Bispecific Antibodies in Lung Cancer: A State-of-the-Art Review
Abstract
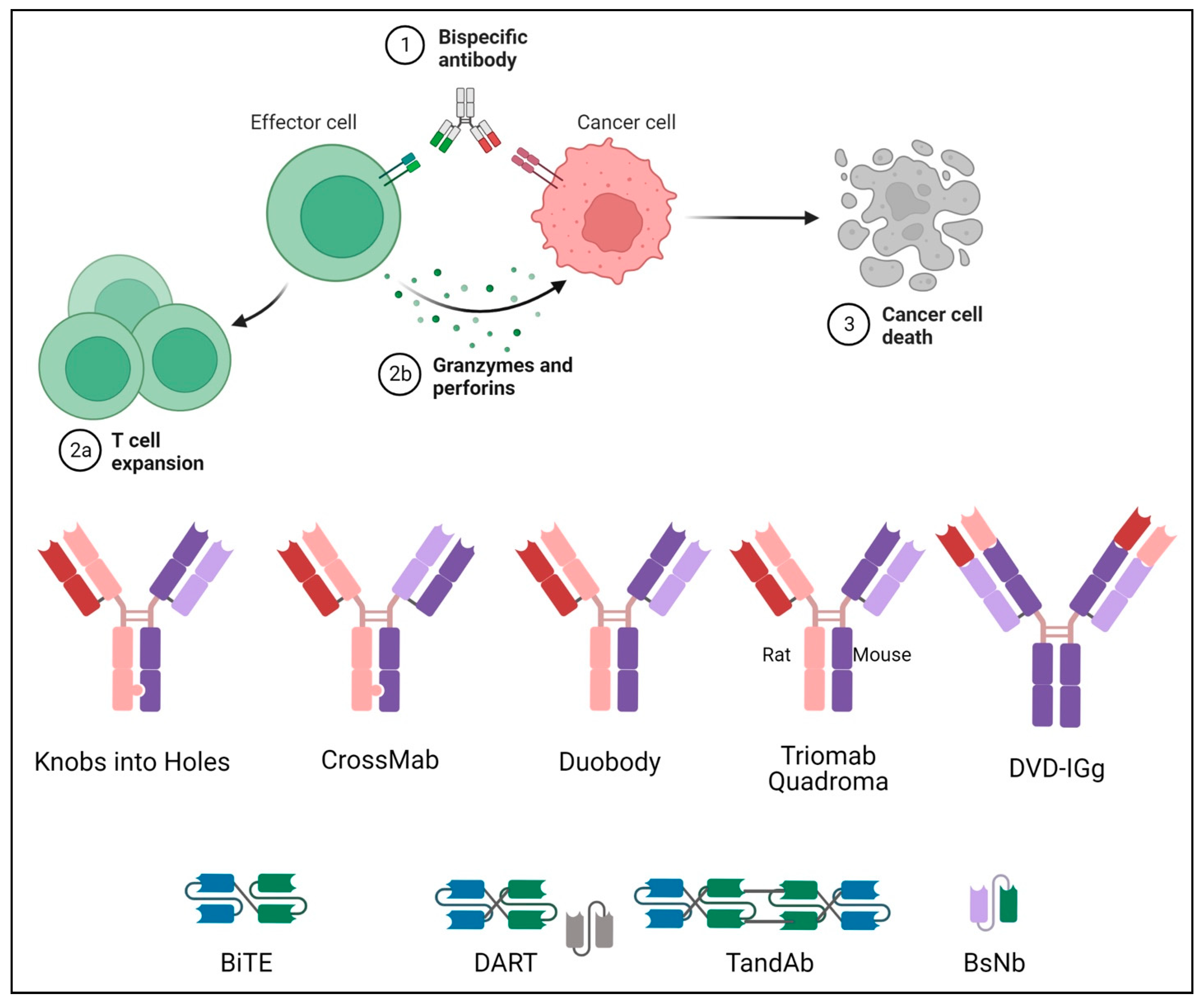
:1. Introduction
2. Structure of Bispecific Antibodies
2.1. Fc-Bearing Subtypes
2.1.1. IgG-like
Knobs-into-Holes
CrossMab
DuoBody
Triomab Quadroma
2.1.2. IgG-Modified
Dual-Variable Domains Ig (DVD-Ig)
2.2. Fc-Free Fragment-Based Subtypes
2.2.1. Bispecific T-Cell Engager (BiTE)
2.2.2. Dual-Affinity Re-Targeting Molecules (DART)
2.2.3. Tandem Diabodies (TandAb)
2.2.4. Bispecific Nanobody (BsNb)
3. Application in Lung Cancer
3.1. MET Targeted
3.1.1. Amivantamab
3.1.2. Bafisontamab (EMB-01)
3.2. DLL3-Targeted
Tarlatamab (AMG 757)
3.3. EpCAM Targeted
3.3.1. Catumaxomab
3.3.2. Solitomab (MT110, AMG 110)
3.4. HER2/HER3 Targeted
3.4.1. Zenocutuzumab (MCLA-128)
3.4.2. MM-111
3.4.3. Izalontamab (SI-B001)
3.4.4. Zanidatamab (ZW25)
3.5. CEA Targeted
MEDI-565 (MT111, AMG211)
3.6. Immune-Checkpoint Targeted
3.6.1. LY3434172
3.6.2. LY3415244
3.6.3. Erfonrilimab (KN046)
3.6.4. Bintrafusp Alfa (M7824)
3.6.5. SHR-1701
3.6.6. Tebotelimab (MGD013)
3.6.7. MEDI5752
3.6.8. AZD2936
3.7. VEGF Targeted
3.7.1. Ivonescimab (AK112)
3.7.2. PM8002
3.7.3. IMM2510
3.7.4. HB0025
3.7.5. Dilpacimab (ABT-165)
4. Challenges and Future Prospects
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- CDC. An Update on Cancer Deaths in the United States 2022. Available online: https://www.cdc.gov/cancer/dcpc/research/update-on-cancer-deaths/index.htm (accessed on 28 February 2022).
