Development of Osthole-Loaded Microemulsions as a Prospective Ocular Delivery System for the Treatment of Corneal Neovascularization: In Vitro and In Vivo Assessments
Abstract
:1. Introduction
2. Results
2.1. Solubility Study
2.2. Screening the Emulsification Abilities of TW-80 and EL-35
2.3. Construction of Pseudoternary Phase Diagrams
2.4. Central Composite Design Response Surface Methodology
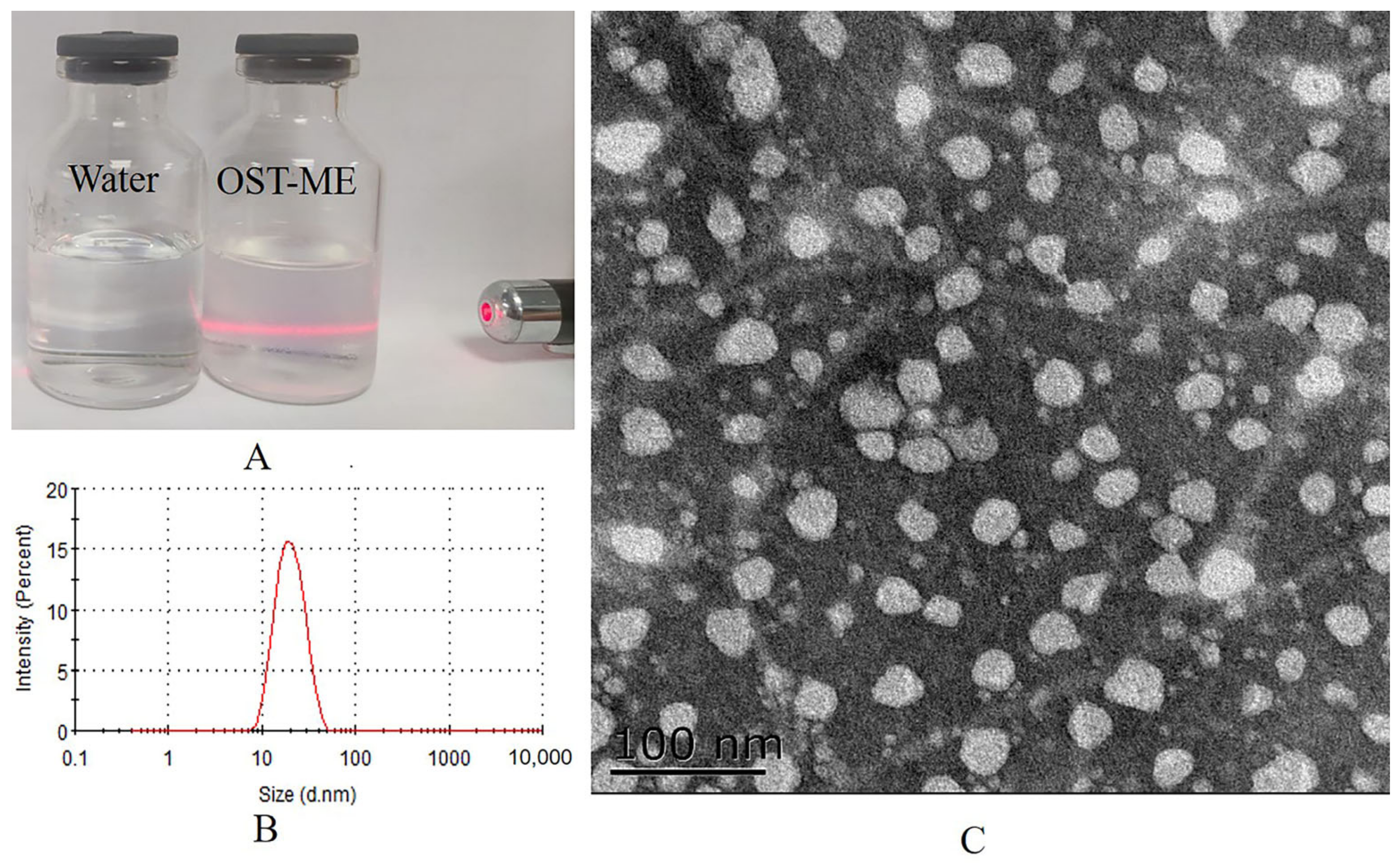
2.5. Preparation and Characterization of the OST-ME
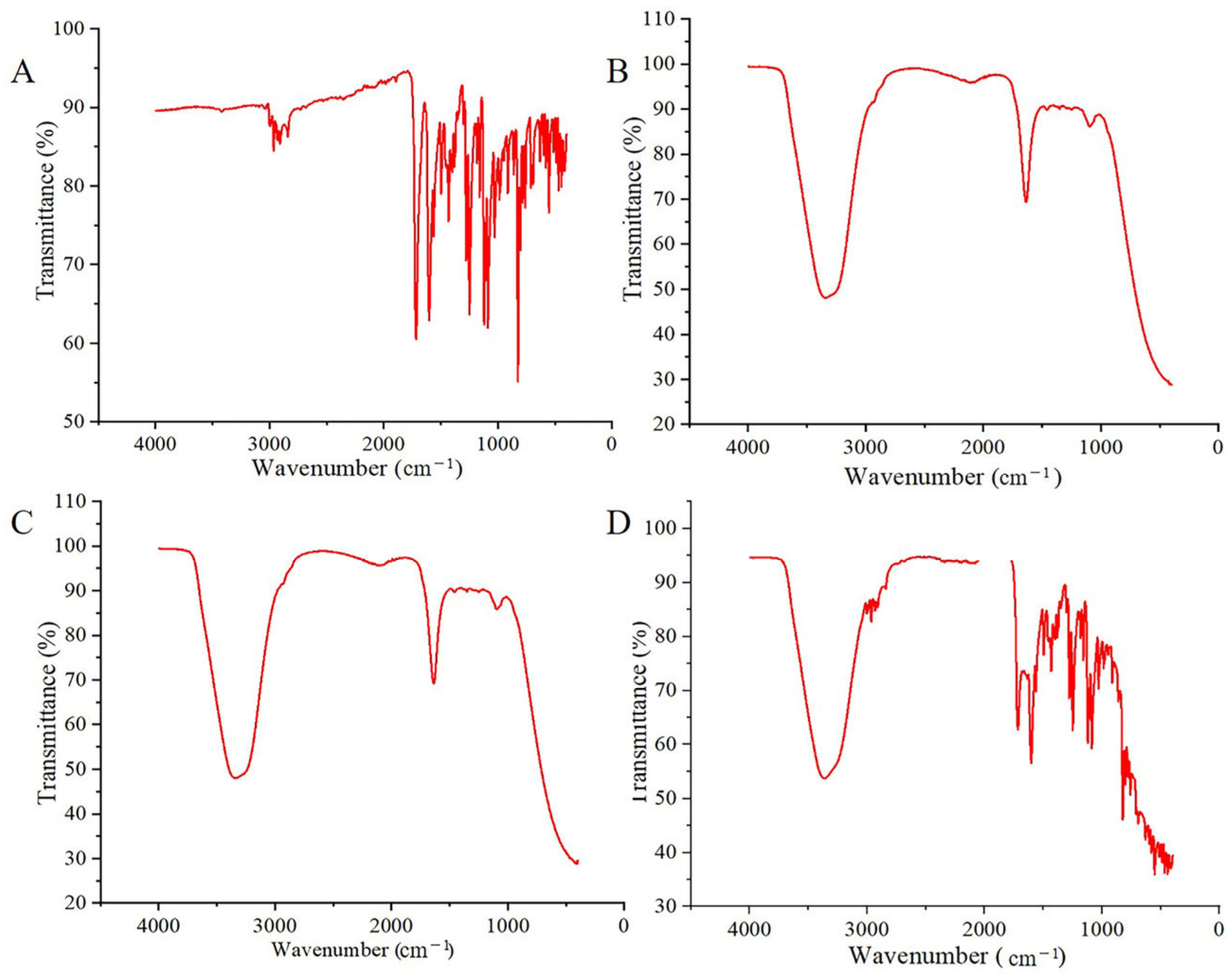
2.6. Fourier Transform Infrared Spectroscopy Analyses
2.7. Short-Term Stability
2.8. In Vitro Drug Release Study
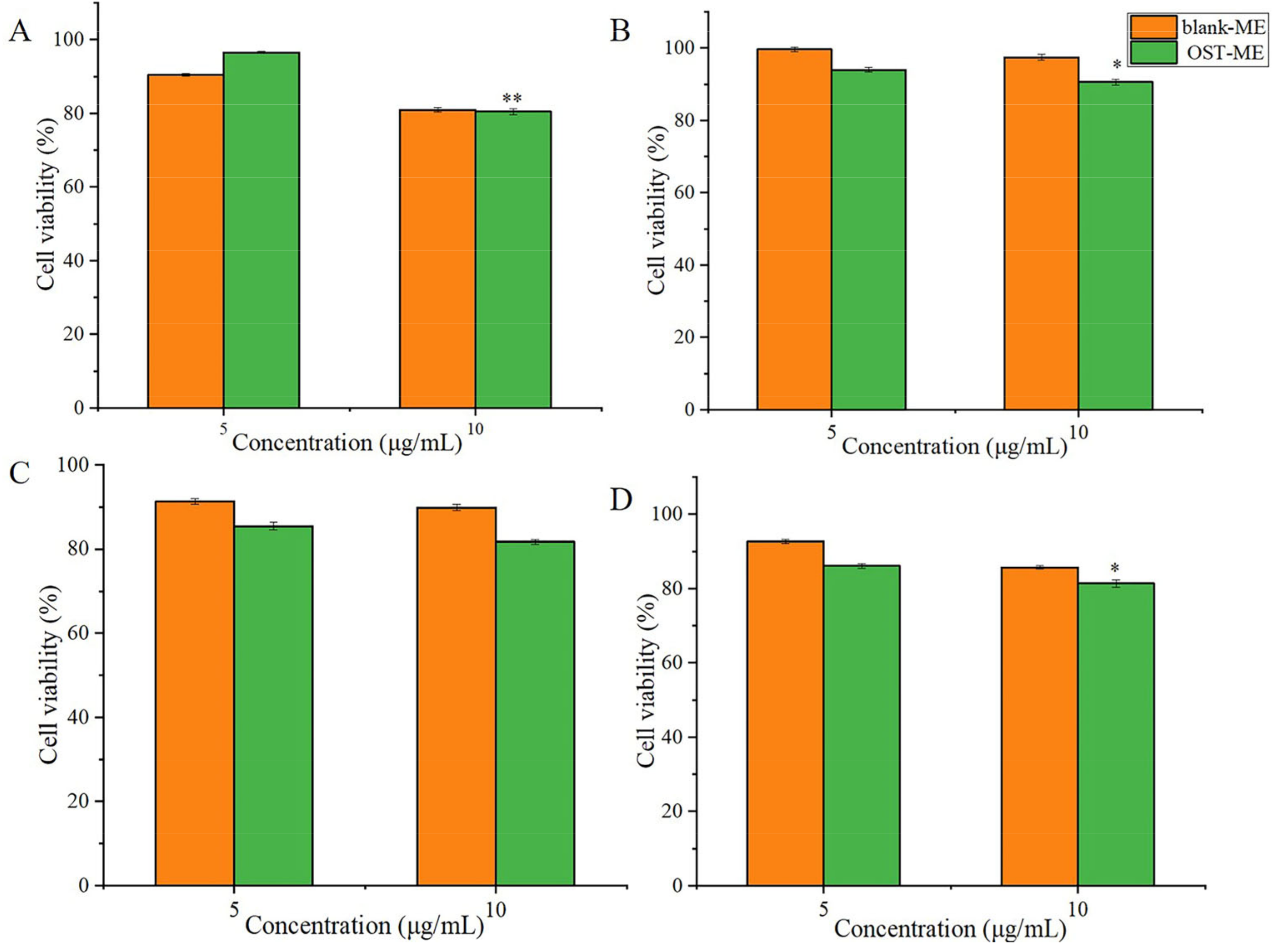
2.9. In vitro Cell Viability Study
2.10. The Ocular Irritation Test
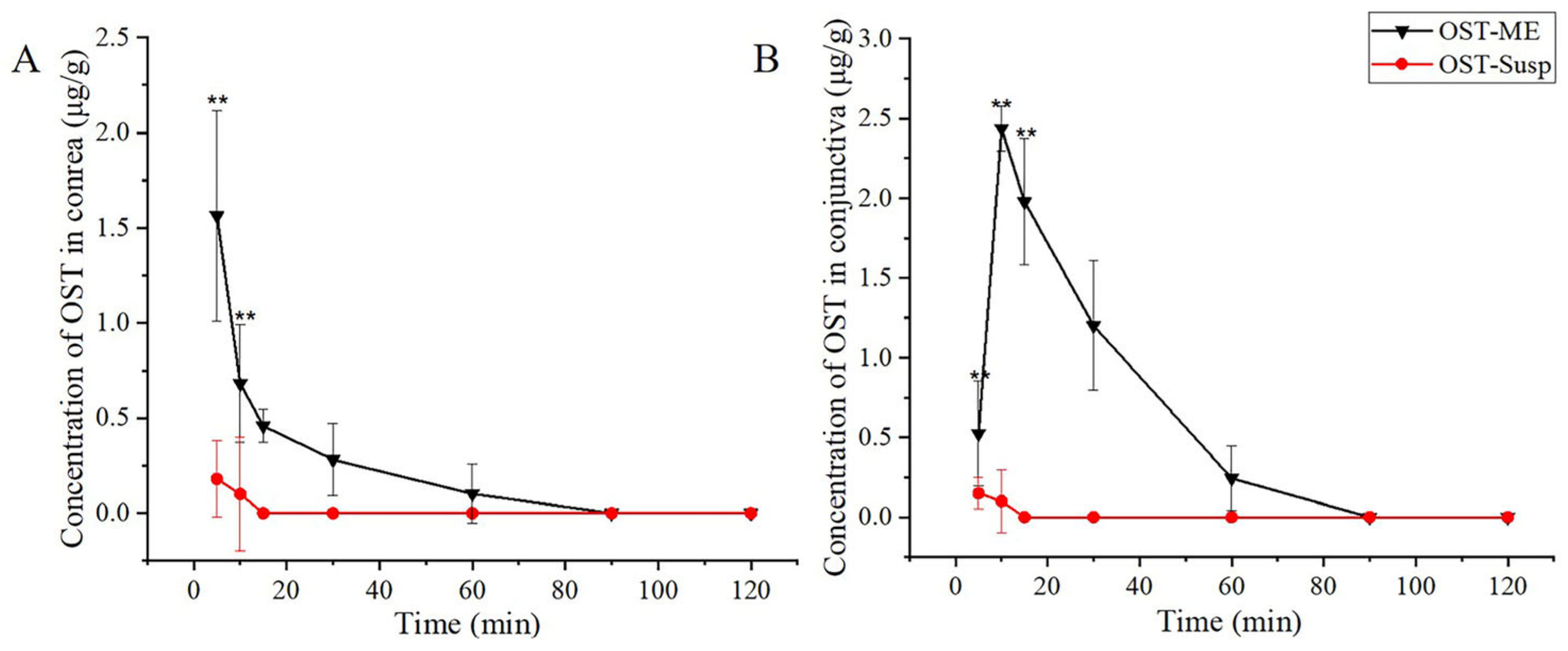
2.11. Ocular Pharmacokinetics Study
2.12. In Vivo Anti-CNV Efficacy in Mice
The Image and Area of CNV
2.13. Histopathological Examination
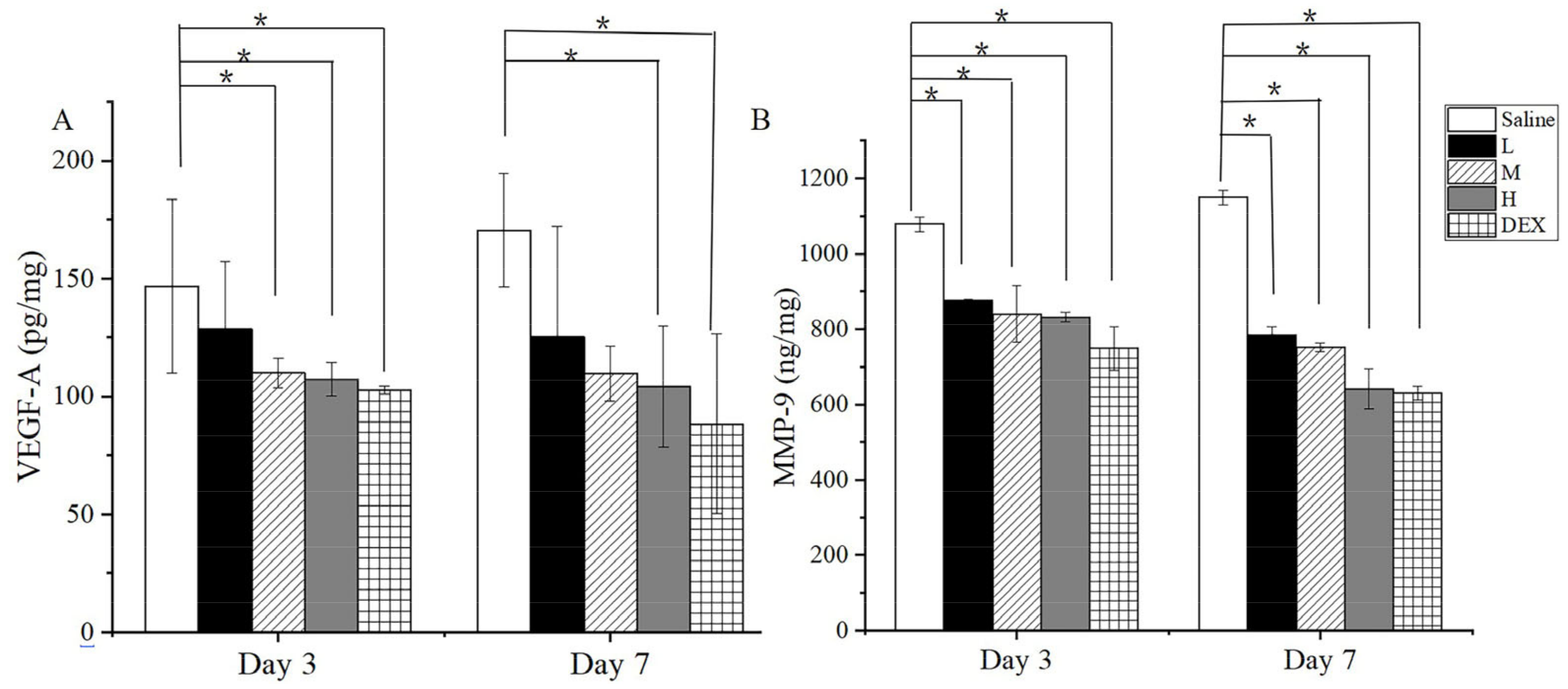
2.14. Enzyme Linked Immunosorbent Assay
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals
4.3. OST Assay by High Performance Liquid Chromatography
4.4. Solubility Study
4.5. Emulsification Ability
4.6. Pseudo-Ternary Phase Diagrams
4.7. Optimization of the OST-ME Formulation
4.8. Preparation of the OST-ME
4.9. Characterization of the OST-ME
4.9.1. Determination of Droplet Size, Zeta Potential and Polydispersity Index
4.9.2. Assessment of Entrapment Efficiency and Drug Loading
4.10. Morphological Observations of the OST-ME
4.11. pH and Osmotic Pressure
4.12. Fourier Transform Infrared Spectroscopy Analyses
4.13. Short-Term Stability
4.14. In vitro Drug Release
4.15. Cytotoxicity
4.16. Ocular Irritation Test
4.17. Ocular Pharmacokinetics
4.17.1. Grouping and Dosing
4.17.2. Analysis of Ocular Tissues
4.18. Anti-CNV Study
Establishment of CNV Model
4.19. Assessment and Quantification of CNV Area
4.20. Histopathological Examination
4.21. Enzyme Linked Immunosorbent Assay
4.22. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mukwaya, A.; Peebo, B.; Xeroudaki, M.; Ali, Z.; Lennikov, A.; Jensen, L.; Lagali, N. Factors regulating capillary remodeling in a reversible model of inflammatory corneal angiogenesis. Sci. Rep. 2016, 6, 32137. [Google Scholar] [CrossRef] [PubMed]
- Skobe, M.; Dana, R. Blocking the path of lymphatic vessels. Nat. Med. 2009, 15, 993–994. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Wang, C.C.; Adamis, A.P. Ocular Neovascularization: An Epidemiologic Review. Surv. Ophthalmol. 1998, 43, 245–269. [Google Scholar] [CrossRef]
