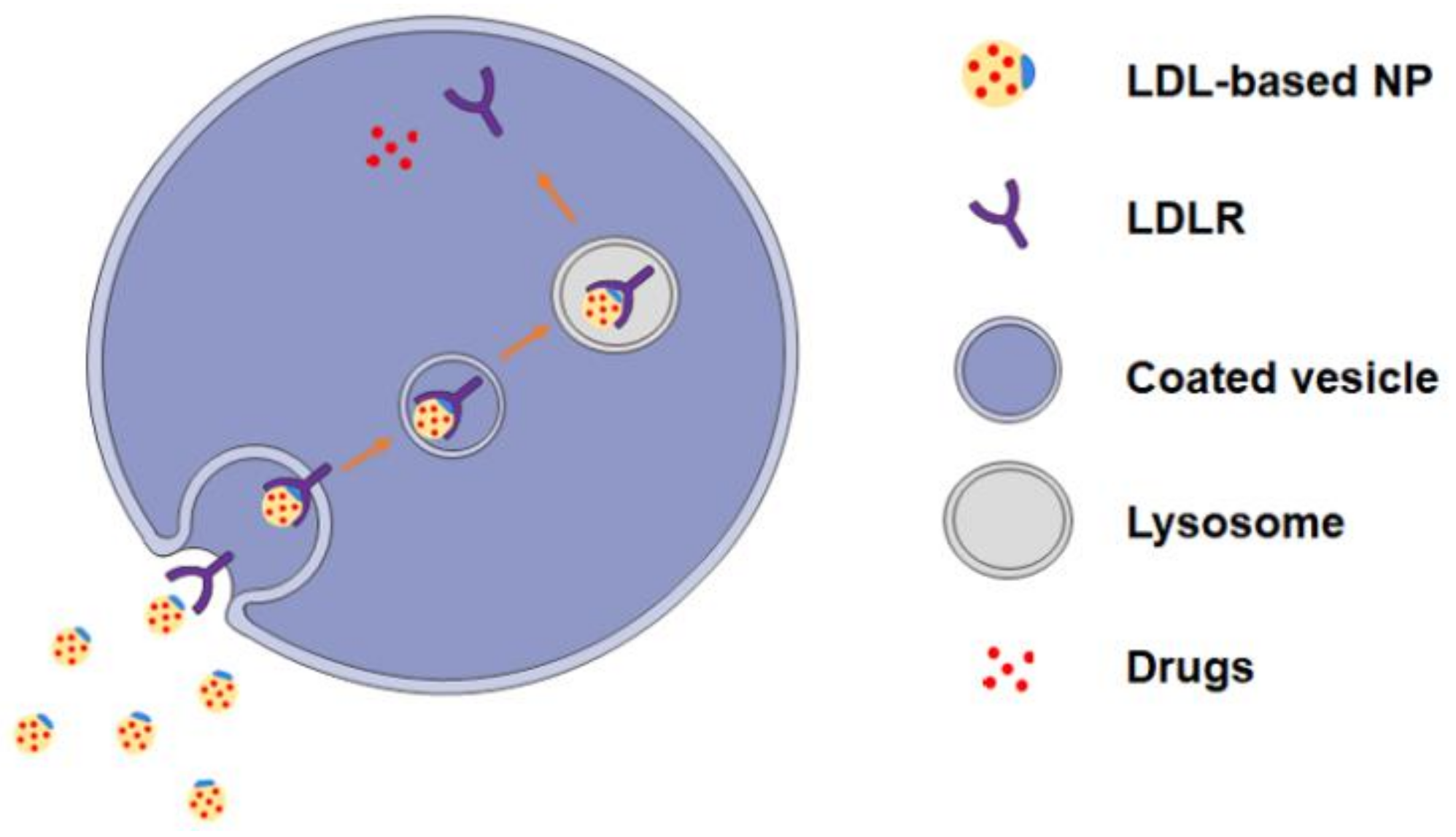
2. LDL and LDL Receptors
3. Categories of LDL-Based NPs
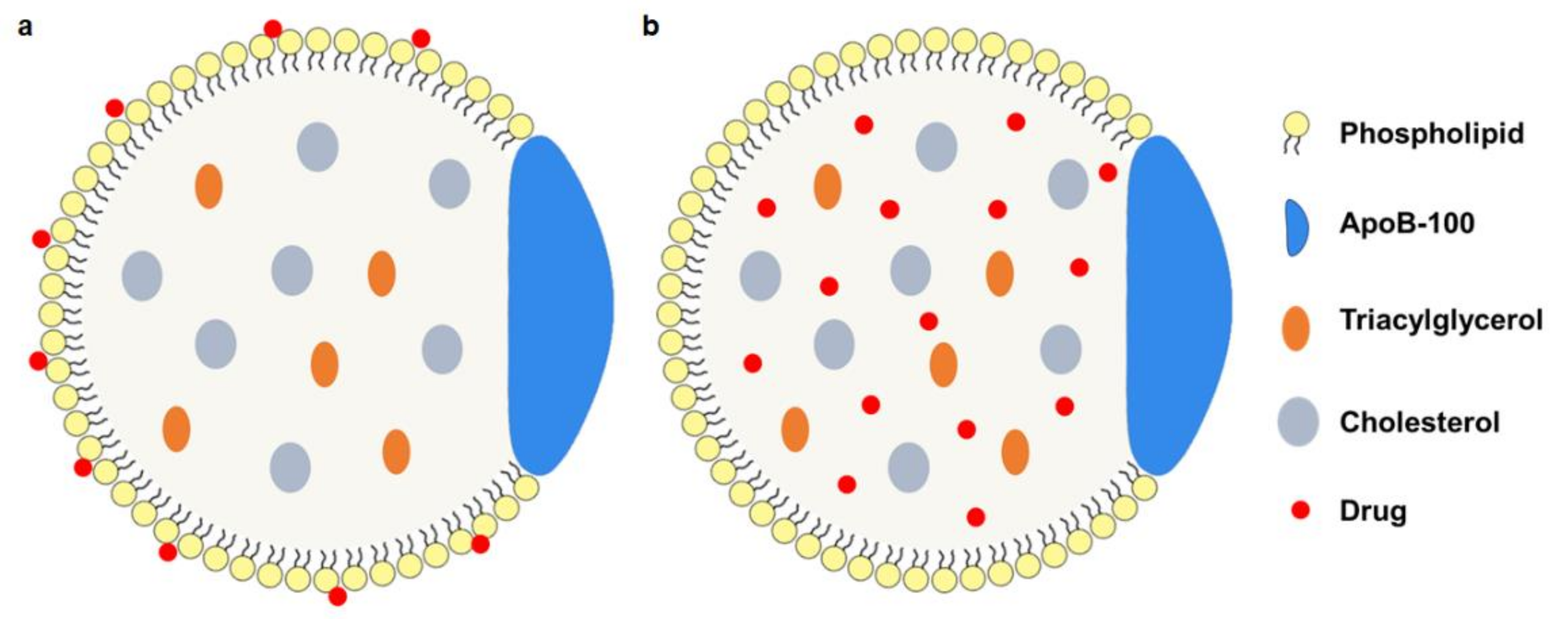
3.1. Native LDL-Drug Particles (nLDL-Drugs)
3.2. Synthesized LDL-Drug Particles (sLDL-Drugs)
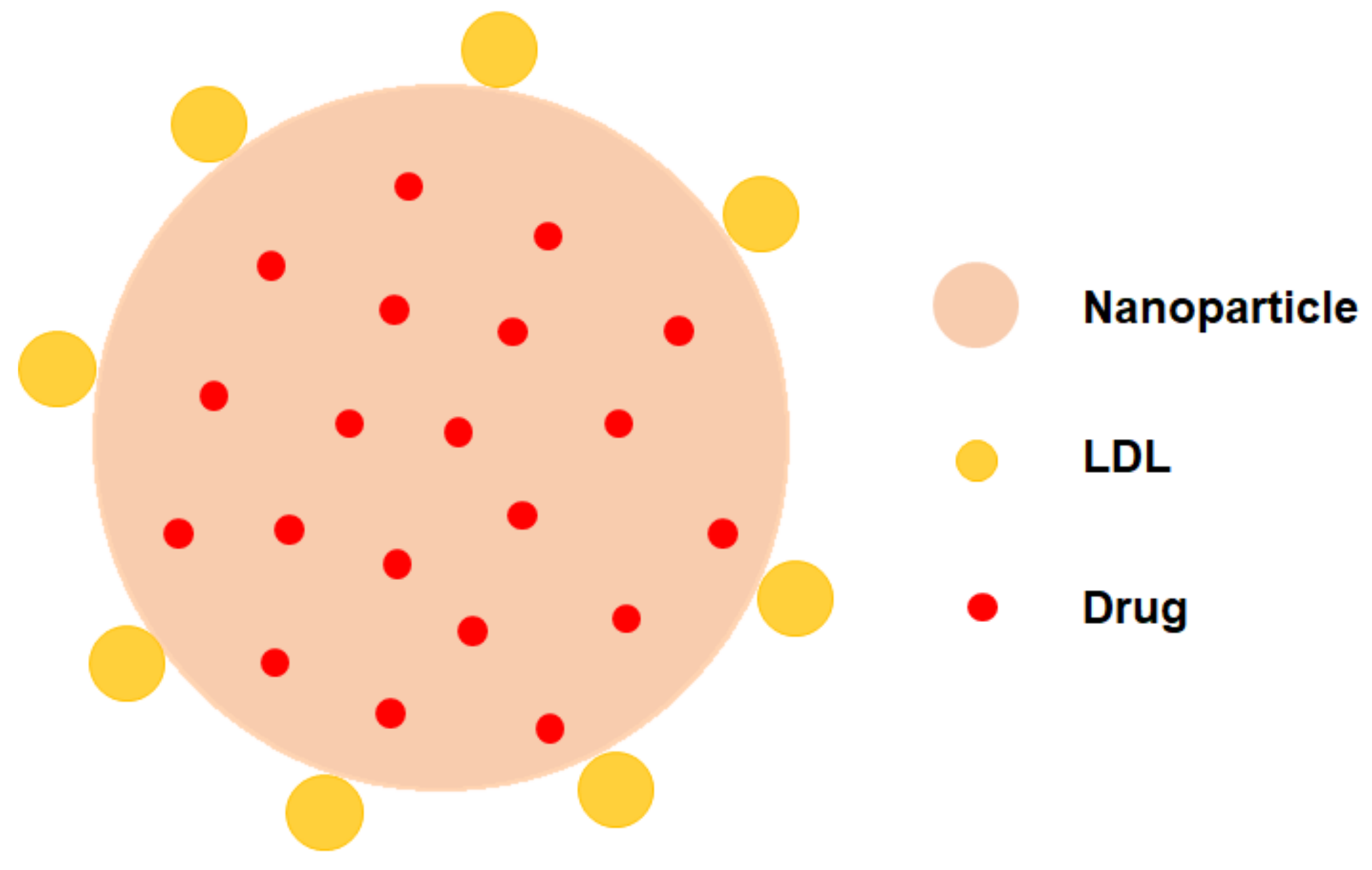
3.3. LDL Decorated LDLR Targeting Nanoparticles NPs (LDL-NPs)
4. Application of LDL-Based NPs in Cancer Therapy
4.1. nLDL-Drugs
| Category | Drugs | Indications | Reference |
|---|---|---|---|
| DNR-LDL | Doxorubicin | Leukemia cells | [26] |
| m-LDL | Cytotoxic compound 25 | Lung fibroblasts | [41] |
| OL-NME-LDL | Elliptinium-oleate | B16 melanoma | [37] |
| Paclitaxel-LDL | Paclitaxel | Leukemia cells | [26] |
| LDL-DOX | Doxorubicin | HepG2 cells | [42] |
| LDL-DOX | Doxorubicin | R-HepG2 cells | [38] |
| r-Pc-LDL-FA | Tetra-t-butyl-silicon phthalocyanine | KB cells, HT-1080 cells, and HepG2 cells | [30] |
| Hyp-LDL | Hypericin | U87-MG cells | [43] |
| LDL-DHA | Docosahexaenoic acid | Hepatoma cells (H4IIE) | [15] |
| LDL-DNA | Docosahexaenoic acid | Fibroblasts | [44] |
| CaP@LDL | STAT3-decoyodns | HepG2 and PLC/PRF/5 cells | [45] |
| DOX-LDL | Doxorubicin | A549 cells | [18] |
| LDL-DHA | Docosahexaenoic acid | HuH-7 and HepG2 cells | [39] |
| LDL-DHA | Docosahexaenoic acid | TIB-75 cells | [8] |
4.2. sLDL-Drugs
| Category | Drugs | Indications | Reference |
|---|---|---|---|
| nLDL-PO | Paclitaxel oleate | GBM cells | [17] |
| siRNA-PEG/SLN | siRNA | PC3 cells | [49] |
| Targeted PtSLNs | Paclitaxel | NCI-H1975, NCI-H1650, NCI-H520, PC9 | [47] |
| FA-mpLNPs | Iron oxide nanocrystals | MCF-7 | [25] |
| PALA-sLDL | Paclitaxel-alpha linolenic acid | U87 MG, HepG2 | [48] |
| FPLM NPs | Paclitaxel | HeLa, A549 | [34] |
| AODN | Pro-doxorubicin | 4T1 | [35] |
| Lf-mNLC | Curcumin | BCECs | [50] |
4.3. LDL-NPs
| Category | Drugs | Indications | Reference |
|---|---|---|---|
| Ost/LDL-NSC-NPs | Osthole | HepG2 cells | [36] |
| Dox-siRNA/LDL-SCS-NPs | Dox siRNA | HepG2, H22 | [51] |
| PTX-siRNA/LDL-NSC-LA micelles | MDR1 siRNA and paclitaxel | MCF-7 cells | [54] |
| LDL/SLN/DTX/TDD | Docetaxel (DTX), Thalidomide (TDD) | HepG2 cells | [52] |
| LDL/SLN/Adr | Adriamycin | Colorectal cancer | [57] |
| LDL-SLN/Sor/Dox | Sorafenib, Doxorubicin | HepG2 cells | [53] |
| LD-SDN | Sorafenib, Dihydroartemisinin | HepG2 cells | [24] |
| LDL/SLN/ICG | Sorafenib, Dihydroartemisinin | MCF-7 cells | [56] |
5. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA. Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Sparano, J.A.; Gray, R.J.; Makower, D.F.; Albain, K.S.; Saphner, T.J.; Badve, S.S.; Wagner, L.I.; Kaklamani, V.G.; Keane, M.M.; Gomez, H.L.; et al. Clinical Outcomes in Early Breast Cancer With a High 21-Gene Recurrence Score of 26 to 100 Assigned to Adjuvant Chemotherapy Plus Endocrine Therapy: A Secondary Analysis of the TAILORx Randomized Clinical Trial. JAMA Oncol. 2020, 6, 367–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moy, B.; Rumble, R.B.; Come, S.E.; Davidson, N.E.; Di Leo, A.; Gralow, J.R.; Hortobagyi, G.N.; Yee, D.; Smith, I.E.; Chavez-MacGregor, M.; et al. Chemotherapy and Targeted Therapy for Patients With Human Epidermal Growth Factor Receptor 2-Negative Metastatic Breast Cancer That Is Either Endocrine-Pretreated or Hormone Receptor-Negative: ASCO Guideline Update. J. Clin. Oncol. 2021, 39, 3938–3958. [Google Scholar] [CrossRef]
- Lu, S.; Wu, L.; Jian, H.; Chen, Y.; Wang, Q.; Fang, J.; Wang, Z.; Hu, Y.; Sun, M.; Han, L.; et al. Sintilimab plus Bevacizumab Biosimilar IBI305 and Chemotherapy for Patients with EGFR-Mutated Non-Squamous Non-Small-Cell Lung Cancer Who Progressed on EGFR Tyrosine-Kinase Inhibitor Therapy (ORIENT-31): First Interim Results from a Randomised, Double-Blind, Multicentre, Phase 3 Trial. Lancet. Oncol. 2022, 23, 1167–1179. [Google Scholar] [PubMed]
- Schwartzberg, L.S.; Modiano, M.R.; Rapoport, B.L.; Chasen, M.R.; Gridelli, C.; Urban, L.; Poma, A.; Arora, S.; Navari, R.M.; Schnadig, I.D. Safety and Efficacy of Rolapitant for Prevention of Chemotherapy-Induced Nausea and Vomiting after Administration of Moderately Emetogenic Chemotherapy or Anthracycline and Cyclophosphamide Regimens in Patients with Cancer: A Randomised, Active-Controlled, Double-Blind, Phase 3 Trial. Lancet. Oncol. 2015, 16, 1071–1078. [Google Scholar]
- An, X.; Zhu, A.; Luo, H.; Ke, H.; Chen, H.; Zhao, Y. Rational Design of Multi-Stimuli-Responsive Nanoparticles for Precise Cancer Therapy. ACS Nano 2016, 10, 5947–5958. [Google Scholar] [CrossRef]
- Aikins, M.E.; Xu, C.; Moon, J.J. Engineered Nanoparticles for Cancer Vaccination and Immunotherapy. Acc. Chem. Res. 2020, 53, 2094–2105. [Google Scholar] [CrossRef]
- Reynolds, L.; Mulik, R.S.; Wen, X.; Dilip, A.; Corbin, I.R. Low-Density Lipoprotein-Mediated Delivery of Docosahexaenoic Acid Selectively Kills Murine Liver Cancer Cells. Nanomedicine 2014, 9, 2123–2141. [Google Scholar] [CrossRef] [Green Version]
