Gallic Acid Treats Hypertrophic Scar in Rabbit Ears via the TGF-β/Smad and TRPC3 Signaling Pathways
Abstract
:1. Introduction
2. Results
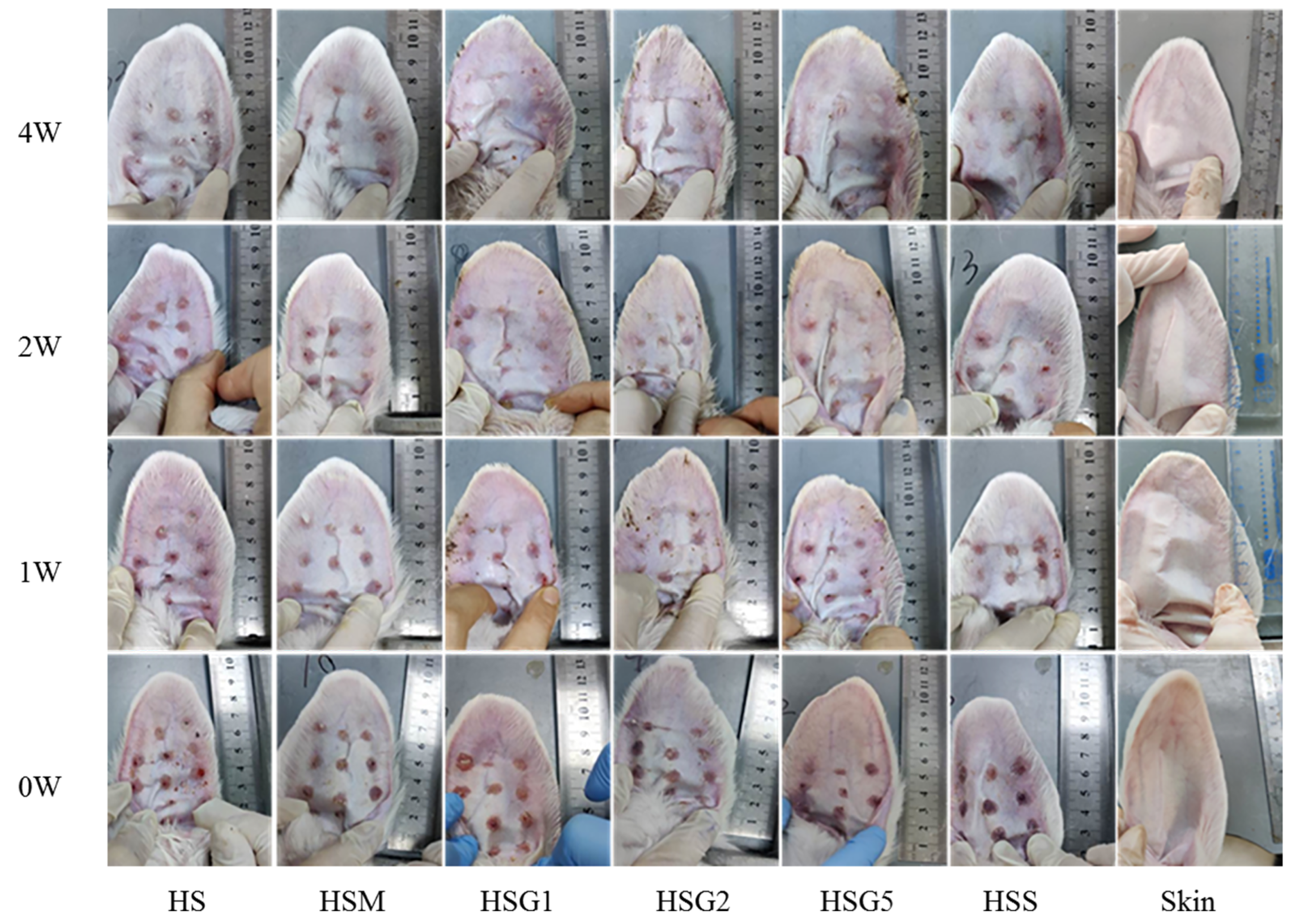
2.1. Effect of GA Treatment on HS
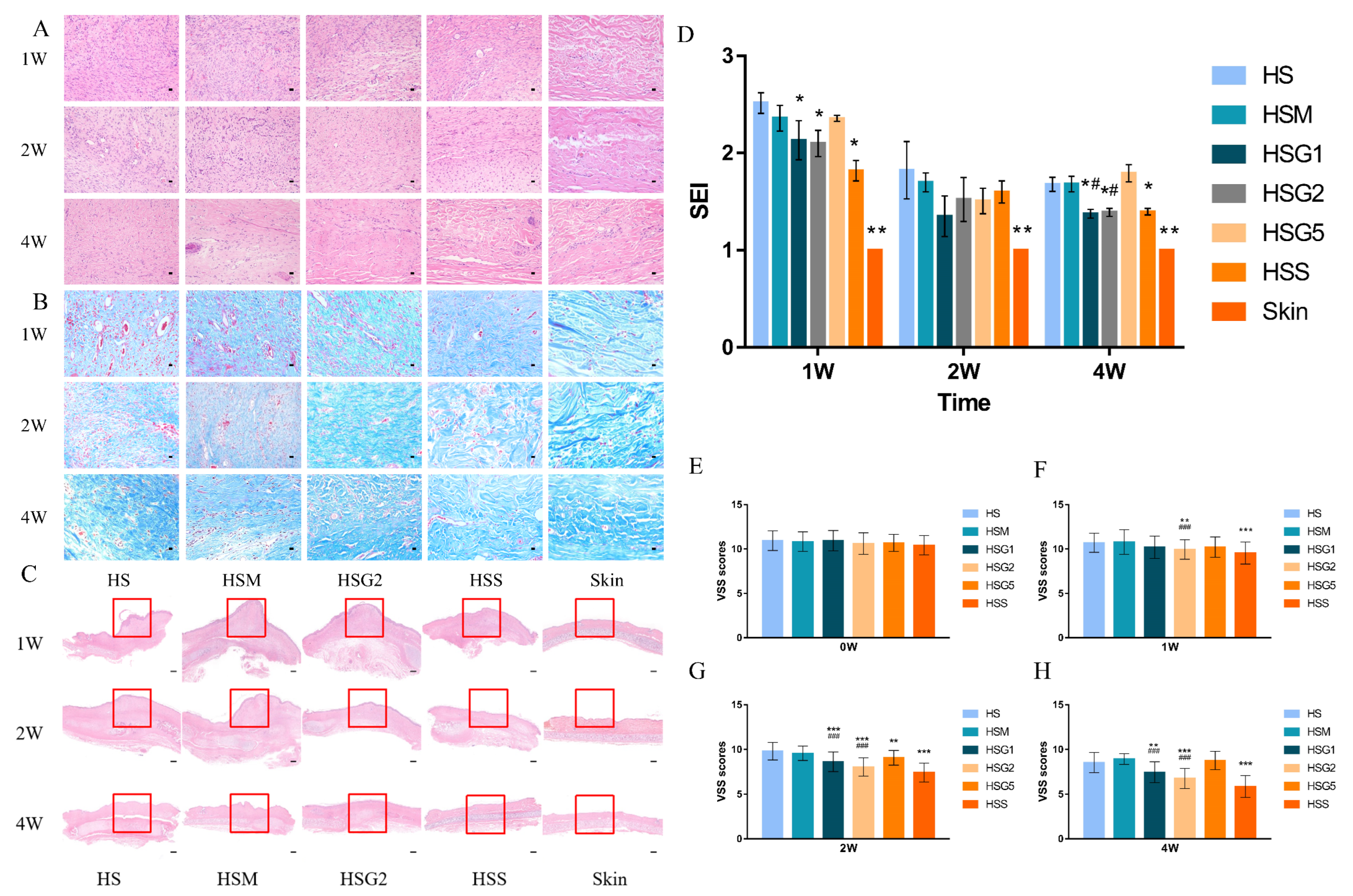
2.2. Effect of GA on the Histological Characteristics of HS
2.3. GA Reduces the SEI Index of HS
2.4. Total VSS Score Shows the Effect of GA on HS
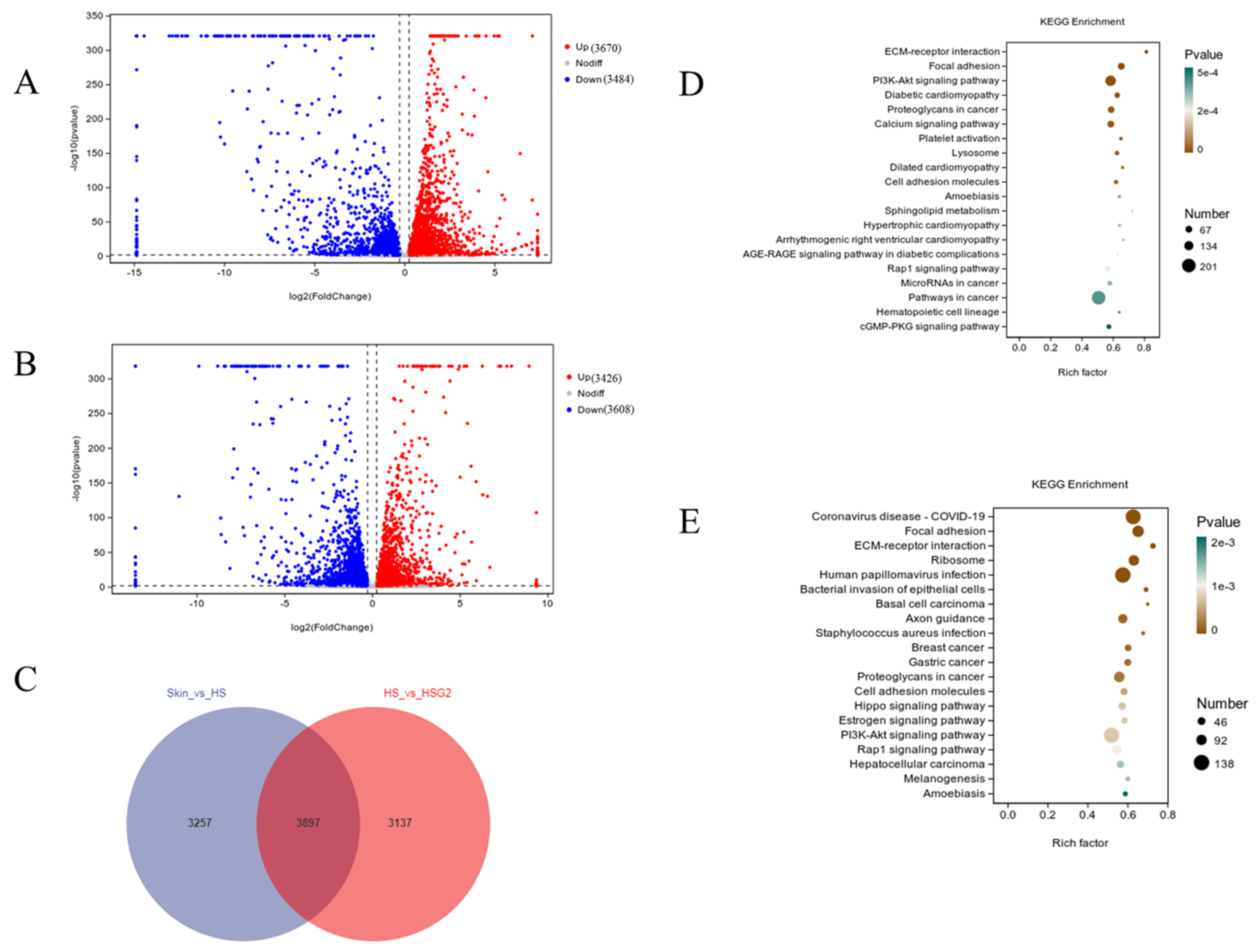
2.5. RNA-Seq Analysis Results
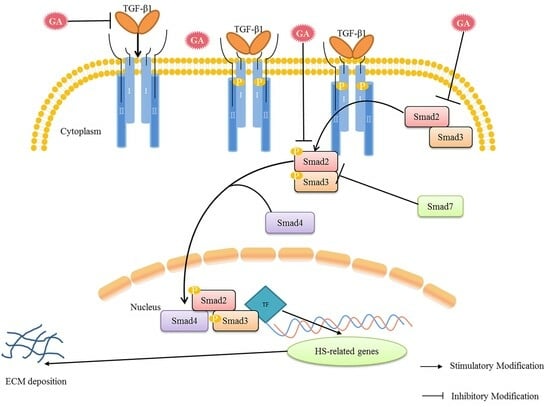
2.6. Effect of GA on the mRNA Expression of Smad2, Smad3, TGF-β1, and TRPC3 in HS
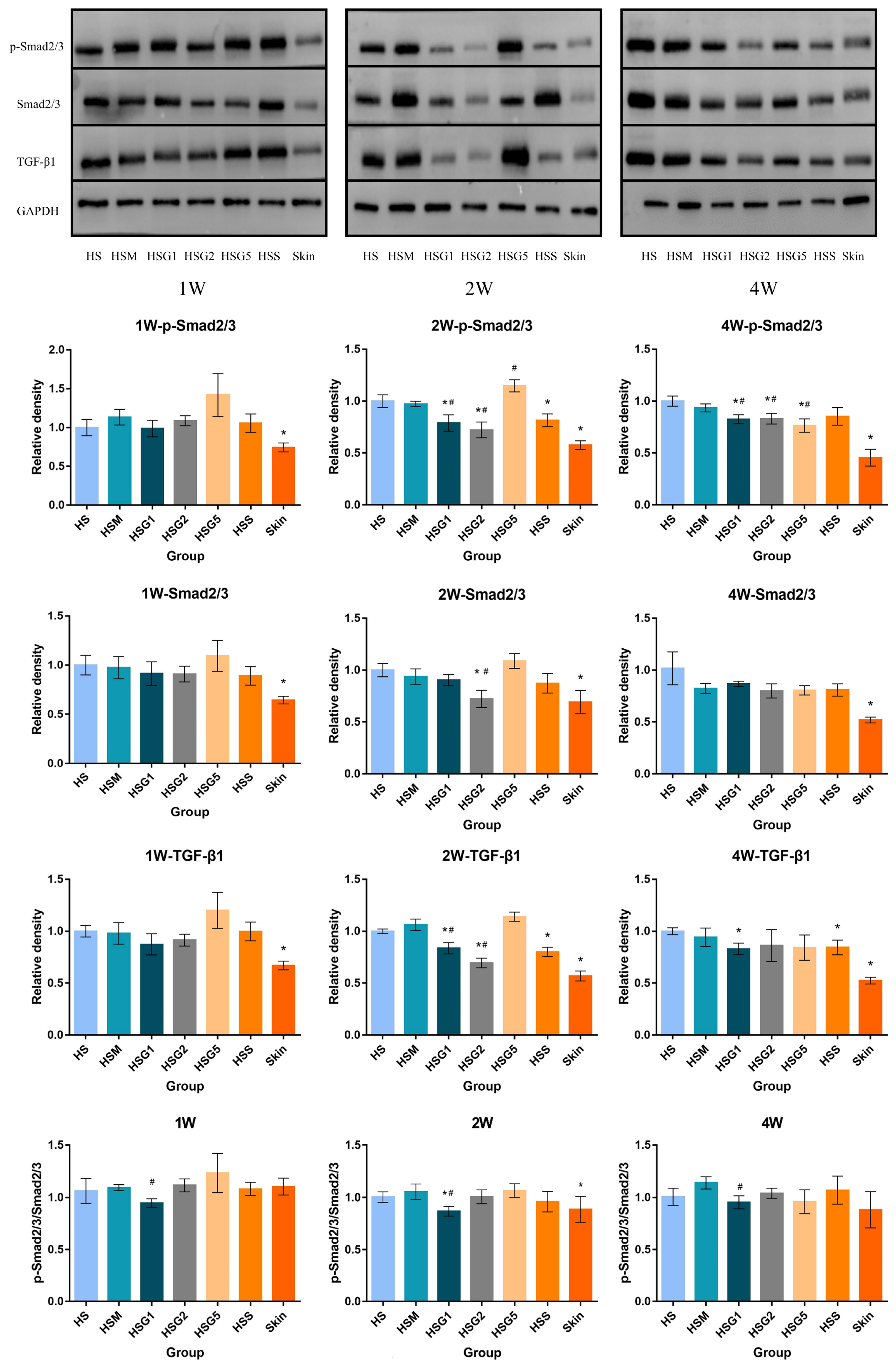
2.7. Effect of GA on p-Smad2/3, Smad2/3 and TGF-β1 Protein Expression in HS
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals
4.3. Preparation of Rabbit Ear HS Models
4.4. Experimental Design and Treatment
4.5. Histological Examination
4.6. Scar Elevation Index and Vancouver Scar Scale Score Evaluation
4.7. RNA Sequencing Analysis
4.8. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
4.9. Western Blot
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, X.; Liu, K.; Ruan, M.; Yang, J.; Gao, Z. Gallic acid inhibits fibroblast growth and migration in keloids through the AKT/ERK signaling pathway. Acta Biochim. Biophys. Sin. 2018, 50, 1114–1120. [Google Scholar] [CrossRef] [PubMed]
- Mousavizadeh, S.M.; Torbati, P.M.; Daryani, A. The effects of kiwifruit dressing on hypertrophic scars in a rabbit ear model. J. Wound Care 2021, 30, XVi–XVvii. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, H.Y.; Wang, D.L.; Zhang, X.D. Effect of pressure therapy for treatment of hypertrophic scar. Medicine 2019, 98, e16263. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Zhou, J.; Huang, L.; Fu, Q.; Ao, M.; Yuan, L.; Luo, G. Hypertrophic scar improvement by early intervention with ablative fractional carbon dioxide laser treatment. Lasers Surg. Med. 2021, 53, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Tunca, M.; Gamsızkan, M.; Yürekli, A.; Göksel, B.A.; Çiçek, A.; Çalışkan, E. Cryosurgery to remove perichondrium for the rabbit ear hypertrophic scar model: A simplified method. Acta Dermatovenerol. Alp. Pannonica Adriat. 2019, 28, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Bi, M.; Sun, P.; Li, D.; Dong, Z.; Chen, Z. Intralesional injection of botulinum toxin type a compared with intralesional injection of corticosteroid for the treatment of hypertrophic scar and keloid: A systematic review and Meta-Analysis. Med. Sci. Monit. 2019, 25, 2950–2958. [Google Scholar] [CrossRef]
- Elsaie, M.L. Update on management of keloid and hypertrophic scars: A systemic review. J. Cosmet. Dermatol. 2021, 20, 2729–2738. [Google Scholar] [CrossRef]
- Chen, D.; Li, Q.; Zhang, H.; Kou, F.; Li, Q.; Lyu, C.; Wei, H. Traditional Chinese medicine for hypertrophic scars-A review of the therapeutic methods and potential effects. Front. Pharmacol. 2022, 13, 1025602. [Google Scholar] [CrossRef]
- Ru, Z.; Hu, Y.; Huang, S.; Bai, L.; Zhang, K.; Li, Y. Bioflavonoid galangin suppresses hypertrophic scar formation by the TGF-beta/Smad signaling pathway. Evid. Based Complement. Alternat. Med. 2021, 2021, 2444839. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, H.L.; Lee, M.H.; You, K.E.; Kwon, B.J.; Seo, H.J.; Park, J.C. Asiaticoside enhances normal human skin cell migration, attachment and growth in vitro wound healing model. Phytomedicine 2012, 19, 1223–1227. [Google Scholar] [CrossRef]