- Senturk, B.; Cubuk, M.O.; Ozmen, M.C.; Aydin, B.; Guler, M.O.; Tekinay, A.B. Inhibition of VEGF mediated corneal neovascularization by anti-angiogenic peptide nanofibers. Biomaterials 2016, 107, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, C.-H.; Huang, S.; Chen, J. Wnt Signaling in vascular eye diseases. Prog. Retin. Eye Res. 2019, 70, 110–133. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yin, Y.; Zhao, J.; Li, Y.; Wang, Y.; Zhang, Z.; Niu, L.; Zheng, Y. An Update on Novel Ocular Nanosystems with Possible Benefits in the Treatment of Corneal Neovascularization. Int. J. Nanomed. 2022, 17, 4911–4931. [Google Scholar] [CrossRef] [PubMed]
- Liarakos, V.S.; Papaconstantinou, D.; Vergados, I.; Douvali, M.; Theodossiadis, P.G. The Effect of Subconjunctival Ranibizumab on Corneal and Anterior Segment Neovascularization: Study on an Animal Model. Eur. J. Ophthalmol. 2014, 24, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Comstock, T.L.; Holland, E.J. Loteprednol and tobramycin in combination: A review of their impact on current treatment regimens. Expert Opin. Pharmacother. 2010, 11, 843–852. [Google Scholar] [CrossRef]
- Fan, Y.; Wei, J.; Guo, L.; Zhao, S.; Xu, C.; Sun, H.; Guo, T. Osthole Reduces Mouse IOP Associated with Ameliorating Extracellular Matrix Expression of Trabecular Meshwork Cell. Investig. Opthalmol. Vis. Sci. 2020, 61, 38. [Google Scholar] [CrossRef]
- Zhang, Q.-Y.; Tao, S.-Y.; Lu, C.; Li, J.-J.; Li, X.-M.; Jiang, Q.; Yan, B. Osthole: A Traditional Chinese Medicine for Ocular Anti-Angiogenic Therapy. Ophthalm. Res. 2020, 63, 483–490. [Google Scholar] [CrossRef]
- Sun, C.; Gui, Y.; Hu, R.; Chen, J.; Wang, B.; Guo, Y.; Lu, W.; Nie, X.; Shen, Q.; Gao, S.; et al. Preparation and Pharmacokinetics Evaluation of Solid Self-Microemulsifying Drug Delivery System (S-SMEDDS) of Osthole. AAPS PharmSciTech 2018, 19, 2301–2310. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Shen, L.; Li, Z.; Zhang, X.; Wu, M.; Zhang, Y.; Liu, J. Design, Preparation, and Evaluation of Osthol Poly-Butyl-Cyanoacrylate Nanoparticles with Improved In Vitro Anticancer Activity in Neuroblastoma Treatment. Molecules 2022, 27, 6908. [Google Scholar] [CrossRef]
- Song, Y.; Wang, X.; Wang, X.; Wang, J.; Hao, Q.; Hao, J.; Hou, X. Osthole-Loaded Nanoemulsion Enhances Brain Target in the Treatment of Alzheimer’s Disease via Intranasal Administration. Oxid. Med. Cell. Longev. 2021, 2021, 8844455. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Li, X.-T.; Ni, Y.-N.; Xiao, H.-H.; Yao, Y.-J.; Wang, Y.-Y.; Ju, R.-J.; Li, H.-Y.; Liu, J.-J.; Fu, M.; et al. Transferrin-Modified Osthole PEGylated Liposomes Travel the Blood-Brain Barrier and Mitigate Alzheimer’s Disease-Related Pathology in APP/PS-1 Mice. Int. J. Nanomed. 2020, 15, 2841–2858. [Google Scholar] [CrossRef] [PubMed]
