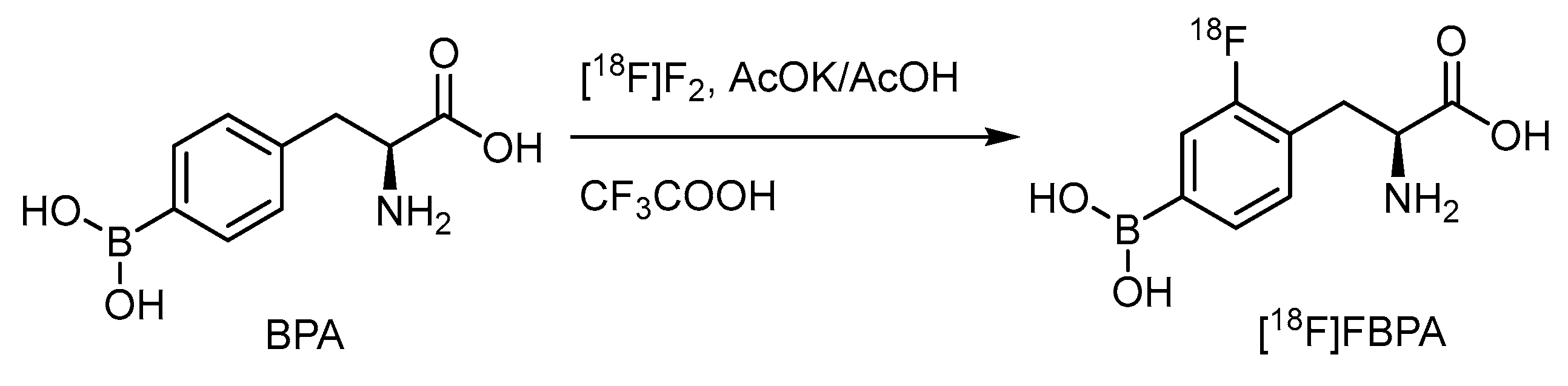Abstract
Boron neutron capture therapy (BNCT) is a binary therapeutic technique employing a boron agent to be delivered to the tumor site followed by the irradiation of neutrons. Biofunctional molecules/nanoparticles labeled with F-18 can provide an initial pharmacokinetic profile of patients to guide the subsequent treatment planning procedure of BNCT. Borono phenylalanine (BPA), recognized by the l-type amino acid transporter, can cross the blood-brain barrier and be accumulated in gliomas. The radiofluoro BNCT agents are reviewed by considering (1) less cytotoxicity, (2) diagnosing and therapeutic purposes, (3) aqueous solubility and extraction route, as well as (4), the trifluoroborate effect. A trifluoroborate-containing amino acid such as fluoroboronotyrosine (FBY) represents an example with both functionalities of imaging and therapeutics. Comparing with the insignificant cytotoxicity of clinical BPA with IC50 > 500 μM, FBY also shows minute toxicity with IC50 > 500 μM. [18F]FBY is a potential diagnostic agent for its tumor to normal accumulation (T/N) ratio, which ranges from 2.3 to 24.5 from positron emission tomography, whereas the T/N ratio of FBPA is greater than 2.5. Additionally, in serving as a BNCT therapeutic agent, the boron concentration of FBY accumulated in gliomas remains uncertain. The solubility of 3-BPA is better than that of BPA, as evidenced by the cerebral dose of 3.4%ID/g vs. 2.2%ID/g, respectively. While the extraction route of d-BPA differs from that of BPA, an impressive T/N ratio of 6.9 vs. 1.5 is noted. [18F]FBPA, the most common clinical boron agent, facilitates the application of BPA in clinical BNCT. In addition to [18F]FBY, [18F] trifluoroborated nucleoside analog obtained through 1,3-dipolar cycloaddition shows marked tumoral uptake of 1.5%ID/g. Other examples using electrophilic and nucleophilic fluorination on the boron compounds are also reviewed, including diboronopinacolone phenylalanine and nonsteroidal anti-inflammatory agents.
1. Introduction
Fluorine-containing small molecule pharmaceuticals have impacted the drug market in the past decades [1,2]. In 2019, 13 new fluoro-pharmaceuticals were approved by the FDA, accounting for 41% of all small-molecule drugs [3]. In view of its high electronegativity as the second-smallest atom after hydrogen, its ability to form hydrogen bonds, the effect on bond strength, and its role as a conformational moderator, fluorine introduction is an efficient approach in tuning the final drug candidates to facilitate the drug discovery process [4,5]. However, fluoro substituents can act as pharmacophore isosteres, such as CF3,to mimic the C=O oxygen group [4]. On the other hand, fluorine compounds can act as diagnostic agents for positron emission tomography (PET) applications [6,7,8]. Its unstable isotope, the radioactive 18F atom, serves as the tool to extract the pharmacokinetic information. This is largely reliant on its unique internal calibrating ability, which is achieved through the emission of two 180° coherent annihilation radiations derived from each event of a radiated positron colliding with the neighboring electron of tissue. Thus, the unique physical feature enables its quantitative access to the distribution profile of the 18F-labeled compound in vivo, regardless of the tissue depth. The imaging data can be reformulated as an expression of counting data over volume, equivalent to a concentration level that can be compared with the initial injection concentration of drugs. The percentage of injection dose per weight of tissue (%ID/g) accumulated in organs of interest would provide straightforwardly the kinetics information of the drug. For example, as one of the most effective PET imaging agents for diagnosing cancers, [18F]FDG has established a gold standard for detecting a variety of tumors, such as lung cancer, head and neck cancer, brain cancer, and pancreatic cancer [9,10].
Additionally, a number of fluoropharmaceuticals were radiolabeled with F-18 to evaluate their pharmacokinetic profiles, e.g., [18F]F-DOPA [11,12] and [18F]F-MISO [13,14]. Equally important is the evaluability of the pharmacokinetics of those potential compound candidates that were readily developed from bench synthesis. Hence, the tool provides a shortcut to evaluating the in vivo performance of a compound for preclinical application.
The two principal radiofluorination sources include nucleophilic fluoride [18F-] and radiofluorine gas [18F]F2 [15,16,17,18]. In view of its short half-life (t1/2 =110 min), the radiofluorination is better arranged at the last step in the whole synthesis. Whereas there are abundant papers reviewing the status of fluorine- and radiofluorine-containing pharmaceuticals, we are interested in presenting our aspect of their application in facilitating the development of borono pharmaceuticals for boron neutron capture therapy (BNCT). This review will be specifically focused on the BNCT agents against brain tumors.
BNCT is a binary therapy combining a nonradioactive 10B atom with a beam line consisting of neutrons [19,20,21,22,23]. As being nonradioactive such as 11B (80%), the 20% abundant 10B is bombarded by neutron beams that are speeded up to an energy level of epithermal range via an accelerator or a linear reactor to reach a 10-cm penetrable depth within tissues. With a significant large cross-section σ in 3840 barns, 10B can capture neutrons to split highly energetic alpha and Li ions via 10B (n, α)7Li. The energy damages hereditary substances such as DNA double strands, thereby resulting in cancerous lethality.
Furthermore, to deliver the potential boron bomb towards the tumor lesions, a targetable molecule/nanoparticle or a biologically recognizable molecule/nanoparticle needs to be introduced. It has been reported that a concentration of 10B at 2 mM (109 atoms/cell or 20–50 μg of 10B/g) is required to sustain a satisfactory BNCT [20,24]. Thus, determination of the concentration of 10B in vivo is a prerequisite to conducting a successful clinical BNCT. Not only the concentration level in the tumor lesions but also the cancerous selectivity of 10B needs to be taken into consideration. whereas a dividing line of 3:1 for the tumor to normal (T/N) accumulating ratio is suggested [25]. Patients with a T/N ratio as low as 2.1 are recruited, and tumor responses are obtained as expected, according to the Taiwanese clinical experience of head and neck cancers or meningiomas [26].
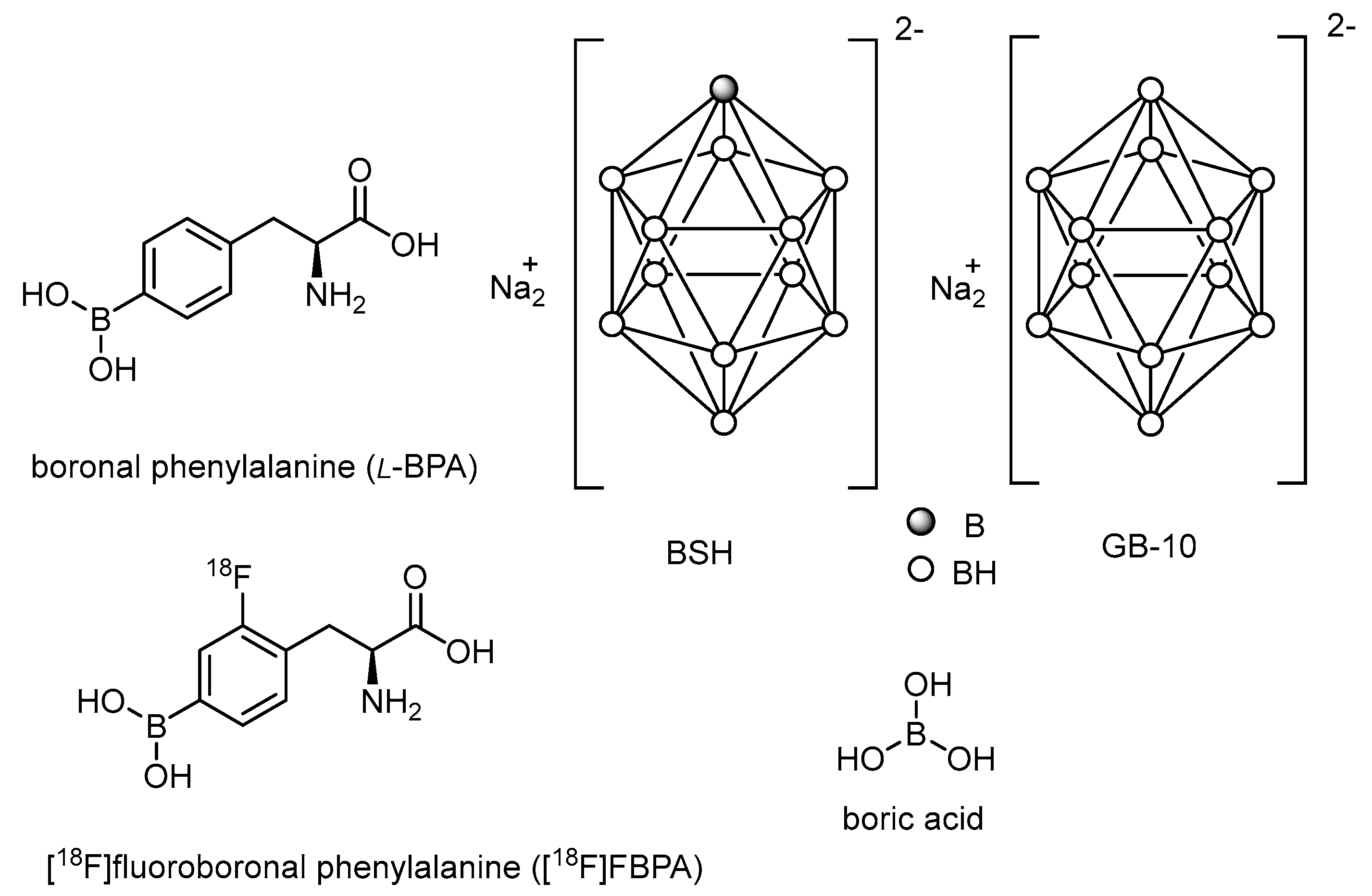
Of the boron-containing agents reported so far, boronophenylalanine (BPA) and borocaptate sodium (BSH) are the two most commonly used boron agents for clinical treatment (Figure 1). In addition, various boron agents have been developed in the past decades, including peptides [27,28], antibody-based delivery systems [29,30], boron compound conjugates [31,32], boron-containing nanoparticles [33,34,35,36,37,38,39,40], boric acid [41,42,43], and decahydrodecaborate (GB-10) [44,45].