- Li, W.; Wang, D.; Li, M.; Li, B. Emodin inhibits the proliferation of papillary thyroid carcinoma by activating AMPK. Exp. Ther. Med. 2021, 22, 1075. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Zhang, Y.; Tang, C.; Hou, Y.; Ai, X.; Chen, X.; Zhang, Y.; Wang, X.; Meng, X. Gallic acid: Pharmacological activities and molecular mechanisms involved in inflammation-related diseases. Biomed. Pharmacother. 2021, 133, 110985. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.J.; Moh, S.H.; Son, D.H.; You, S.; Kinyua, A.W.; Ko, C.M.; Song, M.; Yeo, J.; Choi, Y.H.; Kim, K.W. Gallic acid promotes wound healing in normal and hyperglucidic conditions. Molecules 2016, 21, 899–912. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Sakaguchi, N.; Isuzugawa, K.; Tani, H.; Ogihara, Y. Role of reactive oxygen species in gallic acid-induced apoptosis. Biol. Pharm. Bull. 2000, 23, 1153–1157. [Google Scholar] [CrossRef]
- Chen, C.Y.; Chen, K.C.; Yang, T.Y.; Liu, H.C.; Hsu, S.L. Gallic acid induces a reactive oxygen Species-Provoked c-Jun NH2-Terminal Kinase-Dependent apoptosis in lung fibroblasts. Evid. Based Complement. Alternat. Med. 2013, 2013, 613950. [Google Scholar] [CrossRef]
- Phan, T.T.; Sun, L.; Bay, B.H.; Chan, S.Y.; Lee, S.T. Dietary compounds inhibit proliferation and contraction of keloid and hypertrophic scar-derived fibroblasts in vitro: Therapeutic implication for excessive scarring. J. Trauma 2003, 54, 1212–1224. [Google Scholar] [CrossRef]
- Li, N.; Kong, M.; Ma, T.; Gao, W.; Ma, S. Uighur medicine abnormal savda munzip (ASMq) suppresses expression of collagen and TGF-beta1 with concomitant induce Smad7 in human hypertrophic scar fibroblasts. Int. J. Clin. Exp. Med. 2015, 8, 8551–8560. [Google Scholar]
- He, T.; Bai, X.; Yang, L.; Fan, L.; Li, Y.; Su, L.; Gao, J.; Han, S.; Hu, D. Loureirin b inhibits hypertrophic scar formation via inhibition of the TGF-beta1-ERK/JNK pathway. Cell. Physiol. Biochem. 2015, 37, 666–676. [Google Scholar] [CrossRef]