- Lewis, D.R.; Check, D.P.; Caporaso, N.E.; Travis, W.D.; Devesa, S.S. US lung cancer trends by histologic type. Cancer 2014, 120, 2883–2892. [Google Scholar] [CrossRef]
- Abu Rous, F.; Singhi, E.K.; Sridhar, A.; Faisal, M.S.; Desai, A. Lung Cancer Treatment Advances in 2022. Cancer Investig. 2023, 41, 12–24. [Google Scholar] [CrossRef]
- Gandhi, L.; Rodríguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef]
- Horn, L.; Mansfield, A.S.; Szczęsna, A.; Havel, L.; Krzakowski, M.; Hochmair, M.J.; Huemer, F.; Losonczy, G.; Johnson, M.L.; Nishio, M.; et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2220–2229. [Google Scholar] [CrossRef]
- Pirker, R.; Pereira, J.R.; Szczesna, A.; von Pawel, J.; Krzakowski, M.; Ramlau, R.; Vynnychenko, I.; Park, K.; Yu, C.-T.; Ganul, V.; et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): An open-label randomised phase III trial. Lancet 2009, 373, 1525–1531. [Google Scholar] [CrossRef]
- Desai, A.; Abdayem, P.; Adjei, A.A.; Planchard, D. Antibody-drug conjugates: A promising novel therapeutic approach in lung cancer. Lung Cancer 2022, 163, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Kantarjian, H.; Stein, A.; Gökbuget, N.; Fielding, A.K.; Schuh, A.C.; Ribera, J.M.; Wei, A.; Dombret, H.; Foà, R.; Bassan, R.; et al. Blinatumomab versus Chemotherapy for Advanced Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2017, 376, 836–847. [Google Scholar] [CrossRef]
- Nisonoff, A.; Rivers, M.M. Recombination of a mixture of univalent antibody fragments of different specificity. Arch. Biochem. Biophys. 1961, 93, 460–462. [Google Scholar] [CrossRef] [PubMed]
- Garber, K. Bispecific antibodies rise again. Nat. Rev. Drug Discov. 2014, 13, 799–801. [Google Scholar] [CrossRef] [PubMed]
- Chames, P.; Baty, D. Bispecific antibodies for cancer therapy. Curr. Opin. Drug Discov. Devel. 2009, 12, 276–283. [Google Scholar] [CrossRef]
- Atwell, S.; Ridgway, J.B.; Wells, J.A.; Carter, P. Stable heterodimers from remodeling the domain interface of a homodimer using a phage display library. J. Mol. Biol. 1997, 270, 26–35. [Google Scholar] [CrossRef]
- Ridgway, J.B.; Presta, L.G.; Carter, P. ‘Knobs-into-holes’ engineering of antibody CH3 domains for heavy chain heterodimerization. Protein Eng. 1996, 9, 617–621. [Google Scholar] [CrossRef]
- Merchant, A.M.; Zhu, Z.; Yuan, J.Q.; Goddard, A.; Adams, C.W.; Presta, L.G.; Carter, P. An efficient route to human bispecific IgG. Nat. Biotechnol. 1998, 16, 677–681. [Google Scholar] [CrossRef]
- Klein, C.; Sustmann, C.; Thomas, M.; Stubenrauch, K.; Croasdale, R.; Schanzer, J.; Brinkmann, U.; Kettenberger, H.; Regula, J.T.; Schaefer, W. Progress in overcoming the chain association issue in bispecific heterodimeric IgG antibodies. MAbs 2012, 4, 653–663. [Google Scholar] [CrossRef]
- Kuglstatter, A.; Stihle, M.; Neumann, C.A.; Müller, C.; Schaefer, W.; Klein, C.; Benz, J.; Roche Pharmaceutical Research and Early Development. Structural differences between glycosylated, disulfide-linked heterodimeric Knob-into-Hole Fc fragment and its homodimeric Knob-Knob and Hole-Hole side products. Protein Eng. Des. Sel. 2017, 30, 649–656. [Google Scholar] [PubMed]
- Goulet, D.R.; Orcutt, S.J.; Zwolak, A.; Rispens, T.; Labrijn, A.F.; de Jong, R.N.; Atkins, W.M.; Chiu, M.L. Kinetic mechanism of controlled Fab-arm exchange for the formation of bispecific immunoglobulin G1 antibodies. J. Biol. Chem. 2018, 293, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, Y.; Fan, D.; Xiong, D. The development of bispecific antibodies and their applications in tumor immune escape. Exp. Hematol. Oncol. 2017, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Linke, R.; Klein, A.; Seimetz, D. Catumaxomab: Clinical development and future directions. MAbs 2010, 2, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Ghayur, T. Recent advancements in DVD-Ig based therapeutic development. Drug Discov. Today Technol. 2019, 34, 1–7. [Google Scholar] [CrossRef]
- Ayyar, B.V.; Arora, S.; O’Kennedy, R. Coming-of-Age of Antibodies in Cancer Therapeutics. Trends Pharmacol. Sci. 2016, 37, 1009–1028. [Google Scholar] [CrossRef]
- Demarest, S.J.; Glaser, S.M. Antibody therapeutics, antibody engineering, and the merits of protein stability. Curr. Opin. Drug Discov. Devel. 2008, 11, 675–687. [Google Scholar]
- Baeuerle, P.A.; Kufer, P.; Bargou, R. BiTE: Teaching antibodies to engage T-cells for cancer therapy. Curr. Opin. Mol. Ther. 2009, 11, 22–30. [Google Scholar]