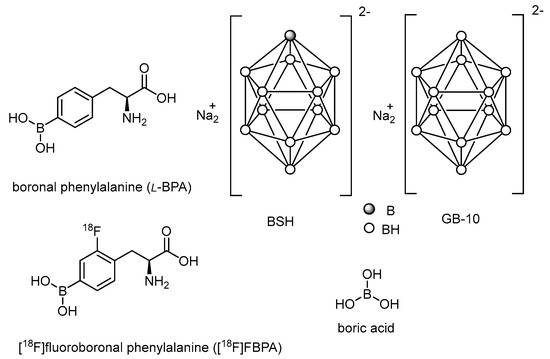
Figure 1.
Most recently developed boron carriers for BNCT.
2. Tyrosine Analogs as Boron Delivery Agents
The partially essential amino acid tyrosine crosses the blood-brain barrier through the l-type amino acid transporter [46,47]. Tumors, especially brain tumors such as gliomas, use tyrosine as one of the essential nutritional sources [47,48,49,50]. The typical nutritional source, such as glucose, is internalized through the glucose transporter in the brain [51]. [18F]FDG is such an example and is still the most successful agent for tumor imaging because of its extraordinary hunger for glucose [50]. This would explain why BPA, the structural isostere of tyrosine, has been so commonly applied in BNCT [52]. As BPA is the most clinically studied boron delivery vehicle, the boron concentration to be determined is mainly through noninvasive imaging techniques such as PET [53,54,55]. Among the most encountered cancer types, head and neck and brain tumors are difficult to resect for biopsy sampling, thus highlighting the precious value of [18F]FBPA [54,56].
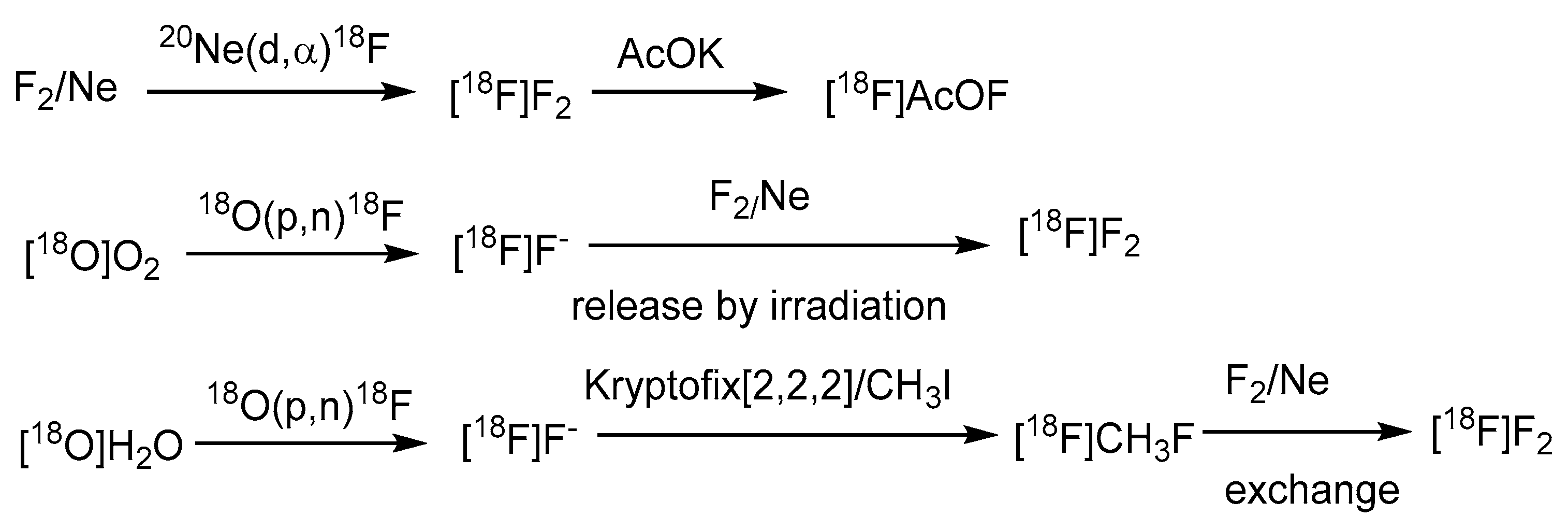
Electrophilic radiofluorination on the meta position of the borono acid substituted aromatic ring produces the radiofluorinated BPA, [18F]FBPA (Scheme 1) [57]. BPA is one of the substrates for the LAT-1-dependent transporter, indicating the main accumulation route in gliomas. Several targets to be bombarded by accelerated particles, including the gaseous Ne (d,α) or O2 (p,n) and the aqueous [18O]H2O (p,n), have produced radiofluorine gas (Scheme 2). Due to the adsorption on the targeting tube, the recovery of radiofluorine through exchange with F2 carrier gas reduces the specific activity of [18F]F2 [52]. Whereas as less as 1/100-1/1000 times specific activity than that of the conventional radiofluoride source (GBq/mmol vs. GBq/umol), fluorine gas-derived boron compounds through the carrier-added fluorine gas better mimic the concentration of ppm level of the therapeutic dosage for BNCT. Hence, [18F]FBPA obtained through electrophilic fluorination is quite suitable as a theranostic agent (Scheme 2).
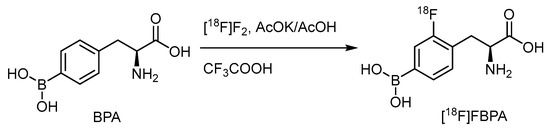
Scheme 1.
Electrophilic radiofluorination of BPA.
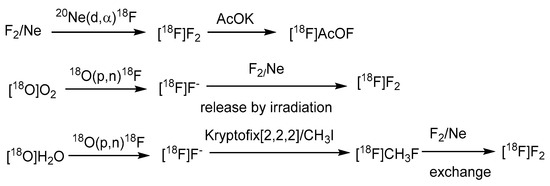
Scheme 2.
The common generation of radiofluorine source [18F]F2 for electrophilic fluorination [52]. In courtesy of the permission of copyright by publisher of Springer Nature.
The radiochemical yield of [18F]FBPA has been optimized by adjusting the reaction conditions, e.g., pre-irradiation before [18F]F2 production, concentration of the carrier F2 in the Ne target, an adequate ratio of BPA to F2, appropriate eluents for separation through high-performance liquid chromatography (HPLC), and enantiomeric purity [57].
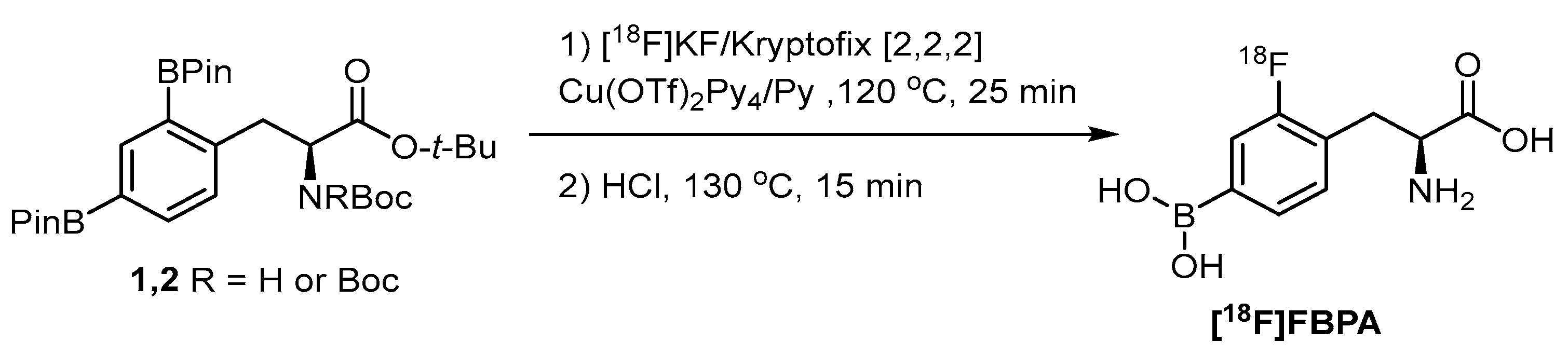
Although the nucleophilic fluorination is incapable of incorporating into a borono compound due to the undesired fluorodeboronation, selective fluorination of one of the diborono groups is a straightforward approach to preserve both the imaging radiofluoro and therapeutic borono groups (Scheme 3) [58]. The copper triflate-mediated fluorination cloned FBPA with high specific activity.
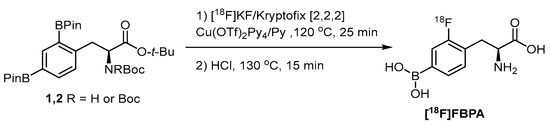
Scheme 3.