- Varga, J.; Abraham, D. Systemic sclerosis: A prototypic multisystem fibrotic disorder. J. Clin. Investig. 2007, 117, 557–567. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, X.F.; Wang, Z.C.; Lou, D.; Fang, Q.Q.; Hu, Y.Y.; Zhao, W.Y.; Zhang, L.Y.; Wu, L.H.; Tan, W.Q. Current potential therapeutic strategies targeting the TGF-beta/Smad signaling pathway to attenuate keloid and hypertrophic scar formation. Biomed. Pharmacother. 2020, 129, 110287. [Google Scholar] [CrossRef]
- Tredget, E.E.; Yang, L.; Delehanty, M.; Shankowsky, H.; Scott, P.G. Polarized Th2 cytokine production in patients with hypertrophic scar following thermal injury. J. Interferon Cytokine Res. 2006, 26, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Qing, C.; Wang, Z.; Song, F.; Wang, X. Dynamic biological changes in fibroblasts during hypertrophic scar formation and regression. Int. Wound J. 2016, 13, 257–262. [Google Scholar]
- Li, Y.; Yu, Z.; Zhao, D.; Han, D. Corilagin alleviates hypertrophic scars via inhibiting the transforming growth factor (TGF)-beta/Smad signal pathway. Life Sci. 2021, 277, 119483. [Google Scholar] [CrossRef]
- Khayyat, N.H.; Tomilin, V.N.; Zaika, O.; Pochynyuk, O. Polymodal roles of TRPC3 channel in the kidney. Channels 2020, 14, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.C.; Zheng, S.Y.; Zhang, Z.G.; Luo, J.H.; Zhu, Z.L.; Li, L.; Chen, L.S.; Lin, X.; Sham, J.; Lin, M.J.; et al. TRPC3 promotes tumorigenesis of gastric cancer via the CNB2/GSK3beta/NFATc2 signaling pathway. Cancer Lett. 2021, 519, 211–225. [Google Scholar] [CrossRef] [PubMed]
- Shimauchi, T.; Numaga-Tomita, T.; Ito, T.; Nishimura, A.; Matsukane, R.; Oda, S.; Hoka, S.; Ide, T.; Koitabashi, N.; Uchida, K. TRPC3-Nox2 complex mediates doxorubicin-induced myocardial atrophy. JCI Insight 2017, 2, e93358. [Google Scholar] [CrossRef]