- Klinger, M.; Benjamin, J.; Kischel, R.; Stienen, S.; Zugmaier, G. Harnessing T cells to fight cancer with BiTE® antibody constructs--past developments and future directions. Immunol. Rev. 2016, 270, 193–208. [Google Scholar] [CrossRef]
- Duell, J.; Lammers, P.E.; Djuretic, I.; Chunyk, A.G.; Alekar, S.; Jacobs, I.; Gill, S. Bispecific Antibodies in the Treatment of Hematologic Malignancies. Clin. Pharmacol. Ther. 2019, 106, 781–791. [Google Scholar] [CrossRef]
- Einsele, H.; Borghaei, H.; Orlowski, R.; Subklewe, M.; Roboz, G.J.; Zugmaier, G.; Kufer, P.; Iskander, K.; Kantarjian, H.M. The BiTE (bispecific T-cell engager) platform: Development and future potential of a targeted immuno-oncology therapy across tumor types. Cancer 2020, 126, 3192–3201. [Google Scholar] [CrossRef]
- Johnson, S.; Burke, S.; Huang, L.; Gorlatov, S.; Li, H.; Wang, W.; Zhang, W.; Tuaillon, N.; Rainey, J.; Barat, B.; et al. Effector cell recruitment with novel Fv-based dual-affinity re-targeting protein leads to potent tumor cytolysis and in vivo B-cell depletion. J. Mol. Biol. 2010, 399, 436–449. [Google Scholar] [CrossRef] [PubMed]
- Maschmeyer, G.; Bullinger, L.; Garcia-Vidal, C.; Herbrecht, R.; Maertens, J.; Menna, P.; Pagano, L.; Thiebaut-Bertrand, A.; Calandra, T. Infectious complications of targeted drugs and biotherapies in acute leukemia. Clinical practice guidelines by the European Conference on Infections in Leukemia (ECIL), a joint venture of the European Group for Blood and Marrow Transplantation (EBMT), the European Organization for Research and Treatment of Cancer (EORTC), the International Immunocompromised Host Society (ICHS) and the European Leukemia Net (ELN). Leukemia 2022, 36, 1215–1226. [Google Scholar] [PubMed]
- Wang, Q.; Chen, Y.; Park, J.; Liu, X.; Hu, Y.; Wang, T.; McFarland, K.; Betenbaugh, M.J. Design and Production of Bispecific Antibodies. Antibodies 2019, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Fu, J.; Zhang, M.; Liu, D. AFM13: A first-in-class tetravalent bispecific anti-CD30/CD16A antibody for NK cell-mediated immunotherapy. J. Hematol. Oncol. 2015, 8, 96. [Google Scholar] [CrossRef]
- Nieto, Y.; Banerjee, P.; Kaur, I.; Bassett, R.; Kerbauy, L.; Basar, R.; Kaplan, M.; Griffin, L.; Esqueda, D.; Ganesh, C.; et al. Abstract CT003: Innate cell engager (ICE®) AFM13 combined with preactivated and expanded cord blood (CB)-derived NK cells for patients with refractory/relapsed CD30+ lymphoma. Cancer Res. 2022, 82 (Suppl. S12), CT003-CT. [Google Scholar] [CrossRef]
- Salvador, J.P.; Vilaplana, L.; Marco, M.P. Nanobody: Outstanding features for diagnostic and therapeutic applications. Anal. Bioanal. Chem. 2019, 411, 1703–1713. [Google Scholar] [CrossRef] [PubMed]
- Ishiwatari-Ogata, C.; Kyuuma, M.; Ogata, H.; Yamakawa, M.; Iwata, K.; Ochi, M.; Hori, M.; Miyata, N.; Fujii, Y. Ozoralizumab, a Humanized Anti-TNFα NANOBODY(®) Compound, Exhibits Efficacy Not Only at the Onset of Arthritis in a Human TNF Transgenic Mouse but Also During Secondary Failure of Administration of an Anti-TNFα IgG. Front. Immunol. 2022, 13, 853008. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, U.; Kontermann, R.E. The making of bispecific antibodies. MAbs 2017, 9, 182–212. [Google Scholar] [CrossRef] [PubMed]
- FDA. Bispecific Antibodies: An Area of Research and Clinical Applications. 2023. Available online: https://www.fda.gov/drugs/news-events-human-drugs/bispecific-antibodies-area-research-and-clinical-applications (accessed on 15 August 2023).
- FDA. FDA Grants Accelerated Approval to Elranatamab-bcmm for Multiple Myeloma. 2023. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-elranatamab-bcmm-multiple-myeloma (accessed on 15 August 2023).
- Dolgin, E. Amivantamab OK’d for EGFR-Mutant NSCLC. Cancer Discov. 2021, 11, 1604. [Google Scholar]
- Shigematsu, H.; Lin, L.; Takahashi, T.; Nomura, M.; Suzuki, M.; Wistuba, I.I.; Fong, K.M.; Lee, H.; Toyooka, S.; Shimizu, N.; et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J. Natl. Cancer Inst. 2005, 97, 339–346. [Google Scholar] [CrossRef]
- Drilon, A.; Cappuzzo, F.; Ou, S.I.; Camidge, D.R. Targeting MET in Lung Cancer: Will Expectations Finally Be MET? J. Thorac. Oncol. 2017, 12, 15–26. [Google Scholar] [CrossRef]
- Engelman, J.A.; Zejnullahu, K.; Mitsudomi, T.; Song, Y.; Hyland, C.; Park, J.O.; Lindeman, N.; Gale, C.M.; Zhao, X.; Christensen, J.; et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 2007, 316, 1039–1043. [Google Scholar] [CrossRef]