Monoradiofluorination of a diboronotyrosine compound to deliver [18F]FBPA [58]. In courtesy of the permission of copyright by the publisher of RSC.
Whereas the structural difference between BPA and [18F]FBPA is minute, variation may arise due to the different injection modes resulting in differential concentration levels [54,59]. For example, in contrast to the imaging dosage that requires only several submicromolarities of [18F]FBPA, therapeutic BNCT needs at least several hundred milligrams of BPA per kilogram of body weight [60,61]. The injection methods may also vary, i.e., bolus injection of [18F]FBPA for imaging purposes and intravenous continuous injection of BPA for BNCT. Additionally, reports have shown comparable results [54,60]. The tumoral heterogeneity and vascularity render the PET imaging inconsistent, thereby lowering the T/N ratio. According to Lo YW et al., T/N greater than 2 is accepted for clinical purposes [62]. Personalized PET imaging of [18F]FBPA to acquire a T/N value and the pharmacokinetics derived thereafter may contribute to a precise treatment planning procedure for BNCT [63].
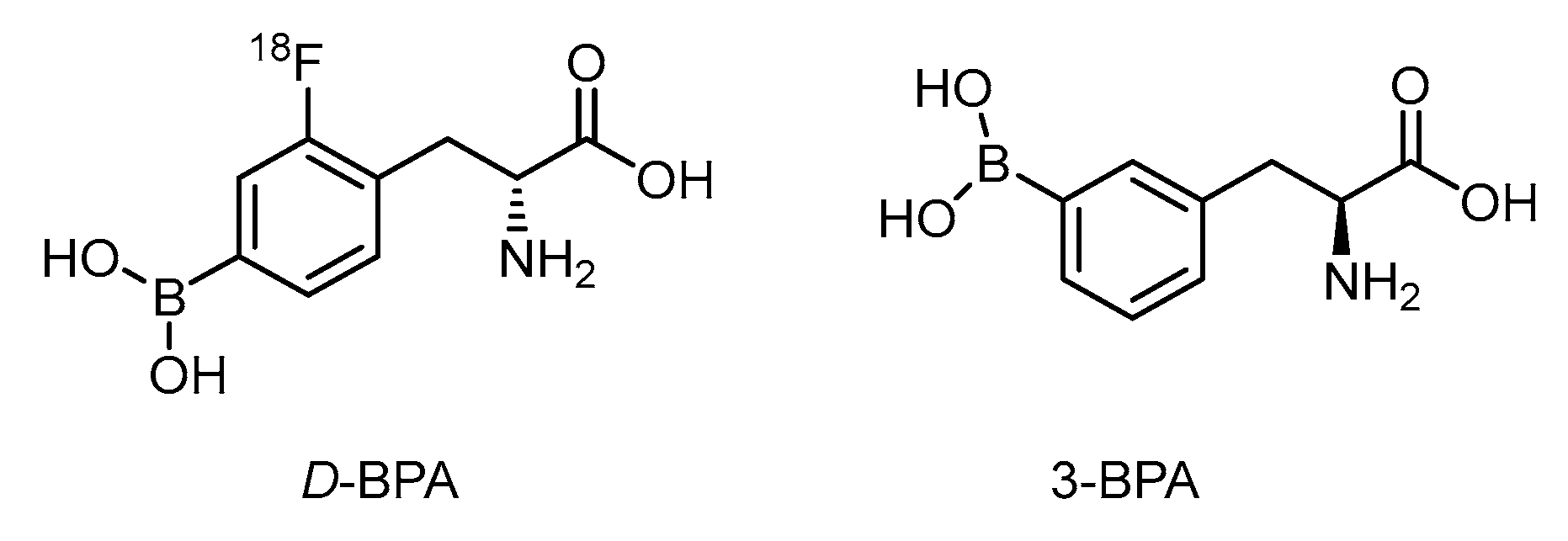
The d-isomer of FBPA is thought to be superior to the l-form on account of its high tumor-to-blood (T/B) ratio of 6.93 vs. 1.45 in the rat glioma model (Figure 2) [64]. The fast washout of d-form improves the contrast ratio. A distinct metabolism of d-FBPA features its major excretion route through the kidney compared with that via the liver for FBPA, however [65]. In spite of the above advantage taken by d-FBPA, data on boron concentration in the glioma lesion is not yet known.
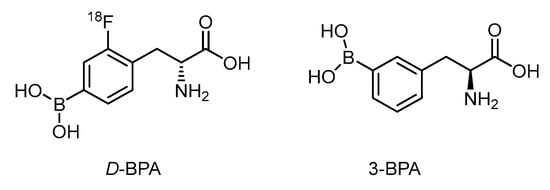
Figure 2.
Structurally related BPA analogs as potential BNCT agents.
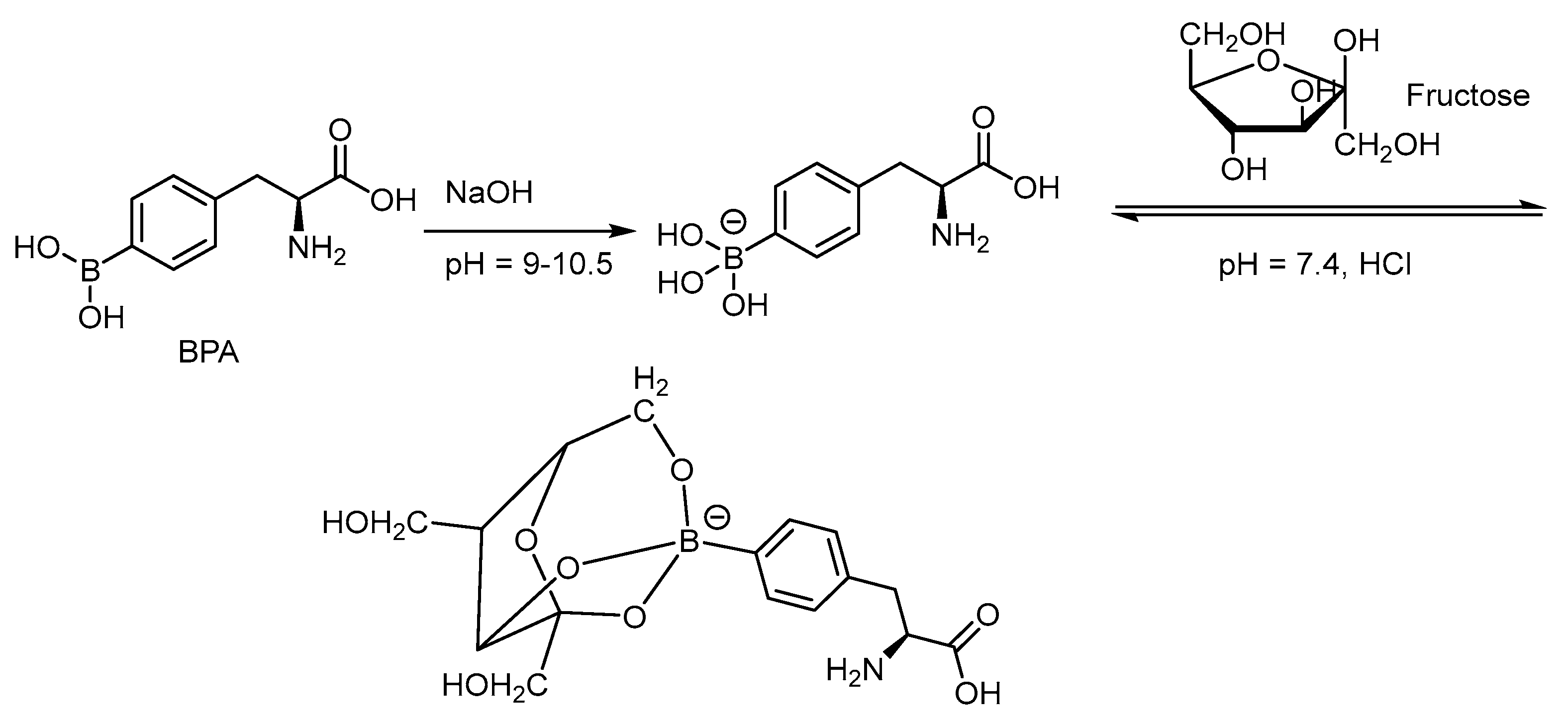
Due to the limited aqueous solubility of BPA, complexation with fructose through dehydration can shift the equilibrium to the more soluble intermediate (Scheme 4) [66]. The 3-borono isomer of BPA has shown to improve the solubility in aqueous solution 100 times better than that of the BPA-fructose complex through conformational assistance (Figure 2) [67]. 3-BPA also distributes better in the glioma of the mouse model than that of 4-BPA as reported from 11B ICP-MS analysis i.e., 3.4%ID/g vs. 2.2%ID/g at 2 h post injection. Until now, no F-18 tagged 3-BPA has been synthesized for PET analysis. The kinetics through PET imaging are useful to answer the metabolic route.
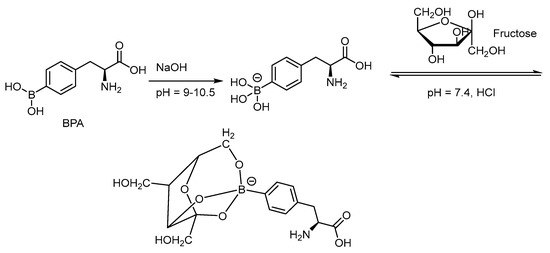
Scheme 4.
Formation of the aqueous solvable BPA-fructose complex through dehydration from the trihydroxyborate and hydroxyl groups of the furanose [66]. In courtesy of the permission of copyright by the publisher of Elsevier.