- Ishise, H.; Larson, B.; Hirata, Y.; Fujiwara, T.; Nishimoto, S.; Kubo, T.; Matsuda, K.; Kanazawa, S.; Sotsuka, Y.; Fujita, K.; et al. Hypertrophic scar contracture is mediated by the TRPC3 mechanical force transducer via NFkB activation. Sci. Rep. 2015, 5, 11620. [Google Scholar] [CrossRef]
- Ogawa, R. The most current algorithms for the treatment and prevention of hypertrophic scars and keloids: A 2020 update of the algorithms published 10 years ago. Plast. Reconstr. Surg. 2022, 149, 79. [Google Scholar] [CrossRef]
- Wei, J.; Wang, Z.; Zhong, C.; Ding, H.; Wang, X.; Lu, S. LncRNA MIR503HG promotes hypertrophic scar progression via miR-143-3p-mediated Smad3 expression. Wound Repair. Regen. 2021, 29, 792–800. [Google Scholar] [CrossRef]
- Sen, C.K.; Gordillo, G.M.; Roy, S.; Kirsner, R.; Lambert, L.; Hunt, T.K.; Gottrup, F.; Gurtner, G.C.; Longaker, M.T. Human skin wounds: A major and snowballing threat to public health and the economy. Wound Repair. Regen. 2009, 17, 763–771. [Google Scholar] [CrossRef]
- Shinno, E.; Shimoji, M.; Imaizumi, N.; Kinoshita, S.; Sunakawa, H.; Aniya, Y. Activation of rat liver microsomal glutathione S-transferase by gallic acid. Life Sci. 2005, 19, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.K.; Kang, N.J.; Jang, Y.J.; Lee, K.W.; Lee, H.J. Gallic acid induces neuronal cell death through activation of c-Jun N-terminal kinase and downregulation of Bcl-2. Ann. N. Y. Acad. Sci. 2009, 1171, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Anti-inflammatory Agents: Present and Future. Cell 2010, 140, 935–950. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Jun, C.D.; Suk, K.; Choi, B.J.; Lim, H.; Park, S.; Lee, S.H.; Shin, H.Y.; Kim, D.K.; Shin, T.Y. Gallic acid inhibits histamine release and pro-inflammatory cytokine production in mast cells. Toxicol. Sci. 2006, 1, 123–131. [Google Scholar] [CrossRef]