- Tabernero, J.; Shapiro, G.I.; LoRusso, P.M.; Cervantes, A.; Schwartz, G.K.; Weiss, G.J.; Paz-Ares, L.; Cho, D.C.; Infante, J.R.; Alsina, M.; et al. First-in-Humans Trial of an RNA Interference Therapeutic Targeting VEGF and KSP in Cancer Patients with Liver Involvement. Cancer Discov. 2013, 3, 406–417. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Wang, L.; Xu, H.Q.; Huang, X.E.; Qian, Y.D.; Xiang, J. Clinical Comparison between Paclitaxel Liposome (Lipusu®) and Paclitaxel for Treatment of Patients with Metastatic Gastric Cancer. Asian Pac. J. Cancer Prev. 2013, 14, 2591–2594. [Google Scholar] [CrossRef] [Green Version]
- Awada, A.; Garcia, A.A.; Chan, S.; Jerusalem, G.H.M.; Coleman, R.E.; Huizing, M.T.; Mehdi, A.; O’Reilly, S.M.; Hamm, J.T.; Barrett-Lee, P.J.; et al. Two Schedules of Etirinotecan Pegol (NKTR-102) in Patients with Previously Treated Metastatic Breast Cancer: A Randomised Phase 2 Study. Lancet. Oncol. 2013, 14, 1216–1225. [Google Scholar] [CrossRef] [PubMed]
- Wicki, A.; Witzigmann, D.; Balasubramanian, V.; Huwyler, J. Nanomedicine in Cancer Therapy: Challenges, Opportunities, and Clinical Applications. J. Control. Release 2015, 200, 138–157. [Google Scholar] [CrossRef] [PubMed]
- McConathy, W.J.; Paranjape, S.; Mooberry, L.; Buttreddy, S.; Nair, M.; Lacko, A.G. Validation of the Reconstituted High-Density Lipoprotein (RHDL) Drug Delivery Platform Using Dilauryl Fluorescein (DLF). Drug Deliv. Transl. Res. 2011, 1, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Taskinen, M.R. LDL-Cholesterol, HDL-Cholesterol or Triglycerides - Which Is the Culprit? Diabetes Res. Clin. Pract. 2003, 61, S19–S26. [Google Scholar] [CrossRef]
- Wen, X.; Reynolds, L.; Mulik, R.S.; Kim, S.Y.; Van Treuren, T.; Nguyen, L.H.; Zhu, H.; Corbin, I.R. Hepatic Arterial Infusion of Low-Density Lipoprotein Docosahexaenoic Acid Nanoparticles Selectively Disrupts Redox Balance in Hepatoma Cells and Reduces Growth of Orthotopic Liver Tumors in Rats. Gastroenterology 2016, 150, 488. [Google Scholar] [CrossRef] [Green Version]
- Nikanjam, M.; Blakely, E.A.; Bjornstad, K.A.; Shu, X.; Budinger, T.F.; Forte, T.M. Synthetic Nano-Low Density Lipoprotein as Targeted Drug Delivery Vehicle for Glioblastoma Multiforme. Int. J. Pharm. 2007, 328, 86–94. [Google Scholar] [CrossRef] [Green Version]
- Nikanjam, M.; Gibbs, A.R.; Hunt, C.A.; Budinger, T.F.; Forte, T.M. Synthetic Nano-LDL with Paclitaxel Oleate as a Targeted Drug Delivery Vehicle for Glioblastoma Multiforme. J. Control. Release 2007, 124, 163–171. [Google Scholar] [CrossRef]