3. Other Non-Glioma-Directed Boron Agents for Radiofluorination
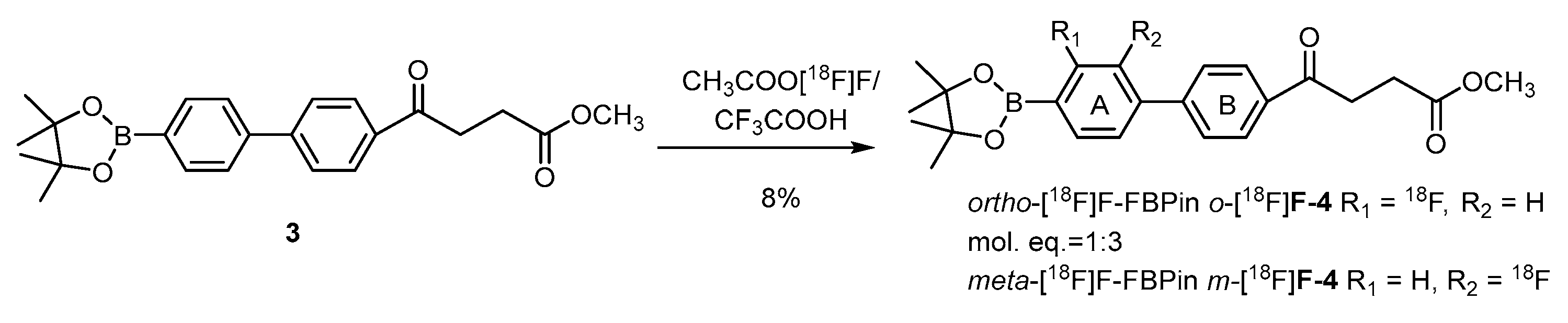
Direct fluorination of the borono compounds is also exemplified by boronofenbufen, a COX-2 inhibitor and a member of the class of nonsteroidal anti-inflammatory drugs (NSAIDs) (Scheme 5) [68]. Cholangiocarcinoma (CCA) that overexpresses COX-2 is a fatal liver cancer with a very low cure rate. CCA is difficult to diagnose due to the foci deep inside the liver lobes. Electrophilic fluorination of fenbufen delivers the meta-radiofluoro borono fenbufen (m-[18F]FFBPin) and the ortho isomer o-[18F]FFBPin in satisfactory radiochemical yields of 6% and 2%, respectively. The fair PET-derived T/N ratio of 1.5 renders it inappropriate as an imaging agent for future BNCT usage.
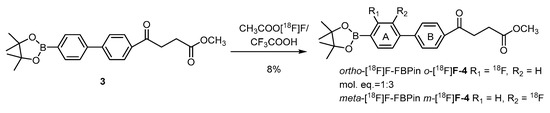
Scheme 5.
Electrophilic radiofluorination of a boronofenbufen for targeting COX-2 overexpressed cholangio carcinoma tumors [68]. In courtesy of the permission of copyright by the publisher of Elsevier.
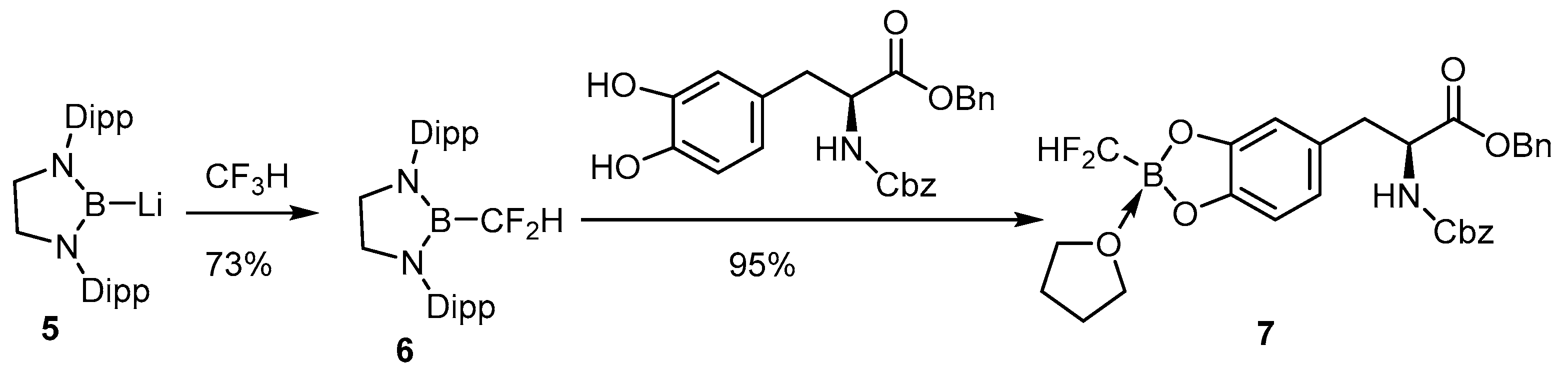
Insertion of the difluoromethyl group into lithium 1,3-bis(2,6-diisopropylphenyl)-1,3,2 diazaborolidinyl-2-uide activates the C-F linkage that can further react with the vicinal dihydroxy phenylalanine derivative to provide the air-stable difluoromethylborono compounds (Scheme 6) [69]. The potential difluoromethylboron tyrosine has not yet been radiofluorinated for in vivo study.
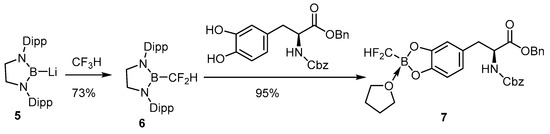
Scheme 6.
Conjugation of a boronodifluoromethylene compound to a tyrosine analog [69]. In courtesy of the permission of copyright by the publisher of JOHN WILEY AND SONS.
4. Facial Radiofluorination through Exchanging 18F for 19F on a Trifluoroborate
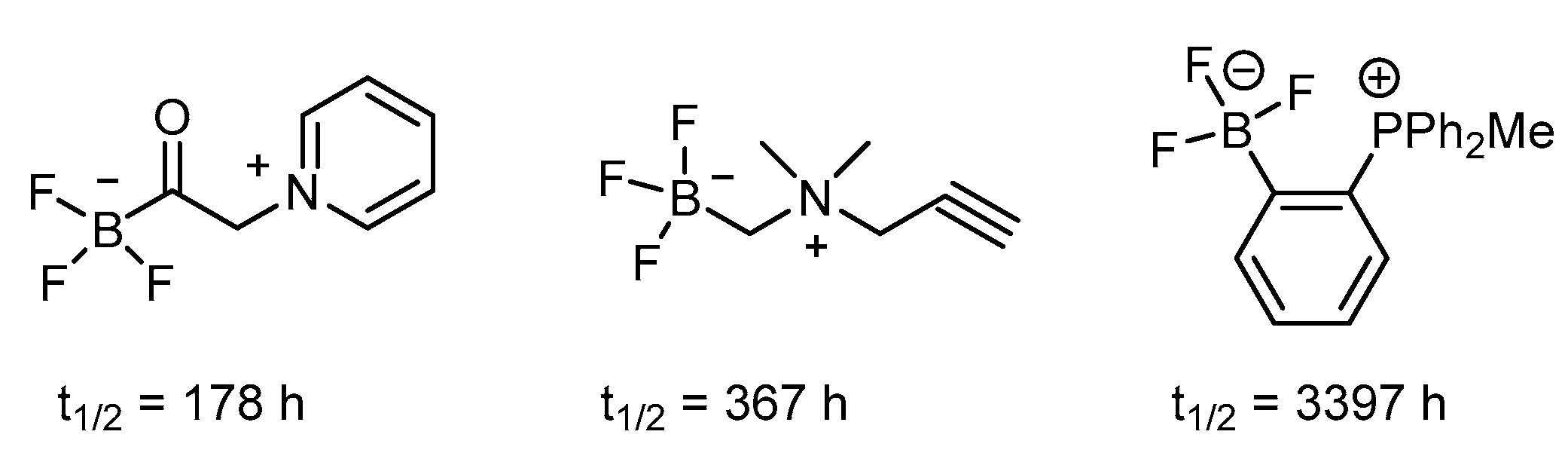
Heteroatomic fluoride exerts a distinct profile from carbon fluoride with respect to bond strength and radiofuorinating ability [70]. Among which, the trifluoroborate emerges as the most promising theranostic boron carrier because of its available functionality for radiofluorination via a facile radiofluoro-fluoro exchange (Figure 3) [70].
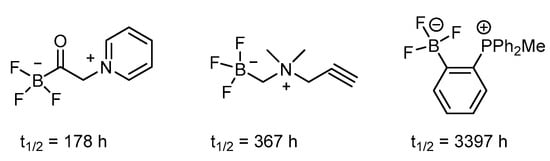
Figure 3.
Trifluoroborates showing stability via electron-withdrawing contribution on an arene ring [70].