- Bachu, R.D.; Chowdhury, P.; Al-Saedi, Z.H.F.; Karla, P.K.; Boddu, S.H.S. Ocular Drug Delivery Barriers—Role of Nanocarriers in the Treatment of Anterior Segment Ocular Diseases. Pharmaceutics 2018, 10, 28. [Google Scholar] [CrossRef] [PubMed]
- Gautam, N.; Kesavan, K. Development of microemulsions for ocular delivery. Ther. Deliv. 2017, 8, 313–330. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Nair, A.B.; Shah, J.; Gupta, S.; Boddu, S.H.S.; Sreeharsha, N.; Joseph, A.; Shinu, P.; Morsy, M.A. Lipid Nanoparticles as a Promising Drug Delivery Carrier for Topical Ocular Therapy—An Overview on Recent Advances. Pharmaceutics 2022, 14, 533. [Google Scholar] [CrossRef]
- Yun, F.; Kang, A.; Shan, J.; Zhao, X.; Bi, X.; Li, J.; Di, L. Preparation of osthole-polymer solid dispersions by hot-melt extrusion for dissolution and bioavailability enhancement. Int. J. Pharm. 2014, 465, 436–443. [Google Scholar] [CrossRef]
- Yang, J.; Liang, Z.; Lu, P.; Song, F.; Zhang, Z.; Zhou, T.; Li, J.; Zhang, J. Development of a Luliconazole Nanoemulsion as a Prospective Ophthalmic Delivery System for the Treatment of Fungal Keratitis: In Vitro and In Vivo Evaluation. Pharmaceutics 2022, 14, 2052. [Google Scholar] [CrossRef]
- Wan, T.; Pan, J.; Long, Y.; Yu, K.; Wang, Y.; Pan, W.; Ruan, W.; Qin, M.; Wu, C.; Xu, Y. Dual roles of TPGS based microemulsion for tacrolimus: Enhancing the percutaneous delivery and anti-psoriatic efficacy. Int. J. Pharm. 2017, 528, 511–523. [Google Scholar] [CrossRef]
- Li, Z.; Liu, R.; Guo, Z.; Chu, D.; Zhu, L.; Zhang, J.; Shuai, X.; Li, J. Celastrol-based nanomedicine promotes corneal allograft survival. J. Nanobiotechnol. 2021, 19, 341. [Google Scholar] [CrossRef] [PubMed]
- Giannaccare, G.; Pellegrini, M.; Bovone, C.; Spena, R.; Senni, C.; Scorcia, V.; Busin, M. Anti-VEGF Treatment in Corneal Diseases. Curr. Drug Targets 2020, 21, 1159–1180. [Google Scholar] [CrossRef] [PubMed]
- Voiculescu, O.B.; Voinea, L.M.; Alexandrescu, C. Corneal neovascularization and biological therapy. J. Med. Life 2015, 8, 444–448. [Google Scholar] [PubMed]
- Torrecilla, J.; Gómez-Aguado, I.; Vicente-Pascual, M.; del Pozo-Rodríguez, A.; Solinís, M.Á.; Rodríguez-Gascón, A. MMP-9 Downregulation with Lipid Nanoparticles for Inhibiting Corneal Neovascularization by Gene Silencing. Nanomaterials 2019, 9, 631. [Google Scholar] [CrossRef]