- Zhu, C.; Pradhan, P.; Huo, D.; Xue, J.; Shen, S.; Roy, K.; Xia, Y. Reconstitution of Low-Density Lipoproteins with Fatty Acids for the Targeted Delivery of Drugs into Cancer Cells. Angew. Chem. Int. Ed. Engl. 2017, 56, 10399–10402. [Google Scholar] [CrossRef]
- Young, S.G. Recent Progress in Understanding Apolipoprotein B. Circulation 1990, 82, 1574–1594. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Sun, T.; Jiang, C. Biomacromolecules as Carriers in Drug Delivery and Tissue Engineering. Acta Pharm. Sin. B 2018, 8, 34–50. [Google Scholar] [CrossRef]
- Damiano, M.G.; Mutharasan, R.K.; Tripathy, S.; McMahon, K.M.; Thaxton, C.S. Templated High Density Lipoprotein Nanoparticles as Potential Therapies and for Molecular Delivery. Adv. Drug Deliv. Rev. 2013, 65, 649–662. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.; Blacklow, S.C. Structure and Physiologic Function of the Low-Density Lipoprotein Receptor. Annu. Rev. Biochem. 2005, 74, 535–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Zhang, J.; Wu, H.; Li, L.; Yang, C.; Song, S.; Peng, P.; Shao, M.; Zhang, M.; Zhao, J.; et al. Lectin-like Oxidized Low-Density Lipoprotein Receptor-1 Facilitates Metastasis of Gastric Cancer through Driving Epithelial-Mesenchymal Transition and PI3K/Akt/GSK3β Activation. Sci. Rep. 2017, 7, 45275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Duan, X.; Lv, Y.; Zhao, Y. Low Density Lipoprotein Receptor (LDLR)-Targeted Lipid Nanoparticles for the Delivery of Sorafenib and Dihydroartemisinin in Liver Cancers. Life Sci. 2019, 239, 117013. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Kim, J.H.; Bae, K.H.; Oh, M.H.; Kim, Y.; Kim, J.S.; Park, T.G.; Park, K.; Lee, J.H.; Nam, Y.S. Low-Density Lipoprotein-Mimicking Nanoparticles for Tumor-Targeted Theranostic Applications. Small 2015, 11, 222–231. [Google Scholar] [CrossRef]
- Masquelier, M.; Vitols, S.; Palsson, M.; Mars, U.; Larsson, B.S.; Peterson, C.O. Low density lipoprotein as a carrier of cytostatics in cancer chemotherapy: Study of stability of drug-carrier complexes in blood. J. Drug Target. 2000, 8, 155–164. [Google Scholar] [CrossRef]
- Caruso, M.G.; Notarnicola, M.; Cavallini, A.; Guerra, V.; Misciagna, G.; Di Leo, A. Demonstration of Low Density Lipoprotein Receptor in Human Colonic Carcinoma and Surrounding Mucosa by Immunoenzymatic Assay. Ital. J. Gastroenterol. 1993, 25, 361–367. [Google Scholar]