- Verma, S.; Singh, A.; Mishra, A. Gallic acid: Molecular rival of cancer. Environ. Toxicol. Pharmacol. 2013, 3, 473–485. [Google Scholar] [CrossRef]
- López-Lázaro, M.; Calderón-Montaño, J.M.; Burgos-Morón, E.; Austin, C.A. Green tea constituents (-)-epigallocatechin-3-gallate (EGCG) and gallic acid induce topoisomerase I- and topoisomerase II-DNA complexes in cells mediated by pyrogallol-induced hydrogen peroxide. Mutagenesis 2011, 4, 489–498. [Google Scholar] [CrossRef]
- Hsieh, S.C.; Wu, C.C.; Hsu, S.L.; Feng, C.H.; Yen, J.H. Gallic acid attenuates TGF-β1-stimulated collagen gel contraction via suppression of RhoA/Rho-kinase pathway in hypertrophic scar fibroblasts. Life Sci. 2016, 161, 19–26. [Google Scholar] [CrossRef]
- Ogawa, R.; Akaishi, S.; Kuribayashi, S.; Miyashita, T. Keloids and hypertrophic scars can now be cured completely: Recent progress in our understanding of the pathogenesis of keloids and hypertrophic scars and the most promising current therapeutic strategy. J. Nippon Med. Sch. 2016, 83, 46–53. [Google Scholar] [CrossRef]
- Zhai, X.X.; Tang, Z.M.; Ding, J.C.; Lu, X.L. Expression of TGF-beta1/mTOR signaling pathway in pathological scar fibroblasts. Mol. Med. Rep. 2017, 15, 3467–3472. [Google Scholar] [CrossRef]
- Cui, J.; Li, Z.; Jin, C.; Jin, Z. Knockdown of fibronectin extra domain B suppresses TGF-β1-mediated cell proliferation and collagen deposition in keloid fibroblasts via AKT/ERK signaling pathway. Biochem. Bioph. Res. Commun. 2020, 526, 1131–1137. [Google Scholar] [CrossRef]
- Fan, C.; Dong, Y.; Xie, Y.; Su, Y.; Zhang, X.; Leavesley, D.; Upton, Z. Shikonin reduces TGF-β1-induced collagen production and contraction in hypertrophic scar-derived human skin fibroblasts. Int. J. Mol. Med. 2015, 36, 985–991. [Google Scholar] [CrossRef] [PubMed]