- Yao, F.; Zhang, L.; Jiang, G.; Liu, M.; Liang, G.; Yuan, Q. Osthole attenuates angiogenesis in an orthotopic mouse model of hepatocellular carcinoma via the downregulation of nuclear factor-κB and vascular endothelial growth factor. Oncol. Lett. 2018, 16, 4471–4479. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Liu, H.; Wang, J.; Feng, S.; Chen, Y.; Shang, Y.; Su, X.; Si, F. Osthole Induces Apoptosis and Inhibits Proliferation, Invasion, and Migration of Human Cervical Carcinoma HeLa Cells. Evid.-Based Complement. Altern. Med. eCAM 2021, 2021, 8885093. [Google Scholar] [CrossRef] [PubMed]
- Ustundag Okur, N.; Çağlar, E.Ş.; Siafaka, P.I. Novel Ocular Drug Delivery Systems: An Update on Microemulsions. J. Ocul. Pharmacol. Ther. 2020, 36, 342–354. [Google Scholar] [CrossRef]
- Kalam, M.A.; Alshamsan, A.; Aljuffali, I.A.; Mishra, A.K.; Sultana, Y. Delivery of gatifloxacin using microemulsion as vehicle: Formulation, evaluation, transcorneal permeation and aqueous humor drug determination. Drug Deliv. 2016, 23, 886–897. [Google Scholar] [CrossRef]
- Gupta, A.; Nayak, K.; Misra, M. Cow ghee fortified ocular topical microemulsion; in vitro, ex vivo, and in vivo evaluation. J. Microencapsul. 2019, 36, 603–621. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Espina, M.; Doktorovova, S.; Souto, E.; García, M. Lipid nanoparticles (SLN, NLC): Overcoming the anatomical and physiological barriers of the eye—Part II—Ocular drug-loaded lipid nanoparticles. Eur. J. Pharm. Biopharm. 2017, 110, 58–69. [Google Scholar] [CrossRef]
- Xi, J.; Chang, Q.; Chan, C.K.; Meng, Z.Y.; Wang, G.N.; Sun, J.B.; Wang, Y.T.; Tong, H.H.Y.; Zheng, Y. Formulation Development and Bioavailability Evaluation of a Self-Nanoemulsified Drug Delivery System of Oleanolic Acid. AAPS PharmSciTech 2009, 10, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Zakkula, A.; Gabani, B.B.; Jairam, R.K.; Kiran, V.; Todmal, U.; Mullangi, R. Preparation and optimization of nilotinib self-micro-emulsifying drug delivery systems to enhance oral bioavailability. Drug Dev. Ind. Pharm. 2020, 46, 498–504. [Google Scholar] [CrossRef]
- Liang, Z.Z.Z.; Zhang, Z.; Yang, J.; Lu, P.; Zhou, T.; Li, J.; Zhang, J. Assessment to the Antifungal Effects in vitro and the Ocular Pharmacokinetics of Solid-Lipid Nanoparticle in Rabbits. Int. J. Nanomed. 2021, 16, 7847–7857. [Google Scholar] [CrossRef] [PubMed]