- Krieger, M.; Goldstein, J.L.; Brown, M.S. Receptor-Mediated Uptake of Low Density Lipoprotein Reconstituted with 25-Hydroxycholesteryl Oleate Suppresses 3-Hydroxy-3-Methylglutaryl-Coenzyme A Reductase and Inhibits Growth of Human Fibroblasts. Proc. Natl. Acad. Sci. USA 1978, 75, 5052–5056. [Google Scholar] [CrossRef] [Green Version]
- Chu, H.L.; Cheng, T.M.; Chen, H.W.; Chou, F.H.; Chang, Y.C.; Lin, H.Y.; Liu, S.Y.; Liang, Y.C.; Hsu, M.H.; Wu, D.S.; et al. Synthesis of Apolipoprotein B Lipoparticles to Deliver Hydrophobic/Amphiphilic Materials. ACS Appl. Mater. Interfaces 2013, 5, 7509–7516. [Google Scholar] [CrossRef]
- Zheng, G.; Chen, J.; Li, H.; Glickson, J.D. Rerouting Lipoprotein Nanoparticles to Selected Alternate Receptors for the Targeted Delivery of Cancer Diagnostic and Therapeutic Agents. Proc. Natl. Acad. Sci. USA 2005, 102, 17757–17762. [Google Scholar] [CrossRef] [Green Version]
- Bergt, C.; Fu, X.; Huq, N.P.; Kao, J.; Heinecke, J.W. Lysine Residues Direct the Chlorination of Tyrosines in YXXK Motifs of Apolipoprotein A-I When Hypochlorous Acid Oxidizes High Density Lipoprotein. J. Biol. Chem. 2004, 279, 7856–7866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Versluis, A.J.; Van Geel, P.J.; Oppelaar, H.; Van Berkel, T.J.C.; Bijsterbosch, M.K. Receptor-Mediated Uptake of Low-Density Lipoprotein by B16 Melanoma Cells in Vitro and in Vivo in Mice. Br. J. Cancer 1996, 74, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Lovell, J.F.; Chen, J.; Ng, K.; Cao, W.; Ding, L.; Zhang, Z.; Zheng, G. Cytosolic Delivery of LDL Nanoparticle Cargo Using Photochemical Internalization. Photochem. Photobiol. Sci. 2011, 10, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Xu, N.; Zhou, X.; Shi, K.; Du, Q.; Yin, X.; Zhao, Z. Low Density Lipoprotein Mimic Nanoparticles Composed of Amphipathic Hybrid Peptides and Lipids for Tumor-Targeted Delivery of Paclitaxel. Int. J. Nanomedicine 2019, 14, 7431–7446. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Fu, J.; Ding, Y.; Liu, D.; Jia, N.; Chen, D.; Hu, H. Low Density Lipoprotein-Inspired Nanostructured Lipid Nanoparticles Containing pro-Doxorubicin to Enhance Tumor-Targeted Therapeutic Efficiency. Acta Biomater. 2019, 96, 456–467. [Google Scholar] [CrossRef]
- Zhang, C.G.; Zhu, Q.L.; Zhou, Y.; Liu, Y.; Chen, W.L.; Yuan, Z.Q.; Yang, S.D.; Zhou, X.F.; Zhu, A.J.; Zhang, X.N.; et al. N-Succinyl-Chitosan Nanoparticles Coupled with Low-Density Lipoprotein for Targeted Osthole-Loaded Delivery to Low-Density Lipoprotein Receptor-Rich Tumors. Int. J. Nanomedicine 2014, 9, 2919–2932. [Google Scholar] [CrossRef] [Green Version]