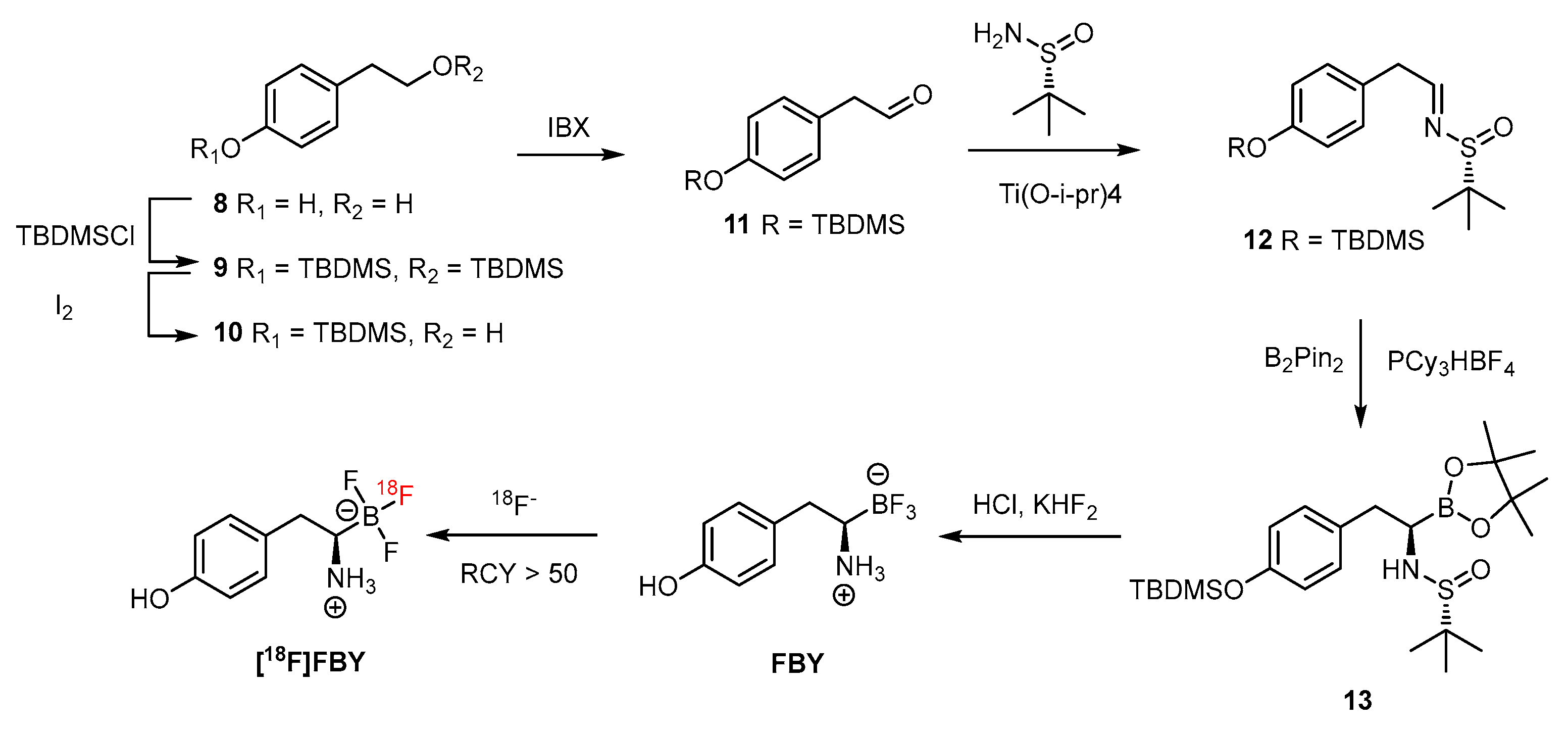
The trifluoroborate could be generated by mixing the precursor, such as boronic acid or boronic ester, with KHF2 under an acidic condition of HCl. The subsequent radiofluorine exchange reaction then provides the target F-18-labeled compound in an efficient way under mild conditions. The reported radiochemical yield of greater than 50% within 15 min demonstrated its usefulness [71]. Additionally, the trifluoroborate group has been introduced to both the aromatic ring and aliphatic chain. The stability of aryl trifluoroborate is dependent on the substituent and is mostly stabilized when the electron-withdrawing group is in the ortho or para position. Further, the presence of the trialkylphosphonium salt can prolong the half-life to 3397 h (Figure 3). The zwitter ion-like nature of an amino acid is retained when the carboxyl group is replaced by the tifluoroboron such as in FBY (Scheme 7) [71].
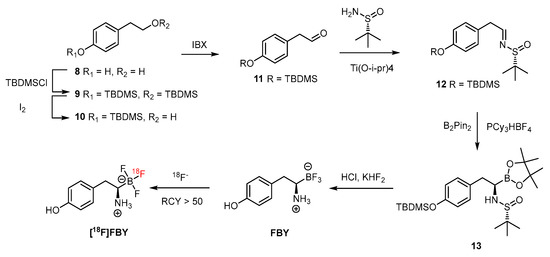
Scheme 7.
FBY and [18F]FBY generated through a facial synthetic route comprising a step of HPLC purification [71]. With the permission of the publisher of ACS publications.
As reported by Li et al., the preparation is facile before the step of introduction of boronopinacolone in the presence of PCy3·HBF4 [71]. After a sophisticated purification with semipreparative HPLC, a relatively low yield (30%) of FBY was obtained. The FBY has shown remarkable stability, with 98% intact form after treatment with H2O2 solution for 4 h, compared with the 99% conversion in 1 h by BPA. A human study by radiofluoro [18F]FBY has shown a high T/N ratio of 2.30 ± 1.26 and 24.56 ± 6.32 in low- and high-grade tumors, respectively [72]. Whereas the concentration of boron in human studies is not available from [18F]FBY, a concentration of 19.59 ppm was reported for melanoma tumors of xenografted mice [71,73]. As a diagnosing agent, the T/N radioactivity ratio is a decisive index, and FBY has shown to be a promising PET imaging agent for diagnosing tumors. For use as a therapeutic agent against tumors, boron concentrations must be as high as possible. In comparison with its high tumoral accumulation [72,74], the uptake of <2%ID/g in brain tissue of the mouse model seems to be surpassed by that of the glioma model in the clinical study. In addition, the preparation of target FBY might constitute a bottom neck in future applications of this BNCT agent because a glioma patient requires an injection dose of BPA of 450 mg/kg equivalent to 32 g for a 70 kg patient, for each irradiation. The aqueous solubility is also needed to be considered. For example, the insufficient solubility of BPA can be improved by complexing with the trihydroxy groups of fructose via condensation. Without auxiliary groups, FBY needs to be water dissolvable through its inherent properties. Nevertheless, the excellent stability of FBY might compensate for the tumoral boron concentration.
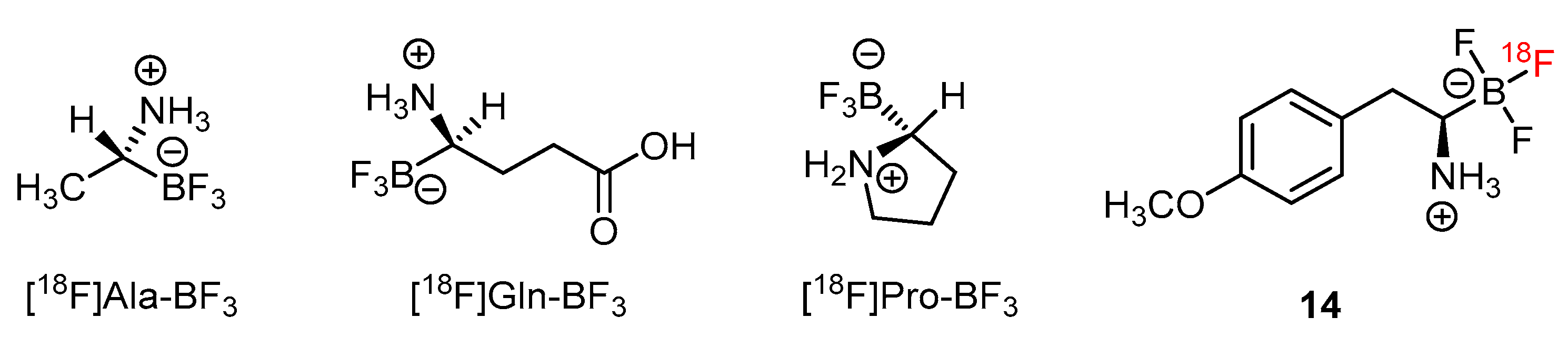
In addition to [18F]FBY, a number of amino acids have been tagged by radiofluoride through the exchange reaction on the trifluoroborate moieties [75]. Examples such as [18F]Ala-BF3, [18F]Gln-BF3 and [18F]Pro-BF3 showed their values as future BNCT theranostic agents (Figure 4).
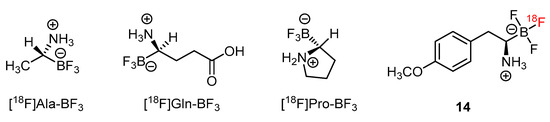
Figure 4.
Radiofluoride-exchanging trifluoroborate-substituted amino acid analogs for tumor imaging [75].
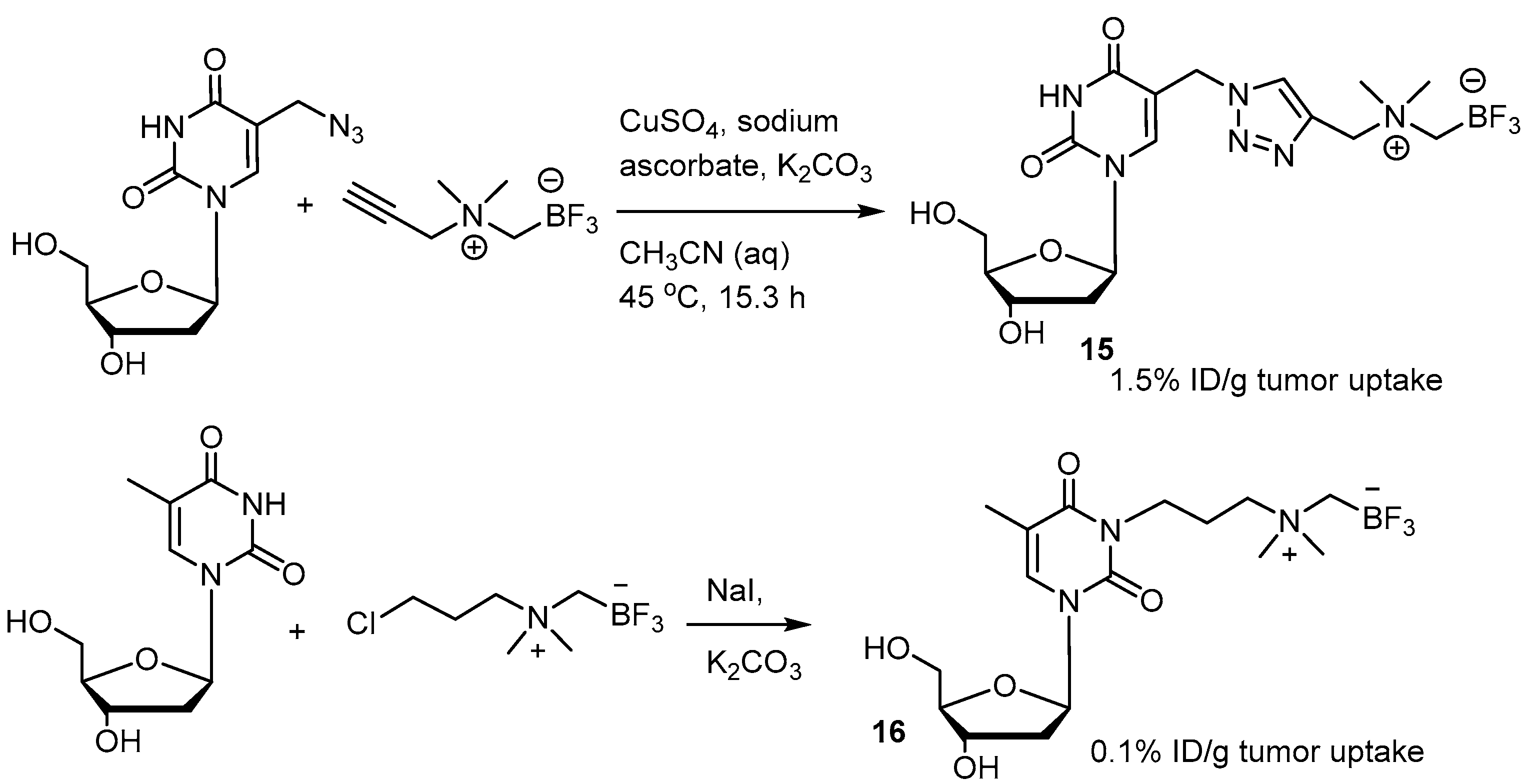
A series of derivative [18F]FBY analogs were developed for studying the uptake of radioactivity in tumors of B16-F10 xenograft mice [73]. Among them, the p-methoxy derivative 14 exerted a tumoral uptake of 3.5%ID/g (Figure 4). 1,3-Dipolar cycloaddition, also known as click chemistry, has been used to combine the trifluoroborate moiety with the nucleoside to give the zwitter ionic nucleoside (Scheme 8) [76]. A tumoral uptake value of 1.5%ID/g for 15 in an U87 Xenograft mouse is much better than that of the n-alkylated trifluoroborated nucleoside, 0.1%ID/g. The uptake value of the brain is not provided.