- Wilhelmus, K.R. The Draize Eye Test. Surv. Ophthalmol. 2001, 45, 493–515. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Miao, Y.; Li, Z.; Lu, P.; Liang, Z.; Yang, J.; He, J.; Xia, H.; Zhang, Z.; Zhang, J. A Comparative Ocular Pharmacokinetics Study of Preservative-Free Latanoprost Unit-Dose Eye Drops and a Benzalkonium Chloride-Preserved Branded Product Following Topical Application to Rabbits. J. Ocul. Pharmacol. Ther. 2020, 36, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Mohammadpour, M.; Hashemi, H.; Jabbarvand, M.; Delrish, E.M. Penetration of Silicate Nanoparticles into the Corneal Stroma and Intraocular Fluids. Cornea 2014, 33, 738–743. [Google Scholar] [CrossRef]
- Rahman, M.A.; Hussain, A.; Hussain, S.; Mirza, M.A.; Iqbal, Z. Role of excipients in successful development of self-emulsifying/microemulsifying drug delivery system (SEDDS/SMEDDS). Drug Dev. Ind. Pharm. 2013, 39, 1–19. [Google Scholar] [CrossRef]
- Moiseev, R.V.; Morrison, P.W.J.; Steele, F.; Khutoryanskiy, V.V. Penetration Enhancers in Ocular Drug Delivery. Pharmaceutics 2019, 11, 321. [Google Scholar] [CrossRef]
- Liu, D.; Li, J.; Cheng, B.; Wu, Q.; Pan, H. Ex Vivo and in Vivo Evaluation of the Effect of Coating a Coumarin-6-Labeled Nanostructured Lipid Carrier with Chitosan-N-acetylcysteine on Rabbit Ocular Distribution. Mol. Pharm. 2017, 14, 2639–2648. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, S.; He, Y.; Yao, B.; Zhang, Y. Regulation of matrix metalloproteinases 2 and 9 in corneal neovascularization. Chem. Biol. Drug Des. 2020, 95, 485–492. [Google Scholar] [CrossRef]
- An, F.; Wang, S.H.; Zhang, D.S.; Zhang, L.; Mu, J.X. Pharmacokinetics of osthole in rabbits. Yao Xue Xue Bao Acta Pharm. Sin. 2003, 38, 571–573. [Google Scholar]
- Li, Y.; Meng, F.; Xiong, Z.; Liu, H.; Li, F. HPLC Determination and Pharmacokinetics of Osthole in Rat Plasma after Oral Administration of Fructus Cnidii Extract. J. Chromatogr. Sci. 2005, 43, 426–429. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, P.; Jain, A.; Srivastava, A.K.; Keshari, M.K. Systematic development and characterization of curcumin-loaded nanogel for topical application. Drug Dev. Ind. Pharm. 2020, 46, 1443–1457. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Yang, J.; Luo, Q.; Shi, J.; Xu, H.; Zhang, J. Preparation and in vitro and in vivo evaluation of an isoliquiritigenin-loaded ophthalmic nanoemulsion for the treatment of corneal neovascularization. Drug Deliv. 2022, 29, 2217–2233. [Google Scholar] [CrossRef] [PubMed]
- Shao, B.; Sun, L.; Xu, N.; Gu, H.; Ji, H.; Wu, L. Development and Evaluation of Topical Delivery of Microemulsions Containing Adapalene (MEs-Ap) for Acne. AAPS PharmSciTech 2021, 22, 125. [Google Scholar] [CrossRef] [PubMed]