- Yun, J.; Lee, S.H.; Kim, S.Y.; Jeong, S.Y.; Kim, J.H.; Pyo, K.H.; Park, C.W.; Heo, S.G.; Yun, M.R.; Lim, S.; et al. Antitumor Activity of Amivantamab (JNJ-61186372), an EGFR-MET Bispecific Antibody, in Diverse Models of EGFR Exon 20 Insertion-Driven NSCLC. Cancer Discov. 2020, 10, 1194–1209. [Google Scholar] [CrossRef]
- Brazel, D.; Nagasaka, M. Spotlight on Amivantamab (JNJ-61186372) for EGFR Exon 20 Insertions Positive Non-Small Cell Lung Cancer. Lung Cancer 2021, 12, 133–138. [Google Scholar] [CrossRef]
- Park, K.; Haura, E.B.; Leighl, N.B.; Mitchell, P.; Shu, C.A.; Girard, N.; Viteri, S.; Han, J.-Y.; Kim, S.-W.; Lee, C.K.; et al. Amivantamab in EGFR Exon 20 Insertion-Mutated Non-Small-Cell Lung Cancer Progressing on Platinum Chemotherapy: Initial Results From the CHRYSALIS Phase I Study. J. Clin. Oncol. 2021, 39, 3391–3402. [Google Scholar] [CrossRef]
- Besse, B.; Baik, C.S.; Marmarelis, M.E.; Sabari, J.K.; Goto, K.; Shu, C.A.; Lee, J.-S.; Ou, S.-H.I.; Cho, B.C.; Waqar, S.N.; et al. Predictive biomarkers for treatment with amivantamab plus lazertinib among EGFR-mutated NSCLC in the post-osimertinib setting: Analysis of tissue IHC and ctDNA NGS. J. Clin. Oncol. 2023, 41 (Suppl. S16), 9013. [Google Scholar] [CrossRef]
- Cho, B.C.; Felip, E.; Hayashi, H.; Thomas, M.; Lu, S.; Besse, B.; Sun, T.; Martinez, M.; Sethi, S.N.; Shreeve, S.M.; et al. MARIPOSA: Phase 3 study of first-line amivantamab + lazertinib versus osimertinib in EGFR-mutant non-small-cell lung cancer. Future Oncol. 2022, 18, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Wu, X.; Yang, D.; Wu, D.; Gong, S.; Zhang, Y.; Lensky, S.; Wu, C. Abstract 528: EMB-01: An innovative bispecific antibody targeting EGFR and cMet on tumor cells mediates a novel mechanism to improve anti-tumor efficacy. Cancer Res. 2020, 80 (Suppl. S16), 528. [Google Scholar] [CrossRef]
- Qing, Z.; Gabrail, N.; Uprety, D.; Rotow, J.; Han, B.; Jänne, P.; Nagasaka, M.; Zheng, M.; Zhang, Y.; Yang, G.; et al. 22P EMB-01: An EGFR-cMET bispecific antibody, in advanced/metastatic solid tumors phase I results. Ann. Oncol. 2022, 33, S39–S40. [Google Scholar] [CrossRef]
- Ramalingam, S.S.; Ahn, M.J.; Akamatsu, H.; Blackhall, F.H.; Borghaei, H.; Hummel, H.D.; Johnson, M.L.; Reck, M.; Zhang, Y.; Jandial, D.; et al. Phase 2 study of tarlatamab, a DLL3-targeting, half life–extended, bispecific T-cell engager (HLE BiTE) immuno-oncology therapy, in relapsed/refractory small cell lung cancer (SCLC). J. Clin. Oncol. 2022, 40 (Suppl. S16), TPS8603-TPS. [Google Scholar] [CrossRef]
- Owonikoko, T.K.; Champiat, S.; Johnson, M.L.; Govindan, R.; Izumi, H.; Lai, W.V.V.; Borghaei, H.; Boyer, M.J.; Boosman, R.J.; Hummel, H.-D.; et al. Updated results from a phase 1 study of AMG 757, a half-life extended bispecific T-cell engager (BiTE) immuno-oncology therapy against delta-like ligand 3 (DLL3), in small cell lung cancer (SCLC). J. Clin. Oncol. 2021, 39 (Suppl. S15), 8510. [Google Scholar] [CrossRef]
- Gires, O.; Pan, M.; Schinke, H.; Canis, M.; Baeuerle, P.A. Expression and function of epithelial cell adhesion molecule EpCAM: Where are we after 40 years? Cancer Metastasis Rev. 2020, 39, 969–987. [Google Scholar] [CrossRef]
- Went, P.; Vasei, M.; Bubendorf, L.; Terracciano, L.; Tornillo, L.; Riede, U.; Kononen, J.; Simon, R.; Sauter, G.; Baeuerle, P.A. Frequent high-level expression of the immunotherapeutic target Ep-CAM in colon, stomach, prostate and lung cancers. Br. J. Cancer 2006, 94, 128–135. [Google Scholar] [CrossRef]
- Sebastian, M.; Passlick, B.; Friccius-Quecke, H.; Jäger, M.; Lindhofer, H.; Kanniess, F.; Wiewrodt, R.; Thiel, E.; Buhl, R.; Schmittel, A. Treatment of non-small cell lung cancer patients with the trifunctional monoclonal antibody catumaxomab (anti-EpCAM x anti-CD3): A phase I study. Cancer Immunol. Immunother. 2007, 56, 1637–1644. [Google Scholar] [CrossRef]
- Wu, Y.; Yi, M.; Zhu, S.; Wang, H.; Wu, K. Recent advances and challenges of bispecific antibodies in solid tumors. Exp. Hematol. Oncol. 2021, 10, 56. [Google Scholar] [CrossRef]
- Brischwein, K.; Schlereth, B.; Guller, B.; Steiger, C.; Wolf, A.; Lutterbuese, R.; Offner, S.; Locher, M.; Urbig, T.; Raum, T.; et al. MT110: A novel bispecific single-chain antibody construct with high efficacy in eradicating established tumors. Mol. Immunol. 2006, 43, 1129–1143. [Google Scholar] [CrossRef] [PubMed]
- Kebenko, M.; Goebeler, M.E.; Wolf, M.; Hasenburg, A.; Seggewiss-Bernhardt, R.; Ritter, B.; Rautenberg, B.; Atanackovic, D.; Kratzer, A.; Rottman, J.B.; et al. A multicenter phase 1 study of solitomab (MT110, AMG 110), a bispecific EpCAM/CD3 T-cell engager (BiTE®) antibody construct, in patients with refractory solid tumors. Oncoimmunology 2018, 7, e1450710. [Google Scholar] [CrossRef] [PubMed]