- Samadi-Baboli, M.; Favre, G.; Canal, P.; Soula, G. Low Density Lipoprotein for Cytotoxic Drug Targeting: Improved Activity of Elliptinium Derivative against B16 Melanoma in Mice. Br. J. Cancer 1993, 68, 319–326. [Google Scholar] [CrossRef] [Green Version]
- Lo, E.H.K.; Ooi, V.E.L.; Fung, K.P. Circumvention of Multidrug Resistance and Reduction of Cardiotoxicity of Doxorubicin in Vivo by Coupling It with Low Density Lipoprotein. Life Sci. 2002, 72, 677–687. [Google Scholar] [CrossRef]
- Yang, J.; Gong, Y.; Sontag, D.P.; Corbin, I.; Minuk, G.Y. Effects of Low-Density Lipoprotein Docosahexaenoic Acid Nanoparticles on Cancer Stem Cells Isolated from Human Hepatoma Cell Lines. Mol. Biol. Rep. 2018, 45, 1023–1036. [Google Scholar] [CrossRef]
- Mulik, R.S.; Bing, C.; Ladouceur-Wodzak, M.; Munaweera, I.; Chopra, R.; Corbin, I.R. Localized Delivery of Low-Density Lipoprotein Docosahexaenoic Acid Nanoparticles to the Rat Brain Using Focused Ultrasound. Biomaterials 2016, 83, 257–268. [Google Scholar] [CrossRef] [Green Version]
- Lundberg, B. Preparation of Drug-Low Density Lipoprotein Complexes for Delivery of Antitumoral Drugs via the Low Density Lipoprotein Pathway. Cancer Res. 1987, 47, 4105–4108. [Google Scholar] [PubMed]
- Chu, A.C.Y.; Tsang, S.Y.; Lo, E.H.K.; Fung, K.P. Low Density Lipoprotein as a Targeted Carrier for Doxorubicin in Nude Mice Bearing Human Hepatoma HepG2 Cells. Life Sci. 2001, 70, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Huntosova, V.; Buzova, D.; Petrovajova, D.; Kasak, P.; Nadova, Z.; Jancura, D.; Sureau, F.; Miskovsky, P. Development of a New LDL-Based Transport System for Hydrophobic/Amphiphilic Drug Delivery to Cancer Cells. Int. J. Pharm. 2012, 436, 463–471. [Google Scholar] [CrossRef]
- Khan, Z.; Hawtrey, A.O.; Ariatti, M. New Cationized LDL-DNA Complexes: Their Targeted Delivery to Fibroblasts in Culture. Drug Deliv. J. Deliv. Target. Ther. Agents 2003, 10, 213–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, K.; Xue, J.; Fang, Y.; Bi, H.; Gao, S.; Yang, D.; Lu, A.; Li, Y.; Chen, Y.; Ke, L. Inorganic Kernel-Reconstituted Lipoprotein Biomimetic Nanovehicles Enable Efficient Targeting “Trojan Horse” Delivery of STAT3-Decoy Oligonucleotide for Overcoming TRAIL Resistance. Theranostics 2017, 7, 4480–4497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baillie, G.; Owens, M.D.; Halbert, G.W. A Synthetic Low Density Lipoprotein Particle Capable of Supporting U937 Proliferation in Vitro. J. Lipid Res. 2002, 43, 69–73. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, Y.; Bae, K.H.; Park, T.G.; Lee, J.H.; Park, K. Tumor-Targeted Delivery of Paclitaxel Using Low Density Lipoprotein-Mimetic Solid Lipid Nanoparticles. Mol. Pharm. 2015, 12, 1230–1241. [Google Scholar] [CrossRef]