- Farghaly, D.A.; Aboelwafa, A.A.; Hamza, M.Y.; Mohamed, M.I. Microemulsion for topical delivery of fenoprofen calcium: In vitro and in vivo evaluation. J. Liposome Res. 2018, 28, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yan, W.; Tian, Y.; Xue, H.; Tang, J.; Zhang, L. Self-Microemulsifying Drug Delivery System of Phillygenin: Formulation Development, Characterization and Pharmacokinetic Evaluation. Pharmaceutics 2020, 12, 130. [Google Scholar] [CrossRef]
- Tang, S.Y.; Manickam, S.; Wei, T.K.; Nashiru, B. Formulation development and optimization of a novel Cremophore EL-based nanoemulsion using ultrasound cavitation. Ultrason. Sonochem. 2012, 19, 330–345. [Google Scholar] [CrossRef]
- Galooyak, S.S.; Dabir, B. Three-factor response surface optimization of nano-emulsion formation using a microfluidizer. J. Food Sci. Technol. 2015, 52, 2558–2571. [Google Scholar] [CrossRef]
- Li, Y.-H.; Wang, Y.-S.; Zhao, J.-S.; Li, Z.-Y.; Chen, H.-H. A pH-sensitive curcumin loaded microemulsion-filled alginate and porous starch composite gels: Characterization, in vitro release kinetics and biological activity. Int. J. Biol. Macromol. 2021, 182, 1863–1873. [Google Scholar] [CrossRef]
- Yang, W.; Li, H.; Pan, T.; Cui, Y.; Li, X.; Gao, J.; Shen, S. Improved oral bioavailability of poorly water-soluble glimepiride by utilizing microemulsion technique. Int. J. Nanomed. 2016, 11, 3777–3788. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yan, J.; Zhou, Z.; Amsden, B.G. Dithiol-PEG-PDLLA Micelles: Preparation and Evaluation as Potential Topical Ocular Delivery Vehicle. Biomacromolecules 2014, 15, 1346–1354. [Google Scholar] [CrossRef] [PubMed]
- Rasoanirina, B.N.V.; Lassoued, M.A.; Miladi, K.; Razafindrakoto, Z.; Chaâbane-Banaoues, R.; Ramanitrahasimbola, D.; Cornet, M.; Sfar, S. Self-nanoemulsifying drug delivery system to improve transcorneal permeability of voriconazole: In-vivo studies. J. Pharm. Pharmacol. 2020, 72, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Teba, H.E.; Khalil, I.A.; El Sorogy, H.M. Novel cubosome based system for ocular delivery of acetazolamide. Drug Deliv. 2021, 28, 2177–2186. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Tao, Q.; Hou, D.; Hu, S.; Tian, S.; Chen, Y.; Gui, R.; Yang, L.; Wang, Y. A novel ion-exchange carrier based upon liposome-encapsulated montmorillonite for ophthalmic delivery of betaxolol hydrochloride. Int. J. Nanomed. 2017, 12, 1731–1745. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ma, Y.; Luo, Q.; Liang, Z.; Lu, P.; Song, F.; Zhang, Z.; Zhou, T.; Zhang, J. Improving the solubility of vorinostat using cyclodextrin inclusion complexes: The physicochemical characteristics, corneal permeability and ocular pharmacokinetics of the drug after topical application. Eur. J. Pharm. Sci. 2022, 168, 106078. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ma, B.; Zhang, Q.; Yang, X.; Sun, J.; Tang, B.; Cui, G.; Yao, D.; Liu, L.; Gu, G.; et al. Simultaneous determination of osthole, bergapten and isopimpinellin in rat plasma and tissues by liquid chromatography–tandem mass spectrometry. J. Chromatogr. B 2014, 970, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Yang, J.; Xu, H.; Shi, J.; Liang, Z.; Zhang, R.; Lu, P.; Pu, G.; Zhao, N.; Zhang, J. Sorafenib-loaded nanostructured lipid carriers for topical ocular therapy of corneal neovascularization: Development, in-vitro and in vivo study. Drug Deliv. 2022, 29, 837–855. [Google Scholar] [CrossRef]
- Irani, Y.D.; Scotney, P.D.; Klebe, S.; Mortimer, L.A.; Nash, A.D.; Williams, K.A. An Anti–VEGF-B Antibody Fragment Induces Regression of Pre-Existing Blood Vessels in the Rat Cornea. Investig. Opthalmol. Vis. Sci. 2017, 58, 3404–3413. [Google Scholar] [CrossRef]
- Li, Z.; Li, J.; Zhu, L.; Zhang, Y.; Zhang, J.; Yao, L.; Liang, D.; Wang, L. Celastrol nanomicelles attenuate cytokine secretion in macrophages and inhibit macrophage-induced corneal neovascularization in rats. Int. J. Nanomed. 2016, 11, 6135–6148. [Google Scholar] [CrossRef]
- Huang, X.; Han, Y.; Shao, Y.; Yi, J.-L. Efficacy of the nucleotide-binding oligomerzation domain 1 inhibitor Nodinhibit-1 on corneal alkali burns in rats. Int. J. Ophthalmol. 2015, 8, 860–865. [Google Scholar] [CrossRef]











| Independent Variables | Value of Response | |||||
|---|---|---|---|---|---|---|
| Actual Value | Predicted Value | |||||
| Formulation | X1(%) | X2 | Y1 (nm) | Y2 | Y1 (nm) | Y2 |
| 1 | 2 | 3 | 14.51 | 0.194 | 14.31 | 0.199 |
| 2 | 4.56066 | 3.70711 | 34.39 | 0.397 | 34.92 | 0.397 |
| 3 | 4.56066 | 2.29289 | 31.14 | 0.369 | 31.93 | 0.362 |
| 4 | 3.5 | 3 | 22.07 | 0.142 | 21.97 | 0.148 |
| 5 | 3.5 | 3 | 20.30 | 0.134 | 20.07 | 0.131 |
| 6 | 3.5 | 3 | 20.36 | 0.139 | 20.01 | 0.135 |
| 7 | 2.43934 | 2.29289 | 16.68 | 0.093 | 17.22 | 0.095 |
| 8 | 3.5 | 2 | 34.67 | 0.387 | 34.85 | 0.392 |
| 9 | 5 | 3 | 34.39 | 0.384 | 34.46 | 0.388 |
| 10 | 3.5 | 4 | 19.84 | 0.083 | 19.88 | 0.087 |
| 11 | 3.5 | 3 | 20.28 | 0.139 | 20.07 | 0.148 |
| 12 | 3.5 | 3 | 21.02 | 0.131 | 21.01 | 0.137 |
| 13 | 2.43934 | 3.70711 | 21.32 | 0.237 | 20.93 | 0.244 |