- Drilon, A.; Somwar, R.; Mangatt, B.P.; Edgren, H.; Desmeules, P.; Ruusulehto, A.; Smith, R.S.; Delasos, L.; Vojnic, M.; Plodkowski, A.J.; et al. Response to ERBB3-Directed Targeted Therapy in NRG1-Rearranged Cancers. Cancer Discov. 2018, 8, 686–695. [Google Scholar] [CrossRef]
- Schultink, A.H.M.d.V.; Doornbos, R.P.; Bakker, A.B.H.; Bol, K.; Throsby, M.; Geuijen, C.; Maussang, D.; Schellens, J.H.M.; Beijnen, J.H.; Huitema, A.D.R. Translational PK-PD modeling analysis of MCLA-128, a HER2/HER3 bispecific monoclonal antibody, to predict clinical efficacious exposure and dose. Investig. New Drugs 2018, 36, 1006–1015. [Google Scholar] [CrossRef] [PubMed]
- Schram, A.M.; Goto, K.; Kim, D.-W.; Martin-Romano, P.; Ou, S.-H.I.; O’Kane, G.M.; O'Reilly, E.M.; Umemoto, K.; Duruisseaux, M.; Neuzillet, C.; et al. Efficacy and safety of zenocutuzumab, a HER2 x HER3 bispecific antibody, across advanced NRG1 fusion (NRG1+) cancers. J. Clin. Oncol. 2022, 40 (Suppl. S16), 105. [Google Scholar] [CrossRef]
- Xue, J.; Kong, D.; Yao, Y.; Yang, L.; Yao, Q.; Zhu, Y.; Ding, Y.; Yang, F.; Gong, J.; Shen, L.; et al. Prediction of Human Pharmacokinetics and Clinical Effective Dose of SI-B001, an EGFR/HER3 Bi-specific Monoclonal Antibody. J. Pharm. Sci. 2020, 109, 3172–3180. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, L.; Fang, W.; Yang, Y.; Huang, Y.; Zou, W.; Wang, Z.; Ding, M.; Peng, Y.; Xiao, S.; et al. SI-B001 plus chemotherapy in patients with locally advanced or metastatic EGFR/ALK wild-type non-small cell lung cancer: A phase II, multicenter, open-label study. J. Clin. Oncol. 2023, 41 (Suppl. S16), 9025. [Google Scholar] [CrossRef]
- Lee, K.-S.; Wang, X.; Im, Y.H.; Zeng, X.; Li, H.; Wang, K.; Li, H.; Zhou, P.; Bao, Y.; Jiang, Z. Zanidatamab (zani), a HER2-targeted bispecific antibody, in combination with docetaxel as first-line (1L) therapy for patients (pts) with advanced HER2-positive breast cancer: Preliminary results from a phase 1b/2 study. J. Clin. Oncol. 2022, 40 (Suppl. S16), 1031. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Beeram, M.; Hamilton, E.; Oh, D.-Y.; Hanna, D.L.; Kang, Y.-K.; Elimova, E.; Chaves, J.; Goodwin, R.; Lee, J.; et al. Zanidatamab, a novel bispecific antibody, for the treatment of locally advanced or metastatic HER2-expressing or HER2-amplified cancers: A phase 1, dose-escalation and expansion study. Lancet Oncol. 2022, 23, 1558–1570. [Google Scholar] [CrossRef]
- Elimova, E.; Ajani, J.A.; Burris, H.A., III; Denlinger, C.S.; Iqbal, S.; Kang, Y.K.; Kim, Y.H.H.; Lee, K.W.; Lin, B.; Mehta, R.; et al. Zanidatamab + chemotherapy as first-line treatment for HER2-expressing metastatic gastroesophageal adenocarcinoma (mGEA). J. Clin. Oncol. 2023, 41 (Suppl. S4), 347. [Google Scholar] [CrossRef]
- Peng, L.; Oberst, M.D.; Huang, J.; Brohawn, P.; Morehouse, C.; Lekstrom, K.; Baeuerle, P.A.; Wu, H.; Yao, Y.; Coats, S.R.; et al. The CEA/CD3-bispecific antibody MEDI-565 (MT111) binds a nonlinear epitope in the full-length but not a short splice variant of CEA. PLoS ONE 2012, 7, e36412. [Google Scholar] [CrossRef] [PubMed]
- You, W.; Yan, L.; Cai, Z.; Xie, L.; Sheng, N.; Wang, G.; Wu, X.; Wang, Z. Clinical Significances of Positive Postoperative Serum CEA and Post-preoperative CEA Increment in Stage II and III Colorectal Cancer: A Multicenter Retrospective Study. Front. Oncol. 2020, 10, 671. [Google Scholar] [CrossRef] [PubMed]
- Grunnet, M.; Sorensen, J.B. Carcinoembryonic antigen (CEA) as tumor marker in lung cancer. Lung Cancer 2012, 76, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, R.D.; Hansen, H.J.; Goldenberg, D.M. Inhibition of adhesion, invasion, and metastasis by antibodies targeting CEACAM6 (NCA-90) and CEACAM5 (Carcinoembryonic Antigen). Cancer Res. 2005, 65, 8809–8817. [Google Scholar] [CrossRef] [PubMed]
- Oberst, M.D.; Fuhrmann, S.; Mulgrew, K.; Amann, M.; Cheng, L.; Lutterbuese, P.; Richman, L.; Coats, S.; Baeuerle, P.A.; Hammond, S.A. CEA/CD3 bispecific antibody MEDI-565/AMG 211 activation of T cells and subsequent killing of human tumors is independent of mutations commonly found in colorectal adenocarcinomas. MAbs 2014, 6, 1571–1584. [Google Scholar] [CrossRef]