- Su, H.T.; Li, X.; Liang, D.S.; Qi, X.R. Synthetic Low-Density Lipoprotein (SLDL) Selectively Delivers Paclitaxel to Tumor with Low Systemic Toxicity. Oncotarget 2016, 7, 51535. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.R.; Kim, I.K.; Bae, K.H.; Lee, S.H.; Lee, Y.; Park, T.G. Cationic Solid Lipid Nanoparticles Reconstituted from Low Density Lipoprotein Components for Delivery of SiRNA. Mol. Pharm. 2008, 5, 622–631. [Google Scholar] [CrossRef]
- Meng, F.; Asghar, S.; Gao, S.; Su, Z.; Song, J.; Huo, M.; Meng, W.; Ping, Q.; Xiao, Y. A Novel LDL-Mimic Nanocarrier for the Targeted Delivery of Curcumin into the Brain to Treat Alzheimer’s Disease. Colloids Surf. B Biointerfaces 2015, 134, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.L.; Zhou, Y.; Guan, M.; Zhou, X.F.; Yang, S.D.; Liu, Y.; Chen, W.L.; Zhang, C.G.; Yuan, Z.Q.; Liu, C.; et al. Low-Density Lipoprotein-Coupled N-Succinyl Chitosan Nanoparticles Co-Delivering SiRNA and Doxorubicin for Hepatocyte-Targeted Therapy. Biomaterials 2014, 35, 5965–5976. [Google Scholar] [CrossRef] [PubMed]
- Ao, M.; Xiao, X.; Ao, Y. Low density lipoprotein modified silica nanoparticles loaded with docetaxel and thalidomide for effective chemotherapy of liver cancer. Braz. J. Med. Biol. Res. Rev. Bras. Pesqui. Med. E Biol. 2018, 51, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Zhang, R.; Chai, W.; Du, X. Low-Density Lipoprotein Decorated Silica Nanoparticles Co-Delivering Sorafenib and Doxorubicin for Effective Treatment of Hepatocellular Carcinoma. Drug Deliv. 2018, 25, 2016–2023. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.D.; Zhu, W.J.; Zhu, Q.L.; Chen, W.L.; Ren, Z.X.; Li, F.; Yuan, Z.Q.; Li, J.Z.; Liu, Y.; Zhou, X.F.; et al. Binary-Copolymer System Base on Low-Density Lipoprotein-Coupled N-Succinyl Chitosan Lipoic Acid Micelles for Co-Delivery MDR1 SiRNA and Paclitaxel, Enhances Antitumor Effects via Reducing Drug. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 1114–1125. [Google Scholar] [CrossRef]