| DS (nm) | PDI | pH | Osmolarity (mOsm/kg) | EE (%) | DL (%) | ZP (mv) |
|---|---|---|---|---|---|---|
| 16.18 ± 0.02 | 0.09 ± 0.00 | 6.61 ± 0.99 | 298.89 ± 1.54 | 99.15 ± 0.66 | 3.70 ± 0.53 | −1.18 ± 0.97 |
| Mathematical Models | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Formulation | Zero-order | First-order | Higuchi | Korsmeyer–Peppas | |||||
| K(h) | R2 | K(h) | R2 | K(h1/2) | R2 | K(h) | n | R2 | |
| OST-ME | 0.77 | 0.92 | 0.25 | 0.86 | 5.42 | 0.99 | 7.62 | 0.42 | 0.98 |
| OST-Susp | 0.09 | 0.13 | 0.51 | 0.94 | 1.23 | 0.38 | 10.14 | 0.16 | 0.14 |
| Tissue | Pharmacokinetic Parameters | Unit | OST-ME | OST-Susp |
|---|---|---|---|---|
| Cornea | Cmax | μg/g | 1.56 ± 0.55 | 0.18 ± 0.21 |
| Tmax | min | 5 | 5 | |
| T1/2 | min | 28 | / | |
| AUC0-t | μg/g·min | 19.74 | 0.7 | |
| Conjunctiva | Cmax | μg/g | 2.44 ± 0.14 | 0.15 ± 0.11 |
| Tmax | min | 10 | 5 | |
| T1/2 | min | 25 | / | |
| AUC0-t | μg/g·min | 63.96 | 0.625 |
| Levels | |||||
|---|---|---|---|---|---|
| Factors (independent variables) | −1.414 | −1 | 0 | 1 | 1.414 |
| X1 (oil concentration, %) | 2 | 2.44 | 3.5 | 4.56 | 5 |
| X2 (Km range) | 2 | 2.29 | 3 | 3.71 | 4 |
| Responses (dependent variables) | Desirability constraints | ||||
| Y1: DS (nm) | Minimize | ||||
| Y2: PDI | Minimize | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Yang, J.; Ji, Y.; Liang, Z.; Wang, Y.; Zhang, J. Development of Osthole-Loaded Microemulsions as a Prospective Ocular Delivery System for the Treatment of Corneal Neovascularization: In Vitro and In Vivo Assessments. Pharmaceuticals 2023, 16, 1342. https://doi.org/10.3390/ph16101342
Zhang Y, Yang J, Ji Y, Liang Z, Wang Y, Zhang J. Development of Osthole-Loaded Microemulsions as a Prospective Ocular Delivery System for the Treatment of Corneal Neovascularization: In Vitro and In Vivo Assessments. Pharmaceuticals. 2023; 16(10):1342. https://doi.org/10.3390/ph16101342
Chicago/Turabian StyleZhang, Yali, Jingjing Yang, Yinjian Ji, Zhen Liang, Yuwei Wang, and Junjie Zhang. 2023. "Development of Osthole-Loaded Microemulsions as a Prospective Ocular Delivery System for the Treatment of Corneal Neovascularization: In Vitro and In Vivo Assessments" Pharmaceuticals 16, no. 10: 1342. https://doi.org/10.3390/ph16101342
APA StyleZhang, Y., Yang, J., Ji, Y., Liang, Z., Wang, Y., & Zhang, J. (2023). Development of Osthole-Loaded Microemulsions as a Prospective Ocular Delivery System for the Treatment of Corneal Neovascularization: In Vitro and In Vivo Assessments. Pharmaceuticals, 16(10), 1342. https://doi.org/10.3390/ph16101342