- Pishvaian, M.; Morse, M.A.; McDevitt, J.; Norton, J.D.; Ren, S.; Robbie, G.J.; Ryan, P.C.; Soukharev, S.; Bao, H.; Denlinger, C.S. Phase 1 Dose Escalation Study of MEDI-565, a Bispecific T-Cell Engager that Targets Human Carcinoembryonic Antigen, in Patients With Advanced Gastrointestinal Adenocarcinomas. Clin. Colorectal Cancer 2016, 15, 345–351. [Google Scholar] [CrossRef]
- Yu, H.; Boyle, T.A.; Zhou, C.; Rimm, D.L.; Hirsch, F.R. PD-L1 Expression in Lung Cancer. J. Thorac. Oncol. 2016, 11, 964–975. [Google Scholar] [CrossRef] [PubMed]
- Kotanides, H.; Li, Y.; Malabunga, M.; Carpenito, C.; Eastman, S.W.; Shen, Y.; Wang, G.; Inigo, I.; Surguladze, D.; Pennello, A.L.; et al. Bispecific Targeting of PD-1 and PD-L1 Enhances T-cell Activation and Antitumor Immunity. Cancer Immunol. Res. 2020, 8, 1300–1310. [Google Scholar] [CrossRef]
- Hellmann, M.D.; Bivi, N.; Calderon, B.; Shimizu, T.; Delafontaine, B.; Liu, Z.T.; Szpurka, A.M.; Copeland, V.; Hodi, F.S.; Rottey, S.; et al. Safety and Immunogenicity of LY3415244, a Bispecific Antibody Against TIM-3 and PD-L1, in Patients With Advanced Solid Tumors. Clin. Cancer Res. 2021, 27, 2773–2781. [Google Scholar] [CrossRef]
- Zhao, H.; Ma, Y.; Zhang, Y.; Hong, S.; Yang, Y.; Fang, W.; Xu, J.; Van, H.; Kong, P.; Yang, F.; et al. The preliminary efficacy and safety data of KN046 in patients failed on prior immune checkpoint inhibitors therapy. J. Clin. Oncol. 2020, 38 (Suppl. S15), 3020. [Google Scholar] [CrossRef]
- Xu, B.; Li, Q.; Zhang, Q.; Zhang, Y.; Ouyang, Q.; Zhang, Y.; Liu, Q.; Sun, T.; Xu, J.; Yang, J.; et al. Abstract 1660: Preliminary safety tolerability & efficacy results of KN046 (an anti-PD-L1/CTLA-4 bispecific antibody) in combination with Nab-paclitaxel in patients with metastatic triple-negative breast cancer (mTNBC). Cancer Res. 2021, 81 (Suppl. S13), 1660. [Google Scholar]
- Lan, Y.; Zhang, D.; Xu, C.; Hance, K.W.; Marelli, B.; Qi, J.; Yu, H.; Qin, G.; Sircar, A.; Hernández, V.M.; et al. Enhanced preclinical antitumor activity of M7824, a bifunctional fusion protein simultaneously targeting PD-L1 and TGF-β. Sci. Transl. Med. 2018, 10, eaan5488. [Google Scholar] [CrossRef] [PubMed]
- Strauss, J.; Heery, C.R.; Schlom, J.; Madan, R.A.; Cao, L.; Kang, Z.; Lamping, E.; Marté, J.L.; Donahue, R.N.; Grenga, I.; et al. Phase I Trial of M7824 (MSB0011359C), a Bifunctional Fusion Protein Targeting PD-L1 and TGFβ, in Advanced Solid Tumors. Clin. Cancer Res. 2018, 24, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- David, J.M.; Dominguez, C.; McCampbell, K.K.; Gulley, J.L.; Schlom, J.; Palena, C. A novel bifunctional anti-PD-L1/TGF-β Trap fusion protein (M7824) efficiently reverts mesenchymalization of human lung cancer cells. Oncoimmunology 2017, 6, e1349589. [Google Scholar] [CrossRef] [PubMed]
- Barlesi, F.; Isambert, N.; Felip, E.; Cho, B.C.; Lee, D.H.; Peguero, J.; Jerusalem, G.; Penel, N.; Saada-Bouzid, E.; Garrido, P.; et al. Bintrafusp Alfa, a Bifunctional Fusion Protein Targeting TGF-β and PD-L1, in Patients With Non-Small Cell Lung Cancer Resistant or Refractory to Immune Checkpoint Inhibitors. Oncologist 2022, 28, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.; Kim, T.M.; Vicente, D.; Felip, E.; Lee, D.H.; Lee, K.H.; Lin, C.-C.; Flor, M.J.; Di Nicola, M.; Alvarez, R.M.; et al. Bintrafusp Alfa, a Bifunctional Fusion Protein Targeting TGF-β and PD-L1, in Second-Line Treatment of Patients With NSCLC: Results From an Expansion Cohort of a Phase 1 Trial. J. Thorac. Oncol. 2020, 15, 1210–1222. [Google Scholar] [CrossRef]
- Shi, M.; Chen, J.; Li, K.; Fang, Y.; Wen, G.; Li, X.; Liu, Y.; Sun, Y.; Zhu, B.; Lin, L.; et al. SHR-1701, a bifunctional fusion protein targeting PD-L1 and TGF-β, for advanced NSCLC with EGFR mutations: Data from a multicenter phase 1 study. J. Clin. Oncol. 2021, 39 (Suppl. S15), 9055. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Zhou, Q.; Pan, Y.; Yang, X.; Zhao, Y.; Han, G.; Pang, Q.; Zhang, Z.; Wang, Q.; Yao, J.; et al. LBA5 A phase II study of neoadjuvant SHR-1701 with or without chemotherapy (chemo) followed by surgery or radiotherapy (RT) in stage III unresectable NSCLC (uNSCLC). Immuno-Oncol. Technol. 2022, 16, 100361. [Google Scholar] [CrossRef]
- Patel, M.; Luke, J.; Hamilton, E.; Chmielowski, B.; Blumenschein, G.; Kindler, H.; Bahadur, S.; Santa-Maria, C.; Koucheki, J.; Sun, J.; et al. 313 A phase 1 evaluation of tebotelimab, a bispecific PD-1 x LAG-3 DART® molecule, in combination with margetuximab in patients with advanced HER2+ neoplasms. J. ImmunoTherapy Cancer 2020, 8 (Suppl. S3), A193. [Google Scholar]