- Zhu, W.J.; Yang, S.D.; Qu, C.X.; Zhu, Q.L.; Chen, W.L.; Li, F.; Yuan, Z.Q.; Liu, Y.; You, B.G.; Zhang, X.N. Low-Density Lipoprotein-Coupled Micelles with Reduction and PH Dual Sensitivity for Intelligent Co-Delivery of Paclitaxel and SiRNA to Breast Tumor. Int. J. Nanomedicine 2017, 12, 3375–3393. [Google Scholar] [CrossRef] [Green Version]
- Pan, H.; Sun, Y.; Cao, D.; Wang, L. Low-Density Lipoprotein Decorated and Indocyanine Green Loaded Silica Nanoparticles for Tumor-Targeted Photothermal Therapy of Breast Cancer. Pharm. Dev. Technol. 2020, 25, 308–315. [Google Scholar] [CrossRef]
- Shi, G.; Li, J.; Yan, X.; Jin, K.; Li, W.; Liu, X.; Zhao, J.; Shang, W.; Zhang, R. Low-Density Lipoprotein-Decorated and Adriamycin-Loaded Silica Nanoparticles for Tumor-Targeted Chemotherapy of Colorectal Cancer. Adv. Clin. Exp. Med. 2019, 28, 479–487. [Google Scholar] [CrossRef]
- Liu, M.; Li, W.; Larregieu, C.A.; Cheng, M.; Yan, B.; Chu, T.; Li, H.; Mao, S.J. Development of Synthetic Peptide-Modified Liposomes with LDL Receptor Targeting Capacity and Improved Anticancer Activity. Mol. Pharm. 2014, 11, 2305–2312. [Google Scholar] [CrossRef]
- Vitols, S.; Gahrton, G.; Ost, A.; Peterson, C. Elevated Low Density Lipoprotein Receptor Activity in Leukemic Cells With Monocytic Differentiation. Blood 1984, 63, 1186–1193. [Google Scholar] [CrossRef] [Green Version]
- Zhou, P.; Hatziieremia, S.; Elliott, M.A.; Scobie, L.; Crossan, C.; Michie, A.M.; Holyoake, T.L.; Halbert, G.W.; Jørgensen, H.G. Uptake of Synthetic Low Density Lipoprotein by Leukemic Stem Cells—A Potential Stem Cell Targeted Drug Delivery Strategy. J. Control. Release 2010, 148, 380–387. [Google Scholar] [CrossRef] [PubMed]


| Strategy | Advantage | Disadvantages |
|---|---|---|
| Phospholipid monolayer loading |
|
|
| Apolipoprotein loading |
|
|
| Core loading |
|
|
| Strategy | Advantages | Disadvantages |
|---|---|---|
| nLDL-drugs |
| |
| sLDL-drugs |
| |
| LDL-NPs |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, B.; Yang, Q. Recent Development of LDL-Based Nanoparticles for Cancer Therapy. Pharmaceuticals 2023, 16, 18. https://doi.org/10.3390/ph16010018
He B, Yang Q. Recent Development of LDL-Based Nanoparticles for Cancer Therapy. Pharmaceuticals. 2023; 16(1):18. https://doi.org/10.3390/ph16010018
Chicago/Turabian StyleHe, Binghong, and Qiong Yang. 2023. "Recent Development of LDL-Based Nanoparticles for Cancer Therapy" Pharmaceuticals 16, no. 1: 18. https://doi.org/10.3390/ph16010018
APA StyleHe, B., & Yang, Q. (2023). Recent Development of LDL-Based Nanoparticles for Cancer Therapy. Pharmaceuticals, 16(1), 18. https://doi.org/10.3390/ph16010018