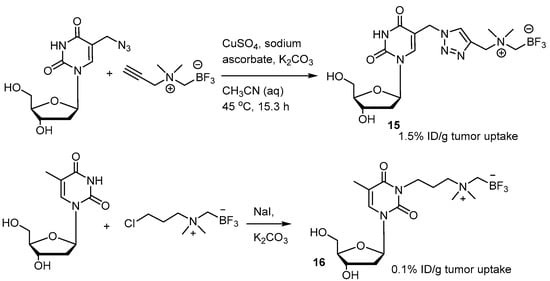
Scheme 8.
Nucleoside trifluoroborate 15 formed from 1,3-dipolar cycloaddition compared with the n-alkylated trifluoroborate nucleoside 16 in tumoral uptake [76]. In courtesy of the permission of copyright by publisher of RSC.
5. Conclusions
5.1. Less Toxicity
Unlike the chemotherapeutic drugs required to be highly cytotoxic against tumors concurrently with minor side effects, BNCT agents merely need to be taken by the tumor in a concentration as high as possible without significant cytotoxicity. Hence, it may be expected that the hit rate of potential clinical boron compounds would be higher than that of the chemotherapeutic agents.
5.2. Diagnostic Purpose and BNCT Therapeutic Purpose
According to the viewpoint of diagnosing gliomas, [18F]FBY is better than [18F]FBPA with respect to the high T/N ratio, ranging from 2.3 to 24.5 vs. greater than 2.5, respectively. In serving as the BNCT therapeutic agent, the concentration of boron in the glioma of FBY has not yet been provided, except for a xenografted melanoma with a concentration of 19.6 ppm. This value may be hampered by the limited cerebral accumulation dose of its radiofluorocongener at 0.4%ID/g. Moreover, subtly released radiofluoride was accumulated on the skull of a glioma patient, implying an exchange of fluoride for the surrounding anion. Thus, compared with BPA, which can reach a concentration of less than 10 ppm and increases to 20–40 ppm through BPA-fructose conjugate coupled with intravenous continuous injection, FBY needs more evidence to support enough boron in gliomas.
5.3. Extraction Route and Solubility
In spite of the fact that d-BPA shows a better T/N of 6.9 than that of BPA, which is 1.5, further animal studies are required because the extraction route is solely through the kidney rather than the partial liver, as is the case with BPA. The 3-BPA has better aqueous solubility than that of BPA, as reflected by the cerebral dose of 3.4%ID/g vs. 2.2%ID/g. Whereas radiofluorinated 3-BPA through electrophilic fluorination remains an obstacle at the present stage, a nucleophilic pathway may be available in the future, thereby enabling the determination of the T/N ratio.
5.4. Trifluoroborate Effect
The fair tumoral uptake of trifluoborate-conjugated natural products such as nucleoside derivatives (1.5%ID/g) is noted. Radiofluorination enables their straightforward assessment of the T/N ratio and boron concentration in vivo.
In spite of the unsatisfactory tumoral uptake and T/N selectivity, BPA remains the most commonly used clinical BNCT agent. PET imaging of F-18-labeled FBPA presents the personalized kinetics of BPA to guide the BNCT treatment planning procedure. Additionally, not only having both characteristics of imaging and therapeutics in a functional group, F-18-labeled trifluoroborate can adequately mimic the COOH group of an amino acid. [18F]FBY appears to be the potential imaging agent for glioma, thus encouraging its nonradioactive FBY to enroll in the clinical trial for BNCT.
Radiofluorination with F-18 is still impacting the development of BNCT agents and other therapeutic agents. As indicated by the roles played by radiofluoroborono compounds in BNCT research, more potentially clinically useful BNCT agents may emerge in due course. The F-18 labeling facilitates the preclinical assessment of those borono compounds just delivered from bench work. The most crucial contribution of F-18 is that an organic chemist can perform a biological assay beyond a cell culture study to save time on the development of boron compounds to meet the features required by metabolic stability, aqueous solubility for injection formulation, lesion targeting ability, and dosage calculation.
Author Contributions
Data curation, J.-P.D.; Investigation, J.-P.D.; Software, J.-P.D.; Conceptualization, C.-S.Y.; Validation, C.-S.Y.; Writing—original draft, C.-S.Y.; Writing—review & editing, C.-S.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science and Technology of Taiwan, NSC-107-2113-M-007-025-, NSC-106-2113-M-007-012-, MOST-108-2113-M-007-023- MY2 and 110-2113-M-007 -014.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Huai-En Yu’s partial typewriting of the manuscript is greatly acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Han, J.L.; Remete, A.M.; Dobson, L.S.; Kiss, L.; Izawa, K.; Moriwaki, H.; Soloshonok, V.A.; O’Hagan, D. Next generation organofluorine containing blockbuster drugs. J. Fluor. Chem. 2020, 239, 109639. [Google Scholar] [CrossRef]
- He, J.R.; Li, Z.Y.; Dhawan, G.; Zhang, W.; Sorochinsky, A.E.; Butler, G.; Soloshonok, V.A.; Han, J.L. Fluorine-containing drugs approved by the FDA in 2021. Chin. Chem. Lett. 2023, 34, 107578. [Google Scholar]
- Inoue, M.; Sumii, Y.; Shibata, N. Contribution of Organofluorine Compounds to Pharmaceuticals. ACS Omega 2020, 5, 10633–10640. [Google Scholar] [CrossRef]
- Han, J.L.; Kiss, L.; Mei, H.B.; Remete, A.M.; Ponikvar-Svet, M.; Sedgwick, D.M.; Roman, R.; Fustero, S.; Moriwaki, H.; Soloshonok, V.A. Chemical Aspects of Human and Environmental Overload with Fluorine. Chem. Rev. 2021, 121, 4678–4742. [Google Scholar] [CrossRef] [PubMed]
- Gillis, E.P.; Eastman, K.J.; Hill, M.D.; Donnelly, D.J.; Meanwell, N.A. Applications of Fluorine in Medicinal Chemistry. J. Med. Chem. 2015, 58, 8315–8359. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.L.; Li, S.F.; Zhang, H.; Xu, H.W. Research progress of F-18 labeled small molecule positron emission tomography (PET) imaging agents. Eur. J. Med. Chem. 2020, 205, 112629. [Google Scholar] [CrossRef]
- Goud, N.S.; Joshi, R.K.; Bharath, R.D.; Kumar, P. Fluorine-18: A radionuclide with diverse range of radiochemistry and synthesis strategies for target based PET diagnosis. Eur. J. Med. Chem. 2020, 187, 111979. [Google Scholar] [CrossRef]
- Jin, C.T.; Luo, X.Y.; Li, X.Y.; Zhou, R.; Zhong, Y.; Xu, Z.J.; Cui, C.Y.; Xing, X.Q.; Zhang, H.; Tian, M. Positron emission tomography molecular imaging-based cancer phenotyping. Cancer 2022, 128, 2704–2716. [Google Scholar] [CrossRef]
- O’Neill, H.; Malik, V.; Johnston, C.; Reynolds, J.V.; O’Sullivan, J. Can the Efficacy of F-18 FDG-PET/CT in Clinical Oncology Be Enhanced by Screening Biomolecular Profiles? Pharmaceuticals 2019, 12, 16. [Google Scholar] [CrossRef]
- Ford, E.C.; Herman, J.; Yorke, E.; Wahl, R.L. F-18-FDG PET/CT for Image-Guided and Intensity-Modulated Radiotherapy. J. Nucl. Med. 2009, 50, 1655–1665. [Google Scholar] [CrossRef]
- Treglia, G.; Muoio, B.; Trevisi, G.; Mattoli, M.V.; Albano, D.; Bertagna, F.; Giovanella, L. Diagnostic Performance and Prognostic Value of PET/CT with Different Tracers for Brain Tumors: A Systematic Review of Published Meta-Analyses. Int. J. Mol. Sci. 2019, 20, 4669. [Google Scholar] [CrossRef] [PubMed]
- Jager, P.L.; Chirakal, R.; Marriott, C.J.; Brouwers, A.H.; Koopmans, K.P.; Gulenchyn, K.Y. 6-L-F-18-fluorodihydroxyphenylalanine PET in neuroendocrine tumors: Basic aspects and emerging clinical applications. J. Nucl. Med. 2008, 49, 573–586. [Google Scholar] [CrossRef] [PubMed]
- Hicks, R.J.; Rischin, D.; Fisher, R.; Binns, D.; Scott, A.M.; Peters, L.J. Utility of FMISO PET in advanced head and neck cancer treated with chemoradiation incorporating a hypoxia-targeting chemotherapy agent. Eur. J. Nucl. Med. Mol. Imaging 2005, 32, 1384–1391. [Google Scholar] [CrossRef] [PubMed]
- Krohn, K.A.; Link, J.M.; Mason, R.P. Molecular imaging of hypoxia. J. Nucl. Med. 2008, 49, 129S–148S. [Google Scholar] [CrossRef]
- Kirk, K.L. Fluorination in medicinal chemistry: Methods, strategies, and recent developments. Org. Process Res. Dev. 2008, 12, 305–321. [Google Scholar] [CrossRef]
- Krasikova, R.N. Nucleophilic Synthesis of 6-l-[18F]FDOPA. Is Copper-Mediated Radiofluorination the Answer? Molecules 2020, 25, 4365. [Google Scholar] [CrossRef]