- Ren, Z.; Guo, Y.; Bai, Y.; Ying, J.; Meng, Z.; Chen, Z.; Gu, S.; Zhang, J.; Liang, J.; Hou, X.; et al. Tebotelimab, a PD-1/LAG-3 bispecific antibody, in patients with advanced hepatocellular carcinoma who had failed prior targeted therapy and/or immunotherapy: An open-label, single-arm, phase 1/2 dose-escalation and expansion study. J. Clin. Oncol. 2023, 41 (Suppl. S4), 578. [Google Scholar] [CrossRef]
- Albiges, L.; Rodriguez, L.M.; Kim, S.-W.; Im, S.-A.; Carcereny, E.; Rha, S.Y.; Tran, B.; Oliveira, J.; Maroto-Rey, P.; Su, W.-C.; et al. Safety and clinical activity of MEDI5752, a PD-1/CTLA-4 bispecific checkpoint inhibitor, as monotherapy in patients (pts) with advanced renal cell carcinoma (RCC): Preliminary results from an FTIH trial. J. Clin. Oncol. 2022, 40 (Suppl. S16), 107. [Google Scholar] [CrossRef]
- Rohrberg, K.S.; Brandão, M.; Alvarez, E.C.; Felip, E.; Gort, E.H.; Hiltermann, T.J.N.; Izumi, H.; Kim, D.-W.; Kim, S.-W.; Paz-Ares, L.G.; et al. Safety, pharmacokinetics (PK), pharmacodynamics (PD) and preliminary efficacy of AZD2936, a bispecific antibody targeting PD-1 and TIGIT, in checkpoint inhibitor (CPI)-experienced advanced/metastatic non-small-cell lung cancer (NSCLC): First report of ARTEMIDE-01. J. Clin. Oncol. 2023, 41 (Suppl. S16), 9050. [Google Scholar]
- Zhou, C.; Ren, S.; Luo, Y.; Wang, L.; Xiong, A.; Su, C.; Zhang, Z.; Li, W.; Zhou, J.; Yu, X.; et al. A phase Ib/II study of AK112, a PD-1/VEGF bispecific antibody, as first- or second-line therapy for advanced non–small cell lung cancer (NSCLC). J. Clin. Oncol. 2022, 40 (Suppl. S16), 9040. [Google Scholar] [CrossRef]
- Fang, W.; Zhao, Y.; Yang, Y.; Zhou, N.; Chen, L.; Huang, Y.; Chen, J.; Zhuang, L.; Du, Y.; Zhuang, W.; et al. Phase II results of ivonescimab (AK112/ SMT112), a novel PD-1/VEGF bispecific, in combination with chemotherapy for first line treatment of advanced or metastatic non-small cell lung cancer (NSCLC) without actionable genomic alterations (AGA) in EGFR/ALK. J. Clin. Oncol. 2023, 41 (Suppl. S16), 9087. [Google Scholar]
- Zhao, Y.; Fang, W.; Yang, Y.; Chen, J.; Zhuang, L.; Du, Y.; Yu, Q.; Zhao, Y.; Zhuang, W.; Zhou, M.; et al. A phase II study of AK112 (PD-1/VEGF bispecific) in combination with chemotherapy in patients with advanced non-small cell lung cancer. J. Clin. Oncol. 2022, 40 (Suppl. S16), 9019. [Google Scholar] [CrossRef]
- Guo, Y.; Guo, J.; Cheng, Y.; Wang, Z.; Li, Y.; Lv, D.; Yin, Y.; Li, G.; Wu, L.; Huang, Y.; et al. Phase Ib/IIa safety and efficacy of PM8002, a bispecific antibody targeting PD-L1 and VEGF-A, as a monotherapy in patients with advanced solid tumors. J. Clin. Oncol. 2023, 41 (Suppl. S16), 2536. [Google Scholar] [CrossRef]
- Cheng, X.; Song, Z.; Zhang, J.; Jin, J.; Gao, S.; Liu, R.; Sun, Y.; Zhang, Y.; Gao, S.; Jia, R.; et al. Preliminary results of a phase I dose escalation study of IMM2510, a PD-L1 and VEGF bispecific fusion protein, in patients with advanced tumors. J. Clin. Oncol. 2023, 41 (Suppl. S16), 2535. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, J.; Sun, M.; Wen, Q.; Zheng, Y.; Tolcher, A.W.; Sommerhalder, D.; Wang, Z.; Li, X.; Gao, Z.; et al. Safety and efficacy of HB0025, an anti-PD-1/VEGF bispecific antibody fusion protein, in patients with advanced solid tumors: Preliminary results from an FIH trial. J. Clin. Oncol. 2023, 41 (Suppl. S16), 2589. [Google Scholar] [CrossRef]
- Gordon, M.S.; Nemunaitis, J.; Barve, M.; Wainberg, Z.A.; Hamilton, E.P.; Ramanathan, R.K.; Sledge, G.W.; Yue, H.; Morgan-Lappe, S.E.; Blaney, M.; et al. Phase I Open-Label Study Evaluating the Safety, Pharmacokinetics, and Preliminary Efficacy of Dilpacimab in Patients with Advanced Solid Tumors. Mol. Cancer Ther. 2021, 20, 1988–1995. [Google Scholar] [CrossRef]

| S No. | NCT | N | Phase | Target | Therapy | Trade Name | Indication | Approval Date | Approved by |
|---|---|---|---|---|---|---|---|---|---|
| 1 | NCT00836654 | 24 | I/II | CD3/EpCAM | Catumaxomab | Removab | Malignant Ascites | April 2009 | EMA |
| 2 | NCT01209286 | 36 | II | CD3/CD19 | Blinatumomab | Blincyto | Relapsed/Refractory B-cell ALL | December 2014 | FDA |
| 3 | NCT02609776 | 362 | I | MET/EGFR | Amivantamab | Rybrevant | EGFR Exon20ins NSCLC | May 2021 | FDA |
| 4 | NCT03070392 | 378 | II | CD3/TCR | Tebentafusp-tebn | Kimmtrak | Metastatic uveal melanoma | January 2022 | FDA |
| 5 | NCT02500407 | 238 | I/II | CD20/CD3 | Mosunetuzumab | Lunsumio | Relapsed/Refractory B-cell lymphoma | June 2022 | FDA |
| 6 | NCT04868708 | 45 | I | PD-1/CTLA-4 | Candonilimab | Recurrent/Metastatic cervical cancer | June 2022 | NMPA | |