- Tzavlaki, K.; Moustakas, A. TGF-beta signaling. Biomolecules 2020, 10, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Armour, A.; Scott, P.G.; Tredget, E.E. Cellular and molecular pathology of HTS: Basis for treatment. Wound Repair Regen. 2007, 15 (Suppl. 1), S6–S17. [Google Scholar] [CrossRef]
- Wu, C.; Jiang, J.; Boye, A.; Jiang, Y.; Yang, Y. Compound Astragalus and Salvia miltiorrhiza extract suppresses rabbits’ hypertrophic scar by modulating the TGF-beta/Smad signal. Dermatology 2014, 229, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.H.; Chen, D.Q.; Wang, Y.N.; Feng, Y.L.; Cao, G.; Vaziri, N.D.; Zhao, Y.Y. New insights into TGF-beta/Smad signaling in tissue fibrosis. Chem. Biol. Interact. 2018, 292, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, S.C.; Wu, C.C.; Hsu, S.L.; Yen, J.H. Molecular mechanisms of gallic acid-induced growth inhibition, apoptosis, and necrosis in hypertrophic scar fibroblasts. Life Sci. 2017, 179, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, N.N.; Li, H.Y.; Jiang, M.; Gao, J.; Bai, G. Active ingredients in rhubarb with anti-proliferative effects on scar fibroblasts. Acta Pharm. Sin. 2012, 47, 1618–1622. [Google Scholar]
- Feng, F.; Liu, M.; Pan, L.; Wu, J.; Wang, C.; Yang, L.; Liu, W.; Xu, W.; Lei, M. Biomechanical regulatory factors and therapeutic targets in keloid fibrosis. Front. Pharmacol. 2022, 13, 906212. [Google Scholar] [CrossRef]
- Wang, D.; Stockard, C.R.; Harkins, L.; Lott, P.; Salih, C.; Yuan, K. Immunohistochemistry in the evaluation of neovascularization in tumor xenografts. Biotech. Histochem. 2008, 83, 179–189. [Google Scholar] [CrossRef]
- Rakocevic, J.; Orlic, D.; Mitrovic-Ajtic, O.; Tomasevic, M. Endothelial cell markers from clinician’s perspective. Exp. Mol. Pathol. 2017, 102, 303–313. [Google Scholar] [CrossRef]