- Vatsadze, S.Z.; Eremina, O.E.; Veselova, I.A.; Kalmykov, S.N.; Nenajdenko, V.G. F-18-Labelled catecholamine type radiopharmaceuticals in the diagnosis of neurodegenerative diseases and neuroendocrine tumours: Approaches to synthesis and development prospects. Russ. Chem. Rev. 2018, 87, 350–373. [Google Scholar] [CrossRef]
- Huang, C.F.; McConathy, J. Fluorine-18 Labeled Amino Acids for Oncologic Imaging with Positron Emission Tomography. Curr. Top. Med. Chem. 2013, 13, 871–891. [Google Scholar] [CrossRef]
- Kiyanagi, Y.; Sakurai, Y.; Kumada, H.; Tanaka, H. Status of Accelerator-Based BNCT Projects Worldwide. In Proceedings of the 25th International Conference on the Application of Accelerators in Research and Industry (CAARI), Grapevine, TX, USA, 12–17 August 2018. [Google Scholar]
- Hughes, A.M. Importance of radiobiological studies for the advancement of boron neutron capture therapy (BNCT). Expert Rev. Mol. Med. 2022, 24, e14. [Google Scholar] [CrossRef]
- Malouff, T.D.; Seneviratne, D.S.; Ebner, D.K.; Stross, W.C.; Waddle, M.R.; Trifiletti, D.M.; Krishnan, S. Boron Neutron Capture Therapy: A Review of Clinical Applications. Front. Oncol. 2021, 11, 601820. [Google Scholar] [CrossRef]
- Moss, R.L. Critical review, with an optimistic outlook, on Boron Neutron Capture Therapy (BNCT). Appl. Radiat. Isot. 2014, 88, 2–11. [Google Scholar] [CrossRef]
- Ali, F.; Hosmane, N.S.; Zhu, Y.H. Boron Chemistry for Medical Applications. Molecules 2020, 25, 828. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M. Boron neutron capture therapy (BNCT): A unique role in radiotherapy with a view to entering the accelerator-based BNCT era. Int. J. Clin. Oncol. 2020, 25, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Barth, R.F.; Mi, P.; Yang, W.L. Boron delivery agents for neutron capture therapy of cancer. Cancer Commun. 2018, 38, 35. [Google Scholar] [CrossRef] [PubMed]
- Lan, T.L.; Chou, F.I.; Lin, K.H.; Pan, P.S.; Lee, J.C.; Huang, W.S.; Liu, Y.M.; Chao, Y.; Chen, Y.W. Using salvage Boron Neutron Capture Therapy (BNCT) for recurrent malignant brain tumors in Taiwan. Appl. Radiat. Isot. 2020, 160, 109105. [Google Scholar] [CrossRef] [PubMed]
- Nakase, I.; Katayama, M.; Hattori, Y.; Ishimura, M.; Inaura, S.; Fujiwara, D.; Takatani-Nakase, T.; Fujii, I.; Futaki, S.; Kirihata, M. Intracellular target delivery of cell-penetrating peptide-conjugated dodecaborate for boron neutron capture therapy (BNCT). Chem. Commun. 2019, 55, 13955–13958. [Google Scholar] [CrossRef]
- Hirase, S.; Aoki, A.; Hattori, Y.; Morimoto, K.; Noguchi, K.; Fujii, I.; Takatani-Nakase, T.; Futaki, S.; Kirihata, M.; Nakase, I. Dodecaborate-Encapsulated Extracellular Vesicles with Modification of Cell-Penetrating Peptides for Enhancing Macropinocytotic Cellular Uptake and Biological Activity in Boron Neutron Capture Therapy. Mol. Pharm. 2022, 19, 1135–1145. [Google Scholar] [CrossRef]
- Nakase, I.; Aoki, A.; Sakai, Y.; Hirase, S.; Ishimura, M.; Takatani-Nakase, T.; Hattori, Y.; Kirihata, M. Antibody-Based Receptor Targeting Using an Fc-Binding Peptide-Dodecaborate Conjugate and Macropinocytosis Induction for Boron Neutron Capture Therapy. ACS Omega 2020, 5, 22731–22738. [Google Scholar] [CrossRef]
- Hu, K.; Yang, Z.M.; Zhang, L.L.; Xie, L.; Wang, L.; Xu, H.; Josephson, L.; Liang, S.H.; Zhang, M.R. Boron agents for neutron capture therapy. Coord. Chem. Rev. 2020, 405, 213139. [Google Scholar] [CrossRef]
- Murphy, N.; McCarthy, E.; Dwyer, R.; Farras, P. Boron clusters as breast cancer therapeutics. J. Inorg. Biochem. 2021, 218, 111412. [Google Scholar] [CrossRef]
- Bednarska-Szczepaniak, K.; Przelazly, E.; Kania, K.D.; Szwed, M.; Litecka, M.; Gruner, B.; Lesnikowski, Z.J. Interaction of Adenosine, Modified Using Carborane Clusters, with Ovarian Cancer Cells: A New Anticancer Approach against Chemoresistance. Cancers 2021, 13, 3855. [Google Scholar] [CrossRef]
- Wu, C.Y.; Lin, J.J.; Chang, W.Y.; Hsieh, C.Y.; Wu, C.C.; Chen, H.S.; Hsu, H.J.; Yang, A.S.; Hsu, M.H.; Kuo, W.Y. Development of theranostic active-targeting boron-containing gold nanoparticles for boron neutron capture therapy (BNCT). Colloids Surf. B Biointerfaces 2019, 183, 110387. [Google Scholar] [CrossRef]
- Heide, F.; McDougall, M.; Harder-Viddal, C.; Roshko, R.; Davidson, D.; Wu, J.D.; Aprosoff, C.; Moya-Torres, A.; Lin, F.; Stetefeld, J. Boron rich nanotube drug carrier system is suited for boron neutron capture therapy. Sci. Rep. 2021, 11, 15520. [Google Scholar] [CrossRef]
- Tamanoi, F.; Chinnathambi, S.; Laird, M.; Komatsu, A.; Birault, A.; Takata, T.; Doan, T.L.H.; Mai, N.X.D.; Raitano, A.; Morrison, K.; et al. Construction of Boronophenylalanine-Loaded Biodegradable Periodic Mesoporous Organosilica Nanoparticles for BNCT Cancer Therapy. Int. J. Mol. Sci. 2021, 22, 2251. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Reina, G.; Kang, H.G.; Chen, X.X.; Zou, Y.J.; Ishikawa, Y.; Suzuki, M.; Komatsu, N. Polyglycerol Functionalized B-10 Enriched Boron Carbide Nanoparticle as an Effective Bimodal Anticancer Nanosensitizer for Boron Neutron Capture and Photothermal Therapies. Small 2022, 18, 2204044. [Google Scholar] [CrossRef]
- Seneviratne, D.; Advani, P.; Trifiletti, D.M.; Chumsri, S.; Beltran, C.J.; Bush, A.F.; Vallow, L.A. Exploring the Biological and Physical Basis of Boron Neutron Capture Therapy (BNCT) as a Promising Treatment Frontier in Breast Cancer. Cancers 2022, 14, 3009. [Google Scholar] [CrossRef]
- Ahmad, P.; Khandaker, M.U.; Muhammad, N.; Rehman, F.; Ullah, Z.; Khan, G.; Khan, M.I.; Haq, S.; Ali, H.; Khan, A.; et al. Synthesis of enriched boron nitride nanocrystals: A potential element for biomedical applications. Appl. Radiat. Isot. 2020, 166, 109404. [Google Scholar] [CrossRef]
- Singh, A.; Kim, B.K.; Mackeyev, Y.; Rohani, P.; Mahajan, S.D.; Swihart, M.T.; Krishnan, S.; Prasad, P.N. Boron-Nanoparticle-Loaded Folic-Acid-Functionalized Liposomes to Achieve Optimum Boron Concentration for Boron Neutron Capture Therapy of Cancer. J. Biomed. Nanotechnol. 2019, 15, 1714–1723. [Google Scholar] [CrossRef]
- Chiang, C.W.; Chien, Y.C.; Yu, W.J.; Ho, C.Y.; Wang, C.Y.; Wang, T.W.; Chiang, C.S.; Keng, P.Y. Polymer-Coated Nanoparticles for Therapeutic and Diagnostic Non-B-10 Enriched Polymer-Coated Boron Carbon Oxynitride (BCNO) Nanoparticles as Potent BNCT Drug. Nanomaterials 2021, 11, 2936. [Google Scholar] [CrossRef]
- Hung, Y.H.; Lin, Y.C.; Lin, Y.T.; Shih, G.W.; Liao, J.W.; Chen, K.S.; Liu, H.M.; Chen, Y.W.; Chuang, Y.J.; Yang, C.M.; et al. Therapeutic Efficacy and Radiobiological Effects of Boric Acid-mediated BNCT in a VX2 Multifocal Liver Tumor-bearing Rabbit Model. Anticancer Res. 2019, 39, 5495–5504. [Google Scholar] [CrossRef]
- Wu, W.C.; Wang, S.H.; Ou, S.T.; Liu, Y.W.H.; Liu, B.H.; Tseng, F.G. Electrosprayed chitosan/alginate/polyvinyl alcohol nanoparticles as boric acid carriers for (10)Boron neutron capture therapy. Nanomedicine 2020, 15, 1067–1077. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.J.; Cho, H.L.; Goudar, V.; Bupphathong, S.; Shu, C.H.; Kung, C.; Tseng, F.G. Boron-enriched polyvinyl-alcohol/boric-acid nanoparticles for boron neutron capture therapy. Nanomedicine 2021, 16, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Garabalino, M.A.; Olaiz, N.; Portu, A.; Saint Martin, G.; Thorp, S.I.; Pozzi, E.C.C.; Curotto, P.; Itoiz, M.E.; Hughes, A.M.; Colombo, L.L.; et al. Electroporation optimizes the uptake of boron-10 by tumor for boron neutron capture therapy (BNCT) mediated by GB-10: A boron biodistribution study in the hamster cheek pouch oral cancer model. Radiat. Environ. Biophys. 2019, 58, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Trivillin, V.A.; Serrano, A.; Garabalino, M.A.; Colombo, L.L.; Pozzi, E.C.; Hughes, A.M.; Curotto, P.M.; Thorp, S.I.; Farias, R.O.; Gonzalez, S.J.; et al. Translational boron neutron capture therapy (BNCT) studies for the treatment of tumors in lung. Int. J. Radiat. Biol. 2019, 95, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Fernstrom, J.D. Large neutral amino acids: Dietary effects on brain neurochemistry and function. Amino Acids 2013, 45, 419–430. [Google Scholar] [CrossRef]