| 7 | NCT03145181 NCT04557098 | 165 | I/II | CD3/BCMA | Teclistamab-cqyv | Tecvayli | Relapsed/Refractory Multiple Myeloma | October 2022 | FDA |
| 8 | NCT03625037 | 148 | I/II | CD3/CD20 | Epcoritamab-bysp | Epkinly | Relapsed/Refractory DLBCL | May 2023 | FDA |
| 9 | NCT03075696 | 132 | I/II | CD3/CD20 | Glofitamab-gxbm | Columvi | Relapsed/Refractory DLBCL or large B-cell lymphoma | June 2023 | FDA |
| 10 | NCT04649359 | 97 | II | CD3/BCMA | Elranatamab-bcmm | Elrexfio | Relapsed/Refractory Multiple Myeloma | August 2023 | FDA |
| S No. | NCT | Study Name | Estimated Number | Phase | Target | Therapy [Platform] | Start Date | Completion Date |
|---|---|---|---|---|---|---|---|---|
| 1 | NCT02609776 | CHRYSALIS | 780 | I | MET/EGFR | Amivantamab [Duobody] | May 2016 | January 2024 |
| 2 | NCT04606381 | PALOMA | 196 | Ib | MET/EGFR | Amivantamab | November 2020 | October 2024 |
| 3 | NCT04077463 | Chrysalis-2 | 460 | I/Ib | MET/EGFR | Amivantamab + Lazertinib | September 2019 | March 2026 |
| 4 | NCT04538664 | PAPILLON | 308 | III | MET/EGFR | Amivantamab + Carboplatin + Pemetrexed | October 2020 | January 2025 |
| 5 | NCT04487080 | MARIPOSA | 1074 | III | MET/EGFR | Amivantamab + Lazertinib | September 2020 | November 2025 |
| 6 | NCT04521179 | - | 30 | II | HER2 | KN026 [Charge Repulsion Induced Bispecific] | December 2020 | October 2023 |
| 7 | NCT02912949 | eNRGy | 250 | I/II | HER2/HER3 | Zenocutuzumab (MCLA-128) [Biclonics] | January 2015 | December 2024 |
| 8 | NCT03821233 | - | 174 | I | HER2 | ZW49 [Azymetric] | April 2019 | August 2025 |
| 9 | NCT02892123 | - | 279 | I | HER2 | ZW25 (Zanidatamab) [Azymetric] | September 2016 | August 2023 |
| 10 | NCT03261011 | - | 153 | 1a/1b | PD-1/CTLA-4 | Cadonilimab (AK104) [Tetrabody] | October 2017 | September 2020 |
| 11 | NCT04647344 | - | 60 | Ib/II | PD-1/CTLA-4 | Cadonilimab (AK104) | November 2020 | April 2023 |
| 12 | NCT04646330 | - | 114 | Ib/II | PD-1/CTLA-4 | Cadonilimab (AK104) + Anlotinib | November 2020 | December 2023 |
| 13 | NCT04544644 | - | 30 | II | PD-1/CTLA-4 | Cadonilimab (AK104) + Anlotinib | September 2020 | September 2023 |
| 14 | NCT03819465 | MAGELLAN | 258 | Ib | PD-1/CTLA-4 | MEDI5752 [Duetmab] | December 2018 | March 2026 |
| 15 | NCT03838848 | - | 120 | II | PD-1/CTLA-4 | KN046 [single-domain antibody] | May 2019 | Terminated |
| 16 | NCT04054531 | - | 50 | II | PD-1/CTLA-4 | KN046 + Platinum chemotherapy | September 2019 | June 2021 |
| 17 | NCT04474119 | ENREACH-L-01 | 482 | III | PD-1/CTLA-4 | KN046 + Paclitaxel + Carboplatin | September 2020 | August 2023 |
| 18 | NCT04900363 | - | 108 | Ib/II | PD-1/VEGF | AK112 [Tetrabody] | May 2021 | May 2024 |
| 19 | NCT04995523 | ARTEMIDE-01 | 192 | I/II | PD-1/TIGIT | AZD2936 | September 2021 | July 2025 |
| 20 | NCT04931654 | - | 81 | I/IIa | PD-1/TIM-3 | AZD7789 | September 2021 | July 2025 |
| 21 | NCT02324257 | - | 149 | I | CD3/CEA | RO6958688 [CrossMab] | December 2014 | September 2019 |
| 22 | NCT02650713 | - | 228 | Ib | CD3/CEA | RO6958688 + Atezolizumab | January 2016 | January 2020 |
| 23 | NCT03337698 | Morpheus Lung | 435 | Ib/II | CD3/CEA | RO6958688 | January 2018 | August 2025 |
| 24 | NCT01221675 | - | 18 | I/II | HSG/CEA | TF2 (IMP288) | June 2011 | April 2016 |
| 25 | NCT04822298 | - | 3 | I | CD3/PSMA | Acapatamab (AMG160) [BiTE] | August 2021 | January 2022 |
| 26 | NCT04496674 | - | 86 | I | CD3/PSMA | CC-1 | February 2022 | September 2025 |
| 27 | NCT04750239 | - | 3 | I/II | CD3/GD2 | Nivatrotamab | August2021 | April 2022 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khosla, A.A.; Jatwani, K.; Singh, R.; Reddy, A.; Jaiyesimi, I.; Desai, A. Bispecific Antibodies in Lung Cancer: A State-of-the-Art Review. Pharmaceuticals 2023, 16, 1461. https://doi.org/10.3390/ph16101461
Khosla AA, Jatwani K, Singh R, Reddy A, Jaiyesimi I, Desai A. Bispecific Antibodies in Lung Cancer: A State-of-the-Art Review. Pharmaceuticals. 2023; 16(10):1461. https://doi.org/10.3390/ph16101461
Chicago/Turabian StyleKhosla, Atulya Aman, Karan Jatwani, Rohit Singh, Aswanth Reddy, Ishmael Jaiyesimi, and Aakash Desai. 2023. "Bispecific Antibodies in Lung Cancer: A State-of-the-Art Review" Pharmaceuticals 16, no. 10: 1461. https://doi.org/10.3390/ph16101461
APA StyleKhosla, A. A., Jatwani, K., Singh, R., Reddy, A., Jaiyesimi, I., & Desai, A. (2023). Bispecific Antibodies in Lung Cancer: A State-of-the-Art Review. Pharmaceuticals, 16(10), 1461. https://doi.org/10.3390/ph16101461