- Shen, Y.; Jin, R.; Liang, X.; Deng, Z.; He, J.; Ding, Y.; Ding, F. Angiogenesis modulation-mediated inhibitory effects of tacrolimus on hypertrophic scar formation. Microvasc. Res. 2023, 145, 104–126. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Liu, P.; Xiong, R.P.; Chen, X.Y.; Zhao, Y.; Lu, W.P. Ski, a modulator of wound healing and scar formation in the rat skin and rabbit ear. J. Pathol. 2011, 223, 659–671. [Google Scholar] [CrossRef] [PubMed]
- Tark, K.C.; Lee, D.W.; Lew, D.H.; Kang, E.H.; Roh, H.; Lee, M.C. Effects of ginsenoside Rb1 on hypertrophic scar remodeling in rabbit model. Eur. J. Pharmacol. 2015, 750, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhou, X.; Xia, L.; Liu, W.; Guo, F.; Liu, J.; Liu, W. Inhibition of hypertrophic scar formation with oral asiaticoside treatment in a rabbit ear scar model. Int. Wound J. 2021, 18, 598–607. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Lyu, C.; Chen, D.; Cai, W.; Kou, F.; Li, Q.; Wei, H.; Zhang, H. Gallic Acid Treats Hypertrophic Scar in Rabbit Ears via the TGF-β/Smad and TRPC3 Signaling Pathways. Pharmaceuticals 2023, 16, 1514. https://doi.org/10.3390/ph16111514
Li Q, Lyu C, Chen D, Cai W, Kou F, Li Q, Wei H, Zhang H. Gallic Acid Treats Hypertrophic Scar in Rabbit Ears via the TGF-β/Smad and TRPC3 Signaling Pathways. Pharmaceuticals. 2023; 16(11):1514. https://doi.org/10.3390/ph16111514
Chicago/Turabian StyleLi, Qiannan, Chunming Lyu, Daqin Chen, Wanling Cai, Fang Kou, Qiang Li, Hai Wei, and Huimin Zhang. 2023. "Gallic Acid Treats Hypertrophic Scar in Rabbit Ears via the TGF-β/Smad and TRPC3 Signaling Pathways" Pharmaceuticals 16, no. 11: 1514. https://doi.org/10.3390/ph16111514
APA StyleLi, Q., Lyu, C., Chen, D., Cai, W., Kou, F., Li, Q., Wei, H., & Zhang, H. (2023). Gallic Acid Treats Hypertrophic Scar in Rabbit Ears via the TGF-β/Smad and TRPC3 Signaling Pathways. Pharmaceuticals, 16(11), 1514. https://doi.org/10.3390/ph16111514