- Kanai, Y. Amino acid transporter LAT1 (SLC7A5) as a molecular target for cancer diagnosis and therapeutics. Pharmacol. Ther. 2022, 230, 107964. [Google Scholar] [CrossRef] [PubMed]
- Puris, E.; Gynther, M.; Auriola, S.; Huttunen, K.M. L-Type amino acid transporter 1 as a target for drug delivery. Pharm. Res. 2020, 37, 88. [Google Scholar] [CrossRef] [PubMed]
- Cappoli, N.; Jenkinson, M.D.; Dello Russo, C.; Dickens, D. LAT1, a novel pharmacological target for the treatment of glioblastoma. Biochem. Pharmacol. 2022, 201, 115103. [Google Scholar] [CrossRef]
- Ancey, P.B.; Contat, C.; Meylan, E. Glucose transporters in cancer—From tumor cells to the tumor microenvironment. FEBS J. 2018, 285, 2926–2943. [Google Scholar] [CrossRef]
- Muth, A.K.; Park, S.Q. The impact of dietary macronutrient intake on cognitive function and the brain. Clin. Nutr. 2021, 40, 3999–4010. [Google Scholar] [CrossRef]
- Ishiwata, K. 4-Borono-2-F-18-fluoro-L-phenylalanine PET for boron neutron capture therapy-oriented diagnosis: Overview of a quarter century of research. Ann. Nucl. Med. 2019, 33, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Sauerwein, W.A.G.; Sancey, L.; Hey-Hawkins, E.; Kellert, M.; Panza, L.; Imperio, D.; Balcerzyk, M.; Rizzo, G.; Scalco, E.; Herrmann, K.; et al. Theranostics in Boron Neutron Capture Therapy. Life 2021, 11, 330. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, M.; Honda, N.; Kurihara, H.; Hiroi, K.; Nakamura, S.; Ito, M.; Shikano, N.; Itami, J.; Fujii, H. Non-invasive estimation of B-10-4-borono-L-phenylalanine-derived boron concentration in tumors by PET using 4-borono-2-F-18-fluoro-phenylalanine. Cancer Sci. 2018, 109, 1617–1626. [Google Scholar] [CrossRef] [PubMed]
- Romanov, V.; Isohashi, K.; Alobthani, G.; Beshr, R.; Horitsugi, G.; Kanai, Y.; Naka, S.; Watabe, T.; Shimosegawa, E.; Hatazawa, J. Evaluation of the total distribution volume of F-18-FBPA in normal tissues of healthy volunteers by non-compartmental kinetic modeling. Ann. Nucl. Med. 2020, 34, 155–162. [Google Scholar] [CrossRef]
- Yoshida, F.; Kurita, T.; Endo, K.; Nakai, K.; Shirakawa, M.; Zaboronok, A.; Tsurubuchi, T.; Ishikawa, E.; Matsumura, A. Difference in BPA uptake between glioma stem-like cells and their cancerous cells. Appl. Radiat. Isot. 2020, 164, 109234. [Google Scholar] [CrossRef] [PubMed]
- Ishiwata, K.; Ebinuma, R.; Watanabe, C.; Hayashi, K.; Toyohara, J. Reliable radiosynthesis of 4-[10B]borono-2-[18F]fluoro-l-phenylalanine with quality assurance for boron neutron capture therapy-oriented diagnosis. Ann. Nucl. Med. 2018, 32, 463–473. [Google Scholar] [CrossRef]
- He, J.; Yan, H.; Du, Y.R.; Ji, Y.; Cai, F.; Fan, W.B.; Huo, L.; Liu, Y.H.; Wang, Z.; Li, S.H. Nucleophilic radiosynthesis of boron neutron capture therapy-oriented PET probe F-18 FBPA using aryldiboron precursors. Chem. Commun. 2021, 57, 8953–8956. [Google Scholar] [CrossRef]
- Morita, T.; Kurihara, H.; Hiroi, K.; Honda, N.; Igaki, H.; Hatazawa, J.; Arai, Y.; Itami, J. Dynamic changes in 18F-borono-L-phenylalanine uptake in unresectable, advanced, or recurrent squamous cell carcinoma of the head and neck and malignant melanoma during boron neutron capture therapy patient selection. Radiat. Oncol. 2018, 13, 4. [Google Scholar] [CrossRef]
- Grunewald, C.; Sauberer, M.; Filip, T.; Wanek, T.; Stanek, J.; Mairinger, S.; Rollet, S.; Kudejova, P.; Langer, O.; Schutz, C.; et al. On the applicability of F-18 FBPA to predict L-BPA concentration after amino acid preloading in HuH-7 liver tumor model and the implication for liver boron neutron capture therapy. Nucl. Med. Biol. 2017, 44, 83–89. [Google Scholar] [CrossRef]
- Watabe, T.; Hanaoka, K.; Naka, S.; Kanai, Y.; Ikeda, H.; Aoki, M.; Shimosegawa, E.; Kirihata, M.; Hatazawa, J. Practical calculation method to estimate the absolute boron concentration in tissues using F-18-FBPA PET. Ann. Nucl. Med. 2017, 31, 481–485. [Google Scholar] [CrossRef]
- Lo, Y.W.; Lee, J.C.; Hu, Y.S.; Li, C.Y.; Chen, Y.L.; Lin, C.S.; Huang, W.S.; Lin, K.H.; Chen, Y.W. The importance of optimal ROIs delineation for FBPA-PET before BNCT. Appl. Radiat. Isot. 2020, 163, 109219. [Google Scholar] [CrossRef] [PubMed]
- Skwierawska, D.; Lopez-Valverde, J.A.; Balcerzyk, M.; Leal, A. Clinical Viability of Boron Neutron Capture Therapy for Personalized Radiation Treatment. Cancers 2022, 14, 2865. [Google Scholar] [CrossRef] [PubMed]
- Tsukada, H.; Sato, K.; Fukumoto, D.; Kakiuchi, T. Evaluation of D-isomers of O-F-18-fluoromethyl, O-F-18-fluoroethyl and O-F-18-fluoropropyl tyrosine as tumour imaging agents in mice. Eur. J. Nucl. Med. Mol. Imaging 2006, 33, 1017–1024. [Google Scholar] [CrossRef]
- Yang, Q.; Zhu, W.J.; Ren, C.; Ji, H.F.; Wang, D.C.; Liu, Y.; Li, F.; Du, Y.R.; Liu, Y.H.; Huo, L. Biodistribution and radiation dosimetry of D-isomer of 4-borono-2- F-18 fluoro-phenylalanine: A comparative PET/CT study with L-isomer in healthy human volunteers. Nucl. Med. Biol. 2021, 94–95, 32–37. [Google Scholar] [CrossRef]
- Kulvik, M.; Vahatalo, J.; Buchar, E.; Farkkila, M.; Jarviluoma, E.; Jaaskelainen, J.; Kriz, O.; Laakso, J.; Rasilainen, M.; Ruokonen, I.; et al. Clinical implementation of 4-dihydroxyborylphenylalanine synthesised by an asymmetric pathway. Eur. J. Pharm. Sci. 2003, 18, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Kondo, N.; Hirano, F.; Temma, T. Evaluation of 3-Borono-l-Phenylalanine as a Water-Soluble Boron Neutron Capture Therapy Agent. Pharmaceutics 2022, 14, 1106. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.N.; Chang, C.W.; Chung, Y.H.; Tien, S.W.; Chen, Y.R.; Chen, T.W.; Huang, Y.C.; Wang, H.E.; Chou, Y.C.; Chen, M.H.; et al. Synthesis and characterization of boron fenbufen and its F-18 labeled homolog for boron neutron capture therapy of COX-2 overexpressed cholangiocarcinoma. Eur. J. Pharm. Sci. 2017, 107, 217–229. [Google Scholar] [CrossRef]
- Yokawa, A.; Hatanaka, M.; Mikami, K. Facile C-F Bond Activation Approach to FAMT-Based Difluoromethyl-BNCT Drug Candidates. Helv. Chim. Acta 2021, 104, e2000211. [Google Scholar] [CrossRef]
- Scroggie, K.R.; Perkins, M.V.; Chalker, J.M. Reaction of F-18 Fluoride at Heteroatoms and Metals for Imaging of Peptides and Proteins by Positron Emission Tomography. Front. Chem. 2021, 9, 472. [Google Scholar] [CrossRef]
- Li, J.Y.; Shi, Y.X.; Zhang, Z.Z.; Liu, H.; Lang, L.X.; Liu, T.; Chen, X.Y.; Liu, Z.B. A Metabolically Stable Boron-Derived Tyrosine Serves as a Theranostic Agent for Positron Emission Tomography Guided Boron Neutron Capture Therapy. Bioconjug. Chem. 2019, 30, 2870–2878. [Google Scholar] [CrossRef]
- Li, Z.; Kong, Z.R.; Chen, J.Y.; Li, J.Y.; Li, N.; Yang, Z.; Wang, Y.; Liu, Z.B. F-18-Boramino acid PET/CT in healthy volunteers and glioma patients. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3113–3121. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Long, R.L.; Hu, M.; Liu, N.; Feng, Y.; Qiu, L.; Li, Z.B.; Chen, Y.; Wang, L. Synthesis and Evaluation of F-18-Labeled Boramino Acids as Potential New Positron Emission Tomography Agents for Cancer Management. Mol. Pharm. 2022, 19, 2191–2202. [Google Scholar] [CrossRef] [PubMed]
- Kong, Z.R.; Li, Z.; Chen, J.Y.; Liu, S.R.; Liu, D.L.; Li, J.Y.; Li, N.; Ma, W.B.; Feng, F.; Wang, Y.; et al. Metabolic characteristics of F-18 fluoroboronotyrosine (FBY) PET in malignant brain tumors. Nucl. Med. Biol. 2022, 106, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.L.; Fan, K.; Cai, W.B. First-in-human study of an F-18-labeled boramino acid: A new class of PET tracers. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3037–3040. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.; Lozada, J.; Lepage, M.L.; Zhang, C.C.; Merkens, H.; Zeisler, J.; Lin, K.S.; Benard, F.; Perrin, D.M. Synthesis and 18F-radiolabeling of thymidine AMBF3 conjugates. RSC Med. Chem. 2020, 11, 569–576. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).